Published online Dec 14, 2020. doi: 10.3748/wjg.v26.i46.7382
Peer-review started: September 23, 2020
First decision: October 27, 2020
Revised: October 29, 2020
Accepted: November 13, 2020
Article in press: November 13, 2020
Published online: December 14, 2020
Processing time: 82 Days and 4.7 Hours
Inflammation plays an important role in tumor progression, and growing evidence has confirmed that the fibrinogen-to-albumin ratio (FAR) is an important prognostic factor for overall survival in malignant tumors.
To investigate the prognostic significance of FAR in patients undergoing radical R0 resection of pancreatic ductal adenocarcinoma (PDAC).
We retrospectively analyzed the data of 282 patients with PDAC who underwent radical R0 resection at The Cancer Hospital of the Chinese Academy of Medical Sciences from January 2010 to December 2019. The surv_cutpoint function of the R package survminer via RStudio software (version 1.3.1073, http://www.rstudio.org) was used to determine the optimal cut-off values of biological markers, such as preoperative FAR. The Kaplan-Meier method and log-rank tests were used for univariate survival analysis, and a Cox regression model was used for multivariate survival analysis for PDAC patients who underwent radical R0 resection.
The optimal cut-off value of FAR was 0.08 by the surv_cutpoint function. Higher preoperative FAR was significantly correlated with clinical symptoms (P = 0.001), tumor location (P < 0.001), surgical approaches (P < 0.001), preoperative plasma fibrinogen concentration (P < 0.001), and preoperative plasma albumin level (P < 0.001). Multivariate analysis showed that degree of tumor differentiation (P < 0.001), number of metastatic lymph nodes [hazard ratio (HR): 0.678, 95% confidence interval (CI): 0.509-0.904, P = 0.008], adjuvant therapy (HR: 1.604, 95%CI: 1.214-2.118, P = 0.001), preoperative cancer antigen 19-9 level (HR: 1.740, 95%CI: 1.288-2.352, P < 0.001), and preoperative FAR (HR: 2.258, 95%CI: 1.720-2.963, P < 0.001) were independent risk factors for poor prognosis in patients with PDAC who underwent radical R0 resection.
The increase in preoperative FAR was significantly related to poor prognosis in patients undergoing radical R0 resection for PDAC. Preoperative FAR can be used clinically to predict the prognosis of PDAC patients undergoing radical R0 resection.
Core Tip: Inflammation plays an important role in the occurrence and development of tumors. Recently, many retrospective studies have reported the significance of the fibrinogen-to-albumin ratio in the prognosis of patients with malignant tumors, but there have been no reports of patients with resectable pancreatic cancer. We studied 282 patients with pancreatic ductal adenocarcinoma who underwent radical R0 resection, and we found that the preoperative fibrinogen-to-albumin ratio is an easily available, cost-effective, and noninvasive prognostic indicator for pancreatic ductal adenocarcinoma patients undergoing radical R0 resection.
- Citation: Zhang LP, Ren H, Du YX, Wang CF. Prognostic value of the preoperative fibrinogen-to-albumin ratio in pancreatic ductal adenocarcinoma patients undergoing R0 resection. World J Gastroenterol 2020; 26(46): 7382-7404
- URL: https://www.wjgnet.com/1007-9327/full/v26/i46/7382.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i46.7382
Of all the malignancies, pancreatic cancer has the worst prognosis, with mortality roughly comparable to the incidence[1]. Pancreatic cancer is the sixth most common cause of cancer-related deaths (2.82%) in China[2]. Globally, pancreatic cancer is the fourth leading cause of cancer-related death[3]. With the annual increase in pancreatic cancer-related deaths, it is estimated that by 2030, just a decade from now, malignant tumors will be the second leading cause of cancer-related deaths in the United States[4]. Based on the latest data from the Surveillance, Epidemiology, and End Results database of the National Cancer Institute, the 5-year overall survival (OS) rate for pancreatic cancer patients is only 10% in the United States[5,6]. At present, radical surgical resection is still an important method, or even the only way, to prolong significantly the survival time of pancreatic cancer patients[7]. Nevertheless the 5-year OS rate of patients undergoing radical resection is still less than 20%[8,9], despite the concept of surgery-based comprehensive treatment being constantly practiced and updated in clinical work[6]. Moreover, only 20% of patients diagnosed with pancreatic cancer have access to surgical treatment[10]. The poor prognosis of pancreatic cancer patients is related to the early asymptomatic nature of the disease, leading to the late stage of disease diagnosis and the high possibility of distant metastasis in the early stage of pancreatic cancer[11]. Since pancreatic cancer has a poor overall prognosis and many patients may choose to refuse treatment, it is particularly important to explore simple and cost-effective biomarkers to help better predict patient OS before treatment, especially for patients who have the opportunity to undergo surgery.
The systemic inflammatory response is a key factor in promoting the occurrence and metastasis of malignant tumors, including pancreatic cancer[12-14]. Pancreatic cancer can also lead to a hypercoagulable state and cause obvious thrombosis in the patient[15], in which fibrinogen plays a vital role. At the same time, the plasma albumin level is also affected by the systemic inflammatory response[16], and it is also a key indicator of nutritional status in malignant tumor patients[17,18].
Recently, growing evidence has confirmed that fibrinogen-to-albumin ratio (FAR), the ratio of preoperative fibrinogen concentration to plasma albumin level, is an important predictor of the OS of patients with esophageal cancer[19], breast cancer[20,21], and liver cancer[22] after radical surgery. However, to date, there have been no reports discussing the association between FAR and the prognosis of patients with pancreatic ductal adenocarcinoma (PDAC) undergoing radical R0 resection. Here, we conducted a retrospective study to explore whether FAR has prognostic value in these patients.
The inclusion criteria were as follows: (1) Positron emission tomography/computed tomography (CT) examination or enhanced magnetic resonance imaging and/or CT scan confirmed no distant metastasis; (2) Radical pancreaticoduodenectomy or distal pancreatectomy with splenectomy was performed; (3) The pathologic diagnosis was PDAC and radical R0 resection (no tumor cells within 1 mm from the resection margins) was performed[23]; and (4) Complete clinical information and follow-up data were available.
The exclusion criteria were as follows: (1) A history of cancer or other malignant tumors at the same time; (2) Received neoadjuvant therapy before surgery; (3) Presence of acute or chronic inflammatory diseases; (4) Presence of anemia or other blood system diseases; (5) Received anticoagulant therapy or albumin infusion before surgery; (6) Abnormal liver function or complicated with other liver diseases; and (7) Death occurred during the perioperative period, 1 mo after the operation.
In total, we retrospectively analyzed the data of 282 patients with PDAC who underwent radical R0 resection at The National Cancer Center/Cancer Hospital of the Chinese Academy of Medical Sciences from January 2010 to December 2019.
All clinicopathological characteristics included age, sex, blood type, clinical symptoms (including jaundice, pain, digestive symptoms, weight loss, fatigue, etc.), diabetes, smoking status, alcohol consumption, family history of cancer, approaches of open surgery, tumor information (including tumor location, degree of differentiation, lymphovascular invasion, perineural invasion, capsular invasion, maximum tumor diameter, number of metastatic lymph nodes), T stage, N stage, Tumor-Node-Metastasis (TNM) stage, adjuvant therapy, preoperative cancer antigen 19-9 ( CA19-9 ) level (U/mL), preoperative plasma fibrinogen concentration (g/L), preoperative plasma albumin level (g/L), and preoperative FAR.
The age of the patients refers to the age at which the primary ductal adenocarcinoma of the pancreas was clearly diagnosed. T staging, N staging, and TNM staging were defined according to the eighth edition of the TNM classification standard of the American Joint Committee on Cancer[24].
The study was conducted according to the ethical standards of the World Medical Association Declaration of Helsinki (9th edition of July 2018) and was approved by the Medical Ethics Committee of The National Cancer Center/Cancer Hospital of the Chinese Academy of Medical Sciences (approval No. 17-168/1424). All patients in the group also signed a written informed consent form.
The patient’s blood samples were collected before breakfast within 7 d before operation and processed quickly (within 2 h). Fibrinogen was processed and measured by a CA7000 automatic coagulation analyzer (Sysmex Corporation, Kobe, Japan). Serum albumin was measured by the bromocresol green dye method. The normal reference value of plasma fibrinogen concentration is 2.0-4.0 g/L, and the normal reference value of plasma albumin is 40.0-55.0 g/L.
FAR was defined as the ratio of the preoperative plasma fibrinogen concentration to the preoperative plasma albumin level.
All patients underwent radical surgery for PDAC, including pancreaticoduodenectomy and distal pancreatectomy with splenectomy, at The National Cancer Center/Cancer Hospital of Chinese Academy of Medical Sciences.
All patients were followed up by reviewing outpatient medical records and by telephone follow-up. The patients were followed up every 3 mo for the first 2 years and every 6 mo thereafter, and we recorded the results of physical, laboratory, and imaging examinations in detail. The date on which the patient underwent surgery was the beginning of the follow-up period, and the OS time was defined from the date of surgery to the date of death or the deadline for follow-up. The last follow-up date was August 16, 2020.
