Published online Jun 14, 2020. doi: 10.3748/wjg.v26.i22.3000
Peer-review started: December 30, 2019
First decision: February 16, 2020
Revised: March 31, 2020
Accepted: May 27, 2020
Article in press: May 27, 2020
Published online: June 14, 2020
Processing time: 166 Days and 14.8 Hours
Non-cirrhotic portal hypertension consists of a group of diseases characterized by signs and complications of portal hypertension, which differ from cirrhosis through histological alterations, hemodynamic characterization and, clinical outcome. Because of the similarities in clinical presentation and imaging signs, frequently these patients, and particularly those with porto-sinusoidal vascular disease (PSVD), are misdiagnosed as having liver cirrhosis and thus raising difficulties in their diagnosis. The most challenging differentiation to be considered is between PSVD and cirrhosis and, although not pathognomonic, liver biopsy is still the standard of diagnosis. Although they still require extended validation before being broadly used, new non-invasive methods for the diagnosis of porto-sinusoidal vascular disease, like transient elastography, contrast-enhanced ultrasound or metabolomic profiling, have shown promising results. Another issue is the differentiation between PSVD and chronic extrahepatic portal vein obstruction, especially now when it is known that 40% of patients suffering from PSVD develop portal vein thrombosis. In this particular case, once the portal vein thrombosis occurred, the diagnosis of PSVD is impossible according to the current guidelines. Moreover, so far, the differentiation between PSVD and sinusoidal obstruction syndrome has not been clear so far in particular circumstances. In this review we highlighted the diagnostic challenges regarding the PSVD, as well as the current techniques used in the evaluation of these patients.
Core tip: Non-cirrhotic portal hypertension consists of a group of diseases characterized by signs and complications of portal hypertension. However, their diagnosis is sometimes difficult due to their similarities in clinical presentation, imaging signs and histological modifications despite advances in their understanding. Moreover, these disorders are sometimes misdiagnosed as cirrhosis. This review highlights their diagnostic challenges, especially with regard to porto sinusoidal vascular disease, extrahepatic portal vein obstruction, sinusoidal obstruction syndrome and cirrhosis and discusses the current available diagnostic tools.
- Citation: Nicoară-Farcău O, Rusu I, Stefănescu H, Tanțău M, Badea RI, Procopeț B. Diagnostic challenges in non-cirrhotic portal hypertension - porto sinusoidal vascular disease. World J Gastroenterol 2020; 26(22): 3000-3011
- URL: https://www.wjgnet.com/1007-9327/full/v26/i22/3000.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i22.3000
Non-cirrhotic portal hypertension is a group of heterogeneous diseases, having in common both the presence of portal hypertension (PHT) as the main clinical feature and the absence of cirrhosis on histology.
The difference between them resides in the pathogenetic mechanism and the level where the PHT develops. Amongst these diseases, the most frequent entities are extrahepatic portal vein obstruction (EHPVO), idiopathic portal hypertension (IPH) and Budd Chiari syndrome – all of which are categorized as vascular liver disorders[1]. If Budd Chiari syndrome in early stages and EHPVO are recognized relatively easily, IPH may be challenging to diagnose because of the imaging and clinical similarities with cirrhosis.
Moreover, in IPH, there are still many unknown data not only concerning the physio-pathological mechanism[2-4], but also concerning its diagnosis and long-term outcome. It is currently known that portal vein thrombosis (PVT) frequently complicates IPH (up to 40% at 5 years)[4], making the diagnosis of IPH impossible according to the current guidelines, if the patient is evaluated after EHPVO has occured.
Therefore, as of recently, as a response to the new concepts that define the disease, new terminology for IPH has been proposed: Porto-sinusoidal vascular disease (PSVD)[5]. PSVD should be considered in patients with or without PHT, in the absence of histologic cirrhosis but with histological signs suggesting of PSVD[5].
However, despite the significant progress made regarding these conditions, there are still unmet needs and challenges, especially concerning their diagnosis. The purpose of this article is to highlight these challenges and present the way they are currently covered.
Since PSVD manifests mainly by clinical signs or complications of PHT in the absence of EHPVO, these patients are often misdiagnosed as cryptogenic cirrhosis[4]. Indeed, 5.4% of all presumed cryptogenic cirrhosis are, in fact, PSVD[6]. If in the case of PSVD the liver function is preserved for a longer time, and the prognosis is relatively good, in the case of cirrhosis, the outcome is entirely different.
PSVD occurs in young patients, mostly under 40 years old in the Western world[7,8] or even earlier in the Eastern world[2], by signs or complications of PHT. It should be noted that ascites may develop by a triggering factor, and is usually transient[1,2] .
Contrary to PSVD, cirrhosis develops usually later in life, depending on the etiological factor and the lasting time of the causal factor, while the most frequent complication is ascites[9]. In patients with hepatitis C virus infection, there are approximately 30 years from the moment of infection until the development of cirrhosis[10]. Therefore, in young patients without apparent etiological factors, the diagnosis of PSVD should not be discarded until performing an extensive workup (including liver biopsy).
