Published online Oct 21, 2020. doi: 10.3748/wjg.v26.i39.6047
Peer-review started: July 1, 2020
First decision: July 28, 2020
Revised: August 13, 2020
Accepted: September 25, 2020
Article in press: September 25, 2020
Published online: October 21, 2020
Processing time: 112 Days and 5.9 Hours
It is well known that an alcohol consumption habit together with inactive heterozygous aldehyde dehydrogenase-2 (ALDH2) is an important risk factor for the development of esophageal squamous cell carcinoma (ESCC). It remains controversial whether human papillomavirus (HPV) infection contributes to the occurrence/development of ESCC. There has been no study in which the relationship between ESCC and HPV in addition to alcohol dehydrogenase-1B (ADH1B) and ALDH2 genotypes was evaluated.
To evaluate relationships between HPV infection and development of esophageal cancer, particularly early esophageal cancer, based on ADH1B/ALDH2 polymorphisms.
We conducted an exploratory retrospective study using new specimens, and we enrolled 145 patients who underwent endoscopic resection for superficial ESCC and had been observed for more than two years by both physical examination and endoscopic examination in Hokkaido University Hospital. Saliva was collected to analyze genetic polymorphisms of ADH1B/ALDH2. We performed in situ hybridization for resected specimens to detect HPV by using an HPV type 16/18 probe.
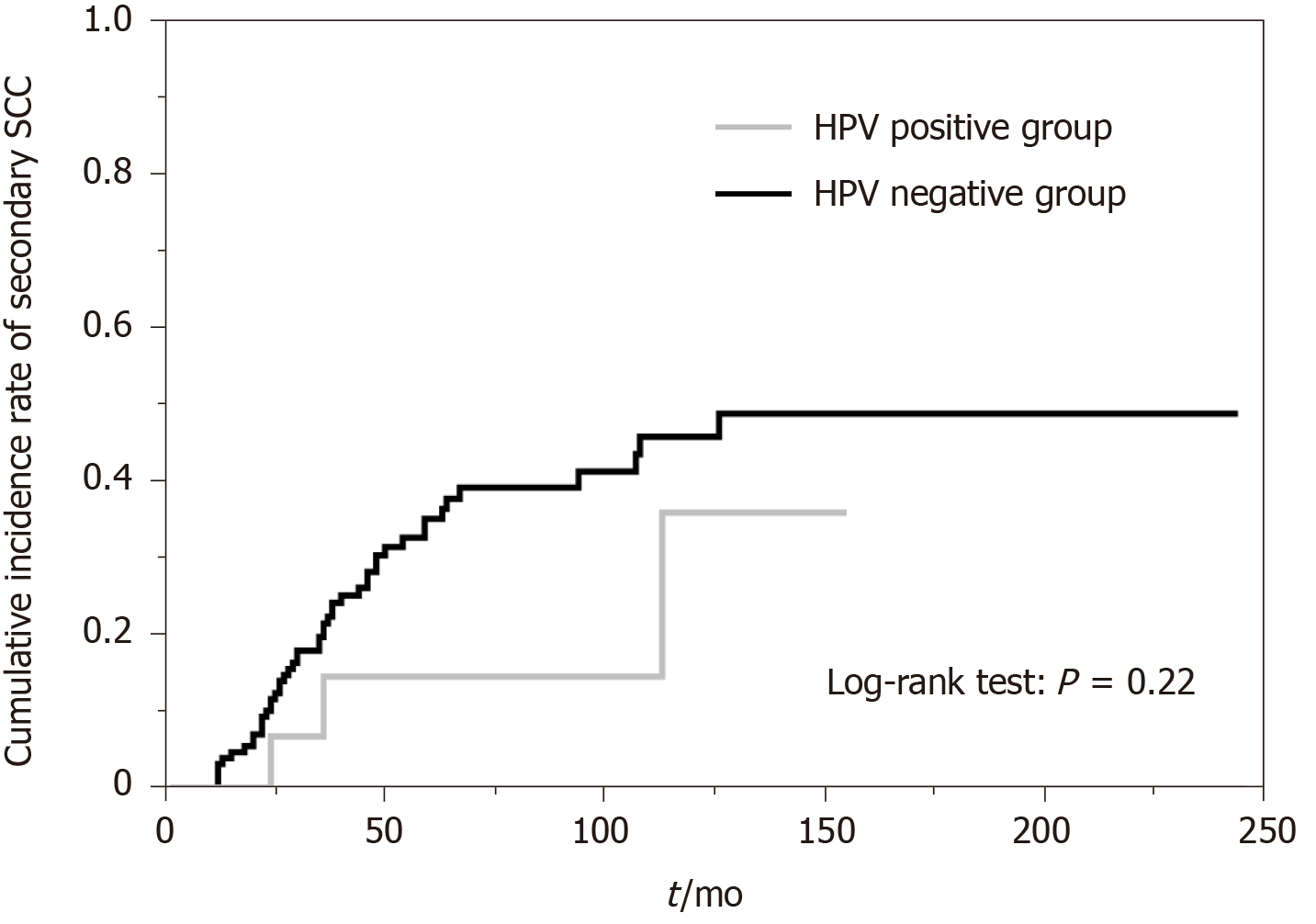
HPV was detected in 15 (10.3%) of the 145 patients with ESCC. HPV-positive rates in inactive ALDH2*1/*2 and ALDH2*1/*1 + *2/*2 were 10.8% and 9.8%, respectively (P = 1.00). HPV-positive rates in slow-metabolizing ADH1B*1/*1 and ADH1B*1/*2 + *2/*2 were 12.0% and 10.0%, respectively (P = 0.72). HPV-positive rates in the heavy or moderate alcohol consumption group and the light or rare consumption group were 11.1% and 8.7%, respectively (P = 0.68). HPV-positive rates in the heavy smoking group and the light or no smoking group were 11.8% and 8.3%, respectively (P = 0.59). The 3-year incidence rates of secondary ESCC or head and neck cancer after initial treatment in the HPV-positive and HPV-negative groups were 14.4% and 21.4% (P = 0.22), respectively.