Continuous data with a normal distribution are shown as the mean ± standard deviation (Kolmogorov-Smirnov test, P > 0.05), and those with a nonnormal distribution are expressed as the median (minimum-maximum). Frequencies and percentages were used for the categorical variables. Statistical analyses were performed using RStudio software (version 1.3.1073, http://www.rstudio.org), SPSS software (version 25.0; IBM Corp., Armonk, NY, United States), and GraphPad Prism software (version 8.02; GraphPad Software, Inc., La Jolla, CA, United States). The surv_cutpoint function was used to determine the optimal cut-off value for preoperative plasma fibrinogen, albumin, and FAR. Clinicopathological characteristics at baseline were compared by the chi-square test and independent samples t-test. The Kaplan-Meier method was used to calculate the cumulative survival rate, and the log-rank test was used to compare survival curves. A multivariate Cox proportional hazards model was used to test further prognostic factors proven statistically significant in the univariate analysis. The results were calculated with 95% confidence intervals (CIs), and a two-tailed P < 0.05 was considered statistically significant.
A total of 282 patients with PDAC who underwent radical R0 resection at The National Cancer Center/Cancer Hospital of the Chinese Academy of Medical Sciences from January 2010 to December 2019 were included in this retrospective study, with a median follow-up of 14.98 mo. A total of 217 patients died during the follow-up period, with an estimated median OS of 17.43 mo (range: 1.30-100.07 mo). The 1-, 2-, 3-, and 5-year postoperative survival rates were 67.5%, 35.9%, 20.4%, and 10.2%, respectively. We analyzed the clinical indicators of all patients who met the inclusion criteria and found that the median age of these patients at diagnosis was 61 years (range: 31-81 years), with 136 (48.2%) of the patients being over 60 years of age. Of these patients, 151 (53.5%) were male, and 225 (79.8%) patients were seen for the presence of clinical symptoms that included jaundice, pain, digestive symptoms, weight loss, and fatigue. A total of 130 (46.1%) patients had tumors located in the head or neck of the pancreas and underwent pancreaticoduodenectomy, and 158 (56.0%) patients received further adjuvant therapy after surgery. A total of 217 (77.0%) patients had a histologic diagnosis of moderate differentiation. Based on the TNM staging criteria, only 29 (10.3%) patients were classified as stage III. Details of the patients' baseline characteristics are shown in Table 1.
| Characteristic | Patients, n = 282 |
| Age in yr | 61 (31-81) |
| > 60 | 136 (48.2) |
| ≤ 60 | 146 (51.8) |
| Sex | |
| Male | 151 (53.5) |
| Female | 131 (46.5) |
| Clinical symptoms | |
| Absent | 57 (20.2) |
| Present | 225 (79.8) |
| Diabetes | |
| Absent | 201 (71.3) |
| Present | 81 (28.7) |
| Smoking status | |
| Absent | 215 (76.2) |
| Present | 67 (23.8) |
| Alcohol consumption | |
| Absent | 235 (83.3) |
| Present | 47 (16.7) |
| Family history of cancer | |
| Absent | 271 (96.1) |
| Present | 11 (3.9) |
| Blood type | |
| A | 87 (30.9) |
| B | 93 (33.0) |
| AB | 22 (7.8) |
| O | 80 (28.4) |
| Tumor location | |
| Head and neck | 130 (46.1) |
| Body and tail | 152 (53.9) |
| Approaches of open surgery | |
| Pancreaticoduodenectomy | 130 (46.1) |
| Distal pancreatectomy with splenectomy | 152 (53.9) |
| Degree of differentiation | |
| Well | 34 (12.1) |
| Moderately | 217 (77.0) |
| Poorly | 31 (11.0) |
| Lymphovascular invasion | |
| Absent | 203 (72.0) |
| Present | 79 (28.0) |
| Perineural invasion | |
| Absent | 70 (24.8) |
| Present | 212 (75.2) |
| Capsular invasion | |
| Absent | 49 (17.4) |
| Present | 233 (82.6) |
| Maximum tumor diameter in cm | |
| > 4 | 88 (31.2) |
| ≤ 4 | 194 (68.8) |
| T stage | |
| T1 | 34 (12.1) |
| T2 | 159 (56.4) |
| T3 | 89 (31.6) |
| Number of metastatic lymph nodes | |
| Absent | 161 (57.1) |
| Present | 121 (42.9) |
| N stage | |
| N0 | 161 (57.1) |
| N1 | 92 (32.6) |
| N2 | 29 (10.3) |
| TNM stage | |
| IA | 20 (7.1) |
| IB | 92 (32.6) |
| IIA | 49 (17.4) |
| IIB | 92 (32.6) |
| III | 29 (10.3) |
| Adjuvant therapy | |
| Absent | 124 (44.0) |
| Present | 158 (56.0) |
| Preoperative CA19-9 level in U/mL | 172.4 (0.6-55412.0) |
| > 336.4 | 77 (27.3) |
| ≤ 336.4 | 205 (72.7) |
| Preoperative plasma fibrinogen concentration in g/L | 3.02 (1.20-6.70) |
| > 3.31 | 141 (50.0) |
| ≤ 3.31 | 141 (50.0) |
| Preoperative plasma albumin level in g/L | 42.6 (23.2-54.0) |
| > 45.2 | 85 (30.1) |
| ≤ 45.2 | 197 (69.9) |
| Preoperative FAR | 0.07 (0.03-0.21) |
| > 0.08 | 126 (44.7) |
| ≤ 0.08 | 156 (55.3) |
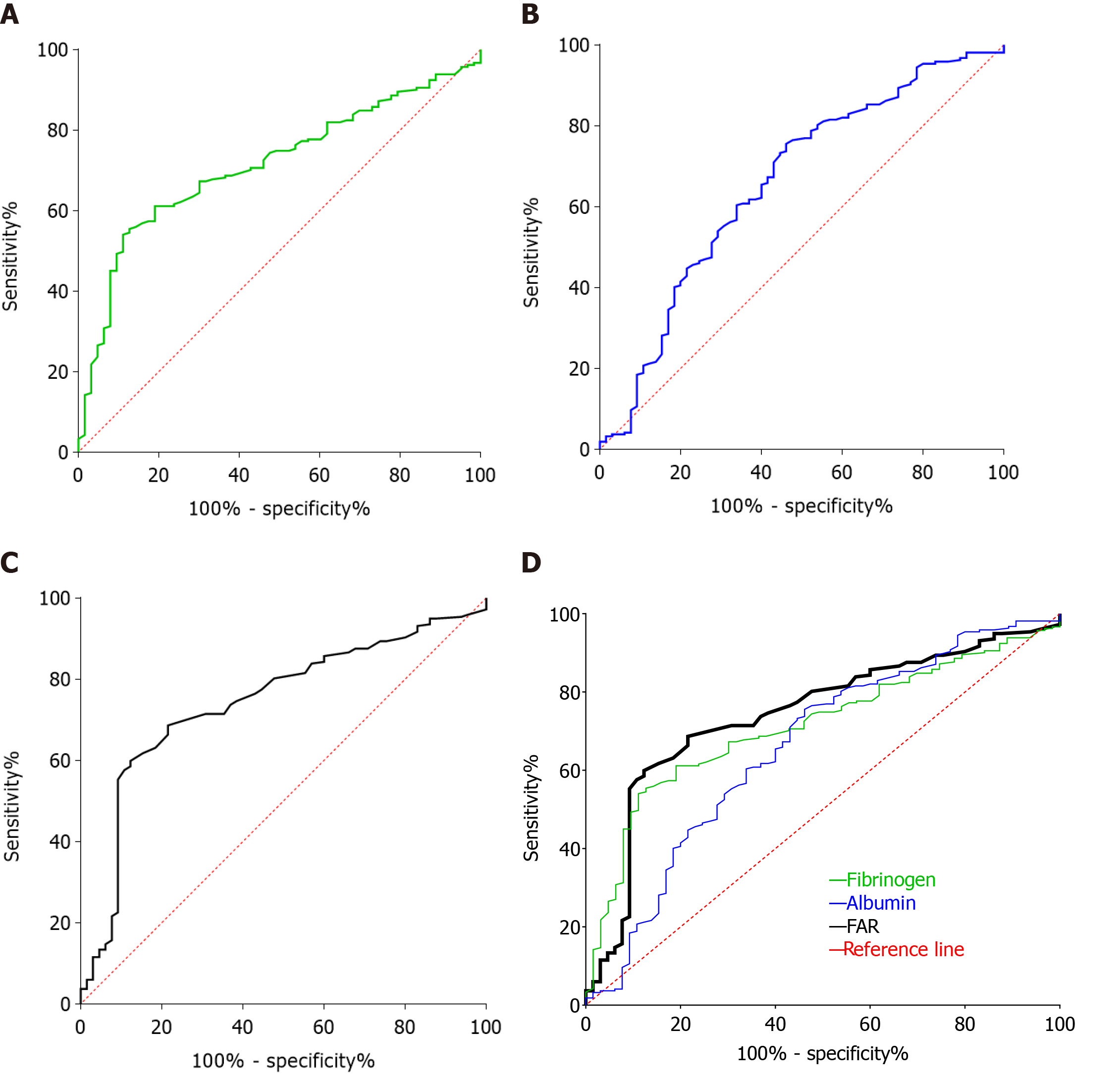
Based on the R programming language, R packages offer a collection of functions and data sets. The R package survminer provides convenient survival analysis and visualization capabilities, in which the surv_cutpoint function determines the optimal cut-off value for one or more continuous variables and plots survival curves for these variables, calculating the hazard ratio and the upper and lower limits of the 95%CIs. We used the surv_cutpoint function to determine the optimal cut-off values for preoperative fibrinogen, albumin, and FAR, which were 3.31 g/L, 45.2 g/L, and 0.08, respectively, and the optimal cut-off values for these indicators were also validated by their respective receiver operating characteristic curves, as shown in Figure 1.
