Published online Sep 14, 2020. doi: 10.3748/wjg.v26.i34.5146
Peer-review started: May 24, 2020
First decision: June 12, 2020
Revised: June 20, 2020
Accepted: August 26, 2020
Article in press: August 26, 2020
Published online: September 14, 2020
Processing time: 107 Days and 22 Hours
Endoscopy-based Kyoto classification for gastritis and pathological topographic distribution of neutrophil infiltration are correlated with gastric cancer risk.
To investigate the association between Kyoto classification and the topographic distribution of neutrophil activity.
Kyoto classification score, ranging from 0 to 8, consisted of atrophy, intestinal metaplasia, enlarged folds, nodularity, and diffuse redness. Neutrophil activity was scored according to the updated Sydney System using biopsy samples obtained from the greater curvature of the corpus and the antrum. The participants were divided into four categories, inactive stomach, antrum-predominant gastritis, pangastritis, and corpus-predominant gastritis, based on the topographic distribution of neutrophil activity. Effects of sex, age, body mass index, drinking habit, smoking habit, family history of gastric cancer, serum Helicobacter pylori (H. pylori) antibody, and Kyoto score on topography of neutrophil infiltration were analyzed.
A total of 327 patients (comprising 50.7% women, with an average age of 50.2 years) were enrolled in this study. H. pylori infection rate was 82.9% with a mean Kyoto score of 4.63. The Kyoto score was associated with the topographic distribution of neutrophil activity. Kyoto scores were significantly higher in the order of inactive stomach, antrum-predominant gastritis, pangastritis, and corpus-predominant gastritis (3.05, 4.57, 5.21, and 5.96, respectively). Each individual score of endoscopic findings (i.e., atrophy, intestinal metaplasia, enlarged folds, nodularity, and diffuse redness) was correlated with the topographic distribution of neutrophil activity. On multivariate analysis, the Kyoto score, age, and serum H. pylori antibody were independently associated with the topographic distribution of neutrophil activity.
The Kyoto classification score was associated with the topographic distribution of neutrophil activity.
Core Tip: We investigated the association between endoscopy-based Kyoto classification of gastritis and pathologically topographic distribution of neutrophil activity which related to gastric cancer risk. Kyoto classification score is consisted of atrophy, intestinal metaplasia, enlarged folds, nodularity, and diffuse redness. The subjects were divided into four categories, inactive stomach, antrum-predominant gastritis, pangastritis, and corpus-predominant gastritis, based on distribution of neutrophil activity. Kyoto scores were higher in the order of inactive stomach, antrum-predominant gastritis, pangastritis, and corpus-predominant gastritis. On multivariate analysis, Kyoto score was independently associated with topographic distribution of neutrophil activity. In conclusion, Kyoto classification score was associated with gastric cancer risk.
- Citation: Toyoshima O, Nishizawa T, Yoshida S, Sakaguchi Y, Nakai Y, Watanabe H, Suzuki H, Tanikawa C, Matsuda K, Koike K. Endoscopy-based Kyoto classification score of gastritis related to pathological topography of neutrophil activity. World J Gastroenterol 2020; 26(34): 5146-5155
- URL: https://www.wjgnet.com/1007-9327/full/v26/i34/5146.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i34.5146
Stomach cancer is the third leading cause of cancer-related mortality in both sexes worldwide according to the 2018 GLOBOCAN estimates[1]. Thus, the key to obtaining a significant effect on the prognosis of gastric adenocarcinoma and its economic burden is to accurately identify at-risk individuals[2-5].
The updated Sydney System is the most widely accepted method for the histological classification and grading of gastritis[6-8]; it can also assess pathologic features related to Helicobacter pylori (H. pylori) infection such as neutrophil activity, chronic inflammation, atrophy, intestinal metaplasia, and gastric cancer[9]. Neutrophil activity is measured by continuing acute inflammation and is linked to tissue damage. The density of intraepithelial neutrophils is correlated with the extent of mucosal damage and intensity of H. pylori infection[6,10]. The topographic distribution of neutrophil activity has been reportedly associated with gastric cancer development[11].
Endoscopy-led risk stratification is preferable since pathology-based evaluation is more invasive. The endoscopic Kyoto classification of gastritis was advocated by the Japan Gastroenterological Endoscopy Society in 2013. The Kyoto classification was established with the aim to unify the endoscopic diagnosis of gastritis in daily practice and match it with the histological diagnosis. The Kyoto classification score consisted of scores in gastric atrophy, intestinal metaplasia, enlarged folds, nodularity, and diffuse redness[12]. Several studies have revealed the association of the Kyoto score with H. pylori infection[13-17] and gastric cancer risk[18-20]; however, the consistency of the Kyoto score with pathological findings has not been clarified. Therefore, this study aimed to investigate the relationship between the Kyoto score and pathological findings.
