Published online Jun 14, 2020. doi: 10.3748/wjg.v26.i22.3087
Peer-review started: January 2, 2020
First decision: February 19, 2020
Revised: February 23, 2020
Accepted: May 13, 2020
Article in press: May 13, 2020
Published online: June 14, 2020
Processing time: 163 Days and 23.7 Hours
In recent decades, an increasing number of patients have received minimally invasive intervention for infected pancreatic necrosis (IPN) because of the benefits in reducing postoperative multiple organ failure and mortality. However, there are limited published data regarding infection recurrence after treatment of this patient population.
To investigate the incidence and prediction of infection recurrence following successful minimally invasive treatment in IPN patients.
Medical records for 193 IPN patients, who underwent minimally invasive treatment between February 2014 and October 2018, were retrospectively reviewed. Patients, who survived after the treatment, were divided into two groups: one group with infection after drainage catheter removal and another group without infection. The morphological and clinical data were compared between the two groups. Significantly different variables were introduced into the correlation and multivariate logistic analysis to identify independent predictors for infection recurrence. Sensitivity and specificity for diagnostic performance were determined.
Of the 193 IPN patients, 178 were recruited into the study. Of them, 9 (5.06%) patients died and 169 patients survived but infection recurred in 13 of 178 patients (7.30%) at 7 (4-10) d after drainage catheters were removed. White blood cell (WBC) count, serum C-reactive protein (CRP), interleukin-6, and procalcitonin levels measured at the time of catheter removal were significantly higher in patients with infection than in those without (all P < 0.05). In addition, drainage duration and length of the catheter measured by computerized tomography scan were significantly longer in patients with infection (P = 0.025 and P < 0.0001, respectively). Although these parameters all correlated positively with the incidence of infection (all P < 0.05), only WBC, CRP, procalcitonin levels, and catheter length were identified as independent predictors for infection recurrence. The sensitivity and specificity for infection prediction were high in WBC count (≥ 9.95 × 109/L) and serum procalcitonin level (≥ 0.05 ng/mL) but moderate in serum CRP level (cut-off point ≥ 7.37 mg/L). The catheter length (cut-off value ≥ 8.05 cm) had a high sensitivity but low specificity to predict the infection recurrence.
WBC count, serum procalcitonin, and CRP levels may be valuable for predicting infection recurrence following minimally invasive intervention in IPN patients. These biomarkers should be considered before removing the drainage catheters.
Core tip: This retrospective study investigated infection recurrence following successful minimally invasive treatment in infected pancreatic necrosis (IPN) patients. Our data demonstrated that infection recurred in 7.30% of IPN patients after drainage catheter removal. This can be predicted by white blood cell count, serum C-reactive protein, and procalcitonin levels measured at the time of catheter removal, and length of the catheter measured by computerized tomography scan. In particular, white blood cell count and serum procalcitonin level were highly sensitive and specific for predicting infection recurrence. These factors should be considered before removing the drainage catheters following minimally invasive treatment in IPN patients.
- Citation: Gao CC, Li J, Cao F, Wang XH, Li A, Wang Z, Li F. Infection recurrence following minimally invasive treatment in patients with infectious pancreatic necrosis. World J Gastroenterol 2020; 26(22): 3087-3097
- URL: https://www.wjgnet.com/1007-9327/full/v26/i22/3087.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i22.3087
Infected pancreatic necrosis (IPN) is a vital condition developed in 40% to 70% of patients in the late stage of acute pancreatitis[1-3]. It is associated with a high mortality rate, ranging from 18% to 28%[4-6] and accounts for 80% of death in patients with acute pancreatitis[7]. Therefore, interventional treatment are required[1,8,9]. Over past decades, new developments in image, radiology, and minimal access technology have offered an increasing number of critically ill IPN patients with opportunities to undergo minimally invasive drainage, debridement and necrosectomy, which provide the benefits of reducing postoperative multiple organ failure and mortality by minimizing tissue damage and a systemic pro-inflammatory response[6,10,11]. Currently, minimally invasive techniques for IPN patients include percutaneous catheter drainage (PCD), video-assisted or laparoscopic-assisted debridement, and laparoscopic pancreatic necrosectomy. They are usually employed as a sole treatment or a step-up approach consisting of PCD, if necessary, followed by other minimally invasive debridement and necrosectomy and finished with a catheter drainage[9,12].
To date, the most commonly reported complications of minimally invasive treatment in IPN patients include fistula, perforation, colonic injury, and pericatheter leaking[13]. There are limited data in clinical guidelines regarding infection recurrence[8,9,14,15]. Freeny et al[16] reported 6.5% of collection recurrence after catheter removal in patients with infected pancreatic fluid collection following percutaneous catheter drainage. However, it is not clear whether or not the recurred collection is due to infection. Evidence for its clinical management and prevention is lacking. Therefore, we conducted a retrospective study to investigate the incidence and predictors of infection recurrence after catheter removal in IPN patients. Our data demonstrated that infection recurred in the local drainage tunnels in 13 patients (7.30%) after catheter removal following catheter removal criteria. It correlated independently with white blood cell (WBC) count, serum levels of C-reactive protein (CRP), and procalcitonin levels, and length of the catheter inside body. This information will be helpful for clinical management of catheter drainage and the prevention of infection recurrence.
