Published online Jan 7, 2021. doi: 10.3748/wjg.v27.i1.92
Peer-review started: October 16, 2020
First decision: November 23, 2020
Revised: December 2, 2020
Accepted: December 11, 2020
Article in press: December 11, 2020
Published online: January 7, 2021
Processing time: 75 Days and 9 Hours
Infantile-onset inflammatory bowel disease (IO-IBD) occurs in very young children and causes severe clinical manifestations, which has poor responses to traditional inflammatory bowel disease (IBD) treatments. At present, there are no simple and reliable laboratory indicators for early screening IO-IBD patients, especially those in whom the disease is caused by monogenic diseases.
To search for valuable indicators for early identifying IO-IBD patients, especially those in whom the disease is caused by monogenic diseases.
A retrospective analysis was performed in 73 patients with IO-IBD admitted to our hospital in the past 5 years. Based on the next-generation sequencing results, they were divided into a monogenic IBD group (M-IBD) and a non-monogenic IBD group (NM-IBD). Forty age-matched patients with allergic proctocolitis (AP) were included in a control group. The clinical manifestations and the inflammatory factors in peripheral blood were evaluated. Logistic regression analysis and receiver operating characteristic (ROC) curve analysis were used to identify the screening factors and cut-off values of IO-IBD as well as monogenic IO-IBD, respectively.
Among the 44 M-IBD patients, 35 carried IL-10RA mutations, and the most common mutations were c.301C>T (p.R101W, 30/70) and the c.537G>A (p.T179T, 17/70). Patients with higher serum tumor necrosis factor (TNF)-α value were more likely to have IBD [odds ratio (OR) = 1.25, 95% confidence interval (CI): 1.05-1.50, P = 0.013], while higher serum albumin level was associated with lower risk of IBD (OR = 0.86, 95%CI: 0.74-1.00, P = 0.048). The cut-off values of TNF-α and albumin were 17.40 pg/mL (sensitivity: 0.78; specificity: 0.88) and 36.50 g/L (sensitivity: 0.80; specificity: 0.90), respectively. The increased ferritin level was indicative of a genetic mutation in IO-IBD patients. Its cut-off value was 28.20 ng/mL (sensitivity: 0.93; specificity: 0.92). When interleukin (IL)-10 level was higher than 33.05 pg/mL (sensitivity: 1.00; specificity: 0.84), or the onset age was earlier than 0.21 mo (sensitivity: 0.82; specificity: 0.94), the presence of disease-causing mutations in IL-10RA in IO-IBD patients was strongly suggested.
Serum TNF-α and albumin level could differentiate IO-IBD patients from allergic proctocolitis patients, and serum ferritin and IL-10 levels are useful indicators for early diagnosing monogenic IO-IBD.
Core Tip: It is very important to identify infantile-onset inflammatory bowel disease (IO-IBD) patients, especially those in whom the disease is caused by monogenic diseases, as early as possible because these patients have poor responses to traditional inflammatory bowel disease treatments. However, there are no simple and reliable laboratory indicators for early differential diagnosis. This is the first study focusing on the laboratory indicators that could be used to distinguish O-IBD patients from allergic enteritis patients and screening IO-IBD patients with monogenic diseases. We believe that these results may be valuable in the initial investigations and diagnosis of IO-IBD.
- Citation: Su W, Yu Y, Xu X, Wang XQ, Huang JB, Xu CD, Xiao Y. Valuable clinical indicators for identifying infantile-onset inflammatory bowel disease patients with monogenic diseases. World J Gastroenterol 2021; 27(1): 92-106
- URL: https://www.wjgnet.com/1007-9327/full/v27/i1/92.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i1.92
Inflammatory bowel disease (IBD) is a chronic recurrent gastrointestinal inflammatory disease, which could be classified into Crohn's disease (CD), ulcerative colitis (UC), and IBD-unclassified (IBD-U)[1]. Common clinical manifestations include diarrhea, hematochezia, abdominal pain, growth retardation, and weight loss. Many studies have reported that the phenotypic characteristics are different in very early onset-IBD (VEO-IBD, the onset age was younger than 6 years old) children from those in adolescent-onset or adult-onset IBD patients[2]. An increasing number of studies[3] have indicated the presence of monogenic defects in VEO-IBD children, especially in infantile patients (< 2 years old). Gene sequencing, especially next generation-sequencing (NGS), could help to find the mutations to explain the cause of the disease; however, this method has high costs, and is time-consuming and not suitable for regular application at the early stage of the disease, particularly in economically underdeveloped areas. Besides, many variants of uncertain significance (VUS) after NGS was applied need laboratory indicators to verify their pathogenicity. However, there have been no reliable clinical indicators reported to identify pediatric IBD patients early, especially those who have potential gene mutations. In this retrospective study, we intended to search for early diagnostic indicators for IO-IBD children with or without the gene mutation, in order to shorten the diagnosis time, reduce medical costs, simplify the analysis of NGS, and administer targeted intervention as early as possible.
This study was approved by the ethics committee of Ruijin Hospital, Shanghai Jiaotong University School of Medicine. All guardians of the enrolled pediatric patients who experienced the gene test signed an informed consent form. This study retrospectively analyzed 73 IO-IBD patients with a disease onset before 2 years of age who were admitted to the Department of Pediatrics, Ruijin Hospital, Shanghai Jiaotong University School of Medicine from January 2014 to February 2019. The diagnostic criteria were based on the Consensus on the Diagnosis and Management of Pediatric Inflammatory Bowel Disease[4] formulated by the Chinese Society of Pediatric Gastroenterology, the Chinese Medical Association. According to the NGS results, the patients were divided into either a monogenic IBD (M-IBD) group, which comprised IO-IBD patients caused by Mendelian diseases, or a non-monogenic IBD (NM-IBD) group, which comprised IO-IBD patients without disease-causing gene mutations. Forty age-matched children who were hospitalized due to diarrhea and hematochezia during the same period and ultimately diagnosed with allergic proctocolitis (AP) were enrolled in the study as a control group.
The clinical data of all patients were collected, including sex, age of onset, body weight and height on admission, average daily frequency of diarrhea, hematochezia, perianal lesions, recurrent fever, and treatment outcomes (remission, non-remission, and death). The results of complete blood count, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), serum albumin, and serum iron levels were collected and analyzed. Tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-10 levels were determined using chemiluminescence immunoassays with a commercial kit (Siemens). The serum ferritin concentration was detected using a double-site enzyme immunoassay with a commercial ferritin kit (ACCES).
Peripheral blood was collected from all IO-IBD patients for the genetic test. A FlexiGene DNA Kit (Qiagen GmbH, D-40724 Hilden) was used to extract DNA according to the manufacturer’s instructions. NGS of targeted genes including primary immunodeficiency diseases and congenital diarrheal diseases (20 cases) was performed by Beijing Mygeno Gene Technology Co., Ltd and whole-exome sequencing (53 cases) was performed by Beijing Berry Genomics Co., Ltd. For the mutations found via NGS, Sanger sequencing was used to retest the corresponding gene sequences of the patients and their parents to verify and confirm the genetic origin. The genetic variations were identified using online databases, such as the Single Nucleotide Polymorphism Database (dbSNP), Clinvar, the Human Gene Mutation Database (HGMD), the 1000 Genomes Project, and Online Mendelian Inheritance in Man (OMIM), to verify whether they were the known pathogenic mutations. For novel mutations not included in the databases, pathogenicity was further evaluated according to the American College of Medical Genetics and Genomics (ACMG) guidelines[5].
