Published online Feb 21, 2020. doi: 10.3748/wjg.v26.i7.725
Peer-review started: November 22, 2019
First decision: December 7, 2019
Revised: January 12, 2020
Accepted: January 19, 2020
Article in press: January 19, 2020
Published online: February 21, 2020
Processing time: 90 Days and 17.1 Hours
Liver resection is an effective treatment for benign and malignant liver tumors. However, a method for preoperative evaluation of hepatic reserve has not yet been established. Previously reported assessments of preoperative hepatic reserve focused only on liver failure in the early postoperative period and did not consider the long-term recovery of hepatic reserve. When determining eligibility for hepatectomy, the underlying pathophysiology needs to be considered to determine if the functional hepatic reserve can withstand both surgery and any postoperative therapy.
To identify pre-hepatectomy factors associated with both early postoperative liver failure and long-term postoperative liver function recovery.
This study was a retrospective cohort study. We retrospectively investigated 215 patients who underwent hepatectomy at our hospital between May 2013 and December 2016. Early post-hepatectomy liver failure (PHLF) was defined using the International Study Group of Liver Surgery’s definition of PHLF. Long-term postoperative recovery of liver function was defined as the time taken for serum total bilirubin and albumin levels to return to levels of < 2 mg/dL and > 2.8 g/dL, respectively, and the time taken for Child-Pugh score to return to Child-Pugh class A.
Preoperative type IV collagen 7S was identified as a significant independent factor associated with both PHLF and postoperative long-term recovery of liver function. Further analysis revealed that the time taken for the recovery of Child-Pugh scores and serum total bilirubin and albumin levels was significantly shorter in patients with type IV collagen 7S ≤ 6 ng/mL than in those with type IV collagen 7S > 6 ng/mL. In additional analyses, similar results were observed in patients without chronic viral hepatitis associated with fibrosis.
Preoperative type IV collagen 7S is a preoperative predictor of PHLF and long-term postoperative liver function recovery. It can also be used in patients without chronic hepatitis virus.
Core tip: In this study, we identified the pre-hepatectomy factor associated with both early postoperative liver failure and long-term postoperative liver function recovery. We found that preoperative type IV collagen 7S is a significant independent factor associated with both post-hepatectomy liver failure and postoperative long-term recovery of liver function. Our analysis revealed that the time required for the recovery of Child-Pugh scores and serum total bilirubin and total bilirubin levels was significantly shorter in patients with type IV collagen 7S ≤ 6 ng/mL than in patients with type IV collagen 7S > 6 ng/mL.
- Citation: Ishii M, Itano O, Shinoda M, Kitago M, Abe Y, Hibi T, Yagi H, Takeuchi A, Tsujikawa H, Abe T, Kitagawa Y. Pre-hepatectomy type IV collagen 7S predicts post-hepatectomy liver failure and recovery. World J Gastroenterol 2020; 26(7): 725-739
- URL: https://www.wjgnet.com/1007-9327/full/v26/i7/725.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i7.725
Liver resection is an effective treatment for benign and malignant liver tumors. Due to advancements in hepatic surgeries and perioperative management, the perioperative mortality in high-volume centers has been reported to be 5% or less[1-5]. However, postoperative liver failure remains a severe complication that can result in death.
In addition to preoperatively assessing whether a patient can tolerate hepatectomy, the application of hepatectomy should be determined with considerations on the underlying pathophysiology to decide whether further postoperative therapies will be required to improve the hepatic reserve. Various methods for hepatic reserve evaluation have been reported, including Child-Pugh score[6], indocyanine green retention rate at 15 min (ICG-R15)[7], galactose elimination capacity[8], maximal removal rate of indocyanine green[9], arterial ketone body ratio[10], and technetium-99m galactosyl human serum albumin scintigraphy[11]. However, the Child-Pugh score and ICG-R15 may be insufficient for accurately determining the hepatic reserve in patients with arteriovenous shunts or transporter abnormalities. To date, there is no established method for preoperative evaluation of hepatic reserve. Furthermore, existing methods for hepatic reserve assessment primarily focus on postoperative liver failure and do not consider the long-term recovery of liver function. From a therapeutic standpoint, there have been significant advancements in chemotherapy for gastrointestinal cancers in recent years, and the need for postoperative multidisciplinary therapy is currently well recognized. In postoperative therapies, long-term recovery of liver function is just as important as early liver failure. Therefore, the present study aimed to identify factors that can predict both early postoperative liver failure and long-term postoperative recovery of liver function in patients undergoing hepatectomy.
This study was a retrospective cohort study. Overall, 230 patients who underwent hepatectomy at Keio University Hospital between May 2013 and December 2016 were recruited. Patient data were extracted from the hospital’s database. Patients with postoperative follow-up < 1 mo (n = 1) and missing data on preoperative liver fibrosis markers (n = 14) were excluded, resulting in a sample size of 215 patients. This included 87 patients with hepatocellular carcinoma, 49 with liver metastasis secondary to colon cancer, 24 with hilar cholangiocarcinoma, 20 with intrahepatic cholangiocarcinoma, 22 with liver metastasis due to non-colon cancers, and 13 with other tumors. There were 148 men (68.8%) and 67 women (31.2%), with an overall median age of 68 years (20-88 years). Additional analyses were performed in 158 patients without chronic viral hepatitis. This study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the ethics committee of our hospital (approval nos. 20120443 and 20140389).
The patients underwent preoperative physical examinations and medical history interviews. Serum levels of aspartate aminotransferase, alanine aminotransferase, total bilirubin (TB), albumin (Alb), cholinesterase, total cholesterol, prothrombin time, C-reactive protein, platelet count, ICG-R15, and the fibrosis markers type IV collagen 7S and hyaluronic acid were estimated in each patient. Acoustic radiation force impulse (ARFI) ultrasonography was performed to measure liver stiffness. ARFI was performed 10 times each over the left and right lobes and the mean value was used in the analyses.
The inclusion criteria for surgery were as follows. It was assumed that the patients were in a generally good condition to tolerate major laparotomy and that the tumors were within the extent of resections. Hepatic reserve was assessed using biochemical tests and ICG-R15 to determine whether hepatectomy could be tolerated[12]. Preoperative computed tomography (Vincent; Fujifilm, Japan) findings were analyzed to calculate the liver volume. The maximum hepatectomy volume was set at 65%.
Early postoperative liver failure was diagnosed based on the criteria established by the International Study Group of Liver Surgery (ISGLS)[13]. The patients were divided into two groups-the PHLF and non-PHLF groups-based on the presence or absence of early postoperative liver failure, respectively.
