Published online Dec 14, 2020. doi: 10.3748/wjg.v26.i46.7405
Peer-review started: August 27, 2020
First decision: November 3, 2020
Revised: November 15, 2020
Accepted: November 21, 2020
Article in press: November 21, 2020
Published online: December 14, 2020
Processing time: 108 Days and 15.5 Hours
Most cholangiocarcinoma patients with malignant obstructive jaundice (MOJ) have varying degrees of malnutrition and immunodeficiency preoperatively. Therefore, perioperative nutritional support has important clinical significance in the treatment of cholangiocarcinoma.
To investigate the effects of postoperative early enteral nutrition (EEN) on immunity function and clinical outcomes of cholangiocarcinoma patients with MOJ.
This prospective clinical study included 60 cholangiocarcinoma patients with MOJ who underwent surgery. The patients were randomly divided into an experimental group and a control group according to the nutrition support modes. The control group received postoperative total parenteral nutrition (TPN), whereas the experimental group received postoperative EEN and parenteral nutrition (PN; EEN + PN). The clinical outcomes, postoperative immune function, incidences of surgical site infection and bile leakage, intestinal function recovery time, average hospitalization days, and hospitalization expenses of the two groups were assessed on postoperative days (PODs) 1, 3, and 7.
The CD3+T, CD4+T, CD8+T, and CD4+T/CD8+T cell count and the immunoglobulin (Ig) G, IgM, and IgA levels in the EEN + PN group were significantly higher than those in the TPN group on PODs 3 and 7 (P < 0.05), whereas no significant differences in the CD3+T, CD4+T, CD8+T, and CD4+T/CD8+T cell counts and IgG, IgM, and IgA levels before operation and on POD 1 were found between the two groups (P > 0.05). The intestinal function recovery time and postoperative hospital stay were shorter (P < 0.001 for both) in the EEN + PN group than in the TPN group. The hospitalization expenses of the EEN + PN group were lower than those of the TPN group (P < 0.001). However, the incidence of abdominal distension was higher than in the EEN + PN group than in the TPN group (P < 0.05). The incidence rates of biliary leakage and surgical site infection were not significantly different between the two groups (P > 0.05).
A postoperative EEN program could reduce the incidence of postoperative complications and improve the clinical outcomes and immune functions of cholangiocarcinoma patients with MOJ and is thus beneficial to patient recovery.
Core Tip: The aim of this prospective clinical trial was to investigate the effects of postoperative early enteral nutrition (EEN) on immunity function and clinical outcomes of cholangiocarcinoma patients with malignant obstructive jaundice (MOJ). Patients were randomly divided into the early enteral nutrition and parenteral nutrition (EEN+PN) and total parenteral nutrition (TPN) groups. As a result, the CD3+T, CD4+T, CD8+T, and CD4+T/CD8+T cell counts, and the immunoglobulin (Ig) G, IgM, and IgA levels in the EEN+PN group were significantly higher than those in the TPNgroup on postoperative days 3 and 7 (P < 0.05). A postoperative EEN program could improve the clinical outcomes and immune functions of cholangiocarcinoma patients with MOJ and is thus beneficial to patient recovery.
- Citation: Ma BQ, Chen SY, Jiang ZB, Wu B, He Y, Wang XX, Li Y, Gao P, Yang XJ. Effect of postoperative early enteral nutrition on clinical outcomes and immune function of cholangiocarcinoma patients with malignant obstructive jaundice. World J Gastroenterol 2020; 26(46): 7405-7415
- URL: https://www.wjgnet.com/1007-9327/full/v26/i46/7405.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i46.7405
Cholangiocarcinoma with malignant obstructive jaundice (MOJ) is a malignant tumor originating from the bile duct epithelium. According to their locations, cholangiocarcinomas are divided into intrahepatic and extrahepatic groups (ECC). This study refers to ECC. Radical surgical resection is the most commonly used method for the treatment of cholangiocarcinoma and is still the only method that can achieve long-term survival of patients.
However, impaired lipid digestion and absorption in patients with cholangiocarcinoma may result from decreased bile flow into the intestine due to bile duct obstruction and inflammation[1]. Most cholangiocarcinoma patients with MOJ have varying degrees of malnutrition and immunodeficiency preoperatively[2,3]; therefore, perioperative nutritional support has important clinical significance in the comprehensive treatment of cholangiocarcinoma.
In this study, the clinical data of 56 cholangiocarcinoma patients with MOJ who underwent surgery were collected. The clinical value of early enteral nutrition (EEN) support in these patients was evaluated using a statistical analysis of the effects of different nutritional support methods on postoperative immune function.
