Published online Dec 28, 2020. doi: 10.3748/wjg.v26.i48.7707
Peer-review started: October 2, 2020
First decision: November 23, 2020
Revised: November 29, 2020
Accepted: December 6, 2020
Article in press: December 6, 2020
Published online: December 28, 2020
Processing time: 76 Days and 21.4 Hours
Primary intestinal lymphangiectasia (PIL), first described in 1961, is a rare disorder of unknown etiology resulting in protein-losing enteropathy. The disease is characterized by dilatation and leakage of intestinal lymph vessels leading to hypoalbuminemia, hypogammaglobulinemia, and lymphopenia. Since the severity and location of lymph vessels being affected can vary considerably, the range of associated symptoms is wide from mild lower-limb edema to generalized edema, abdominal and/or pleural effusion, and recurrent diarrhea, among others. Although usually developing in early childhood, we present the case of a 34-year-old woman with PIL. Moreover, we performed a literature review systematically assessing clinical presentation, and provide a practical approach to facilitate diagnosis and therapy of PIL in adults.
Our patient presented with unspecific symptoms of abdominal discomfort, fatigue, nausea, and recurrent edema of the lower limbs. Interestingly, a striking collinearity of clinical symptoms with female hormone status was evident. Additionally, polyglobulia, hypoalbuminemia, hypogammaglobulinemia, and transient lymphocytopenia were evident. Due to suspicion of a bone marrow disease, an extensive diagnostic investigation was carried out excluding secondary causes of polyglobulinemia and hypoalbuminemia. The diagnosis of primary intestinal lymphangiectasia was established after 22 wk by histological analysis of biopsy samples obtained via enteroscopy. Consecutively, the patient was put on a high-protein and low-fat diet with medium-chain triglycerides supplementation leading to significant improvement of clinical symptoms until 2 years of follow-up.
PIL can be the reason for cryptogenic hypoalbuminemia, hypogammaglobulinemia, and lymphopenia in adulthood. Due to difficulty in correct diagnosis, treatment initiation is often delayed despite being effective and well-tolerated. This leads to a significant disease burden in affected patients. PIL is increasingly been recognized in adults since the majority of case reports were published within the last 10 years, pointing towards an underestimation of the true prevalence. The association with female hormone status warrants further investigation.
Core Tip: Although Primary intestinal lymphangiectasia usually develops in early childhood, we present the case of a 34-year-old woman. We observed a striking collinearity with female hormone status in our patient, presenting a potential area of future research. Moreover, we performed a literature review of all published case reports so far and systematically assessed clinical presentation to provide a practical approach to facilitate diagnosis and therapy of primary intestinal lymphangiectasia in adults for the first time.
- Citation: Huber R, Semmler G, Mayr A, Offner F, Datz C. Primary intestinal lymphangiectasia in an adult patient: A case report and review of literature. World J Gastroenterol 2020; 26(48): 7707-7718
- URL: https://www.wjgnet.com/1007-9327/full/v26/i48/7707.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i48.7707
Primary intestinal lymphangiectasia (PIL) has been first described by Waldmann et al[1] in 1961 as a rare disorder of intestinal lymphangiectasia that results in protein-losing enteropathy (PLE)[1]. PIL is caused by a diffuse or localized dilatation and/or rupture of intestinal lymphatic vessels in the mucosa, submucosa, or subserosa due to high pressure in lymphatic vessels[2]. Importantly, prevalence and etiology are yet unknown. However, genetic associations are been discussed since the diagnosis is generally established in childhood with very rare cases in adults[3].
Symptoms largely relate to the severity of lymph loss and consecutive loss of proteins resulting in hypoproteinemia, lymphopenia, and decreased serum levels of immunoglobulins. Among others, these symptoms include pitting edemas of the lower limbs, generalized edema, as well as pleural, epicardial, or often chylous abdominal effusion[4]. Here, we present the case of PIL in a 34-year-old female patient, together with a literature review of all case reports on PIL in adulthood (using the terms “Primary intestinal lymphangiectasia” and “Waldman’s disease”), focusing on clinical presentation, and providing a diagnostic and therapeutic overview for clinicians to enhance recognition and facilitate diagnosis.
In October 2018, a 34-year-old woman presented with recurring nausea independent of food intake, episodes of abdominal discomfort, fatigue, occasional episodes of diarrhea as well as a feeling of increased susceptibility to opportunistic infections.
After the end of pregnancy earlier this year, she reported on more frequently observed limb edema. The other medical history including comorbidities and drug intake were unremarkable.
Interestingly, temporary facial edema were occasionally reported already during childhood starting at the age of 12. After puberty, pronounced edema of the lower limbs, recurring nausea and fatigue were continuously reported, but vanished when using oral contraceptives or during pregnancy.
The physical examination at the initial contact did not reveal any abnormalities. Especially, no edema of the upper or lower limb were observed and weight was stable with a body mass index of 18.3 kg/m².