If in cirrhosis, the prognosis is mainly related to the liver function and the degree of PHT, in PSVD the liver function remains normal for a more extended period, which will lead to better toleration of complications. The outcome of patients also depends on the associated diseases and varies within different cohorts between 56% and 82% at ten years[8,11]. Also, even if 19% of the patients will develop liver impairment (and some of them will require liver transplantation[7,11] ), the survival rate of patients with PSVD is situated between that of the general population and that of patients who have cirrhosis[12].
Nonetheless, both conditions tend to develop PVT during disease[4,13]. Compared to cirrhosis, PSVD patients develop PVT more frequently, up to 40% at five years[4], therefore having a negative impact over life expectancy[14-17]. In both conditions, alterations of coagulation factors are present, which may contribute to PVT occurrence. PSVD is frequently associated with thrombotic disorders, as protein C and S deficiency, factor V Leiden mutation, or antiphospholipid syndrome[4,7,14,15]. In cirrhosis, protein C, S, and antithrombin III are all decreased whereas factor VIII and von Willebrand factor, strong promoters of coagulation, are increased[18]. However, the exact mechanism of PVT development in cirrhosis is unknown.
Therefore, based only on clinical features, it is impossible to distinguish between PSVD and cirrhosis. However, the differentiation between the two conditions is essential for the prognostic and for liver transplantation reference. Consequently, an extensive workup might be necessary to differentiate between the two conditions. If, in the case of liver cirrhosis, the diagnosis can be made based on non-invasive tools, the diagnosis of PSVD demands the exclusion of other causes of PHT[1] using a liver biopsy which is the definitive diagnostic tool.
The first step in evaluating a patient with clinical signs of PHT is by imaging workup. Ultrasound (US) examination is frequently the first examination performed in patients with suspicion of PHT. In PSVD patients, the liver aspect may be either normal or inhomogeneous with an irregular surface (nodular transformation), therefore rendering it very difficult to differentiate it from cirrhosis. In both conditions, the dominating findings are splenomegaly, portal venous axis dilatation, and the presence of spontaneous shunts. Interestingly, in PSVD, the spleen is often larger in comparison with cirrhosis[19,20]. In PSVD, the portal vein and its intrahepatic branches may have atypical thickened (> 3 mm) and have hyperechoic walls. This sign could indicate periportal fibrosis manifested as a “layered” pattern. These changes are accompanied by a sudden narrowing or cutoff of intrahepatic second- and third-degree portal vein branches like a ‘‘withered tree appearance’’[20]. Using contrast-enhanced US, in PSVD, the parenchymal enhancement is more heterogeneous due to delayed periportal enhancement[21]. Also, the time-intensity curves were different between PSVD and cirrhosis[22].
Cross-sectional imaging methods come in addition to the US and have better performance to assess its extension and duration, mainly if thrombosis of the portal vein is found in the US. Although there is no specific sign for PSDV, the presence of subcapsular atrophy, heterogeneous hepatic enhancement, or paucity of the medium size portal branches may suggest the diagnosis[23].
Liver stiffness measurement (LSM) proved to have excellent results in both the diagnosis of advanced liver diseases and in the usage of identifying and selecting patients at risk to have clinical significant portal hypertension[24]. As such, in patients with splenomegaly or low platelet count (high pretest probability of advanced liver disease), an LSM higher than 20 kPa would be a definitive diagnostic for cirrhosis complicated with clinically significant PHT. In patients with PSDV, liver stiffness is normal or slightly elevated in comparison with patients with cirrhosis, 8.4 ± 3.3 kPa vs 40.9 ± 20.5 kPa respectively[25], results that were further confirmed by other studies as well[26] .
Interestingly, there is a subgroup of patients with PSVD in which the LSM does not help differentiate them from those with cirrhosis. These patients have histological signs of nodular regenerative hyperplasia (NRH), and their LSM ranges from 3.5 kPa to 22.0 kPa[27,28] making it difficult to suspect PSVD if the LSM is above 12.5 kPa, which is close to the threshold seen in liver cirrhosis[29,30]. Moreover, it seems not to correlate with the degree of liver fibrosis on histological examination[27]. According to the new definition, NRH is a specific histological sign of PSVD[5]. NRH is a nodular transformation of the hepatic parenchyma without fibrosis surrounding the nodules. Obliterative portal venopathy is the stimulus of this abnormal arrangement of hepatocytes. These alterations of the portal flow lead to the atrophy of tributary hepatocytes and the hyperplasia of the hepatocytes with normal portal vascularization[31]. Moreover, perisinusoidal, centrolobular and some degree of portal and periportal fibrosis may be observed in NRH and this might contribute to the increased LSM[27].
Despite the modest performances for non-cirrhotic PHT diagnosis, LSM has an important role in ruling out cirrhosis. Therefore, in patients with clinical signs of PHT but with low LSM, non-cirrhotic PHT should be strongly suspected.
Hemodynamic studies are used to evaluate the severity of PHT indirectly. The hepatic venous pressure gradient (HVPG) measurement behaves as a risk prediction method in patients with cirrhosis, because it is associated with the development of esophageal varices, variceal bleeding, ascites or hepatocellular carcinoma[32].
Since PSVD patients have a presinusoidal type of PHT, such correlations cannot be made for these patients. Still, hepatic hemodynamic studies are essential for the distinction between the two disorders and cirrhosis.
