Published online Sep 14, 2020. doi: 10.3748/wjg.v26.i34.5130
Peer-review started: April 24, 2020
First decision: May 15, 2020
Revised: May 19, 2020
Accepted: August 20, 2020
Article in press: August 20, 2020
Published online: September 14, 2020
Processing time: 138 Days and 4 Hours
Reliable biomarkers of cirrhosis, hepatocellular carcinoma (HCC), or progression of chronic liver diseases are missing. In this context, Golgi protein-73 (GP73) also called Golgi phosphoprotein-2, was originally defined as a resident Golgi type II transmembrane protein expressed in epithelial cells. As a result, GP73 expression was found primarily in biliary epithelial cells, with only slight detection in hepatocytes. However, in patients with acute or chronic liver diseases and especially in HCC, the expression of GP73 is significantly up-regulated in hepatocytes. So far, few studies have assessed GP73 as a diagnostic or prognostic marker of liver fibrosis and disease progression.
To assess serum GP73 efficacy as a diagnostic marker of cirrhosis and/or HCC or as predictor of liver disease progression.
GP73 serum levels were retrospectively determined by a novel GP73 ELISA (QUANTA Lite® GP73, Inova Diagnostics, Inc., Research Use Only) in a large cohort of 632 consecutive patients with chronic viral and non-viral liver diseases collected from two tertiary Academic centers in Larissa, Greece (n = 366) and Debrecen, Hungary (n = 266). Aspartate aminotransferase (AST)/Platelets (PLT) ratio index (APRI) was also calculated at the relevant time points in all patients. Two hundred and three patients had chronic hepatitis B, 183 chronic hepatitis C, 198 alcoholic liver disease, 28 autoimmune cholestatic liver diseases, 15 autoimmune hepatitis, and 5 with other liver-related disorders. The duration of follow-up was 50 (57) mo [median (interquartile range)]. The development of cirrhosis, liver decompensation and/or HCC during follow-up were assessed according to internationally accepted guidelines. In particular, the surveillance for the development of HCC was performed regularly with ultrasound imaging and alpha-fetoprotein (AFP) determination every 6 mo in cirrhotic and every 12 mo in non-cirrhotic patients.
Increased serum levels of GP73 (> 20 units) were detected at initial evaluation in 277 out of 632 patients (43.8%). GP73-seropositivity correlated at baseline with the presence of cirrhosis (96.4% vs 51.5%, P < 0.001), decompensation of cirrhosis (60.3% vs 35.5%, P < 0.001), presence of HCC (18.4% vs 7.9%, P < 0.001) and advanced HCC stage (52.9% vs 14.8%, P = 0.002). GP73 had higher diagnostic accuracy for the presence of cirrhosis compared to APRI score [Area under the curve (AUC) (95%CI): 0.909 (0.885-0.934) vs 0.849 (0.813-0.886), P = 0.003]. Combination of GP73 with APRI improved further the accuracy (AUC: 0.925) compared to GP73 (AUC: 0.909, P = 0.005) or APRI alone (AUC: 0.849, P < 0.001). GP73 levels were significantly higher in HCC patients compared to non-HCC [22.5 (29.2) vs 16 (20.3) units, P < 0.001) and positively associated with BCLC stage [stage 0: 13.9 (10.8); stage A: 17.1 (16.8); stage B: 19.6 (22.3); stage C: 32.2 (30.8); stage D: 45.3 (86.6) units, P < 0.001] and tumor dimensions [very early: 13.9 (10.8); intermediate: 19.6 (18.4); advanced: 29.1 (33.6) units, P = 0.004]. However, the discriminative ability for HCC diagnosis was relatively low [AUC (95%CI): 0.623 (0.570-0.675)]. Kaplan-Meier analysis showed that the detection of GP73 in patients with compensated cirrhosis at baseline, was prognostic of higher rates of decompensation (P = 0.036), HCC development (P = 0.08), and liver-related deaths (P < 0.001) during follow-up.
GP73 alone appears efficient for detecting cirrhosis and superior to APRI determination. In combination with APRI, its diagnostic performance can be further improved. Most importantly, the simple GP73 measurement proved promising for predicting a worse outcome of patients with both viral and non-viral chronic liver diseases.
Core Tip: This retrospective study evaluated the utility of serum Golgi protein-73 (GP73) determination as a diagnostic marker of cirrhosis and/or hepatocellular carcinoma (HCC) or as a predictor of liver disease progression in a large cohort of 632 consecutive patients suffering from diverse liver diseases diagnosed and followed in two tertiary care hospitals in Greece and Hungary. We found that GP73 was quite efficient for detecting cirrhosis and superior to Aspartate aminotransferase (AST)/Platelets (PLT) ratio index (APRI) determination. In combination with APRI score, its diagnostic performance can be further improved. Most importantly, however, the simple GP73 measurement proved promising for predicting a worse outcome during follow-up in patients with both viral and non-viral chronic liver diseases.
- Citation: Gatselis NK, Tornai T, Shums Z, Zachou K, Saitis A, Gabeta S, Albesa R, Norman GL, Papp M, Dalekos GN. Golgi protein-73: A biomarker for assessing cirrhosis and prognosis of liver disease patients. World J Gastroenterol 2020; 26(34): 5130-5145
- URL: https://www.wjgnet.com/1007-9327/full/v26/i34/5130.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i34.5130
Hepatocellular carcinoma (HCC) is the second leading cause of cancer-associated mortality and the fifth most common cancer globally. However, its early detection is difficult as confounding factors such as the coexistence of inflammation and cirrhosis, may lead to limited predictive values of the existing serological and radiological methods of surveillance[1]. Since the 1970s, alpha-fetoprotein (AFP) has been widely applied as a primary biomarker for HCC screening in cirrhotics. However, its value as a primary screening tool for HCC has been questioned due to its low sensitivity and specificity, especially for early HCC detection[1]. Therefore, novel serum biomarkers for assessing cirrhosis and HCC risk with higher diagnostic accuracy and reasonable costs are still urgently required[2,3].
In this context, Golgi protein-73 (GP73), also called Golgi phosphoprotein-2 (GOLPH2), was originally defined as a resident Golgi type II transmembrane protein expressed in epithelial cells[4]. The N-terminal region of GP73 contains a transmembrane domain that can be cleaved by a proprotein convertase, resulting in the release of GP73 into the extracellular space, which may then be detected in serum and utilized as a novel biomarker[5]. In normal human liver, GP73 expression was found primarily in biliary epithelial cells, with only slight detection in hepatocytes. However, in patients with acute or chronic liver diseases and especially in HCC, the expression of GP73 was significantly up-regulated in hepatocytes[4,5].
So far, there have been several studies examining serum GP73 as a tumor marker for HCC with conflicting results[6-9]. Moreover, few studies have assessed GP73 as a diagnostic marker for liver fibrosis[4,10-12] or as a prognostic factor of disease progression from chronic hepatitis to cirrhosis and subsequent development of liver decompensation and/or HCC development[8,10,13]. Therefore, we designed the present study in order to specifically assess the efficacy of GP73 as a potential diagnostic biomarker for detecting liver cirrhosis and HCC development, and also to evaluate its performance for predicting the progression of chronic liver disease by investigating a large cohort of 632 consecutive patients with chronic viral and non-viral liver diseases collected from two Academic centers in Greece and Hungary.
Stored serum samples at -20 oC from 632 consecutive Greek and Hungarian patients with chronic liver diseases who were prospectively diagnosed and followed at the Department of Medicine and Research Laboratory of Internal Medicine, National Expertise Center of Greece in Autoimmune Liver Diseases, General University Hospital of Larissa, Larissa, Greece (n = 366) and the Department of Internal Medicine, Division of Gastroenterology, University of Debrecen, Debrecen, Hungary (n = 266) were analyzed retrospectively at the time point of initial evaluation. Two hundred and three patients had chronic hepatitis B (CHB), 183 chronic hepatitis C (CHC), 198 alcoholic liver disease, 28 autoimmune cholestatic liver diseases [(26 primary biliary cholangitis (PBC) and 2 primary sclerosing cholangitis (PSC)], 15 autoimmune hepatitis (AIH), and 5 with other liver-related disorders. The baseline demographic, clinical, biochemical, and histological characteristics of the patients are shown in Table 1.