During several routine blood tests after pregnancy, polyglobulia [hemoglobin 17.4 g/dL (normal range: 12.3-15.3 g/dL), blood count 5.82 (normal range: 3.60-5.00)], normal leukocyte count (4.48, normal range: 4.40-11.30) with diminished lymphocyte count (14.6%, normal range: 19.3%-51.7%) following differential blood count, and reduced total serum protein (4.9 g/dL, normal range: 6.2-8.2 g/dL) with hypoalbuminemia (2.8 g/dL, normal range: 3.4-5.0 g/dL) were observed. Additionally, quantitative immunoglobulin analysis displayed hypogammaglobulinemia of 7.3% (normal range: 11.1%-18.8%) with a pronounced deficiency of the IgG class (268 mg/dL, normal range: 700-1600 mg/dL), moderate deficiency of IgA class (54 mg/dL (normal range: 70-500 mg/dL), and reduced IgG-1 and IgG-2 subclasses (193 mg/dL, normal range: 405-1011 mg/dL; 93 mg/dL, normal range: 169-786 mg/dL). Additionally, the CD4: CD8 T-cell ratio was reduced [0.9 (normal range: 1.0–3.6) and kappa and lambda light-chains were diminished [75 mg/dL (normal range: 173-383 mg/dL), 46 mg/dL (normal range: 81-192 mg/dL)]. The urinary sediment showed no proteinuria and no signs of renal, hepatic, pancreatic, or cardiac disease. Since the laboratory constellation pointed towards a cellular and humoral immune defect, exhaustive investigations were started. Negative results for JAK2 mutations (JAK2-exon 12 sequencing and JAK2-mutation V617F) and negative BCR/ABL ratio ruled out polycythemia vera. Additionally, bone marrow analysis neither showed myeloid neoplasia nor infiltration by lymphoma, and ß2-microglobulin was within the normal range ruling out a hematogenous disease. Autoantibody screening and virus serology including hepatitis viridae, cytomegalovirus, Epstein-Bar virus, and human immunodeficiency virus were negative, and pancreatic insufficiency was excluded.
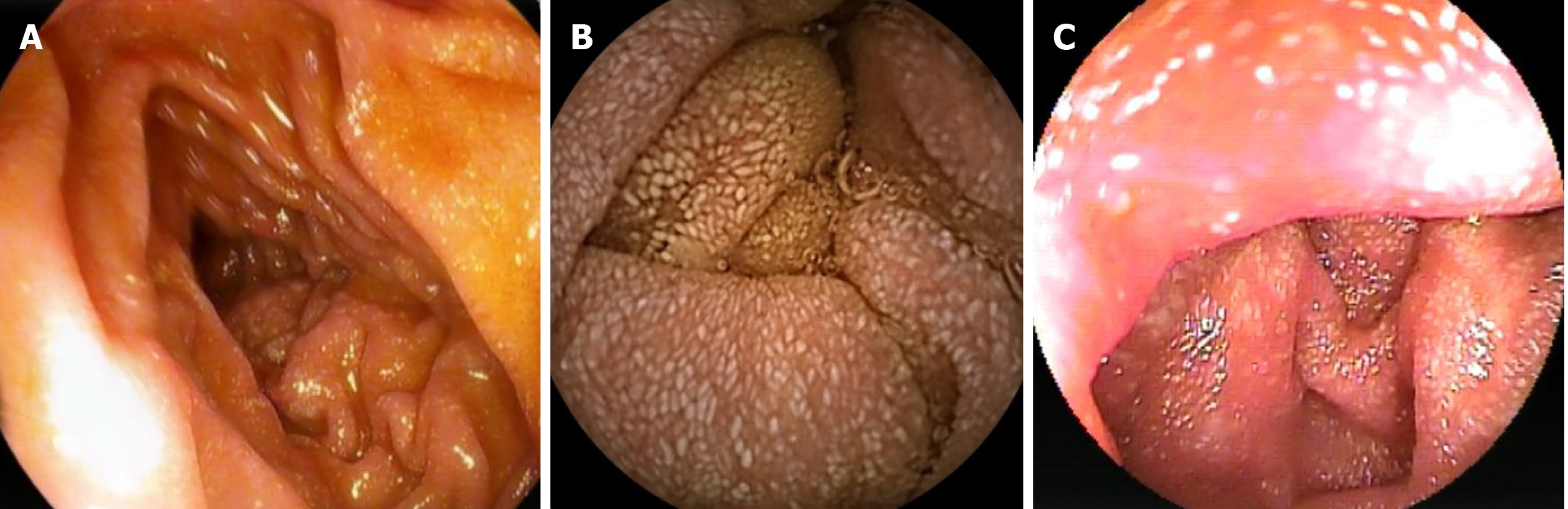
Incidentally, abdominopelvic computerized tomography (CT) showed a thickened wall of the ileum and jejunum with enlarged mesenteric lymph nodes up to 32 mm localized in the lower abdomen. Therefore, an ileocolonoscopy with exploration of > 8 cm of the ileum was performed showing a normal result. After CT-findings were confirmed on magnetic resonance imaging, a gastroduodenoscopy with standard intubation to the mid-descending duodenum revealed creamy white spots of the duodenal mucosa, suggesting lymphedema (Figure 1A). However, the histological evaluation did not show evidence for dilated lymph vessels or PIL, giardiasis, celiac disease, Whipple disease, or intestinal bowel disease, which was additionally excluded by normal calprotectin levels. Following video capsule endoscopy that showed a snowflake appearance of the mucosa (Figure 1B), double-balloon enteroscopy exploring approximately 70 cm of the jejunum verified mucosal lesions compatible with lymphangiectasia macroscopically (Figure 1C).
