Published online Dec 14, 2020. doi: 10.3748/wjg.v26.i46.7325
Peer-review started: August 6, 2020
First decision: October 18, 2020
Revised: October 31, 2020
Accepted: November 9, 2020
Article in press: November 9, 2020
Published online: December 14, 2020
Processing time: 129 Days and 18.9 Hours
Combined hepatocellular-cholangiocarcinoma (CHC) is a rare type of primary liver cancer. Due to its complex histopathological characteristics, the imaging features of CHC can overlap with those of hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC).
To investigate the possibility and efficacy of differentiating CHC from HCC and ICC by using contrast-enhanced ultrasound (CEUS) Liver Imaging Reporting and Data System (LI-RADS) and tumor biomarkers.
Between January 2016 and December 2019, patients with histologically confirmed CHC, ICC and HCC with chronic liver disease were enrolled. The diagnostic formula for CHC was as follows: (1) LR-5 or LR-M with elevated alpha-fetoprotein (AFP) and carbohydrate antigen 19-9 (CA19-9); (2) LR-M with elevated AFP and normal CA19-9; or (3) LR-5 with elevated CA19-9 and normal AFP. The sensitivity, specificity, accuracy and area under the receiver operating characteristic curve were calculated to determine the diagnostic value of the criteria.
After propensity score matching, 134 patients (mean age of 51.4 ± 9.4 years, 108 men) were enrolled, including 35 CHC, 29 ICC and 70 HCC patients. Based on CEUS LI-RADS classification, 74.3% (26/35) and 25.7% (9/35) of CHC lesions were assessed as LR-M and LR-5, respectively. The rates of elevated AFP and CA19-9 in CHC patients were 51.4% and 11.4%, respectively, and simultaneous elevations of AFP and CA19-9 were found in 8.6% (3/35) of CHC patients. The sensitivity, specificity, positive predictive value, negative predictive value, accuracy and area under the receiver operating characteristic curve of the aforementioned diagnostic criteria for discriminating CHC from HCC and ICC were 40.0%, 89.9%, 58.3%, 80.9%, 76.9% and 0.649, respectively. When considering the reported prevalence of CHC (0.4%-14.2%), the positive predictive value and NPV were revised to 1.6%-39.6% and 90.1%-99.7%, respectively.
CHCs are more likely to be classified as LR-M than LR-5 by CEUS LI-RADS. The combination of the CEUS LI-RADS classification with serum tumor markers shows high specificity but low sensitivity for the diagnosis of CHC. Moreover, CHC could be confidently excluded with high NPV.
Core Tip: The imaging features of combined hepatocellular-cholangiocarcinoma (CHC) are complicated due to its complex histopathological characteristics. In addition, biopsy may misguide the correct diagnosis of CHC due to sampling error or tissue insufficiency. This study investigated the diagnostic value of the contrast-enhanced ultrasound Liver Imaging Reporting and Data System classification in association with serological tumor markers in differentiating CHC from hepatocellular carcinoma and intrahepatic cholangiocarcinoma. The results showed that the combined diagnostic criteria had high specificity and negative predictive value but low sensitivity for the diagnosis of CHC. These findings could help radiologists and clinical investigators confidently exclude CHC lesions in the clinical setting.
- Citation: Yang J, Zhang YH, Li JW, Shi YY, Huang JY, Luo Y, Liu JB, Lu Q. Contrast-enhanced ultrasound in association with serum biomarkers for differentiating combined hepatocellular-cholangiocarcinoma from hepatocellular carcinoma and intrahepatic cholangiocarcinoma. World J Gastroenterol 2020; 26(46): 7325-7337
- URL: https://www.wjgnet.com/1007-9327/full/v26/i46/7325.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i46.7325
Combined hepatocellular-cholangiocarcinoma (CHC) is increasingly recognized in cirrhotic liver, with a reported prevalence of 0.4%-14.2% of all primary liver carcinomas[1-3]. CHC is the second most common primary liver cancer in cirrhotic liver, followed by intrahepatic cholangiocarcinoma (ICC), excluding perihilar cholangiocarcinoma[4]. According to the 2010 World Health Organization classification[5], this special type of tumor requires the presence of both unambiguously differentiated hepatocellular and biliary components. CHC can have various imaging features overlapping with hepatocellular carcinoma (HCC), ICC and liver metastasis for complex histopathological components[6-8]. However, the prognosis and treatment of CHC differ from those of HCC and ICC[9], and therefore the accurate diagnosis of this tumor type is of great importance for appropriate patient management.
A few studies have reported the imaging features of CHC lesions on contrast-enhanced ultrasound (CEUS)[10-13]. Theoretically, due to the mixed elements of CHC, both HCC-like and ICC-like imaging features would be visualized. In addition, serum biomarkers, especially alpha-fetoprotein (AFP) and carbohydrate antigen 19-9 (CA19-9), have been shown to be helpful in the diagnosis of CHC[10,11,13,14]. The combination of CEUS and serum biomarkers was reported to improve specificity for the differentiation between CHC and HCC or ICC in a study population not limited to patients at risk for HCC[10].
The American College of Radiology released CEUS Liver Imaging Reporting and Data System (LI-RADS) for standardizing CEUS diagnosis of liver nodules in high-risk patients[15]. Although not fully validated, CEUS LI-RADS has been reported to be effective for the diagnosis of HCC[16,17]. However, challenges still exist for the differential diagnosis of highly suspicious HCC and other malignant entities, such as CHC and ICC. The purpose of this study was to investigate whether the combination of CEUS LI-RADS and serum biomarkers is helpful for differentiating CHC from HCC and ICC in patients with chronic liver disease.