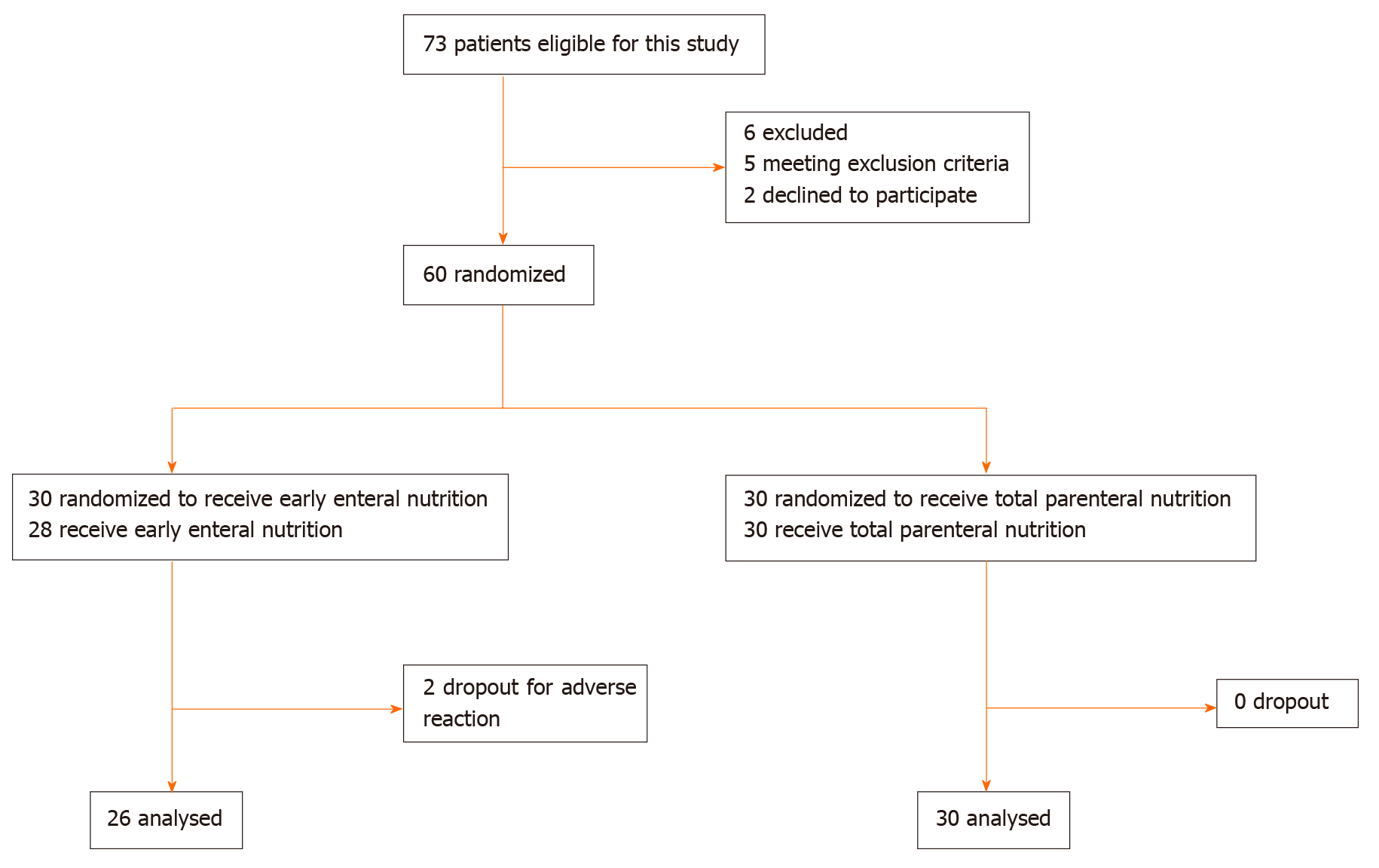
This was a prospective, single-center, randomized clinical trial. The patients were randomly assigned to either the EEN and parenteral nutrition (EEN + PN) or total parenteral nutrition (TPN) group after surgery. We used simple or complete randomization, which is a grouping method based on the remaining random numbers. After a random number was generated, the remaining grouping method (divided by two) was performed in a computer to determine which group the patient was assigned to, either the EEN + PN or TPN group. The treatment allocation was concealed from the patients included in the study and was not deleted from the study after it was made known to the patients. The main difference between the two groups was the nutritional support mode, so blinding the treating physicians to the treatment allocation was not possible. As the included patients had no knowledge of the treatment allocation, this study was considered a single-blind clinical trial. This study was approved by the ethics committee of Gansu Provincial Hospital (approval No. 2016-036), and all the programs were performed after the patients or their relatives signed the informed consent form. This study was registered at the Chinese Clinical Trial Registry, under registration identification No. ChiCTR2000034561. Figure 1 shows the flowchart of the participants.
All adult patients (aged 18-70 years) who were admitted to the Department of General Surgery of Gansu Provincial Hospital between November 2016 and June 2019 under the diagnosis of ECC were included in this prospective clinical study.
The inclusion criteria were as follows: (1) Patients whose
The exclusion criteria were as follows: (1) Patients who could not cooperate to complete the relevant investigation or examination; (2) Patients who accepted palliative operation; (3) Patients with cardiovascular, liver, kidney, brain, lung, or other serious diseases or those with poor control of hypertension or diabetes; (4) Pregnant or lactating patients; (5) Extremely weak patients with malignant fluids in the late stage of cancer; (6) Patients with mental illness; (7) Patients who participated in other clinical trials within 1 mo prior to participation in this study; and (8) Patients who were unsuitable to participate in this clinical study on the basis of the evaluation performed by the researchers.
Preoperative parenteral nutrition was given according to the patient's nutritional status. Patients with preoperative total bilirubin greater than 200 µmol/L were treated with preoperative biliary drainage therapy, such as percutaneous transhepatic cholangial drainage or endoscopic nasobiliary drainage[4]. The bilirubin of patients with cholangiocarcinoma dropped to around 200 µmol/L, and patients with Child-Pugh liver function was class A or B, so surgical treatment can be considered.