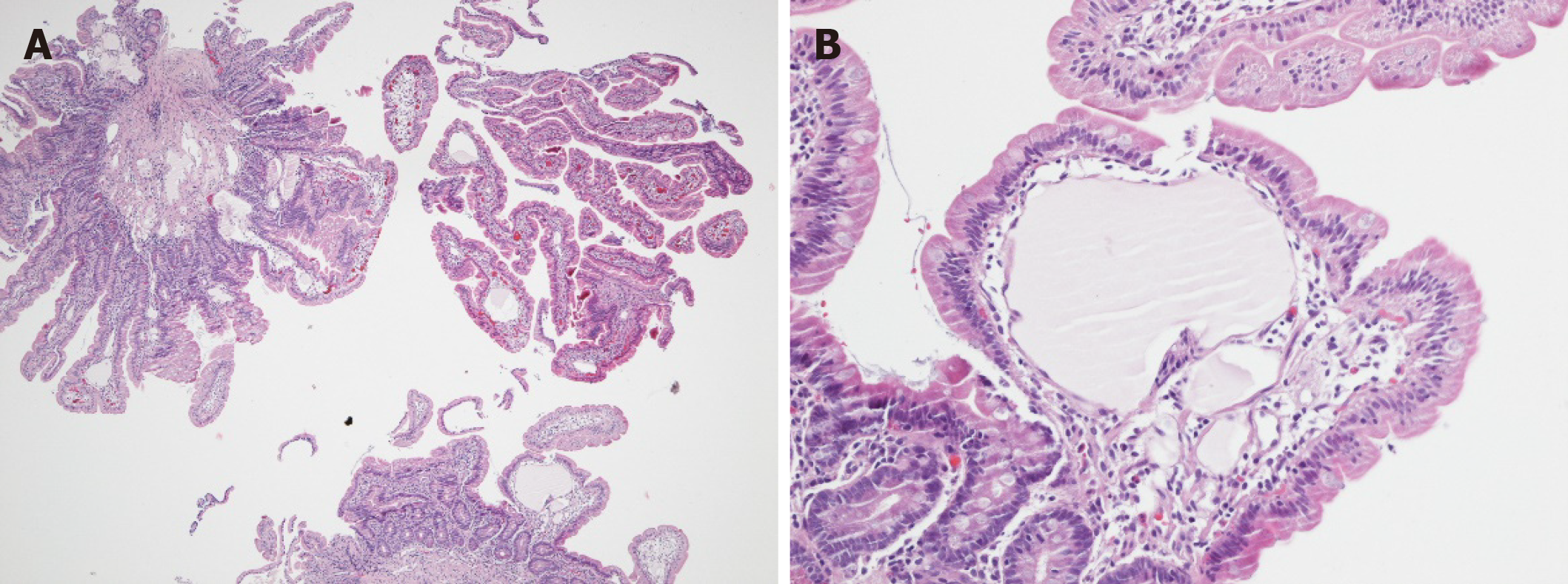
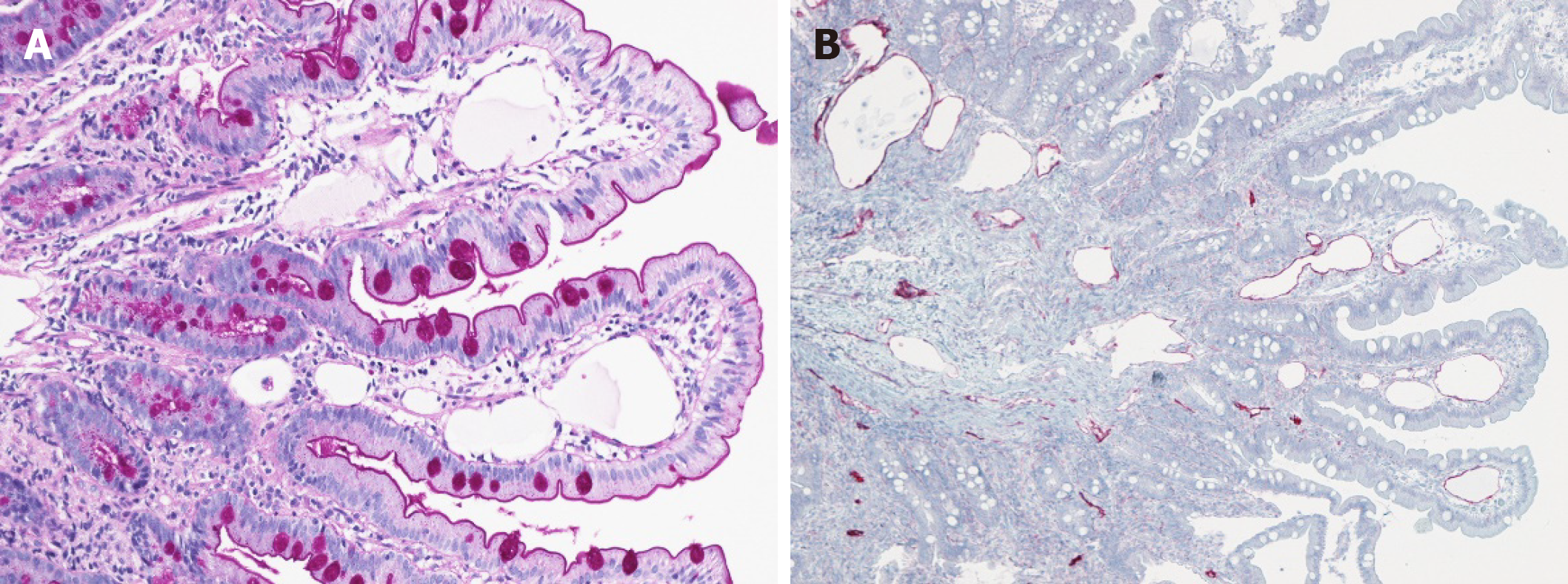
Finally, PIL was confirmed on histological and immunohistological analyses from jejunal biopsies (Figures 2 and 3).
After putting the patient on a medium-chain triglyceride (MCT) diet rich in protein, the clinical condition of the patient significantly improved within 4 wk.
Until 2 years after diagnosis, mild lower limb edema were only observed between the end of breastfeeding period and a second pregnancy, and abdominal discomfort, fatigue and nausea significantly improved. Laboratory improvement was characterized by increase in total serum protein, albumin, and quantitative immunoglobulin levels.