Z scores for height and weight were calculated using WHO Anthro V3.2.2 software. All data were statistically analyzed using IBM SPSS Statistics 25.0. Measurement data with a normal distribution are expressed as the mean ± standard deviation (SD), and nonnormally distributed data are expressed as the median and interquartile range (IQR). Data with a normal distribution and homogeneity of variance were analyzed using ANOVA analysis, and abnormally distributed data were analyzed by nonparametric Kruskal-Wallis analysis. Categorical data were analyzed using the chi-square test, or Fisher's exact Chi-square test. Multivariate binary logistic regression analysis was performed to identify risk factors for IO-IBD and monogenic IO-IBD. Receiver operating characteristic (ROC) curve analysis was performed to assess the diagnostic value of the identified indicators. P values were adjusted using the Bonferroni method for pairwise comparison. P < 0.05 was considered statistically significant. The statistical methods of this study were reviewed by Li J from the Clinical Research Center, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine.
Among the 73 IO-IBD patients, 24 did not carry any disease-causing mutations in IBD-associated genes, and 49 had the gene mutations related to the disease. Among 49 patients with mutations, 39 carried IL-10RA mutations, 2 carried CYBB mutations, and WAS, IKBKG, SLC37A4, CD40LG, LIG4, CARD11, PIK3CD, and CXCR4 mutations were observed in one patient each (Table 1). Four of them carried the IL-10RA heterozygous mutation which did not meet the criteria of Mendelian disease, and one of them had a heterozygous CXCR4 mutation which was inherited from his father with ankylosing spondylitis and recognized as a VUS according to the ACMG guidelines because of absence of typical manifestations of autosome dominant WHIM syndrome. Therefore, these five patients were included in the NM-IBD group. There were 14 different missense mutations found in IL-10RA among M-IBD patients. Two variations, the c.301C>T (p.R101W) and the c.537G>A (p.T179T), were considered hotspot mutations in the M-IBD patient cohort, the mutation frequencies were 42.86% (30/70) and 24.29% (17/70) respectively. We also found nine novel mutations: c.109G>T, c.302G>A, c.569T>G, and c.787C>T in IL-10RA gene; c.674+2T>C in CYBB gene; c.267delC in CD40LG gene; c.1144_1145delCT in LIG4 gene; c.155T>C in CARD11 gene; and c.1001G>A in CXCR4 gene. The amino acid substitutions and pathogenicity of these variations that were assessed according to the ACMG guidelines are summarized in Table 1.
| Patient | Gene | Variant (allele 1) | Amino acid | ACMG (P/LP) | Variant (allele 2) | Amino acid | ACMG (P/LP) |
| 1 | IL10RA | c.301C>T | R101W | P (PS1+PS3+PM1+PM2+PP3+PP4) | c.301C>T | R101W | P (PS1+PS3+PM1+PM2+PP3+PP4) |
| 2 | IL10RA | c.299T>G | V100G | P (PS1+PS3+PM2+PP3+PP4) | c.301C>T | R101W | P (PS1+PS3+PM1+PM2+PP3+PP4) |
| 3 | IL10RA | c.191A>G | Y64C | LP (PM1+PM2+PM3+PP3+PP4) | c.537G>A | T179T | P (PS1+PS3+PM2+PP3+PP4) |
| 4 | IL10RA | c.301C>T | R101W | P (PS1+PS3+PM1+PM2+PP3+PP4) | c.537G>A | T179T | P (PS1+PS3+PM2+PP3+PP4) |
| 5 | IL10RA | c.301C>T | R101W | P (PS1+PS3+PM1+PM2+PP3+PP4) | c.301C>T | R101W | P (PS1+PS3+PM1+PM2+PP3+PP4) |
| 6 | IL10RA | c.493C>T | R165X | P (PVS1+PS1+PM2+PP4) | c.301C>T | R101W | P (PS1+PS3+PM1+PM2+PP3+PP4) |
| 7 | IL10RA | c.301C>T | R101W | P (PS1+PS3+PM1+PM2+PP3+PP4) | c.537G>A | T179T | P (PS1+PS3+PM2+PP3+PP4) |
| 8 | IL10RA | c.350G>A | R117H | P (PS1+PM1+PM2+PP3+PP4) | c.493C>T | R165X | P (PVS1+PS1+PM2+PP4) |
| 9 | IL10RA | c.301C>T | R101W | P (PS1+PS3+PM1+PM2+PP3+PP4) | c.436delC | P146fs | P (PVS1+PS1+PM2+PP4) |
| 10 | IL10RA | c.301C>T | R101W | P (PS1+PS3+PM1+PM2+PP3+PP4) | c.537G>A | T179T | P (PS1+PS3+PM2+PP3+PP4) |
| 11 | IL10RA | c.421G>A | G141R | P (PS1+PM2+PM3+PP3+PP4) | c.537G>A | T179T | P (PS1+PS3+PM2+PP3+PP4) |
| 12 | IL10RA | c.301C>T | R101W | P (PS1+PS3+PM1+PM2+PP3+PP4) | c.301C>T | R101W | P (PS1+PS3+PM1+PM2+PP3+PP4) |
| 13 | IL10RA | c.301C>T | R101W | P (PS1+PS3+PM1+PM2+PP3+PP4) | c.350G>A | R117H | P (PS1+PM1+PM2+PP3+PP4) |
| 14 | IL10RA | c.537G>A | T179T | P (PS1+PS3+PM2+PP3+PP4) | c.537G>A | T179T | P (PS1+PS3+PM2+PP3+PP4) |
| 15 | IL10RA | c.301C>T | R101W | P (PS1+PS3+PM1+PM2+PP3+PP4) | c.537G>A | T179T | P (PS1+PS3+PM2+PP3+PP4) |
| 16 | IL10RA | c.99G>A | W33X | P (PVS1+PS1+PM2+PP4) | c.301C>T | R101W | P (PS1+PS3+PM1+PM2+PP3+PP4) |
| 17 | IL10RA | c.493C>T | R165X | P (PVS1+PS1+PM2+PP4) | c.493C>T | R165X | P (PVS1+PS1+PM2+PP4) |
| 18 | IL10RA | c.109G>T | E37X | LP (PVS1+PM2+PM3+PP4) | c.301C>T | R101W | P (PS1+PS3+PM1+PM2+PP3+PP4) |
| 19 | IL10RA | c.301C>T | R101W | P (PS1+PS3+PM1+PM2+PP3+PP4) | c.537G>A | T179T | P (PS1+PS3+PM2+PP3+PP4) |
| 20 | IL10RA | c.301C>T | R101W | P (PS1+PS3+PM1+PM2+PP3+PP4) | c.537G>A | T179T | P (PS1+PS3+PM2+PP3+PP4) |
| 21 | IL10RA | c.301C>T | R101W | P (PS1+PS3+PM1+PM2+PP3+PP4) | c.537G>A | T179T | P (PS1+PS3+PM2+PP3+PP4) |
| 22 | IL10RA | c.301C>T | R101W | P (PS1+PS3+PM1+PM2+PP3+PP4) | c.350G>A | R117H | P (PS1+PM1+PM2+PP3+PP4) |
| 23 | IL10RA | c.99G>A | W33X | P (PVS1+PS1+PM2+PP4) | c.299T>G | V100G | P (PS1+PS3+PM2+PP3+PP4) |
| 24 | IL10RA | c.301C>T | R101W | P (PS1+PS3+PM1+PM2+PP3+PP4) | c.537G>A | T179T | P (PS1+PS3+PM2+PP3+PP4) |
| 25 | IL10RA | c.301C>T | R101W | P (PS1+PS3+PM1+PM2+PP3+PP4) | c.537G>A | T179T | P (PS1+PS3+PM2+PP3+PP4) |