This retrospective study was approved by the institutional review board, and informed consent was obtained from all patients. From January 2016 to December 2019, patients with pathologically confirmed primary liver cancer after liver resection were retrospectively selected through a review of our Ultrasonic Information System. Inclusion criteria included: (1) CEUS performed within 1 mo before liver resection; (2) Patients with risk factors for HCC, including cirrhosis and chronic hepatitis B; and (3) Testing of AFP and CA19-9 levels within 7 d before curative resection. Lesions with neoplastic vascular thrombi were excluded from this study. Eventually, we included 35 CHC, 29 ICC and 1051 HCC patients. After one-to-two (CHC:HCC = 1:2) propensity score matching by tumor size, age and gender, 70 HCC lesions were selected for analysis. A flow chart for the study population selection is presented in Figure 1.
All patients underwent B-mode ultrasound and CEUS examination by an ultrasound system (IU22, Philips Medical Solutions; Mountain View, CA, United States) equipped with a C5-1 abdominal convex transducer (frequency range of 1-5 MHz). The CEUS examination was performed according to technical recommendations following the World Federation for Ultrasound in Medicine and Biology-European Federation of Societies for Ultrasound in Medicine and Biology guidelines after a conventional ultrasound study[18]. After activation of the contrast-specific imaging mode, 1.2-2.4 mL of contrast agent (SonoVue, Bracco, Milan, Italy) was injected intravenously and flushed with 5 mL of 0.9% saline solution through a 20-gauge angio-catheter needle placed in the antecubital vein. The imaging timer was started immediately upon completion of SonoVue injection. The set of images was stored on the hard disk of the ultrasound system and copied to a portable hard disk for later evaluation.
According to the CEUS LI-RADS (2017 version), a hepatic nodule is categorized from LR-1 to LR-5 or LR-M and LR-TIV according to the likelihood of HCC[17]. The following imaging features were used to categorize each nodule: Nodule size, pattern of arterial phase enhancement, presence, timing and degree of washout and tumor-in-vein (Supplementary Appendix Table 1).
| CHC, n = 35 | Non-CHC | P1 | P2 | ||
| ICC, n = 29 | HCC, n = 70 | ||||
| Tumor size in cm | 5.6 ± 3.7 | 6.9 ± 3.3 | 5.1 ± 3.1 | 0.158 | 0.432 |
| Age in yr | 49.3 ± 9.5 | 55.0 ± 9.3 | 50.5 ± 9.2 | 0.018 | 0.555 |
| Numbers of male | 31 (88.6) | 24 (82.8) | 46 (76.7) | 0.720 | |
| HBV (+) | 30 (85.7) | 21 (72.4) | 62 (88.6) | 0.224 | 0.756 |
| HCV (+) | 3 (8.6) | 3 (10.3) | 0 (0.0) | 1.000 | 0.035 |
| Liver cirrhosis | 23 (65.7) | 12 (41.4) | 42 (60.0) | 0.077 | 0.671 |
| AFP, > 20 ng/mL | 18 (51.4) | 3 (10.3) | 41 (58.6) | 0.001 | 0.535 |
| CA19-9, > 100 U/mL | 4 (11.4) | 9 (31.0) | 1 (1.4) | 0.066 | 0.041 |
| AC | 3 (8.6) | 1 (3.4) | 1 (1.4) | 0.620 | 0.107 |
The CEUS images were reviewed independently by two certified radiologists (Li JW and Shi YY with more than 5 and 3 years of experience with liver CEUS, respectively) who were blinded to the pathology results and serum biomarker levels and assigned a category to each nodule according to CEUS LI-RADS (2017 version)[15,17]. In case of discordant interpretations between the reviewers, arbitration from an expert radiologist with more than 10 years of experience (Lu Q) was performed.
The diagnostic criteria of CHC were defined as follows: (1) LR-5 or LR-M lesion with simultaneously elevated AFP and CA19-9 (AFP > 20 ng/mL and CA19-9 > 100 units/mL) levels[19-21]; (2) LR-M lesion with elevated AFP levels and normal CA19-9 Levels; or (3) LR-5 lesion with elevated CA19-9 levels and normal AFP levels. The diagnostic test was performed with pathological results as the reference standard.
Quantitative data were expressed as the mean ± standard deviation. Qualitative data were presented as numbers and percentages. Differences in quantitative variables were tested by the independent sample t-test. Comparison of the rates of imaging characteristics was performed by using the χ2 test or Fisher’s exact test. The area under the receiver operating characteristic curve (AUC) was used to analyze the performance of the diagnostic criteria. The sensitivity, specificity, accuracy, positive predictive value (PPV) and negative predictive value (NPV) for CHC were calculated by using standard procedures[22].
Kappa values were evaluated to measure intrareader agreement of CEUS features and CEUS LI-RADS classification of the nodules. The strength of agreement was interpreted according to the classification scales for kappa: 0.00-0.20 poor, 0.21-0.40 fair, 0.41-0.60 moderate, 0.61-0.80 substantial and 0.81-1.00 almost perfect[23]. Propensity score matching was performed with R software version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria; www.r-project.org). Statistical analyses were performed with the statistical software package SPSS, version 25.0; IBM, Armonk, NY, United States). Significance was defined as two-sided P < 0.05.
After propensity score matching, a total of 35 CHC, 29 ICC and 70 HCC patients were included for analysis. The clinical characteristics of the 134 patients are shown in Table 1. In the CHC group, the mean nodule size was 5.6 ± 3.7 cm (range 2.0-17.0 cm) in diameter. Elevated AFP levels were found in 51.4% (18/35) of CHC patients, in contrast to 10.3% (3/29) of ICC patients and 58.6% (41/70) of HCC patients (P = 0.001 and 0.535, respectively). In addition, elevated CA19-9 levels were found in 11.4% (4/35) of CHC patients compared with 31.0% (9/29) of ICC patients and 1.4% (1/70) of HCC patients (P = 0.066 and 0.041, respectively). Simultaneous elevations in AFP and CA19-9 were found in 8.6% (3/35) of CHC, 3.4% (1/29) of ICC and 1.4% (1/70) of HCC patients (P > 0.05).