In the present situation, HPV status is considered to be less important than other risk factors, such as alcohol consumption, smoking habit, ADH1B/ALDH2 polymorphisms, and HPV status would therefore have no effect on ESCC risk management.
Core Tip: We examined esophageal squamous cell carcinoma (ESCC) tissues obtained by endoscopic mucosal resection or endoscopic submucosal dissection for human papillomavirus (HPV) infection. Genotyping of alcohol dehydrogenase-1B (ADH1B)/ aldehyde dehydrogenase-2 (ALDH2) by using saliva sampling was performed. As a result, significant differences were not found between HPV infection and ADH1B/ALDH2. However, results of investigations including investigation of genetic polymorphisms in alcohol metabolism were shown for the first time, and there has so far been study on only early ESCC. We therefore consider the results of our study to be important.
- Citation: Inoue M, Shimizu Y, Ishikawa M, Abiko S, Shimoda Y, Tanaka I, Kinowaki S, Ono M, Yamamoto K, Ono S, Sakamoto N. Relationships of early esophageal cancer with human papillomavirus and alcohol metabolism. World J Gastroenterol 2020; 26(39): 6047-6056
- URL: https://www.wjgnet.com/1007-9327/full/v26/i39/6047.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i39.6047
Esophageal cancer is a prevalent cancer and has a high mortality rate with a 5-year relative survival rate of only about 20% despite recent technological advances in surgery and chemoradiotherapy[1]. It is well known that smoking and alcohol consumption are risk factors for esophageal squamous cell carcinoma (ESCC). However, some ESCC patients have had no smoking or alcohol consumption habit until they are diagnosed with ESCC. This indicates that there might be other risk factors for the development of ESCC including polycyclic aromatic hydrocarbons, high-temperature foods, diet, oral health, and microbiome and human papillomavirus (HPV) infection[2]. The hypothesis of a relationship between HPV infection and development of ESCC was first proposed in 1982[3]. After that, various studies were performed, and the results of some studies supported the hypothesis, while the results of other studies did not support the hypothesis[4-13]. It remains controversial whether HPV contributes to the development of ESCC. In head and neck squamous cell carcinoma (HNSCC), which is the same squamous cell carcinoma as ESCC is, it has been shown that HPV infection promotes the development of cancer, and HPV has been recognized as a carcinogenic agent[14]. Moreover, it has been reported that HPV infection in oropharyngeal squamous cell carcinoma is a significant prognostic factor for good survival. Notably, it has been reported that the incidence of second primary tumors is significantly lower in patients with HPV-positive oropharyngeal squamous cell carcinoma[15]. Subsequent investigations revealed that not only the prognosis of oropharyngeal squamous cell carcinoma (SCC) but also that of other head and neck cancers such as hypopharyngeal, laryngeal, oral cavity cancer is related to HPV status[16].
Alcohol consumption is known to be the most important risk factor for ESCC. Ethanol, which is one type of alcohol, itself does not cause the occurrence of ESCC, but acetaldehyde produced by alcohol has been reported to have carcinogenicity[17,18]. Ethanol is broken down by an enzyme called alcohol dehydrogenase 1B (ADH1B), resulting in the formation of acetaldehyde. Acetaldehyde is mostly metabolized in the liver by an enzyme called aldehyde dehydrogenase 2 (ALDH2), resulting in the formation of harmless acetic acid[19]. ADH1B and ALDH2 have active and inactive forms in the metabolism to acetaldehyde, and the combination of these forms is associated with not only advanced ESCC but also early stage ESCC[20,21]. Many studies have shown an association between ADH1B/ALDH2 and incidence of ESCC[21,22]. As for metachronous ESCC, Abiko et al[23] demonstrated that superficial ESCC patients with inactive ALDH2 have a high incidence of secondary ESCC.
There has been no study in which the risk of ESCC depending on the HPV status and the genetic polymorphisms of ADH1B/ALDH2 was investigated. In this study, we therefore investigated the relationship between HPV infection and development of esophageal cancer, particularly early esophageal cancer, based on ADH1B/ALDH2 polymorphisms.