| Total cohort (n = 632) | |
| Greek/Hungarian, n (%) | 366/266 (57.9/42.1) |
| Age, median (IQR), yr | 56.5 (17) |
| Sex, male/female, n (%) | 376/256 (59.5/40.5) |
| CHB/CHC/Alcoholic/PBC+PSC/AIH/other, n | 203/183/198/28/15/5 |
| GP73, median (IQR), units, (ULN: 20 units) | 17.3 (20.4) |
| GP73, pos/neg, n (%) | 277/355 (43.8/56.2) |
| INR, median (IQR), (normal: 0.85-1.15) | 1.15 (0.33) |
| Platelets, median (IQR), x103/μL | 153 (114) |
| AST, median (IQR), (ULN: 40 U/L) | 50 (54) |
| ALT, median (IQR), (ULN: 40 U/L) | 40 (44) |
| γ-GT, median (IQR), (ULN: 37 U/L) | 62 (113) |
| ALP, median (IQR), (ULN: 104 U/L) | 95 (77) |
| Bilirubin, median (IQR), (ULN: 1.1 mg/dL) | 1.2 (1.7) |
| Albumin, median (IQR), (normal: 3.5-5.2 g/dL) | 3.9 (1.3) |
| AFP, median (IQR), (ULN: 10 ng/ml) | 4 (6.1) |
| Fibrosis METAVIR stage, (F0, F1/≥ F2, n (%) | 96/100 (49/51) |
| Cirrhosis, yes/no, n (%) | 450/182 (71.2/28.8) |
| Decompensation of cirrhosis, yes/no, n (%) | 226/224 (50.2/49.8) |
| HCC, yes/no, n (%) | 79/553 (12.5/87.5) |
| HCC BCLC stage, 0/A/B/C/D1, n (%) | 2/23/22/21/10 (2.6/29.5/28.2/26.9/12.8) |
| HCC BCLC stage, 0-A-B/C-D1, n (%) | 47/31 (60.3/39.7) |
| HCC dimensions (very early/intermediate/advanced) | 2/32/44 (2.6/41/56.4) |
| Follow-up ≥ 6 mo, yes/no, n (%) | 479/153 (75.8/24.2) |
| Duration of follow-up, median (IQR), mo | 50 (57) |
At baseline, 450 out of 632 patients (71.2%) had cirrhosis according to our previous published criteria[2,3,14-19]. Briefly, for patients without available biopsy, the diagnosis of cirrhosis was based on either ultrasonography (nodules in liver, spleen > 12 cm, portal vein > 16 mm), or elastography (liver stiffness > 12 kPa), or endoscopic findings of cirrhosis (varices, portal gastropathy), and/or clinical findings of decompensation (ascites, variceal bleeding, encephalopathy)[17-19]. HCC diagnosis was based on standard histological and/or radiological findings[1]. At initial evaluation, 226 out of 450 cirrhotic patients (50.2%) had already experienced at least one episode of decompensation in the past. Liver biopsy was available for 196 Greek patients and fibrosis was assessed using the METAVIR scoring system[20].
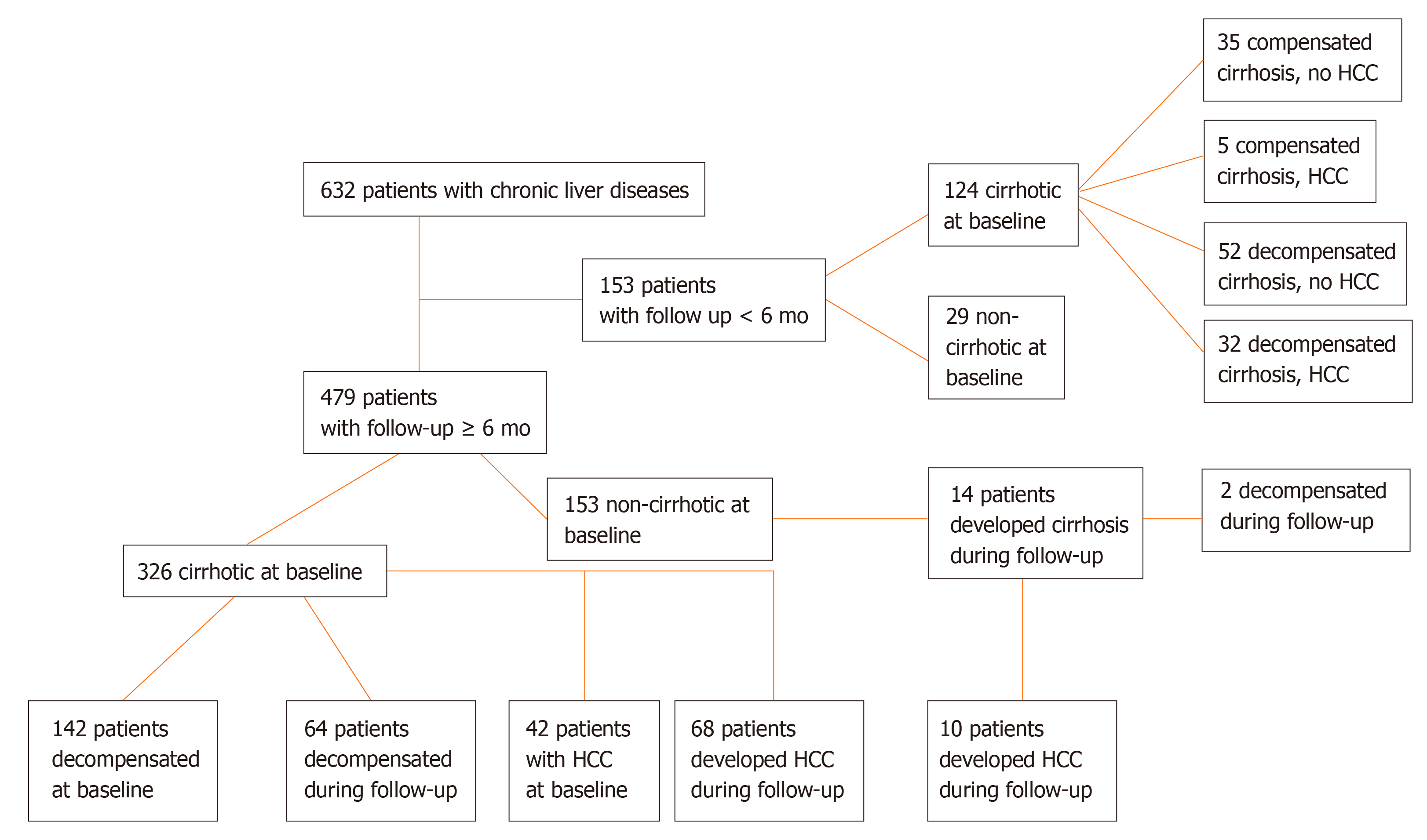
Four hundred and seventy-nine patients were followed ≥ 6 mo for a median [interquartile range (IQR)] 50 (57) mo (Figure 1). HCC surveillance was performed regularly with ultrasound imaging and AFP determination every 6 mo in cirrhotic and every 12 mo in non-cirrhotic patients. Development of cirrhosis, liver decompensation and HCC was assessed according to international criteria and in case of death, the cause was recorded[1,21]. Three hundred and twenty-six out of the 479 patients were cirrhotic at baseline, while 14 out of the remaining 153 non-cirrhotic patients developed cirrhosis during follow-up. One hundred and forty-two out of the 326 cirrhotic patients at baseline, had already experienced an event of decompensation prior to initial evaluation, while 66 out of 198 patients (patients with baseline cirrhosis n = 184 and patients who developed cirrhosis during follow-up n = 14) suffered from at least one episode of decompensation during follow-up for the first time (Figure 1).
In total, the diagnosis of HCC was established in 157 subjects; 79 were diagnosed at baseline and 78 during follow-up (Figure 1). Serum samples at the time of HCC diagnosis were available in 130 patients (79 at baseline and 51 at follow-up). Patients with available serum sample at the time of HCC diagnosis were classified according to the Barcelona Clinic Liver Cancer (BCLC) staging system as very early (stage 0, n = 8), early (stage A, n = 41), intermediate (stage B, n = 35), advanced (stage C, n = 34) or terminal stage (stage D, n = 11)[22]. They were also classified according to the tumor dimensions as very early (one single lesion < 2 cm in diameter, n = 8), intermediate (one single lesion 2-5 cm or less than three lesions with diameter < 3 cm each, n = 56), and advanced (n = 65). One patient could not be classified, as HCC diagnosis was established at autopsy. Paired samples for the estimation of GP73 alterations before and after the diagnosis of the tumor were available for 51 patients. Two hundred and twenty-two out of 632 patients (35.1%) died because of liver-related causes (65 patients during the first 6-mo from the initial presentation).
The ethical committees of the two Universities Hospitals approved the protocol which conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution's human research committee. Patients agreed to the use of their data and serum samples for anonymous retrospective analysis by written consent at the time of initial evaluation. In case patients were not able to give consent, a first-degree relative authorized to give consent.