This study was approved by the institutional review board at the Institute of Medical Science, University of Tokyo on September 21, 2013 (approval No. 25-34-0921). All participants provided written informed consent.
This cohort study consisted of participants who underwent esophagogastroduo-denoscopy at Toyoshima Endoscopy Clinic from December 2013 to January 2016. Esophagogastroduodenoscopies were performed either for screening, evaluation of present symptoms, or surveillance of previous esophagogastroduodenal diseases. Inclusion criteria were as follows: Patients aged ≥ 20 years without history of gastric neoplasia, surgical gastrectomy, or H. pylori eradication. Exclusion criteria involved a withdrawal of concent.
Demographic characteristics including age, sex, body mass index, smoking history, habitual drinking, and first-degree family history of gastric cancer were obtained[21]. A score of at least 400 on the Brinkman index was defined as positive smoking history. Consumption of at least one alcoholic drink per day was defined as habitual drinking.
Endoscopic Kyoto classification score for gastritis, from 0 to 8, is based on the total scores of the following five endoscopic findings: Atrophy, intestinal metaplasia, enlarged folds, nodularity, and diffuse redness. A high score represents increased risk for gastric cancer and H. pylori infection[12].
Pathological atrophy is defined as the loss of normal glandular tissue of the gastric mucosa. Endoscopic atrophy was classified according to the extent of mucosal atrophy, as described by Kimura et al[22,23]. Non-atrophy and C1 were scored as Atrophy score 0, C2 and C3 as Atrophy score 1, and O1 to O3 as Atrophy score 2.
Pathological intestinal metaplasia is defined as a phenotypic change from the normal epithelial cell of the gastric mucosa to an intestinal phenotype. Endoscopically, intestinal metaplasia typically appears as grayish-white and slightly elevated plaques surrounded by mixed patchy pink and pale areas of the mucosa, forming an irregular uneven surface. Villous appearance, whitish mucosa, and rough mucosal surface are useful indicators for endoscopic diagnosis of intestinal metaplasia[24]. Intestinal metaplasia score 0 is defined as the absence of intestinal metaplasia; Intestinal metaplasia score 1 as the presence of intestinal metaplasia within the antrum; and Intestinal metaplasia score 2 as intestinal metaplasia extending into the corpus. The Intestinal metaplasia score is calculated based on the diagnosis using the white light imaging. Intestinal metaplasia diagnosis based on image-enhanced endoscopy and chromo-endoscopy is not included in the Intestinal metaplasia score.
An enlarged fold is defined as a width of ≥ 5 mm that is not flattened or is only partially flattened by stomach insufflation. The absence and presence of enlarged folds were scored as Enlarged folds score 0 and 1, respectively.
Nodular gastritis is characterized by a miliary pattern resembling “goose flesh” mainly located in the antrum. The absence and presence of nodularity was scored as Nodularity score 0 and 1, respectively.
Diffuse redness refers to uniform redness with continuous expansion observed in non-atrophic mucosa mainly in the corpus[22]. Regular arrangement of collecting venules (RAC) is a condition where collecting venules are arranged in the corpus. From a distance, it appears like numerous dots; up close, it has the appearance of a regular pattern of starfish-like shapes. The absence of diffuse redness, presence of mild diffuse redness or diffuse redness with RAC, and severe diffuse redness or diffuse redness without RAC were scored as Diffuse redness score 0, 1, and 2, respectively.
We obtained biopsy specimens from two sites, the greater curvature of the corpus and the antrum[25]. One experienced gastrointestinal pathologist diagnosed neutrophil activity score based on the updated Sydney System in hematoxylin and eosin staining. Neutrophil infiltration was graded on a scale of 0-3 (none, 0; mild, 1; moderate, 2; severe, 3).
Based on the topographic distribution of neutrophil infiltration, the patients were divided into four categories: “inactive stomach,” “antrum-predominant gastritis,” “pangastritis,” and “corpus-predominant gastritis.” When neutrophil activity was null for the antrum and the corpus, the diagnosis was “inactive stomach.” When the antrum score was larger than that of the corpus, the diagnosis was “antrum-predominant gastritis.” When neutrophil activity was positive, and the antrum score was equal to that of the corpus, the diagnosis was “pangastritis.” When the corpus score was larger than that of the antrum, the diagnosis was “corpus-predominant gastritis[11,26].”