Long-term postoperative recovery of liver function was analyzed based on the time to recovery (days) of Child-Pugh class A and serum TB < 2 mg/dL and Alb > 2.8 g/dL. When blood transfusions or blood products were used, recovery was identified based on two consecutive samples without blood transfusions or use of blood products between the samples. The patients were divided into two groups - serum type IV collagen 7S ≤ 6 ng/mL and that with serum type IV collagen 7S > 6 ng/mL-, because type IV collagen 7S ≤ 6 ng/mL is defined as within normal limit in many laboratory companies which measure type IV collagen 7S. The minimum time to recovery was 1 d. For example, if serum TB < 2 mg/dL was observed on postoperative day 3, the time to recovery was 3 d. Even in cases with preoperative serum Alb < 2.8 g/dL, the time to recovery was from the day of surgery to the postoperative day when the levels rose above 2.8 g/dL. All patients were followed up every 1-3 mo after surgery.
A pathologist evaluated fibrosis of the background liver using the METAVIR score in 91 patients between May 2013 and December 2014[14]. We analyzed the pathological data of these patients. Additionally, Elastica van Gieson (EVG) staining of the background liver was performed to determine the areas of fibrosis. For pathological evaluation, 3-μm liver specimen slices were fixed with formalin and stained with EVG. A NanoZoomer HT (Hamamatsu Photonics, Hamamatsu, Japan) was used to obtain an overall tissue image from virtual slides (whole-slide images). The analysis was performed at 0.46 μm/pixel in 20 fields of view. Based on this analysis, whole-slide images were placed into five categories-four tissue components (collagen, elastin, nuclei, and cytoplasm) and one non-tissue component-and the amount of each tissue component was measured[15].
Continuous variables are expressed as mean ± SD; either the χ2 test or Kruskal-Wallis test was used for comparisons. We identified factors associated with significant differences in early postoperative liver failure and long-term postoperative recovery of liver function using multivariate logistic regression and Cox regression. The relationship between collagen and elastin levels and the presence or absence of postoperative early liver failure was examined using the Mann-Whitney U test.
We also examined the relationship between collagen and elastin levels and the other parameters. Spearman’s correlation coefficient was used for correlations with collagen and elastin levels. Long-term postoperative recovery of liver function was compared between the group of patients with serum type IV collagen 7S ≤ 6 ng/mL and that with serum type IV collagen 7S > 6 ng/mL using the Kaplan-Meier method. All statistical analyses were performed using SPSS version 25 (IBM Japan, Tokyo, Japan) with P < 0.05 considered as statistically significant.
Data regarding ICG-R15 and ARFI, which are factors that affect liver function, were missing in 10.2% and 29.8% of cases, respectively. As this harms the reliability and accuracy of the analyses, widens the confidence intervals (CIs), and can result in a bias in the calculation of odds ratios (ORs), we hence compensated for the missing values with multiple imputations using preoperative levels of serum TB, ALB, and cholinesterase and prothrombin time to predict the missing ICG-R15 values and preoperative levels of serum aspartate aminotransferase, type IV collagen 7S, and hyaluronic acid to predict the missing ARFI values. Data were generated for 20 ICG-R15 and ARFI values, which were analyzed separately and combined with the results to calculate the ORs[16,17].
Table 1 summarizes the patient characteristics, preoperative liver function parameters, and operative procedures. Based on the ISGLS definition, there were 27 cases of early post-hepatectomy liver failure (PHLF) and 188 cases without early PHLF (non-PHLF). Of these, nine were of grade A and 18 were of grade B. There were no intraoperative deaths in this study.
| Overall (n = 215) | No postoperative liver failure (n = 188) | Postoperative liver failure (n = 27) | P value | ||
| Age, yr (median) | 68 | 68 | 72 | 0.107 | |
| Sex | Men | 148 | 125 | 23 | 0.073 |
| Women | 67 | 63 | 4 | ||
| Child-Pugh score | 5 | 180 | 162 | 18 | 0.01 |
| 6 | 27 | 22 | 5 | ||
| 7 | 7 | 3 | 4 | ||
| 8 | 1 | 1 | 0 | ||
| Liver tumor | Hepatocellular carcinoma | 87 | 75 | 12 | 0.030 |
| Colorectal metastases | 49 | 46 | 3 | ||
| Hilar cholangiocarcinoma | 24 | 17 | 7 | ||
| Intrahepatic cholangiocarcinoma | 20 | 16 | 4 | ||
| Metastatic liver tumor (other than colorectal cancer) | 22 | 21 | 1 | ||
| Other tumors | 13 | 13 | 0 | ||
| Surgery | Partial resection | 112 | 103 | 9 | 0.041 |
| Sectionectomy | 23 | 22 | 1 | ||
| Lobectomy (+extended) | 73 | 58 | 15 | ||
| Three sectionectomy | 7 | 5 | 2 |
Tables 2 and 3 summarize the results of the analyses of preoperative liver function parameters between the PHLF and non-PHLF groups. In multivariate analysis, preoperative type IV collagen 7S level was a significant independent factor associated with early postoperative liver failure (OR = 1.543; 95%CI: 1.258-1.892; P < 0.001).
