Published online Oct 21, 2020. doi: 10.3748/wjg.v26.i39.6037
Peer-review started: May 28, 2020
First decision: June 18, 2020
Revised: August 28, 2020
Accepted: September 5, 2020
Article in press: September 5, 2020
Published online: October 21, 2020
Processing time: 146 Days and 1.6 Hours
Detailed information on metastatic patterns in of patients with esophageal and gastric cancer is limited. Early recognition of metastases is important to avoid futile locoregional treatments. Furthermore, knowledge on metastatic patterns is necessary for further development of personalized treatment modalities.
To gain insight into the metastatic pattern of gastroesophageal cancer.
A nationwide retrospective autopsy study of 3876 patients with adenocarcinoma (AC) or squamous cell carcinoma (SCC) of the esophagus or stomach between 1990 and 2017 was performed. Only patient with metastases were included for analysis. The metastatic pattern was analyzed according to the primary tumor location and histological subtype.
Metastatic disease was found in 268 esophageal and 331 gastric cancer patients. In esophageal cancer, the most common metastatic locations were liver (56%), distant lymph nodes (53%) and lung (50%). Esophageal AC showed more frequently metastases to the peritoneum and bone compared with esophageal SCC. In gastric cancer, the most common metastatic locations were distant lymph nodes (56%), liver (53%) and peritoneum (51%). Intestinal-type AC of the stomach showed metastases to the liver more frequently, whereas metastases to the bone, female reproductive organs and colorectum were observed more frequently in diffuse-type gastric AC.
This study showed differences in metastatic patterns of patients with esophageal and gastric cancer according to the primary tumor location and histological subtype.
Core tip: In this nationwide retrospective autopsy study, the metastatic pattern of 599 patients with metastases of primary adenocarcinoma or squamous cell carcinoma of the esophagus or stomach were evaluated. Differences in metastatic patterns of these patients were found according both to the primary tumor location as well as the histological subtype. This study provides robust data regarding metastatic patterns in esophageal and gastric cancer patients. Knowledge of metastatic patterns is helpful during preoperative staging, follow-up and in future research.
- Citation: Verstegen MHP, Harker M, van de Water C, van Dieren J, Hugen N, Nagtegaal ID, Rosman C, van der Post RS. Metastatic pattern in esophageal and gastric cancer: Influenced by site and histology. World J Gastroenterol 2020; 26(39): 6037-6046
- URL: https://www.wjgnet.com/1007-9327/full/v26/i39/6037.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i39.6037
In 2018, over 570000 esophageal cancer and over 1000000 gastric cancer cases occurred globally, respectively the 6th and 3rd leading cause of cancer related death[1,2]. Over the last decade, peri-operative treatment modalities for patients with potentially curable esophageal or gastric cancer have improved. In the Netherlands, most patients with locally advanced esophageal cancer are treated with neo-adjuvant chemoradiotherapy, followed by resection[3]. Patients with potentially curable gastric cancer are treated with peri-operative chemotherapy and resection[4,5]. Despite the fact that these treatment modalities resulted in improved overall survival, the prognosis of patients with gastric or esophageal cancer is still dismal mainly due to the high incidence of locoregional recurrence and distant metastases. Moreover, up to 50% of gastro-esophageal cancer patients present with metastatic disease at time of diagnosis[6,7]. Early recognition of metastases is important to avoid futile locoregional treatments. There is a wide variability in the timing, location and extent of metastatic disease. In order to optimize pretreatment evaluation of patients and to ensure adequate surveillance, it is essential to know more about the metastatic pattern occurring in this patient group.
Metastases of esophageal cancer are most frequently seen in the liver, lung and distant lymph nodes[8-10]. The most common metastatic sites of gastric cancer are the liver, peritoneum and distant lymph nodes[11,12]. There are limited studies reporting on differences of the metastatic spreading according to the primary tumor location, for example, upper esophageal vs distal esophageal cancer or cardia vs non-cardia gastric cancer[9,12]. Few studies reported data on differences in metastatic site according to histological subtype[8,9,11,12].
As upper gastrointestinal tract tumors are a heterogenous group, further improvement of survival probably lies within a more personalized treatment strategy. Therefore, it is important to attain deeper knowledge on the different patterns of metastatic spreading and the factors that are instrumental in the determination of these patterns. The aim of this study is to gain insight into the location of metastases and the metastatic pattern according to the primary tumor site and the histology of the primary tumor.
A nationwide retrospective review was conducted of pathological records of patients diagnosed with esophageal or gastric cancer who underwent autopsy between 1990 and 2017. Patients were selected from the nationwide network and registry of histopathology and cytopathology in the Netherlands (PALGA)[13]. In the Netherlands, post-mortem examination is performed at the request of the family or treating physician with consent of the family and is carried out by a pathologist. All autopsies included in this study were performed in order to obtain information on the medical status of the deceased or to determine the exact cause of death. This type of study does not require approval from an ethics committee under Dutch law.