The postoperative nutritional support was based on a 40-mL/(kg·day) liquid volume and total energy levels of 10, 20, 30, and 35 kcal/kg on the first, second, third, and following days, respectively (Table 1). In the EEN + PN group, a 10-F nasojejunal feeding tube (Flocare, Nutricia Ltd, Paris, France) was placed as needed. EN (Peptisorb, Nutricia Ltd) was used on postoperative day (POD) 2, and PN was used as a supplement in the case of insufficient EN. In the TPN group, a 10-F nasojejunal feeding tube was placed as well, whereas patients were completely supplied by PN via a central venous catheter, and the calorie-to-nitrogen ratio was defined as 120-150:1. Of the total energy intake, 50% to 70% was provided by glucose, while the supply of lipids was based on the serum triglyceride levels. In addition, electrolytes, adequate insulin, vitamins, and trace elements were supplemented.
| Group | Nutritional support | POD 1 | POD 2 | POD 3 | POD 4 | POD 5 | POD 6 |
| TPN group | PN | 10 kcal/kg | 20 kcal/kg | 30 kcal/kg | 35 kcal/kg | 35 kcal/kg | 35 kcal/kg |
| EN | 0 | 0 | 0 | 0 | 0 | 0 | |
| EEN + PN group | PN | 10 kcal/kg | 10 kcal/kg | 10 kcal/kg | 5 kcal/kg | 0 | 0 |
| EN | 0 | 10 kcal/kg | 20 kcal/kg | 30 kcal/kg | 35 kcal/kg | 35 kcal/kg |
On admission, the baseline data, including sex, age, NRS score, total bilirubin level, and jaundice duration, were collected. The general postoperative indicators, namely bile leakage, surgical site infection, abdominal distention, intestinal function recovery time, length of postoperative hospital stay, and total cost of hospitalization, in all the patients were recorded and compared. Multiple indicators of immune function and nutritional status were evaluated in all the patients before surgery and on PODs 1, 3, and 7. Immune function was assessed by measuring the counts of the blood lymphocyte subsets (CD3+T, CD4+T, CD8+T, and CD4+T/CD8+T cells) and serum immunoglobulin (Ig) G, IgM, and IgA levels (g/L). T-cell subpopulations (CD3+T, CD4+T, and CD8+T) and serum IgG, IgM, and IgA were detected with commercially available human Quantikine enzyme-linked immunosorbent assay kits (R and D Systems, Bio-Techne Corporation, Minneapolis, MN, United States) in accordance with the manufacturer’s instructions.
GraphPad Prism 8 (San Diego, CA, United States) and SPSS 22.0 package (SPSS Inc., Armonk, NY, United States) was used to perform the analysis. Continuous variables were expressed as the mean ± standard deviation. An independent samples t-test was used to compare differences between the two groups. Categorical variables were expressed as absolute and relative frequencies. The Chi-square test or Fisher’s exact test were used to compare the differences in proportions between the two groups. A two-sided P value < 0.05 was considered statistically significant.