Due to the rarity of this disease, the worldwide incidence of PIL in humans is unknown[3]. However, a genetic predisposition is been discussed since if mostly affects children below 3 years of age[5]. This is supported by familial forms of specific syndromes that have been associated with PIL, including the yellow-nail syndrome, von Recklinghausen’s disease, Turner, Noonan, Klippel-Trenaunay or Hennekam syndrome[3,6,7]. Nevertheless, cases in adult patients exist. We performed a literature review and could identify 49 cases from 46 case reports of PIL in adults in which the onset of symptoms occurred after the 18th birthday (Table 1, Supplementary Table 1[8-53]). Notably, 27/46 (58.7%) were published since 2010, indicating that this entity is increasingly been recognized and the prevalence might be underestimated. Median age at diagnosis was 43 (range: 20-83) years while median time from onset of symptoms to final diagnosis was 3 (range: 0-40) years, highlighting the difficulty in the correct diagnosis of this entity. Although a literature review of PIL cases existed reporting a mean age of 13.3 years at symptom onset and 8.5 years until diagnosis, it has to be pointed out that 75% of cases in this review included patients with symptom onset before 20 years of age[54]. In terms of gender distribution, 22 male (44.9%) and 27 female (55.1%) cases were reported.
| Clinical presentation | n | |
| Patient characteristics | Number of adult patients with PIL | 49 |
| Age at diagnosis, yr | 43 (20-83) | |
| Time to final diagnosis, yr | 3 (0-40) | |
| Male, n (%) | 21 (42.9) | |
| Female, n (%) | 28 (57.1) | |
| Symptoms, n (%) | Edema | 40/48 (83.3) |
| Recurrent diarrhea | 20/48 (41.7) | |
| Abdominal effusion | 13/48 (27.1) | |
| Pleural effusion | 10/48 (20.8) | |
| Abdominal pain | 9/48 (18.8) | |
| Laboratory findings, n (%) | Hypogammaglobulinemia | 35/48 (72.9) |
| Hypoalbuminemia | 35/48 (72.9) | |
| Lymphocytopenia | 30/48 (62.5) | |
| Hypoproteinemia | 26/48 (54.2) | |
| Hypocalcemia | 12/48 (25.0) | |
| α1-antitrypsin (stool) | 10/48 (20.8)2 | |
| CT-scan, n (%) | Normal | 8/27 (29.6) |
| Thickened wall of small bowel | 11/27 (40.7) | |
| Diagnosis possible, n (%) | Gastro-duodenoscopy | 21/35 (60.0) |
| Ileo-colonoscopy | 5/21 (23.8) | |
| Enteroscopy | 13/13 (100) | |
Our case describes a 34-year-old female being diagnosed with PIL around 22 years after the first occurrence of edema, and 22 wk after the first contact at our institution. She presented with nausea, abdominal discomfort, diarrhea, and bilateral limb edemas. From 48 patients reporting on symptoms in the literature, 40 patients (83.3%) reported the presence of any peripheral/generalized edema while only 2 patients did not present with edema and 6 case reports did not report on this symptom. The majority (n = 27, 56.3%) only reported bilateral edema of the lower limb. 9/48 patients (18.8%) presented with abdominal pain, 13 patients (27.1%) presented with (chylous) ascites, 10 patients (20.8%) with pleural, and 4 patients (8.3%) with pericardial effusion. Diarrhea was present in 20/48 patients (41.7%). Other rare unspecific symptoms include changes in weight, nausea, general weakness, pallor, and gastrointestinal bleeding. These findings go in line with a former literature review of 84 PIL cases (including predominantly children), reporting limb edema, diarrhea, ascites, and lymphedema in 78%, 62%, 41%, and 22% respectively[54]. These symptoms and their varying extent can largely be explained as a consequence of lymphatic/ protein and subsequently watery loss due to lower oncotic pressure in interstitial fluid.
The fact that pregnancy and oral contraception led to the vanishing of edemas in our patient is indeed surprising as this has not yet been reported. Of note, symptoms completely resolved when taking oral contraceptives and re-appeared during pill-free days in between. One may hypothesize that differences in estradiol might influence the severity of lymphedema: Morfoisse et al[55], who explored the role of estrogens on lymphatic endothelial cells, found that estradiol is protective of lymphedema, and blockage of the estrogen receptor is associated with stronger lymphatic leakage. However, this was only shown in an animal model of secondary lymphedema, and no other studies providing further evidence on this mechanism are available.
Among the most frequently observed laboratory findings in literature were anemia in 16 patients (33.3%), lymphocytopenia in 30 (62.5%), hypoproteinemia in 26 (54.2%), and hypoalbuminemia and hypogammaglobulinemia/reduced serum IgG level in 35 (72.9%) while no patients specifically reported the absence of the latter two laboratory findings. However, these numbers might be underestimated since not all case reports reported on these features. 12 patients (25.0%) specifically reported reduced serum levels of calcium and 5 patients (10.4%) reduced levels of magnesium, leading to occasional muscle seizures in several patients. Other findings include hypoglobulinemia with reduced numbers of IgM, IgA, and IgG, and reduced numbers of CD3+ and CD4+ cells. Profound hypoproteinemia, hypoalbuminemia, and hypogammaglobulinemia and reduced CD4:CD8 ratio could also be confirmed in our patient. However, lymphocytopenia was only transient and leucocyte count was within the normal range indicating that these parameters might be very unspecific and significantly influenced by temporary inflammatory processes in the body. This is especially true since an increased susceptibility to opportunistic infections based on lymphocytopenia and hypogammaglobulinemia could be present. This was reported in our patient, 2 case reports of adult patients with additional 2 patients suffering from cryptococcal meningitis at initial presentation, and 3 case reports of children[5]. This humoral and cellular immunodeficiency is assumed to be due to a lymphatic loss of B- and T- lymphocytes. Interestingly, 4 patients in the literature report an extensive presence of warts, probably representing the end-stage of acquired immunodeficiency.
Notably, fecal α1-antitrypsin levels or α1-antitrypsin-clearance seem to be a good indicator for the presence of PLE/PIL in these patients with positive results in all patients who reported on this feature (10/48, 20.8%). Since α1-antitrypsin is resistant to degradation by digestive enzymes, it indicates the presence of blood proteins in the intestinal tract[56].