Patients with a history of esophageal or gastric cancer who underwent autopsy were selected from the Dutch pathology registry (PALGA). Only patients with a history of esophageal or gastric cancer with metastases, or those who were diagnosed with metastatic esophageal or gastric cancer during autopsy, were included. Patients with a primary diagnosis of a premalignant lesion, (i.e. dysplasia and in-situ carcinoma), neuroendocrine neoplasm, mesenchymal tumor, lymphoma or metastases from elsewhere to the esophagus or stomach were excluded. Incomplete autopsies were excluded. Patients were excluded if the location of the metastases could not be retrieved from the records.
Gender, date of autopsy, age at autopsy, type of autopsy (body or body and brain), location of primary cancer (proximal esophagus, mid esophagus, distal esophagus [incl. gastro-esophageal junction (GEJ)], proximal stomach (i.e. cardia and fundus), stomach corpus, distal stomach (i.e. antrum, pylorus and linitis plastica), number of metastases and location of metastases were recorded. The histological type of carcinoma was recorded according to the World Health Organization and Laurén classification. In case of adenocarcinoma not otherwise specified, the carcinoma was assigned to the intestinal-type adenocarcinoma group. In case of metastases to the abdominal organs, records where thoroughly screened whether it was a peritoneal metastasis rather than an organ specific metastasis.
Statistical analyses were performed with the statistical software package IBM SPSS Statistics for Windows, version 22.0 (IBM Corp., Armonk, NY, United States). For dichotomous data, frequencies are presented. Continuous data are presented as mean and range. Values are compared by the chi2-squared test. All tests of significance were two-tailed: P values of < 0.05 were considered to be significant.
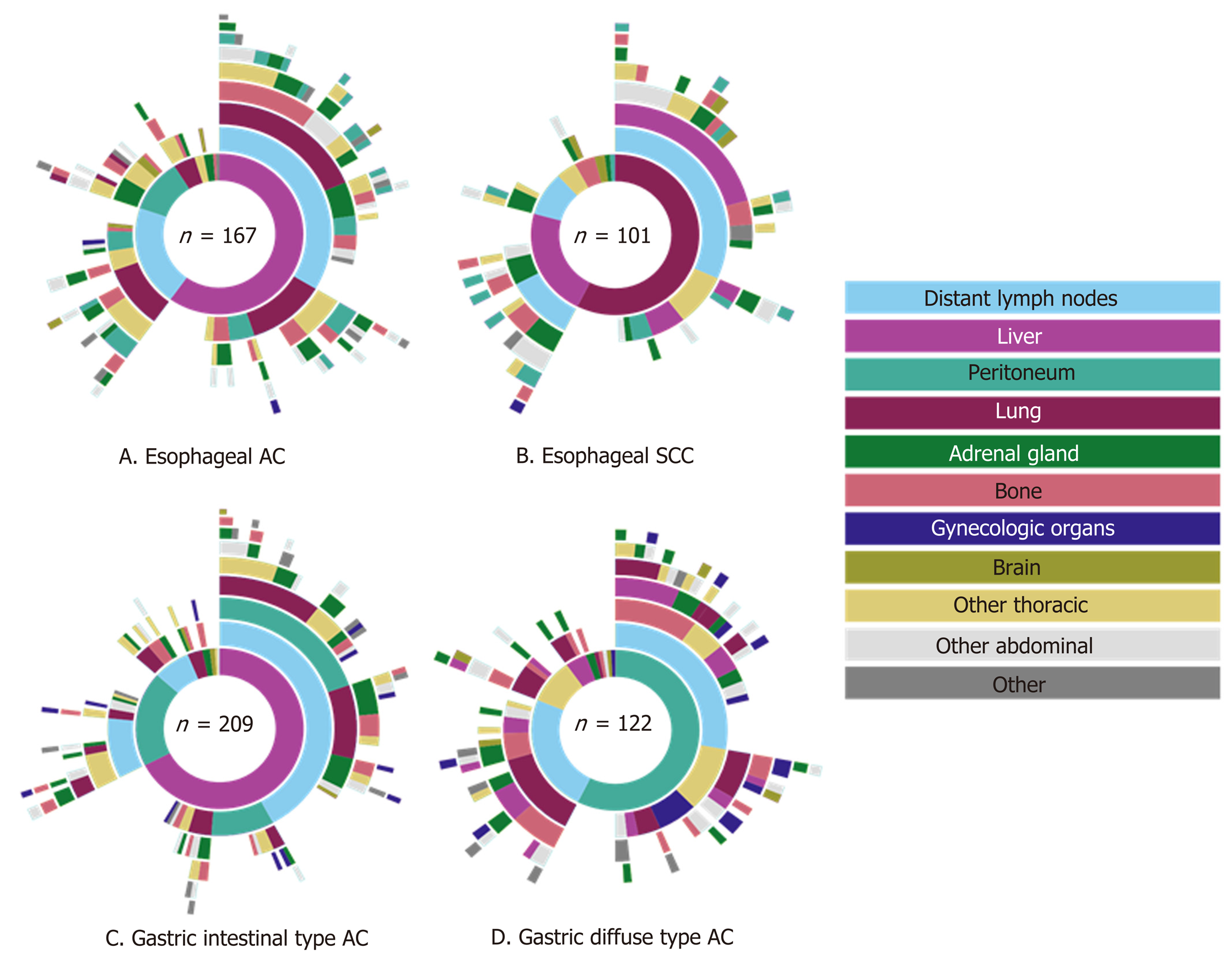
A total of 3876 autopsy records were initially retrieved in this study: Esophageal cancer was diagnosed in 1686 cases, gastric cancer in 2190 cases. We excluded 3277 patients from further analysis, since they did not have metastatic disease (n = 2919), tumor type was not specified (n = 7) or patients did not have a primary gastroesophageal carcinoma (n = 351). The remaining 599 patients were included in our analysis; 268 (45%) patients had primary esophageal and 331 (55%) gastric carcinoma. Two hundred four patients (34.1%) underwent a resection. The mean age of patients at diagnosis was 66 years (range 25-94 years) and age of patients at death was 67 years (range 25-94 years). In only 28 (5%) cases the neurocranium was examined. The mean number of metastatic locations per patient was 3 (SD 1.86) and 21% of the patients had only one metastasis at the time of death.