This was a single-center retrospective study. All IPN patients, who underwent minimally invasive treatment at the Department of General, Xuanwu Hospital, Capital Medical University (Beijing, China) between February 2014 and October 2018, were identified using a computerized database. The inclusion criteria were as follows: (1) Age > 18 years; (2) Confirmed diagnosis of infectious pancreatic necrosis; (3) Onset of IPN > 4 wk; (4) Minimally invasive treatment including percutaneous catheter drainage (PCD), video-assisted retroperitoneal debridement (VARD), laparoscopic-assisted transomental debridement (LATOD), and laparoscopic pancreatic debridement; and (5) Placement of drainage catheters for necrotic collection following the procedures. The exclusion criteria were as follows: (1) Traumatic pancreatitis; (2) Infection caused by pancreatic fistula following pancreatic surgery; and (3) Having surgical treatment for complications such as digestive tract or biliary obstruction, digestive tract fistula, or pseudoaneurysm rupture. The study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the ethics committee of Xuanwu Hospital. Due to the nature of a retrospective study, written informed consent was omitted.
All data collected from medical records included general information and clinical variables. General information included age, gender, smoke and alcohol use, medical history, and body mass index. Clinical variables included the onset, etiology, complications, severity of IPN, laboratory test results before treatment and at the time of catheter removal, type of minimally invasive treatment, length of the drainage catheter measured by computerized tomography (CT) scan, and duration of drainage and outcomes. The severity of pancreatitis was evaluated by the Bedside Index for Severity in Acute Pancreatitis score[17] and Chronic Health Evaluation score[18]. Blood tests were performed before treatment and at the time of catheter removal. The normal range of blood test was WBC (4.0 × 109/L to 10.0 × 109/L), blood urea nitrogen (BUN) (1.7-8.3 mmol/L), creatinine (17.7-104.0 µmol/L), CRP (< 8.0 mg/L), interleukin-6 (IL-6) (0-7 pg/mL), and procalcitonin (0.10–0.49 ng/mL).
According to the revised Atlanta classification system[1] and clinical guidelines[9,14], IPN was diagnosed by persistent sepsis, progressive clinical deterioration despite maximal support in the intensive care unit, serum lipase level or amylase level at least 3 times greater than the upper limit of normality, the presence of gas bubbles within the necrotic tissue or peripancreatic collection observed on contrast-enhanced CT scans, or a positive fine-needle aspiration culture.
After admission, all patients received aggressive intravenous fluid resuscitation and nutritional support, broad spectrum antibiotics, and minimally invasive interventions. The choice of interventional approach was determined by the location of the necrotic collection relative to the stomach, colon, liver, spleen, and kidney.
In PCD procedure, 16-20 F drainage catheters were introduced using a direct transperitoneal approach under CT or ultrasound guidance and left in place until the catheter removal criteria was met.
VARD procedure was previously described[19]. In brief, a subcostal 3-4 cm incision was made. After the collection wall was opened, a laparoscope was introduced through the incision and the ring forceps was used parallel to the laparoscope to remove the necrosis under full laparoscopic vision. Several 30-36 F drainage catheters were placed for continuous drainage.
The LATOD procedure through the transomental approach was used for patients with necrotic collection located on the pancreatic head or near the duodenum. Following an upper midline 3-4 cm incision, a small incision on the gastrocolic ligament was made, and then the ligament was sutured with parietal peritoneum circumferentially to establish a debridement passageway. Once the collection was confirmed by fine-needle aspiration, a laparoscope was introduced and the necrosis was removed under full laparoscopic vision. Several 30-36 F drainage catheters were placed to continue the drainage of the collection.
Laparoscopic pancreatic debridement was previously described[20]. Briefly, patients were placed in a left or right lateral position. A hand access device port and two standard laparoscopic ports were established. The access into the lesser sac was gained through the greater omentum between the stomach and the colon. Using this procedure, the necrotic tissue in the lesser sac, the left paracolic gutter, and the head of the pancreas were removed. Finally, several 30-36 F drainage catheters were placed to continue the drainage of the collection. In 2 patients with retrogastric pancreatic necrosis, debridement was performed via the transgastric approach as described by Worhunsky et al[21].
Following treatment, drainage catheters were flushed with 20 mL saline, three times daily in order to keep the drain open. If the catheter was blocked, a replacement was inserted under local anesthesia. Before removal, catheters were temperately closed and a CT scan was performed. Catheter removal criteria included: (1) Patients were asymptomatic for at least 2 wk; (2) No fistula and peripancreatic cavity were present in repeat CT scan; (3) The output of drainage was clear and less than 20 mL/d; (4) Amylase level in the drainage was less than 100 IU/L; and (5) The results of repeat cultures of drainage were negative. If all criteria are fulfilled, the drainage catheters could be removed sequentially; otherwise, the catheter was reopened to continue the drainage with further follow-up.
The data were analyzed using SPSS version 22.0 (IBM Corp, Armonk, NY, United States). Comparative analyses were performed with the chi-square test for categorical variables and the Student’s t-test for continuous variables. Pearson’s correlation and multivariate logistic tests were performed to identify independent predictors for infection recurrence. Receiver operating characteristic curves, the respective areas under the curve, and the cut-off values were calculated. Sensitivity and specificity of different parameters were determined. P values less than 0.05 were considered statistically significant.