The median preoperative fibrinogen concentration for all patients included in this study was 3.02 g/L (range: 1.20-6.70 g/L) (Table 1), with an optimal cut-off value of 3.31 g/L. As shown in Figure 1A, the area under the curve (AUC) of preoperative fibrinogen was 0.714 (95%CI: 0.649-0.779), and the sensitivity and specificity at the maximum Youden’s Index were 61.14% and 79.37%, respectively. Based on this cut-off value, 141 patients had preoperative fibrinogen concentrations > 3.31/L, as detailed in Table 2.
| Characteristics | Preoperative plasma fibrinogen concentration | P value | |
| > 3.31 g/L, n = 141 | ≤ 3.31 g/L, n = 141 | ||
| Age in yr | 0.812 | ||
| > 60 | 72 (25.5) | 74 (26.2) | |
| ≤ 60 | 69 (24.5) | 67 (23.8) | |
| Sex | 0.283 | ||
| Male | 71 (25.2) | 80 (28.4) | |
| Female | 70 (24.8) | 61 (21.6) | |
| Clinical symptoms | < 0.001 | ||
| Absent | 15 (5.3) | 42 (14.9) | |
| Present | 126 (44.7) | 99 (35.1) | |
| Diabetes | 0.357 | ||
| Absent | 97 (34.4) | 104 (36.9) | |
| Present | 44 (15.6) | 37 (13.1) | |
| Smoking status | 0.889 | ||
| Absent | 107 (37.9) | 108 (38.3) | |
| Present | 34 (12.1) | 33 (11.7) | |
| Alcohol consumption | 0.263 | ||
| Absent | 121 (42.9) | 114 (40.4) | |
| Present | 20 (7.1) | 27 (9.6) | |
| Family history of cancer | 0.356 | ||
| Absent | 137 (48.6) | 134 (47.5) | |
| Present | 4 (1.4) | 7 (2.5) | |
| Blood type | 0.565 | ||
| A | 39 (13.8) | 48 (17.0) | |
| B | 51 (18.1) | 42 (14.9) | |
| AB | 10 (3.5) | 12 (4.3) | |
| O | 41 (14.5) | 39 (13.8) | |
| Tumor location | < 0.001 | ||
| Head and neck | 80 (28.4) | 50 (17.7) | |
| Body and tail | 61 (21.6) | 91 (32.3) | |
| Approaches of open surgery | < 0.001 | ||
| Pancreaticoduodenectomy | 80 (28.4) | 50 (17.7) | |
| Distal pancreatectomy with splenectomy | 61 (21.6) | 91 (32.3) | |
| Degree of differentiation | 0.079 | ||
| Well | 20 (7.1) | 14 (5.0) | |
| Moderately | 111 (39.4) | 106 (37.6) | |
| Poorly | 10 (3.5) | 21 (7.4) | |
| Lymphovascular invasion | 0.233 | ||
| Absent | 97 (34.4) | 106 (37.6) | |
| Present | 44 (15.6) | 35 (12.4) | |
| Perineural invasion | 0.054 | ||
| Absent | 28 (9.9) | 42 (14.9) | |
| Present | 113 (40.1) | 99 (35.1) | |
| Capsular invasion | 0.271 | ||
| Absent | 21 (7.4) | 28 (9.9) | |
| Present | 120 (42.6) | 113 (40.1) | |
| Maximum tumor diameter in cm | 1.000 | ||
| > 4 | 44 (15.6) | 44 (15.6) | |
| ≤ 4 | 97 (34.4) | 97 (34.4) | |
| T stage | 0.991 | ||
| T1 | 17 (6.0) | 17 (6.0) | |
| T2 | 80 (28.4) | 79 (28.0) | |
| T3 | 44 (15.6) | 45 (16.0) | |
| Number of metastatic lymph nodes | 0.718 | ||
| Absent | 82 (29.1) | 79 (28.0) | |
| Present | 59 (20.9) | 62 (22.0) | |
| N stage | 0.110 | ||
| N0 | 82 (29.1) | 79 (28.0) | |
| N1 | 40 (14.2) | 52 (18.4) | |
| N2 | 19 (6.7) | 10 (3.5) | |
| TNM stage | 0.099 | ||
| IA | 8 (2.8) | 12 (4.3) | |
| IB | 44 (15.6) | 48 (17.0) | |
| IIA | 30 (10.6) | 19 (6.7) | |
| IIB | 40 (14.2) | 52 (18.4) | |
| III | 19 (6.7) | 10 (3.5) | |
| Adjuvant therapy | 0.631 | ||
| Absent | 64 (22.7) | 60 (21.3) | |
| Present | 77 (27.3) | 81 (28.7) | |
| Preoperative CA19-9 level in U/mL | 0.023 | ||
| > 336.4 | 47 (16.7) | 30 (10.6) | |
| ≤ 336.4 | 94 (33.3) | 111 (39.4) | |
| Preoperative plasma albumin level in g/L | 0.006 | ||
| > 45.2 | 32 (11.3) | 53 (18.8) | |
| ≤ 45.2 | 109 (38.2) | 88 (31.2) | |
| Preoperative FAR | < 0.001 | ||
| > 0.08 | 114 (40.4) | 12 (4.3) | |
| ≤ 0.08 | 27 (9.6) | 129 (45.7) | |
The median preoperative albumin level for all patients enrolled in this study was 42.6 g/L (range: 23.2-54.0 g/L) (Table 1), and the optimal cut-off value was 45.2 g/L. As shown in Figure 1B, the AUC of preoperative albumin was 0.659 (95%CI: 0.580-0.738), and the sensitivity and specificity at the maximum Youden’s Index were 75.58% and 53.85%, respectively. Based on this cut-off value, 197 patients had a preoperative albumin level ≤ 45.2 g/L, and 85 patients had a preoperative albumin level > 45.2 g/L, the details of which are shown in Table 3.