Of the 268 esophageal cancer patients with metastatic disease, 62% had AC (167 cases) and 38% SCC (101 cases). AC was subdivided into intestinal-type (144 cases, 86%) and diffuse-type AC (23 cases, 14%). The 331 gastric adenocarcinoma patients were categorized as diffuse-type (including signet-ring cell carcinoma) in 37% (122 cases) or intestinal-type AC in 63% (209 cases). Clinicopathological data of metastatic esophageal and gastric cancer patients are presented in Table 1.
| Esophageal carcinoma | Gastric adenocarcinoma | |||
| AC | SCC | Intestinal-type AC | Diffuse-type AC | |
| n = 167 | n = 101 | n = 209 | n = 122 | |
| Sex | ||||
| Female | 31 (18.6%) | 32 (31.7%) | 54 (25.8%) | 50 (41.0%) |
| Men | 136 (81.4%) | 69 (68.3%) | 155 (74.2%) | 72 (59.0%) |
| Year of diagnosis | ||||
| 1987-1993 | 24 (14.4%) | 20 (19.8%) | 40 (19.1%) | 22 (18.0%) |
| 1994-1999 | 38 (22.8%) | 30 (29.7%) | 69 (33.0%) | 28 (23.0%) |
| 2000-2005 | 28 (16.8%) | 19 (18.8%) | 41 (19.6%) | 23 (18.9%) |
| 2006-2011 | 34 (20.4%) | 17 (16.8%) | 30 (14.4%) | 20 (16.4%) |
| 2011-2017 | 43 (25.7%) | 15 (14.9%) | 29 (13.9%) | 29 (23.8%) |
| Mean age at diagnosis (years) | 66 | 64 | 69 | 64 |
| Mean age at death (years) | 67 | 65 | 70 | 65 |
| Location of primary tumor | ||||
| Proximal | 1 (0.6%) | 12 (11.9%) | 73 (34.9%) | 29 (23.8%) |
| Mid | 2 (1.2%) | 10 (9.9%) | 25 (12.0%) | 20 (16.4%) |
| Distal | 132 (79.0%) | 42 (41.6%) | 43 (20.6%) | 18 (14.8%) |
| NOS | 32 (19.2%) | 37 (36.6%) | 68 (32.5%) | 44 (36.1%) |
| Linitis plastica | - | - | - | 11 (9.0%) |
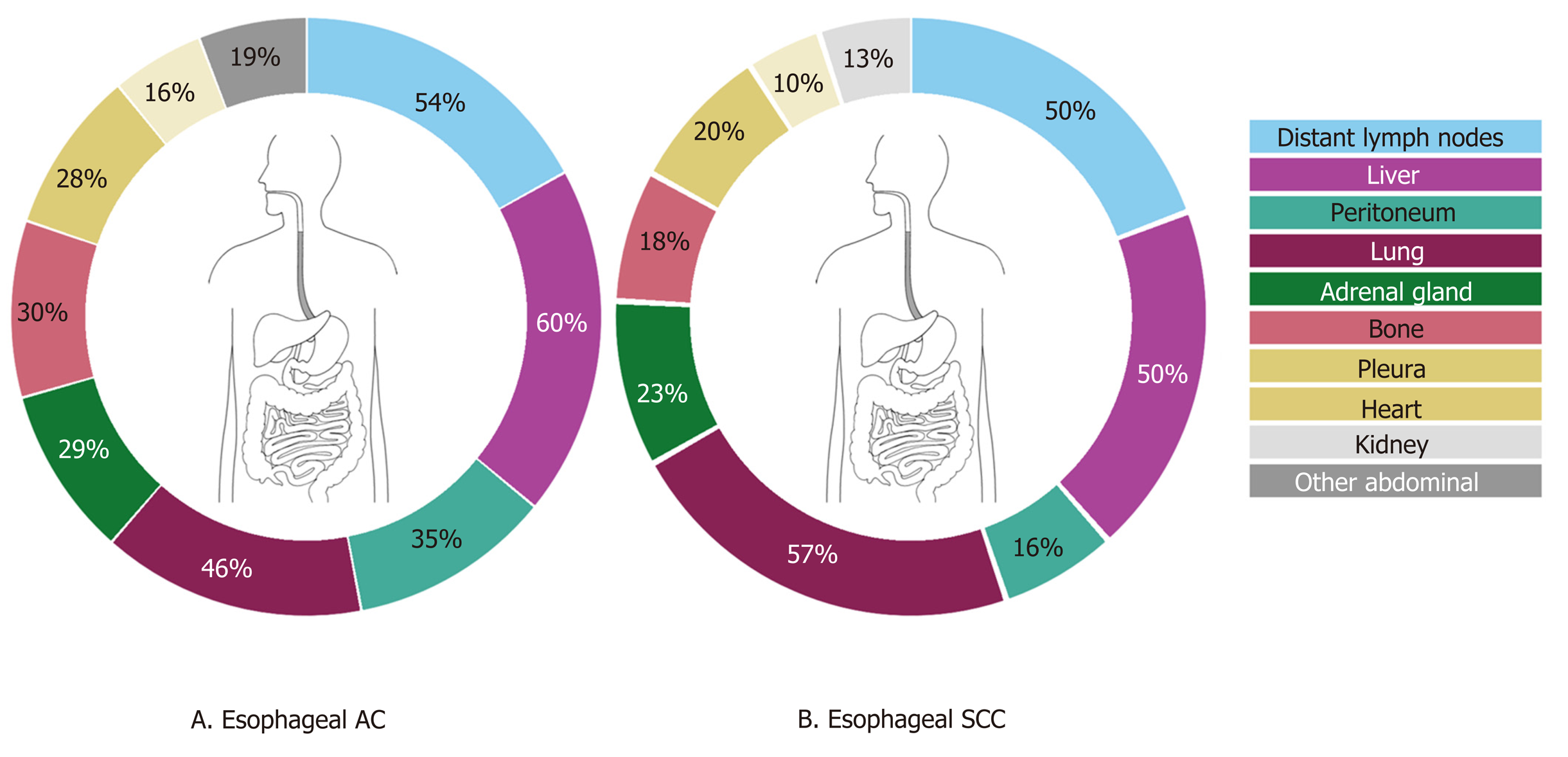