The subjects for this study were registered during the period from July 2016 to August 2017. The subjects were patients who underwent endoscopic mucosal dissection (ESD) or endoscopic mucosal resection (EMR) for ESCC at Hokkaido University Hospital and received follow-up endoscopic examinations for 2 years or more. Endoscopic examinations were performed in all patients using iodine staining at 3 mo, 6 mo, and 1 year after the EMR/ESD and every 6 mo to 1 year thereafter. All of the patients underwent annual physical examinations including laryngoscopy conducted by otolaryngologists. Inclusion criteria for this study were (1) pathological diagnosis of ESCC in resected specimens; (2) complete endoscopic resection by EMR or ESD; (3) written informed consent obtained from the patient; and (4) possibility of collecting saliva samples. Exclusion criteria were (1) surgical resection after EMR/ESD; (2) unsuitability as subjects; (3) age < 20 years; and (4) current pregnancy. After exclusion, 158 patients were included in the study. No patients had lymph node metastasis. This study was a retrospective study in which newly obtained specimens were used. Written informed consent was obtained from the study subjects.
Information on alcohol consumption and smoking before and after EMR/ESD was obtained by using a questionnaire. Information on past treatment for esophageal cancer or head and neck cancer by EMR/ESD and histological diagnosis was obtained from electronic health records. Before the endoscopic examination, approximately 1 mL of saliva was obtained by using a pipette or a cotton swab to examine two single nucleotide polymorphisms (SNPs) in ADH1B and ALDH2 genotyping.
This study was approved by the Medical Ethics and Human Clinical Trial Committee of Hokkaido University Hospital.
Subjects whose alcohol consumption was less than 1 unit/wk were classified as rare drinkers, those whose alcohol consumption was 1 to 8.9 units/wk were classified as light drinkers, those whose alcohol consumption was 9 to 17.9 units/wk were classified as moderate drinkers, and those whose alcohol consumption was 18 or more units/wk were classified as heavy drinkers. We defined 1 unit of alcohol as 22 g of ethanol, which is contained in 500 mL of beer or 1/4 bottle of wine. Subjects who did not smoke or smoked rarely were classified as rare smokers (nonsmokers). Current smokers with a smoking history of 30 pack years were classified as light smokers, while subjects who had a smoking history of ≥ 30 pack years were classified as heavy smokers. Thirty pack years = one package of cigarettes (20 cigarettes) daily for 30 years[24,25].
We conducted in situ hybridization to detect HPV using the ENZO PATHO-GENE HPV type 16/18 probe according to the protocol of manufacturer (Morpho Technology Co., Ltd, Sapporo, Japan). Fixed specimens were deparaffinized and rehydrated through a graded ethanol series and then washed with phosphate buffered saline. The deparaffinized tissue sections were incubated with pronase, rinsed in deionized water, immersed in 0.3% H2O2 in methanol for 20 min, and rinsed in deionized water again. A drop of HPV probe was added to the air-dried sections. After heart denaturation at 90 °C, hybridization was done in a pre-warmed humid chamber at 37 °C for 16 to 18 h. The slides were washed in Tris-buffered saline with Tween 20 (TBST). Hybridized probes were detected by streptavidin. HPV-positive cervical cancer was used as a positive control. All pathological diagnoses including whether HPV is positive or negative were made by a pathologist.
SNPs of ADH1B and ALDH2 genes were genotyped using the TaqMan assay on an ABI 7300 Real-Time polymerase chain reaction (PCR) System (Applied Biosystems)[26]. The ADH1B*1 allele encodes the slow-metabolizing form of ADH1B, while the ALDH2*2 allele encodes the inactive form of ALDH2. The ADH1B and ALDH2 genotype combinations were classified into five categories according to the classification proposed by Yokoyama et al[27]: A group, ADH1B*1/*1 + ALDH2*1/*1; B group, ADH1B*2 carrier + ALDH2*1/*1; C group, ADH1B*1/*1 + ALDH2*1/*2; D group, ADH1B*2 carrier + ALDH2*1/*2; E group, ADH1B any + ALDH2*2/*2.
Analyses of data were performed by using JMP® Pro 14.0.1 (SAS Institute, Inc., Cary, NC, United States). Age is expressed as mean ± SD values. Differences in frequency distributions were tested using Fisher’s exact test, and quantitative data were examined with Student’s t-test. Differences were considered statistically significant at P < 0.05. The time to development of metachronous ESCC/HNSCC was defined as the period from the day of EMR/ESD to the day of endoscopic diagnosis of metachronous ESCC/HNSCC. The Kaplan–Meier method and log-rank test were used for analysis of the development of metachronous ESCC as well as HNSCC.