GP73 antigen was measured using a novel GP73 ELISA (QUANTA Lite® GP73, Inova Diagnostics, Inc., Research Use Only) according to the manufacturer’s instructions. The assay was constructed using proprietary anti-GP73 monoclonal and rabbit polyclonal antibodies developed specifically against a highly purified proprietary full-length GP73 antigen engineered to maintain glycosylation and conformational integrity. Arbitrary units were calculated using a low positive control as a calibrator. In brief, samples were diluted 1:101 in proprietary diluent and incubated for 1 h at room temperature. Wells were aspirated and washed 3 times with wash buffer followed by addition of 100 uL of horseradish peroxidase conjugated rabbit anti-GP73 antibody. Samples were incubated for 30 min, aspirated, and 100 uL of tetramethyl-benzidine was then added. After 30 min of incubation the reaction was terminated by addition of 100 uL of stop solution and plates were then read at 450 nm. Arbitrary units were calculated using a low positive control as a calibrator. According to the manufacturer’s instructions, levels above 20 units were considered positive for GP73. This cut-off was established using a cohort of 518 healthy and disease controls (anti-phospholipid syndrome, n = 25; rheumatoid arthritis, n = 55; scleroderma, n = 25; inflammatory bowel disease, n = 10; breast cancer, n = 46; colorectal cancer, n = 50; prostate cancer, n = 45; non-cirrhotic CHC, n = 14; non-cirrhotic CHB, n = 25; human immunodeficiency virus infection, n = 8; syphilis, n = 40, healthy controls, n = 175) along with 719 cirrhotic and 254 HCC patients (Supplementary Figure 1).
Aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transpeptidase (γ-GT), alkaline phosphatase (ALP), bilirubin, albumin, platelets (PLT), international normalized ratio (INR), and AFP levels were determined using standard techniques. AST/PLT ratio index (APRI) was assessed at the relevant time points[23].
Normality of the distribution of variables was assessed by Kolmogorov-Smirnov test. Quantitative values are expressed as median (IQR). Data were analyzed by Mann-Whitney U, χ2, Kruskal-Wallis, Wilcoxon signed ranks, Spearman correlation and binary logistic regression analysis, where applicable. Significant interactions and multicollinearity between independent variables were examined using variance inflation factors. Logistic regression predicted value probability was used to calculate the combined effect of two diagnostic markers. Receiver operating characteristic (ROC) curves were constructed, and comparisons were done by the log-rank test. Youden indexes (sensitivity+specificity-1) were calculated to establish optimal cut-offs for GP73. Survival was evaluated with Kaplan-Meier plots for GP73-positive or GP73-negative patients up to the end of follow-up or when patients reached one of the end-points (development of cirrhosis, liver decompensation, development of HCC, liver-related death). Two-sided P values less than 0.05 were considered statistically significant. The statistical review of the study was performed by NKG and KZ who have MSc in biostatistics. All data analyses were performed using the statistical software SPSS version 20.0.
GP73-positive levels (> 20 units) were found in 277/632 (43.8%) patients. GP73-positivity was significantly related to older age (P < 0.001), increased AST (P < 0.001), γ-GT (P < 0.001), ALP (P < 0.001), INR (P < 0.001), bilirubin (P < 0.001), and lower PLT (P < 0.001) and albumin (P < 0.001) (Table 2). Moreover, GP73 positivity correlated with higher AFP (P < 0.001) and APRI score (P < 0.001), advanced fibrosis (P < 0.001), presence of cirrhosis (P < 0.001), liver decompensation (P < 0.001), HCC presence (P < 0.001) and advanced HCC stage (P = 0.002) (Table 2).
| GP73-positive (n = 277) | GP73-negative (n = 355) | P value | |
| Age, median (IQR), yr | 59 (15) | 55 (22) | < 0.001 |
| Sex, male/female, n (%) | 169/108 (61/39) | 207/148 (58.3/41.7) | 0.545 |
| INR, median (IQR) | 1.28 (0.36) | 1.09 (0.23) | < 0.001 |
| Platelets, median (IQR), x103/μL | 125 (99.3) | 180 (113.7) | < 0.001 |
| AST, median (IQR), U/L | 59 (67) | 40 (44) | < 0.001 |
| ALT, median (IQR), U/L | 43 (45) | 39 (43) | 0.127 |
| γ-GT, median (IQR), U/L | 105 (171) | 45 (79) | < 0.001 |
| ALP, median (IQR), U/L | 111 (80) | 85 (66) | < 0.001 |
| Bilirubin, median (IQR), mg/dL | 1.9 (3.1) | 0.9 (0.9) | < 0.001 |
| Albumin, median (IQR), g/dL | 3.4 (1.1) | 4.3 (0.9) | < 0.001 |