Among the 193 patients, who underwent minimally invasive treatment for IPN at our department between February 2014 and October 2018, 15 patients were excluded including 1 with traumatic pancreatitis, 1 with pancreatic fistula following pancreatic surgery, and 13 patients undergoing surgical treatment for complications such as bleeding and colon fistula following minimally invasive procedures. Finally, 178 patients were recruited into the study. Intravenous fluid resuscitation, nutritional support, and broad-spectrum antibiotics were administered to all patients. Details of minimally invasive approaches are summarized in Table 1, including sole treatment with PCD, VARD, or LATOD in 88 patients (49.43%) and combined treatment with VARD and LATOD, or a step-up treatment starting with PCD in 90 patients (50.57%). Drainage catheters were installed following the procedures and kept for a medium of 60 (2-161) d.
| Treatment | Number of patients | Percentage |
| PCD | 35 | 19.66 |
| VARD | 35 | 19.66 |
| LATOD | 18 | 10.11 |
| PCD + VARD/LATOD | 78 | 43.82 |
| VARD + LATOD | 4 | 2.25 |
| PCD + LPD | 3 | 1.69 |
| PCD + VARD + LATOD | 5 | 2.81 |
| Total | 178 | 100 |
After minimally invasive treatment, 9 (5.06%) patients died due to severe bleeding (3/178, 1.69%), uncontrolled sepsis (5/178, 2.81%), or multiple organ failure (1, 0.56%). The remaining 169 patients became asymptomatic and had drainage catheters removed strictly following the catheter removal criteria. However, only 156 patients (87.64%) were cured and 13 patients (7.30%) became symptomatic again with fever (8 patients) and abdominal pain (5 patients) after 7 (4-10) d. Repeat CT scans showed effusion in catheter tunnels, indicating infection recurrence. The cultures of the effusion showed Enterococcus faecium in 4 patients, Escherichia coli in 4 patients, Staphylococcus in 3 patients, Acinetobacter baumannii in 1 patient, and Klebsiella pneumoniae in 1 patient. Finally, 10 patients were managed successfully with needle puncture aspiration and antibiotics. The other 3 patients underwent additional PCD and subsequently cured.
To investigate predictive factors for infection recurrence following catheter removal, we compared demographics and clinical characteristics between the survived patients with (13 patients) and without (156 patients) infection. No difference was found in the patients’ demographics (Table 2) between the two groups. As shown in Table 3, clinical characteristics of patients such as the time onset and causes of IPN, severity of IPN assessed by Bedside Index for Severity in Acute Pancreatitis score and Chronic Health Evaluation score, mental status and systemic inflammatory response syndrome were not significantly different between the two groups (all P = not significant [NS]). Minimally invasive approaches performed in both groups were similar (P = NS). Pre-operative WBC count, serum BUN, creatinine, CRP, IL-6, and procalcitonin levels were abnormally higher than clinical normal range in all patients but no significant difference was found between the two groups (all P = NS). Repeat blood tests at the time of catheter removal showed that in patients without infection recurrence, WBC count, serum BUN, creatinine, CRP, IL-6, and procalcitonin levels returned to the normal range. In patients with infection recurrence, all of these parameters were significantly reduced from the preoperative levels but serum CRP and IL-6 levels were still significantly higher than the normal range. Comparing the two groups, there was no significant difference in terms of BUN and creatine levels (all P = NS) but WBC count, serum CRP, IL-6, and procalcitonin levels were significantly higher in patients with infection than in those without (all P < 0.05). In addition, drainage duration and the length of catheter inside the body measured by CT scan were significantly longer in patients with infection than in those without (P = 0.025 and P < 0.0001, respectively).