| Factor | No postoperative liver failure | Postoperative liver failure | Univariate analysis | |
| (n = 188) | (n = 27) | OR (95%CI) | P value | |
| Age, yr (median) | 68 | 72 | 0.095 | |
| Sex, male/female | 125/63 | 4/23 | 0.064 | |
| Preoperative biliary drainage | 15 | 7 | 0.005 | |
| Child-Pugh score | 5.16 ± 0.45 | 5.48 ± 0.75 | 2.505 (1.345-4.665) | 0.004 |
| Blood platelet (× 104/μL) | 19.5 ± 7.7 | 16.7 ± 6.6 | 0.099 | |
| Total bilirubin (mg/dL) | 0.80 ± 0.33 | 0.90 ± 0.39 | 0.099 | |
| PT-INR (INR) | 1.00 ± 0.12 | 1.09 ± 0.13 | 63.56 (2.533-1595.002) | 0.012 |
| Albumin (g/dL) | 4.03 ± 0.45 | 3.64 ± 0.54 | 0.210 (0.091-0.484) | < 0.001 |
| Aspartate aminotransferase (IU/L) | 30.9 ± 23.6 | 40.6 ± 24.8 | 1.012 (0.999-1.025) | 0.071 |
| Alanine aminotransferase (IU/L) | 27.3 ± 20.7 | 32.6 ± 24.2 | 0.171 | |
| Cholinesterase (IU/L) | 284.2 ± 82.1 | 194.6 ± 69.9 | 0.982 (0.975-0.990) | < 0.001 |
| Total cholesterol (mg/dL) | 188.2 ± 39.2 | 165.8 ± 37.1 | 0.984 (0.972-0.996) | 0.011 |
| ICG-R15 (%) | 10.9 ± 9.01 | 14.8 ± 11.1 | 1.034 (0.998-1.071) | 0.068 |
| Type IV collagen 7S (ng/mL) | 5.50 ± 1.91 | 8.80 ± 3.06 | 1.601 (1.340-1.914) | < 0.001 |
| Hyaluronic acid (ng/mL) | 88.5 ± 100.6 | 256.5 ± 216.5 | 1.005 (1.001-1.009) | 0.018 |
| ARFITM (v/s) | 1.56 ± 0.57 | 2.01 ± 0.69 | 2.785 (1.431-5.422) | 0.003 |
| CRP (mg/dL) | 0.39 ± 1.01 | 0.82 ± 1.33 | 1.319 (0.995-1.747) | 0.054 |
| Extent of hepatic resection[26] | 0.81 ± 0.97 | 1.37 ± 1.04 | 1.688 (1.114-2.557) | 0.013 |
| Factors | Multivariate analysis | |
| OR (95%CI) | P value | |
| Sex, male/female | 2.802 (0.813-9.653) | 0.0513 |
| ICG-R15 (%) | 0.998 (0.951-1.047) | 0.466 |
| Type IV collagen 7S (ng/mL) | 1.543 (1.258-1.892) | < 0.001 |
| ARFITM (v/s) | 1.525 (0.673-3.457) | 0.156 |
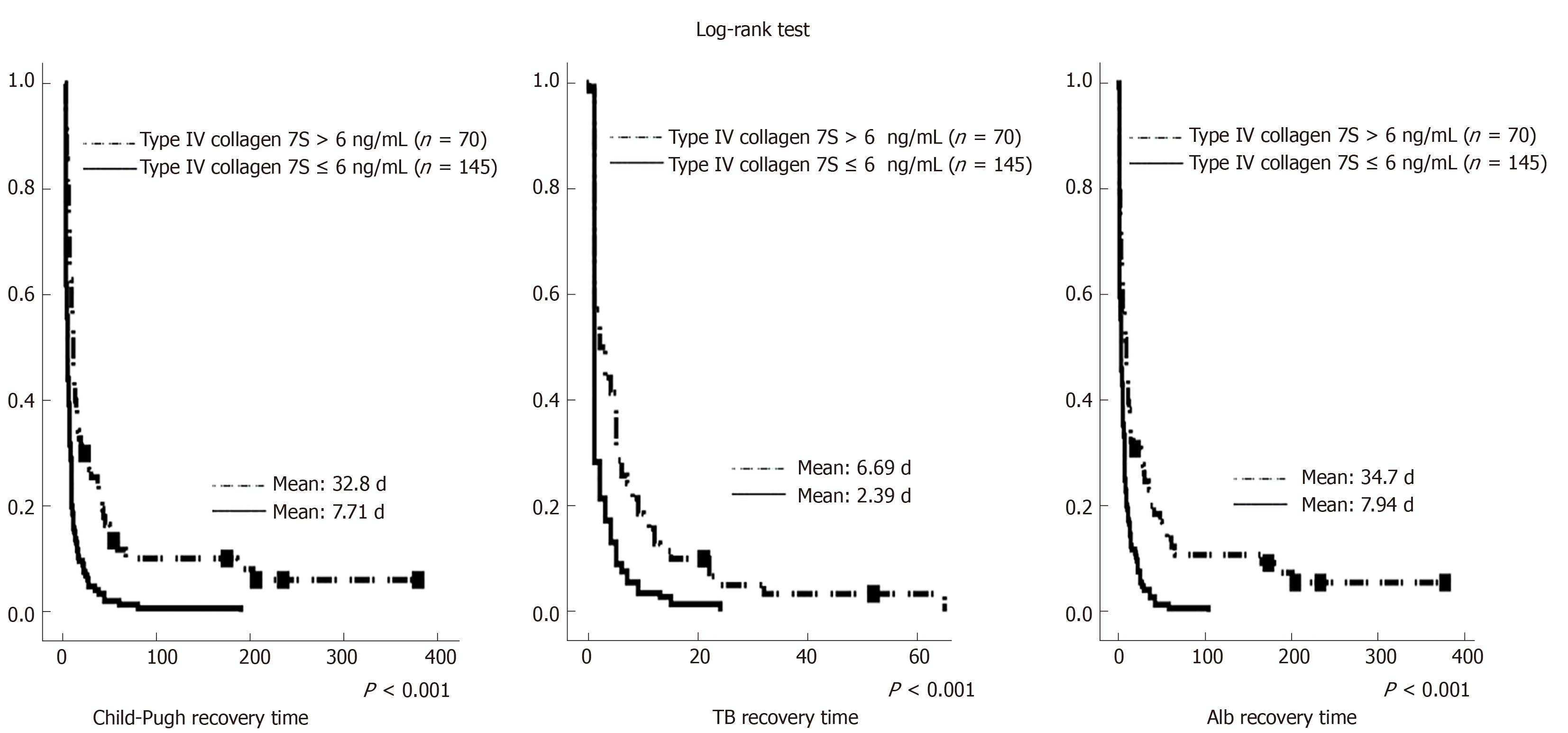
Tables 4, 5, and 6 summarize the results of Cox regression analyses of time to recovery. Preoperative type IV collagen 7S level was a significant independent factor associated with the number of days until recovery to these levels in Cox regression analyses. Figure 1 illustrates the differences in time to recovery. The time to recovery was significantly shorter in the group with preoperative type IV collagen 7S ≤ 6 ng/mL than in the group with preoperative type IV collagen 7S > 6 ng/mL.