| AFP, median (IQR), ng/mL | 5 (14.4) | 3.5 (5) | < 0.001 |
| APRI score, median (IQR) | 1.4 (2.1) | 0.6 (1) | < 0.001 |
| Fibrosis stage (METAVIR) F0, F1 / ≥ F2, n (%) | 4/28 (12.5/87.5) | 92/72 (56.1/43.9) | < 0.001 |
| Cirrhosis, yes/no, n (%) | 267/10 (96.4/3.6) | 183/172 (51.5/48.5) | < 0.001 |
| Decompensation of cirrhosis, yes/no, n (%) | 161/106 (60.3/39.7) | 65/118 (35.5/64.5) | < 0.001 |
| HCC, yes/no, n (%) | 51/226 (18.4/81.6) | 28/327 (7.9/92.1) | < 0.001 |
| HCC BCLC stage, 0/A/B/C/D, n (%) | 1/12/11/18/9 (2/23.5/21.6 /35.3/17.6) | 1/11/11/3/11 (3.7/40.7/40.7 /11.1/3.7) | 0.029 |
| HCC BCLC stage, 0-A-B/ C-D, n (%) | 24/27 (47.1/52.9) | 23/41 (85.2/14.8) | 0.002 |
| HCC dimensions (very early/intermediate/advanced), n (%) | 1/19/31 (2/37.3/60.8) | 1/13/131 (3.7/48.1/48.1) | 0.543 |
Multivariate analysis revealed that GP73-positivity was independently associated with lower albumin (OR = 0.540, 95%CI: 0.375-0.778, P = 0.001) and presence of cirrhosis at baseline (OR = 7.608, 95%CI: 3.310-17.488; P < 0.001; Table 3). A second multivariate model including only cirrhotic patients, revealed that GP73-positivity was associated with decompensation of liver disease at baseline (OR = 1.812, 95%CI: 1.059-3.101; P = 0.030), but not to the presence of HCC (OR = 1.302, 95%CI: 0.687-2.467; P = 0.419; Table 4). No significant multicollinearity was found between the independent variables.
| Variables | Adjusted OR | 95%CI | Adjusted P value |
| Age | 0.990 | 0.971-1.010 | 0.336 |
| INR | 2.122 | 0.824-5.467 | 0.119 |
| Platelets | 1.000 | 1.000-1.000 | 0.160 |
| AST | 1.002 | 0.998-1.006 | 0.322 |
| γ-GT | 1.001 | 0.999-1.002 | 0.229 |
| ALP | 1.000 | 0.997-1.003 | 0.960 |
| Bilirubin | 0.999 | 0.926-1.078 | 0.978 |
| Albumin | 0.540 | 0.375-0.778 | 0.001 |
| AFP | 1.000 | 1.000-1.000 | 0.187 |
| Presence of cirrhosis | 7.608 | 3.310-17.488 | < 0.001 |
| Presence of HCC | 1.329 | 0.704-2.510 | 0.381 |
| Variables | Adjusted OR | 95%CI | Adjusted P value |
| Age | 0.996 | 0.975-1.018 | 0.733 |
| INR | 2.242 | 0.820-6.128 | 0.116 |
| Platelets | 1.000 | 1.000-1.000 | 0.719 |
| AST | 1.002 | 0.997-1.006 | 0.405 |
| γ-GT | 1.001 | 0.999-1.002 | 0.278 |
| ALP | 1.000 | 0.997-1.003 | 0.959 |
| Bilirubin | 1.002 | 0.929-1.081 | 1.002 |
| Albumin | 0.713 | 0.478-1.064 | 0.098 |
| AFP | 1.000 | 1.000-1.000 | 0.282 |
| Presence of decompensation | 1.812 | 1.059-3.101 | 0.030 |
| Presence of HCC | 1.302 | 0.687-2.467 | 0.419 |
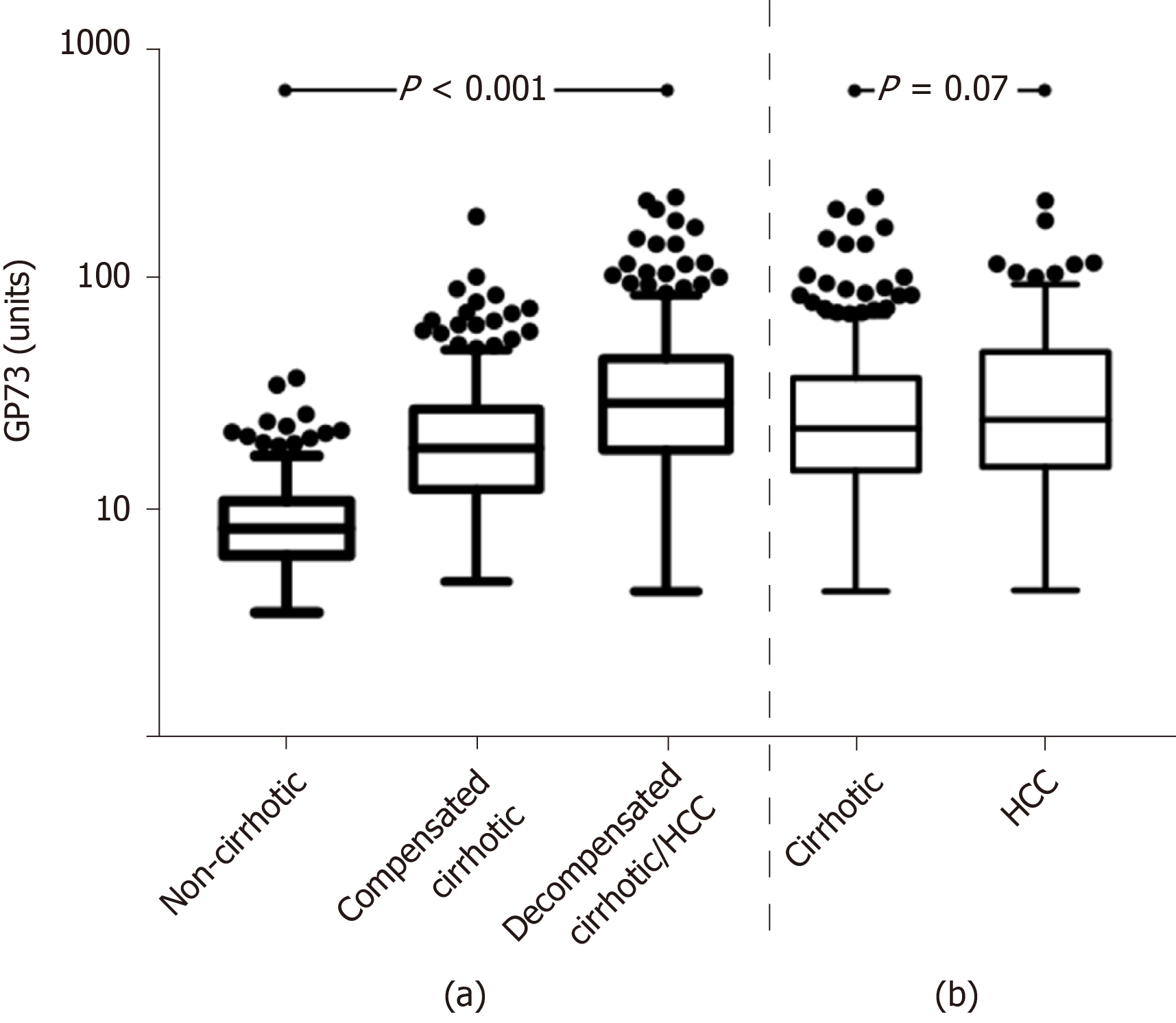
Of note, GP73 levels progressively increased from non-cirrhotic patients to patients with compensated cirrhosis and finally to patients with decompensated cirrhosis and/or HCC [8.4 (4.6) vs 18.7 (15.3) vs 29.5 (27.6) units; P < 0.001], as well as between the total number of cirrhotic patients (compensated and decompensated) and HCC patients [22.8 (22.9) vs 25 (33.7) units; P = 0.06] (Figure 2). This pattern remained constant among the different groups of liver diseases analyzed (Supplementary Figure 2).
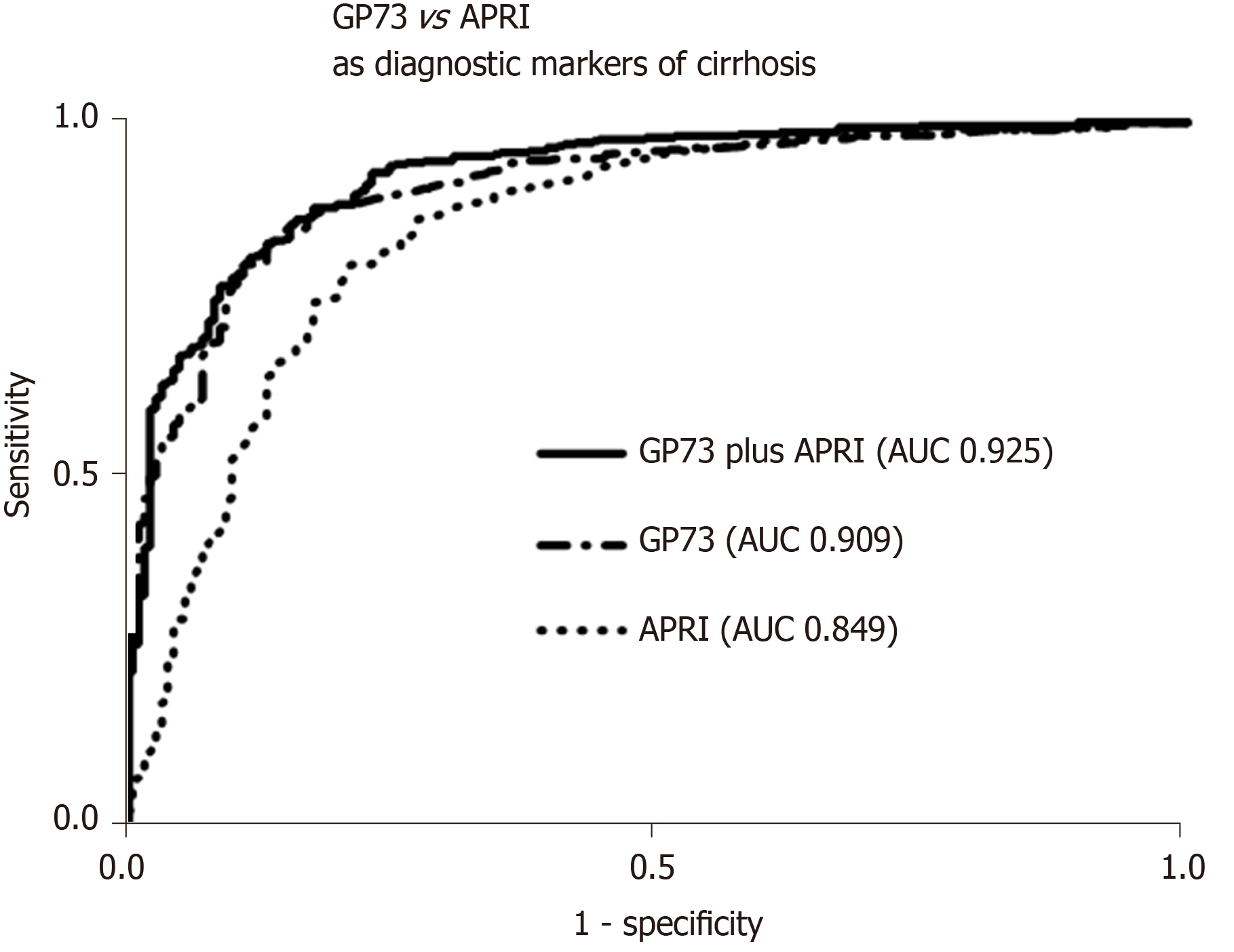
GP73 levels were significantly higher in cirrhotics than in non-cirrhotics [23.3 (23.9) vs 8.4 (4.6) units; P < 0.001]. GP73 had a high discriminative ability for detecting cirrhosis [AUC (95%CI): 0.909 (0.885-0.934)], which was superior to the APRI score [0.849 (0.813-0.886); P = 0.003]. The cut-off of 20 units had 59% sensitivity and 95% specificity for diagnosing cirrhosis (PPV: 96%, NPV: 48%). Youden index revealed an optimal cut-off for detecting cirrhosis at 12.7 units with sensitivity and specificity of 85% for both (PPV: 93%, NPV: 70%). The AUC (95%CI) of GP73 for detecting cirrhosis was 0.873 (0.838-0.908) for patients with viral liver diseases and 0.965 (0.929-1.000) for non-viral liver diseases. The AUC (95%) for the diagnosis of cirrhosis in a sub-analysis of patients with available liver biopsy (n = 196; F4 = 66; F3 = 34; F0-F2 = 96) was 0.850 (0.793-0.908) for GP73 and 0.806 (0.745-0.868) for APRI score (P = 0.276).
The combination of GP73 and APRI, further improved the diagnostic accuracy for detecting cirrhosis [AUC (95%): 0.925 (0.903-0.948)], compared to the respective AUCs of GP73 (P = 0.005) or APRI alone (P < 0.001; Figure 3). In a minority of 118 patients (64 with CHB, 46 CHC, 1 AIH, 7 PBC) with available liver stiffness measurements at the same time point with serum samples, a positive correlation was found between liver stiffness and GP73 values (r = 0.433; P < 0.001).