As shown in Figure 1, 60 patients were enrolled in this randomized clinical trial during the study period. Of these patients, 28 received EEN + PN, and 30 received TPN. There were two patients in the EEN + PN group who dropped out for adverse reactions of early enteral feeding, and 56 patients were analyzed in this study in the end. The comparison of the basic data before surgery and on POD 1 between the two groups revealed no significant differences between the two groups in terms of sex, age, preoperative NRS score, serum total bilirubin level, disease duration of jaundice, or the proportion of patients who underwent percutaneous transhepatic cholangial drainage (all P > 0.05), which were comparable (Table 2). We found no significant differences in operation methods, operation time, intraoperative blood loss, and NRS score on POD 1 between the two groups (all P > 0.05), as shown in Table 3.
| Group | Number of cases | Age in yr, mean ± SD | Gender of cases, M/F | NRS score, mean ± SD | Total bilirubin as µmol/L, mean ± SD | Jaundice duration in d, mean ± SD | PTCD cases |
| TPN group | 30 | 58.90 ± 7.42 | 18/12 | 4.03 ± 0.78 | 100.26 ± 19.18 | 21.26 ± 9.16 | 16 |
| EEN + PN group | 26 | 59.08 ± 7.64 | 15/11 | 4.08 ± 0.78 | 96.50 ± 18.01 | 18.96 ± 8.89 | 18 |
| t or χ2 | 0.089 | 0.031 | 0.239 | 0.753 | 0.950 | 1.476 | |
| P value | 0.9291 | 0.8612 | 0.8121 | 0.4551 | 0.3461 | 0.2242 |
| Basic data | EEN + PN group, n = 26 | TPN group, n = 30 | t or χ2 | P value |
| Operation methods by case | ||||
| Pancreatoduodenectomy | 12 | 20 | 2.393 | 0.1222 |
| Choledochojejunostomy | 14 | 10 | ||
| Operative duration in h, mean ± SD | 3.27 ± 0.88 | 2.84 ± 0.90 | 1.802 | 0.0771 |
| Amount of bleeding in mL, mean ± SD | 476.00 ± 187.71 | 524.00 ± 169.61 | 1.005 | 0.3191 |
| NRS score on the first day after operation by score, mean ± SD | 4.25 ± 0.74 | 4.21 ± 0.88 | 0.183 | 0.8561 |
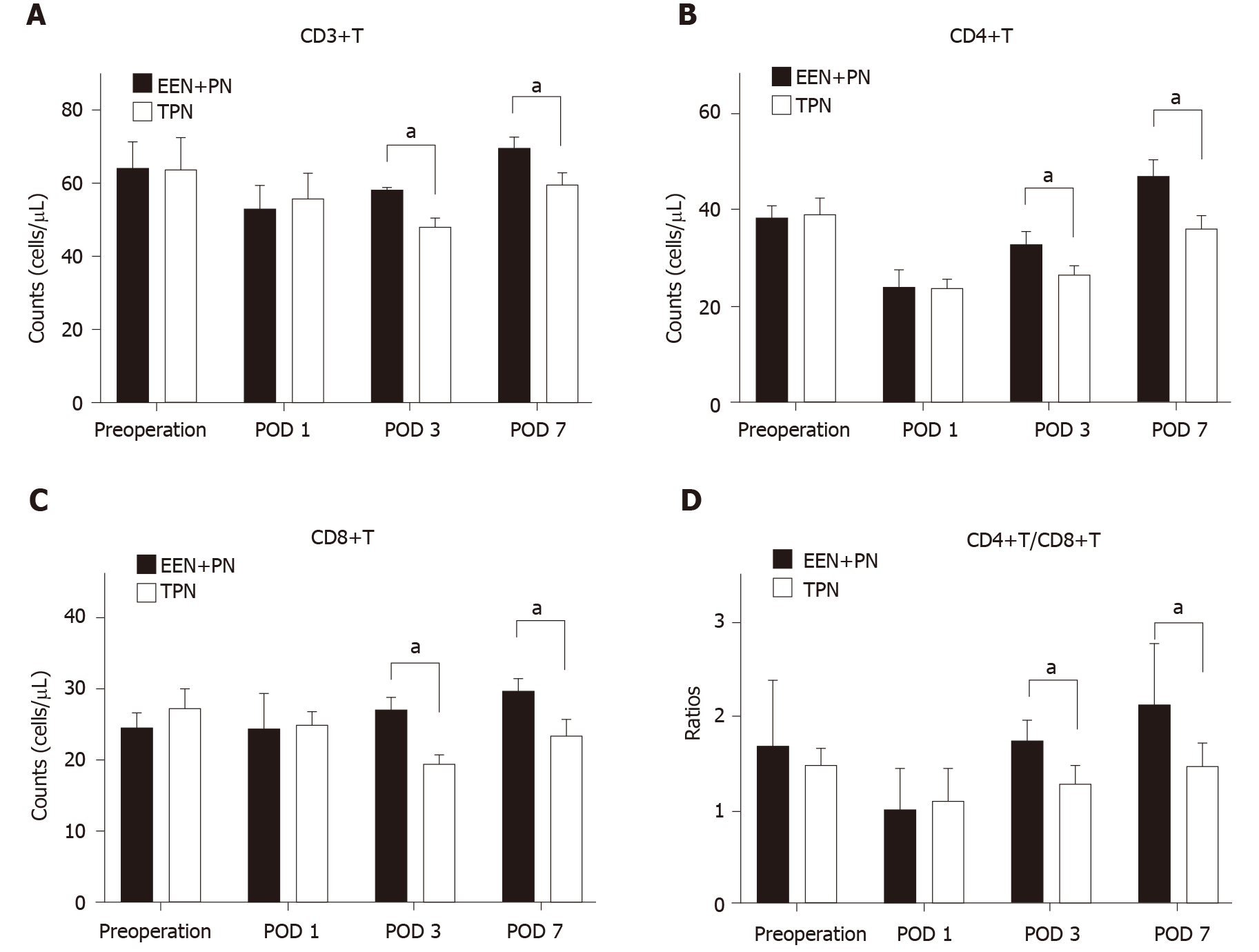
In the EEN + PN group, compared with the preoperative counts, the CD3+T, CD4+T, and CD4+T/CD8+T cell counts were all decreased on POD 1 (P < 0.05; Figure 2) and then began to increase on POD 3 and were all higher on POD 7 (P < 0.05), but the change in CD8+T cell count was not significant (P > 0.05; Figure 2C). In the TPN group, the CD3+T, CD4+T, and CD4+T/CD8+T cell counts decreased on POD 1 (P < 0.05). The CD3+T and CD8+T cell counts continued to decrease on POD 3 and began to increase on POD 7 but were both lower than the preoperative counts (P < 0.05; Figure 2A). The CD4+T and CD4+T/CD8+T cell counts increased from PODs 3 to 7 but were still lower than the preoperative counts (P < 0.05; Figure 2B and Figure D). No significant differences in the CD3+T, CD4+T, CD8+T, and CD4+T/CD8+T cell counts before surgery and on POD 1 were found between the two groups (P > 0.05). The CD3+T, CD4+T, CD8+T, and CD4+T/CD8+T cell counts in the EEN + PN group were significantly higher than those in the TPN group on PODs 3 and 7 (P < 0.05).