Compared with cirrhosis, in PSVD patients with signs of PHT, the HVPG is normal or slightly elevated (7.8 ± 3.6 mmHg in IPH vs 17.0 ± 3.0 mmHg in cirrhosis)[25]. Although PSVD often has vein-to-vein communications, which might preclude to obtain an adequate wedge hepatic venous pressure, if the balloon is inflated below the vein-to vein shunt, HVPG might be accurately measured[25] .
Nonetheless, the challenge arises when the hemodynamic studies in patients with PSVD show values similar to the sinusoidal type of PHT. Indeed, 70% of the patients with NRH with clinical signs of PHT (varices/ascites) had HVPG < 10 mmHg suggesting pre-sinusoidal PHT, which was confirmed by a portal vein pressure higher than 12 mm Hg[33]. However, 30% of patients had an HVPG > 10 mmHg (WHVP ranged from 16 -34 mmHg), suggesting sinusoidal PHT[33]. These findings indicate that both types of PHT may coexist in NRH (pre-sinusoidal and sinusoidal) depending on the predominant mechanism causing the PHT: OPV- pre-sinusoidal or compression of the sinusoids by the regenerative nodules- sinusoidal[33,34]. Nevertheless, PSVD is mainly a presinusoidal type of PHT as demonstrated by the increase in the splenic-to-free hepatic pressure gradient[35]. However, the treatment with non-selective beta-blockers decreases portal pressure in patients with PSVD as well as EHPVO[36].
PSVD shows a hyperdynamic circulation comparable with compensated cirrhosis proven by the increased cardiac index and low systemic and pulmonary vascular resistance[25,37].
When performing venography, contrary to cirrhosis, PSVD shows frequent vein-to-vein communications, and the angles between large veins and their tributaries are narrower[38]. Also, the middle-sized branches are smooth, but sometimes with irregularities giving a general appearance of “weeping willow”[38].
Transjugular liver biopsy is performed most frequently in PSVD patients because of severe thrombocytopenia. The transjugular route also allows for HVPG measurement. In case of clinical signs of PHT (esophageal varices, splenomegaly and low platelet count) finding an HVPG less than 10 mmHg strongly weights against sinusoidal PHT and should raise the suspicion of non-cirrhotic PHT. Finally, the result of the liver biopsy will lead to a definitive diagnosis. Indeed, the current proposed definition of histological findings clearly states that the diagnosis of PSVD needs the exclusion of liver cirrhosis on a good quality specimen[5].
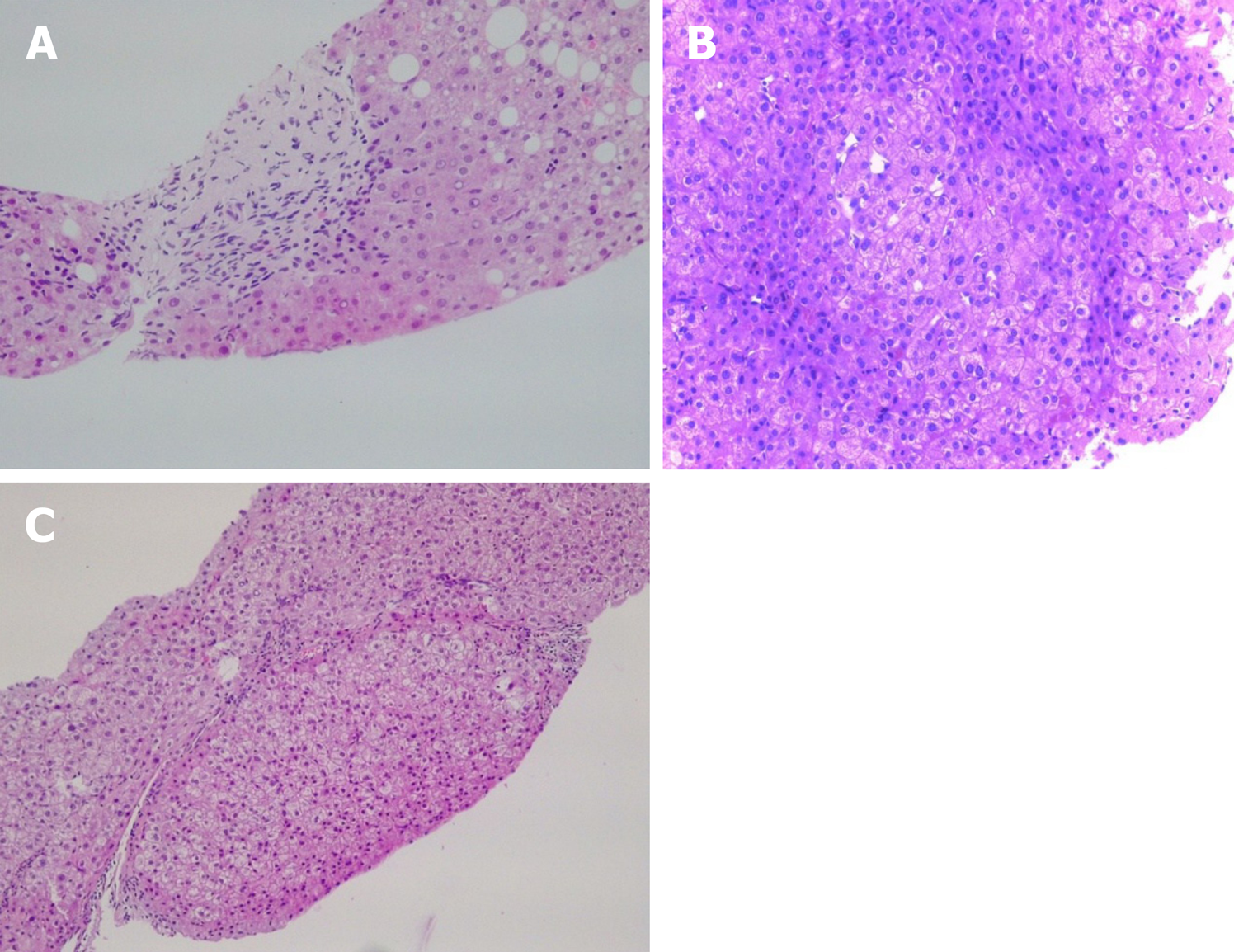
Classified according to their localization, the most frequent findings are: (1) Portal tracts: Phlebosclerosis or obliterative portal venopathy of the portal veins, characterized by luminal narrowing and sclerosis (Figure 1A); the absence of the portal vein radicle (vanishing); aberrant portal vessels herniated into the periportal parenchyma; abnormal thin shunt vessels adjacent to the portal tracts that open into the sinusoids; various of thin-walled vessels inside the portal tract; portal tracts remnants (hypoplastic portal tract), and incomplete septal fibrosis. Immuno-histochemistry analysis may not show pathognomonic features. However, there seems to be differences between the different protein expressions in PSVD and cirrhosis. For example, the connective tissue growth factor is highly expressed in portal mononuclear cells around the bile ducts in PSVD patients. Still, it showed a low expression of matrix metalloproteinase 9 as opposed to what was seen in cirrhotic livers[39]. This pattern might explain the periductal and periportal fibrosis in PSVD[39]. In PSVD patients, the expression of CD34 (a marker of sinusoidal endothelial cells) was reduced, while pSmad2 was increased in the peripheral portal veins, as opposed to what was seen in cirrhosis[40]; (2) Hepatic parenchyma: Parenchymal collapse in the subcapsular region, atrophic hepatocytes with focal nodular regenerative hyperplasia and sinusoidal dilatation with eventually perisinusoidal fibrosis; and (3) Centrolobular vein: Central vein dilatation with or without phlebosclerosis[3,41,42].