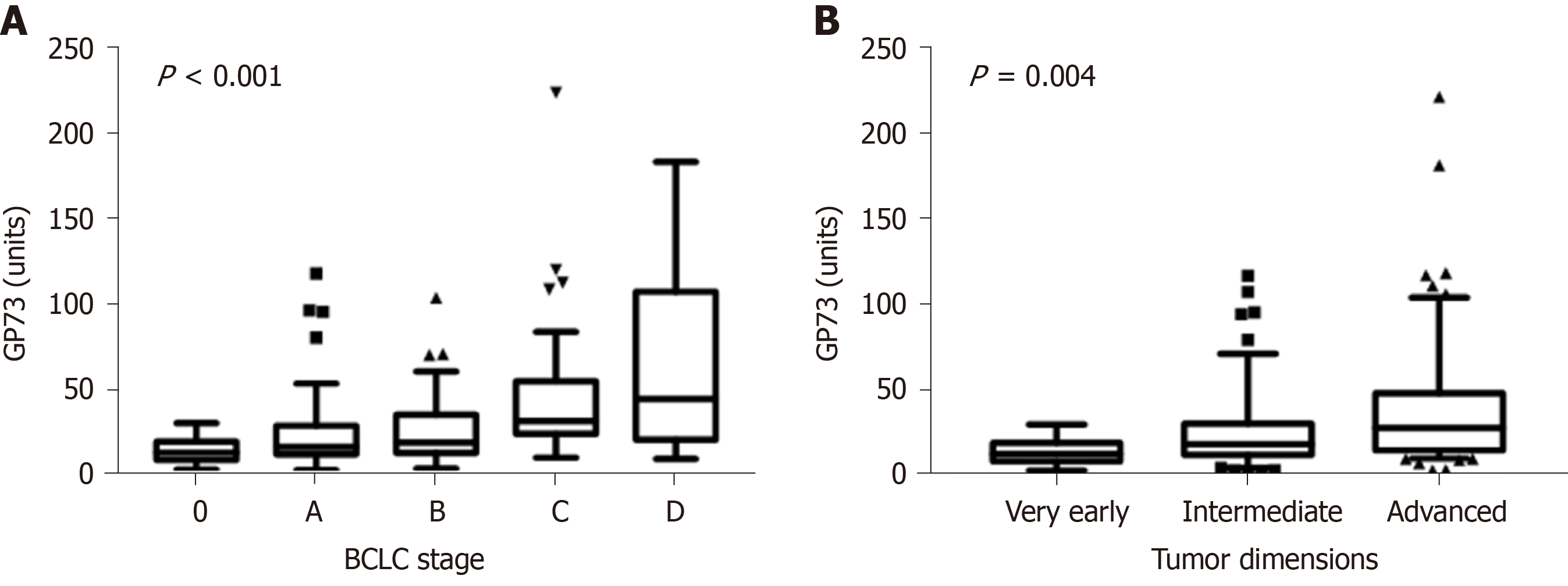
One hundred and thirty patients had available samples at HCC diagnosis (79 from patients who were diagnosed at presentation and 51 from those who were diagnosed at follow-up visits). GP73 levels were higher in HCC patients compared to non-HCC (n = 502) [22.5 (29.2) vs 16 (20.3) units; P < 0.001]. Furthermore, GP73 levels were positively associated with the Barcelona Clinic Liver Cancer (BCLC) stage (P < 0.001) and tumor dimensions (P = 0.004; Figure 4). HCC patients with underlying cirrhosis (n = 124) had also higher GP73 levels compared to HCC without cirrhosis (n = 6) [23.8 (29.9) vs 15.5 (16.2) units; P = 0.08].
However, the discriminative ability of GP73 for detecting HCC was relatively low [AUC (95%CI): 0.623 (0.570-0.675)] and significantly lower than that of AFP [AUC (95%CI): 0.767 (0.714-0.821); P = 0.004]. The cut-off of 20 units for GP73 had a sensitivity of 56% and specificity of 59% for diagnosing HCC, with a PPV of 26% and NPV of 83%. According to Youden Index, the optimal cut-off of GP73 for diagnosing HCC was calculated at 12.7 units with a sensitivity of 84% and specificity of 40% (PPV: 27% and NPV: 90%).
The combination of GP73 and AFP did not improve the diagnostic accuracy for detecting HCC in the total population [AUC (95%CI): 0.759 (0.709-0.808)] (Supplementary Figure 3). Superiority of AFP compared to GP73 for detecting HCC remained stable after exclusion of non-cirrhotic patients [AUC (95%CI): 0.738 (0.678-0.798) vs 0.500 (0.438-0.562); P < 0.001] and patients with advanced stages of HCC (BCLC stages B, C and D) [AUC (95%CI): 0.644 (0.544-0.744) vs 0.376 (0.284-0.468); P < 0.001]. No significant difference was found in GP73 levels before [17.3 (15.6) units] and after HCC development [17.5 (20.7) units] in the 51 patients with available paired samples during follow-up (median: 53; range 8-163 mo; Supplementary Figure 4).
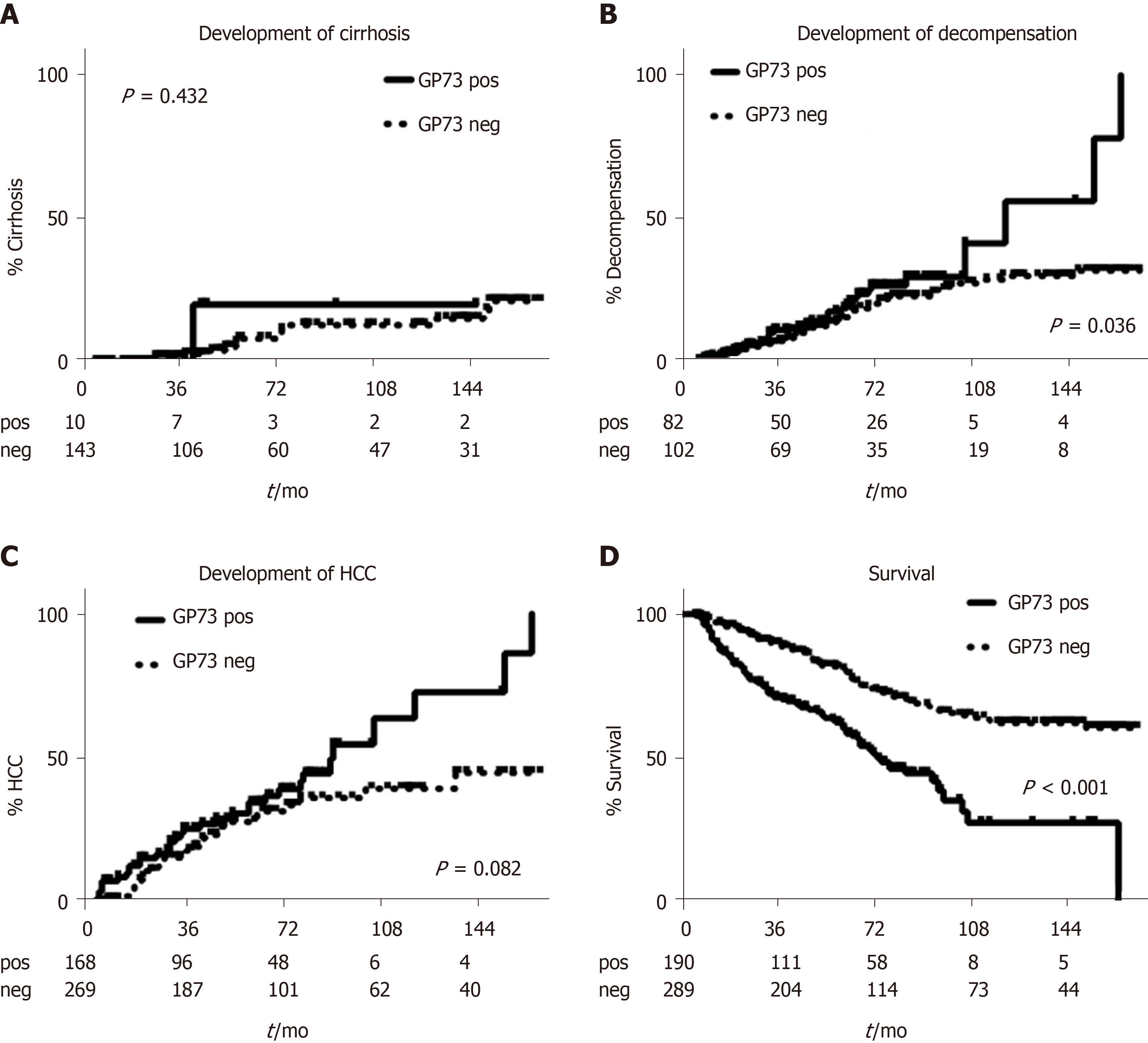
Kaplan-Meier analysis in patients with available follow-up ≥ 6 mo, showed no differences regarding the development of cirrhosis between GP73-positive and –negative patients (P = 0.432; Figure 5A). In contrast, GP73-positive patients with compensated cirrhosis at baseline and without any previous episode of decompensation had higher rates of liver decompensation (P = 0.036, Figure 5B) and HCC development (total group of patients; P = 0.08; Figure 5C). Of note, liver-related deaths were more common in GP73-positive patients (P < 0.001, Figure 5D) either in the subgroup with available long-term follow-up (≥ 6 mo), or in the group with follow-up of any duration (P < 0.001; data not shown).