| Patients without recurrent infection, n = 156 | Patients with recurrent infection, n = 13 | P value | |
| Age in yr | 53 ± 1 | 56 ± 4 | 0.435 |
| Male sex, n (%) | 96 (61.54) | 6 (46.15) | 0.128 |
| Smoke, n (%) | 54 (34.62) | 5 (38.46) | 0.224 |
| Alcohol, n (%) | 24 (15.38) | 0 (0) | 0.126 |
| Medical history, n (%) | |||
| Pancreatitis | 27 (17.31) | 1 (7.69) | 0.243 |
| Cardiovascular Disease | 10 (6.41) | 2 (15.38) | 0.180 |
| Diabetes | 3 (1.92) | 1 (7.69) | 0.246 |
| Renal disease | 8 (5.13) | 0 (0) | 0.520 |
| Liver disease | 14 (8.97) | 0 (0) | 0.311 |
| BMI in kg/m2 | 23.63 ± 0.13 | 23.99 ± 0.43 | 0.430 |
| Patients without recurrent infection, n = 156 | Patients with recurrent infection, n = 13 | P value | |
| Time onset of IPN in d | 53 ± 1 | 55 ± 5 | 0.796 |
| Cause of IPN, n (%) | |||
| Biliary | 92 (58.97) | 6 (46.15) | 0.543 |
| Alcohol | 23 (14.74) | 3 (23.08) | 0.136 |
| Idiopathic | 5 (3.21) | 1 (7.69) | 0.386 |
| Hypertriglyceridemia | 36 (23.08) | 3 (23.08) | 1.000 |
| BISAP score | 3.53 ± 0.05 | 3.62 ± 0.14 | 0.632 |
| APACHE II score | 9.56 ± 0.18 | 8.92 ± 0.40 | 0.328 |
| Impaired mental status, n (%) | 21 (13.46) | 2 (15.38) | 0.302 |
| SIRS, n (%) | 146 (93.59) | 12 (92.31) | 0.395 |
| Pre-operative blood test | |||
| WBC as 109/L | 14.59 ± 0.281 | 15.32 ± 0.601 | 0.466 |
| BUN in mmol/L | 10.45 ± 0.501 | 9.09 ± 0.971 | 0.440 |
| Creatinine in µmol/L | 156.86 ± 9.091 | 115.61 ± 10.601 | 0.195 |
| CRP in mg/L | 66.47 ± 5.631 | 42.66 ± 10.441 | 0.230 |
| IL-6 in pg/mL | 62.25 ± 7.791 | 53.05 ± 8.451 | 0.735 |
| Procalcitonin in ng/mL | 2.44 ± 0.191 | 2.29 ± 0.551 | 0.823 |
| Minimally invasive treatment | 0.157 | ||
| Sole treatment with PCD/VARD/LATOD, n (%) | 86 (55.13) | 4 (30.77) | |
| Combined treatment, n (%) | 70 (44.87) | 9 (69.23) | |
| Post treatment blood test | |||
| WBC as 109/L | 7.37 ± 0.21 | 11.00 ± 0.61 | 0.000 |
| BUN in mmol/L | 6.89 ± 0.21 | 6.92 ± 0.75 | 0.974 |
| Creatinine in µmol/L | 86.82 ± 2.78 | 74.85 ± 7.46 | 0.227 |
| CRP in mg/L | 7.68 ± 0.54 | 18.97 ± 3.841 | 0.000 |
| IL-6 in pg/mL | 8.28 ± 0.68 | 18.76 ± 4.241 | 0.000 |
| Procalcitonin in ng/mL | 0.04 ± 0.01 | 0.09 ± 0.01 | 0.000 |
| Duration of drainage in d | 79 ± 3 | 104 ± 13 | 0.025 |
| Catheter length in cm | 7.13 ± 0.26 | 10.68 ± 0.66 | 0.000 |
Bivariate correlation and multivariable logistic regression analysis revealed that WBC count, serum CRP and procalcitonin levels, and length of the catheter were independent predictors for infection recurrence (all P < 0.05) (Table 4).
| Variable | Pearson's correlation coefficient | B | Wald | P value |
| WBC | 0.328 | 0.661 | 8.622 | 0.003 |
| CRP | 0.265 | 0.205 | 7.74 | 0.005 |
| IL-6 | 0.192 | 0.075 | 2.247 | 0.134 |
| Procalcitonin | 0.327 | 24.779 | 4.533 | 0.033 |
| Duration of drainage | 0.159 | 0.011 | 0.712 | 0.399 |
| Catheter length | 0.277 | 0.589 | 6.032 | 0.014 |
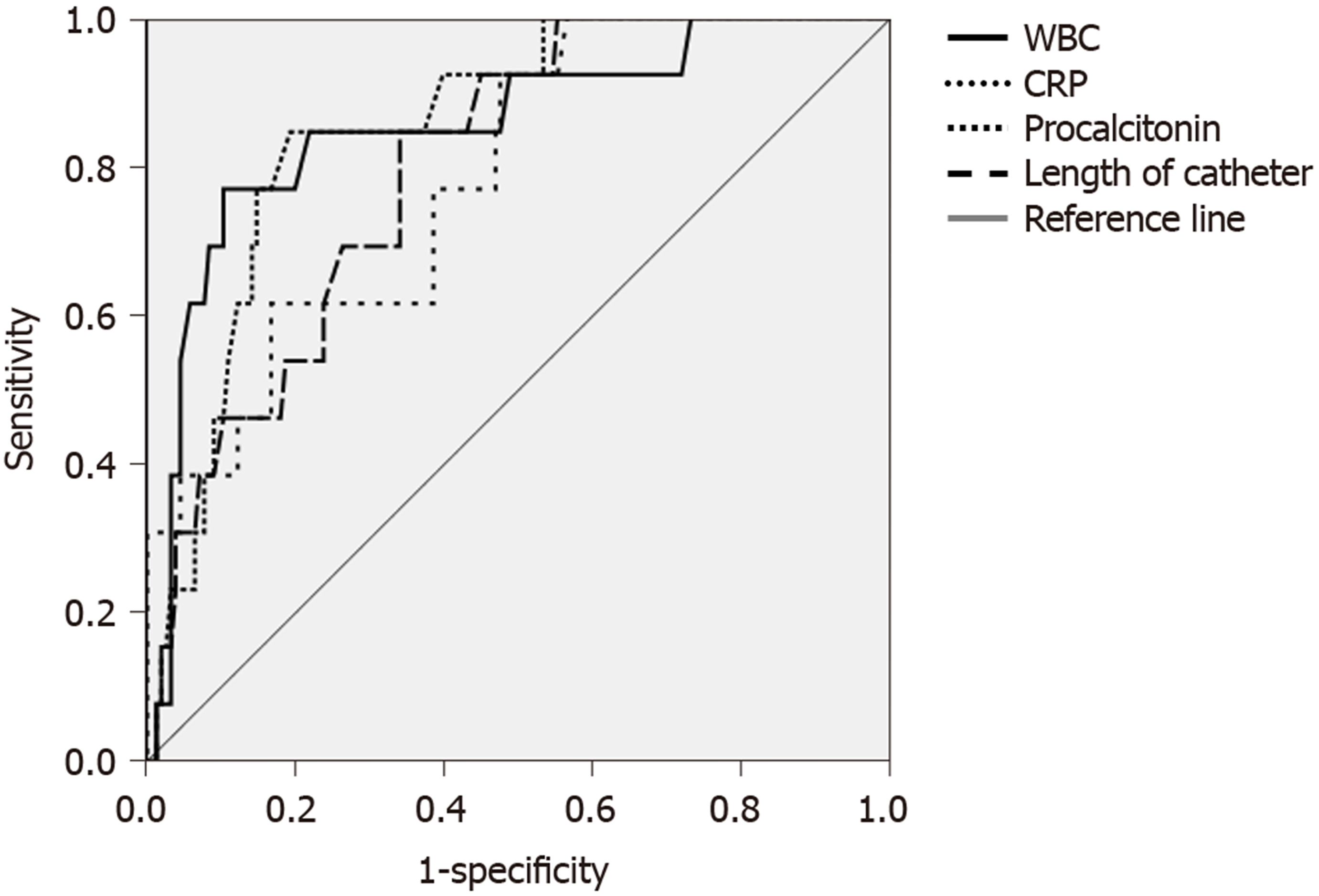
Receiver operating characteristic curves for the observed values of WBC, serum CRP and procalcitonin levels and catheter length are shown in Figure 1. Only WBC count (cut-off value ≥ 9.95 × 109/L) and serum procalcitonin level (≥ 0.05 ng/mL) had high sensitivity and specificity to predict infection, while the sensitivity and specificity of serum CRP level (cut-off point ≥ 7.37 mg/L) were moderate for the prediction of infection. If using 8.05 cm as a cut-off value, the catheter length measured by CT scan was highly sensitive but not very specific to predict the infection recurrence after catheter removal (Table 5).
| Area under curve (95%CI) | P value | Cut-off | Sensitivity | Specificity | |
| WBC as 109/L | × 0.856 (0.739-0.972) | 0.000 | 9.95 | 0.77 | 0.83 |
| CRP in mg/L | 0.787 (0.670-0.904) | 0.001 | 7.37 | 0.77 | 0.62 |