| Factors | Univariate analysis | Multivariate analysis | ||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Age, yr (mean) | 0.984 (0.972-0.996) | 0.007 | 0.837 | |
| Sex, male/female | 0.688 | 0.656 | ||
| Child-Pugh score | 0.577 (0.427-0.780) | < 0.001 | 0.123 | |
| Cholinesterase (IU/L) | 1.002 (1.001-1.003) | 0.001 | 0.346 | |
| Total cholesterol (mg/dL) | 1.004 (1.001-1.007) | 0.024 | 0.932 | |
| ICG-R15 (%) | 0.980 (0.964-0.997) | 0.024 | 0.756 | |
| Type IV collagen 7S (ng/mL) | 0.837 (0.784-0.894) | < 0.001 | 0.779 (0.682-0.890) | < 0.001 |
| Hyaluronic acid (ng/mL) | 0.996 (0.994-0.999) | 0.003 | 0.179 | |
| ARFITM (v/s) | 0.651 (0.490-0.866) | 0.003 | 0.625 | |
| CRP (mg/dL) | 0.792 (0.669-0.936) | 0.006 | 0.142 | |
| Extent of hepatic resection[26] | 0.813 (0.707-0.936) | 0.004 | 0.64 | |
| Factors | Univariate analysis | Multivariate analysis | ||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Age, yr (mean) | 0.275 | 0.53 | ||
| Sex, male/female | 0.127 | 0.222 | ||
| Child-Pugh score | 0.749 (0.565-0.994) | < 0.001 | 0.21 | |
| PT-INR (INR) | 0.195 (0.47-0.803) | 0.024 | 0.155 | |
| Cholinesterase (IU/L) | 1.002 (1.001-1.004) | 0.003 | 0.106 | |
| ICG-R15 (%) | 0.165 | |||
| Type IV collagen 7S (ng/mL) | 0.905 (0.851-0.926) | 0.001 | 0.906 (0.851-0.962) | 0.001 |
| Hyaluronic acid (ng/mL) | 0.552 | |||
| ARFITM (v/s) | 0.056 | |||
| CRP (mg/dL) | 0.118 | |||
| Extent of hepatic resection[26] | 0.159 | |||
| Factors | Univariate analysis | Multivariate analysis | ||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Age, yr (mean) | 0.985 (0.973-0.996) | 0.008 | 0.18 | |
| Sex, male/female | 0.059 | 0.457 | ||
| Child-Pugh score | 0.573 (0.424-0.776) | < 0.001 | 0.611 | |
| Cholinesterase (IU/L) | 1.002 (1.001-1.003) | 0.001 | 0.39 | |
| ICG-R15 (%) | 0.979 (0.962-0.996) | 0.008 | 0.983 | |
| Type IV collagen 7S (ng/mL) | 0.841 (0.787-0.899) | < 0.001 | 0.759 (0.659-0.875) | < 0.001 |
| Hyaluronic acid (ng/mL) | 0.996 (0.994-0.999) | 0.004 | 0.292 | |
| ARFITM (v/s) | 0.723 (0.545-0.959) | 0.024 | 0.774 | |
| CRP (mg/dL) | 0.808 (0.685-0.953) | 0.011 | 0.192 | |
| Extent of hepatic resection[26] | 0.830 (0.722-0.955) | 0.009 | 0.636 | |
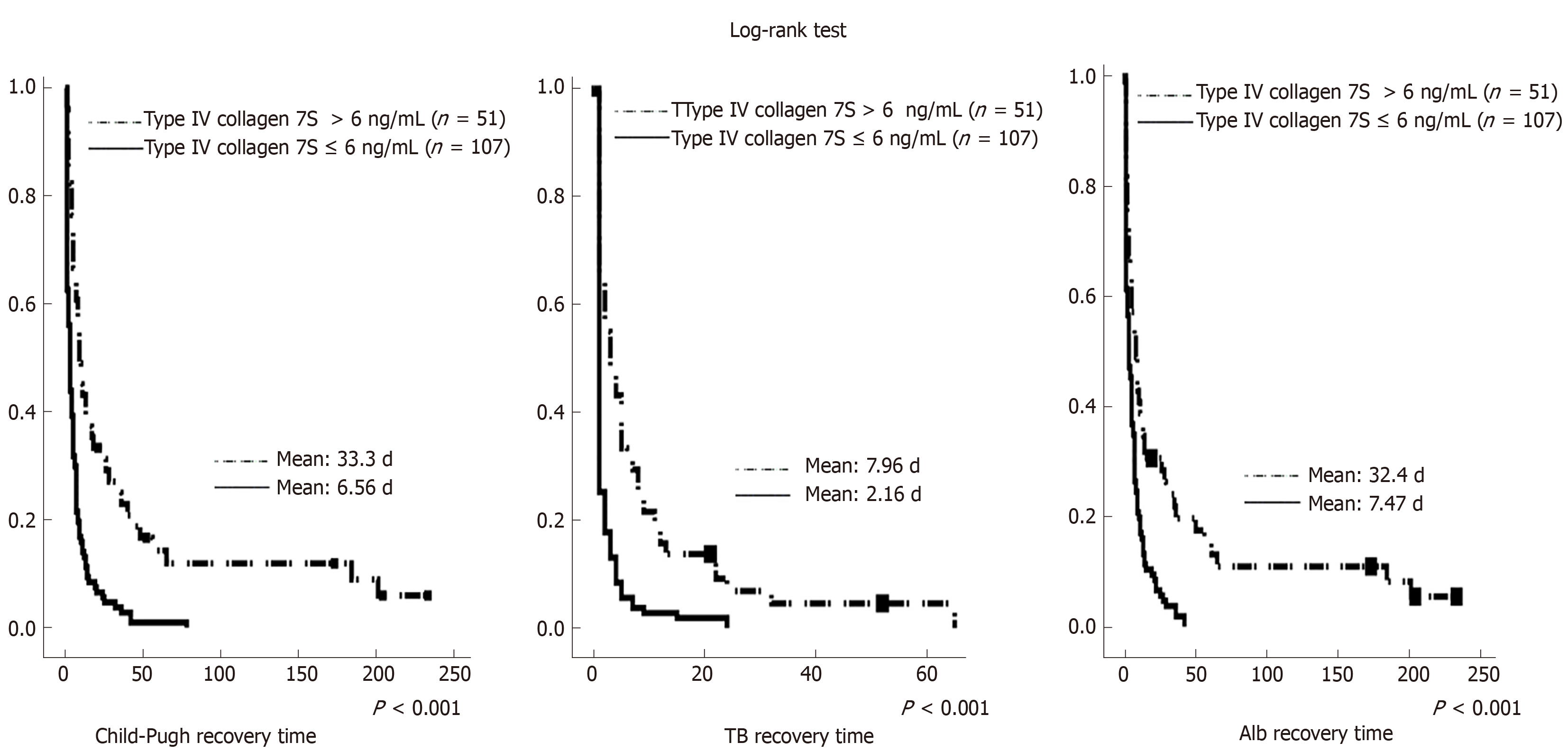
When assessing the presence or absence of fibrosis, there can be bias due to the presence or absence of chronic viral hepatitis, which makes the liver prone to failure. Therefore, an additional analysis of 158 patients without chronic hepatitis virus was performed. Tables 7 and 8 summarize these results. In these analyses, preoperative type IV collagen 7S was a significant factor associated with early postoperative liver failure (OR = 1.423; 95%CI: 1.074-1.886; P < 0.001). Figure 2 illustrates the time to recovery in 158 patients without chronic hepatitis virus. For long-term postoperative recovery of liver function as well, the time to recovery was significantly shorter in the group with preoperative type IV collagen 7S ≤ 6 ng/mL than in the group with preoperative type IV collagen 7S > 6 ng/mL.