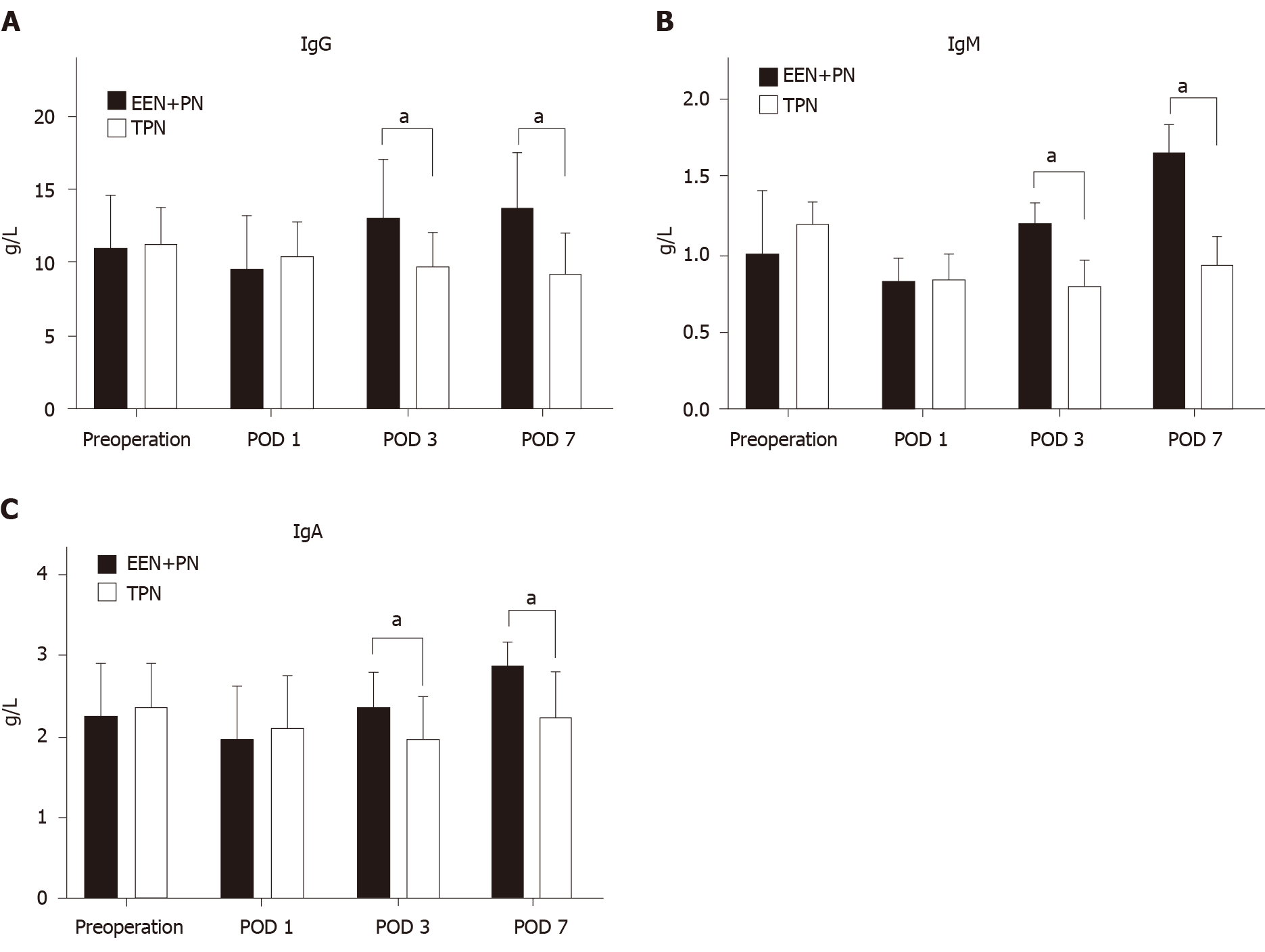
In the EEN + PN group, the IgG, IgA, and IgM levels decreased on POD 1 as compared with the preoperative levels (P < 0.05) and gradually increased on POD 3 and were significantly higher on POD 7 as compared with the preoperative levels (P < 0.05; Figure 3). In the TPN group, as compared with the preoperative levels, the IgG, IgA, and IgM levels began to decrease on POD 1 (P < 0.05) and slightly increased on POD 7 but were still lower than those before operation (P < 0.05).
No significant differences in the IgG, IgM, and IgA levels before operation and on POD 1 were found between the two groups (P > 0.05). The IgG, IgM, and IgA levels in the EEN + PN group were significantly higher than those in the TPN group on PODs 3 and 7 (P < 0.05; Figure 3).
The incidence of abdominal distention in the EEN + PN group was significantly higher than that in the TPN group (P < 0.05), which mostly occurred on PODs 1 to 3. In the TPN group, two patients had abdominal distention until POD 9. No significant differences in the incidence rates of bile leakage and infections at the surgical site were found between the two groups (P > 0.05), as shown in Table 4.
| Cases | Abdominal distention | Bile leakage | Surgical site infection | |
| TPN group | 30 | 4 (13.33) | 4 (13.33) | 5 (16.67) |
| EEN + PN group | 26 | 11 (42.31) | 2 (7.96) | 2 (7.96) |
| Total | 56 | 15 (26.79) | 6 (10.71) | 7 (12.50) |
| P value | 0.018 | 0.675 | 0.431 |
The recovery time of intestinal function in the EEN + PN group was significantly shorter than that in the TPN group (P < 0.001). The hospitalization time and total cost in the EEN + PN group were shorter or lower than those in the TPN group (P < 0.01), as shown in Table 5.