Patients’ blood samples were obtained on the day of esophagogastroduodenoscopy. The serum antibody titer was measured by an enzyme-linked immunoassay kit using antigens derived from Japanese individuals: E-plate Eiken H. pylori antibody II kit (Eiken Chemical, Tokyo, Japan). We defined a cut-off value of 10 U/mL was identified for H. pylori positivity as the manufacturer recommended[13-15].
We tested the association between the Kyoto classification score, including Atrophy, Intestinal metaplasia, Enlarged folds, Nodularity, and Diffuse redness score, and the four categories of topographic distribution of neutrophil activity by Kruskal–Wallis and Steel–Dwass analysis. A multinomial logistic regression analysis was performed using the four categories of gastritis as objective variables. Sex, age, body mass index, drinking, smoking habit, first-degree family history of gastric cancer, serum H. pylori antibody, and the Kyoto score were used as explanatory variables. A P value of < 0.05 was defined as statistical significance. Calculations were carried out using the statistical software Ekuseru-Toukei 2015 (Social Survey Research Information Co., Ltd., Tokyo, Japan).
A total of 327 patients (comprising 50.7% women, with an average age of 50.2 years) were enrolled in this study. H. pylori infection rate was 82.9% with a mean Kyoto score of 4.63 (Table 1).
| Baseline characteristics of patients | |
| Number | 327 |
| Female sex, % | 50.7 |
| Age, mean (± SD), yr | 50.2 (12.3) |
| Body mass index, mean (± SD), kg/m2 | 22.4 (3.1) |
| Drinking, % | 26.0 |
| Smoking, % | 8.3 |
| Family history of gastric cancer, % | 16.8 |
| Positive Helicobacter pylori antibody, % | 82.9 |
| Kyoto score, mean (± SD) | 4.63 (1.89) |
| Atrophy score, mean (± SD) | 1.35 (0.69) |
| Intestinal metaplasia score, mean (± SD) | 0.61 (0.88) |
| Enlarged folds score, mean (± SD) | 0.47 (0.50) |
| Nodularity score, mean (± SD) | 0.40 (0.49) |
| Diffuse redness score, mean (± SD) | 1.71 (0.64) |
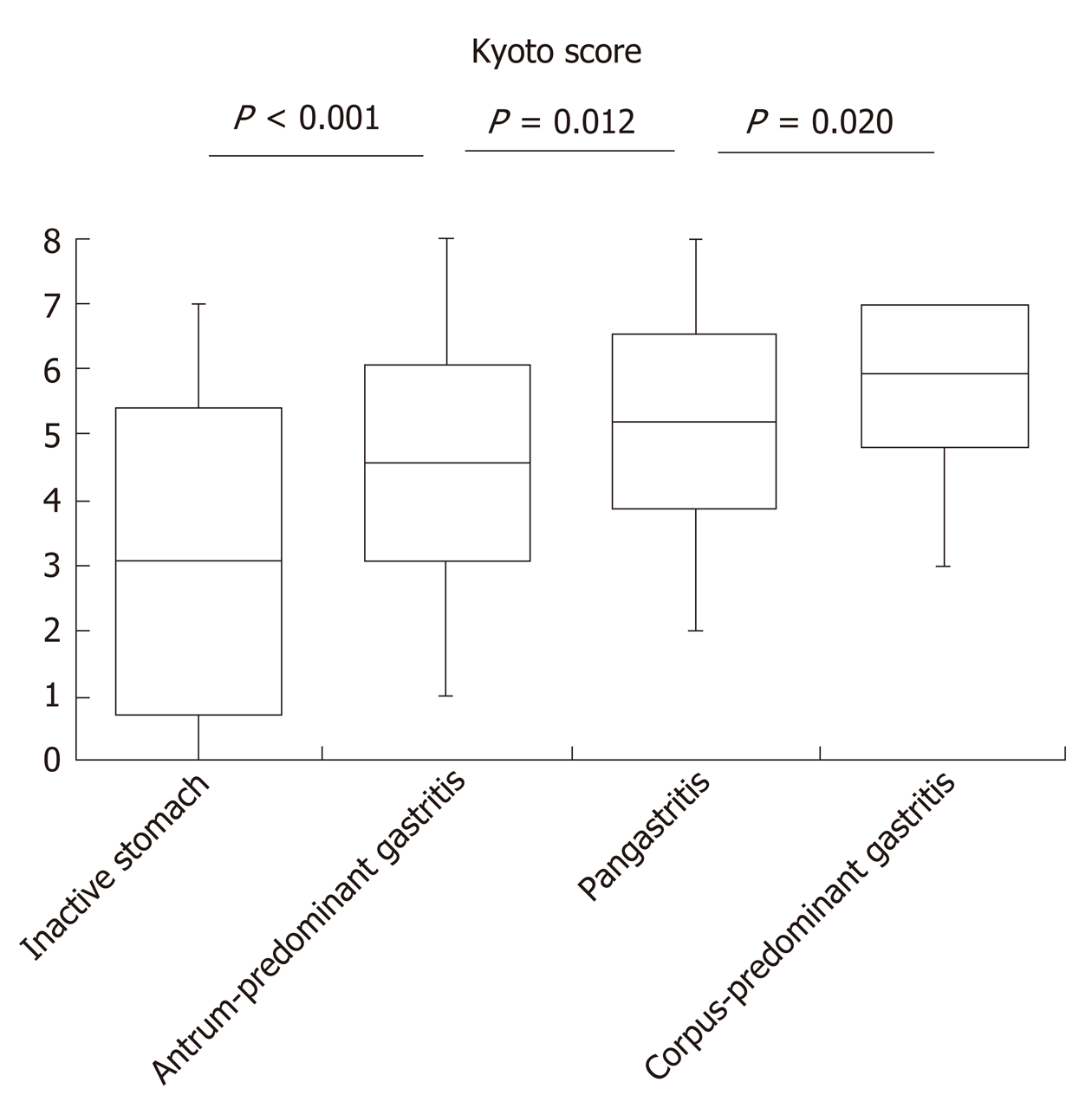