Of the 268 esophageal cancer patients, 54.5% had multiple (3 or more) metastases, 23.9% had 2 metastases and 21.6% had only one metastasis. Overall, esophageal cancer patients presented most frequently with liver, distant lymph node and lung metastases (56.0%, 52.6%, and 50.0% respectively) (Figure 1). Metastases to the liver were most common in AC (59.9%), whereas metastases to the lungs were most frequently seen in SCC (57.4%). There were three major differences in metastatic pattern between histological subtypes (Figure 2A and B). AC more frequently had metastases to the peritoneum and bone compared with SCC, 34.7% vs 15.8% (P < 0.01) and 29.9% vs 17.8% (P < 0.05), respectively. Lung metastases were observed more frequently in SCC (57.4%) compared with AC (45.5%, P = 0.059). Patients with a single metastatic location predominantly seem to have a liver metastasis in AC and a liver or lung metastasis in SCC esophageal cancer (Figure 2A and B).
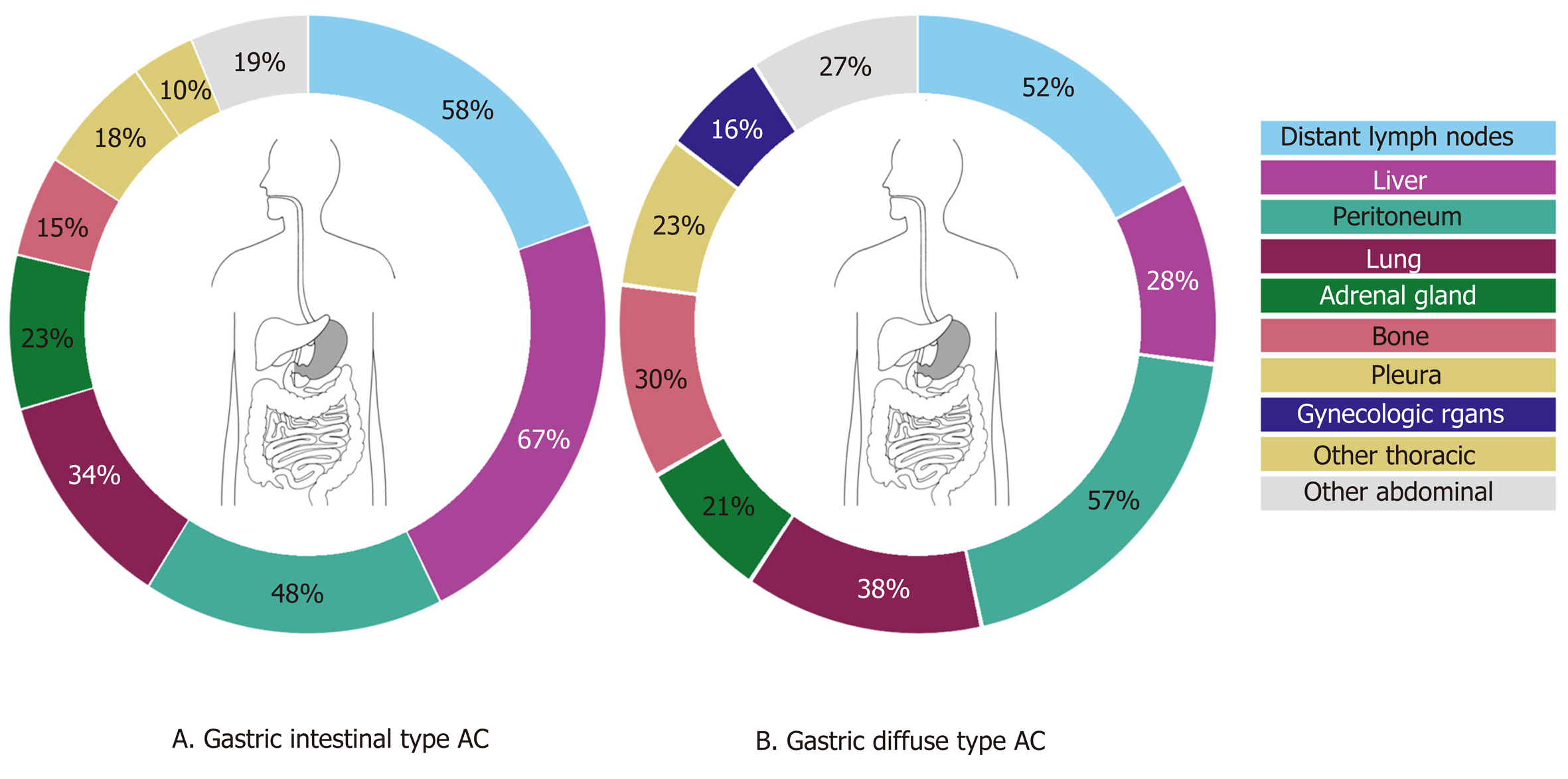
Of the gastric cancer patients, 54.1% had multiple (3 or more) metastases, 26.3% had 2 metastases and 19.6% had only one metastasis. Overall, gastric cancer patients presented most frequently with distant lymph node, liver and peritoneal metastases (55.9%, 52.9%, and 51.4% respectively) (Figure 3). Intestinal-type AC showed predominantly metastases to the liver (67.5%), whereas diffuse-type AC more likely present with metastases to the peritoneum (57.4%). Significant differences were found in the occurrence of metastases to the liver (67.5% in intestinal-type vs 27.9% in diffuse-type, P < 0.0001), bone (15.3% in intestinal-type vs 29.5% in diffuse-type, P < 0.01), female reproductive organs (5.7% in intestinal-type vs 16.4% in diffuse-type, P < 0.01) and colorectum (1.0% in intestinal-type vs 5.7% in diffuse-type, P < 0.05). In diffuse-type gastric cancer, patients with metastases to the female productive organs often were simultaneously diagnosed with metastases to the peritoneal cavity (Figure 2C and D).