Because of persistently diminished IgG and IgA, an abdominal/pelvic CT scan was performed to rule out lymphoma or thymoma in our patient. This incidentally revealed a thickened wall in the jejunum and ileum with enlarged lymph nodes. Interestingly – when looking into the literature – 11/27 of patients (40.7%) undergoing a CT scan reported abnormalities in the small bowel wall while 8/27 (29.6%) had a completely normal result. However, other imaging modalities such as lymphangioscintigraphy or technetium-labeled human serum albumin (99TmTc-HSA) scintigraphy might be of higher accuracy pointing towards the diagnosis of PIL, demonstrating abnormal lymphatic, or protein leakage.
When comparing endoscopic findings in these patients, 21/35 (60.0%) gastroduodenoscopies performed in symptomatic PIL patients revealed an endoscopic view suggestive for PIL with “snowflake appearance” of the duodenal mucosa indicating lymphatic dilations while in 2 patients the diagnosis could be made histologically despite normal macroscopic appearance. On the contrary, ileocolonoscopy was inferior with only 5/21 (23.8%) showing characteristic features in the terminal ileum that led to the diagnosis. However, it has to be mentioned that considering the improvement in technology, this rate might be underestimated due to the large number of studies performed > 10 years ago. Enteroscopy, which was performed in 14 patients including our patient to establish the diagnosis, is highly sensitive and should be regarded as the gold-standard for diagnosis. Video capsule endoscopy could be used to help with diagnosis, being similarly sensitive to detect lymphedema in the small bowel.
In our case, although a gastroduodenoscopy was performed showing an endoscopic snowflake appearance of the duodenal mucosa indicating lymphatic dilations, the histological report was normal. To our opinion, two possible explanations exist for this phenomenon: On the one hand, the dilated lymphatic endothelial cells can be distributed in different locations in the intestine to a different extent, hence the histology specimens might not show dilated lymphatic endothelial cells despite endoscopic and macroscopic appearance. On the other hand, the lack of experience of pathologists with this entity could result in an insufficient evaluation of the histological specimens and delay of diagnosis, as this was the case in our patient.
Lifelong adherence to a diet rich in protein with substitution of MCT remains the cornerstone in the therapy of PIL. Because MCTs are directly absorbed into the portal venous system bypassing the lymphatic system, they can be used to overcome chronic malnutrition. The need for dietary control in people appears to be permanent because clinical and biochemical findings seem to reappear after low-fat dietary withdrawal. 16/26 patients (61.5%) receiving MCT alone reported significant improvement in symptoms while 2 patients reported only moderate improvement. Octreotide can be regarded as the preferred treatment in patients in whom dietary changes fail to achieve significant improvement. Octreotide is a long-acting somatostatin analogue that suppresses gastrointestinal motility and hormone secretion in the pituitary gland, pancreas, and intestine. Although the mechanism of action of octreotide in diminishing protein loss through the gastrointestinal tract is unclear, theorized mechanisms of octreotide’s action in PIL include decreased intestinal fat absorption, inhibition of gastrointestinal vasoactive peptides, and stimulation of the autonomic nervous system[57-59]. Octreotide is usually given at doses of 150-200 µg subcutaneously twice daily[14]. From all 29 cases that reported efficacy of therapy, octreotide was added to MCT in 6 patients and started as initial treatment in one patient, with 2 patients having an insufficient response and 2 patients report in recurrence of symptoms after discontinuation of octreotide with otherwise good response. Other medical therapeutic options include propranolol, which is thought to downregulate the RAF mitogen-activated protein kinase signaling pathway with reduced expression of VEGF, and everolimus, which is an mTOR inhibitor. mTOR is a serine/threonine kinase, representing a key enzyme for numerous cellular processes including angiogenesis and cell growth. Ozeki et al[60] found significant mTOR expression in tissues affected by PIL and applied everolimus (1.6 mg/m²/day) as a treatment of PIL improving diarrhea and hypoproteinemia. However, no case report on an adult patient with PIL exists using these two substances. Surgical resection seems to be the last option both for diagnosis and therapy of PIL. In 6/49 patients, diagnosis of PIL was established after surgical resection, however in most cases without performing an enteroscopy before. All 7 patients that reported surgical resection as the form of treatment – sometimes after the failure of conservative therapy – described improvement of the clinical condition after surgery. However, long-term follow-up does not exist in these patients.
Nevertheless, long-term follow-up is needed since lymphoma have been described as long-term complications in patients with PIL. Laharie et al[40] reported on 12 cases of lymphoma after PIL, which was adopted for the present case report and completed by literature review of additional cases until 2020[61]. So far, 13 cases have been published with lymphoma occurring after a median of 14 years (range: 0-39) after PIL diagnosis (Supplementary Table 2).