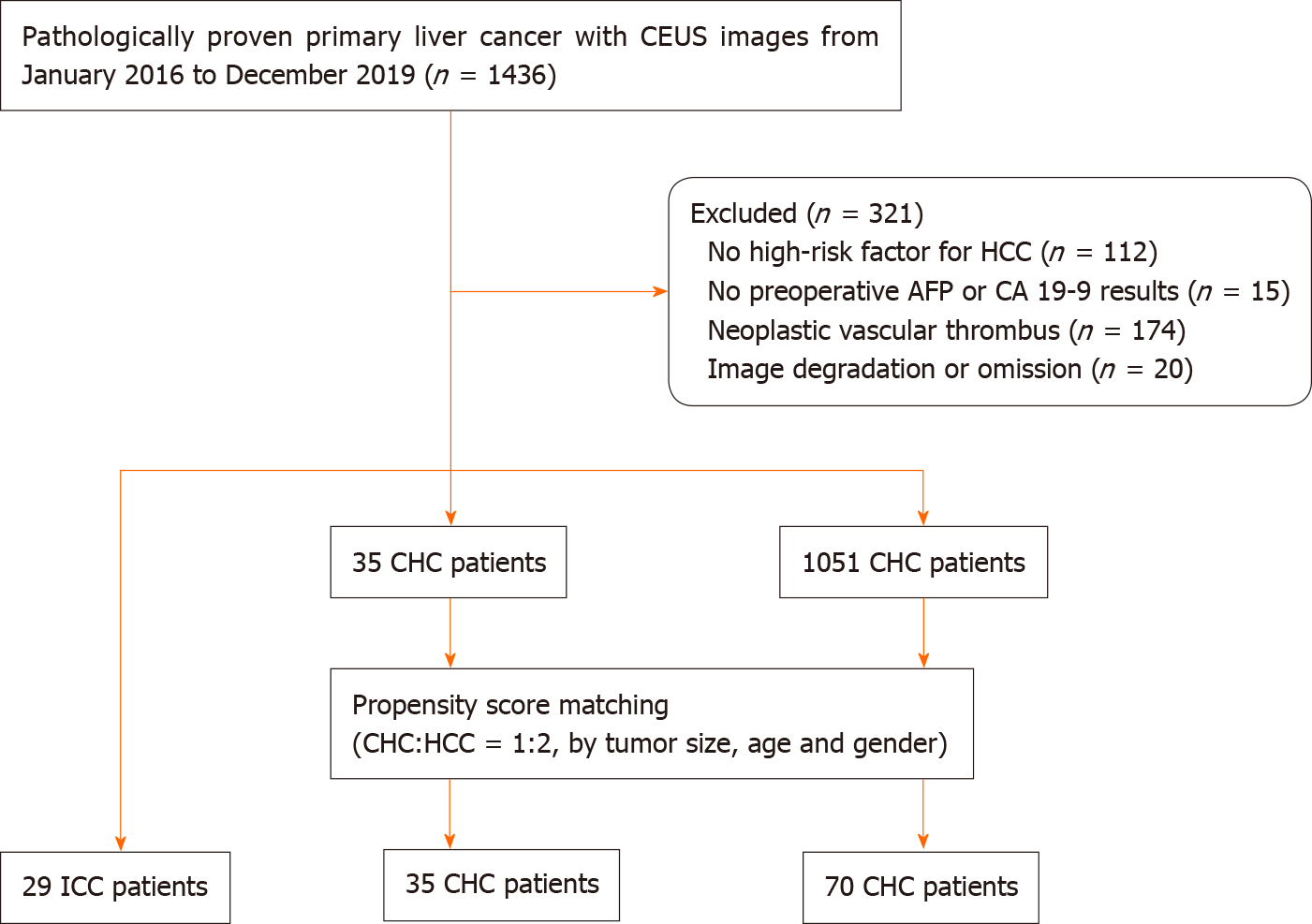
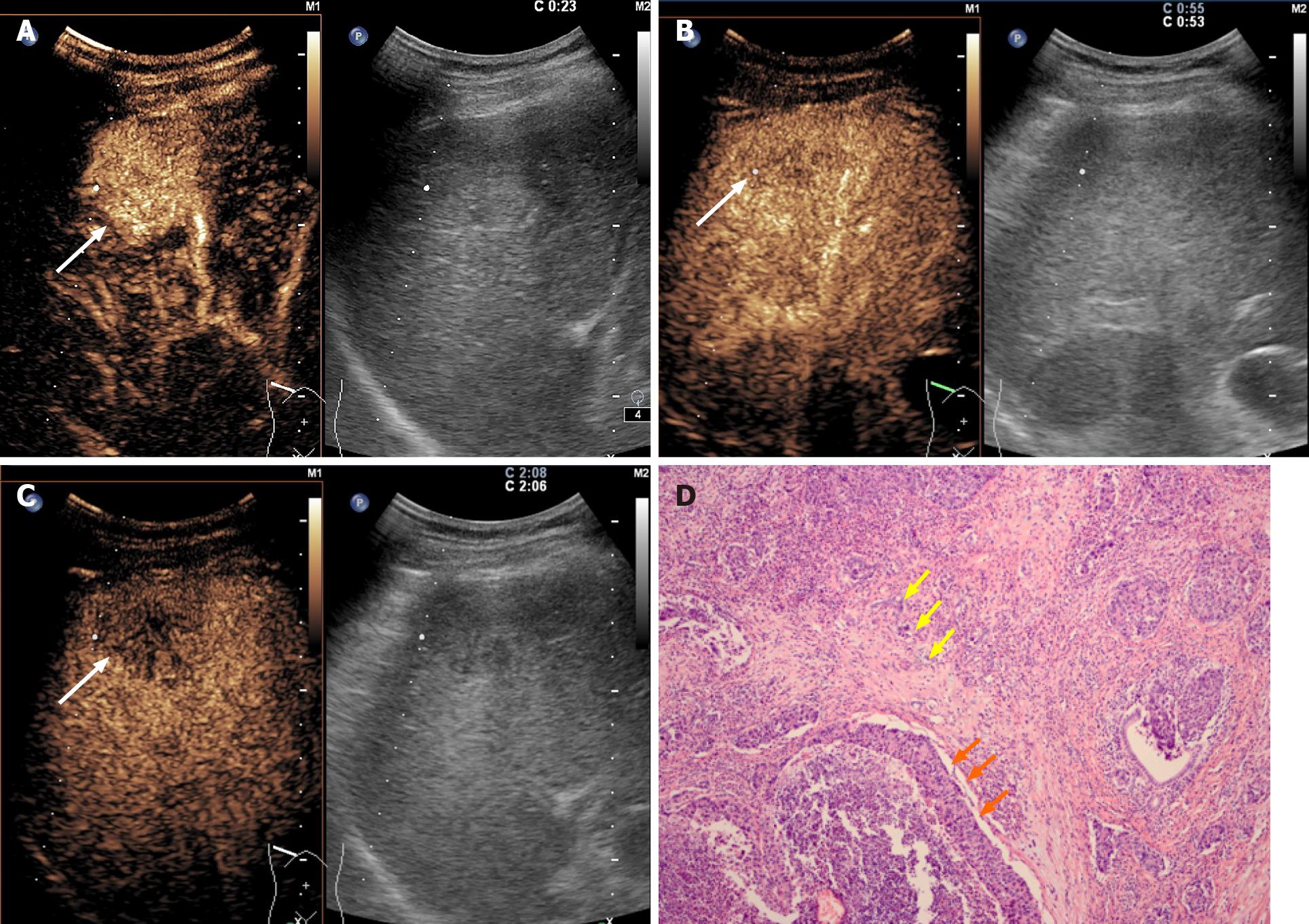
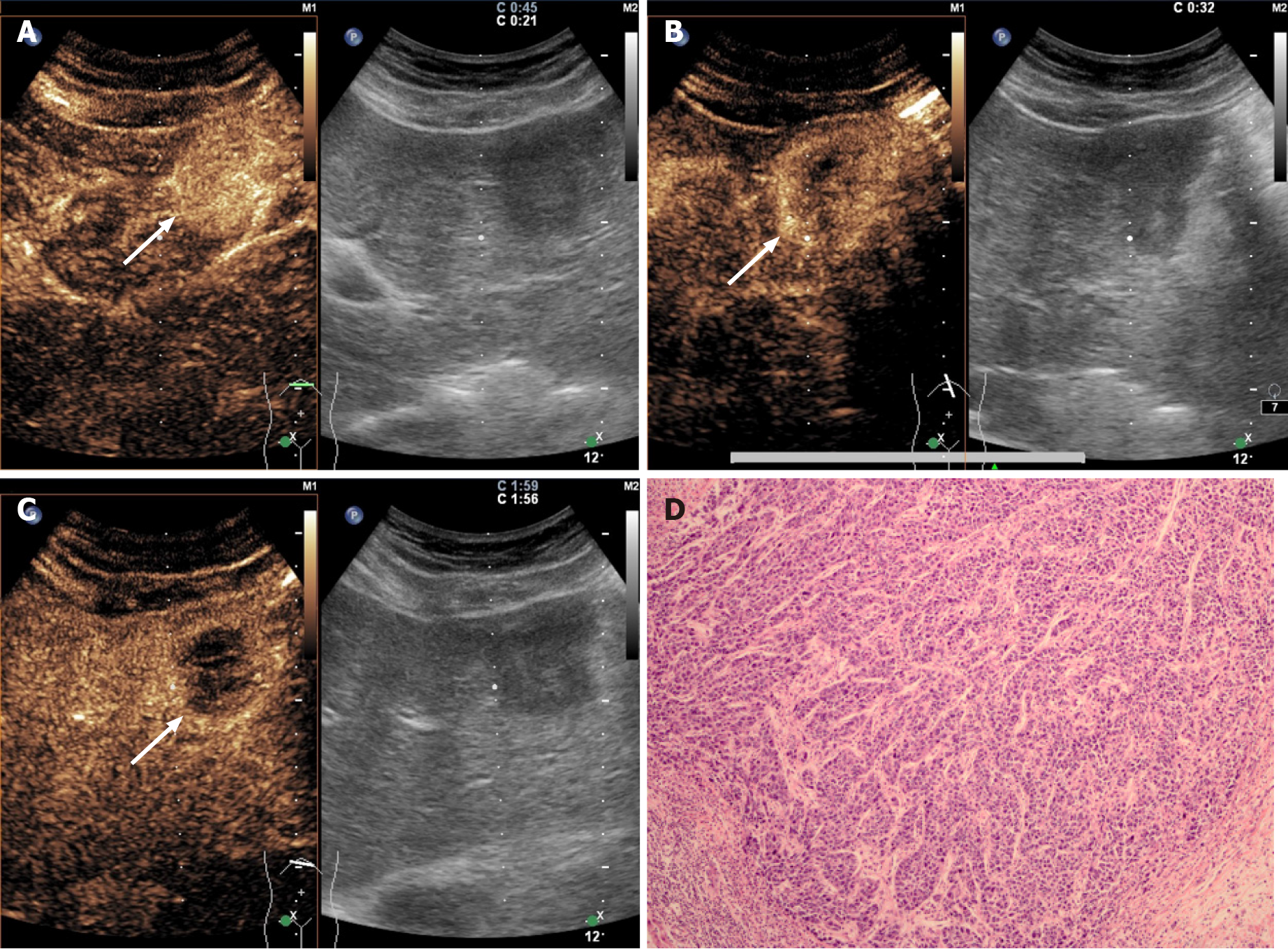
All the CHC lesions illustrated hyperenhancement in the arterial phase and hypo-enhancement in the portal or late phase. In the arterial phase, peripheral irregular rim-like and non-rim-like hyperenhancement were demonstrated in 17.1% (6/35) and 82.9% (29/35) of CHC lesions, respectively. In the portal phase, early washout was present in 74.3% (26/35) of CHC lesions compared with 96.6% (28/29) of ICC lesions (P = 0.017) and 18.6% (13/70) of HCC patients (P < 0.001). Marked washout within 120 s was found in 5.7% (2/35) of CHC lesions, in contrast to 3.4% (1/29) of ICC lesions (P = 1.000) and none of the HCC lesions (P = 0.109). According to CEUS LI-RADS, 25.7% (9/35) and 74.3% (26/35) of CHCs were classified as LR-5 and LR-M, respectively, in contrast to none and 100% in ICC (P = 0.003) and 81.4% (57/70) and 18.6% (13/70) in HCC (P < 0.001). The pre-contrast and contrast-enhanced ultrasonographic imaging features are presented in Table 2.
| CHC, n = 35 | Non-CHC | P1 | P2 | ||
| ICC, n = 29 | HCC, n = 70 | ||||
| Echogenic degree | 0.692 | 0.099 | |||
| Hypo- | 32 (91.4) | 25 (86.2) | 55 (78.6) | ||
| Hyper- | 3 (8.6) | 4 (13.8) | 15 (21.4) | ||