| Factors | No postoperative liver failure | Postoperative liver failure | Univariate analysis | |
| (n = 136) | (n = 22) | OR (95%CI) | P value | |
| Age, yr (mean) | 65.3 | 70.7 | 0.09 | |
| Sex, male/female | 87/49 | 4/18 | 0.109 | |
| Preoperative biliary drainage | 9 | 4 | 0.045 | |
| Child-Pugh score | 5.15 ± 0.39 | 5.59 ± 0.80 | 3.694 (1.766-7.729) | 0.001 |
| Platelets (× 104/μL) | 21.1 ± 7.7 | 17.1 ± 7.0 | 0.991 | |
| Total bilirubin (mg/dL) | 0.79 ± 0.33 | 0.91 ± 0.40 | 0.111 | |
| PT-INR (INR) | 0.99 ± 0.13 | 1.1 ± 0.14 | 123.581 (2.637-5791.435) | 0.014 |
| Albumin (g/dL) | 4.03 ± 0.44 | 3.57 ± 0.60 | 0.157 (0.059-0.414) | < 0.001 |
| Aspartate aminotransferase (IU/L) | 30.8 ± 26.6 | 41.5 ± 25.9 | 0.121 | |
| Alanine aminotransferase (IU/L) | 28.0 ± 22.9 | 33.6 ± 25.5 | 0.296 | |
| Cholinesterase (IU/L) | 293.1 ± 85.1 | 192.2 ± 67.9 | 0.979 (0.970-0.988) | < 0.001 |
| Total cholesterol (mg/dL) | 192.2 ± 42.2 | 168.4 ± 40.2 | 0.985 (0.973-0.998) | 0.02 |
| ICG-15 clearance rate (%) | 10.3 ± 9.11 | 15.0 ± 11.8 | 1.034 (0.998-1.081) | 0.064 |
| Type IV collagen 7S (ng/mL) | 5.36 ± 1.85 | 9.15 ± 3.13 | 1.731 (1.383-2.167) | < 0.001 |
| Hyaluronic acid (ng/mL) | 78.7 ± 99.2 | 261.9 ± 207.3 | 1.005 (1.004-1.011) | < 0.001 |
| ARFITM (v/s) | 1.49 ± 0.53 | 1.98 ± 0.67 | 2.785 (1.546-7.887) | 0.003 |
| CRP (mg/dL) | 0.47 ± 1.14 | 1.00 ± 1.42 | 0.056 | |
| Extent of hepatic resection[26] | 0.90 ± 1.0 | 1.41 ± 1.05 | 1.638 (1.037-2.587) | 0.034 |
| Factors | Multivariate analysis | |
| OR (95%CI) | P value | |
| Cholinesterase (IU/L) | 0.987 (0.977-0.998) | 0.0083 |
| ICG-R15 (%) | 0.959 (0.896-1.026) | 0.11 |
| Type IV collagen 7S (ng/mL) | 1.423 (1.074-1.886) | 0.002 |
| Hyaluronic acid (ng/mL) | 1.004 (1.000-1.008) | 0.021 |
| ARFITM (v/s) | 1.144 (0.406-3.220) | 0.6 |
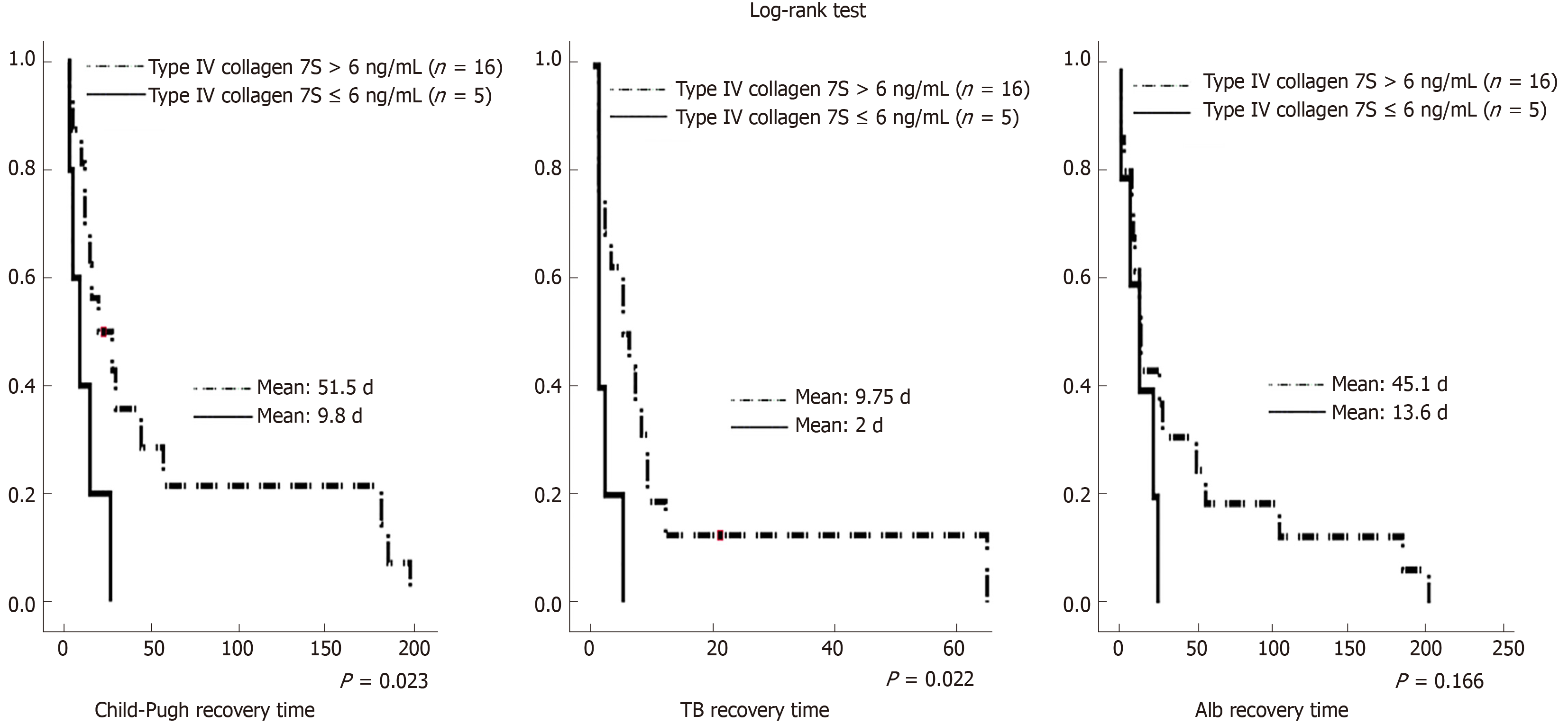
Similarly, there can be bias due to the presence or absence of preoperative jaundice, which makes the liver prone to failure. Therefore, an additional analysis of 21 patients without preoperative jaundice was performed. Table 9 summarizes the results of the sub-analysis of 21 patients with preoperative jaundice. Preoperative type IV collagen 7S level was a significant independent factor associated with early postoperative liver failure (OR = 1.540; 95%CI: 1.036-2.288; P = 0.033). Further, Figure 3 illustrates the time to recovery. For long-term postoperative recovery of liver function as well, the time to recovery was significantly shorter in the group with preoperative type IV collagen 7S ≤ 6 ng/mL than in the group with preoperative type IV collagen 7S > 6 ng/mL. The time to recovery of Alb levels tended to be shorter in the group with preoperative type IV collagen 7S ≤ 6 ng/mL than in the group with preoperative type IV collagen 7S > 6 ng/mL; however, the difference was not significant.