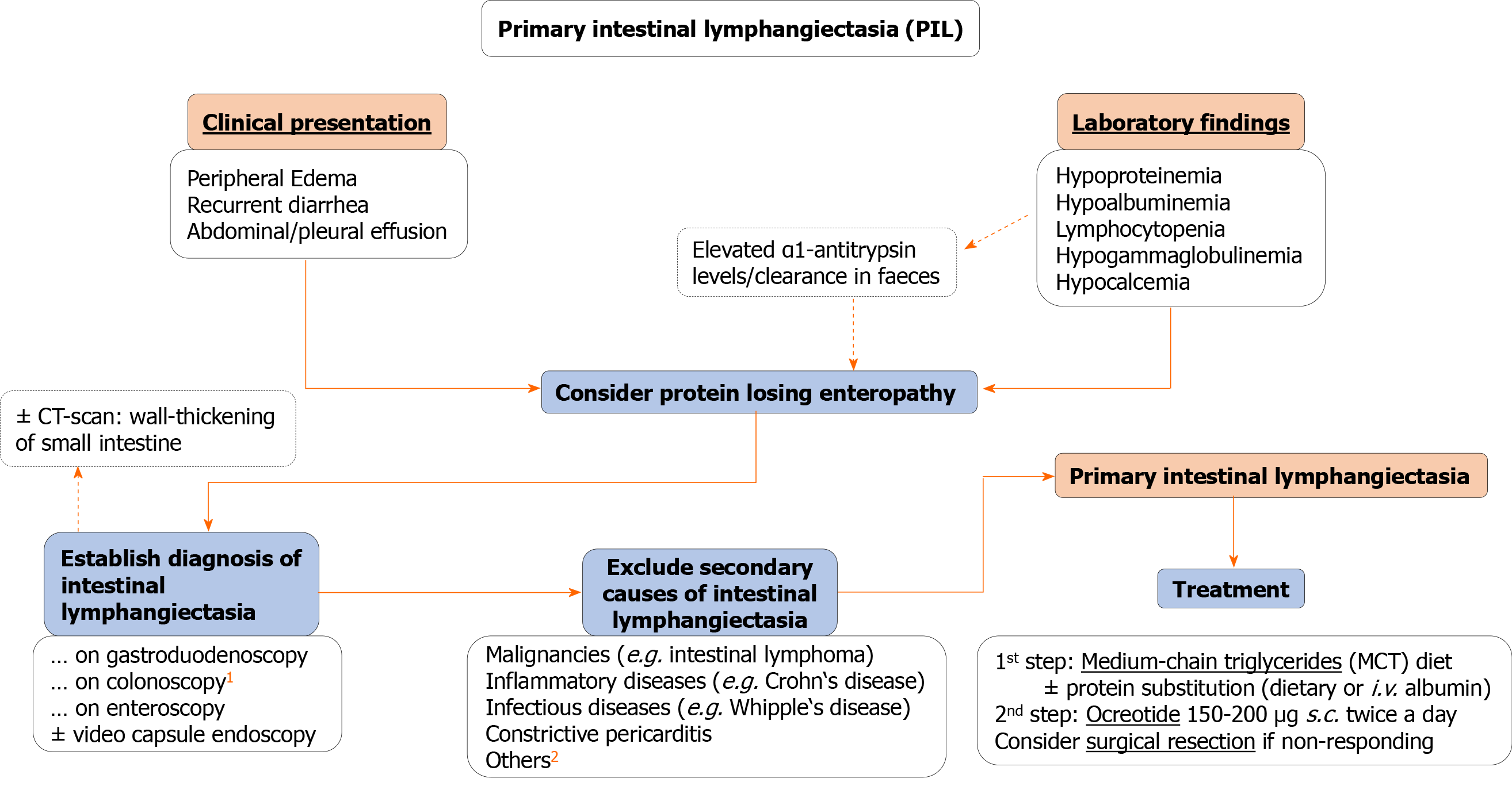
In conclusion, PIL can be a rare cause of PLE in adults. Unspecific symptoms and a wide range of clinical manifestations can significantly hamper establishing the definite diagnosis, leading to a “diagnostic roller coaster” for the individual patient. Despite good treatment options, low recognition of this entity leads to significant morbidity in these patients. Following review of published case reports, we present a practical overview of symptoms, laboratory findings, the accuracy of diagnostic modalities, and a potential treatment approach to facilitate diagnosis, and management of these patients. Finally, we highlight the striking collinearity with female hormone status in our patient, presenting a potential area of future research (Figure 4).
| 1. | Waldmann TA, Steinfeld JL, Dutcher TF, Davidson JD, Gordon RS Jr. The role of the gastrointestinal system in "idiopathic hypoproteinemia". Gastroenterology. 1961;41:197-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 182] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Levitt DG, Levitt MD. Protein losing enteropathy: comprehensive review of the mechanistic association with clinical and subclinical disease states. Clin Exp Gastroenterol. 2017;10:147-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (1)] |
| 3. | Vignes S, Bellanger J. Primary intestinal lymphangiectasia (Waldmann's disease). Orphanet J Rare Dis. 2008;3:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 164] [Article Influence: 9.1] [Reference Citation Analysis (37)] |
| 4. | Freeman HJ, Nimmo M. Intestinal lymphangiectasia in adults. World J Gastrointest Oncol. 2011;3:19-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (39)] |
| 5. | Lopez RN, Day AS. Primary intestinal lymphangiectasia in children: A review. J Paediatr Child Health. 2020;56:1719-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (32)] |
| 6. | Samman PD, White WF. The “Yellow Nail” Syndrome. Brit J Dermatol. 1964; 76:153-157. [RCA] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 241] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Hennekam RCM, Geerdink RA, Hamel BCJ, Hennekam FAM, Kraus P, Rammeloo JA, Tillemans AAW. Autosomal recessive intestinal lymphangiectasia and lymphedema, with facial anomalies and mental retardation. Am J Med Genet. 1989;34:593-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 126] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Singh AK, Zameer A, Sood R, Verma S, Samanta J, Bal A, Sinha SK, Kochhar R. Chronic diarrhea with white colon: Primary Intestinal lymphangiectasia. QJM. 2020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Crutzen B, Poncelet PA. Protein-Losing Enteropathy in Primary Lymphangiectasia. J Belg Soc Radiol. 2020;104:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Tseng YJ, Ding WQ, Luo ZG. Protein-losing enteropathy and primary intestinal lymphangiectasia. QJM. 2020;113:224-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Tominaga K, Tsuchiya A, Kawata Y, Yokoyama J, Terai S. Novel Magnified Single-Balloon Enteroscopy Enables Observation of Jejunal White Spots Associated with Lymphangiectasia. Dig Dis. 2019;37:170-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Zafar Y, Gondi KT, Tamimi T, Colson J, Lankachandra K, El-Halawany H. Primary Intestinal Lymphangiectasia Causing Intussusception and Small Bowel Obstruction. ACG Case Rep J. 2019;6:e00233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Cappell MS, Edhi A, Amin M. Case report of primary intestinal lymphangiectasia diagnosed in an octogenarian by ileal intubation and by push enteroscopy after missed diagnosis by standard colonoscopy and EGD. Medicine (Baltimore). 2018;97:e9649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Altın Z, Atabay Y, Özer S, Karakoyun M, Ekmekçi S, Yürekli EY, Akar H. Primary intestinal lymphangiectasia and a review of the current literature. Turk J Gastroenterol. 2018;29:714-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Martins CR, Gagnaire A, Rostain F, Lepage C. Waldmann's disease: a rare cause of protein losing enteropathy in an adult patient. Rev Esp Enferm Dig. 2017;109:385-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (35)] |
| 16. | Lu J, Zhai H. Exacerbation of primary intestinal lymphangiectasia during late pregnancy and recovery after delivery: A case report and literature review. Medicine (Baltimore). 2017;96:e7928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Huber X, Degen L, Muenst S, Trendelenburg M. Primary intestinal lymphangiectasia in an elderly female patient: A case report on a rare cause of secondary immunodeficiency. Medicine (Baltimore). 2017;96:e7729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Balaban VD, Popp A, Grasu M, Vasilescu F, Jinga M. Severe Refractory Anemia in Primary Intestinal Lymphangiectasia. A Case Report. J Gastrointestin Liver Dis. 2015;24:369-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (37)] |