| Characteristics | Preoperative plasma albumin level | P value | |
| > 45.2 g/L, n = 85 | ≤ 45.2 g/L, n = 197 | ||
| Age in yr | 0.602 | ||
| > 60 | 42 (14.9) | 104 (36.9) | |
| ≤ 60 | 43 (15.2) | 93 (33.0) | |
| Sex | 0.699 | ||
| Male | 47 (16.7) | 104 (36.9) | |
| Female | 38 (13.5) | 93 (33.0) | |
| Clinical symptoms | 0.012 | ||
| Absent | 25 (8.9) | 32 (11.3) | |
| Present | 60 (21.3) | 165 (58.5) | |
| Diabetes | 0.458 | ||
| Absent | 58 (20.6) | 143 (50.7) | |
| Present | 27 (9.6) | 54 (19.1) | |
| Smoking status | 0.806 | ||
| Absent | 64 (22.7) | 151 (53.5) | |
| Present | 21 (7.4) | 46 (16.3) | |
| Alcohol consumption | 0.523 | ||
| Absent | 69 (24.5) | 166 (58.9) | |
| Present | 16 (5.7) | 31 (11.0) | |
| Family history of cancer | 0.832 | ||
| Absent | 82 (29.1) | 189 (67.0) | |
| Present | 3 (1.1) | 8 (2.8) | |
| Blood type | 0.169 | ||
| A | 33 (11.7) | 54 (19.1) | |
| B | 22 (7.8) | 71 (25.2) | |
| AB | 8 (2.8) | 14 (5.0) | |
| O | 22 (7.8) | 58 (20.6) | |
| Tumor location | < 0.001 | ||
| Head and neck | 24 (8.5) | 106 (37.6) | |
| Body and tail | 61 (21.6) | 91 (32.3) | |
| Approaches of open surgery | < 0.001 | ||
| Pancreaticoduodenectomy | 24 (8.5) | 106 (37.6) | |
| Distal pancreatectomy with splenectomy | 61 (21.6) | 91 (32.3) | |
| Degree of differentiation | 0.567 | ||
| Well | 8 (2.8) | 26 (9.2) | |
| Moderately | 66 (23.4) | 151 (53.5) | |
| Poorly | 11 (3.9) | 20 (7.1) | |
| Lymphovascular invasion | 0.814 | ||
| Absent | 62 (22.0) | 141 (50.0) | |
| Present | 23 (8.2) | 56 (19.9) | |
| Perineural invasion | 0.741 | ||
| Absent | 20 (7.1) | 50 (17.7) | |
| Present | 65 (23.0) | 147 (52.1) | |
| Capsular invasion | 0.673 | ||
| Absent | 16 (5.7) | 33 (11.7) | |
| Present | 69 (24.5) | 164 (58.2) | |
| Maximum tumor diameter in cm | 0.330 | ||
| > 4 | 30 (10.6) | 58 (20.6) | |
| ≤ 4 | 55 (19.5) | 139 (49.3) | |
| T stage | 0.530 | ||
| T1 | 8 (2.8) | 26 (9.2) | |
| T2 | 47 (16.7) | 112 (39.7) | |
| T3 | 30 (10.6) | 59 (20.9) | |
| Number of metastatic lymph nodes | 0.363 | ||
| Absent | 52 (18.4) | 109 (38.7) | |
| Present | 33 (11.7) | 88 (31.2) | |
| N stage | 0.127 | ||
| N0 | 52 (18.4) | 109 (38.7) | |
| N1 | 29 (10.3) | 63 (22.3) | |
| N2 | 4 (1.4) | 25 (8.9) | |
| TNM stage | 0.145 | ||
| IA | 4 (1.4) | 16 (5.7) | |
| IB | 34 (12.1) | 58 (20.6) | |
| IIA | 14 (5.0) | 35 (12.4) | |
| IIB | 29 (10.3) | 63 (22.3) | |
| III | 4 (1.4) | 25 (8.9) | |
| Adjuvant therapy | 0.003 | ||
| Absent | 26 (9.2) | 98 (34.8) | |
| Present | 59 (20.9) | 99 (35.1) | |
| Preoperative CA19-9 level in U/mL | 0.416 | ||
| > 336.4 | 26 (9.2) | 51 (18.1) | |
| ≤ 336.4 | 59 (20.9) | 146 (51.8) | |
| Preoperative plasma fibrinogen concentration in g/L | 0.006 | ||
| > 3.31 | 32 (11.3) | 109 (38.7) | |
| ≤ 3.31 | 53 (18.8) | 88 (31.2) | |
| Preoperative FAR | < 0.001 | ||
| > 0.08 | 15 (5.3) | 111 (39.4) | |
| ≤ 0.08 | 70 (24.8) | 86 (30.5) | |
The median preoperative FAR for all patients included in this retrospective study was 0.07 (range: 0.03-0.21) (Table 1), and the optimal cut-off value was 0.08. As shown in Figure 1C, the AUC of preoperative FAR was 0.743 (95%CI: 0.677-0.810), with a sensitivity and specificity of 59.91% and 87.69%, respectively, at the maximum Youden’s Index. Based on this optimal cut-off value, 156 patients had a preoperative FAR ≤ 0.08, and 126 patients had a preoperative FAR > 0.08, as detailed in Table 4.
| Characteristic | Preoperative FAR | P value | |
| > 0.08, n = 126 | ≤ 0.08, n = 156 | ||
| Age in yr | 0.854 | ||
| > 60 | 66 (23.4) | 80 (28.4) | |
| ≤ 60 | 60 (21.3) | 76 (27.0) | |
| Sex | 0.724 | ||
| Male | 66 (23.4) | 85 (30.1) | |
| Female | 60 (21.3) | 71 (25.2) | |
| Clinical symptoms | 0.001 | ||
| Absent | 14 (5.0) | 43 (15.2) | |
| Present | 112 (39.7) | 113 (40.1) | |
| Diabetes | 0.831 | ||
| Absent | 89 (31.6) | 112 (39.7) | |
| Present | 37 (13.1) | 44 (15.6) | |
| Smoking status | 0.765 | ||
| Absent | 95 (33.7) | 120 (42.6) | |
| Present | 31 (11.0) | 36 (12.8) | |
| Alcohol consumption | 0.335 | ||
| Absent | 108 (38.3) | 127 (45.0) | |
| Present | 18 (6.4) | 29 (10.3) | |
| Family history of cancer | 0.571 | ||
| Absent | 122 (43.3) | 149 (52.8) | |
| Present | 4 (1.4) | 7 (2.5) | |
| Blood type | 0.817 | ||
| A | 36 (12.8) | 51 (18.1) | |
| B | 45 (16.0) | 48 (17.0) | |
| AB | 10 (3.5) | 12 (4.3) | |
| O | 35 (12.4) | 45 (16.0) | |
| Tumor location | < 0.001 | ||
| Head and neck | 77 (27.3) | 53 (18.8) | |
| Body and tail | 49 (17.4) | 103 (36.5) | |
| Approaches of open surgery | < 0.001 | ||
| Pancreaticoduodenectomy | 77 (27.3) | 53 (18.8) | |
| Distal pancreatectomy with splenectomy | 49 (17.4) | 103 (36.5) | |
| Degree of differentiation | 0.208 | ||
| Well | 20 (7.1) | 14 (5.0) | |
| Moderately | 93 (33.0) | 124 (44.0) | |
| Poorly | 13 (4.6) | 18 (6.4) | |
| Lymphovascular invasion | 0.729 | ||
| Absent | 92 (32.6) | 111 (39.4) | |
| Present | 34 (12.1) | 45 (16.0) | |
| Perineural invasion | 0.082 | ||
| Absent | 25 (8.9) | 45 (16.0) | |
| Present | 101 (35.8) | 111 (39.4) | |
| Capsular invasion | 0.360 | ||
| Absent | 19 (6.7) | 30 (10.6) | |
| Present | 107 (37.9) | 126 (44.7) | |
| Maximum tumor diameter in cm | 0.391 | ||
| > 4 | 36 (12.8) | 52 (18.4) | |
| ≤ 4 | 90 (31.9) | 104 (36.9) | |
| T stage | 0.462 | ||
| T1 | 13 (4.6) | 21 (7.4) | |
| T2 | 76 (27.0) | 83 (29.4) | |
| T3 | 37 (13.1) | 52 (18.4) | |
| Number of metastatic lymph nodes | 0.988 | ||
| Absent | 72 (25.5) | 89 (31.6) | |
| Present | 54 (19.1) | 67 (23.8) | |
| N stage | 0.902 | ||
| N0 | 72 (25.5) | 89 (31.6) | |
| N1 | 40 (14.2) | 52 (18.4) | |
| N2 | 14 (5.0) | 15 (5.3) | |
| TNM stage | 0.494 | ||
| IA | 6 (2.1) | 14 (5.0) | |
| IB | 40 (14.2) | 52 (18.4) | |
| IIA | 26 (9.2) | 23 (8.2) | |
| IIB | 40 (14.2) | 52 (18.4) | |
| III | 14 (5.0) | 15 (5.3) | |
| Adjuvant therapy | 0.531 | ||
| Absent | 58 (20.6) | 66 (23.4) | |
| Present | 68 (24.1) | 90 (31.9) | |
| Preoperative CA19-9 level in U/mL | 0.132 | ||