Among the 158 patients, 13 patients were excluded because their primary lesions were not large enough to assess HPV. Finally, 145 patients were assessed. Characteristics of the patients and lesions are shown in Table 1. Specimens from 15 (10.3%) of the 145 patients were HPV-positive. There was no significant difference between HPV-positive patients and HPV-negative patients in sex, age or tumor location. The main macroscopic types were 0-Is in 1 patient (0.7%), 0-IIa in 16 patients (15.2%), 0-IIb in 43 patients (29.7%), and 0-IIc in 85 patients (58.6%). The main macroscopic type was related to HPV infection, and the number of 0-IIa cases was significantly larger in HPV-positive ESCC cases (P = 0.045). The mean tumor size was 22.4 ± 10.6 mm. There was no difference between the HPV-positive and the HPV-negative groups in depth of invasion or vascular invasion.
| Total (n = 145) | HPV positive (n = 15) | HPV negative (n = 130) | P value | |
| Sex | ||||
| Male | 119 (82.1) | 12 (80.0) | 107 (82.3) | 0.73 |
| Female | 26 (17.9) | 3 (20.0) | 23 (17.7) | |
| Age (yr), mean ± SD | 66.3 ± 7.8 | 63.9 ± 9.3 | 66.6 ± 7.6 | 0.21 |
| Tumor location | ||||
| Cervical | 1 (0.7) | 1 (6.7) | 0 (0) | 0.059 |
| Upper | 22 (15.2) | 0 (0) | 22 (16.9) | |
| Middle | 85 (58.6) | 11 (73.3) | 74 (56.9) | |
| Lower | 36 (24.8) | 3 (20.0) | 33 (25.4) | |
| Abdominal | 1 (0.7) | 0 (0) | 1 (0.8) | |
| Main macroscopic type | ||||
| 0-Is | 1 (0.7) | 0 (0) | 1 (0.8) | 0.045 |
| 0-IIa | 16 (11.0) | 5 (33.3) | 11 (8.5) | |
| 0-IIb | 43 (29.7) | 4 (26.7) | 39 (30.0) | |
| 0-IIc | 85 (58.6) | 6 (40.0) | 79 (60.8) | |
| Tumor size (mm), mean (SD) | 22.4 (10.6) | 26.5 (12.7) | 21.9 (10.3) | 0.11 |
| Depth of invasion | ||||
| EP | 53 (36.6) | 5 (33.3) | 48 (36.9) | 0.74 |
| LPM | 66 (45.5) | 7 (46.7) | 59 (45.4) | |
| MM | 19 (13.1) | 2 (13.3) | 17 (13.1) | |
| SM1 | 4 (2.8) | 1 (6.7) | 3 (2.3) | |
| SM2 | 3 (2.1) | 0 (0) | 3 (2.3) | |
| Vascular invasion | ||||
| Yes | 6 (4.1) | 2 (13.3) | 4 (3.1) | 0.12 |
| No | 139 (95.9) | 13 (86.7) | 126 (96.9) | |
The relationships of HPV status with risk factors for ESCC are summarized in Table 2. HPV-positive rates in inactive ALDH2*1/*2 and ALDH2*1/*1 + *2/*2 were 10.8% and 9.6%, respectively (P = 1.00). HPV-positive rates in slow-metabolizing ADH1B*1/*1 and ADH1B*1/*2 + *2/*2 were 12.0% and 10.0%, respectively (P = 0.72). HPV-positive rates in the heavy or moderate alcohol consumption group and the light or rare consumption group were 11.1% and 8.7%, respectively (P = 0.68). HPV-positive rates in the heavy smoking group and the light or no smoking group were 11.8% and 8.3%, respectively (P = 0.59). Genotype combinations did not show significant differences in HPV-positive rates (group A, 0%; group B, 10.9%; group C, 15.0%; group D, 9.6%; group E, 0.0%; P = 0.87). There were no significant differences in HPV-positive rates according to either ADH1B/ALDH2 genotype or smoking and alcohol consumption histories. The median follow-up period was 73 mo (range, 24-244 mo). The 3-year incidence rates of secondary ESCC or HNSCC after initial treatment in the HPV-positive and HPV-negative groups were 14.4% and 21.4% (P = 0.22), respectively (Figure 1).