Apart from these lesions, two other important findings represent major diagnostic arguments for PSVD: Nodular regenerative hyperplasia and incomplete septal fibrosis[43].
Nodular regenerative hyperplasia is a nodular transformation of the hepatic parenchyma characterized by hyperplastic hepatocytes arranged in few cell-thick plates and surrounded by zones of atrophic hepatocytes without fibrosis (Figure 1B).
Compared with PSVD, in cirrhosis, aside from the presence of regenerative nodules with fibrosis, there is a reduction in the portal vascular bed with a compensatory increase of the hepatic arteries and intrahepatic shunt formation between the portal veins and hepatic veins or hepatic arteries and hepatic veins or portal veins. In PSVD, the hepatic arterial flow fails to compensate for the reduction of the portal venous index leading to atrophy - especially in the subcapsular area[3,44]. These histological modifications have been distributed into different stages according to the severity of the disease: Stage I, with the absence of peripheral parenchymal atrophy, stage II, with the presence of peripheral parenchymal atrophy in a non-atrophic liver, stage III with the presence of peripheral parenchymal atrophy in an atrophic liver and stage IV with the presence of obstructive thrombosis in intrahepatic large branches or trunk of portal vein[45].
Incomplete septal fibrosis is characterized by thin fibrous septa that originate from the portal tract and end blindly inside the hepatic parenchyma without bridging with other septa (Figure 1C)[43]. Recently, increasing evidence of cirrhosis regression emerged, and one of the most challenging tasks is to differentiate cirrhosis regression[46] from incomplete septal fibrosis (related to PSVD)[47]. Following the regression of cirrhosis, vasculature anomalies can persist for many years, which is probably the reason for PHT findings in these patients. “Hepatic repair complex” represents histological prove of cirrhosis regression. It includes isolated thick fibrous septa, periportal fibrous spikes, portal tracts remnants, aberrant parenchymal vessels or regenerative nodules[46]. Together with a very well documented clinical history and etiological workup it is essential to differentiate between the two pathological entities[43].
It should be noted that esophageal varices or variceal bleeding is the most frequent symptom in patients with PSVD. It has been proven that in cirrhosis, HVPG ≥ 10 mmHg, which defines clinically significant PHT (CSPH), increases the risk of esophageal varices that need treatment. Consequently, in compensated cirrhosis, all efforts should be made to identify those patients with CSPH, preferably using non-invasive means. Thus, according to the last Baveno consensus, in the case of cirrhotic patients if platelets are above 150000/mm3 and liver stiffness measured by transient elastography is less than 20 kPa[48], then screening endoscopies can be avoided. However, these recommendations cannot be made for non-cirrhotic portal hypertensive patients.