| > 336.4 | 40 (14.2) | 37 (13.1) | |
| ≤ 336.4 | 86 (30.5) | 119 (42.2) | |
| Preoperative plasma fibrinogen concentration in g/L | < 0.001 | ||
| > 3.31 | 114 (40.4) | 27 (9.6) | |
| ≤ 3.31 | 12 (4.3) | 129 (45.7) | |
| Preoperative plasma albumin level in g/L | < 0.001 | ||
| > 45.2 | 15 (5.3) | 70 (24.8) | |
| ≤ 45.2 | 111 (39.4) | 86 (30.5) | |
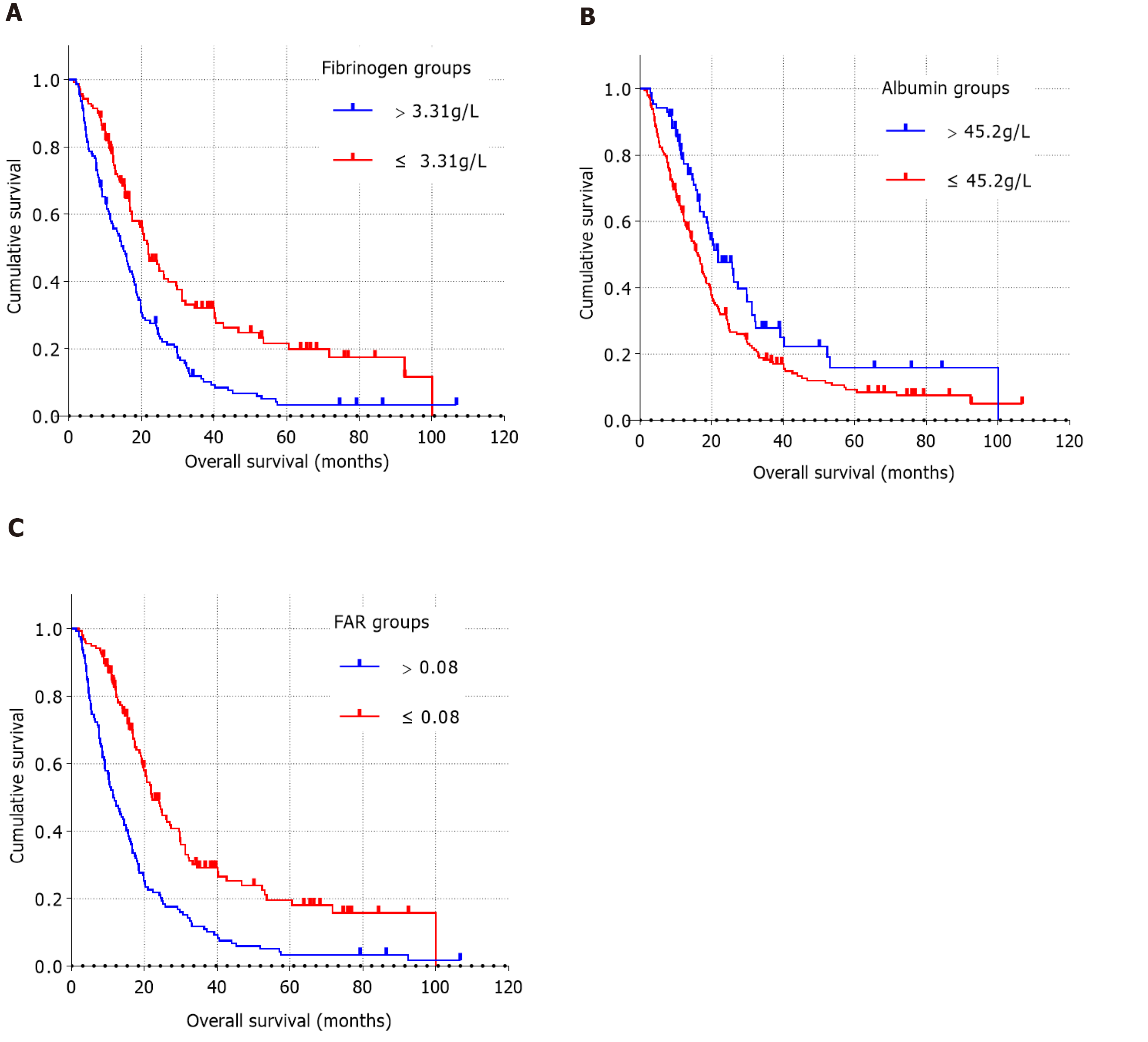
As shown in Table 2, all patients could be categorized into low or high value groups based on the optimal cut-off value of preoperative fibrinogen concentration. The increase in preoperative plasma fibrinogen concentration was significantly correlated with clinical symptoms, tumor location, surgical approaches, preoperative CA19-9 level, preoperative plasma albumin level, and preoperative FAR. However, preoperative fibrinogen concentration did not significantly correlate with age, sex, blood type, diabetes, smoking status, alcohol consumption, family history of cancer, degree of tumor differentiation, lymphovascular invasion, perineural invasion, capsular invasion, maximum tumor diameter, number of metastatic lymph nodes, T stage, N stage, TNM stage, or adjuvant therapy. The survival curves for preoperative fibrinogen concentrations showed shorter OS times in patients with preoperative fibrinogen concentrations > 3.31 g/L than in patients with preoperative fibrinogen concentrations ≤ 3.31 g/L (Figure 2A).
As shown in Table 3, all patients could be categorized into a low value group (≤ 45.2 g/L) or a high value group (> 45.2 g/L) based on the optimal cut-off value of preoperative plasma albumin level. The increase in preoperative albumin level was significantly correlated with clinical symptoms (P = 0.012), tumor location (P < 0.001), surgical approaches (P < 0.001), adjuvant therapy (P = 0.003), preoperative fibrinogen concentration (P = 0.006), and preoperative FAR (P < 0.001). The survival curves for preoperative albumin level showed longer OS times in patients with preoperative plasma albumin level > 45.2 g/L than in those with preoperative albumin level ≤ 45.2 g/L (Figure 2B).
As shown in Table 4, according to the optimal cut-off value of preoperative FAR, all patients can be divided into a low-value group (≤ 0.08) or a high-value group (> 0.08). Higher preoperative FAR was significantly associated with clinical symptoms (P = 0.001), tumor location (P < 0.001), surgical approaches (P < 0.001), preoperative plasma fibrinogen concentration (P < 0.001), and preoperative plasma albumin level (P < 0.001). The survival curves for preoperative FAR showed shorter OS times in patients with FAR > 0.08 than in patients with FAR ≤ 0.08 (Figure 2C).
In the univariate Cox analysis (Table 5), age [hazard ratio (HR): 1.358, 95%CI: 1.036-1.780, P = 0.027], clinical symptoms (HR: 0.600, 95%CI: 0.424-0.848, P = 0.004), degree of tumor differentiation (P < 0.001), capsular invasion (HR: 0.609, 95%CI: 0.420- 0.885, P = 0.009), maximum tumor diameter (HR: 1.403, 95%CI: 1.058-1.862, P = 0.019), T stage (P = 0.035), number of metastatic lymph nodes (HR: 0.590, 95%CI: 0.449-0.775, P < 0.001), N stage (P = 0.001), TNM stage (P = 0.003), adjuvant therapy (HR: 1.625, 95%CI: 1.244-2.123, P < 0.001), preoperative CA19-9 level (HR: 1.971, 95%CI: 1.469-2.644, P < 0.001), preoperative plasma fibrinogen concentration (HR: 1.888, 95%CI: 1.438-2.479, P < 0.001), preoperative plasma albumin level (HR: 0.650, 95%CI: 0.475-0.890, P = 0.007), and preoperative FAR (HR: 2.257, 95%CI: 1.725-2.952, P < 0.001) were important prognostic risk factors for OS in patients with PDAC who underwent radical R0 resection.
| Characteristic | HR (95%CI) | P value |
| Age in yr | 1.358 (1.036-1.780) | 0.027 |
| > 60 | ||
| ≤ 60 | ||
| Sex | 1.281 (0.979-1.675) | 0.071 |
| Male | ||
| Female | ||
| Clinical symptoms | 0.600 (0.424-0.848) | 0.004 |
| Absent | ||
| Present | ||
| Diabetes | 0.903 (0.676-1.206) | 0.491 |
| Absent | ||
| Present | ||
| Smoking status | 0.866 (0.635-1.181) | 0.363 |
| Absent | ||
| Present | ||
| Alcohol consumption | 1.083 (0.754-1.556) | 0.667 |
| Absent | ||
| Present | ||
| Family history of cancer | 1.251 (0.617-2.537) | 0.535 |
| Absent | ||
| Present | ||
| Blood type | — | 0.579 |
| A | ||
| B | ||
| AB | ||
| O | ||
| Tumor location | 0.954 (0.729-1.249) | 0.731 |
| Head and neck | ||
| Body and tail | ||
| Approaches of open surgery | 0.954 (0.729-1.249) | 0.731 |
| Pancreaticoduodenectomy | ||
| Distal pancreatectomy with splenectomy | ||
| Degree of differentiation | — | < 0.001 |
| Well | ||
| Moderately | ||
| Poorly | ||
| Lymphovascular invasion | 0.793 (0.590-1.065) | 0.123 |
| Absent | ||
| Present | ||
| Perineural invasion | 0.905 (0.666-1.231) | 0.525 |
| Absent | ||
| Present | ||
| Capsular invasion | 0.609 (0.420-0.885) | 0.009 |
| Absent | ||
| Present | ||
| Maximum tumor diameter in cm | 1.403 (1.058-1.862) | 0.019 |
| > 4 | ||
| ≤ 4 | ||
| T stage | — | 0.035 |
| T1 | ||
| T2 | ||
| T3 | ||
| Number of metastatic lymph nodes | 0.590 (0.449-0.775) | < 0.001 |
| Absent | ||
| Present | ||
| N stage | 0.001 | |
| N0 | ||
| N1 | ||
| N2 | ||
| TNM stage | 0.003 | |
| IA | ||
| IB | ||
| IIA | ||
| IIB | ||
| III | ||
| Adjuvant therapy | 1.625 (1.244-2.123) | < 0.001 |
| Absent | ||
| Present | ||
| Preoperative CA19-9 level in U/mL | 1.971 (1.469-2.644) | < 0.001 |
| > 336.4 | ||
| ≤ 336.4 | ||
| Preoperative plasma fibrinogen concentration in g/L | 1.888 (1.438-2.479) | < 0.001 |
| > 3.31 | ||
| ≤ 3.31 | ||
| Preoperative plasma albumin level in g/L | 0.650 (0.475-0.890) | 0.007 |
| > 45.2 | ||
| ≤ 45.2 | ||
| Preoperative FAR | 2.257 (1.725-2.952) | < 0.001 |
| > 0.08 | ||
| ≤ 0.08 |
However, sex, blood type, diabetes, smoking status, alcohol consumption, family history of cancer, surgical approaches, tumor location, lymphovascular invasion, and perineural invasion were not prognostic risk factors for OS in patients with PDAC who underwent radical R0 resection (P > 0.05).