In the current study, we showed that GP73-positivity is strongly associated with the presence of cirrhosis, while increased GP73 appear to identify patients at high risk of disease progression to liver decompensation, HCC development, and liver-related mortality. Importantly, multivariate analysis revealed that GP73-positivity was independently associated only with the presence of cirrhosis irrespective of the levels of aminotransferases. Furthermore, the ROC curves confirmed the high diagnostic accuracy of GP73 for detecting cirrhosis and its superiority to the APRI score. In addition, GP73 was associated with the presence of HCC, BCLC stage, and tumor dimensions, although its diagnostic accuracy for HCC was lower than that of AFP.
GP73 is a 73-kDa transmembrane glycoprotein that resides in epithelial cells of many human tissues, within the cis-Golgi complex[4,5]. Wright et al[24] described significant hepatocellular injury, including microvesicular hepatic steatosis, hepatocyte nuclear membrane irregularities and intranuclear inclusions in a transgenic mice model of GP73 deficiency, indicating that GP73 is essential for normal survival. Recent knock-down genetic studies in HepG2.2.15 cell lines provide further support as they proved that GP73 inhibition is related to a reduction of the inside surface area of the Golgi complex[25,26]. Additionally, previous reports showed that GP73 promotes HCC cell migration[27] and invasion[28], while GP73 N-glycosylation reduces HCC cell functions like motility and invasiveness[29,30], suggesting GP73 as a potential target of novel therapies for the inhibition of tumor proliferation and metastasis.
Our finding of a strong association of GP73-positivity with cirrhosis is in line with the initial manufacturer’s performance data which showed a sensitivity of 50.2% with a high specificity (96.9%) and also with previous studies which showed that GP73 is not overexpressed only in hepatocytes and liver cancer cells, but also in activated hepatic stellate cells which are the primary drivers of hepatic fibrosis[4,31,32]. In addition, previous studies have shown greater GP73 concentrations in cirrhosis rather than in HCC[33]. Our results provide strong support indicating that GP73 could be used as a tool to evaluate the presence of cirrhosis irrespective of the etiology of liver disease and very importantly, in contrast to other complex and more expensive models for staging fibrosis such as FibroMeter®, FibroTest® and HepaScore®[34], it is a single marker which can be rapidly determined by a simple ELISA, using equipment found in every clinical laboratory worldwide. Moreover, GP73 could also be combined with other novel "easy-to-use" markers of fibrosis like cartilage oligomeric matrix protein (COMP) already published by our group[2,3,35]. Indeed, in a subgroup of 288 patients with available measurements of both GP73 and COMP, the combination of GP73 with COMP had the higher discriminative ability to detect cirrhosis [AUC (95%CI): 0.881 (0.840-0.922)] compared to GP73 [AUC (95%CI): 0.846 (0.799-0.893)], APRI [AUC (95%CI): 0.834 (0.788-0.880) or COMP [AUC (95%CI): 0.767 (0.710-0.823)] alone, while the addition of APRI did not change the diagnostic performance of detecting cirrhosis [GP73 + COMP + APRI AUC (95%CI): 0.886 (0.846-0.926); Dalekos, Gatselis data in preparation].
Recently, Cao et al[11,12] found a gradual increase of GP73 through liver fibrosis stages and proposed that serum GP73 might be a complementary tool to transient elastography in an attempt to estimate significant fibrosis in CHB patients. In this context, a proposed algorithm including GP73 values and liver stiffness has been recently suggested to have better diagnostic accuracy than currently available approaches[12]. Our study verified a positive correlation between liver stiffness and GP73 values in a subset of patients with available measurements, although the retrospective nature of the study did not allow us for further statistical analysis due to the low number of patients with simultaneous elastography determinations and liver biopsy. Our findings of a gradual increase of GP73 levels from chronic hepatitis to cirrhosis and finally to decompensation of liver disease along with the predictive ability of GP73-positivity for a worse outcome, suggest that GP73 might be implicated in a central cellular pathway of liver diseases and not only in HCC development.
Indeed, recently Yao et al[36] found that GP73 can be induced by interleukin-6 through the STAT3 signal pathway, implicating a positive role of GP73 in liver regeneration and that enhanced GP73 expression may reflect the proliferative potential for hepatic parenchymal cells. In line with our findings, Xu et al[10] demonstrated that changes in serum GP73 levels were closely associated with changes in liver injury severity, and therefore, GP73 may be an effective biomarker for the evaluation of disease progression. Further support of the role of GP73 as an important promoter of HCC progression was just published by Wei et al[37], where they suggest that increased serum GP73 contributes to HCC development in a mouse model. The induction of GP73 expression in cell lines transfected by adenovirus, HBV and HCV, and a high-level of GP73 expression in a variety of acute and chronic liver diseases has also been described[4,29,32,38]. Sai et al[39] showed a shorter survival in HCC patients with higher GP73 levels than that of patients with low GP73 expression and proposed that an increased GP73 level in liver cancer cells might be associated with cell survival by protecting them from apoptosis. Furthermore, other studies have evaluated the utility of GP73 for monitoring the efficacy of several therapeutic procedures in HCC or disease recurrence such as, the radiofrequency ablation, surgical resection or transcatheter arterial chemoembolization[7,40,41]. Indeed, further research is needed to define the diagnostic value of GP73 in cirrhosis, including also patients with non-alcoholic fatty liver disease.
In our patients, we failed to detect any advantage of GP73 in HCC diagnosis over AFP, even when both markers were combined. Moreover, we did not manage to detect any benefit regarding the efficacy of GP73 at early detection of HCC, although GP73 levels were positively associated with BCLC stage and the tumor dimensions. Our findings are in accordance with a recent study from China which showed that AFP remained the best biomarker for the very early diagnosis of HBV-associated HCC, and the combination of one or more markers including GP73 did not significantly improve the diagnostic accuracy for HCC[42]. In this context, multiple studies have investigated the utility of GP73 for detecting HCC with conflicting results[13,33,43-47]. A recent meta-analysis reviewed the high heterogeneity of the studies with opposing or ambiguous results[9]. Of interest, Liu et al[48] demonstrated that serum GP73 increased in cirrhotic HCC patients, but not in those without cirrhosis, suggesting that the underlying cirrhosis, but not HCC per se, was related to the GP73 upregulation. Further support to this assumption raised from another study which showed that serum GP73 did not change after tumor resection in contrast to AFP levels[49]. In support of these findings, we failed to detect any difference before and after HCC development, while GP73 levels in our HCC patients were higher in cirrhotic HCC patients compared to non-cirrhotic. Furthermore, other studies found elevated GP73 levels or protein expression in liver tumor samples from patients with benign liver lesions such as, hepatocellular adenoma, focal nodal hyperplasia and hepatic cysts[7,8]. Taken together, these findings indicate that while GP73 is unlikely to be useful as a specific marker for HCC diagnosis, it may be of significant value to identify patients with cirrhosis and those potentially progressing towards HCC.
It is likely that technical issues contribute significantly to the conflicting results reported by various studies, as different detection methods and importantly, different antigens and monoclonal reagents have been used in various studies. Methodological approaches followed in each study may also differ, especially the patient inclusion criteria. Since our results suggest the importance of GP73 in disease progression, differences in the time of specimen collection in relation to the patient’s disease course may also impact the strength of the observed results. Importantly, many studies used healthy subjects as the control group, rather than patients with chronic hepatitis or liver cirrhosis. Discordances between studies may also be due to variant differentiation of HCC cells with different capacity to produce and secrete GP73 protein. In addition, different regulatory mechanisms of GP73 between benign and malignant liver diseases may be implicated[13]. Sun et al[6] showed that only tissue GP73 protein levels and not serum levels were positively associated with aggressive behaviour of HCC, while Wang et al[40] and Mao et al[7] found that serum levels of GP73 in HCC patients were not consistently influenced by tumor size and differentiation. On the other hand, Shan et al[13] found that both tissue and serum GP73 levels in HCC patients were lower than that in patients with cirrhosis and negatively associated with tumor size and tumor-node-metastasis stage, while tissue GP73 levels were positively correlated with tumor differentiation. In addition, they observed that serum GP73 levels contribute little to the prognosis of HCC, because the majority of HCC patients are already cirrhotic, resulting in simultaneous secretion of GP73 from both tumorous and cirrhotic tissue.
In conclusion, although GP73 does not appear to be a specific marker of HCC, its determination by a low cost, easy to perform, non-invasive blood test method that can be done in any clinical laboratory, appears to offer better diagnostic accuracy than the APRI score for detecting liver cirrhosis in patients with both viral and non-viral chronic liver diseases. Unlike APRI, GP73 is not affected by transaminase levels and very importantly, unlike transient elastography, the assay needs no specialized equipment. This is a critically important point as special equipment may not be available in regions where testing is most needed. Of note, GP73 in combination with APRI score further improved the diagnostic accuracy for diagnosing cirrhosis compared to GP73 or APRI alone. Most importantly however, GP73 proved efficient to predict a worse outcome of patients with chronic liver diseases as attested by the increased risk of progression to decompensated liver disease, HCC development and liver-related mortality. While our results, indicate a potential role of GP73 testing in patient management, future large-scale studies with prospectively collected serum samples will be needed to validate these findings.