| 26 | IL10RA | c.301C>T | R101W | P(PS1+PS3+PM1+PM2+PP3+PP4) | c.493C>T | R165X | P (PVS1+PS1+PM2+PP4) |
| 27 | IL10RA | c.301C>T | R101W | P (PS1+PS3+PM1+PM2+PP3+PP4) | c.537G>A | T179T | P (PS1+PS3+PM2+PP3+PP4) |
| 28 | IL10RA | c.493C>T | R165X | P (PVS1+PS1+PM2+PP4) | c.537G>A | T179T | P (PS1+PS3+PM2+PP3+PP4) |
| 29 | IL10RA | c.301C>T | R101W | P (PS1+PS3+PM1+PM2+PP3+PP4) | c.301C>T | R101W | P (PS1+PS3+PM1+PM2+PP3+PP4) |
| 30 | IL10RA | c.302G>A | R101Q | LP (PM1+PM2+PM3+PM5+PP3+PP4) | c.349C>T | R117C | P (PS1+PM1+PM2+PP3+PP4) |
| 31 | IL10RA | c.301C>T | R101W | P (PS1+PS3+PM1+PM2+PP3+PP4) | c.301C>T | R101W | P (PS1+PS3+PM1+PM2+PP3+PP4) |
| 32 | IL10RA | c.301C>T | R101W | P (PS1+PS3+PM1+PM2+PP3+PP4) | c.301C>T | R101W | P (PS1+PS3+PM1+PM2+PP3+PP4) |
| 33 | IL10RA | c.299T>G | V100G | P (PS1+PS3+PM2+PP3+PP4) | c.569T>G | F190C | LP (PS1+PM2+PM3_VeryStrong+PP3+PP4) |
| 34 | IL10RA | c.537G>A | T179T | P (PS1+PS3+PM2+PP3+PP4) | c.537G>A | T179T | P (PS1+PS3+PM2+PP3+PP4) |
| 35 | IL10RA | c.299T>G | V100G | P (PS1+PS3+PM2+PP3+PP4) | c.299T>G | V100G | P (PS1+PS3+PM2+PP3+PP4) |
| 36 | WAS | IVS8: +3- +6 GAGT del | LP (PS1+PM2+PP3+PP4) | ||||
| 37 | IKBKG | c.1217A>T | D406V | P (PS1+PM1+PM2+PP3+PP4) | |||
| 38 | SLC37A4 | c.310 ins T | P (PVS1+PM2+PP4) | c.1014-1120 del107 | P (PVS1+PM2+PP4) | ||
| 39 | CYBB | c.674+2T>C | P (PVS1+PM2+PP4) | ||||
| 40 | CYBB | c.1272G>A | W424X | P (PVS1+PS1+PM2+PP4) | |||
| 41 | CD40LG | c.267delC | Q90Sfs*6 | LP (PVS1+PM2+PP4) | |||
| 42 | LIG4 | c.833G>T | R278L | LP (PM1+PM2+PM3+PM5+PP3+PP4) | c.1144_1145delCT | L382Efs*5 | LP (PVS1+PS1+PM2+PP4) |
| 43 | CARD11 | c.155T>C | I52T | LP (PS2+PM2+PP4) | |||
| 44 | PIK3CD | c.3061G>A | E1021K | P (PS1+PS3+PM2+PP3+PP4) | |||
| 45 | IL10RA | c.350G>A | R117H | P (PS1+PM1+PM2+PP3+PP4) | |||
| 46 | IL10RA | c.537G>A | T179T | P (PS1+PS3+PM2+PP3+PP4) | |||
| 47 | IL10RA | c.787C>T | R263X | LP (PVS1+PM2+PP4) | |||
| 48 | IL10RA | c.301C>T | R101W | P (PS1+PS3+PM1+PM2+PP3+PP4) | |||
| 49 | CXCR4 | c.1001G>A | R334Q | VUS (PM2+PP3+PP4:BS4) |
As shown in Table 2, the proportions of male patients in the AP group, the M-IBD group, and the NM-IBD group was higher than those of female patients; however, no significant difference was observed among the three groups. The median onset age of patients in the M-IBD group was 0.51 (0.04-1.79) mo, which was much younger than that in the AP group [4.44 (1.25-11.76) mo; P = 0.003] and the NM-IBD group [3.99 (3-9) mo; P = 0.001]. The proportions of all IO-IBD patients who had severe diarrhea (more than 8 times/d, 86.4% in the M-IBD group and 58.6% in the NM-IBD group) and recurrent fever (63.6% in the M-IBD group and 41.4% in the NM-IBD group) were significantly higher than those in AP patients (25% of patients had severe diarrhea [P < 0.001] and 5% of patients had a recurrent fever [P = 0.01]). The percentages of the patients who had severe diarrhea and perianal lesions in the M-IBD group (86.4% and 79.5%, respectively) were much higher than those in the NM-IBD group (58.6% [P = 0.007] and 24.1% [P = 0.005]). The anthropometric results showed that the Z scores for body weight (-1.95 ± 0.26 for the M-IBD group and -1.15 ± 0.29 for the NM-IBD group) and body height (-1.95 ± 0.25 for the M-IBD group and -1.32 ± 0.31 for the NM-IBD group) of the IBD patients were all lower than those of the children in the AP group (-0.05 ± 0.21 for weight [P < 0.001] and 0.17 ± 0.19 for height [P < 0.001]), while there was no significant difference between the M-IBD group and the NM-IBD group. Regarding the outcomes of treatment, mortality in the M-IBD group was significantly higher than that in the NM-IBD group (20.5% vs 3.4%, P = 0.001), whereas no death occurred in the AP group.
| AP | M-IBD | NM-IBD | P value | |
| Total number | 40 | 44 | 29 | |
| Male (%) | 25 (62.50) | 27 (61.36) | 21 (72.41) | 0.591 |
| Median onset age (mo, IQR) | 4.44 (1.25,11.76) | 0.51 (0.04,1.79)1 | 3.99 (3.00,9.00) | < 0.001 |
| Diarrhea (≥ 8 times/d, %) | 10 (25.00) 1 | 38 (86.40) 1 | 17 (58.60) | < 0.001 |
| Diarrhea with blood (%) | 30 (75.00) | 29 (65.90) | 24 (82.80) | 0.320 |
| Perianal disease (%) | 0 (0) | 35 (79.54) 1 | 7 (24.14) | 0.005 |
| Fever (%) | 2 (5.00)1 | 28 (63.63) | 12 (41.38) | < 0.001 |
| Death (%) | 0 (0.00) | 9 (20.45) | 1 (3.45)2 | 0.001 |
| Z scores for weight (mean ± SD) | -0.05 ± 0.211 | -1.95 ± 0.26 | -1.15 ± 0.29 | < 0.001 |
| Z scores for height (mean ± SD) | 0.19 ± 0.191 | -1.95 ± 0.25 | -1.32 ± 0.31 | < 0.001 |
| Z scores for BMI (mean ± SD) | -0.21 ± 0.242 | -1.30 ± 0.28 | -0.52 ± 0.27 | 0.026 |
As shown in Table 3, the blood test results showed that peripheral white blood cell count (WBC), platelet count (PLT), CRP, ESR, TNF-α, and IL-6 levels in the IO-IBD group were significantly higher than those in the AP group (P < 0.01 for all), while serum albumin level was lower than that in the AP group (P < 0.001). Differences in serum iron levels were not significant among the three groups. Notably, serum IL-10 [85.50 (42.75-127.25) pg/mL] and ferritin [55.90 (36.10-231.20) ng/mL] levels in the M-IBD group were significantly higher than those in the AP group [IL-10: 5.00 (5.00-5.00) pg/mL, P < 0.001; ferritin: 22.90 (14.90-37.05) ng/mL, P = 0.002] and in the NM-IBD group [IL-10: 6.37 (5.00-14.80) pg/mL, P < 0.001; ferritin: 15.30 (9.40-27.60) ng/mL, P < 0.001].