| Poor boundary | 23 (65.7) | 22 (75.9) | 36 (51.4) | 0.422 | 0.212 |
| Irregular shape | 23 (65.7) | 20 (69.0) | 30 (42.9) | 1.000 | 0.038 |
| APHE pattern | 0.757 | 0.001 | |||
| Rim-like | 6 (17.1) | 6 (20.7) | 0 (0.0) | ||
| Non-rim-like | 29 (82.9) | 23 (79.3) | 70 (100) | ||
| Timing of washout onset | 0.017 | < 0.001 | |||
| Early, < 60 s | 26 (74.3) | 28 (96.6) | 13 (18.6) | ||
| Late, > 60 s | 9 (25.7) | 1 (3.4) | 57 (81.4) | ||
| Degree of washout | 1.000 | 0.109 | |||
| Marked, as observed within first 120 s | 2 (5.7) | 1 (3.4) | 0 (0.0) | ||
| Mild | 33 (94.3) | 28 (96.6) | 70 (100.0) | ||
| CEUS LI-RADS | 0.003 | < 0.001 | |||
| LR-5 | 9 (25.7) | 0 (0.0) | 57 (81.4) | ||
| LR-M | 26 (74.3) | 29 (100.0) | 13 (18.6) | ||
The interobserver agreement of CEUS features for the CHC and non-CHC groups is summarized in Table 3. In the CHC group, the k values of interobserver agreements on the arterial phase hyperenhancement pattern, early washout and marked washout were 0.624, 0.608 and 0.635, respectively. In the non-CHC group, the k values of interobserver agreements on arterial enhancement pattern, early washout and marked washout were 0.524, 0.875 and 0.662, respectively. The k values of CEUS LI-RADS categorization in the two groups were 0.663 and 0.876, respectively.