Golgi protein-73 (GP73) is a resident Golgi type II transmembrane protein expressed in epithelial cells. Regarding liver, GP73 is expressed mainly in biliary epithelial cells, and to a lesser extent in hepatocytes. Nevertheless, its expression is significantly up-regulated in hepatocytes in patients with liver diseases and particularly in those with hepatocellular carcinoma (HCC).
Although a considerable number of studies with conflicting results have assessed the importance of GP73 in HCC patients, very few have investigated whether serum GP73 levels can be used as reliable diagnostic markers of liver fibrosis and disease progression from chronic hepatitis to cirrhosis, liver decompensation and HCC development.
To specifically evaluate GP73 efficacy as a biomarker for detecting cirrhosis and HCC development, and also to assess its performance in predicting liver disease progression.
Six hundred and thirty-two consecutive patients with chronic viral and non-viral liver diseases who were diagnosed, treated and followed for 50 (57) mo in two Academic centers in Greece (n = 366) and Hungary (n = 266) were retrospectively investigated for GP73 serum levels by a novel GP73 ELISA. APRI score was also determined in all patients. The development of cirrhosis, decompensation of liver disease and/or HCC development during follow-up were evaluated according to well-known international guidelines.
At baseline, 43.8% of patients had high GP73 levels (> 20 units) which were significantly associated with the presence of cirrhosis (P < 0.001), decompensated liver disease (P < 0.001), HCC presence (P < 0.001) and advanced stage of HCC (P = 0.002). Interestingly, GP73 had higher diagnostic accuracy for detecting cirrhosis [AUC, 95%CI: 0.909 (0.885-0.934)] compared to APRI (P = 0.003). Combination of GP73 with APRI improved further the accuracy compared to GP73 or APRI alone. GP73 was significantly higher in HCC patients compared to non-HCC (P < 0.001) and correlated positively with HCC stage (P < 0.001) and tumor dimensions (P = 0.004). However, the discriminative ability for detecting HCC was relatively low. Kaplan-Meier analysis showed that the group of patients with compensated cirrhosis at baseline having GP73 values above the cut-off, had significantly higher rates of decompensation, HCC development, and liver-related deaths compared to those with normal levels.
Although serum GP73 does not seem to be a specific marker for detecting HCC, we showed that its determination by an easy to perform, non-invasive blood test assay which can be performed in any laboratory, is efficient for detecting liver cirrhosis and superior to APRI score. Most importantly however, GP73 proved promising in predicting a worse outcome of patients with both viral and non-viral liver diseases.
As GP73 proved efficient to predict prognosis of patients with chronic liver diseases, GP73 testing by using a simple assay could be used in clinical practice as an additional and very important tool for the initial evaluation and follow-up of these patients. However, future large-scale multicenter studies with prospectively collected serum samples are needed in an attempt to definitely validate our findings.
| 1. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6413] [Article Influence: 801.6] [Reference Citation Analysis (9)] |
| 2. | Norman GL, Gatselis NK, Shums Z, Liaskos C, Bogdanos DP, Koukoulis GK, Dalekos GN. Cartilage oligomeric matrix protein: A novel non-invasive marker for assessing cirrhosis and risk of hepatocellular carcinoma. World J Hepatol. 2015;7:1875-1883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Zachou K, Gabeta S, Shums Z, Gatselis NK, Koukoulis GK, Norman GL, Dalekos GN. COMP serum levels: A new non-invasive biomarker of liver fibrosis in patients with chronic viral hepatitis. Eur J Intern Med. 2017;38:83-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Kladney RD, Bulla GA, Guo L, Mason AL, Tollefson AE, Simon DJ, Koutoubi Z, Fimmel CJ. GP73, a novel Golgi-localized protein upregulated by viral infection. Gene. 2000;249:53-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 212] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 5. | Kim HJ, Lv D, Zhang Y, Peng T, Ma X. Golgi phosphoprotein 2 in physiology and in diseases. Cell Biosci. 2012;2:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Sun Y, Yang H, Mao Y, Xu H, Zhang J, Li G, Lu X, Sang X, Zhao H, Zhong S, Huang J, Zhang H. Increased Golgi protein 73 expression in hepatocellular carcinoma tissue correlates with tumor aggression but not survival. J Gastroenterol Hepatol. 2011;26:1207-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Mao Y, Yang H, Xu H, Lu X, Sang X, Du S, Zhao H, Chen W, Xu Y, Chi T, Yang Z, Cai J, Li H, Chen J, Zhong S, Mohanti SR, Lopez-Soler R, Millis JM, Huang J, Zhang H. Golgi protein 73 (GOLPH2) is a valuable serum marker for hepatocellular carcinoma. Gut. 2010;59:1687-1693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 200] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 8. | Bröker ME, Ijzermans JN, Witjes CD, van Vuuren HJ, de Man RA. The predictive value of Golgi protein 73 in differentiating benign from malignant liver tumors. PLoS One. 2014;9:e100187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Dai M, Chen X, Liu X, Peng Z, Meng J, Dai S. Diagnostic Value of the Combination of Golgi Protein 73 and Alpha-Fetoprotein in Hepatocellular Carcinoma: A Meta-Analysis. PLoS One. 2015;10:e0140067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Xu Z, Pan X, Wei K, Ding H, Wei M, Yang H, Liu Q. Serum Golgi protein 73 levels and liver pathological grading in cases of chronic hepatitis B. Mol Med Rep. 2015;11:2644-2652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Cao Z, Li Z, Wang Y, Liu Y, Mo R, Ren P, Chen L, Lu J, Li H, Zhuang Y, Liu Y, Wang X, Zhao G, Tang W, Xiang X, Wang H, Cai W, Liu L, Zhu C, Bao S, Xie Q. Assessment of serum Golgi protein 73 as a biomarker for the diagnosis of significant fibrosis in patients with chronic HBV infection. J Viral Hepat. 2017;24 Suppl 1:57-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Cao Z, Li Z, Wang H, Liu Y, Xu Y, Mo R, Ren P, Chen L, Lu J, Li H, Zhuang Y, Liu Y, Wang X, Zhao G, Tang W, Xiang X, Cai W, Liu L, Bao S, Xie Q. Algorithm of Golgi protein 73 and liver stiffness accurately diagnoses significant fibrosis in chronic HBV infection. Liver Int. 2017;37:1612-1621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Shan SG, Gao YT, Xu YJ, Huang Y, Zhang Q, Zhai DK, Li JB, Wang FM, Jing X, Du Z, Wang YJ. Gradually increased Golgi protein 73 expression in the progression of benign liver diseases to precancerous lesions and hepatocellular carcinoma correlates with prognosis of patients. Hepatol Res. 2013;43:1199-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Gatselis NK, Georgiadou SP, Tassopoulos N, Zachou K, Liaskos C, Hatzakis A, Dalekos GN. Impact of parietal cell autoantibodies and non-organ-specific autoantibodies on the treatment outcome of patients with hepatitis C virus infection: a pilot study. World J Gastroenterol. 2005;11:482-487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Gatselis NK, Rigopoulou E, Stefos A, Kardasi M, Dalekos GN. Risk factors associated with HCV infection in semi-rural areas of central Greece. Eur J Intern Med. 2007;18:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Stefos A, Gatselis N, Zachou K, Rigopoulou E, Hadjichristodoulou C, Dalekos GN. Descriptive epidemiology of chronic hepatitis B by using data from a hepatitis registry in Central Greece. Eur J Intern Med. 2009;20:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Papatheodoridis GV, Manolakopoulos S, Touloumi G, Nikolopoulou G, Raptopoulou-Gigi M, Gogos C, Vafiadis-Zouboulis I, Karamanolis D, Chouta A, Ilias A, Drakoulis C, Mimidis K, Ketikoglou I, Manesis E, Mela M, Hatzis G, Dalekos GN; HepNet. Greece Study Group. Hepatocellular carcinoma risk in HBeAg-negative chronic hepatitis B patients with or without cirrhosis treated with entecavir: HepNet.Greece cohort. J Viral Hepat. 2015;22:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Papatheodoridis G, Dalekos G, Sypsa V, Yurdaydin C, Buti M, Goulis J, Calleja JL, Chi H, Manolakopoulos S, Mangia G, Gatselis N, Keskin O, Savvidou S, de la Revilla J, Hansen BE, Vlachogiannakos I, Galanis K, Idilman R, Colombo M, Esteban R, Janssen HL, Lampertico P. PAGE-B predicts the risk of developing hepatocellular carcinoma in Caucasians with chronic hepatitis B on 5-year antiviral therapy. J Hepatol. 2016;64:800-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 424] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 19. | Papatheodoridis GV, Sypsa V, Dalekos GN, Yurdaydin C, Van Boemmel F, Buti M, Calleja JL, Chi H, Goulis J, Manolakopoulos S, Loglio A, Voulgaris T, Gatselis N, Keskin O, Veelken R, Lopez-Gomez M, Hansen BE, Savvidou S, Kourikou A, Vlachogiannakos J, Galanis K, Idilman R, Esteban R, Janssen HLA, Berg T, Lampertico P. Hepatocellular carcinoma prediction beyond year 5 of oral therapy in a large cohort of Caucasian patients with chronic hepatitis B. J Hepatol. 2020;72:1088-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (1)] |