| AP | M-IBD | NM-IBD | P value | |
| WBC (× 109) | 8.77 (6.78, 10.75)1 | 14.88 (9.84, 19.37) | 13.77 (8.87,18.51) | < 0.001 |
| Hemoglobin (mg/L) | 119 (115, 126)1 | 98 (90, 113) | 100 (80.50,116) | < 0.001 |
| PLT (× 109) | 304 (254, 383)1 | 447 (306.75, 591.50) | 477 (323.50, 601.50) | 0.002 |
| CRP (mg/L) | 1 (0.20, 1.00)1 | 48 (7.98, 75.23)1 | 21 (1.00, 44.35) | < 0.001 |
| ESR (mm/h) | 6 (4, 8)1 | 18.50 (8, 39) | 21 (6, 42) | < 0.001 |
| TNF-α (pg/mL) | 14.60 (10.68, 17.03)1 | 21.50 (17.58, 27.88) | 22.90 (14.48, 61.28) | < 0.001 |
| IL-6 (pg/mL) | 2.85 (2.00, 3.35)1 | 21.00 (10.20, 50.30) | 14.55 (6.81, 30.00) | < 0.001 |
| IL-10 (pg/mL) | 500 (5, 5) | 85.50 (42.75, 127.25)1 | 6.37 (5.00, 14.80) | < 0.001 |
| Ferritin (ng/mL) | 22.90 (14.90, 37.05) | 55.90 (36.10, 231.20)1 | 15.50 (10.60, 27.72) | < 0.001 |
| Albumin (g/L) | 41 (38, 43)1 | 31 (27, 35) | 33 (27.00, 36.50) | < 0.001 |
| Serum iron (μmol/L) | 8.00 (5.90, 10.20) | 3.90 (2.60, 6.88) | 5.70 (4.35, 11.38) | 0.069 |
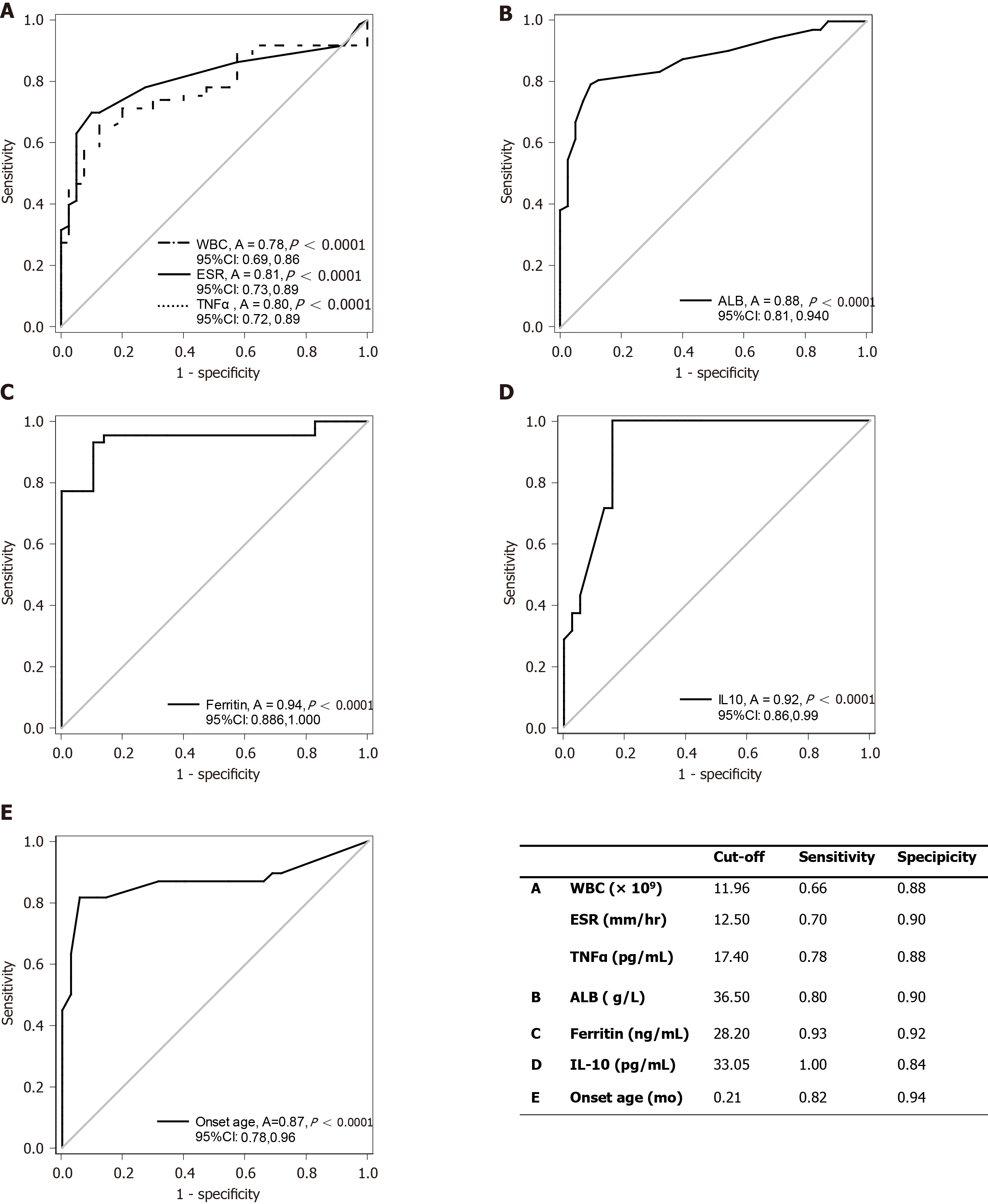
Clinical indicators for risk of IO-IBD: Binary logistic regression analysis was performed for the parameters with significant differences between the AP group and the IO-IBD group [including diarrhea (> 8 times/d), recurrent fever, Z-scores for weight, Z-scores for height, WBC, hemoglobin (Hb), PLT, CRP, ESR, TNF-α, IL-6, and albumin levels]. The results showed that increased peripheral WBC (odds ratio [OR] = 1.19, 95% confidence interval [CI]: 1.01-1.40, P = 0.040), ESR (OR = 1.10, 95%CI: 1.01-1.20, P = 0.037) and levels of TNF-α (OR =1.25, 95%CI: 1.05-1.50, P =0.013), as well as reduced albumin levels were risk factors (OR = 0.86, 95%CI: 0.74-1.00, P = 0.048) for the diagnosis of IO-IBD (Table 4). We further investigated these four indicators using ROC curve analysis to explore their diagnostic value. The areas under the curves (AUCs) and the cut-off values are shown in Figure 1A and B. For WBC, the AUC was 0.78 (95%CI: 0.69-0.86, P < 0.001) and the cut-off value was 11.96 × 109/L (sensitivity: 0.66; specificity: 0.88). For ESR, the AUC was 0.81 (95%CI: 0.73-0.89, P < 0.001), and the cut-off value was 12.50 mm/h (sensitivity: 0.70; specificity: 0.90). For TNF-α, the AUC was 0.80 (95%CI: 0.72-0.89, P < 0.001) and the cut-off value was 17.40 pg/mL (sensitivity: 0.78; specificity: 0.88). For albumin, the AUC was 0.88 (95%CI: 0.81-0.94, P < 0.001), and the cut-off value was 36.50 g/L (sensitivity: 0.80; specificity: 0.90). Based on the results, WBC and ESR had a low sensitivity for recognizing IO-IBD. While TNF-α and albumin level had a higher sensitivity and specificity, and we speculated they may be used for early identifying IO-IBD patients.