In the multivariate Cox regression analysis (Table 6), degree of tumor differentiation (P < 0.001), number of metastatic lymph nodes (HR: 0.678, 95%CI: 0.509-0.904, P = 0.008), adjuvant therapy (HR: 1.604, 95%CI: 1.214-2.118, P = 0.001), preoperative CA19-9 level (HR: 1.740, 95%CI: 1.288-2.352, P < 0.001), and preoperative FAR (HR: 2.258, 95%CI: 1.720-2.963, P < 0.001) were independent risk factors for poor prognosis in patients with PDAC who underwent radical R0 resection.
| Characteristics | HR (95%CI) | Wald | P value |
| Age in yr | 1.289 (0.971-1.710) | 3.076 | 0.079 |
| > 60 | |||
| ≤ 60 | |||
| Degree of differentiation | 31.261 | < 0.001 | |
| Poorly/well | 5.209 (2.837-9.562) | 28.353 | < 0.001 |
| Moderately/well | 1.912 (1.184-3.085) | 7.037 | 0.008 |
| Capsular invasion | 0.697 (0.475-1.022) | 3.409 | 0.065 |
| Absent | |||
| Present | |||
| Maximum tumor diameter in cm | 1.296 (0.959-1.751) | 2.843 | 0.092 |
| > 4 | |||
| ≤ 4 | |||
| Number of metastatic lymph nodes | 0.678 (0.509-0.904) | 7,027 | 0.008 |
| Absent | |||
| Present | |||
| Adjuvant therapy | 1.604 (1.214-2.118) | 11.058 | 0.001 |
| Absent | |||
| Present | |||
| Preoperative CA19-9 level in U/mL | 1.740 (1.288-2.352) | 13.003 | < 0.001 |
| > 336.4 | |||
| ≤ 336.4 | |||
| Preoperative FAR | 2.258 (1.720-2.963) | 34.468 | < 0.001 |
| > 0.08 | |||
| ≤ 0.08 |
Our results showed that preoperative FAR was closely related to OS in patients with PDAC after radical R0 resection. To our knowledge, this is the first report of FAR in patients with PDAC who underwent radical R0 resection. A higher preoperative FAR predicted poor prognosis in these patients. Further multivariate analysis showed that preoperative FAR (P < 0.001), degree of differentiation (P < 0.001), number of metastatic lymph nodes (P = 0.008), adjuvant therapy (P = 0.001), and preoperative CA19-9 level (P < 0.001) were independent prognostic factors for the OS of patients with PDAC after radical R0 resection.
As a newly discovered tumor prognostic marker, the prognostic value of preoperative FAR in patients with malignant tumors undergoing radical surgery is increasing. The prognostic role of FAR has been confirmed in patients who underwent radical resection of tumors, such as esophageal cancer[19], breast cancer[20,21] and liver cancer[22]. It has also been reported that FAR (HR: 1.634; 95%CI: 1.359-1.964; P < 0.001) is an important prognostic factor for locally advanced or metastatic pancreatic cancer[25]. With regard to the optimal cut-off value of FAR, the value in patients who underwent radical operation for esophageal cancer was the same as ours, whereas that in patients who underwent radical operation for liver cancer was 0.062, which was lower than our value. The optimal cut-off value of FAR in patients with locally advanced or metastatic pancreatic cancer was 0.079, which was similar to our value. However, the reported optimal cut-off value of preoperative FAR in patients who underwent radical mastectomy was between 0.071 and 0.084, indicating that the optimal cut-off value may be different even in different patient groups with the same tumor type. The reasons for the above differences may be related to the different biological behaviors of different tumors or the selection bias of different patient groups with the same tumor type, so more basic and clinical studies are needed to verify this. Although preoperative FAR has a clear role in evaluating the prognosis of patients undergoing radical resection of malignant tumors[19-22], the molecular mechanisms related to the occurrence and development of tumors are still not completely clear.
Fibrinogen is a soluble glycoprotein with a relative molecular mass of 340 kDa that is composed of three different polypeptide chains, namely α, β, and γ. Normally, fibrinogen is synthesized by the liver and released into the bloodstream[26], where it plays an important role in both the inflammatory response and blood coagulation.
Inflammation is an important marker of cancer[14], and numerous clinical and experimental studies have convincingly supported the notion that inflammation is an important component of tumor progression[12,13]. The link between colitis and colon cancer has been fully confirmed, and researchers have directly tested the hypothesis of a link between fibrinogen and colitis-associated colon cancer in mice through animal experiments[27]. Preoperative hyperfibrinogenemia has been reported to be significantly associated with shorter OS (HR: 3.138, 95%CI: 1.077-9.139, P = 0.036) in patients with nonmetastatic colon cancer[28]. Hyperfibrinogenemia is associated with systemic inflammation and can predict poor prognosis in advanced pancreatic cancer (HR: 2.184, 95%CI: 1.574-3.032, P < 0.001)[29]. It has also been reported that fibrinogen concentration is positively correlated with the stage of pancreatic cancer, and the increase in plasma fibrinogen concentration before surgery may be a useful indicator for predicting the distant metastasis of pancreatic cancer (distant metastasis group vs no distant metastasis group: 4.41 ± 0.84 g/L vs 3.76 ± 1.04 g/L, P < 0.01)[30]. Survival analyses reported in the literature show that plasma fibrinogen concentration is an independent prognostic factor for operable patients with non-small cell lung cancer. Patients with high fibrinogen concentrations (fibrinogenemia) had a higher risk of disease progression (HR: 1.49, 95%CI: 1.07-2.05, P < 0.05) and death (HR: 1.64, 95%CI: 1.06-2.53, P < 0.05) than those with normal fibrinogen concentrations[31]. In a large-scale observation study of 2563 patients with nonmetastatic primary nasopharyngeal carcinoma, a combined increase in Epstein-Barr virus deoxyribonucleic acid copy number and fibrinogen concentration was found to be significantly associated with reduced disease-free survival (HR: 8.51, 95%CI: 5.34-13.56, P < 0.001) and OS (HR 12.77, 95%CI: 5.08-32.12, P < 0.001)[32]. Thus, elevated plasma fibrinogen concentrations before treatment were associated with a significant reduction in the survival of patients with solid tumors, which also suggests that clinical trials are needed to determine whether plasma fibrinogen can be included in the cancer staging system and whether reducing the concentration of plasma fibrinogen has a beneficial effect on disease recurrence and mortality[33].
Pancreatic cancer has also been found to cause hypercoagulability, which may lead to significant thromboembolism in patients with pancreatic cancer, with a reported prevalence of venous thromboembolism of 12%-36%[34,35]. Fibrinogen not only plays a key role in hemostasis but also plays an important role in tumor biology. Fibrin deposition provides a temporary medium-like matrix rich in growth factors that promote the growth of cancer cells. The importance of fibrinogen in tumor biology has been demonstrated in studies of fibrinogen-deficient mice[36]. Studies have shown that fibrinogen can promote platelet adhesion to tumor cells; at the same time, platelets promote the accumulation of more fibrinogen around tumor cells by forming thrombin, and these components cooperate with each other to protect tumor cells from natural killer cells[37]. Fibrinogen can be cleaved by fibrinolytic enzymes, and its protein hydrolysate can promote angiogenesis, which is the premise of tumor growth and metastasis[38]. Fibrinogen has the ability to bind directly to vascular endothelial growth factor (VEGF), platelet-derived growth factor, fibroblast growth factor family members, and transforming growth factor-β, which also reflects the important role fibrinogen plays in angiogenesis, tumor cell proliferation, the hematogenous metastasis of malignant tumors, and epithelial-mesenchymal transformation[39-41]. By inducing epithelial-mesenchymal transformation, fibrinogen promotes the migration of tumor cells and enhances the invasion and metastasis of tumor cells[42].
In cancer patients, the diagnosis of malnutrition is very common and has a very negative impact on prognosis[17]. Progressive malnutrition is closely related to the occurrence and development of cachexia, especially in patients with pancreatic cancer and gastric cancer[43]. Epidemiological studies on the relationship between the level of plasma albumin and the survival of cancer patients have found that the decrease in plasma albumin level is associated with reduced survival in patients[18]. The relationship between pretreatment plasma albumin level and prognosis has been reported in head and neck cancer[44], non-small cell lung cancer[45], kidney cancer[46], gastric cardia adenocarcinoma[47], and ovarian cancer[48]. Inflammation is known to play a critical role in the development, progression, and prognosis of colorectal tumors, and plasma albumin concentration has been shown to be a valuable prognostic factor for colorectal tumors[49]. Tumor cells produce and release proinflammatory cytokines (interleukin (IL)-1, IL-6, tumor necrosis factor-α, interferon-γ) and hormones that are associated with the pathogenesis of both malnutrition and cachexia[43,50], and these inflammatory cytokines can interfere with the normal synthesis of albumin in the liver[16]. Among them, IL-6 has been shown to regulate the secretion of VEGF in glioblastoma cells, while VEGF can lead to an increase in vascular permeability, which indirectly leads to a decrease in plasma albumin level[51].