| CHC | K1 | Non-CHC | K2 | |||
| Reader 1 | Reader 2 | Reader 1 | Reader 2 | |||
| APHE pattern | 0.624 | 0.524 | ||||
| Rim-like | 6 | 3 | 8 | 3 | ||
| Non-rim-like | 29 | 32 | 91 | 96 | ||
| Timing of washout onset | 0.608 | 0.875 | ||||
| Early, < 60 s | 26 | 22 | 42 | 40 | ||
| Late, > 60 s | 9 | 13 | 57 | 59 | ||
| Degree of washout | 0.635 | 0.662 | ||||
| Marked, as observed within first 120 s | 3 | 3 | 2 | 1 | ||
| Mild | 32 | 32 | 97 | 98 | ||
| CEUS LI-RADS | 0.663 | 0.876 | ||||
| LR-5 | 9 | 12 | 56 | 58 | ||
| LR-M | 26 | 23 | 43 | 41 | ||
Serum tumor biomarkers (AFP and CA19-9) and the CEUS LI-RADS categorization of the 134 patients are shown in Table 4 (Figure 2-4). Table 5 shows the performance of the diagnostic criteria. The AUC, sensitivity, specificity, PPV, NPV, and accuracy were 0.649, 40.0%, 89.9%, 58.3%, 80.9% and 76.9%, respectively. In this study, CHC accounted for 26.1% (35/134) of the study population, yielding an adjusted PPV of 58.3% and NPV of 80.9%. However, the actual prevalence of CHC is much lower, reportedly accounting for 0.4%-14.2% of primary hepatic cancers[2,24,25]. Considering eighth reported prevalence of CHC (0.4%-14.2%), the PPV and NPV were modified to 1.6%-39.6% and 90.1%-99.7%, respectively.