| AP (median, IQR) | IO-IBD (median, IQR) | Adjusted OR (95%CI) | P value | |
| WBC (× 109) | 8.77 (6.78, 10.75) | 14.12 (8.85, 18.27) | 1.19 (1.01, 1.40) | 0.040 |
| ESR (mm/h) | 6 (4, 8) | 19.00 (8.00, 39.00) | 1.10 (1.01, 1.20) | 0.037 |
| TNF-α (pg/mL) | 14.60 (10.68, 17.03) | 21.65 (17.33, 29.45) | 1.25 (1.05, 1.50) | 0.013 |
| Albumin (g/L) | 41 (38, 43) | 32 (27, 36) | 0.86 (0.74, 1.00) | 0.048 |
Clinical indicators for risk of M-IBD: Binary logistic regression analysis was performed using the parameters with significant differences between the M-IBD group and the NM-IBD group [including median onset age, diarrhea (> 8 times/d), perianal disease, CRP, IL-10, and ferritin levels]. The results showed that very early onset age (OR = 3.47, 95%CI: 1.29-9.29, P = 0.013], presence of perianal diseases (OR = 11.42, 95%CI: 1.71-76.23, P = 0.012), and high levels of serum ferritin (OR = 1.14, 95%CI: 1.06-1.24, P = 0.001) and IL-10 (OR = 1.04, 95%CI: 1.01-1.08, P = 0.005) were the main risk factors for M-IBD diagnosis (Table 5). Because the IL-10RA mutations in this study accounted for a dominant proportion (79.6%) in the M-IBD group, to avoid bias caused by sample selection, we further compared the above high-risk factors among patients with IL-10RA mutations or non-IL-10RA mutations in the M-IBD group, and those without any disease-causing mutations in the NM-IBD group. The results showed that only serum ferritin could discriminate M-IBD and NM-IBD regardless of genotypes. The level of serum ferritin was significantly lower in the NM-IBD group than in the other two groups (Table 6) and could be used as an indicator for recognizing IO-IBD children with genetic mutations. The ROC curve analysis was also performed to explore the indicators for the diagnosis of M-IBD. Using gene sequencing results as the gold standard, all IO-IBD patients were included in the analysis. The AUC of ferritin for diagnosing M-IBD was 0.94 (95%CI: 0.87-1.00, P < 0.001), with a cut-off value of 28.20 ng/mL (sensitivity: 0.93; specificity: 0.92; Figure 1C), which showed high diagnostic value. Besides, we further analyzed the value of serum IL-10 level and onset age for early noticing IL-10RA mutation in M-IBD patients. The AUC for IL-10 was 0.91 (95%CI: 0.86-0.99, P < 0.001), and the cut-off value was 33.05 pg/mL (sensitivity: 1.00; specificity: 0.84); the AUC for onset age was 0.87 (95%CI: 0.78-0.96, P < 0.001), and the cut-off value was 0.21 mo (sensitivity: 0.82; specificity: 0.94; Figure 1D and E). These results indicated that the two indicators had high diagnostic value and may be used in clinical practice. All above results suggested that, when serum ferritin levels were higher than 28.20 ng/mL, it might indicate the existence of pathogenic gene mutations in IO-IBD patients. If the infant got IO-IBD within 1 wk after the birth or their serum IL-10 levels were higher than 33.05 pg/mL, it might strongly suggest the presence of IL-10RA gene mutations.
| M-IBD (median, IQR) | NM-IBD (median, IQR) | Ajusted OR (95%CI) | P value | |
| Ferritin (ng/mL) | 55.90 (36.10, 231.20) | 15.30 (9.40, 27.60) | 1.14 (1.06, 1.24) | 0.001 |
| IL-10 (pg/mL) | 85.20 (43.30, 126.50) | 5.00 (5.00, 9.11) | 1.04 (1.01, 1.08) | 0.005 |
| Median onset age (mo) | 0.51 (0.04, 1.79) | 3.99 (3.00, 9.00) | 3.47 (1.29, 9.29) | 0.013 |
| Perianal disease (%) | 35 (79.50) | 7 (24.10) | 11.42 (1.71, 76.23) | 0.012 |
| IL-10RA M-IBD | Non-IL-10RA M-IBD | NM-IBD | P value | |
| Ferritin (ng/mL) | 55.90 (53.12, 74.73) | 45.25 (16.58, 68.70) | 15.30 (14.55, 17.95)1 | < 0.001 |
| IL-10 (pg/mL) | 85.20 (66.20, 125.00)1 | 8.90 (5.00, 85.20) | 5.00 (5.00, 9.11) | < 0.001 |
| Median onset age (mo) | 0.04 (0.00, 0.08)1 | 2 (0.41, 4.08) | 3.99 (3, 9) | < 0.001 |
| Perianal disease (%) | 32 (91.43)1 | 3 (33.33) | 7 (24.14) | 0.002 |
Approximately 15% of pediatric patients were categorized as having VEO-IBD[2], and approximately 1% of pediatric patients developed symptoms during infancy (IO-IBD). Over the past decade, the prevalence of VEO-IBD had increased by more than 50%[6,7]. Increasing studies believed that genetic factors, such as monogenic diseases, play important roles in the development of VEO-IBD, especially IO-IBD. Currently, there is no epidemiological data for IO-IBD patients in China. However, in the past 5 years, more than 200 cases of VEO-IBD have been reported by our hospital and other hospitals in China[8-13], and more than half of the patients presented genetic defects. Among the 73 pediatric patients in this study, 49 (67.12%) carried disease-related gene mutations, which was much higher than the reported proportions in European and American countries[14,15] but similar to the proportion reported by Huang et al[10], suggesting that the presence of monogenic diseases is more common in Chinese VEO-IBD patients.
A total of ten mutated genes were identified in this study, among which the IL-10RA mutation was the most common (39/49). This finding was similar to those reported by other researchers in China[8], while IL-10RB and IL-10 mutations were not observed. Among the 14 IL-10RA variants, ten have been reported previously, among which c.537G>A, c.493C>T, c.436delC, c.299T>G, and c.191A>G mutations were observed in children only in China or other Asian countries[11,16,17] but have not been reported in Western countries, suggesting a genetic founder effect in IL-10RA. The four identified novel variants were c.109G>T, c.302G>A, c.569T>G, and c.787C>T, which were likely pathogenic mutations according to the ACMG guidelines. In this study, the most common IL-10RA mutation locus was c.301C>T, followed by c.537G>A, which was consistent with the previous reports in Asian children[8]. Notably, c.537G>A mutation was located in exon 4, which was always missed in the reports as it was mistaken for a synonymous mutation; however, it had been confirmed as a splicing mutation that has a deleterious effect on IL-10 receptor[11]. For IBD children who are highly suspected of having abnormal IL-10 signaling pathways, especially those who have a heterozygous IL-10RA mutation after gene sequencing, clinicians should be aware of the sequencing report and not to miss the c.537G>A mutation.
In addition to IL-10RA, the other eight genetic mutations that could cause immunodeficiency diseases were also observed in this study. These included CYBB mutation, which caused X-linked chronic granulomatous disease[18], IKBKG mutation, which caused ectodermal dysplasia and immunodeficiency[19,20], PIK3CD mutation, which caused activated phosphoinositide 3-kinase δ syndrome[21], CD40LG mutation, which caused X-linked hyper-IgM syndrome[22], SLC37A4 mutation, which caused glycogen storage disease type 1B[23], LIG4 mutation, which caused LIG4 syndrome[24], WAS mutation, which caused Wiskott-Aldrich syndrome[25], and CARD11 mutation, which caused immunodeficiency type 11B with atopic dermatitis[26]. These diseases have been confirmed to cause IBD-like colitis in infants, such as recurrent diarrhea, hematochezia, infections, perianal abscess, and growth retardation.
Because of an insufficient understanding of IO-IBD, especially that caused by monogenic diseases, most patients could not be diagnosed as early as possible. It has been reported that the median time from the occurrence of symptoms to disease diagnosis was approximately 6 mo[15]. The most common misdiagnosis was allergic diseases, therefore, this study included a group of age-matched patients who were admitted to the hospital due to diarrhea and hematochezia and were eventually diagnosed as allergic proctocolitis. Although the AP children had diarrhea, hematochezia, or other similar symptoms with the IBD children, there was less onset of failure to thrive, perianal lesions, and death in AP patients; meanwhile, the daily frequency of diarrhea was significantly higher in IO-IBD children than in AP children. Inflammatory markers in peripheral blood such as WBC, CRP, ESR, PLT, TNF-α, and IL-6 in IO-IBD children were significantly higher than those in AP children, while the nutritional indicators such as albumin and hemoglobin were lower in IBD patients than in AP patients. Logistic regression analysis further confirmed that increased levels of WBC, ESR, and TNF-α, as well as lower serum albumin level were associated with a higher likelihood of IBD. Although it has been reported that hematological examinations had little value in the differential diagnosis of food allergy and IBD[15],the ROC curve analysis in this study showed that TNF-α and serum albumin level had moderate value for differentiating IBD from allergic disease. Moreover, the high sensitivities and specificities of cut-off values for TNF-α and serum albumin may help clinicians, especially those in areas with limited medical resources, to identify IO-IBD patients earlier.