Therefore, preoperative FAR, as an indicator that reflects the ratio between the fibrinogen concentration and albumin level, can be considered a risk factor for the prognosis of patients undergoing radical R0 resection of PDAC.
This study has some limitations. First, this study is a single-center, retrospective study with a small sample, which may have selection bias. In the future, it is necessary to design more multicenter, prospective studies with a large sample to verify the current conclusions. Second, due to the relatively small number of patients, we did not divide the patients into a training group and test group for statistical verification, and there was a lack of external verification, which needs further study. In our study, we focused on the prognostic significance of preoperative FAR, while the relationship between the change in FAR after surgery and the prognosis of these patients was not been studied. In addition, many patients received multiple treatments due to tumor recurrence during the follow-up period, which also affected OS.
In conclusion, in this first report of preoperative FAR in PDAC, we have found that preoperative FAR, defined as the ratio of the fibrinogen concentration to the albumin level, is a useful indicator for assessing the prognosis of patients undergoing radical R0 resection of PDAC. Its practicability, convenience, and low cost make FAR a promising serum biomarker for predicting the prognosis of patients with PDAC. It may provide important help in treatment decision-making for patients with resectable PDAC in the future. However, more relevant studies are needed to validate further the relevant conclusions.
Pancreatic cancer has the worst prognosis of all the malignancies, with only 20% of pancreatic cancer patients having access to surgical treatment. The poor prognosis of pancreatic cancer patients is related to the early asymptomatic nature of the disease, leading to the late stage of disease diagnosis and the high possibility of distant metastasis in the early stage of pancreatic cancer. Inflammation plays an important role in tumor progression, and growing evidence has confirmed that the fibrinogen-to-albumin ratio (FAR) is an important prognostic factor for overall survival (OS) in malignant tumors.
We speculate that FAR, as an easily available, cost-effective, and noninvasive prognostic indicator for pancreatic cancer patients, could help to select and identify pancreatic cancer patients suitable for surgical resection. This will benefit pancreatic cancer patients.
The main objective of our study was to investigate the prognostic significance of FAR in pancreatic ductal adenocarcinoma (PDAC) patients undergoing radical R0 resection.
A total of 282 PDAC patients undergoing radical R0 resection were included in this retrospective study. We used the surv_cutpoint function and receiver operating characteristic curves to determine the optimal cut-off values for preoperative fibrinogen, albumin, and FAR. We analyzed the patients’ clinicopathological data, the Kaplan-Meier method and log-rank tests were used for univariate survival analysis, and a Cox regression model was used for multivariate survival analysis for these patients.
The optimal cut-off value of FAR was 0.08 in our study. Higher preoperative FAR was significantly correlated with clinical symptoms, tumor location, surgical approaches, preoperative plasma fibrinogen concentration, and preoperative plasma albumin level (all P < 0.05). Multivariate analysis showed that preoperative FAR (HR: 2.258, 95%CI: 1.720-2.963, P < 0.001) was an independent prognostic factor in PDAC patients undergoing radical R0 resection.
Preoperative FAR, as an indicator that reflects the ratio between the fibrinogen concentration and albumin level, is an important predictor of the OS in PDAC patients undergoing radical R0 resection. A higher preoperative FAR predicted poor prognosis in these patients.
In the future, it is necessary to design more multicenter, prospective studies with a large sample to verify the current conclusions. Additionally, more basic experiments exploring the potential mechanisms are necessary.
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13300] [Cited by in RCA: 15623] [Article Influence: 2231.9] [Reference Citation Analysis (11)] |
| 2. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13323] [Article Influence: 1332.3] [Reference Citation Analysis (4)] |
| 3. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56660] [Article Influence: 7082.5] [Reference Citation Analysis (134)] |
| 4. | Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913-2921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5379] [Cited by in RCA: 5355] [Article Influence: 446.3] [Reference Citation Analysis (0)] |
| 5. | Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA. SEER Cancer Statistics Review, 1975-2017, National Cancer Institute, based on November 2019 SEER data submission, posted to the SEER web site, April 2020, cited 2020-09-20. Available from: https://seer.cancer.gov/csr/1975_2017/. |
| 6. | Grossberg AJ, Chu LC, Deig CR, Fishman EK, Hwang WL, Maitra A, Marks DL, Mehta A, Nabavizadeh N, Simeone DM, Weekes CD, Thomas CR Jr. Multidisciplinary standards of care and recent progress in pancreatic ductal adenocarcinoma. CA Cancer J Clin. 2020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 348] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 7. | Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388:73-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1397] [Cited by in RCA: 1787] [Article Influence: 178.7] [Reference Citation Analysis (1)] |
| 8. | Kulemann B, Hoeppner J, Wittel U, Glatz T, Keck T, Wellner UF, Bronsert P, Sick O, Hopt UT, Makowiec F, Riediger H. Perioperative and long-term outcome after standard pancreaticoduodenectomy, additional portal vein and multivisceral resection for pancreatic head cancer. J Gastrointest Surg. 2015;19:438-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | Hoem D, Viste A. Improving survival following surgery for pancreatic ductal adenocarcinoma--a ten-year experience. Eur J Surg Oncol. 2012;38:245-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Gillen S, Schuster T, Meyer Zum Büschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7:e1000267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1243] [Cited by in RCA: 1193] [Article Influence: 74.6] [Reference Citation Analysis (3)] |
| 11. | Groot VP, Gemenetzis G, Blair AB, Rivero-Soto RJ, Yu J, Javed AA, Burkhart RA, Rinkes IHMB, Molenaar IQ, Cameron JL, Weiss MJ, Wolfgang CL, He J. Defining and Predicting Early Recurrence in 957 Patients With Resected Pancreatic Ductal Adenocarcinoma. Ann Surg. 2019;269:1154-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 293] [Article Influence: 48.8] [Reference Citation Analysis (1)] |
| 12. | Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883-899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8437] [Cited by in RCA: 8412] [Article Influence: 525.8] [Reference Citation Analysis (8)] |
| 13. | Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10123] [Cited by in RCA: 11509] [Article Influence: 479.5] [Reference Citation Analysis (2)] |
| 14. | Mantovani A. Cancer: Inflaming metastasis. Nature. 2009;457:36-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 493] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 15. | Campello E, Ilich A, Simioni P, Key NS. The relationship between pancreatic cancer and hypercoagulability: a comprehensive review on epidemiological and biological issues. Br J Cancer. 2019;121:359-371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 16. | Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8581] [Cited by in RCA: 8551] [Article Influence: 475.1] [Reference Citation Analysis (0)] |
| 17. | Virizuela JA, Camblor-Álvarez M, Luengo-Pérez LM, Grande E, Álvarez-Hernández J, Sendrós-Madroño MJ, Jiménez-Fonseca P, Cervera-Peris M, Ocón-Bretón MJ. Nutritional support and parenteral nutrition in cancer patients: an expert consensus report. Clin Transl Oncol. 2018;20:619-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 18. | Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 2010;9:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1011] [Cited by in RCA: 1087] [Article Influence: 67.9] [Reference Citation Analysis (0)] |