| 19. | Lee SJ, Song HJ, Boo SJ, Na SY, Kim HU, Hyun CL. Primary intestinal lymphangiectasia with generalized warts. World J Gastroenterol. 2015;21:8467-8472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | El-Etreby SA, Altonbary AY, Sorogy ME, Elkashef W, Mazroa JA, Bahgat MH. Anaemia in Waldmann's disease: A rare presentation of a rare disease. World J Gastrointest Endosc. 2015;7:567-572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Huber T, Paschold M, Eckardt AJ, Lang H, Kneist W. Surgical therapy of primary intestinal lymphangiectasia in adults. J Surg Case Rep. 2015;2015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Raithel M, Rau TT, Hagel AF, Albrecht H, de Rossi T, Kirchner T, Hahn EG. Jejunitis and brown bowel syndrome with multifocal carcinogenesis of the small bowel. World J Gastroenterol. 2015;21:10461-10467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Troskot R, Jurčić D, Bilić A, Gomerčić Palčić M, Težak S, Brajković I. How to treat an extensive form of primary intestinal lymphangiectasia? World J Gastroenterol. 2015;21:7320-7325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Milazzo L, Peri AM, Lodi L, Gubertini G, Ridolfo AL, Antinori S. Intestinal lymphangiectasia and reversible high liver stiffness. Hepatology. 2014;60:759-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Ersoy O, Akin E, Demirezer A, Yilmaz E, Solakoglu T, Irkkan C, Yurekli OT, Buyukasik S. Evaluation of primary intestinal lymphangiectasia by capsule endoscopy. Endoscopy. 2013;45 Suppl 2 UCTN:E61-E62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Jabeen SA, Murthy A, Kandadai RM, Meena AK, Borgohain R, Uppin MS. Cryptoccocal menigitis as a primary manifestation in a patient with intestinal lymphangictasia. Ann Indian Acad Neurol. 2012;15:218-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Suehiro K, Morikage N, Murakami M, Yamashita O, Hamano K. Late-onset primary intestinal lymphangiectasia successfully managed with octreotide: a case report. Ann Vasc Dis. 2012;5:96-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Maamer AB, Baazaoui J, Zaafouri H, Soualah W, Cherif A. Primary intestinal lymphangiectasia or Waldmann's disease: a rare cause of lower gastrointestinal bleeding. Arab J Gastroenterol. 2012;13:97-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 29. | Kneist W, Drescher DG, Hansen T, Kreitner KF, Lang H. [Surgical therapy of segmental jejunal, primary intestinal lymphangiectasia]. Z Gastroenterol. 2013;51:576-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Koçak G, Koçak E, Akbal E, Duranay M, Köklü S. A rare cause of severe hypoalbuminemia in a patient with primary hypoparathyroidism: intestinal lymphangiectasia. Acta Clin Belg. 2011;66:246-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Choi EH, Reidel W, Coyle W. Forty years of shortness of breath and lower extremity edema. Diagnosis: Primary intestinal lymphangiectasia (Waldmann's disease). Gastroenterology. 2011;141:e6-e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Wiedermann CJ, Kob M, Benvenuti S, Carella R, Lucchin L, Piazzi L, Chilovi F, Mazzoleni G. Digital clubbing in primary intestinal lymphangiectasia: a case report. Wien Med Wochenschr. 2010;160:431-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Steines JC, Larson JH, Wilkinson N, Kirby P, Goodheart MJ. Intestinal lymphangiectasia mimicking primary peritoneal carcinoma. Am J Obstet Gynecol. 2010;203:e9-e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 34. | Zhu LH, Cai XJ, Mou YP, Zhu YP, Wang SB, Wu JG. Partial enterectomy: treatment for primary intestinal lymphangiectasia in four cases. Chin Med J (Engl). 2010;123:760-764. [PubMed] |
| 35. | Paggi S, Ferrero S, Braidotti P, de Rai P, Conte D, Basilisco G. Neuromuscular alterations in the dilated ileum of an adult patient with segmental lymphangiectasia. Eur J Gastroenterol Hepatol. 2008;20:935-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 36. | Herfarth H, Hofstädter F, Feuerbach S, Jürgen Schlitt H, Schölmerich J, Rogler G. A case of recurrent gastrointestinal bleeding and protein-losing gastroenteropathy. Nat Clin Pract Gastroenterol Hepatol. 2007;4:288-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | Cole SL, Ledford DK, Lockey RF, Daas A, Kooper J. Primary gastrointestinal lymphangiectasia presenting as cryptococcal meningitis. Ann Allergy Asthma Immunol. 2007;98:490-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 38. | Fang YH, Zhang BL, Wu JG, Chen CX. A primary intestinal lymphangiectasia patient diagnosed by capsule endoscopy and confirmed at surgery: a case report. World J Gastroenterol. 2007;13:2263-2265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 39. | Chamouard P, Nehme-Schuster H, Simler JM, Finck G, Baumann R, Pasquali JL. Videocapsule endoscopy is useful for the diagnosis of intestinal lymphangiectasia. Dig Liver Dis. 2006;38:699-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 40. | Laharie D, Degenne V, Laharie H, Cazorla S, Belleannee G, Couzigou P, Amouretti M. Remission of protein-losing enteropathy after nodal lymphoma treatment in a patient with primary intestinal lymphangiectasia. Eur J Gastroenterol Hepatol. 