Genetic diagnosis is currently the gold standard for the diagnosis of IO-IBD caused by gene defects. However, the economic and time costs of this detection are relatively high in China. Most parents are not willing to choose this method at initial visits, and this would delay the diagnosis and treatment. The binary logistic regression analysis showed the serum ferritin (OR = 1.14) and IL-10 (OR = 1.04) may indicate the presence of gene defects in IO-IBD children; however, in view of the high proportion of IL-10RA mutations in our cohort, we further compared these factors among patients with IL-10RA mutations, those with non-IL-10RA mutations, and those without any disease-causing mutations. The final results showed that serum ferritin was abnormally elevated in IO-IBD patients with gene mutations and was not affected by the genotypes. Further ROC curve analysis showed that serum ferritin might be a valuable diagnostic indicator for monogenic IBD. Serum ferritin was acknowledged as an inflammatory marker, which would reflect the acute and chronic inflammatory state in infectious diseases, immunological diseases, hematological diseases, and malignant diseases[27]. A variety of cells could synthesize and secrete ferritin, of which macrophages were the main source and could induce diseases[27]. The role of ferritin in inflammatory responses is still unclear. It was hypothesized that in the inflammatory state, the Toll-like receptor (TLR) 9 signaling pathway is activated, IL-1β and IL-18 levels increased, then macrophages were activated, and synthesized more ferritin, which could further amplify the inflammatory response through the TLR9 signaling pathway, thereby forming a positive feedback loop and resulting in continuous aggravation of inflammation[28-32]. Some researchers believed that serum ferritin could bind to T and B lymphocytes directly[32,33], then inhibit T cell proliferation, B cell maturation, and immunoglobulin synthesis[33-35]. In addition, serum ferritin could also promote regulatory T cell differentiation through the IL-10 signaling pathway and play an immunoregulatory role[36]. In our study, the serum ferritin level was significantly elevated in M-IBD children, but whether it plays a pro-inflammatory or immunomodulatory role needs further study.
Both in our study and other studies in China, IL-10RA is the most common mutated gene in Chinese VEO-IBD patients. We also found that compared to the patients with other gene mutations, patients with the IL-10RA deficiency had unique features. First, the age of onset was particularly early. The median onset age was 0.04-mo-old, while it was 2-mo-old in patients with other gene mutations, indicating that diarrhea and hematochezia existed from birth in IL-10RA mutated children. Second, serum IL-10 level in these patients was particularly high. IL-10 is an important anti-inflammatory factor secreted by a variety of immune cells. After binding to IL-10R, IL-10 can activate a series of cascade reactions to maintain immune homeostasis by inhibiting inflammatory factors such as TNF-α[37]. IL-10R is composed of two subunits, IL-10RA and IL-10RB, and IL-10RA only binds to IL-10[38]. It was previously believed that the increased expression of IL-10 in IBD patients indicated a good steroid response and prognosis[39]. However, when IL-10R is deficient, the anti-inflammatory function of IL-10 could not be achieved. Glocker et al[40] confirmed that human peripheral blood mononuclear cells (PBMCs) could secrete large amounts of TNF-α after stimulation with lipopolysaccharide (LPS) in vitro, but its level decreased significantly when exogenous IL-10 was added into the culture medium, while this phenomenon could not be observed in the PBMC culture supernatant from IL-10RA deficient children, indicating the important role of the IL-10 signaling pathway in anti-inflammatory processes. In our study, both high IL-10 and TNF-α levels were observed in patients with IL-10RA mutation, which indicated that even such a high level of IL-10 could not inhibit TNF-α release and alleviate the inflammation in these children. These findings were similar to the in vitro research mentioned above. Therefore, we speculated that IL-10 level could be used as an in vivo detective indicator to verify the function of novel mutations of the IL-10RA gene. In this study, ROC curve analysis also confirmed that serum IL-10 level had a high sensitivity and specificity for identifying the presence of IL-10RA mutations, which suggested that if IO-IBD patients have a remarkably elevated IL-10 level, the gene sequencing is strongly recommended to identify the existence of IL-10RA deficiency. In fact, we did further investigation by whole-genome sequencing (WGS) in two patients of this cohort, whose clinical manifestations were similar to those of IL-10RA mutated patients, especially the increased significantly IL-10 level but whole-exsome sequencing only found heterozygous mutations in IL-10RA. However, the results of WGS showed that there was another heterozygous deletion of 333bp in IL-10RA which led to exon 1 absence. This result further supported that serum IL-10 level may help distinguish IL-10RA mutated patients.
IO-IBD patients, especially those caused by gene mutations, are rare. Although the cohort included in this study was larger than many published studies, the absolute number was not large, which may lead to statistical bias. Besides, we needed further prospective studies to evaluate the diagnostic value of the indicators identified in this study.
Children with IO-IBD have a higher proportion of Mendelian diseases. For Chinese Han pediatric patients, IL-10RA mutation is the most common pathogenic gene. A complete medical history and clinical evaluation are necessary to differentiate IBD and allergic proctocolitis earlier. Diarrhea frequency, body weight, height, serum albumin, and TNF-α level could help distinguish IO-IBD and allergy. Elevated serum ferritin level could be used for identifying IO-IBD caused by gene mutations, and the remarkably increased serum IL-10 level strongly suggests the presence of IL-10RA gene mutation. These clinical characteristics and hematological parameters would help to shorten the time of diagnosis so that the patients are able to receive timely and correct treatment and then reduce social and financial burdens.
Infantile-onset inflammatory bowel disease (IO-IBD) causes severe clinical manifestations and responds poorly to traditional inflammatory bowel disease (IBD) treatments. At present, there are no simple and reliable laboratory indicators for early screening IO-IBD patients, especially those in whom the disease is caused by monogenic diseases.
Because of an insufficient understanding of IO-IBD, especially that caused by monogenic diseases, most patients could not be diagnosed as early as possible. It is hard to persuade the parents to accept endoscopic examination or genetic diagnosis at initial visits, especially those in areas with limited medical resources.
We intended to search for early diagnostic indicators for IO-IBD children with or without gene mutations, in order to shorten the diagnosis time, reduce medical costs, simplify the analysis of next generation-sequencing, and administer targeted intervention as early as possible.
A retrospective analysis was performed in 73 patients with IO-IBD admitted to our hospital in the past 5 years. Based on the next-generation sequencing results, they were divided into either a monogenic IBD group (M-IBD) or non-monogenic IBD group (NM-IBD). Forty age-matched patients with allergic proctocolitis (AP) were included as a control group. The clinical manifestations and the inflammatory factors in peripheral blood were evaluated. Logistic regression analysis and receiver operating characteristic (ROC) curve analysis were performed to find the screening factors and cut-off values of IO-IBD as well as monogenic IO-IBD.