| PCT in ng/mL | 0.854 (0.767-0.941) | 0.000 | 0.05 | 0.85 | 0.75 |
| Catheter length in cm | 0.800 (0.699-0.900) | 0.000 | 8.05 | 0.85 | 0.61 |
In the present study, we investigated the incidence and predictors of infection recurrence after minimally invasive treatment for IPN. Our data demonstrated that infection recurred in 7.30% of IPN patients at 7 (4-10) d after catheter removal following successful minimally invasive procedures. Of them, 10 patients were managed successfully with needle puncture aspiration and antibiotics. Other patients required additional PCD and recovered successfully. WBC count, serum levels of CRP and procalcitonin, and length of the drainage catheter were identified as independent predictors for infection recurrence. Of these, WBC and serum procalcitonin level were highly sensitive and specific.
There are limited published data available regarding long-term infection recurrence following minimally invasive treatment for IPN patients. Seewald et al[22] reported 6.25% of recurrent fluid collections after endoscopic drainage and necrosectomy in patients with symptomatic pancreatic fluid collection. In this study, only 36 of 80 patients had infected walled-off necrosis. Freeny et al[16] also reported 6.5% of collection recurrence after catheter removal in 23 patients with infected pancreatic fluid collections following the PCD procedure. In a study by Zerem et al[23], 19 of 86 (22.1%) patients with IPN had infection recurrence following step-up procedure. In this study, most patients were critically ill but infection recurrence rate was only 7.30%. If we include all IPN patients who were treated in our department, the incidence of infection will be even lower. Nevertheless, our findings indicate that long-term infection complication remains in a small proportion of critical IPN patients even if minimally invasive procedures are successful. In order to prevent it, further exploration into the prediction of infection recurrence is certainly worthwhile for clinical practice.
To date, there is no standard criterion for catheter removal following minimally invasive procedures in IPN patients. The Italian Association for the Study of the Pancreas recommends removing catheters after PCD procedure for patients with severe acute pancreatitis when drainage is less than 10 mL in a 24 h period and a fistula or a peri-pancreative cavity is ruled out by CT scan[9]. In a study by Baudin et al[24], the catheter removal criteria not only included the above indicators but also included stable return of WBC count and CRP level to normal range. Unfortunately, this study did not evaluate long-term complications after catheter removal. Therefore, it is not clear whether infection can be prevented by following these criteria. According to our criteria, patients should be asymptomatic for at least 2 wk without fistula and peripancreatic cavity presented in repeat CT scan, and drainage is negative in culture and less than 20 mL/d with low amylase level (< 100 IU/L). However, it still failed to completely prevent infection in severe IPN patients. Therefore, additional risk factors or predictors should be considered. We found that WBC count, serum CRP and procalcitonin levels, and the length of the catheter inside the body can predict infection recurrence, of which WBC count and serum procalcitonin level had a high sensitivity and specificity particularly. It is well known that WBCs play a very important role in fighting viruses and bacteria. We found that using a cut-off value of WBC count ≥ 9.95 × 109/L (normal up limit in clinical practice is 10.00 × 109/L) provided 77% sensitivity and 83% specificity for the prediction of infection recurrence after catheter removal. As a routine test in clinical practice with low cost, monitoring WBC prior to catheter removal is cost-effective for the prevention of infection.