| Factors | Univariate analysis | Multivariate analysis | ||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Age, yr (mean) | 0.74 | 0.817 | ||
| Sex, male/female | 0.105 | 0.237 | ||
| Child-Pugh score | 0.22 | |||
| Cholinesterase (IU/L) | 0.979 (0.970-0.988) | 0.066 | 0.157 | |
| Total cholesterol (mg/dL) | 0.652 | |||
| ICG-R15 (%) | 0.371 | |||
| Type IV collagen 7S (ng/mL) | 1.540 (1.036-2.288) | 0.033 | 1.540 (1.036-2.288) | 0.033 |
| Hyaluronic acid (ng/mL) | 0.187 | |||
| ARFI TM (v/s) | 0.171 | |||
| CRP (mg/dL) | 0.533 | |||
| Extent of hepatic resection[26] | 0.481 | |||
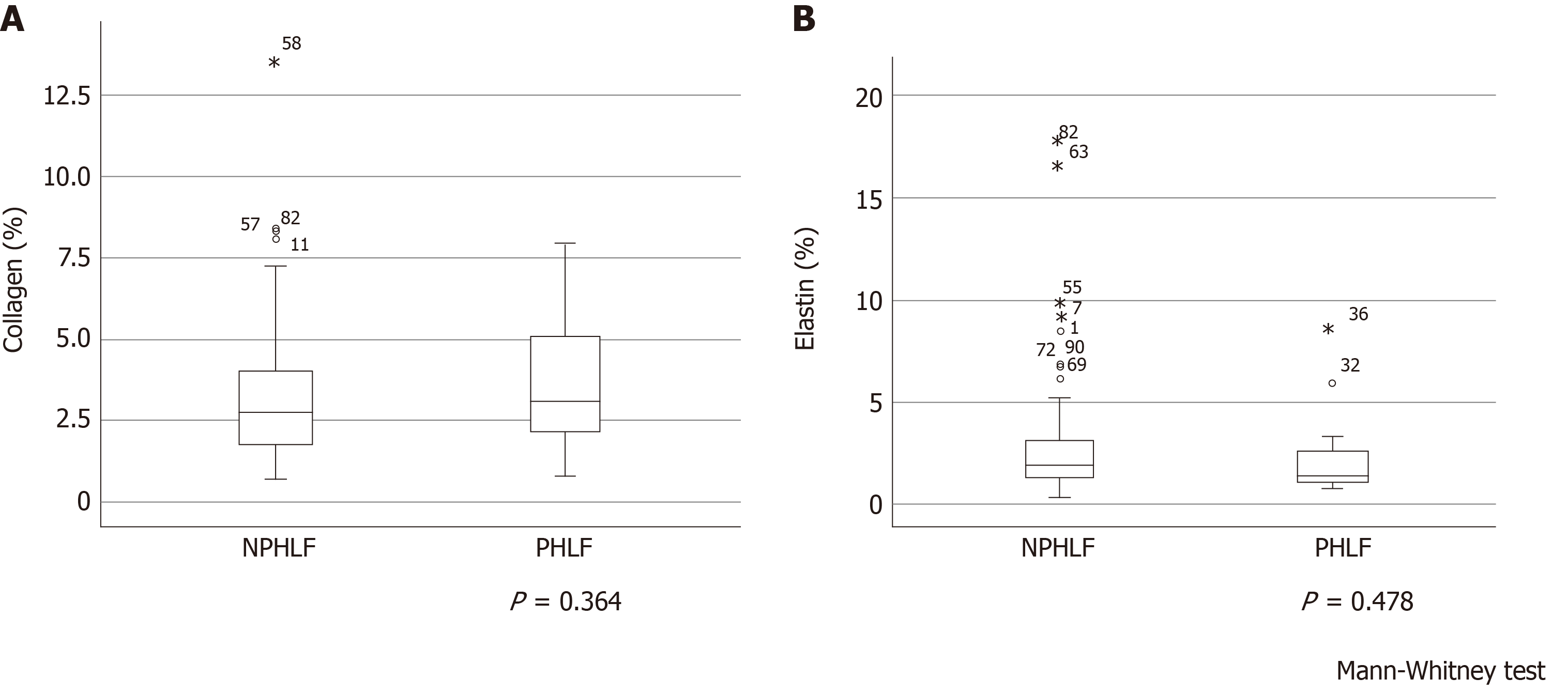
A pathologist evaluated fibrosis of the background liver using the METAVIR score in 91 patients between May 2013 and December 2014. METAVIR score was not a significant factor associated with postoperative early liver failure (P = 0.801) (Table 10). Figure 4 illustrates the relationships between collagen and elastin levels and early postoperative liver failure. Collagen and elastin levels were no different between patients who experienced early postoperative liver failure and those who did not. Furthermore, when the correlations of collagen and elastin with other data were examined, the preoperative type IV collagen 7S level was correlated with collagen (ρ = 0.281, P = 0.007) but not with elastin (ρ = 0.167, P = 0.114). ARFI correlated with both collagen (ρ = 0.405, P < 0.001) and elastin (ρ = 0.316, P = 0.002).
| Factors | Univariate analysis | Multivariate analysis | ||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Age, yr (mean) | 0.083 | 0.065 | ||
| Sex, male/female | 0.462 | 0.557 | ||
| Child-Pugh score | 0.347 | |||
| Platelets (× 104/μL) | 0.509 | |||
| PT-INR (INR) | 0.064 | |||
| Aspartate aminotransferase (IU/L) | 0.087 | |||
| Alanine aminotransferase (IU/L) | 0.468 | |||
| Cholinesterase (IU/L) | 0.986 (0.977-0.995) | 0.003 | 0.348 | |
| Total cholesterol (mg/dL) | 0.128 | |||
| ICG-R15 (%) | 0.643 | |||
| Type IV collagen 7S (ng/mL) | 1.514 (0.784-0.894) | < 0.001 | 2.178 (0.684-0.890) | 0.005 |
| Hyaluronic acid (ng/mL) | 1.005 (1.001-1.009) | 0.018 | 0.674 | |
| ARFITM (v/s) | 0.145 | |||
| CRP (mg/dL) | 0.495 | |||
| METAVIR score | 0.801 | |||
| Extent of hepatic resection[26] | 2.296 (0.707-0.936) | 0.009 | 0.86 | |
The purpose of this study was to analyze predictive factors for early postoperative liver failure and long-term postoperative recovery of liver function. The results indicated that preoperative type IV collagen 7S was a significant independent factor associated with early postoperative liver failure. Additionally, the time to recovery of Child-Pugh classification and serum TB and Alb-which are indicators of long-term postoperative recovery of liver function-was significantly shorter in the group with preoperative type IV collagen 7S ≤ 6 ng/mL than in the group with preoperative type IV collagen 7S > 6 ng/mL. Similar results were observed irrespective of multiple imputations used in the analysis of postoperative liver failure.