| HPV | ALDH2 genotype | P value | |||||
| *1/*2 (n = 93) | *1/*1 + *2/*2 (n = 52) | ||||||
| Positive (n = 15) | 10 (10.8) | 5 (9.6) | 1.00 | ||||
| Negative (n = 130) | 83 (89.3) | 47 (90.4) | |||||
| ADH1B genotype | |||||||
| *1/*1 (n = 25) | *2 carrier (n = 120) | ||||||
| Positive (n = 15) | 3 (12.0) | 12 (10.0) | 0.72 | ||||
| Negative (n = 130) | 22 (88.0) | 108 (90.0) | |||||
| Alcohol consumption | |||||||
| Light – Rare (n = 46) | Heavy – Moderate (n = 99) | ||||||
| Positive (n = 15) | 4 (8.7) | 11 (11.1) | 0.68 | ||||
| Negative (n = 130) | 42 (91.3) | 88 (88.9) | |||||
| Smoking habits | |||||||
| Light – Rare (n = 60) | Heavy (n = 85) | ||||||
| Positive (n = 15) | 5 (8.3) | 10 (11.8) | 0.59 | ||||
| Negative (n = 130) | 55 (91.7) | 75 (88.2) | |||||
| Genotype combinations | |||||||
| A | B | C | D | E | |||
| Positive (n = 15) | 0 (0.0) | 5 (10.9) | 3 (15.0) | 7 (9.6) | 0 (0.0) | 0.87 | |
| Negative (n = 130) | 5 (100.0) | 41 (89.1) | 17 (85.0) | 66 (90.4) | 1 (100.0) | ||
This study is the first study that focused on HPV infection in cases of early ESCC based on ADH1B/ALDH2 polymorphisms. Many studies have been carried out to determine whether the hypothesis that HPV contributes to the occurrence of ESCC is true, but the results have not been consistent[4-13]. Even in a recent meta-analysis and systematic review, the conclusions are still confusing. Petrick et al[13] conducted a meta-analysis including 124 studies with a total of 13832 ESCC cases and reported that the highest HPV prevalence was found in Africa and Asia, notably among Chinese studies from provinces with high ESCC incidence rates. Halec et al[10] conducted a study on HPV-transformation of ESCC by using tissues from high-incidence ESCC regions and a meta-analysis of 14 other similar studies. They concluded that the results of the studies did not support an etiological role of HPV in ESCC carcinogenesis. Although the effects of HPV infection on the occurrence of ESCC with consideration of smoking and drinking habits were examined in many previous studies, there has been no study focusing on ADH1B/ALDH2. Assuming that HPV infection is involved in the development of ESCC, we thought that patients who do not have ESCC risk factors, particularly inactive ALDH2, would tend to show high HPV-positive rates. However, we could not find any significant difference between HPV infection and any of the ESCC risk factors. From another point of view, early ESCC after endoscopic resection often causes metachronous recurrence. After examining the possibility that HPV infection contributes to less prevalence of metachronous recurrence of ESCC, no significant difference was found between HPV-positive and HPV-negative groups in the incidence of secondary SCC. As for the characteristics of HPV-positive ESCC, in our analyses, type IIa lesions, which have mild protrusion up to about 1 mm, tended to be detected in HPV-positive lesions. In many previous studies, significant clinicopathological differences between HPV-positive ESCC and HPV-negative ESCC were not revealed[28-33]. Since the relationship between HPV infection and development of ESCC is not clear, it is debatable whether HPV infection contributes to upward growth. The influence of HPV infection on tumor growth and morphology in the esophagus remains unknown.
In a past review, it was shown that HPV-positive rates in patients with ESCC range from 11.7% to 38.9%[9]. Some differences in HPV-positive rates have been observed in previous studies, and the HPV-positive rate of 10.3% in our study is slightly lower than the rates in previous studies. There are various possible reasons for the differences, but geographic variation has been reported to be the main reason for the differences in HPV-positive rates[34]. Studies showing high HPV prevalence rates were studies conducted in Asia, while studies showing low HPV prevalence rates were studies conducted in North America and Europe[8,34,35]. Previous studies including meta-analyses in which the relationship between HPV infection and esophageal cancer was investigated included many studies conducted in Asia, which is considered to be a region with a high incidence of HPV. Such publication bias has been an issue of discussion[9]. For example, Syrjänen conducted a meta-analysis including 10234 ESCC cases and reported that the overall HPV prevalence was 30.6%. However, when regions were limited to North America, which is a region with a low incidence of HPV, the rate fell to 10.1%[36]. Japan is a country in Asia, but considering HPV prevalence regions, it is not clear whether Japan, like other Asian countries, can be regarded as a region with a high incidence of HPV. There have been only a few reports about the prevalence of HPV in Japan. Goto et al[37] reported that the HPV-positive rate in patients with esophageal carcinoma in Asia including Japan was 9.4%, and they also showed that HPV-positive rates varied depending on the location in Japan. These regional differences might be caused by environmental risk factors, genetic background, and histological types. We therefore cannot simply compare HPV-positive rates in our region with HPV-positive rates in previous reports. However, there would not be large differences. Moreover, even if HPV infection rate varies depending on the region, the role HPV in ESCC would not change. HPV-positive rates are also affected by the quality of patient samples and the methods used for evaluating HPV status. Sampling by endoscopic biopsies might result in inadequate or incorrect tissue samples[38]. EMR/ESD for ESCC can provide precise tissue samples of almost exclusively ESCC without the problem of excessive or insufficient tissue samples. We analyzed early ESCC specimens, all of which were endoscopically resected. In the most past studies, the association of HPV with ESCC was investigated using samples derived from endoscopic biopsy or parts of surgical specimens. There is no information about HPV-positive rates only in early ESCC specimens derived from EMR/ESD. The use of specimens obtained from EMR/ESD would reduce sampling errors such errors caused by inaccurate endoscopic biopsy and would enable accurate evaluation of ESCC cases of the same stage. We therefore consider that HPV-ESCC prevalence was evaluated with a high level of accuracy and under the most possible uniform condition in our study. With regard to methodological issues, another concern is the method used for inspection of tissue samples. In most recent studies, ISH or PCR was used for detecting HPV infection. It has been reported that HPV infection rate varied depending on the method used for detecting HPV[34,36,39,40]. In general, ISH has higher specificity but lower sensitivity than those of PCR for detection of HPV[41]. In our study, lower HPV prevalence might have resulted from the characteristics of ISH.