The Kyoto score was associated with the topographic distribution of neutrophil activity (P < 0.001 calculated by Kruskal–Wallis test). Kyoto scores were significantly higher in the order of inactive stomach, antrum-predominant gastritis, pangastritis, and corpus-predominant gastritis (3.05, 4.57, 5.21, and 5.96, respectively; Figure 1). Table 2 shows that each individual score of endoscopic findings (i.e., atrophy, intestinal metaplasia, enlarged folds, nodularity, and diffuse redness) was correlated with the topographic distribution of neutrophil activity. On multivariate analysis, the Kyoto score, age, and serum H. pylori antibody were independently associated with the topographic distribution of neutrophil activity (Table 3).
| Inactive stomach | Antrum-predominant gastritis | Pangastritis | Corpus-predominant gastritis | P value | |
| Atrophy score, mean (± SD) | 1.03 (0.80) | 1.30 (0.64) | 1.46 (0.61) | 1.76 (0.52) | < 0.001 |
| Intestinal metaplasia score, mean (± SD) | 0.53 (0.83) | 0.54 (0.83) | 0.57 (0.88) | 1.36 (0.91) | < 0.001 |
| Enlarged folds score, mean (± SD) | 0.18 (0.39) | 0.54 (0.50) | 0.57 (0.50) | 0.56 (0.51) | < 0.001 |
| Nodularity score, mean (± SD) | 0.27 (0.45) | 0.32 (0.47) | 0.53 (0.50) | 0.28 (0.46) | < 0.001 |
| Diffuse redness score, mean (± SD) | 0.97 (0.85) | 1.83 (0.49) | 1.97 (0.25) | 1.96 (0.20) | < 0.001 |
| Inactive stomach | Antrum-predominant gastritis | Pangastritis | Corpus-predominant gastritis | P value | |
| No. | 73 | 82 | 147 | 25 | |
| Female sex, % | 50.7 | 42.7 | 52.4 | 48.0 | 0.646 |
| Age, mean (± SD), yr | 53.6 (14.1) | 46.8 (10.4) | 48.7 (11.3) | 60.4 (10.9) | < 0.001 |
| Body mass index, mean (± SD), kg/m2 | 22.8 (3.1) | 22.9 (3.6) | 22.0 (2.8) | 21.8 (3.2) | 0.254 |
| Drinking, % | 27.4 | 23.2 | 25.2 | 36.0 | 0.758 |
| Smoking, % | 9.6 | 8.5 | 5.4 | 20.0 | 0.083 |
| Family history of gastric cancer, % | 13.7 | 18.3 | 15.0 | 32.0 | 0.162 |
| Positive Helicobacter pylori antibody, % | 45.2 | 92.7 | 95.2 | 88.0 | < 0.001 |
| Kyoto score, mean (± SD) | 3.05 (2.36) | 4.57 (1.52) | 5.21 (1.35) | 5.96 (1.17) | < 0.001 |
Our study revealed that the Kyoto score was associated with the topographic distribution of neutrophil activity. Uemura et al[11] reported the significance of gastritis topography, and relative risks of gastric cancer for pangastritis and corpus-predominant gastritis were 15.6 and 34.5, respectively, as compared with antrum-predominant gastritis. Namely, the risk of gastric cancer increased in the order of antrum-predominant gastritis, pangastritis, and corpus-predominant gastritis. In this study, Kyoto score also increased in a similar order.