Esophageal vs gastric carcinomas: For both esophageal (including GEJ) and gastric cancer (all histological types), the liver was the most frequent metastatic site (56.0% and 52.9%, respectively). Esophageal cancer was more likely to show metastases to the lung (50.0% vs 35.3%, P < 0.0001) and heart (13.4% vs 7.0%, P < 0.01). Metastases to the peritoneum (e.g. peritoneum, mesentery, omentum, abdominal cavity not further specified) (27.6% vs 51.4%, P < 0.0001), female reproductive organs (1.1% vs 9.7%, P < 0.0001) and the urinary bladder (0.4% vs 2.4%, P < 0.05) were seen more often in gastric cancer.
Proximal and mid esophageal carcinomas vs distal esophageal and GEJ carcinomas: A sub-analysis for the metastatic site of proximal and mid vs distal esophageal cancer (including GEJ carcinoma) was impeded due to the small number of upper esophageal carcinomas (n = 25). Upper esophageal carcinomas tend to show metastases to the spleen more often (3 out of 25 patients (12%) vs 2 out of 174 patients (1.1%), P < 0.01), while distal esophageal carcinomas more often showed metastases to the peritoneum (3 out of 25 patients (12%) vs 57 vs 174 patients (32.8%), P < 0.05).
Distal esophageal, GEJ and cardia carcinomas: Comparing distal esophageal, GEJ and cardia adenocarcinomas (intestinal- and diffuse-type) vs non-cardia gastric adenocarcinomas showed differences in the metastatic pattern. Metastases to the peritoneum and female reproductive organs were seen more frequently in non-cardia gastric cancer (56.3% vs 37.6%, P < 0.001 and 12.2% vs 2.1%, P < 0.001, respectively). Distal esophageal, GEJ and cardia carcinomas more often showed metastases to the liver (61.5% vs 47.2%, P < 0.05), pleura (27.8% vs 17.9%, P < 0.05) and heart (12.0% vs 6.1%, P < 0.05) compared with non-cardia gastric carcinomas. Lung metastases were more frequently found when comparing only distal esophageal and GEJ carcinomas with cardia adenocarcinomas (49.2% vs 36.2%, P < 0.05).