Even so, it is worth noting that while in PSVD the esophageal varices are often large and gastric varices are more common than in the case of cirrhosis[7,49,50] the course of esophageal varices development and variceal growth is very similar between patients with PSDV and cirrhosis[7,12]. Recent studies have shown that patients with PSVD without varices develop them at rates of 10%, 20%, and 65%, and those with small ones show progression at rates of 13%, 35%, and 44% at 1, 2, and 5 years, respectively[11,12] .
Recently, plasma global metabolic profiling in patients with PSVD was able to distinguish between PSVD and cirrhosis based on 28 metabolites with an AUROC of 0.99[51]. Using targeted analysis, the same group identified three lipid metabolites (fatty acid, lysophosphatidylethanolamine, and triacylglycerol) to differentiate PSVD from cirrhosis. Two lipid metabolites (bile acid and lysophosphatidylethanolamine) would discriminate PSDV from healthy volunteers[52]. New biomarkers of PSVD may offer opportunities for diagnosis of PSVD in preclinical stages.
The EHPVO is considered to be a childhood disease in the developing countries, whereas in the Western world, it is the second most frequent cause of PHT in adults, and a prothrombotic state frequently causes it[53].
Similar to PSVD, liver structure and function remain preserved until late in the course of the disease, and the most important clinical presentation is recurrent episodes of gastroesophageal variceal bleeding, which are often well controlled. The hallmark of chronic EHPVO is the cavernous transformation of the portal vein, which is easily detectable on different imaging methods. As previously mentioned, up to 40% of PSVD patients will develop PVT during the disease[4], and thus, some patients may become symptomatic only after PVT occurrence. According to the current guidelines, these patients cannot be correctly diagnosed. However, the most recent definition does not exclude the diagnosis of PSVD in the context of PVT[5]. Still, when a cavernoma reveals the signs of PHT, the diagnosis of PSVD cannot be made[5]. This is a diagnostic challenge, nonetheless, because the cavernous transformation can occur soon after the acute thrombosis[54]. Thus, it cannot exclude the presence of a PVT lying on a PSVD. When PVT develops in PSVD, it is mostly restricted in the main trunk or the intrahepatic branches of the portal vein. However, the extension to the superior mesenteric vein (SMV) or splenic vein (SV) is possible and might lead to the progression of the disease[8-15]. Isolated SV and SMV thrombosis are excluded from the definition of EHPVO. However, the extension to these vessels is possible as well in this setting. At presentation, one-third of the patients may already have an extended PV obstruction[55]. Indeed, apart from the possible thrombophilia factors associated with PSVD and EHPVO, alike in cirrhosis, the splenic vein endothelium suffers anomalies due to the increase in portal hypertension. These injuries could be a reason for splenic thrombosis[56].
Because in both conditions prothrombotic abnormalities are frequently associated and because both have similar clinical features, for the moment, it is impossible to differentiate between PSVD complicated with PVT and EHPVO.
In reality, the situation is even more complicated because chronic EHPVO may lead to a dysmorphic liver and a mosaic pattern of parenchymal enhancement in the arterial phase[57] and, therefore, in some cases it is impossible to be differentiated from cirrhosis or PSDV. These morphological abnormalities have a histological correspondence, which is similar in EHPVO and PSDV.
Probably the most relevant question to be answered is whether the distinction of these disorders has an impact on the clinical practice. At the moment, both diseases are managed similarly as cirrhosis, namely, to control PHT related complications[1]. As previously stated, the diagnosis of EHPVO is based on imaging findings. The hallmark of the US is the cavernous transformation of the portal vein defined by serpiginous vascular channels replacing the portal vein. Despite the patency of these collaterals, they are not able to maintain an efficient portal inflow and thus determining the development of PHT. Similar to PSVD and cirrhosis, there are signs of PTH as splenomegaly and portosystemic collaterals. Usually, the liver surface is smooth, but with the progression of the disease, it may become irregular – therefore simulating cirrhosis.
Sometimes signs of portal biliopathy may be seen[5,58], and using the contrast-enhanced US the diagnosis is more accurate[59]. There is no imaging technique or sign that may be used to distinguish PSVD complicated with PVT from EHPVO.
LSM by transient elastography shows lower values than those observed in cirrhosis and interestingly, lower also than those found in PSVD[30]. As in most cases of PSVD, in pure EHPVO, the HVPG is also below 10 mmHg[16,25].
In EHPVO, as in PSVD, 71% of the patients are already diagnosed with large varices at the first presentation. If no varices are found at initial endoscopy, the esophageal varices development rate is 2%, 22% and 22% at 1, 3 and 5 years, respectively. Also, small varices grow at a rate of 13%, 40%, and 54% at 1, 3 and 5 years, respectively[60]. This similar natural history between these conditions led to the same management[1], although the level of evidence for PSDV and EHPVO is quite low.