Our data also showed that age, endoscopic atrophy score, and endoscopic intestinal metaplasia score increased in the order of pathologically antrum-predominant active gastritis, pangastritis, and corpus-predominant gastritis. Correa’s cascade in H. pylori-associated gastritis follows the following consecutive steps: (1) Normal gastric mucosa; (2) Nonatrophic antrum-predominant active gastritis; (3) Atrophy; (4) Intestinal metaplasia; (5) Dysplasia; and (6) Cancer[27]. Atrophic gastritis progresses from the antrum to the corpus[22,23]. In the early phase of atrophic gastritis, neutrophils are mainly infiltrated in the antrum. This condition could correspond to antrum-predominant active gastritis. When atrophic gastritis progresses from the antrum to the corpus, neutrophil infiltration in antrum and corpus would be similar. This condition could correspond to pangastritis. Along with the atrophic gastritis progression, intestinal metaplasia occurs, especially in the antrum. Since intestinal metaplasia could be a harsh environment for H. pylori, topographic distribution of H. pylori could alter from the antrum to the corpus[28]. The density of H. pylori was also correlated with neutrophil activity[10,29-31]. After the emergence of antral intestinal metaplasia, neutrophil activity decreases in the antrum, and the status could be categorized as corpus-predominant gastritis. Namely, pathologically active gastritis could progress in the order of antrum-predominant gastritis, pangastritis, and corpus-predominant gastritis (Figure 2). Imagawa et al[32] also suggested that gastritis topography changes in the order of antrum-predominant gastritis, pangastritis, and corpus-predominant gastritis as age advances. In our study, endoscopic atrophy and intestinal metaplasia were associated with the development of pathologically active gastritis.
A number of studies have shown that pathological topography of neutrophil infiltration was correlated with gastric cancer risk[32]. Sakitani et al[33] demonstrated that neutrophil infiltration in the corpus was a risk factor for gastric cancer, especially for the diffuse-type cancer. Matsuhisa et al[34] showed that among H. pylori-positive patients, corpus-predominant gastritis was common in elderly Japanese and Chinese whose prevalence of gastric cancer were high, whereas antrum-predominant gastritis was prevalent in Thailand and Vietnam, which had low incidence of gastric cancer. We previously reported that corpus-predominant gastritis and pangastritis were associated with the risk allele of Prostate Stem Cell Antigen gene, a gastric cancer-related single nucleotide polymorphism. The patients with corpus-predominant gastritis and the pangastritis had low expression of Prostate Stem Cell Antigen in the gastric mucosa, as did the stomach cancer patients did[26]. It has also been reported that the presence of neutrophil infiltration in the corpus is related to metachronous gastric cancer[35] and that activated neutrophils generate reactive oxygen and nitrogen species, which are mutagenic and carcinogenic[36,37]. Although gastritis topography for gastric cancer risk has ample evidence, Kyoto score for gastric cancer risk has not been fully assessed yet[18,38]. Thus, this study provides evidentiary support that Kyoto score is useful for assessing gastric cancer risk.
This study has several limitations. First, this was a retrospective, single-center study. Second, interobserver agreement and reproducibility of the Kyoto classification have not been demonstrated. Third, the category of inactive stomach included subjects who had not been infected with H. pylori and those with spontaneous disappearance of H. pylori. Since cancer risks of the two types of subjects are markedly different, it is advisable that these patients will be investigated separately in the future[39,40].
In conclusion, the Kyoto classification score was associated with topographic distribution of neutrophil activity.
The pathological topographic distribution of neutrophil activity in the gastric mucosa correlates to gastric cancer development. Endoscopy-based Kyoto classification of gastritis has also been reported to be associated with gastric cancer risk.