Among the 44 M-IBD patients, 35 carried IL-10RA mutations, and the most common mutations were c.301C>T (p.R101W, 30/70) and c.537G>A (p.T179T, 17/70). Patients with higher serum tumor necrosis factor (TNF)-α value were more likely have IBD [odds ratio (OR) = 1.25, 95% confidence interval (CI): 1.05-1.50, P = 0.013), while higher serum albumin level was associated with lower risk of IBD (OR = 0.86, 95%CI: 0.74-1.00, P = 0.048). The cut-off values of TNF-α and albumin were 17.40 pg/mL (sensitivity: 0.78; specificity: 0.88) and 36.50 g/L (sensitivity: 0.80; specificity: 0.90), respectively. The increased ferritin level was indicative of a genetic mutation in IO-IBD patients. Its cut-off value was 28.20 ng/mL (sensitivity: 0.93; specificity: 0.92). When interleukin (IL)-10 level was higher than 33.05 pg/mL (sensitivity: 1.00; specificity: 0.84), or the onset age was earlier than 0.21 mo (sensitivity: 0.82; specificity: 0.94), the presence of disease-causing mutations in IL-10RA in IO-IBD patients was strongly suggested.
Serum TNF-α and albumin level could differentiate IO-IBD patients from allergic proctocolitis patients, and serum ferritin and IL-10 level are useful indicators for early diagnosing monogenic IO-IBD.
Using serum TNF-α and albumin level may contribute to distinguishing IO-IBD which needs further investigations, such as gastrointestinal endoscopy. High levels of serum ferritin and IL-10 can infer a monogenic disease in IO-IBD patients, especially the mutations in the IL-10RA gene. The diagnostic value of the indicators identified in this study should be evaluated by prospective studies.
We thank Li J at the Ruijin Hospital for statistics assistance and Chen XY at the Ruijin Hospital for pathological diagnosis.
| 1. | Levine A, Koletzko S, Turner D, Escher JC, Cucchiara S, de Ridder L, Kolho KL, Veres G, Russell RK, Paerregaard A, Buderus S, Greer ML, Dias JA, Veereman-Wauters G, Lionetti P, Sladek M, Martin de Carpi J, Staiano A, Ruemmele FM, Wilson DC; European Society of Pediatric Gastroenterology; Hepatology; and Nutrition. ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr. 2014;58:795-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 822] [Cited by in RCA: 1059] [Article Influence: 88.3] [Reference Citation Analysis (0)] |
| 2. | Heyman MB, Kirschner BS, Gold BD, Ferry G, Baldassano R, Cohen SA, Winter HS, Fain P, King C, Smith T, El-Serag HB. Children with early-onset inflammatory bowel disease (IBD): analysis of a pediatric IBD consortium registry. J Pediatr. 2005;146:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 370] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 3. | Chandrakasan S, Venkateswaran S, Kugathasan S. Nonclassic Inflammatory Bowel Disease in Young Infants: Immune Dysregulation, Polyendocrinopathy, Enteropathy, X-Linked Syndrome, and Other Disorders. Pediatr Clin North Am. 2017;64:139-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Pediatircs' Inflammation bowl diesease collaborating group attached to the Chinese society of pediatric gastroenterology of Chinese Medical Association, Chen J, Xu CD, Huang ZH, Gong ST, Dong YS, Dong M, Sun M, Ye LY, Huang YK, Wang BX, Wang LL, Xu XW, Jiang MZ, Yang WL, Zhu CM, You JY, Wu QB, Jiang LR, Li ZL, Shao CH, Huang Y, Zhang YL, Xu XH, Liu FL, Mang M. Consensus on the Diagnosis and Management of Pediatric Inflammatory Bowel Disease. Zhongguo Shiyong Erke Zazhi. 2010;25:263-265. |
| 5. | Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL; ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26738] [Cited by in RCA: 24707] [Article Influence: 2246.1] [Reference Citation Analysis (0)] |
| 6. | Benchimol EI, Guttmann A, Griffiths AM, Rabeneck L, Mack DR, Brill H, Howard J, Guan J, To T. Increasing incidence of paediatric inflammatory bowel disease in Ontario, Canada: evidence from health administrative data. Gut. 2009;58:1490-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 311] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 7. | Benchimol EI, Mack DR, Nguyen GC, Snapper SB, Li W, Mojaverian N, Quach P, Muise AM. Incidence, outcomes, and health services burden of very early onset inflammatory bowel disease. Gastroenterology 2014; 147: 803-813. quiz e14-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 201] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 8. | Zheng C, Huang Y, Hu W, Shi J, Ye Z, Qian X, Huang Z, Xue A, Wang Y, Lu J, Tang Z, Wu J, Wang L, Peng K, Zhou Y, Miao S, Sun H. Phenotypic Characterization of Very Early-Onset Inflammatory Bowel Disease with Interleukin-10 Signaling Deficiency: Based on a Large Cohort Study. Inflamm Bowel Dis. 2019;25:756-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Su W, Xu CD, Wang XQ, Yu Y, Guo Y, Xu X, Xiao Y. Analysis of clinical phenotype and genotype of 30 Chinese pediatric patients suffering very early onset inflammatory bowel disease. Zhonghua Yanxingchangbing Zazhi. 2018;2:83-88. [DOI] [Full Text] |
| 10. | Huang Z, Peng K, Li X, Zhao R, You J, Cheng X, Wang Z, Wang Y, Wu B, Wang H, Zeng H, Yu Z, Zheng C, Wang Y, Huang Y. Mutations in Interleukin-10 Receptor and Clinical Phenotypes in Patients with Very Early Onset Inflammatory Bowel Disease: A Chinese VEO-IBD Collaboration Group Survey. Inflamm Bowel Dis. 2017;23:578-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 11. | Xu YB, Chen YB, Zeng P, Chen HS, Zeng HS. Interleukin-10 receptor mutations in children with neonatal onset inflammatory bowel disease: genetic diagnosis and pathogenesis. Zhonghua Erke Zazhi. 2015;53:348-354. [DOI] [Full Text] |
| 12. | Liu LL, Jiang Y, HOU XL, Tang ZZ, Zhou CL, Sun GW. Clinical characteristics and genetic diagnosis of infantile inflammatory bowel disease within four months after birth: seven case report. Zhongguo Xunzheng Erke Zazhi. 2016;11:285-289. [DOI] [Full Text] |
| 13. | Xiao Y, Wang XQ, Yu Y, Guo Y, Xu X, Gong L, Zhou T, Li XQ, Xu CD. Comprehensive mutation screening for 10 genes in Chinese patients suffering very early onset inflammatory bowel disease. World J Gastroenterol. 2016;22:5578-5588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Kotlarz D, Beier R, Murugan D, Diestelhorst J, Jensen O, Boztug K, Pfeifer D, Kreipe H, Pfister ED, Baumann U, Puchalka J, Bohne J, Egritas O, Dalgic B, Kolho KL, Sauerbrey A, Buderus S, Güngör T, Enninger A, Koda YK, Guariso G, Weiss B, Corbacioglu S, Socha P, Uslu N, Metin A, Wahbeh GT, Husain K, Ramadan D, Al-Herz W, Grimbacher B, Sauer M, Sykora KW, Koletzko S, Klein C. Loss of interleukin-10 signaling and infantile inflammatory bowel disease: implications for diagnosis and therapy. Gastroenterology. 2012;143:347-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 353] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 15. | Kammermeier J, Dziubak R, Pescarin M, Drury S, Godwin H, Reeve K, Chadokufa S, Huggett B, Sider S, James C, Acton N, Cernat E, Gasparetto M, Noble-Jamieson G, Kiparissi F, Elawad M, Beales PL, Sebire NJ, Gilmour K, Uhlig HH, Bacchelli C, Shah N. Phenotypic and Genotypic Characterisation of Inflammatory Bowel Disease Presenting Before the Age of 2 years. J Crohns Colitis. 2017;11:60-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 16. | Yanagi T, Mizuochi T, Takaki Y, Eda K, Mitsuyama K, Ishimura M, Takada H, Shouval DS, Griffith AE, Snapper SB, Yamashita Y, Yamamoto K. Novel exonic mutation inducing aberrant splicing in the IL10RA gene and resulting in infantile-onset inflammatory bowel disease: a case report. BMC Gastroenterol. 2016;16:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Oh SH, Baek J, Liany H, Foo JN, Kim KM, Yang SC, Liu J, Song K. A Synonymous Variant in IL10RA Affects RNA Splicing in Paediatric Patients with Refractory Inflammatory Bowel Disease. J Crohns Colitis. 2016;10:1366-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Schäppi MG, Smith VV, Goldblatt D, Lindley KJ, Milla PJ. Colitis in chronic granulomatous disease. Arch Dis Child. 