The liver histology is not characteristic in EHPVO. As such, portal fibrosis can be found in about 40% of the cases. Compared with EHPVO, PSDV showed phlebosclerosis, portal tract remnants, and nodular regeneration more frequently[42]. Table 1 resumes the comparison between different histological findings in PSDV, EHPVO, and cirrhosis. A summary of the most relevant diagnostic tools differentiating PSVD from EHPVO and cirrhosis is presented in Table 2.
| PSVD | EHPVO | Cirrhosis | ||
| Portal and periportal zone | ||||
| Small portal vein obliteration (“hepato-portal sclerosis", phlebosclerosis) | ++ | +/- | - | |
| Periportal shunt vessel | ++ | + | - | |
| Fibrous septa | +/-; without bridging1 | - | ++; with bridging delimitating nodules | |
| Inflammation | - | - | ++ | |
| Portal biliopathy | - | +/- | - | |
| Liver lobule (parenchyma) | ||||
| Perisinusoidal fibrosis | + | - | +/- | |
| Sinusoidal dilatation | ++ | ++ | - | |
| Nodular regenerative hyperplasia | + | + | - | |
| Hepatocyte atrophy | + | + | - | |
| Centrilobular zone | ||||
| Central vein dilatation | ++ | + | - | |
| Perivenular fibrosis | ++ | + | - | |
| Diagnostic tools | PSVD | EHPVO | Cirrhosis |
| Invasive tools | |||
| Liver biopsy | Predominant vascular anomalies | Predominant vascular anomalies | Predominant architectural changes and fibrosis |
| Hepatic hemodinamics | FHVP N | FHVP N | FHVP N |
| WHVP ↓ | WHVP ↓ | WHVP ↑ | |
| HVPG N or slightly ↑ | HVPG N | HVPG ↑ | |
| Frequent vein-to-vein communications | Infrequent vein-to-vein communications | Infrequent vein-to-vein communications | |
| Hyperdynamic circulatory state | Hyperdynamic circulatory state | Hyperdynamic circulatory state | |
| Endoscopic findings | Esophageal varices | Esophageal varices | Esophageal varices |
| Gastric varices (GOV1, GOV2) more common | Gastric varices more common (especially IGV1, IGV2) | Gastric varices less common | |
| Portal hypertensive gastropathy less common | Portal hypertensive gastropathy less common | Portal hypertensive gastropathy more common | |
| Non invasive tools | |||
| Ultrasound examination | |||
| Liver | Normal/irregular surface | Normal/irregular surface | Irregular surface |
| Homogeneous/innhomogenous parenchima | Homogeneous/innhomogenous parenchima | Innhomogenous | |
| Focal liver lesions | |||
| Portal veins | Dilatated, hyperechoic with thickened walls | Chronic thrombosis | Dilatated |
| +/- spontaneous shunts | +/- cavernomatous transformation | +/- thrombosis | |
| +/- thrombosis | +/- portal biliopathy | ||
| Spleen | Often giant splenomegaly | Often giant splenomegaly | Splenomegaly |
| Porto systemic collaterals, +/- ascites | |||
| CEUS examination | Delayed periportal enhancement | Homogenous/heterogenous/delayed periportal enhancement | |
| Cross-sectional imaging | Heterogeneous hepatic enhancement or paucity of the medium size portal branches | (1) Better characterization of the level and extension of thrombus; and (2) Better characterization of the portal biliopathy | Better characterization of the focal liver lesions |
| Elastography | |||
| Liver stiffenss | N or slightly ↑ | N or slightly ↑ (less then in the PSVD) | ↑ (has prognostic value) |
| Spleen stiffness | ↑ | ↑ | ↑ (less then in PSVD or EHPVO) |
Recently, increased attention has focused on the ability to discern between the healthy population and those with histological signs of PSVD but no clinical signs of PHT. Indeed, 20% of the patients with obliterative portal venopathy without signs of PHT, present initially alterations in liver enzymes without any other etiology[17]. This is important since 40% of the these patients will developed PHT during the follow up[61]. Until present, no laboratory findings (either serum tests, autoantibody, or pro-thrombotic conditions) may predict which patient will develop PHT[61]. Naturally, this raised the question whether obliterative portal venopathy can be considered an early stage of PSDV and whether the diagnostic could be made sooner (before the development of PHT)[17,61]. Indeed, the new definition of PSVD includes patients with histological signs of PSVD but without clinical signs of PHT[5].
However, currently there are not enough data regarding either the natural history or what should be the appropriate management of these patients.
The definition of PSVD excludes patients with sinusoidal obstruction syndrome (SOS) and those following bone marrow transplantation. However, so far, data regarding the distinction between the two entities is scarce, and sometimes these two conditions are confounded. Indeed, the diagnosis of SOS is simple, according to the clinical Seattle and Baltimore criteria[62]. SOS is defined by hepatomegaly, fluid retention and weight gain, with elevated serum bilirubin that follows cytoreductive therapy, in the absence of other explanations for these signs and symptoms. It occurs between 0 and 20 d following the administration of the toxic drug[62]. The challenge arises when SOS become evident long after the administration of the causative agent[63,64], especially after oxaliplatin or azathioprine, which are two drugs that could contribute to the development of PSVD[62].