The consistency of the Kyoto classification score with the topographic distribution of neutrophil activity was not clear.
To investigate the association between endoscopic findings of gastritis based on the Kyoto classification and pathological topography of neutrophil activity.
This study consisted of participants who underwent esophagogastroduodenoscopy at the Toyoshima Endoscopy Clinic from December 2013 to January 2016. We obtained two-points biopsy samples from the greater curvature of corpus and antrum. Based on the pathological topographic distribution of neutrophil activity, the subjects were divided into four categories: Inactive stomach, antrum-predominant gastritis, pangastritis, and corpus-predominant gastritis. We tested the association between the Kyoto classification score, including Atrophy, Intestinal metaplasia, Enlarged folds, Nodularity, and Diffuse redness score, and the four categories of topographic distribution of neutrophil activity.
We enrolled 327 patients. The Kyoto scores were significantly higher in the order of inactive stomach, antrum-predominant gastritis, pangastritis, and corpus-predominant gastritis (3.05, 4.57, 5.21, and 5.96, respectively). Especially, Atrophy score and Intestinal metaplasia score were correlated with the topographic distribution of neutrophil activity. On multivariate analysis, the Kyoto score, age, and serum Helicobacter pylori antibody were independently associated with the topographical distribution of neutrophil activity.
Endoscopic findings of gastritis based on the Kyoto classification were associated with the pathological topographic distribution of neutrophil activity and showed the stepwise increase in the order of inactive stomach, antrum-predominant gastritis, pangastritis, and corpus-predominant gastritis.
Our study supports the hypothesis that endoscopic findings based on the Kyoto score are useful for the assessment of gastric cancer risk. However, further studies are warranted to clarify the association between the Kyoto classification of gastritis and gastric cancer risk.
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56643] [Article Influence: 7080.4] [Reference Citation Analysis (134)] |
| 2. | Banks M, Graham D, Jansen M, Gotoda T, Coda S, di Pietro M, Uedo N, Bhandari P, Pritchard DM, Kuipers EJ, Rodriguez-Justo M, Novelli MR, Ragunath K, Shepherd N, Dinis-Ribeiro M. British Society of Gastroenterology guidelines on the diagnosis and management of patients at risk of gastric adenocarcinoma. Gut. 2019;68:1545-1575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 425] [Cited by in RCA: 453] [Article Influence: 64.7] [Reference Citation Analysis (1)] |
| 3. | Quach DT, Hiyama T, Gotoda T. Identifying high-risk individuals for gastric cancer surveillance from western and eastern perspectives: Lessons to learn and possibility to develop an integrated approach for daily practice. World J Gastroenterol. 2019;25:3546-3562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Negovan A, Iancu M, Fülöp E, Bănescu C. Helicobacter pylori and cytokine gene variants as predictors of premalignant gastric lesions. World J Gastroenterol. 2019;25:4105-4124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Sugimoto M, Murata M, Yamaoka Y. Chemoprevention of gastric cancer development after Helicobacter pylori eradication therapy in an East Asian population: Meta-analysis. World J Gastroenterol. 2020;26:1820-1840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (1)] |
| 6. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Dinis-Ribeiro M, Areia M, de Vries AC, Marcos-Pinto R, Monteiro-Soares M, O'Connor A, Pereira C, Pimentel-Nunes P, Correia R, Ensari A, Dumonceau JM, Machado JC, Macedo G, Malfertheiner P, Matysiak-Budnik T, Megraud F, Miki K, O'Morain C, Peek RM, Ponchon T, Ristimaki A, Rembacken B, Carneiro F, Kuipers EJ; European Society of Gastrointestinal Endoscopy; European Helicobacter Study Group; European Society of Pathology; Sociedade Portuguesa de Endoscopia Digestiva. Management of precancerous conditions and lesions in the stomach (MAPS): guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED). Endoscopy. 2012;44:74-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 500] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 8. | Matsuo Y, Kido Y, Akada J, Shiota S, Binh TT, Trang TT, Dung HD, Tung PH, Tri TD, Thuan NP, Tam LQ, Nam BC, Khien VV, Yamaoka Y. Novel CagA ELISA exhibits enhanced sensitivity of Helicobacter pylori CagA antibody. World J Gastroenterol. 2017;23:48-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Chey WD, Wong BC; Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102:1808-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 819] [Cited by in RCA: 837] [Article Influence: 44.1] [Reference Citation Analysis (3)] |