2001;84:147-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 93] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Mizukami T, Obara M, Nishikomori R, Kawai T, Tahara Y, Sameshima N, Marutsuka K, Nakase H, Kimura N, Heike T, Nunoi H. Successful treatment with infliximab for inflammatory colitis in a patient with X-linked anhidrotic ectodermal dysplasia with immunodeficiency. J Clin Immunol. 2012;32:39-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Orange JS, Jain A, Ballas ZK, Schneider LC, Geha RS, Bonilla FA. The presentation and natural history of immunodeficiency caused by nuclear factor kappaB essential modulator mutation. J Allergy Clin Immunol. 2004;113:725-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 133] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Nunes-Santos CJ, Uzel G, Rosenzweig SD. PI3K pathway defects leading to immunodeficiency and immune dysregulation. J Allergy Clin Immunol. 2019;143:1676-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 113] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 22. | Levy J, Espanol-Boren T, Thomas C, Fischer A, Tovo P, Bordigoni P, Resnick I, Fasth A, Baer M, Gomez L, Sanders EA, Tabone MD, Plantaz D, Etzioni A, Monafo V, Abinun M, Hammarstrom L, Abrahamsen T, Jones A, Finn A, Klemola T, DeVries E, Sanal O, Peitsch MC, Notarangelo LD. Clinical spectrum of X-linked hyper-IgM syndrome. J Pediatr. 1997;131:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 432] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 23. | Gerin I, Veiga-da-Cunha M, Achouri Y, Collet J-F, Van Schaftingen E. Sequence of a putative glucose 6-phosphate translocase, mutated in glycogen storage disease type Ib1. FEBS Lett. 1997;419:235-238. [RCA] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 152] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 24. | Felgentreff K, Perez-Becker R, Speckmann C, Schwarz K, Kalwak K, Markelj G, Avcin T, Qasim W, Davies EG, Niehues T, Ehl S. Clinical and immunological manifestations of patients with atypical severe combined immunodeficiency. Clin Immunol. 2011;141:73-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 124] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 25. | Catucci M, Castiello MC, Pala F, Bosticardo M, Villa A. Autoimmunity in wiskott-Aldrich syndrome: an unsolved enigma. Front Immunol. 2012;3:209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 26. | Dorjbal B, Stinson JR, Ma CA, Weinreich MA, Miraghazadeh B, Hartberger JM, Frey-Jakobs S, Weidinger S, Moebus L, Franke A, Schäffer AA, Bulashevska A, Fuchs S, Ehl S, Limaye S, Arkwright PD, Briggs TA, Langley C, Bethune C, Whyte AF, Alachkar H, Nejentsev S, DiMaggio T, Nelson CG, Stone KD, Nason M, Brittain EH, Oler AJ, Veltri DP, Leahy TR, Conlon N, Poli MC, Borzutzky A, Cohen JI, Davis J, Lambert MP, Romberg N, Sullivan KE, Paris K, Freeman AF, Lucas L, Chandrakasan S, Savic S, Hambleton S, Patel SY, Jordan MB, Theos A, Lebensburger J, Atkinson TP, Torgerson TR, Chinn IK, Milner JD, Grimbacher B, Cook MC, Snow AL. Hypomorphic caspase activation and recruitment domain 11 (CARD11) mutations associated with diverse immunologic phenotypes with or without atopic disease. J Allergy Clin Immunol. 2019;143:1482-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 112] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 27. | Kernan KF, Carcillo JA. Hyperferritinemia and inflammation. Int Immunol. 2017;29:401-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 420] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 28. | Gray CP, Arosio P, Hersey P. Heavy chain ferritin activates regulatory T cells by induction of changes in dendritic cells. Blood. 2002;99:3326-3334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 91] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Carcillo JA, Sward K, Halstead ES, Telford R, Jimenez-Bacardi A, Shakoory B, Simon D, Hall M; Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network Investigators. A Systemic Inflammation Mortality Risk Assessment Contingency Table for Severe Sepsis. Pediatr Crit Care Med. 2017;18:143-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 30. | Fautrel B, Le Moël G, Saint-Marcoux B, Taupin P, Vignes S, Rozenberg S, Koeger AC, Meyer O, Guillevin L, Piette JC, Bourgeois P. Diagnostic value of ferritin and glycosylated ferritin in adult onset Still's disease. J Rheumatol. 2001;28:322-329. [PubMed] |
| 31. | Garcia PC, Longhi F, Branco RG, Piva JP, Lacks D, Tasker RC. Ferritin levels in children with severe sepsis and septic shock. Acta Paediatr. 2007;96:1829-1831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 99] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 32. | Recalcati S, Invernizzi P, Arosio P, Cairo G. New functions for an iron storage protein: the role of ferritin in immunity and autoimmunity. J Autoimmun. 2008;30:84-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 205] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 33. | Fargion S, Fracanzani AL, Brando B, Arosio P, Levi S, Fiorelli G. Specific binding sites for H-ferritin on human lymphocytes: modulation during cellular proliferation and potential implication in cell growth control. Blood. 1991;78:1056-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 34. | Broxmeyer HE, Williams DE, Geissler K, Hangoc G, Cooper S, Bicknell DC, Levi S, Arosio P. Suppressive effects in vivo of purified recombinant human H-subunit (acidic) ferritin on murine myelopoiesis. Blood. 1989;73:74-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 35. | Yamashita M, Harada G, Matsumoto SE, Aiba Y, Ichikawa A, Fujiki T, Udono M, Kabayama S, Yoshida T, Zhang P, Fujii H, Shirahata S, Katakura Y. Suppression of immunoglobulin production in human peripheral blood mononuclear cells by monocytes via secretion of heavy-chain ferritin. Immunobiology. 2014;219:149-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Li R, Luo C, Mines M, Zhang J, Fan GH. Chemokine CXCL12 induces binding of ferritin heavy chain to the chemokine receptor CXCR4, alters CXCR4 signaling, and induces phosphorylation and nuclear translocation of ferritin heavy chain. J Biol Chem. 2006;281:37616-37627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 98] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 37. | Hutchins AP, Diez D, Miranda-Saavedra D. The IL-10/STAT3-mediated anti-inflammatory response: recent developments and future challenges. Brief Funct Genomics. 2013;12:489-498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 333] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 38. | Commins S, Steinke JW, Borish L. The extended IL-10 superfamily: IL-10, IL-19, IL-20, IL-22, IL-24, IL-26, IL-28, and IL-29. J Allergy Clin Immunol. 2008;121:1108-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 247] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 39. | Santaolalla R, Mañé J, Pedrosa E, Lorén V, Fernández-Bañares F, Mallolas J, Carrasco A, Salas A, Rosinach M, Forné M, Espinós JC, Loras C, Donovan M, Puig P, Mañosa M, Gassull MA, Viver JM, Esteve M. Apoptosis resistance of mucosal lymphocytes and IL-10 deficiency in patients with steroid-refractory Crohn's disease. Inflamm Bowel Dis. 2011;17:1490-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Glocker EO, Kotlarz D, Boztug K, Gertz EM, Schäffer AA, Noyan F, Perro M, Diestelhorst J, Allroth A, Murugan D, Hätscher N, Pfeifer D, Sykora KW, Sauer M, Kreipe H, Lacher M, Nustede R, Woellner C, Baumann U, Salzer U, Koletzko S, Shah N, Segal AW, Sauerbrey A, Buderus S, Snapper SB, Grimbacher B, Klein C. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med. 2009;361:2033-2045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1149] [Cited by in RCA: 1119] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tanabe S S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Wang LL