This nationwide autopsy study provides insight into the metastatic patterns in patients with esophageal or gastric cancer (n = 599). The two most common metastatic sites for esophageal and gastric cancer are liver and distant lymph nodes. Lung metastases are more frequently observed in patients with esophageal cancer while peritoneal metastases are more common in gastric cancer patients. Furthermore, differences in metastatic pattern according to primary tumor location and histological subtype were observed.
Overall, the most common metastatic sites of esophageal and gastric cancer found in this study are comparable to the literature[8-12]. Metastases to the liver are frequently observed in both esophageal and gastric cancer. The venous drainage of the distal esophagus is partly provided by the left gastric vein and by the gastroepiploic veins for the stomach which both drain directly into the portal vein. This may explain a high frequency of liver metastases in both groups. The other part of the venous drainage of the distal esophagus, as well as the mid and proximal part of the esophagus, is provided by the azygos vein which directly drains to the superior vena cava which probably explains the high frequency of lung metastases[14]. In addition to anatomical factors which promote specific spreading, organ specific tropism of circulating tumor cells as suggested by the seed and soil hypothesis[15] may also account for frequent metastases to the liver and lung. In esophageal and gastric cancer patients with metastatic disease, we surprisingly also observed frequent metastases in the adrenal gland (27% and 23%), heart (13% and 7%) and kidney (10% and 7%). In literature, metastases to the adrenal gland, heart and kidney are sparsely described. This can probably be explained by the fact that our study is based on autopsy cases, and these distant metastases might be later occurrences, that are also part of a more widespread disease[9,16-18]. Major differences in metastatic sites between histological subtypes were found. While esophageal AC had a predilection for peritoneal and bone metastases, SCC more often spread to the lungs, as is in line with previous findings[8,9]. Underlying mechanisms for differences in metastatic patterns between histological esophageal cancer subtypes are not clear, although the tumor location can be a confounding factor as ACs are generally located in the distal esophagus and SCC generally represent the more proximal tumors. For gastric intestinal adenocarcinoma, more metastases were found in the liver while diffuse-type gastric cancer spreads more often to bone, colorectum, peritoneum and female reproductive organs. The preference of signet-ring cell carcinoma for metastases to the female reproductive organs and peritoneum has been described before in colorectal cancer and may be organ specific tropism of circulating signet-ring cells[19]. Furthermore, previous research reported on the affinity of diffuse-type gastric cancer for peritoneal seeding[12,20]. However, subclassifying of adenocarcinomas into intestinal- and diffuse-type cancers may vary between pathologists since the subclassification of these carcinomas was ill-defined until very recently[21].
This study has limitations due to the retrospective nature of the study. Post-mortem studies offer a unique opportunity to examine the extent and location of metastases and can been seen as the gold standard in the study of cancer metastatic pattern. An autopsy study can lead to a biased population in which patients are included who have died postoperatively, had an unexpected clinical course, or died of other causes than esophageal or gastric cancer. However, previous studies confirmed the validity of data from autopsy studies in other cancer types where independent clinical trial and population-based cohorts showed identical patterns[19,22], illustrating that the bias is indeed limited. Unfortunately, the PALGA database does not include a broad spectrum of patient characteristics regarding, for example, comorbidities and demographics and therefore the exact external validity of this study is unclear. Another limitation is the problem of classifying cardia cancers, since the cardia is an ill-defined region of the stomach[23]. This may explain the differences in the metastatic pattern of proximal stomach (cardia) carcinomas in our cohort compared to literature[12].
Over the last decades, considerable improvements of diagnostic techniques in preoperative staging have been made[24]. Still, early recognition of metastases is necessary to avoid futile locoregional treatments. For example, diffuse-type gastric cancer has the tendency to spread to the peritoneal cavity, and therefore a diagnostic laparoscopy for the detection of peritoneal metastases is now common practice in the staging of gastric cancer[25]. Interestingly, this study shows that peritoneal metastases are also frequently observed in patients with metastatic distal esophageal and GEJ AC (38%). Few clinical studies do show the added value of a diagnostic laparoscopy for occult metastatic disease in distal esophageal and GEJ adenocarcinoma patients, whilst other studies report a relatively low rate of positive findings for routine laparoscopy in these patients. Further clinical studies are warranted to investigate if a pre-treatment diagnostic laparoscopy could also be of value for these patients[26,27]. Furthermore, knowledge of the preference location of metastases may help to develop individualized treatment strategies in case of metastatic disease such as Selective liver Internal Radiation therapy (SIRT) for liver metastases or adjuvant pressurized intraperitoneal aerosol chemotherapy or hyperthermic intraperitoneal chemotherapy for peritoneal metastases[28].
In conclusion, this autopsy study shows differences in the spread of distant metastases based on the primary tumor location and their histological subtypes in a large national cohort. These results should be taken into account during preoperative staging, during follow-up and in future research.
Patients with gastric or esophageal cancer have a high incidence of locoregional recurrence and distant metastases and therefore have limited survival. There are limited studies reporting on differences of the metastatic spreading according to the primary tumor location and histological subtype.