CHC is the second most common type of primary liver cancer in cirrhotic liver[4]. A definitive diagnosis of CHC requires both unambiguously differentiated hepatocellular and biliary components in the same tumor in pathological analysis as per the World Health Organization classification[5]. However, biopsy may misguide the correct diagnosis of CHC due to sampling error or tissue insufficiency[26,27], which highlights the importance of surgical specimens and imaging diagnosis. Our study indicated that a higher percentage of CHC was classified as LR-M than LR-5 (74.3% vs 25.7%, P < 0.05). Simultaneous elevation of AFP and CA19-9 was present in only 8.6% (3/35) of CHC patients. The combination of CEUS LI-RADS and serum AFP and CA19-9 levels showed a specificity of 89.9% and accuracy of 76.9% for the diagnosis of CHC in patients with chronic liver disease.
In our study, all CHC lesions showed typical manifestations of liver cancer (i.e. the enhancement mode of “rapid wash in and out”)[10-12,28]. However, 74.3% (26/35) of CHC cases were assessed as LR-M, which is higher than the rates reported by Choi et al[29] (28.0%) and Jeon et al[30] (61.4%) based on magnetic resonance imaging (MRI) LI-RADS. This discrepancy may be explained by the fact that early washout is more easily observed by CEUS due to higher temporal resolution than MRI. In this study, the other 25.7% (9/35) of CHC lesions were classified as LR-5. In other words, 100% of CHC lesions were correctly classified as malignant by CEUS LI-RADS, which is in line with the findings of Sagrini et al[12], where CEUS correctly suggested a condition of malignancy in a higher number of cases than CT and MRI for the diagnosis of CHC.
Elevated AFP and CA19-9 levels have been reported as potential diagnostic indicators for HCC and ICC, respectively[19,31]. Prior studies have demonstrated that the combination of AFP and CA19-9 with radiologic characteristics may aid the diagnosis of CHC[10,11,13,14]. Huang et al[10] reported that the sensitivity and specificity of diagnosing CHC based on their criteria (i.e. simultaneous elevation of AFP and CA19-9 or with a tumor marker elevation in discordance with HCC-like or ICC-like pattern on CEUS), were 32.5% and 92.8%, respectively, which is comparable to the result of our study. Compared with Huang’s study, HCC lesions were selected by propensity score matching in this study, which reduced patient selection bias. Moreover, CEUS LI-RADS was adopted in our criteria, which makes it more standardized to use in the clinical setting.
The diagnostic criteria for CHC showed high specificity (89.9%) and modified NPV (90.1% to 99.7%), which indicated that CHC could be effectively ruled out by using CEUS LI-RADS. Atypical HCC or ICC would be classified as LR-M, a category indicating malignancy but not specific for HCC. Biopsy is recommended for the management of LR-M lesions. However, for CHC, the value of biopsy may be limited due to sampling error or tissue insufficiency. In such a scenario, combining CEUS LI-RADS with serum biomarkers could confidently exclude the possibility of CHC if the lesion does not meet the criteria. However, the performance of the diagnostic criteria in this study indicated that differentiation between CHC and HCC or ICC remains challenging.
There are a few limitations to our study. First, this was a single-center retrospective case-control study, which may have potential selection bias. Additional multicenter prospective studies are needed to validate the diagnostic criteria. Second, the sample size was small because of the relatively low incidence of CHC tumors. Third, this study mainly enrolled patients with chronic hepatitis B. Therefore, our results may not be reproducible in patients with other etiologies, especially liver cirrhosis.
In conclusion, CHC could be accurately diagnosed as malignant by CEUS LI-RADS, with the majority of the lesions in the LR-M category. The combination of CEUS LI-RADS classification with serum tumor markers shows high specificity but low sensitivity for the diagnosis of CHC. These findings could help radiologists and clinical investigators confidently exclude CHC lesions in the clinical setting.
Combined hepatocellular-cholangiocarcinoma (CHC) is a rare type of primary liver cancer. Due to its complex histopathological characteristics, the imaging features of CHC may overlap with those of hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC).
The contrasted-enhanced ultrasound (CEUS) Liver Imaging Reporting and Data System (LI-RADS) released by the American College of Radiology has been reported to be effective for the diagnosis of HCC. However, CHC lesions meeting the criteria for LR-5 classification may compromise the high specificity of LR-5 for the diagnosis of HCC if we only take the imaging features into consideration. Serum biomarkers, especially alpha-fetoprotein (AFP) and carbohydrate antigen 19-9 (CA19-9), have been shown to be helpful in the diagnosis of CHC. However, whether combining CEUS LI-RADS with serum biomarkers is helpful for differentiating CHC from HCC and ICC in at-risk patients has not been fully evaluated.
The purpose of this study was to investigate whether the combination of CEUS LI-RADS and serum biomarkers is helpful for differentiating CHC from HCC and ICC in patients with chronic liver disease.
Patients with histologically confirmed CHC, ICC and HCC with chronic liver disease between January 2016 and December 2019 were enrolled in this retrospective case control study. HCC patients were finally enrolled after one-to-two (CHC:HCC = 1:2) propensity score matching by tumor size, age and sex. Differences in quantitative variables were tested by the independent sample t-test. The rates of imaging characteristics were compared by using the χ2 test or Fisher’s exact test. Receiver operating characteristic curve analysis was used to investigate the potential of CEUS LI-RADS and serum tumor markers for differentiating CHC from HCC and ICC.