2005;17:1417-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Goktan C, Pekindil G, Orguc S, Coskun T, Serter S. Bilateral Breast Edema in Intestinal Lymphangiectasia. Breast J. 2005;11:360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 42. | Cammarota G, Cianci R, Gasbarrini G. High-resolution magnifying video endoscopy in primary intestinal lymphangiectasia: a new role for endoscopy? Endoscopy. 2005;37:607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 43. | Filik L, Oguz P, Koksal A, Koklu S, Sahin B. A case with intestinal lymphangiectasia successfully treated with slow-release octreotide. Dig Liver Dis. 2004;36:687-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 44. | Lynn J, Knight AK, Kamoun M, Levinson AI. A 55-year-old man with hypogammaglobulinemia, lymphopenia, and unrelenting cutaneous warts. J Allergy Clin Immunol. 2004;114:409-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 45. | Chen CP, Chao Y, Li CP, Lo WC, Wu CW, Tsay SH, Lee RC, Chang FY. Surgical resection of duodenal lymphangiectasia: a case report. World J Gastroenterol. 2003;9:2880-2882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 46. | Strehl J, Schepke M, Wardelmann E, Caselmann WH, Sauerbruch T. [Chronic diarrhea in a 43-year-old patient]. Internist (Berl). 2003;44:626-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 47. | Klingenberg RD, Homann N, Ludwig D. Type I intestinal lymphangiectasia treated successfully with slow-release octreotide. Dig Dis Sci. 2003;48:1506-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 48. | Kuroiwa G, Takayama T, Sato Y, Takahashi Y, Fujita T, Nobuoka A, Kukitsu T, Kato J, Sakamaki S, Niitsu Y. Primary intestinal lymphangiectasia successfully treated with octreotide. J Gastroenterol. 2001;36:129-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 49. | Keberle M, Mörk H, Jenett M, Hahn D, Scheurlen M. Computed tomography after lymphangiography in the diagnosis of intestinal lymphangiectasia with protein-losing enteropathy in Noonan's syndrome. Eur Radiol. 2000;10:1591-1593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 50. | Alfano V, Tritto G, Alfonsi L, Cella A, Pasanisi F, Contaldo F. Stable reversal of pathologic signs of primitive intestinal lymphangiectasia with a hypolipidic, MCT-enriched diet. Nutrition. 2000;16:303-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 51. | Bouhnik Y, Etienney I, Nemeth J, Thevenot T, Lavergne-Slove A, Matuchansky C. Very late onset small intestinal B cell lymphoma associated with primary intestinal lymphangiectasia and diffuse cutaneous warts. Gut. 2000;47:296-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 52. | Gumà J, Rubió J, Masip C, Alvaro T, Borràs JL. Aggressive bowel lymphoma in a patient with intestinal lymphangiectasia and widespread viral warts. Ann Oncol. 1998;9:1355-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 53. | Lenzhofer R, Lindner M, Moser A, Berger J, Schuschnigg C, Thurner J. Acute jejunal ileus in intestinal lymphangiectasia. Clin Investig. 1993;71:568-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 54. | Wen J, Tang Q, Wu J, Wang Y, Cai W. Primary intestinal lymphangiectasia: four case reports and a review of the literature. Dig Dis Sci. 2010;55:3466-3472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 55. | Morfoisse F, Tatin F, Chaput B, Therville N, Vaysse C, Métivier R, Malloizel-Delaunay J, Pujol F, Godet AC, De Toni F, Boudou F, Grenier K, Dubuc D, Lacazette E, Prats AC, Guillermet-Guibert J, Lenfant F, Garmy-Susini B. Lymphatic Vasculature Requires Estrogen Receptor-α Signaling to Protect From Lymphedema. Arterioscler Thromb Vasc Biol. 2018;38:1346-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 56. | Florent C, L'Hirondel C, Desmazures C, Aymes C, Bernier JJ. Intestinal clearance of alpha 1-antitrypsin. A sensitive method for the detection of protein-losing enteropathy. Gastroenterology. 1981;81:777-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 120] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 57. | Reubi JC, Laissue JA, Waser B, Steffen DL, Hipkin RW, Schonbrunn A. Immunohistochemical detection of somatostatin sst2a receptors in the lymphatic, smooth muscular, and peripheral nervous systems of the human gastrointestinal tract: facts and artifacts. J Clin Endocrinol Metab. 1999;84:2942-2950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 58. | Ballinger AB, Farthing MJ. Octreotide in the treatment of intestinal lymphangiectasia. Eur J Gastroenterol Hepatol. 1998;10:699-702. [PubMed] |
| 59. | Sari S, Baris Z, Dalgic B. Primary intestinal lymphangiectasia in children: is octreotide an effective and safe option in the treatment? J Pediatr Gastroenterol Nutr. 2010;51:454-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 60. | Ozeki M, Hori T, Kanda K, Kawamoto N, Ibuka T, Miyazaki T, Fukao T. Everolimus for Primary Intestinal Lymphangiectasia With Protein-Losing Enteropathy. Pediatrics. 2016;137:e20152562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 61. | Patel KV, Goel RM, Wong T. Diffuse large B-cell lymphoma recurrence complicating primary intestinal lymphangiectasia. Clin Gastroenterol Hepatol. 2013;11:e86-e87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Austria
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li J, Mao Y, Wang ZJ, Yang JS S-Editor: Zhang L L-Editor: A P-Editor: Wang LL