As upper gastrointestinal tract tumors are a heterogenous group, further improvement of survival probably lies within a more personalized treatment strategy. Also, early recognition of metastases is important to avoid futile locoregional treatments. Therefore, it is important to attain deeper knowledge on the different patterns of metastatic spreading and the factors that are instrumental in the determination of these patterns.
The aim of this study is to gain insight into the metastatic pattern of gastroesophageal cancer.
A nationwide retrospective autopsy study of patients with adenocarcinoma or squamous cell carcinoma (SCC) of the esophagus or stomach with metastases between 1990 and 2017 was performed. The metastatic pattern was analyzed according to the primary tumor location and histological subtype.
Metastatic disease was found in 268 esophageal and 331 gastric cancer patients that underwent an autopsy. In esophageal cancer, the most common metastatic locations were liver (56%), distant lymph nodes (53%) and lung (50%). Esophageal adenocarcinoma showed more frequently metastases to the peritoneum and bone compared with esophageal SCC. In gastric cancer, the most common metastatic locations were distant lymph nodes (56%), liver (53%) and peritoneum (51%). Intestinal-type adenocarcinoma of the stomach showed metastases to the liver more frequently, whereas metastases to the bone, female reproductive organs and colorectum were observed more frequently in diffuse-type gastric adenocarcinoma.
This autopsy study provides novel data on differences in the spread of distant metastases based on the primary tumor location and their histological subtypes in a large national autopsy cohort.
These results should be taken into account during preoperative staging, during follow-up and in future research.
| 1. | Torre LA, Siegel RL, Ward EM, Jemal A. Global Cancer Incidence and Mortality Rates and Trends--An Update. Cancer Epidemiol Biomarkers Prev. 2016;25:16-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2004] [Cited by in RCA: 2568] [Article Influence: 233.5] [Reference Citation Analysis (0)] |
| 2. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56651] [Article Influence: 7081.4] [Reference Citation Analysis (134)] |
| 3. | van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, Cuesta MA, Blaisse RJ, Busch OR, ten Kate FJ, Creemers GJ, Punt CJ, Plukker JT, Verheul HM, Spillenaar Bilgen EJ, van Dekken H, van der Sangen MJ, Rozema T, Biermann K, Beukema JC, Piet AH, van Rij CM, Reinders JG, Tilanus HW, van der Gaast A; CROSS Group. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074-2084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3288] [Cited by in RCA: 4253] [Article Influence: 303.8] [Reference Citation Analysis (3)] |
| 4. | Cats A, Jansen EPM, van Grieken NCT, Sikorska K, Lind P, Nordsmark M, Meershoek-Klein Kranenbarg E, Boot H, Trip AK, Swellengrebel HAM, van Laarhoven HWM, Putter H, van Sandick JW, van Berge Henegouwen MI, Hartgrink HH, van Tinteren H, van de Velde CJH, Verheij M; CRITICS investigators. Chemotherapy vs chemoradiotherapy after surgery and preoperative chemotherapy for resectable gastric cancer (CRITICS): an international, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19:616-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 397] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 5. | Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, Kopp HG, Mayer F, Haag GM, Luley K, Lindig U, Schmiegel W, Pohl M, Stoehlmacher J, Folprecht G, Probst S, Prasnikar N, Fischbach W, Mahlberg R, Trojan J, Koenigsmann M, Martens UM, Thuss-Patience P, Egger M, Block A, Heinemann V, Illerhaus G, Moehler M, Schenk M, Kullmann F, Behringer DM, Heike M, Pink D, Teschendorf C, Löhr C, Bernhard H, Schuch G, Rethwisch V, von Weikersthal LF, Hartmann JT, Kneba M, Daum S, Schulmann K, Weniger J, Belle S, Gaiser T, Oduncu FS, Güntner M, Hozaeel W, Reichart A, Jäger E, Kraus T, Mönig S, Bechstein WO, Schuler M, Schmalenberg H, Hofheinz RD; FLOT4-AIO Investigators. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel vs fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393:1948-1957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2086] [Cited by in RCA: 1812] [Article Influence: 258.9] [Reference Citation Analysis (0)] |
| 6. | Dikken JL, Lemmens VE, Wouters MW, Wijnhoven BP, Siersema PD, Nieuwenhuijzen GA, van Sandick JW, Cats A, Verheij M, Coebergh JW, van de Velde CJ. Increased incidence and survival for oesophageal cancer but not for gastric cardia cancer in the Netherlands. Eur J Cancer. 2012;48:1624-1632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 7. | Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1956] [Cited by in RCA: 2001] [Article Influence: 153.9] [Reference Citation Analysis (5)] |
| 8. | Ai D, Zhu H, Ren W, Chen Y, Liu Q, Deng J, Ye J, Fan J, Zhao K. Patterns of distant organ metastases in esophageal cancer: a population-based study. J Thorac Dis. 2017;9:3023-3030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 9. | Quint LE, Hepburn LM, Francis IR, Whyte RI, Orringer MB. Incidence and distribution of distant metastases from newly diagnosed esophageal carcinoma. Cancer. 1995;76:1120-1125. [PubMed] |