| 20. | Boozari B, Potthoff A, Mederacke I, Hahn A, Reising A, Rifai K, Wedemeyer H, Bahr M, Kubicka S, Manns M, Gebel M. Evaluation of sound speed for detection of liver fibrosis: prospective comparison with transient dynamic elastography and histology. J Ultrasound Med. 2010;29:1581-1588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3143] [Article Influence: 392.9] [Reference Citation Analysis (3)] |
| 22. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2915] [Article Influence: 108.0] [Reference Citation Analysis (1)] |
| 23. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2762] [Cited by in RCA: 3339] [Article Influence: 145.2] [Reference Citation Analysis (0)] |
| 24. | Wright LM, Yong S, Picken MM, Rockey D, Fimmel CJ. Decreased survival and hepato-renal pathology in mice with C-terminally truncated GP73 (GOLPH2). Int J Clin Exp Pathol. 2009;2:34-47. [PubMed] |
| 25. | Ba MC, Long H, Tang YQ, Cui SZ. GP73 expression and its significance in the diagnosis of hepatocellular carcinoma: a review. Int J Clin Exp Pathol. 2012;5:874-881. [PubMed] |
| 26. | Zhang YL, Zhang YC, Han W, Li YM, Wang GN, Yuan S, Wei FX, Wang JF, Jiang JJ, Zhang YW. Effect of GP73 silencing on proliferation and apoptosis in hepatocellular cancer. World J Gastroenterol. 2014;20:11287-11296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Chen X, Wang Y, Tao J, Shi Y, Gai X, Huang F, Ma Q, Zhou Z, Chen H, Zhang H, Liu Z, Sun Q, Peng H, Chen R, Jing Y, Yang H, Mao Y, Zhang H. mTORC1 Up-Regulates GP73 to Promote Proliferation and Migration of Hepatocellular Carcinoma Cells and Growth of Xenograft Tumors in Mice. Gastroenterology. 2015;149:741-52. e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 28. | Jin D, Tao J, Li D, Wang Y, Li L, Hu Z, Zhou Z, Chang X, Qu C, Zhang H. Golgi protein 73 activation of MMP-13 promotes hepatocellular carcinoma cell invasion. Oncotarget. 2015;6:33523-33533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Jiang K, Li W, Zhang Q, Yan G, Guo K, Zhang S, Liu Y. GP73 N-glycosylation at Asn144 reduces hepatocellular carcinoma cell motility and invasiveness. Oncotarget. 2016;7:23530-23541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Liu Y, Zhang X, Sun T, Jiang J, Li Y, Chen M, Wei Z, Jiang W, Zhou L. Knockdown of Golgi phosphoprotein 2 inhibits hepatocellular carcinoma cell proliferation and motility. Oncotarget. 2016;7:21404-21415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 31. | Kladney RD, Cui X, Bulla GA, Brunt EM, Fimmel CJ. Expression of GP73, a resident Golgi membrane protein, in viral and nonviral liver disease. Hepatology. 2002;35:1431-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 181] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 32. | Iftikhar R, Kladney RD, Havlioglu N, Schmitt-Gräff A, Gusmirovic I, Solomon H, Luxon BA, Bacon BR, Fimmel CJ. Disease- and cell-specific expression of GP73 in human liver disease. Am J Gastroenterol. 2004;99:1087-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 33. | Li B, Li B, Guo T, Sun Z, Li X, Li X, Chen L, Zhao J, Mao Y. Artificial neural network models for early diagnosis of hepatocellular carcinoma using serum levels of α-fetoprotein, α-fetoprotein-L3, des-γ-carboxy prothrombin, and Golgi protein 73. Oncotarget. 2017;8:80521-80530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Leroy V, Sturm N, Faure P, Trocme C, Marlu A, Hilleret MN, Morel F, Zarski JP. Prospective evaluation of FibroTest®, FibroMeter®, and HepaScore® for staging liver fibrosis in chronic hepatitis B: comparison with hepatitis C. J Hepatol. 2014;61:28-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 35. | Zachou K, Gabeta S, Gatselis NK, Norman GL, Dalekos GN. Cartilage oligomeric matrix protein on the spot for liver fibrosis evaluation: Too early or too late? Eur J Intern Med. 2017;43:e48-e49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 36. | Yao M, Wang L, Leung PSC, Li Y, Liu S, Wang L, Guo X, Zhou G, Yan Y, Guan G, Chen X, Bowlus CL, Liu T, Jia J, Gershwin ME, Ma X, Zhao J, Lu F. The Clinical Significance of GP73 in Immunologically Mediated Chronic Liver Diseases: Experimental Data and Literature Review. Clin Rev Allergy Immunol. 2018;54:282-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 37. | Wei C, Yang X, Liu N, Geng J, Tai Y, Sun Z, Mei G, Zhou P, Peng Y, Wang C, Zhang X, Zhang P, Geng Y, Wang Y, Zhang X, Liu X, Zhang Y, Wu F, He X, Zhong H. Tumor Microenvironment Regulation by the Endoplasmic Reticulum Stress Transmission Mediator Golgi Protein 73 in Mice. Hepatology. 2019;70:851-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 38. | Hu L, Yao W, Wang F, Rong X, Peng T. GP73 is upregulated by hepatitis C virus (HCV) infection and enhances HCV secretion. PLoS One. 2014;9:e90553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 39. | Sai W, Wang L, Zheng W, Yang J, Pan L, Cai Y, Qiu L, Zhang H, Wu W, Yao D. Abnormal Expression of Golgi Protein 73 in Clinical Values and Their Role in HBV-Related Hepatocellular Carcinoma Diagnosis and Prognosis. Hepat Mon. 2015;15:e32918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Wang NY, Wang C, Li W, Wang GJ, Cui GZ, He H, Zhao HJ. Prognostic value of serum AFP, AFP-L3, and GP73 in monitoring short-term treatment response and recurrence of hepatocellular carcinoma after radiofrequency ablation. Asian Pac J Cancer Prev. 2014;15:1539-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 41. | Ai N, Liu W, Li ZG, Ji H, Li B, Yang G. High expression of GP73 in primary hepatocellular carcinoma and its function in the assessment of transcatheter arterial chemoembolization. Oncol Lett. 2017;14:3953-3958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 42. | Wu M, Liu Z, Li X, Zhang A, Li N. Dynamic Changes in Serum Markers and Their Utility in the Early Diagnosis of All Stages of Hepatitis B-Associated Hepatocellular Carcinoma. Onco Targets Ther. 2020;13:827-840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 43. | Zhou Y, Yin X, Ying J, Zhang B. Golgi protein 73 versus alpha-fetoprotein as a biomarker for hepatocellular carcinoma: a diagnostic meta-analysis. BMC Cancer. 2012;12:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 44. | Witjes CD, van Aalten SM, Steyerberg EW, Borsboom GJ, de Man RA, Verhoef C, Ijzermans JN. Recently introduced biomarkers for screening of hepatocellular carcinoma: a systematic review and meta-analysis. Hepatol Int. 2013;7:59-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 45. | Gao G, Dong F, Xu X, Hu A, Hu Y. Diagnostic value of serum Golgi protein 73 for HBV-related primary hepatic carcinoma. Int J Clin Exp Pathol. 2015;8:11379-11385. [PubMed] |
| 46. | Jing JS, Ye W, Jiang YK, Ma J, Zhu MQ, Ma JM, Zhou H, Yu LQ, Yang YF, Wang SC. The Value of GPC3 and GP73 in Clinical Diagnosis of Hepatocellular Carcinoma. Clin Lab. 2017;63:1903-1909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 47. | Qiao Y, Chen J, Li X, Wei H, Xiao F, Chang L, Zhang R, Hao X, Wei H. Serum gp73 is also a biomarker for diagnosing cirrhosis in population with chronic HBV infection. Clin Biochem. 2014;47:216-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 48. | Liu T, Yao M, Liu S, Wang L, Wang L, Hou J, Ma X, Jia J, Zhao J, Zhuang H, Lu F. Serum Golgi protein 73 is not a suitable diagnostic marker for hepatocellular carcinoma. Oncotarget. 2017;8:16498-16506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 49. | Yamamoto K, Imamura H, Matsuyama Y, Kume Y, Ikeda H, Norman GL, Shums Z, Aoki T, Hasegawa K, Beck Y, Sugawara Y, Kokudo N. AFP, AFP-L3, DCP, and GP73 as markers for monitoring treatment response and recurrence and as surrogate markers of clinicopathological variables of HCC. J Gastroenterol. 2010;45:1272-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 160] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Norton PA S-Editor: Wang DM L-Editor: A P-Editor: Ma YJ