| Cases | Recovery time of intestinal function after operation in h | Postoperative hospital stay in d | Cost of hospitalization in RMB | |
| TPN group | 30 | 72.0 ± 12.9 | 9.7 ± 1.5 | 109036.69 ± 7949.71 |
| EEN + PN group | 26 | 51.2 ± 4.4 | 8.7 ± 0.8 | 87852.37 ± 9127.04 |
| t | 7.828 | 3.043 | 9.285 | |
| P value | < 0.001 | 0.004 | < 0.001 |
Many studies have found that EN can improve the metabolism of patients, promote the early recovery of liver and gastrointestinal functions, and significantly reduce the incidence of infection in perioperative patients[5,6]. At the same time, in a study with patients in the intensive care unit[7], EN was found to show a stronger immune improvement effect on patients and lower concurrent infection rate. These results suggest that EN support may have a regulatory effect on the immune function of the body. Immune surveillance of tumors is mainly dependent on cellular immunity. As one of the main effector cells, T lymphocytes play an important role in tumor immunity. They are divided into CD4+T and CD8+T cells, and a decreased ratio of CD4+T/CD8+T cells represents a decrease in immunity[8]. CD4+T cells, as helper T lymphocytes, play an important role in tumor immune surveillance. Humoral immunity is mediated by antibodies in vivo or substances with antibody activity. IgG, IgM, and IgA are non-specific antibodies, which are the main substances of mediated liquid immunity[9], and humoral immunity mainly prevents infection. After surgery, EN can effectively stimulate the immune system, rapidly increase the ratios of CD3+T, CD4+T, CD8+T cells, and CD4+T/CD8+T cells in peripheral blood[10], and increase the plasma immunoglobulin level[11].
At present, many studies have been conducted on postoperative nutritional support in patients with digestive system tumors such as gastric, colorectal, and esophageal cancers. Owing to the specific location of cholangiocarcinoma, the early diagnosis rate is low, the root treatment surgery is traumatic, the complication rates are high, and patients are often accompanied by malnutrition risk or malnutrition[12], which is not only a complication of cancer but also a disease condition related to one of the reasons for general health deterioration[13]. In recent years, with the development of nutritional support, more in-depth research on nutritional support has been conducted. TPN has been found to cause possibly liver function damage such as intrahepatic cholestasis[14] and increase the risk of infection in patients, while EN has no such complications[15]. In a study in patients with esophageal cancer[16], immune-enhanced EN not only corrected malnutrition but also regulated the immune function impairment and enhanced the anti-tumor effect of T lymphocytes.
The human immune system mainly includes cellular and humoral immunities. However, patients with cholangiocarcinoma have more trauma during operation, and most of them have obstructive jaundice and incomplete liver function preoperatively. In addition, their food intake before operation is insufficient, which may lead to low immune function, intestinal mucosa barrier damage, and increased postoperative complication and mortality rates[17]. When patients with cholangiocarcinoma developed obstructive jaundice, the total number of lymphocytes decreased, the risk of infection increased[18], the immune function of the intestinal mucosa decreased significantly[19], the reticuloendothelial system of the liver was damaged so that bacteria and endotoxins in the blood could not be effectively removed[20], and sepsis was ultimately more likely to occur. The enteral nutrient solution is absorbed in the intestine, enters the liver through the portal vein, and finally transforms into the liver, which not only supplies nutrition to the body but also provides comprehensive demand for the gastrointestinal mucosa. In addition, it activates the intestinal neuroendocrine system, promotes the growth of the gastrointestinal mucosa, maintains the normal immune functions of the intestine and body, maintains the integrity of the intestinal mucosa function, and blocks the heterotopia of the flora[21]. EEN can promote the recovery of intestinal function, reduce the degree of abdominal distention, and shorten the time of abdominal distention[22]. Moreover, studies have shown that EN is more reasonable within 24 h after operation[23]. In a study on postoperative abdominal surgery, PN with postoperative EEN not only improved the nutritional status of patients with tumors but also reduced the incidence of postoperative complications[24,25]. The gastrointestinal tract function is not complete after the operation for obstructive jaundice and the absorption capacity of nutrition is reduced[26]. EN not only has a protective effect on the immune function of the intestinal mucosa[27] but also is conducive to the recovery of liver function[28], which can improve the postoperative nutrition of patients, promote the recovery of intestinal function, and reduce the incidence of bile leakage[29].
In this study, we found that the immunological indexes began to recover on the third day after radical operation of cholangiocarcinoma, and the CD3+T, CD4+T, CD8+T, and CD4+T/CD8+T cell counts and IgG, IgM, and IgA levels in the patients with EEN support were significantly higher than those in the patients with TPN support. Combined with the literature analysis, our findings showed that the causes of this phenomenon are not only related to postoperative cholangiocarcinoma, which affects the participation of bile in enterohepatic circulation and the balance of nutrient absorption after the release of obstruction[30], but also more closely related to the maintenance of the intestinal mucosal barrier and improvement of immune function[31,32]. The use of postoperative EEN had significant advantages over TPN in terms of recovery time of intestinal function and total hospitalization cost (P < 0.05), but the incidence of abdominal distention in the EEN + PN group (42.31%) was higher than that in the TPN group (13.33%; P < 0.05). However, abdominal distention also occurred in the TPN group, and the duration of abdominal distention in the EEN + PN group was longer than that in the TPN group. We found no significant differences in the incidence rates of infections in the surgical site and bile leakage and length of stay after surgery (P > 0.05).