| 10. | Fiocca R, Villani L, Luinetti O, Gianatti A, Perego M, Alvisi C, Turpini F, Solcia E. Helicobacter colonization and histopathological profile of chronic gastritis in patients with or without dyspepsia, mucosal erosion and peptic ulcer: a morphological approach to the study of ulcerogenesis in man. Virchows Arch A Pathol Anat Histopathol. 1992;420:489-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3246] [Article Influence: 129.8] [Reference Citation Analysis (1)] |
| 12. | Toyoshima O, Nishizawa T, Koike K. Endoscopic Kyoto classification of Helicobacter pylori infection and gastric cancer risk diagnosis. World J Gastroenterol. 2020;26:466-477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 101] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (2)] |
| 13. | Toyoshima O, Nishizawa T, Arita M, Kataoka Y, Sakitani K, Yoshida S, Yamashita H, Hata K, Watanabe H, Suzuki H. Helicobacter pylori infection in subjects negative for high titer serum antibody. World J Gastroenterol. 2018;24:1419-1428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 14. | Toyoshima O, Nishizawa T, Sakitani K, Yamakawa T, Takahashi Y, Yamamichi N, Hata K, Seto Y, Koike K, Watanabe H, Suzuki H. Serum anti-Helicobacter pylori antibody titer and its association with gastric nodularity, atrophy, and age: A cross-sectional study. World J Gastroenterol. 2018;24:4061-4068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Nishizawa T, Sakitani K, Suzuki H, Yamakawa T, Takahashi Y, Yamamichi N, Watanabe H, Seto Y, Koike K, Toyoshima O. A combination of serum anti-Helicobacter pylori antibody titer and Kyoto classification score could provide a more accurate diagnosis of H pylori. United European Gastroenterol J. 2019;7:343-348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Yoshii S, Mabe K, Watano K, Ohno M, Matsumoto M, Ono S, Kudo T, Nojima M, Kato M, Sakamoto N. Validity of endoscopic features for the diagnosis of Helicobacter pylori infection status based on the Kyoto classification of gastritis. Dig Endosc. 2020;32:74-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 17. | Toyoshima O, Nishizawa T, Sakitani K, Yamakawa T, Takahashi Y, Kinoshita K, Torii A, Yamada A, Suzuki H, Koike K. Helicobacter pylori eradication improved the Kyoto classification score on endoscopy. JGH Open. 2020;. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Sugimoto M, Ban H, Ichikawa H, Sahara S, Otsuka T, Inatomi O, Bamba S, Furuta T, Andoh A. Efficacy of the Kyoto Classification of Gastritis in Identifying Patients at High Risk for Gastric Cancer. Intern Med. 2017;56:579-586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 19. | Nishizawa T, Toyoshima O, Kondo R, Sekiba K, Tsuji Y, Ebinuma H, Suzuki H, Tanikawa C, Matsuda K, Koike K. The simplified Kyoto Classification Score is Consistent with the ABC Method of Classification. J Clin Biochem Nutr. 2020;. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Toyoshima O, Nishizawa T, Sekiba K, Matsuno T, Kondo R, Watanabe H, Suzuki H, Tanikawa C, Koike K, Matsuda K. A single nucleotide polymorphism in Prostate Stem Cell Antigen is associated with endoscopic grading in Kyoto classification of gastritis. J Clin Biochem Nutr. 2020;. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Nishizawa T, Suzuki H, Sakitani K, Yamashita H, Yoshida S, Hata K, Kanazawa T, Fujiwara N, Kanai T, Yahagi N, Toyoshima O. Family history is an independent risk factor for the progression of gastric atrophy among patients with Helicobacter pylori infection. United European Gastroenterol J. 2017;5:32-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969;3:87-97. [RCA] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 777] [Article Influence: 43.2] [Reference Citation Analysis (5)] |