This study has some limitations. First, participants in this study were all Japanese patients, and the study was conducted in a single institution. Therefore, geographic bias, especially for HPV prevalence, could not be completely excluded. Second, methodological bias must be considered for accurate estimation of HPV-positive rates. HPV-positive rate would differ depending on the sensitivity and accuracy of the method used for detecting HPV infection. However, in our study conducted with EMR/ESD samples and using ISH for detection of HPV, it is thought that bias was reduced as much as possible. Third, this study was conducted with a restricted sample size. To improve statistical reliability, further studies with large sample sizes are required.
In conclusion, our study in which genetic polymorphisms of ADH1B/ALDH2 were considered suggested that HPV did not have an association with ESCC. In the present situation, HPV status is considered to be less important than other risk factors, such as alcohol consumption, smoking habit, and ADH1B/ALDH2 polymorphisms, and HPV status would therefore have no effect on ESCC risk management.
There has been no study in which the relationship between superficial esophageal squamous cell carcinoma (ESCC) and human papillomavirus (HPV) in addition to alcohol metabolism was evaluated.
We aimed to clarify whether HPV infection together with alcohol metabolism genes affects the carcinogenesis of ESCC.
We enrolled 145 patients who underwent endoscopic submucosal dissection (ESD) or endoscopic mucosal resection (EMR) for ESCC.
We analyzed patients' genetic polymorphisms of alcohol dehydrogenase-1B (ADH1B)/ aldehyde dehydrogenase-2 (ALDH2) and performed in situ hybridization for resected specimens to detect HPV by using an HPV type 16/18 probe.
There were no significant differences in HPV-positive rates according to either ADH1B/ALDH2 genotype or smoking and alcohol consumption histories.
HPV status is considered to be less important than other risk factors, such as alcohol consumption, smoking habit, ADH1B/ALDH2 polymorphisms and HPV status would therefore have no effect on ESCC risk management.
We are planning a multicenter study of patients with superficial pharyngeal cancer evaluating ADH1B/ALDH2 and HPV status.
| 1. | Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang XS, Bannon F, Ahn JV, Johnson CJ, Bonaventure A, Marcos-Gragera R, Stiller C, Azevedo e Silva G, Chen WQ, Ogunbiyi OJ, Rachet B, Soeberg MJ, You H, Matsuda T, Bielska-Lasota M, Storm H, Tucker TC, Coleman MP; CONCORD Working Group. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet. 2015;385:977-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1738] [Cited by in RCA: 1780] [Article Influence: 161.8] [Reference Citation Analysis (0)] |
| 2. | Abnet CC, Arnold M, Wei WQ. Epidemiology of Esophageal Squamous Cell Carcinoma. Gastroenterology. 2018;154:360-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 935] [Cited by in RCA: 1249] [Article Influence: 156.1] [Reference Citation Analysis (1)] |
| 3. | Syrjänen KJ. Histological changes identical to those of condylomatous lesions found in esophageal squamous cell carcinomas. Arch Geschwulstforsch. 1982;52:283-292. [PubMed] |
| 4. | Dong HC, Cui XB, Wang LH, Li M, Shen YY, Zhu JB, Li CF, Hu JM, Li SG, Yang L, Zhang WJ, Chen YZ, Li F. Type-specific detection of human papillomaviruses in Kazakh esophageal squamous cell carcinoma by genotyping both E6 and L1 genes with MALDI-TOF mass spectrometry. Int J Clin Exp Pathol. 2015;8:13156-13165. [PubMed] |
| 5. | Türkay DÖ, Vural Ç, Sayan M, Gürbüz Y. Detection of human papillomavirus in esophageal and gastroesophageal junction tumors: A retrospective study by real-time polymerase chain reaction in an instutional experience from Turkey and review of literature. Pathol Res Pract. 2016;212:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Li X, Gao C, Yang Y, Zhou F, Li M, Jin Q, Gao L. Systematic review with meta-analysis: the association between human papillomavirus infection and oesophageal cancer. Aliment Pharmacol Ther. 2014;39:270-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 7. | Liyanage SS, Rahman B, Ridda I, Newall AT, Tabrizi SN, Garland SM, Segelov E, Seale H, Crowe PJ, Moa A, Macintyre CR. The aetiological role of human papillomavirus in oesophageal squamous cell carcinoma: a meta-analysis. PLoS One. 2013;8:e69238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Yong F, Xudong N, Lijie T. Human papillomavirus types 16 and 18 in esophagus squamous cell carcinoma: a meta-analysis. Ann Epidemiol. 2013;23:726-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Ludmir EB, Stephens SJ, Palta M, Willett CG, Czito BG. Human papillomavirus tumor infection in esophageal squamous cell carcinoma. J Gastrointest Oncol. 