Previous studies have identified inflammatory cytokines such as IL-6, CRP, and procalcitonin for early prediction of necrosis infection in acute necrotizing pancreatitis[25,26]. Similarly, others studies have also shown that a cut-off procalcitonin level > 0.5 ng/mL has 80% sensitivity and 91% specificity for prediction of infected pancreatic necrosis[27]. These evaluations have been performed during the developing period of IPN, which is different from the present study that evaluates cytokines after treatment with interventional approaches and antibiotics. The cut-off serum levels of inflammatory cytokines are therefore different. Our results demonstrated that a cut-off value of procalcitonin ≥ 0.05 ng/mL has high sensitivity and specificity to predict infection recurrence after catheter removal. However, when interpreting the change of serum procalcitonin level, it is important to remember that procalcitonin is only a nonspecific marker for bacterial infection[28,29]. It is valuable for the prediction of infection but not for treatment purposes. According to our analysis, the sensitivity and specificity of serum CRP level were moderate at a cut-off point ≥ 7.37 mg/L. It may be due to a delayed response of CRP to infection progress[30]. Interestingly, we found that serum level of IL-6 significantly correlated with infection recurrence but multivariate logistic analysis failed to identify it as an independent predictor. The discrepancy between the present study and aforementioned studies may be due to two factors. First, the role of IL-6 in the inflammatory process is complicated[31]; thus, the change in IL-6 level may be less sensitive to predict the onset of infection. Second, the serum level of IL-6 in our study at the time of catheter removal may be too low to predict inflammation compared to other reports[32]. Nevertheless, assessing serum procalcitonin and CRP levels before catheter removal may help to predict infection recurrence. It is noteworthy that for all prediction evaluation, the cut-off point is increased for the specificity while the sensitivity will be not preferable. In this context, high specificity for infection prediction seems to be more meaningful.
Another independent predictor for infection recurrence identified in this study is the length of the catheter inside the body. The length of the catheter measured by CT scan (≥ 8.05 cm) was highly sensitive but not very specific to predict the infection recurrence after catheter removal. There hasn’t been any previous report regarding this issue. It is possible that an increased length in the drainage catheter will increase the chance of catheter blockage and residual infectious fluid after catheter removal. In addition, the delayed occlusion of the catheter tunnel will also provide opportunities for residual bacteria to grow after catheter removal. Therefore, we strongly suggest that when patients meet the removal criteria, the drainage catheters should be gradually withdrawn over a few days in order to continuously drain the residual fluid remaining in the tunnel.
In our study, there were only nine deaths in 193 IPN patients undergoing minimally invasive treatment. The mortality rate was lower than that in previous reports.
There were some limitations for the present study. First, it was difficult to recruit a big cohort of patients in a single-center study. As a result, the sample size for infection analysis is small. Moreover, patients with other complications such as fistula were excluded, which further reduced the study population. Second, the retrospective nature of the study may result in lower sensitivity and specificity of each individual parameter. Third, we defined drainage less than 20 mL in a 24 h period as one of the criteria for catheter removal, which is bigger than other studies. We did not evaluate whether this had any relationship to the infection incidence. Finally, our data were collected from the procedures performed within a large teaching hospital with a high volume and diverse patient population. Given the availability of the advanced technological facilities and experts at the hospital, our results may not apply to small medical centers without the same dedicated resources. This may also be the reason why the mortality rate is very low in our center (9 of 193 IPN patients treated with minimally invasive approaches). Nevertheless, with a large patient population, our findings still provide meaningful information for the prediction of infection recurrence after successful minimally invasive treatment for IPN.
In conclusion, WBC count, serum procalcitonin, and CRP measurements may be valuable for predicting infection recurrence after drainage catheter removal. Including these biomarkers in catheter removal criteria may help to prevent infection recurrence following successful minimally invasive treatment in IPN patients. In addition, gradually removing the drainage catheters over a few days may also help to reduce opportunities for infection caused by residual bacteria.
Infected pancreatic necrosis (IPN) is a vital condition. Without interventional treatment, its mortality rate is high. In recent decades, the development of minimally invasive interventional therapies provides benefits in reducing postoperative multiple organ failure and mortality. Therefore, they have been applied to an increasing number of IPN patients. There are limited data in clinical guidelines regarding infection recurrence.
To date, the most commonly reported complications of minimally invasive treatment in IPN patients include fistula, perforation, colonic injury, and pericatheter leaking. However, the infection recurrence after treatment in this patient population is not clear. The study in this aspect will certainly provide evidence for its clinical management and prevention.
This study investigated the incidence and prediction of infection recurrence following successful minimally invasive treatment in IPN patients.
Medical records for IPN patients who underwent minimally invasive treatment were retrospectively reviewed. Patients, who survived after the treatment, were divided into two groups: one group with infection after drainage catheter removal and another group without infection. The morphological and clinical data were compared between the two groups. Significantly different variables were introduced into the correlation and multivariate logistic analysis to identify independent predictors for infection recurrence. Sensitivity and specificity for diagnostic performance were determined.