| 23. | Toyoshima O, Yamaji Y, Yoshida S, Matsumoto S, Yamashita H, Kanazawa T, Hata K. Endoscopic gastric atrophy is strongly associated with gastric cancer development after Helicobacter pylori eradication. Surg Endosc. 2017;31:2140-2148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 24. | Fukuta N, Ida K, Kato T, Uedo N, Ando T, Watanabe H, Shimbo T; Study Group for Investigating Endoscopic Diagnosis of Gastric Intestinal Metaplasia. Endoscopic diagnosis of gastric intestinal metaplasia: a prospective multicenter study. Dig Endosc. 2013;25:526-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Toyoshima O, Nishizawa T, Sakitani K, Yamakawa T, Watanabe H, Yoshida S, Nakai Y, Hata K, Ebinuma H, Suzuki H, Koike K. Nodularity-like appearance in the cardia: novel endoscopic findings for Helicobacter pylori infection. Endosc Int Open. 2020;8:E770-E774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Toyoshima O, Tanikawa C, Yamamoto R, Watanabe H, Yamashita H, Sakitani K, Yoshida S, Kubo M, Matsuo K, Ito H, Koike K, Seto Y, Matsuda K. Decrease in PSCA expression caused by Helicobacter pylori infection may promote progression to severe gastritis. Oncotarget. 2018;9:3936-3945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Correa P, Houghton J. Carcinogenesis of Helicobacter pylori. Gastroenterology. 2007;133:659-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 514] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 28. | Shi H, Xiong H, Qian W, Lin R. Helicobacter pylori infection progresses proximally associated with pyloric metaplasia in age-dependent tendency: a cross-sectional study. BMC Gastroenterol. 2018;18:158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Yamamura F, Yoshikawa N, Akita Y, Mitamura K, Miyasaka N. Relationship between Helicobacter pylori infection and histologic features of gastritis in biopsy specimens in gastroduodenal diseases, including evaluation of diagnosis by polymerase chain reaction assay. J Gastroenterol. 1999;34:461-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Kalebi A, Rana F, Mwanda W, Lule G, Hale M. Histopathological profile of gastritis in adult patients seen at a referral hospital in Kenya. World J Gastroenterol. 2007;13:4117-4121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Fu H, Ma Y, Yang M, Zhang C, Huang H, Xia Y, Lu L, Jin W, Cui D. Persisting and Increasing Neutrophil Infiltration Associates with Gastric Carcinogenesis and E-cadherin Downregulation. Sci Rep. 2016;6:29762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Imagawa S, Yoshihara M, Ito M, Yoshida S, Wada Y, Tatsugami M, Takamura A, Tanaka S, Haruma K, Chayama K. Evaluation of gastric cancer risk using topography of histological gastritis: a large-scaled cross-sectional study. Dig Dis Sci. 2008;53:1818-1823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Sakitani K, Hirata Y, Watabe H, Yamada A, Sugimoto T, Yamaji Y, Yoshida H, Maeda S, Omata M, Koike K. Gastric cancer risk according to the distribution of intestinal metaplasia and neutrophil infiltration. J Gastroenterol Hepatol. 2011;26:1570-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 34. | Matsuhisa T, Matsukura N, Yamada N. Topography of chronic active gastritis in Helicobacter pylori-positive Asian populations: age-, gender-, and endoscopic diagnosis-matched study. J Gastroenterol. 2004;39:324-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Sugimoto T, Yamaji Y, Sakitani K, Isomura Y, Yoshida S, Yamada A, Hirata Y, Ogura K, Okamoto M, Koike K. Neutrophil infiltration and the distribution of intestinal metaplasia is associated with metachronous gastric cancer following endoscopic submucosal dissection. Can J Gastroenterol Hepatol. 2015;29:321-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Cerutti PA. Prooxidant states and tumor promotion. Science. 1985;227:375-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1907] [Cited by in RCA: 1729] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 37. | Tamir S, Tannenbaum SR. The role of nitric oxide (NO.) in the carcinogenic process. Biochim Biophys Acta. 1996;1288:F31-F36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 52] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 38. | Shichijo S, Hirata Y, Niikura R, Hayakawa Y, Yamada A, Koike K. Association between gastric cancer and the Kyoto classification of gastritis. J Gastroenterol Hepatol. 2017;32:1581-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 39. | Sakitani K, Nishizawa T, Arita M, Yoshida S, Kataoka Y, Ohki D, Yamashita H, Isomura Y, Toyoshima A, Watanabe H, Iizuka T, Saito Y, Fujisaki J, Yahagi N, Koike K, Toyoshima O. Early detection of gastric cancer after Helicobacter pylori eradication due to endoscopic surveillance. Helicobacter. 2018;23:e12503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 40. | Kishikawa H, Ojiro K, Nakamura K, Katayama T, Arahata K, Takarabe S, Miura S, Kanai T, Nishida J. Previous Helicobacter pylori infection-induced atrophic gastritis: A distinct disease entity in an understudied population without a history of eradication. Helicobacter. 2020;25:e12669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
P-Reviewer: Rostami K S-Editor: Yan JP L-Editor: A P-Editor: Zhang YL