2015;6:287-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 10. | Halec G, Schmitt M, Egger S, Abnet CC, Babb C, Dawsey SM, Flechtenmacher C, Gheit T, Hale M, Holzinger D, Malekzadeh R, Taylor PR, Tommasino M, Urban MI, Waterboer T, Pawlita M, Sitas F; InterSCOPE Collaboration. Mucosal alpha-papillomaviruses are not associated with esophageal squamous cell carcinomas: Lack of mechanistic evidence from South Africa, China and Iran and from a world-wide meta-analysis. Int J Cancer. 2016;139:85-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Gao GF, Roth MJ, Wei WQ, Abnet CC, Chen F, Lu N, Zhao FH, Li XQ, Wang GQ, Taylor PR, Pan QJ, Chen W, Dawsey SM, Qiao YL. No association between HPV infection and the neoplastic progression of esophageal squamous cell carcinoma: result from a cross-sectional study in a high-risk region of China. Int J Cancer. 2006;119:1354-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Koshiol J, Wei WQ, Kreimer AR, Chen W, Gravitt P, Ren JS, Abnet CC, Wang JB, Kamangar F, Lin DM, von Knebel-Doeberitz M, Zhang Y, Viscidi R, Wang GQ, Gillison ML, Roth MJ, Dong ZW, Kim E, Taylor PR, Qiao YL, Dawsey SM. No role for human papillomavirus in esophageal squamous cell carcinoma in China. Int J Cancer. 2010;127:93-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Petrick JL, Wyss AB, Butler AM, Cummings C, Sun X, Poole C, Smith JS, Olshan AF. Prevalence of human papillomavirus among oesophageal squamous cell carcinoma cases: systematic review and meta-analysis. Br J Cancer. 2014;110:2369-2377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 14. | D'Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, Westra WH, Gillison ML. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944-1956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1930] [Cited by in RCA: 1852] [Article Influence: 97.5] [Reference Citation Analysis (0)] |
| 15. | Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, Westra WH, Chung CH, Jordan RC, Lu C, Kim H, Axelrod R, Silverman CC, Redmond KP, Gillison ML. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5387] [Cited by in RCA: 5137] [Article Influence: 321.1] [Reference Citation Analysis (0)] |
| 16. | Bryant AK, Sojourner EJ, Vitzthum LK, Zakeri K, Shen H, Nguyen C, Murphy JD, Califano JA, Cohen EEW, Mell LK. Prognostic Role of p16 in Nonoropharyngeal Head and Neck Cancer. J Natl Cancer Inst. 2018;110:1393-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 17. | Seitz HK, Stickel F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat Rev Cancer. 2007;7:599-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 745] [Cited by in RCA: 791] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 18. | Brooks PJ, Zakhari S. Acetaldehyde and the genome: beyond nuclear DNA adducts and carcinogenesis. Environ Mol Mutagen. 2014;55:77-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 19. | Zakhari S. Overview: how is alcohol metabolized by the body? Alcohol Res Health. 2006;29:245-254. [PubMed] |
| 20. | Matejcic M, Gunter MJ, Ferrari P. Alcohol metabolism and oesophageal cancer: a systematic review of the evidence. Carcinogenesis. 2017;38:859-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 21. | Cui R, Kamatani Y, Takahashi A, Usami M, Hosono N, Kawaguchi T, Tsunoda T, Kamatani N, Kubo M, Nakamura Y, Matsuda K. Functional variants in ADH1B and ALDH2 coupled with alcohol and smoking synergistically enhance esophageal cancer risk. Gastroenterology. 2009;137:1768-1775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 272] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 22. | Tanaka F, Yamamoto K, Suzuki S, Inoue H, Tsurumaru M, Kajiyama Y, Kato H, Igaki H, Furuta K, Fujita H, Tanaka T, Tanaka Y, Kawashima Y, Natsugoe S, Setoyama T, Tokudome S, Mimori K, Haraguchi N, Ishii H, Mori M. Strong interaction between the effects of alcohol consumption and smoking on oesophageal squamous cell carcinoma among individuals with ADH1B and/or ALDH2 risk alleles. Gut. 2010;59:1457-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 23. | Abiko S, Shimizu Y, Miyamoto S, Ishikawa M, Matsuda K, Tsuda M, Mizushima T, Yamamoto K, Ono S, Kudo T, Ono K, Sakamoto N. Risk assessment of metachronous squamous cell carcinoma after endoscopic resection for esophageal carcinoma based on the genetic polymorphisms of alcoholdehydrogense-1B aldehyde dehydrogenase-2: temperance reduces the risk. J Gastroenterol. 2018;53:1120-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Yokoyama A, Katada C, Yokoyama T, Yano T, Kaneko K, Oda I, Shimizu Y, Doyama H, Koike T, Takizawa K, Hirao M, Okada H, Yoshii T, Konishi K, Yamanouchi T, Tsuda T, Omori T, Kobayashi N, Suzuki H, Tanabe S, Hori K, Nakayama N, Kawakubo H, Ishikawa H, Muto M. Alcohol abstinence and risk assessment for second esophageal cancer in Japanese men after mucosectomy for early esophageal cancer. PLoS One. 2017;12:e0175182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Yokoyama T, Yokoyama A, Kato H, Tsujinaka T, Muto M, Omori T, Haneda T, Kumagai Y, Igaki H, Yokoyama M, Watanabe H, Yoshimizu H. Alcohol flushing, alcohol and aldehyde dehydrogenase genotypes, and risk for esophageal squamous cell carcinoma in Japanese men. Cancer Epidemiol Biomarkers Prev. 2003;12:1227-1233. [PubMed] |