Various reports have described existing methods for predicting early postoperative liver failure. Among them, the Child-Pugh classification is widely used worldwide. ICG-R15 is also commonly used in Asia and has been recognized in several other parts of the world[18]. However, in the present study, the Child-Pugh classification and ICG-R15 were not sufficient predictors of early postoperative liver failure. Additionally, the Child-Pugh classification, ICG-R15, and other liver function tests only focus on early postoperative liver failure and do not consider the long-term postoperative recovery of liver function.
The present study used the time to recovery of the Child-Pugh classification and serum TB and Alb as indicators of long-term postoperative recovery of liver function. Postoperative recovery of liver function is particularly clinically important when adjuvant therapy is used. In our study, preoperative type IV collagen 7S was a significant independent factor associated with the time to recovery. Furthermore, the time to recovery was significantly shorter in the group with preoperative type IV collagen 7S ≤ 6 ng/mL than in the group with preoperative type IV collagen 7S > 6 ng/mL. This suggests that type IV collagen 7S may also be useful in predicting the long-term postoperative recovery of liver function.
There can be bias due to the presence or absence of chronic viral hepatitis or preoperative jaundice, which makes the liver prone to failure. In additional analyses of patients without chronic viral hepatitis associated with fibrosis, preoperative type IV collagen 7S was also a significant factor associated with early postoperative liver failure and long-term postoperative recovery of liver function. Similarly, in cases of jaundice, type IV collagen 7S was also a significant independent factor associated with early postoperative liver failure and long-term postoperative recovery of liver function. In cases without chronic viral hepatitis associated with fibrosis and cases with jaundice, early postoperative liver failure and postoperative long-term recovery of liver function were also associated with preoperative type IV collagen 7S.
The METAVIR score-which represents histological liver fibrosis-was not significantly associated with early postoperative liver failure. Additionally, we calculated the amount of collagen and elastin fibers in the samples using EVG staining but found no histological differences between patients with early postoperative liver failure and those without (Figure 4).
We evaluated two markers of liver fibrosis-type IV collagen 7S[19] and hyaluronic acid[20]. In our study, preoperative type IV collagen 7S was a significant independent factor associated with early PHLF and long-term postoperative recovery of liver function. Type IV collagen is a basement membrane protein that is located around the sinusoids in the liver and is an immunohistochemical marker of the basement membrane[21]. In a normal liver, sinusoids have no basement membrane; however, as the liver undergoes fibrosis, sinusoidal capillarization occurs and the basement membrane appears. The presence of type IV collagen 7S in the basement membrane is believed to reflect in the form of increased levels in the blood[22].
There are two possible reasons for the association of type IV collagen 7S with early postoperative liver failure and long-term postoperative recovery of liver function. Type IV collagen 7S is affected by factors other than fibrosis. Alternatively, it may reflect a type of fibrosis that does not exhibit histological differences from non-fibrotic tissue. In recent years, type IV collagen 7S has been believed to reflect fibrogenesis-an indicator of collagen production during this process. Therefore, fibrogenesis is different from the histological appearance of fibrosis. Fibrogenesis reflects the current damage to the liver caused by fibrosis and not liver damage that has accumulated over many years, which could be why the histological differences were not observed in the present study. According to Parola et al[23], fibrogenesis is a reaction to liver damage that involves growth of extracellular matrices (ECM) for liver regeneration. Increased ECM in the space of Disse reduces liver function[24]. Therefore, fibrogenesis is believed to be a reaction that reduces liver function. The reason why type IV collagen 7S is associated with early postoperative liver failure and postoperative long-term recovery of liver function may be because it reflects fibrosis that does not exhibit histological differences. This suggests that instead of fibrosis—which manifests histologically-fibrogenesis affects the early and long-term postoperative recovery of liver function.
This study has some limitations. Our study was a retrospective analysis at a single center, which limits the validity of the results. Additionally, there is the possibility of bias because only patients that were able to undergo hepatectomy were included. Additionally, a new marker of liver fibrosis-M2BPGi[25]-was listed for insurance coverage in Japan in January 2015; however, it was not examined in this study. Going forward, multicenter, prospective studies are required to investigate the application of preoperative serum type IV collagen 7S levels in predicting the postoperative long-term course and early liver failure.
In conclusion, preoperative type IV collagen 7S is a useful predictive factor for early postoperative liver failure and long-term postoperative recovery of liver function. In particular, similar results were obtained in patients without chronic hepatitis virus in the background liver and in those with jaundice. Therefore, preoperative type IV collagen could be useful in predicting early liver failure and long-term postoperative recovery of liver function in different subsets of patients undergoing hepatectomy.
Liver resection is an effective treatment for benign and malignant liver tumors. However, a method for preoperative evaluation of hepatic reserve has not yet been established. Previously reported assessments of preoperative hepatic reserve focused only on liver failure in the early postoperative period and did not consider the long-term recovery of hepatic reserve.
When determining eligibility for hepatectomy, the underlying pathophysiology needs to be considered to determine if the functional hepatic reserve can withstand both surgery and any postoperative therapy.
To identify pre-hepatectomy factors associated with both early postoperative liver failure and long-term postoperative liver function recovery.
This study was a retrospective cohort study. We retrospectively investigated 215 patients who underwent hepatectomy at our hospital between May 2013 and December 2016. Early post-hepatectomy liver failure (PHLF) was defined using the International Study Group of Liver Surgery’s definition of PHLF. Long-term postoperative recovery of liver function was defined as the time taken for serum total bilirubin and albumin levels to return to levels of < 2 mg/dL and > 2.8 g/dL, respectively, and the time taken for Child-Pugh score to return to Child-Pugh class A.
Preoperative type IV collagen 7S was identified as a significant independent factor associated with both PHLF and postoperative long-term recovery of liver function. Further analysis revealed that the time taken for the recovery of Child-Pugh scores and serum total bilirubin and albumin levels was significantly shorter in patients with type IV collagen 7S ≤ 6 ng/mL than in those with type IV collagen 7S > 6 ng/mL. In additional analyses, similar results were observed in patients without chronic viral hepatitis associated with fibrosis.
Preoperative type IV collagen 7S is a useful predictive factor for early postoperative liver failure and long-term postoperative recovery of liver function. In particular, similar results were obtained in patients without chronic hepatitis virus in the background liver.