| 19. | Tan Z, Zhang M, Han Q, Wen J, Luo K, Lin P, Zhang L, Yang H, Fu J. A novel blood tool of cancer prognosis in esophageal squamous cell carcinoma: the Fibrinogen/Albumin Ratio. J Cancer. 2017;8:1025-1029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 20. | Hwang KT, Chung JK, Roh EY, Kim J, Oh S, Kim YA, Rhu J, Kim S. Prognostic Influence of Preoperative Fibrinogen to Albumin Ratio for Breast Cancer. J Breast Cancer. 2017;20:254-263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 21. | Zheng Y, Wu C, Yan H, Chen S. Prognostic value of combined preoperative fibrinogen-albumin ratio and platelet-lymphocyte ratio score in patients with breast cancer: A prognostic nomogram study. Clin Chim Acta. 2020;506:110-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Xu Q, Yan Y, Gu S, Mao K, Zhang J, Huang P, Zhou Z, Chen Z, Zheng S, Liang J, Lin Z, Wang J, Yan J, Xiao Z. A Novel Inflammation-Based Prognostic Score: The Fibrinogen/Albumin Ratio Predicts Prognoses of Patients after Curative Resection for Hepatocellular Carcinoma. J Immunol Res. 2018;2018:4925498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 23. | Bockhorn M, Uzunoglu FG, Adham M, Imrie C, Milicevic M, Sandberg AA, Asbun HJ, Bassi C, Büchler M, Charnley RM, Conlon K, Cruz LF, Dervenis C, Fingerhutt A, Friess H, Gouma DJ, Hartwig W, Lillemoe KD, Montorsi M, Neoptolemos JP, Shrikhande SV, Takaori K, Traverso W, Vashist YK, Vollmer C, Yeo CJ, Izbicki JR; International Study Group of Pancreatic Surgery. Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2014;155:977-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 654] [Cited by in RCA: 687] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 24. | Chun YS, Pawlik TM, Vauthey JN. 8th Edition of the AJCC Cancer Staging Manual: Pancreas and Hepatobiliary Cancers. Ann Surg Oncol. 2018;25:845-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 583] [Article Influence: 64.8] [Reference Citation Analysis (1)] |
| 25. | Fang L, Yan FH, Liu C, Chen J, Wang D, Zhang CH, Lou CJ, Lian J, Yao Y, Wang BJ, Li RY, Han SL, Bai YB, Yang JN, Li ZW, Zhang YQ. Systemic Inflammatory Biomarkers, Especially Fibrinogen to Albumin Ratio, Predict Prognosis in Patients with Pancreatic Cancer. Cancer Res Treat. 2020;Online ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 26. | Adams RA, Schachtrup C, Davalos D, Tsigelny I, Akassoglou K. Fibrinogen signal transduction as a mediator and therapeutic target in inflammation: lessons from multiple sclerosis. Curr Med Chem. 2007;14:2925-2936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 130] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 27. | Steinbrecher KA, Horowitz NA, Blevins EA, Barney KA, Shaw MA, Harmel-Laws E, Finkelman FD, Flick MJ, Pinkerton MD, Talmage KE, Kombrinck KW, Witte DP, Palumbo JS. Colitis-associated cancer is dependent on the interplay between the hemostatic and inflammatory systems and supported by integrin alpha(M)beta(2) engagement of fibrinogen. Cancer Res. 2010;70:2634-2643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 28. | Son HJ, Park JW, Chang HJ, Kim DY, Kim BC, Kim SY, Park SC, Choi HS, Oh JH. Preoperative plasma hyperfibrinogenemia is predictive of poor prognosis in patients with nonmetastatic colon cancer. Ann Surg Oncol. 2013;20:2908-2913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (1)] |
| 29. | Qi Q, Geng Y, Sun M, Chen H, Wang P, Chen Z. Hyperfibrinogen Is Associated With the Systemic Inflammatory Response and Predicts Poor Prognosis in Advanced Pancreatic Cancer. Pancreas. 2015;44:977-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 30. | Guo Q, Zhang B, Dong X, Xie Q, Guo E, Huang H, Wu Y. Elevated levels of plasma fibrinogen in patients with pancreatic cancer: possible role of a distant metastasis predictor. Pancreas. 2009;38:e75-e79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 31. | Sheng L, Luo M, Sun X, Lin N, Mao W, Su D. Serum fibrinogen is an independent prognostic factor in operable nonsmall cell lung cancer. Int J Cancer. 2013;133:2720-2725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 32. | Tang LQ, Chen QY, Guo SS, Chen WH, Li CF, Zhang L, Lai XP, He Y, Xu YX, Hu DP, Wen SH, Peng YT, Liu H, Liu LT, Yan SM, Guo L, Zhao C, Cao KJ, Liu Q, Qian CN, Ma J, Guo X, Zeng MS, Mai HQ. The impact of plasma Epstein-Barr virus DNA and fibrinogen on nasopharyngeal carcinoma prognosis: an observational study. Br J Cancer. 2014;111:1102-1111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 33. | Perisanidis C, Psyrri A, Cohen EE, Engelmann J, Heinze G, Perisanidis B, Stift A, Filipits M, Kornek G, Nkenke E. Prognostic role of pretreatment plasma fibrinogen in patients with solid tumors: A systematic review and meta-analysis. Cancer Treat Rev. 2015;41:960-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 147] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 34. | Ishigaki K, Nakai Y, Isayama H, Saito K, Hamada T, Takahara N, Mizuno S, Mohri D, Kogure H, Matsubara S, Yamamoto N, Tada M, Koike K. Thromboembolisms in Advanced Pancreatic Cancer: A Retrospective Analysis of 475 Patients. Pancreas. 2017;46:1069-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Epstein AS, Soff GA, Capanu M, Crosbie C, Shah MA, Kelsen DP, Denton B, Gardos S, O'Reilly EM. Analysis of incidence and clinical outcomes in patients with thromboembolic events and invasive exocrine pancreatic cancer. Cancer. 2012;118:3053-3061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 36. | Palumbo JS, Potter JM, Kaplan LS, Talmage K, Jackson DG, Degen JL. Spontaneous hematogenous and lymphatic metastasis, but not primary tumor growth or angiogenesis, is diminished in fibrinogen-deficient mice. Cancer Res. 2002;62:6966-6972. [PubMed] |
| 37. | Zheng S, Shen J, Jiao Y, Liu Y, Zhang C, Wei M, Hao S, Zeng X. Platelets and fibrinogen facilitate each other in protecting tumor cells from natural killer cytotoxicity. Cancer Sci. 2009;100:859-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 147] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 38. | Zhao C, Su Y, Zhang J, Feng Q, Qu L, Wang L, Liu C, Jiang B, Meng L, Shou C. Fibrinogen-derived fibrinostatin inhibits tumor growth through anti-angiogenesis. Cancer Sci. 2015;106:1596-1606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 39. | Staton CA, Brown NJ, Lewis CE. The role of fibrinogen and related fragments in tumour angiogenesis and metastasis. Expert Opin Biol Ther. 2003;3:1105-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 96] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 40. | Witsch E, Sela M, Yarden Y. Roles for growth factors in cancer progression. Physiology (Bethesda). 2010;25:85-101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 294] [Cited by in RCA: 313] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 41. | Martino MM, Briquez PS, Ranga A, Lutolf MP, Hubbell JA. Heparin-binding domain of fibrin(ogen) binds growth factors and promotes tissue repair when incorporated within a synthetic matrix. Proc Natl Acad Sci USA. 2013;110:4563-4568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 359] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 42. | Shu YJ, Weng H, Bao RF, Wu XS, Ding Q, Cao Y, Wang XA, Zhang F, Xiang SS, Li HF, Li ML, Mu JS, Wu WG, Liu YB. Clinical and prognostic significance of preoperative plasma hyperfibrinogenemia in gallbladder cancer patients following surgical resection: a retrospective and in vitro study. BMC Cancer. 2014;14:566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 43. | Sadeghi M, Keshavarz-Fathi M, Baracos V, Arends J, Mahmoudi M, Rezaei N. Cancer cachexia: Diagnosis, assessment, and treatment. Crit Rev Oncol Hematol. 2018;127:91-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 147] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 44. | Danan D, Shonka DC Jr, Selman Y, Chow Z, Smolkin ME, Jameson MJ. Prognostic value of albumin in patients with head and neck cancer. Laryngoscope. 2016;126:1567-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 45. | Espinosa E, Feliu J, Zamora P, González Barón M, Sánchez JJ, Ordón ez A, Espinosa J. Serum albumin and other prognostic factors related to response and survival in patients with advanced non-small cell lung cancer. Lung Cancer. 1995;12:67-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 100] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 46. | Chen Z, Shao Y, Wang K, Cao W, Xiong Y, Wu R, Luo S, Xu X, He X. Prognostic role of pretreatment serum albumin in renal cell carcinoma: a systematic review and meta-analysis. Onco Targets Ther. 2016;9:6701-6710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 47. | Lien YC, Hsieh CC, Wu YC, Hsu HS, Hsu WH, Wang LS, Huang MH, Huang BS. Preoperative serum albumin level is a prognostic indicator for adenocarcinoma of the gastric cardia. J Gastrointest Surg. 2004;8:1041-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 138] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 48. | Asher V, Lee J, Bali A. Preoperative serum albumin is an independent prognostic predictor of survival in ovarian cancer. Med Oncol. 2012;29:2005-2009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 49. | González-Trejo S, Carrillo JF, Carmona-Herrera DD, Baz-Gutiérrez P, Herrera-Goepfert R, Núñez G, Ochoa-Carrillo FJ, Gallardo-Rincón D, Aiello-Crocifoglio V, Oñate-Ocaña LF. Baseline serum albumin and other common clinical markers are prognostic factors in colorectal carcinoma: A retrospective cohort study. Medicine (Baltimore). 2017;96:e6610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 50. | Van Cutsem E, Arends J. The causes and consequences of cancer-associated malnutrition. Eur J Oncol Nurs. 2005;9 Suppl 2:S51-S63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 404] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 51. | Loeffler S, Fayard B, Weis J, Weissenberger J. Interleukin-6 induces transcriptional activation of vascular endothelial growth factor (VEGF) in astrocytes in vivo and regulates VEGF promoter activity in glioblastoma cells via direct interaction between STAT3 and Sp1. Int J Cancer. 2005;115:202-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 174] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Isaji S S-Editor: Huang P L-Editor: Filipodia P-Editor: Wang LL