Of the 193 IPN patients, 178 were recruited into the study. Of them, 9 (5.06%) patients died and 169 patients survived but infection recurred in 13 of 178 patients (7.30%) at 7 (4-10) d after drainage catheters were removed. WBC count, serum CRP, IL-6, and procalcitonin levels measured at the time of catheter removal were significantly higher in patients with infection than in those without (all P < 0.05). In addition, drainage duration and length of the catheter measured by computerized tomography scan were significantly longer in patients with infection (P = 0.025 and P < 0.0001, respectively). Although these parameters all correlated positively with the incidence of infection (all P < 0.05), only white blood cell (WBC), C-reactive protein (CRP), procalcitonin levels, and catheter length were identified as independent predictors for infection recurrence. The sensitivity and specificity for infection prediction were high in WBC count (≥ 9.95 109/L) and serum procalcitonin level (≥ 0.05 ng/mL) but moderate in serum CRP level (cut-off point ≥ 7.37 mg/L). The length of catheter (cut-off value ≥ 8.05 cm) had a high sensitivity but low specificity to predict the infection recurrence.
This study confirmed that WBC count, serum procalcitonin, and CRP levels may be valuable for predicting infection recurrence following minimally invasive intervention in IPN patients. These biomarkers should be considered before removing the drainage catheters.
This is the first study to unveil the high sensitivity and specificity of WBC count and serum procalcitonin level for predicting infection recurrence following minimally invasive treatment in IPN patients. Our findings suggest that these factors should be considered before removing the drainage catheters in clinical practice. Further study in a big patient population is required.
| 1. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4721] [Article Influence: 363.2] [Reference Citation Analysis (48)] |
| 2. | Hartwig W, Werner J, Uhl W, Büchler MW. Management of infection in acute pancreatitis. J Hepatobiliary Pancreat Surg. 2002;9:423-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Beger HG, Bittner R, Block S, Büchler M. Bacterial contamination of pancreatic necrosis. A prospective clinical study. Gastroenterology. 1986;91:433-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 626] [Cited by in RCA: 530] [Article Influence: 13.3] [Reference Citation Analysis (4)] |
| 4. | Guo Q, Li A, Xia Q, Liu X, Tian B, Mai G, Huang Z, Chen G, Tang W, Jin X, Chen W, Lu H, Ke N, Zhang Z, Hu W. The role of organ failure and infection in necrotizing pancreatitis: a prospective study. Ann Surg. 2014;259:1201-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 5. | Werge M, Novovic S, Schmidt PN, Gluud LL. Infection increases mortality in necrotizing pancreatitis: A systematic review and meta-analysis. Pancreatology. 2016;16:698-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 144] [Article Influence: 14.4] [Reference Citation Analysis (1)] |
| 6. | van Santvoort HC, Bakker OJ, Bollen TL, Besselink MG, Ahmed Ali U, Schrijver AM, Boermeester MA, van Goor H, Dejong CH, van Eijck CH, van Ramshorst B, Schaapherder AF, van der Harst E, Hofker S, Nieuwenhuijs VB, Brink MA, Kruyt PM, Manusama ER, van der Schelling GP, Karsten T, Hesselink EJ, van Laarhoven CJ, Rosman C, Bosscha K, de Wit RJ, Houdijk AP, Cuesta MA, Wahab PJ, Gooszen HG; Dutch Pancreatitis Study Group. A conservative and minimally invasive approach to necrotizing pancreatitis improves outcome. Gastroenterology. 2011;141:1254-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 485] [Article Influence: 32.3] [Reference Citation Analysis (2)] |
| 7. | Maher MM, Lucey BC, Gervais DA, Mueller PR. Acute pancreatitis: the role of imaging and interventional radiology. Cardiovasc Intervent Radiol. 2004;27:208-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13:e1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1080] [Cited by in RCA: 1095] [Article Influence: 84.2] [Reference Citation Analysis (10)] |
| 9. | Italian Association for the Study of the Pancreas (AISP), Pezzilli R, Zerbi A, Campra D, Capurso G, Golfieri R, Arcidiacono PG, Billi P, Butturini G, Calculli L, Cannizzaro R, Carrara S, Crippa S, De Gaudio R, De Rai P, Frulloni L, Mazza E, Mutignani M, Pagano N, Rabitti P, Balzano G. Consensus guidelines on severe acute pancreatitis. Dig Liver Dis. 2015;47:532-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 112] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 10. | Raraty MG, Halloran CM, Dodd S, Ghaneh P, Connor S, Evans J, Sutton R, Neoptolemos JP. Minimal access retroperitoneal pancreatic necrosectomy: improvement in morbidity and mortality with a less invasive approach. Ann Surg. 2010;251:787-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 199] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 11. | van Santvoort HC, Besselink MG, Bollen TL, Buskens E, van Ramshorst B, Gooszen HG; Dutch Acute Pancreatitis Study Group. Case-matched comparison of the retroperitoneal approach with laparotomy for necrotizing pancreatitis. World J Surg. 2007;31:1635-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | van Grinsven J, van Santvoort HC, Boermeester MA, Dejong CH, van Eijck CH, Fockens P, Besselink MG; Dutch Pancreatitis Study Group. Timing of catheter drainage in infected necrotizing pancreatitis. Nat Rev Gastroenterol Hepatol. 2016;13:306-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 13. | van Santvoort HC, Besselink MG, Bakker OJ, Hofker HS, Boermeester MA, Dejong CH, van Goor H, Schaapherder AF, van Eijck CH, Bollen TL, van Ramshorst B, Nieuwenhuijs VB, Timmer R, Laméris JS, Kruyt PM, Manusama ER, van der Harst E, van der Schelling GP, Karsten T, Hesselink EJ, van Laarhoven CJ, Rosman C, Bosscha K, de Wit RJ, Houdijk AP, van Leeuwen MS, Buskens E, Gooszen HG; Dutch Pancreatitis Study Group. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med. 2010;362:1491-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1038] [Cited by in RCA: 1079] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 14. | Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400-15; 1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1232] [Cited by in RCA: 1429] [Article Influence: 109.9] [Reference Citation Analysis (3)] |