After propensity score matching, 134 patients (mean age of 51.4 ± 9.4 years, 108 men) were enrolled, including 35 CHC, 29 ICC and 70 HCC patients. Based on the CEUS LI-RADS classification, 74.3% (26/35) and 25.7% (9/35) of CHC lesions were assessed as LR-M and LR-5, respectively. The rates of elevated AFP and CA19-9 levels in CHC patients were 51.4% and 11.4%, respectively. Simultaneous elevation of AFP and CA19-9 was found in 8.6% (3/35) of CHC patients. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), accuracy and AUC of the aforementioned diagnostic criteria for discriminating CHC from HCC and ICC were 40.0%, 89.9%, 58.3%, 80.9%, 76.9% and 0.649, respectively. When the reported prevalence rate of CHC (0.4%-14.2%) was taken into account, the PPV and NPV were revised to 1.6%-39.6% and 90.1%-99.7%, respectively.
CHCs are more likely to be classified as LR-M than LR-5 by CEUS LI-RADS. The combination of the CEUS LI-RADS classification with serum tumor markers shows high specificity but low sensitivity for the diagnosis of CHC. Moreover, CHC could be confidently excluded with a high NPV.
The imaging features of CHC are complicated due to its complex histopathological characteristics. In addition, biopsy may misguide the correct diagnosis of CHC due to sampling error or tissue insufficiency. This study investigated the diagnostic value of the CEUS LI-RADS classification combined with serological tumor markers in differentiating CHC from HCC and ICC. The results showed that the combined diagnostic criteria had high specificity and NPV but low sensitivity for the diagnosis of CHC. These findings could help radiologists and clinical investigators confidently exclude CHC lesions in the clinical setting.
We thank all medical staff and technicians of dialysis centers who agreed to participate in this study.
| 1. | Wang J, Wang F, Kessinger A. Outcome of combined hepatocellular and cholangiocarcinoma of the liver. J Oncol. 2010;2010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Jarnagin WR, Weber S, Tickoo SK, Koea JB, Obiekwe S, Fong Y, DeMatteo RP, Blumgart LH, Klimstra D. Combined hepatocellular and cholangiocarcinoma: demographic, clinical, and prognostic factors. Cancer. 2002;94:2040-2046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 260] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 3. | ALLEN RA, LISA JR. Combined liver cell and bile duct carcinoma. Am J Pathol. 1949;25:647-655. [PubMed] |
| 4. | Kim MJ, Lee S, An C. Problematic lesions in cirrhotic liver mimicking hepatocellular carcinoma. Eur Radiol. 2019;29:5101-5110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Bosman FT. WHO classification of tumours of the digestive system 4th ed: Lyon, International Agency for Research on Cancer, 2010. |
| 6. | Fowler KJ, Sheybani A, Parker RA 3rd, Doherty S, M Brunt E, Chapman WC, Menias CO. Combined hepatocellular and cholangiocarcinoma (biphenotypic) tumors: imaging features and diagnostic accuracy of contrast-enhanced CT and MRI. AJR Am J Roentgenol. 2013;201:332-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 161] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 7. | Sammon J, Fischer S, Menezes R, Hosseini-Nik H, Lewis S, Taouli B, Jhaveri K. MRI features of combined hepatocellular- cholangiocarcinoma versus mass forming intrahepatic cholangiocarcinoma. Cancer Imaging. 2018;18:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Wells ML, Venkatesh SK, Chandan VS, Fidler JL, Fletcher JG, Johnson GB, Hough DM, Roberts LR. Biphenotypic hepatic tumors: imaging findings and review of literature. Abdom Imaging. 2015;40:2293-2305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Rogers JE, Bolonesi RM, Rashid A, Elsayes KM, Elbanan MG, Law L, Kaseb A, Shroff RT. Systemic therapy for unresectable, mixed hepatocellular-cholangiocarcinoma: treatment of a rare malignancy. J Gastrointest Oncol. 2017;8:347-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Huang XW, Huang Y, Chen LD, Wang Z, Yang Z, Liu JY, Xie XY, Lu MD, Shen SL, Wang W. Potential diagnostic performance of contrast-enhanced ultrasound and tumor markers in differentiating combined hepatocellular-cholangiocarcinoma from hepatocellular carcinoma and cholangiocarcinoma. J Med Ultrason (2001). 2018;45:231-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Li R, Yang D, Tang CL, Cai P, Ma KS, Ding SY, Zhang XH, Guo DY, Yan XC. Combined hepatocellular carcinoma and cholangiocarcinoma (biphenotypic) tumors: clinical characteristics, imaging features of contrast-enhanced ultrasound and computed tomography. BMC Cancer. 2016;16:158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 12. | Sagrini E, Iavarone M, Stefanini F, Tovoli F, Vavassori S, Maggioni M, Renzulli M, Salvatore V, Stefanescu H, Colombo M, Bolondi L, Piscaglia F. Imaging of combined hepatocellular-cholangiocarcinoma in cirrhosis and risk of false diagnosis of hepatocellular carcinoma. United European Gastroenterol J. 2019;7:69-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Ye J, Xie X, Liu B, Zhang X, Wang W, Huang X, Lu M, Huang G. Imaging Features on Contrast-Enhanced Ultrasound and Clinical Characteristics of Hepatitis B Virus-Related Combined Hepatocellular-Cholangiocarcinoma: Comparison with Hepatitis B Virus-Related Hepatocellular Carcinoma. Ultrasound Med Biol. 2017;43:2530-2536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Kassahun WT, Hauss J. Management of combined hepatocellular and cholangiocarcinoma. Int J Clin Pract. 