Histologically, SOS is characterized by sinusoidal destruction and microscopic hemorrhage; however, this pattern is present in 51% of cases in one series[63]. Nevertheless, 15%-33% of patients will also develop NRH induced-oxaliplatin, which might be related to the severity of sinusoidal injury[65-67].
In patients treated with oxaliplatin, the depletion of glutathione transferase could be the initial mechanism of SOS[63,68]. This depletion leads to toxic insult to sinusoidal endothelial cells and it compromises the integrity of the vascular wall, followed by activation of the hepatic stellate cells and the deposition of matrix in the sinusoids. Obstruction is caused by erythrocytes sloughing, and blebs, characterized by free fragments of cytoplasmic processes, occasionally containing cellular organelles. Finally, oxaliplatin may cause fibrotic obliteration of the small vessels, hepatocyte plate disruption, and parenchymal extinction lesions along with hyperperfused regenerative areas[65,69,70]. Chronic hypoxia of the centrilobular areas caused by intrahepatic blood flow impairment may lead to NRH[63]. However, signs of HNR can be found outside of the spectrum of SOS[65].
Some patients treated with azathioprine will also develop signs of SOS/NRH. However, the pathophysiological mechanisms are not very clear[62,71] .
As both SOS and NRH can appear after treatment with oxaliplatin and azathioprine, the distinction between the two is very important. This is relevant because, although in patients with SOS most cases (70%-90%) will resolve spontaneously, a few may progress to NRH[62].
Moreover, it is important since a significant feature of NRH is that it can become clinically evident long after the finalization of chemotherapy[63,72].
In the pediatric population, EHPVO is the most frequent cause of portal hypertension. It is caused mostly by phlebitis after umbilical catheterization or omphalitis, previous surgery, dehydration or a prothrombotic state[73]. Rarely, it is caused by non-cirrhotic portal fibrosis which accounts for 4.6% of all causes of non-cirrhotic PHT in children[74]. This entity is compatible with what is described in adults as PSVD[50,75,76]. However, it is important to notice some clinical differences. It occurs secondary to malignancy or after chemotherapy[50] or may have a genetic component[77]. The clinical presentation occurs by the age of 13 years and is found predominantly in males. Frequently, the diagnosis is revealed by splenomegaly, symptomatic hypersplenism, or variceal bleeding[11] . Unlike the adult population, the growth retardation is seen in 73% of children[74], although this feature has been described in EHPVO as well[78]. Due to the low number of patients, little is known so far about the long term prognosis or these patients, but in the mid-term, it seems to be favorable[74].
In conclusion, with all available diagnostic tools, the diagnosis of PSVD with or without PH remains challenging, and liver biopsy is still indispensable. PSVD frequently complicates with PVT and, if the diagnosis is not previously done, the diagnosis is currently virtually impossible. This is because there are no pathognomonic features for PSVD on liver biopsy and, moreover, PSVD and EHPVO share many histological findings. To overcome these drawbacks, evidence from the metabolomics field are promising, yet require further studies.
| 1. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Vascular diseases of the liver. J Hepatol. 2016;64:179-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 565] [Article Influence: 56.5] [Reference Citation Analysis (3)] |
| 2. | Sarin SK, Kapoor D. Non-cirrhotic portal fibrosis: current concepts and management. J Gastroenterol Hepatol. 2002;17:526-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 104] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Okudaira M, Ohbu M, Okuda K. Idiopathic portal hypertension and its pathology. Semin Liver Dis. 2002;22:59-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 97] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Hernández-Gea V, Baiges A, Turon F, Garcia-Pagán JC. Idiopathic Portal Hypertension. Hepatology. 2018;68:2413-2423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | De Gottardi A, Rautou PE, Schouten J, Rubbia-Brandt L, Leebeek F, Trebicka J, Murad SD, Vilgrain V, Hernandez-Gea V, Nery F, Plessier A, Berzigotti A, Bioulac-Sage P, Primignani M, Semela D, Elkrief L, Bedossa P, Valla D, Garcia-Pagan JC; VALDIG group. Porto-sinusoidal vascular disease: proposal and description of a novel entity. Lancet Gastroenterol Hepatol. 2019;4:399-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 195] [Article Influence: 32.5] [Reference Citation Analysis (1)] |
| 6. | Jain M, Venkataraman J, Varghese J, Vij M, Reddy MS, Rela M. Explant liver evaluation decodes the mystery of cryptogenic cirrhosis! JGH Open. 2020;4:39-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Hillaire S, Bonte E, Denninger MH, Casadevall N, Cadranel JF, Lebrec D, Valla D, Degott C. Idiopathic non-cirrhotic intrahepatic portal hypertension in the West: a re-evaluation in 28 patients. Gut. 2002;51:275-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 209] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 8. | Siramolpiwat S, Seijo S, Miquel R, Berzigotti A, Garcia-Criado A, Darnell A, Turon F, Hernandez-Gea V, Bosch J, Garcia-Pagán JC. Idiopathic portal hypertension: natural history and long-term outcome. Hepatology. 2014;59:2276-2285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 148] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 9. | D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1892] [Cited by in RCA: 2216] [Article Influence: 110.8] [Reference Citation Analysis (3)] |