Preoperative type IV collagen could be useful in predicting early liver failure and long-term postoperative recovery of liver function in different subsets of patients undergoing hepatectomy in this study. Multicenter and prospective studies are required to investigate the application of preoperative serum type IV collagen 7S levels in predicting the postoperative early liver failure and long-term postoperative recovery of liver function.
| 1. | Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, Corvera C, Weber S, Blumgart LH. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397-406; discussion 406-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 374] [Reference Citation Analysis (0)] |
| 2. | Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, Wong J. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg. 1999;229:322-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 559] [Article Influence: 20.7] [Reference Citation Analysis (2)] |
| 3. | Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, Wong J. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg. 2004;240:698-708; discussion 708-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 533] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 4. | Das BC, Isaji S, Kawarada Y. Analysis of 100 consecutive hepatectomies: risk factors in patients with liver cirrhosis or obstructive jaundice. World J Surg. 2001;25:266-72; discussion 272-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 47] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Virani S, Michaelson JS, Hutter MM, Lancaster RT, Warshaw AL, Henderson WG, Khuri SF, Tanabe KK. Morbidity and mortality after liver resection: results of the patient safety in surgery study. J Am Coll Surg. 2007;204:1284-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 173] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 6. | Albers I, Hartmann H, Bircher J, Creutzfeldt W. Superiority of the Child-Pugh classification to quantitative liver function tests for assessing prognosis of liver cirrhosis. Scand J Gastroenterol. 1989;24:269-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 184] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Li H, Li B, Wei Y. Potential factors dedicated to postoperative liver dysfunction in patients with normal preoperative ICG-15 clearance rate. Dig Dis Sci. 2013;58:1163-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Tygstrup N. Determination of the hepatic elimination capacity (Lm) of galactose by single injection. Scand J Clin Lab Invest Suppl. 1966;18:118-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 105] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Mizumoto R, Kawarada Y, Noguchi T. Preoperative estimation of operative risk in liver surgery, with special reference to functional reserve of the remnant liver following major hepatic resection. Jpn J Surg. 1979;9:343-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Mori K, Ozawa K, Yamamoto Y, Maki A, Shimahara Y, Kobayashi N, Yamaoka Y, Kumada K. Response of hepatic mitochondrial redox state to oral glucose load. Redox tolerance test as a new predictor of surgical risk in hepatectomy. Ann Surg. 1990;211:438-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 62] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Kwon AH, Matsui Y, Kaibori M, Ha-Kawa SK. Preoperative regional maximal removal rate of technetium-99m-galactosyl human serum albumin (GSA-Rmax) is useful for judging the safety of hepatic resection. Surgery. 2006;140:379-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Miyagawa S, Makuuchi M, Kawasaki S, Kakazu T. Criteria for safe hepatic resection. Am J Surg. 1995;169:589-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 265] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 13. | Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, Koch M, Makuuchi M, Dematteo RP, Christophi C, Banting S, Usatoff V, Nagino M, Maddern G, Hugh TJ, Vauthey JN, Greig P, Rees M, Yokoyama Y, Fan ST, Nimura Y, Figueras J, Capussotti L, Büchler MW, Weitz J. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149:713-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1224] [Cited by in RCA: 1824] [Article Influence: 121.6] [Reference Citation Analysis (1)] |
| 14. | Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2860] [Cited by in RCA: 3130] [Article Influence: 104.3] [Reference Citation Analysis (1)] |
| 15. | Abe T, Hashiguchi A, Yamazaki K, Ebinuma H, Saito H, Kumada H, Izumi N, Masaki N, Sakamoto M. Quantification of collagen and elastic fibers using whole-slide images of liver biopsy specimens. Pathol Int. 2013;63:305-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Larsen C, Bousquet V, Delarocque-Astagneau E, Pioche C, Roudot-Thoraval F; HCV Surveillance Steering Committee; HCV Surveillance Group, Desenclos JC. Hepatitis C virus genotype 3 and the risk of severe liver disease in a large population of drug users in France. J Med Virol. 2010;82:1647-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Hamidou Z, Chibaudel B, Hebbar M, Hug de Larauze M, André T, Louvet C, Brusquant D, Garcia-Larnicol ML, de Gramont A, Bonnetain F. Time to Definitive Health-Related Quality of Life Score Deterioration in Patients with Resectable Metastatic Colorectal Cancer Treated with FOLFOX4 versus Sequential Dose-Dense FOLFOX7 followed by FOLFIRI: The MIROX Randomized Phase III Trial. PLoS One. 2016;11:e0157067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Fan ST. Liver functional reserve estimation: state of the art and relevance for local treatments: the Eastern perspective. J Hepatobiliary Pancreat Sci. 2010;17:380-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Murawaki Y, Koda M, Okamoto K, Mimura K, Kawasaki H. Diagnostic value of serum type IV collagen test in comparison with platelet count for predicting the fibrotic stage in patients with chronic hepatitis C. J Gastroenterol Hepatol. 2001;16:777-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Murawaki Y, Ikuta Y, Koda M, Nishimura Y, Kawasaki H. Clinical significance of serum hyaluronan in patients with chronic viral liver disease. J Gastroenterol Hepatol. 1996;11:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Mak KM, Chen LL, Lee TF. Codistribution of collagen type IV and laminin in liver fibrosis of elderly cadavers: immunohistochemical marker of perisinusoidal basement membrane formation. Anat Rec (Hoboken). 2013;296:953-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Murawaki Y, Ikuta Y, Koda M, Kawasaki H. Serum type III procollagen peptide, type IV collagen 7S domain, central triple-helix of type IV collagen and tissue inhibitor of metalloproteinases in patients with chronic viral liver disease: relationship to liver histology. Hepatology. 1994;20:780-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 97] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Parola M, Pinzani M. Hepatic wound repair. Fibrogenesis Tissue Repair. 2009;2:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Selden C, Khalil M, Hodgson HJ. What keeps hepatocytes on the straight and narrow? Maintaining differentiated function in the liver. Gut. 1999;44:443-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Kuno A, Ikehara Y, Tanaka Y, Ito K, Matsuda A, Sekiya S, Hige S, Sakamoto M, Kage M, Mizokami M, Narimatsu H. A serum "sweet-doughnut" protein facilitates fibrosis evaluation and therapy assessment in patients with viral hepatitis. Sci Rep. 2013;3:1065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 291] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 26. | Makuuchi M, Arii S, Kanematsu T, Kudo M, Takayasu K, Nakanuma Y, Sakamoto M. General Rules for the Clinical and Pathological Study of Primary Liver Cancer Liver cancer study of Japan. 2010;24. |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gumerova A, Jin B S-Editor: Ma YJ L-Editor: A E-Editor: Ma YJ