2008;62:1271-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 111] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 15. | Kono Y, Lyshchik A, Cosgrove D, Dietrich CF, Jang HJ, Kim TK, Piscaglia F, Willmann JK, Wilson SR, Santillan C, Kambadakone A, Mitchell D, Vezeridis A, Sirlin CB. Contrast Enhanced Ultrasound (CEUS) Liver Imaging Reporting and Data System (LI-RADS®): the official version by the American College of Radiology (ACR). Ultraschall Med. 2017;38:85-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 129] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 16. | Huang JY, Li JW, Lu Q, Luo Y, Lin L, Shi YJ, Li T, Liu JB, Lyshchik A. Diagnostic Accuracy of CEUS LI-RADS for the Characterization of Liver Nodules 20 mm or Smaller in Patients at Risk for Hepatocellular Carcinoma. Radiology. 2020;294:329-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 96] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 17. | Lyshchik A, Kono Y, Dietrich CF, Jang HJ, Kim TK, Piscaglia F, Vezeridis A, Willmann JK, Wilson SR. Contrast-enhanced ultrasound of the liver: technical and lexicon recommendations from the ACR CEUS LI-RADS working group. Abdom Radiol (NY). 2018;43:861-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 18. | Claudon M, Dietrich CF, Choi BI, Cosgrove DO, Kudo M, Nolsøe CP, Piscaglia F, Wilson SR, Barr RG, Chammas MC, Chaubal NG, Chen MH, Clevert DA, Correas JM, Ding H, Forsberg F, Fowlkes JB, Gibson RN, Goldberg BB, Lassau N, Leen EL, Mattrey RF, Moriyasu F, Solbiati L, Weskott HP, Xu HX; World Federation for Ultrasound in Medicine; European Federation of Societies for Ultrasound. Guidelines and good clinical practice recommendations for Contrast Enhanced Ultrasound (CEUS) in the liver - update 2012: A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound Med Biol. 2013;39:187-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 506] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 19. | Patel AH, Harnois DM, Klee GG, LaRusso NF, Gores GJ. The utility of CA 19-9 in the diagnoses of cholangiocarcinoma in patients without primary sclerosing cholangitis. Am J Gastroenterol. 2000;95:204-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 288] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 20. | Sherman M, Peltekian KM, Lee C. Screening for hepatocellular carcinoma in chronic carriers of hepatitis B virus: incidence and prevalence of hepatocellular carcinoma in a North American urban population. Hepatology. 1995;22:432-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3145] [Article Influence: 393.1] [Reference Citation Analysis (3)] |
| 22. | Bender R, Lange S, Freitag G, Trampisch HJ. Variation of sensitivity, specificity, likelihood ratios and predictive values with disease prevalence by H. Brenner and O. Stat Med. 1998;17:946-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med. 2005;37:360-363. [PubMed] |
| 24. | Brunt E, Aishima S, Clavien PA, Fowler K, Goodman Z, Gores G, Gouw A, Kagen A, Klimstra D, Komuta M, Kondo F, Miksad R, Nakano M, Nakanuma Y, Ng I, Paradis V, Nyun Park Y, Quaglia A, Roncalli M, Roskams T, Sakamoto M, Saxena R, Sempoux C, Sirlin C, Stueck A, Thung S, Tsui WMS, Wang XW, Wee A, Yano H, Yeh M, Zen Y, Zucman-Rossi J, Theise N. cHCC-CCA: Consensus terminology for primary liver carcinomas with both hepatocytic and cholangiocytic differentation. Hepatology. 2018;68:113-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 289] [Cited by in RCA: 271] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 25. | Koh KC, Lee H, Choi MS, Lee JH, Paik SW, Yoo BC, Rhee JC, Cho JW, Park CK, Kim HJ. Clinicopathologic features and prognosis of combined hepatocellular cholangiocarcinoma. Am J Surg. 2005;189:120-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 158] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 26. | Yeh MM. Pathology of combined hepatocellular-cholangiocarcinoma. J Gastroenterol Hepatol. 2010;25:1485-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 27. | Gigante E, Ronot M, Bertin C, Ciolina M, Bouattour M, Dondero F, Cauchy F, Soubrane O, Vilgrain V, Paradis V. Combining imaging and tumour biopsy improves the diagnosis of combined hepatocellular-cholangiocarcinoma. Liver Int. 2019;39:2386-2396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 28. | Wakizaka K, Yokoo H, Kamiyama T, Ohira M, Kato K, Fujii Y, Sugiyama K, Okada N, Ohata T, Nagatsu A, Shimada S, Orimo T, Kamachi H, Taketomi A. Clinical and pathological features of combined hepatocellular-cholangiocarcinoma compared with other liver cancers. J Gastroenterol Hepatol. 2019;34:1074-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 29. | Choi SH, Lee SS, Park SH, Kim KM, Yu E, Park Y, Shin YM, Lee MG. LI-RADS Classification and Prognosis of Primary Liver Cancers at Gadoxetic Acid-enhanced MRI. Radiology. 2019;290:388-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 148] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 30. | Jeon SK, Joo I, Lee DH, Lee SM, Kang HJ, Lee KB, Lee JM. Combined hepatocellular cholangiocarcinoma: LI-RADS v2017 categorisation for differential diagnosis and prognostication on gadoxetic acid-enhanced MR imaging. Eur Radiol. 2019;29:373-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 99] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 31. | Trevisani F, D'Intino PE, Morselli-Labate AM, Mazzella G, Accogli E, Caraceni P, Domenicali M, De Notariis S, Roda E, Bernardi M. Serum alpha-fetoprotein for diagnosis of hepatocellular carcinoma in patients with chronic liver disease: influence of HBsAg and anti-HCV status. J Hepatol. 2001;34:570-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 524] [Article Influence: 21.0] [Reference Citation Analysis (4)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Izbicki JR, Sempokuya T S-Editor: Gao CC L-Editor: Filipodia P-Editor: Liu JH