| 15. | Leppäniemi A, Tolonen M, Tarasconi A, Segovia-Lohse H, Gamberini E, Kirkpatrick AW, Ball CG, Parry N, Sartelli M, Wolbrink D, van Goor H, Baiocchi G, Ansaloni L, Biffl W, Coccolini F, Di Saverio S, Kluger Y, Moore E, Catena F. 2019 WSES guidelines for the management of severe acute pancreatitis. World J Emerg Surg. 2019;14:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 620] [Cited by in RCA: 481] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 16. | Freeny PC, Lewis GP, Traverso LW, Ryan JA. Infected pancreatic fluid collections: percutaneous catheter drainage. Radiology. 1988;167:435-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 81] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Wu BU, Johannes RS, Sun X, Tabak Y, Conwell DL, Banks PA. The early prediction of mortality in acute pancreatitis: a large population-based study. Gut. 2008;57:1698-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 573] [Article Influence: 31.8] [Reference Citation Analysis (5)] |
| 18. | LeGall JR, Loirat P, Alpérovitch A. APACHE II--a severity of disease classification system. Crit Care Med. 1986;14:754-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 57] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | García-Ureña MÁ, López-Monclús J, Melero-Montes D, Blázquez-Hernando LA, Castellón-Pavón C, Calvo-Durán E, Gordo-Vidal F, Aguilera-del Hoyo LF. Video-assisted laparoscopic débridement for retroperitoneal pancreatic collections: a reliable step-up approach. Am Surg. 2013;79:429-433. [PubMed] |
| 20. | Parekh D. Laparoscopic-assisted pancreatic necrosectomy: A new surgical option for treatment of severe necrotizing pancreatitis. Arch Surg. 2006;141:895-902; discussion 902-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 21. | Worhunsky DJ, Qadan M, Dua MM, Park WG, Poultsides GA, Norton JA, Visser BC. Laparoscopic transgastric necrosectomy for the management of pancreatic necrosis. J Am Coll Surg. 2014;219:735-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Seewald S, Ang TL, Richter H, Teng KY, Zhong Y, Groth S, Omar S, Soehendra N. Long-term results after endoscopic drainage and necrosectomy of symptomatic pancreatic fluid collections. Dig Endosc. 2012;24:36-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Zerem E, Imamović G, Sušić A, Haračić B. Step-up approach to infected necrotising pancreatitis: a 20-year experience of percutaneous drainage in a single centre. Dig Liver Dis. 2011;43:478-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Baudin G, Chassang M, Gelsi E, Novellas S, Bernardin G, Hébuterne X, Chevallier P. CT-guided percutaneous catheter drainage of acute infectious necrotizing pancreatitis: assessment of effectiveness and safety. AJR Am J Roentgenol. 2012;199:192-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 25. | Riché FC, Cholley BP, Laisné MJ, Vicaut E, Panis YH, Lajeunie EJ, Boudiaf M, Valleur PD. Inflammatory cytokines, C reactive protein, and procalcitonin as early predictors of necrosis infection in acute necrotizing pancreatitis. Surgery. 2003;133:257-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 96] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 26. | Chen HZ, Ji L, Li L, Wang G, Bai XW, Cheng CD, Sun B. Early prediction of infected pancreatic necrosis secondary to necrotizing pancreatitis. Medicine (Baltimore). 2017;96:e7487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 27. | Mofidi R, Suttie SA, Patil PV, Ogston S, Parks RW. The value of procalcitonin at predicting the severity of acute pancreatitis and development of infected pancreatic necrosis: systematic review. Surgery. 2009;146:72-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 134] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 28. | Gogos CA, Drosou E, Bassaris HP, Skoutelis A. Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: a marker for prognosis and future therapeutic options. J Infect Dis. 2000;181:176-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 620] [Cited by in RCA: 641] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 29. | Jensen JU, Heslet L, Jensen TH, Espersen K, Steffensen P, Tvede M. Procalcitonin increase in early identification of critically ill patients at high risk of mortality. Crit Care Med. 2006;34:2596-2602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 251] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 30. | Cardoso FS, Ricardo LB, Oliveira AM, Canena JM, Horta DV, Papoila AL, Deus JR. C-reactive protein prognostic accuracy in acute pancreatitis: timing of measurement and cutoff points. Eur J Gastroenterol Hepatol. 2013;25:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 31. | Nishimoto N, Kishimoto T. Interleukin 6: from bench to bedside. Nat Clin Pract Rheumatol. 2006;2:619-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 480] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 32. | Rao SA, Kunte AR. Interleukin-6: An Early Predictive Marker for Severity of Acute Pancreatitis. Indian J Crit Care Med. 2017;21:424-428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jha AK, Tomažič A S-Editor: Yu XQ L-Editor: Filipodia E-Editor: Liu JH