| 26. | Hayashida M, Ota T, Ishii M, Iwao-Koizumi K, Murata S, Kinoshita K. Direct detection of single nucleotide polymorphism (SNP) by the TaqMan PCR assay using dried saliva on water-soluble paper and hair-roots, without DNA extraction. Anal Sci. 2014;30:427-429. [PubMed] |
| 27. | Yokoyama A, Brooks PJ, Yokoyama T, Mizukami T, Shiba S, Nakamoto N, Maruyama K. Recovery from anemia and leukocytopenia after abstinence in Japanese alcoholic men and their genetic polymorphisms of alcohol dehydrogenase-1B and aldehyde dehydrogenase-2. Jpn J Clin Oncol. 2017;47:306-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Abdirad A, Eram N, Behzadi AH, Koriyama C, Parvaneh N, Akiba S, Kato T, Kahn N, Ghofrani M, Sadigh N. Human papillomavirus detected in esophageal squamous cell carcinoma in Iran. Eur J Intern Med. 2012;23:e59-e62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Herrera-Goepfert R, Lizano M, Akiba S, Carrillo-García A, Becker-D'Acosta M. Human papilloma virus and esophageal carcinoma in a Latin-American region. World J Gastroenterol. 2009;15:3142-3147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Castillo A, Aguayo F, Koriyama C, Torres M, Carrascal E, Corvalan A, Roblero JP, Naquira C, Palma M, Backhouse C, Argandona J, Itoh T, Shuyama K, Eizuru Y, Akiba S. Human papillomavirus in esophageal squamous cell carcinoma in Colombia and Chile. World J Gastroenterol. 2006;12:6188-6192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Yao PF, Li GC, Li J, Xia HS, Yang XL, Huang HY, Fu YG, Wang RQ, Wang XY, Sha JW. Evidence of human papilloma virus infection and its epidemiology in esophageal squamous cell carcinoma. World J Gastroenterol. 2006;12:1352-1355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Antonsson A, Nancarrow DJ, Brown IS, Green AC, Drew PA, Watson DI, Hayward NK, Whiteman DC; Australian Cancer Study. High-risk human papillomavirus in esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2010;19:2080-2087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 33. | Shuyama K, Castillo A, Aguayo F, Sun Q, Khan N, Koriyama C, Akiba S. Human papillomavirus in high- and low-risk areas of oesophageal squamous cell carcinoma in China. Br J Cancer. 2007;96:1554-1559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Syrjänen KJ. HPV infections and oesophageal cancer. J Clin Pathol. 2002;55:721-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 210] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 35. | Hardefeldt HA, Cox MR, Eslick GD. Association between human papillomavirus (HPV) and oesophageal squamous cell carcinoma: a meta-analysis. Epidemiol Infect. 2014;142:1119-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 36. | Syrjänen K. Geographic origin is a significant determinant of human papillomavirus prevalence in oesophageal squamous cell carcinoma: systematic review and meta-analysis. Scand J Infect Dis. 2013;45:1-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 37. | Goto A, Li CP, Ota S, Niki T, Ohtsuki Y, Kitajima S, Yonezawa S, Koriyama C, Akiba S, Uchima H, Lin YM, Yeh KT, Koh JS, Kim CW, Kwon KY, Nga ME, Fukayama M. Human papillomavirus infection in lung and esophageal cancers: analysis of 485 Asian cases. J Med Virol. 2011;83:1383-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 38. | Löfdahl HE, Du J, Näsman A, Andersson E, Rubio CA, Lu Y, Ramqvist T, Dalianis T, Lagergren J, Dahlstrand H. Prevalence of human papillomavirus (HPV) in oesophageal squamous cell carcinoma in relation to anatomical site of the tumour. PLoS One. 2012;7:e46538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 39. | Poljak M, Cerar A, Seme K. Human papillomavirus infection in esophageal carcinomas: a study of 121 lesions using multiple broad-spectrum polymerase chain reactions and literature review. Hum Pathol. 1998;29:266-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 54] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 40. | Matsha T, Erasmus R, Kafuko AB, Mugwanya D, Stepien A, Parker MI; CANSA/MRC Oesophageal Cancer Research Group. Human papillomavirus associated with oesophageal cancer. J Clin Pathol. 2002;55:587-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 41. | Kimple AJ, Torres AD, Yang RZ, Kimple RJ. HPV-associated head and neck cancer: molecular and nano-scale markers for prognosis and therapeutic stratification. Sensors (Basel). 2012;12:5159-5169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Xu YW S-Editor: Zhang H L-Editor: A P-Editor: Li JH