In conclusion, from the results of this study, as compared with TPN support alone, early use of EN support in ECC patients with MOJ not only can promote the recovery of immune function but also can significantly promote the recovery of gastrointestinal function, reduce the total treatment cost, and be used safely. However, long-term follow-up is needed, and statistical data from a large number of cases is needed to determine whether EN support has an impact on the survival time of patients with cholangiocarcinoma after surgery. Its role in immune monitoring for tumors needs further study.
Most cholangiocarcinoma patients with malignant obstructive jaundice (MOJ) have varying degrees of malnutrition and immunodeficiency preoperatively.
Perioperative nutritional support has important clinical significance in the comprehensive treatment of cholangiocarcinoma.
We aimed to investigate the effects of postoperative early enteral nutrition (EEN) on immunity function and clinical outcomes of cholangiocarcinoma patients with MOJ.
The patients were randomly divided into an experimental group and a control group according to the nutrition support modes. The control group received postoperative total parenteral nutrition (TPN), whereas the experimental group received postoperative EEN and parenteral nutrition (PN; EEN + PN). The clinical outcomes, postoperative immune function, incidences of surgical site infection and bile leakage, intestinal function recovery time, average hospitalization days, and hospitalization expenses of the two groups were assessed on postoperative days (PODs) 1, 3, and 7.
The CD3+T, CD4+T, CD8+T, and CD4+T/CD8+T cell counts and the immunoglobulin (Ig) G, IgM, and IgA levels in the EEN + PN group were significantly higher than those in the TPN group on PODs 3 and 7 (P < 0.05), whereas no significant differences in the CD3+T, CD4+T, CD8+T, and CD4+T/CD8+T cell counts and IgG, IgM, and IgA levels before operation and on POD 1 were found between the two groups (P > 0.05). The intestinal function recovery time and postoperative hospital stay were shorter (P < 0.001 for both) in the EEN + PN group than in the TPN group. The hospitalization expenses of the EEN + PN group were lower than those of the TPN group (P < 0.001). However, the incidence of abdominal distension was higher than in the EEN + PN group than in the TPN group (P < 0.05). The incidence rates of biliary leakage and surgical site infection were not significantly different between the two groups (P > 0.05).
A postoperative EEN program could reduce the incidence of postoperative complications and improve the clinical outcomes and immune functions of cholangiocarcinoma patients with MOJ and is thus beneficial to patient recovery.
Long-term follow-up is needed, and statistical data from a large number of cases are needed to determine whether EN support has an impact on the survival time of patients with cholangiocarcinoma after surgery.
| 1. | Hussain I, Ahmad Z, Garg A. Extreme hypercholesterolemia presenting with pseudohyponatremia - a case report and review of the literature. J Clin Lipidol. 2015;9:260-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Kimura F, Miyazaki M, Suwa T, Sugiura T, Shinoda T, Itoh H, Nagakawa K, Ambiru S, Shimizu H, Yoshitome H. Anti-inflammatory response in patients with obstructive jaundice caused by biliary malignancy. J Gastroenterol Hepatol. 2001;16:467-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Ljungdahl M, Osterberg J, Ransjö U, Engstrand L, Haglund U. Inflammatory response in patients with malignant obstructive jaundice. Scand J Gastroenterol. 2007;42:94-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Nimura Y. Preoperative biliary drainage before resection for cholangiocarcinoma (Pro). HPB (Oxford). 2008;10:130-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Xie TP, Zhao YF, Peng L, Zhu J, Li Q. Effect of early enteral nutrition on liver functions in patients following esophageal cancer surgery. Zhongguo Linchuang Yingyang Zazhi. 2007;15:95-98. |
| 6. | Jiang W, Zhang J, Geng Q, Xu X, Lv X, Chen Y, Liu X, Tang W. Early enteral nutrition in neonates with partial gastrectomy: a multi-center study. Asia Pac J Clin Nutr. 2016;25:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 7. | Zhang Y, Wang J, Zhu HL, Liang Y, Wu J. Effects of bifidobacterium-containing enteral nutrition intervention on the nutritional status and intestinal flora disturbance in patients with the severe cerebral infarction. Hainan Yixueyuan Xuebao. 2017;23:151-154. [DOI] [Full Text] |
| 8. | Yao D, Zheng L, Wang J, Guo M, Yin J, Li Y. Perioperative Alanyl-Glutamine-Supplemented Parenteral Nutrition in Chronic Radiation Enteritis Patients With Surgical Intestinal Obstruction: A Prospective, Randomized, Controlled Study. Nutr Clin Pract. 2016;31:250-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Chuntrasakul C, Siltham S, Sarasombath S, Sittapairochana C, Leowattana W, Chockvivatanavanit S, Bunnak A. Comparison of a immunonutrition formula enriched arginine, glutamine and omega-3 fatty acid, with a currently high-enriched enteral nutrition for trauma patients. J Med Assoc Thai. 2003;86:552-561. [PubMed] |
| 10. | Zhou WC, Li YM, Zhang H, Li X, Zhang L, Meng WB, Zhu KX, Zhang QB, He MY. Therapeutic effects of endoscopic therapy combined with enteral nutrition on acute severe biliary pancreatitis. Chin Med J (Engl). 2011;124:2993-2996. [PubMed] |