| 10. | Shiozaki H, Sudo K, Xiao L, Wadhwa R, Elimova E, Hofstetter WL, Skinner HD, Lee JH, Weston B, Bhutani MS, Blum MA, Maru DM, Ajani JA. Distribution and timing of distant metastasis after local therapy in a large cohort of patients with esophageal and esophagogastric junction cancer. Oncology. 2014;86:336-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Mori M, Sakaguchi H, Akazawa K, Tsuneyoshi M, Sueishi K, Sugimachi K. Correlation between metastatic site, histological type, and serum tumor markers of gastric carcinoma. Hum Pathol. 1995;26:504-508. [PubMed] |
| 12. | Riihimäki M, Hemminki A, Sundquist K, Sundquist J, Hemminki K. Metastatic spread in patients with gastric cancer. Oncotarget. 2016;7:52307-52316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 324] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 13. | Casparie M, Tiebosch AT, Burger G, Blauwgeers H, van de Pol A, van Krieken JH, Meijer GA. Pathology databanking and biobanking in The Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cell Oncol. 2007;29:19-24. [PubMed] |
| 14. | Viadana E, Bross ID, Pickren JW. The metastatic spread of cancers of the digestive system in man. Oncology. 1978;35:114-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 46] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8:98-101. [PubMed] |
| 16. | Grise P, Botto H, Camey M. Esophageal cancer metastatic to kidney: report of 2 cases. J Urol. 1987;137:274-276. [PubMed] |
| 17. | Nakahashi C, Kinoshita T, Konishi M, Nakagohri T, Inoue K, Oda T, Yoshida J, Hasebe T, Ochiai A. Long-term survival achieved by repeated resections of metachronous pulmonary and adrenal metastases of alpha-fetoprotein-producing gastric cancer: report of a case. Surg Today. 2004;34:784-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Shaheen O, Ghibour A, Alsaid B. Esophageal Cancer Metastases to Unexpected Sites: A Systematic Review. Gastroenterol Res Pract. 2017;2017:1657310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 19. | Hugen N, van de Velde CJH, de Wilt JHW, Nagtegaal ID. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Ann Oncol. 2014;25:651-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 369] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 20. | Honoré C, Goéré D, Messager M, Souadka A, Dumont F, Piessen G, Elias D, Mariette C; FREGAT Working Group – FRENCH. Risk factors of peritoneal recurrence in eso-gastric signet ring cell adenocarcinoma: results of a multicentre retrospective study. Eur J Surg Oncol. 2013;39:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Mariette C, Carneiro F, Grabsch HI, van der Post RS, Allum W, de Manzoni G; European Chapter of International Gastric Cancer Association. Consensus on the pathological definition and classification of poorly cohesive gastric carcinoma. Gastric Cancer. 2019;22:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 151] [Article Influence: 21.6] [Reference Citation Analysis (1)] |
| 22. | Knijn N, van Erning FN, Overbeek LI, Punt CJ, Lemmens VE, Hugen N, Nagtegaal ID. Limited effect of lymph node status on the metastatic pattern in colorectal cancer. Oncotarget. 2016;7:31699-31707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | von Rahden BH, Feith M, Stein HJ. Carcinoma of the cardia: classification as esophageal or gastric cancer? Int J Colorectal Dis. 2005;20:89-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Rubenstein JH, Shaheen NJ. Epidemiology, Diagnosis, and Management of Esophageal Adenocarcinoma. Gastroenterology 2015; 149: 302-17. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 272] [Article Influence: 24.7] [Reference Citation Analysis (1)] |
| 25. | Hori Y; SAGES Guidelines Committee. Diagnostic laparoscopy guidelines : This guideline was prepared by the SAGES Guidelines Committee and reviewed and approved by the Board of Governors of the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES), November 2007. Surg Endosc. 2008;22:1353-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Yoon HH, Lowe VJ, Cassivi SD, Romero Y. The role of FDG-PET and staging laparoscopy in the management of patients with cancer of the esophagus or gastroesophageal junction. Gastroenterol Clin North Am. 2009;38:105-120, ix. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Mehta K, Bianco V, Awais O, Luketich JD, Pennathur A. Minimally invasive staging of esophageal cancer. Ann Cardiothorac Surg. 2017;6:110-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Koemans WJ, van der Kaaij RT, Boot H, Buffart T, Veenhof AAFA, Hartemink KJ, Grootscholten C, Snaebjornsson P, Retel VP, van Tinteren H, Vanhoutvin S, van der Noort V, Houwink A, Hahn C, Huitema ADR, Lahaye M, Los M, van den Barselaar P, Imhof O, Aalbers A, van Dam GM, van Etten B, Wijnhoven BPL, Luyer MDP, Boerma D, van Sandick JW. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy vs palliative systemic chemotherapy in stomach cancer patients with peritoneal dissemination, the study protocol of a multicentre randomised controlled trial (PERISCOPE II). BMC Cancer. 2019;19:420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Pathology
Country/Territory of origin: Netherlands
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ilson DH S-Editor: Wang DM L-Editor: A P-Editor: Zhang YL