| 11. | Zou XP, Chen M, Wei W, Cao J, Chen L, Tian M. Effects of enteral immunonutrition on the maintenance of gut barrier function and immune function in pigs with severe acute pancreatitis. JPEN J Parenter Enteral Nutr. 2010;34:554-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15:95-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1052] [Cited by in RCA: 1206] [Article Influence: 150.8] [Reference Citation Analysis (0)] |
| 13. | Braga M, Gianotti L, Nespoli L, Radaelli G, Di Carlo V. Nutritional approach in malnourished surgical patients: a prospective randomized study. Arch Surg. 2002;137:174-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 287] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 14. | Ferreira LG, Anastácio LR, Lima AS, Correia MI. [Malnutrition and inadequate food intake of patients in the waiting list for liver transplant]. Rev Assoc Med Bras (1992). 2009;55:389-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Hajdú N, Belágyi T, Issekutz A, Bartek P, Gartner B, Oláh A. [Intravenous glutamine and early nasojejunal nutrition in severe acute pancreatitis -- a prospective randomized clinical study]. Magy Seb. 2012;65:44-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Miyata H, Yano M, Yasuda T, Yamasaki M, Murakami K, Makino T, Nishiki K, Sugimura K, Motoori M, Shiraishi O, Mori M, Doki Y. Randomized study of the clinical effects of ω-3 fatty acid-containing enteral nutrition support during neoadjuvant chemotherapy on chemotherapy-related toxicity in patients with esophageal cancer. Nutrition. 2017;33:204-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Van Cutsem E, Arends J. The causes and consequences of cancer-associated malnutrition. Eur J Oncol Nurs. 2005;9 Suppl 2:S51-S63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 404] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 18. | Wu F, Duan H, Xie Y. Preventive Effects of Dexmedetomidine on Renal Dysfunction and Hemodynamic Stability in Malignant Obstructive Jaundice Patients During Peri-Operative Period. Med Sci Monit. 2019;25:6782-6787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Tian YF, Li Y, Zhao Q, Fan LQ, Zhao WJ, Xu BL, Song ZC, Kuang G, Dong ZM, Zhang QF. [Effect of ulinastatin on intestinal mucosal barrier function of rats with obstructive jaundice]. Nanfang Yike Daxue Xuebao. 2007;27:987-990. [PubMed] |
| 20. | Bleier JI, Katz SC, Chaudhry UI, Pillarisetty VG, Kingham TP 3rd, Shah AB, Raab JR, DeMatteo RP. Biliary obstruction selectively expands and activates liver myeloid dendritic cells. J Immunol. 2006;176:7189-7195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Ouyang H, Xu B, Zhao H. Application of early enteral nutrition through nasojejunostomy in children with acute pancreatitis. J Clin Pediatr Surg. 2016;15:510-512, 517. |
| 22. | Reis AMD, Fruchtenicht AV, Loss SH, Moreira LF. Use of dietary fibers in enteral nutrition of critically ill patients: a systematic review. Rev Bras Ter Intensiva. 2018;30:358-365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Wheble GA, Knight WR, Khan OA. Enteral vs total parenteral nutrition following major upper gastrointestinal surgery. Int J Surg. 2012;10:194-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Zheng R, Devin CL, Pucci MJ, Berger AC, Rosato EL, Palazzo F. Optimal timing and route of nutritional support after esophagectomy: A review of the literature. World J Gastroenterol. 2019;25:4427-4436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 25. | Hu Y. Clinical value of early enteral nutrition with three lumen gastrointestinal tract in patients with hilar cholangiocarcinoma. Guoji Yiyao Weisheng Daobao. 2017;23:123-126. [DOI] [Full Text] |
| 26. | Chen D, Liang LJ, Peng BG, Zhou Q, Li SQ, Tang D, Huang L, Huang JF. [Effect of preoperative biliary drainage on liver function changes in patients with malignant obstructive jaundice in the low bile duct before and after pancreaticoduodenectomy]. Ai Zheng. 2008;27:78-82. [PubMed] |
| 27. | Xu Y, Guo Z, Huang L, Gong J, Li Y, Gu L, Shen W, Zhu W. A nomogram for predicting the response to exclusive enteral nutrition in adult patients with isolated colonic Crohn's disease. Therap Adv Gastroenterol. 2019;12:1756284819881301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Wan SL, Li YC. Early administration of enteral nutrition after surgical resection of hilar cholangiocarcinoma. Zhongguo Putong Waike Zazhi. 2012;. |
| 29. | Song P, Mao L, Bian XJ, Zhou T, Fan YY, Zhang J, Xie M, Qiu YD. [Curative effect analysis of bile reinfusion combined with enteral nutrition support before surgery of hilar cholangiocarcinoma]. Zhonghua Wai Ke Za Zhi. 2018;56:367-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 30. | Pavlidis ET, Pavlidis TE. Pathophysiological consequences of obstructive jaundice and perioperative management. Hepatobiliary Pancreat Dis Int. 2018;17:17-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 31. | Stefanov CS, Boyadzhiev NP, Uchikov AP, Dimov R, Mandev P, Terzhumanov R. Enteral nutrition in sepsis patients. Folia Med (Plovdiv). 2005;47:11-20. [PubMed] |
| 32. | Sun JK, Zhang WH, Chen WX, Wang X, Mu XW. Effects of early enteral nutrition on Th17/Treg cells and IL-23/IL-17 in septic patients. World J Gastroenterol. 2019;25:2799-2808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dinç T, Ooi L S-Editor: Gao CC L-Editor: Filipodia P-Editor: Ma YJ