| 10. | Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2199] [Cited by in RCA: 2169] [Article Influence: 74.8] [Reference Citation Analysis (0)] |
| 11. | Schouten JN, Nevens F, Hansen B, Laleman W, van den Born M, Komuta M, Roskams T, Verheij J, Janssen HL. Idiopathic noncirrhotic portal hypertension is associated with poor survival: results of a long-term cohort study. Aliment Pharmacol Ther. 2012;35:1424-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 12. | Sarin SK, Kumar A, Chawla YK, Baijal SS, Dhiman RK, Jafri W, Lesmana LA, Guha Mazumder D, Omata M, Qureshi H, Raza RM, Sahni P, Sakhuja P, Salih M, Santra A, Sharma BC, Sharma P, Shiha G, Sollano J; Members of the APASL Working Party on Portal Hypertension. Noncirrhotic portal fibrosis/idiopathic portal hypertension: APASL recommendations for diagnosis and treatment. Hepatol Int. 2007;1:398-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 124] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 13. | Tsochatzis EA, Senzolo M, Germani G, Gatt A, Burroughs AK. Systematic review: portal vein thrombosis in cirrhosis. Aliment Pharmacol Ther. 2010;31:366-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 226] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 14. | Gioia S, Nardelli S, Pasquale C, Pentassuglio I, Nicoletti V, Aprile F, Merli M, Riggio O. Natural history of patients with non cirrhotic portal hypertension: Comparison with patients with compensated cirrhosis. Dig Liver Dis. 2018;50:839-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 15. | Matsutani S, Maruyama H, Akiike T, Kobayashi S, Yoshizumi H, Okugawa H, Fukuzawa T, Kimura K, Saisho H. Study of portal vein thrombosis in patients with idiopathic portal hypertension in Japan. Liver Int. 2005;25:978-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Khanna R, Sarin SK. Idiopathic portal hypertension and extrahepatic portal venous obstruction. Hepatol Int. 2018;12:148-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 17. | Cazals-Hatem D, Hillaire S, Rudler M, Plessier A, Paradis V, Condat B, Francoz C, Denninger MH, Durand F, Bedossa P, Valla DC. Obliterative portal venopathy: portal hypertension is not always present at diagnosis. J Hepatol. 2011;54:455-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 126] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 18. | Tripodi A, Primignani M, Chantarangkul V, Dell'Era A, Clerici M, de Franchis R, Colombo M, Mannucci PM. An imbalance of pro- vs anti-coagulation factors in plasma from patients with cirrhosis. Gastroenterology. 2009;137:2105-2111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 404] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 19. | Working Subgroup for Clinical Practice Guideline for Aberrant Portal Hemodynamics*,**. Diagnosis and treatment guidelines for aberrant portal hemodynamics: The Aberrant Portal Hemodynamics Study Group supported by the Ministry of Health, Labor and Welfare of Japan. Hepatol Res. 2017;47:373-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Rajesh S, Mukund A, Sureka B, Bansal K, Ronot M, Arora A. Non-cirrhotic portal hypertension: an imaging review. Abdom Radiol (NY). 2018;43:1991-2010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Maruyama H, Okugawa H, Kobayashi S, Yoshizumi H, Takahashi M, Ishibashi H, Yokosuka O. Non-invasive portography: a microbubble-induced three-dimensional sonogram for discriminating idiopathic portal hypertension from cirrhosis. Br J Radiol. 2012;85:587-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Maruyama H, Shimada T, Ishibashi H, Takahashi M, Kamesaki H, Yokosuka O. Delayed periportal enhancement: a characteristic finding on contrast ultrasound in idiopathic portal hypertension. Hepatol Int. 2012;6:511-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Glatard AS, Hillaire S, d'Assignies G, Cazals-Hatem D, Plessier A, Valla DC, Vilgrain V. Obliterative portal venopathy: findings at CT imaging. Radiology. 2012;263:741-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Berzigotti A. Non-invasive evaluation of portal hypertension using ultrasound elastography. J Hepatol. 2017;67:399-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 198] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 25. | Seijo S, Reverter E, Miquel R, Berzigotti A, Abraldes JG, Bosch J, García-Pagán JC. Role of hepatic vein catheterisation and transient elastography in the diagnosis of idiopathic portal hypertension. Dig Liver Dis. 2012;44:855-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 26. | Sharma P, Agarwal R, Dhawan S, Bansal N, Singla V, Kumar A, Arora A. Transient Elastography (Fibroscan) in Patients with Non-cirrhotic Portal Fibrosis. J Clin Exp Hepatol. 2017;7:230-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 27. | Laharie D, Vergniol J, Bioulac-Sage P, Diris B, Poli J, Foucher J, Couzigou P, Drouillard J, de Lédinghen V. Usefulness of noninvasive tests in nodular regenerative hyperplasia of the liver. Eur J Gastroenterol Hepatol. 2010;22:487-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Vuppalanchi R, Mathur K, Pyko M, Samala N, Chalasani N. Liver Stiffness Measurements in Patients with Noncirrhotic Portal Hypertension-The Devil Is in the Details. Hepatology. 2018;68:2438-2440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Lédinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1796] [Cited by in RCA: 1861] [Article Influence: 88.6] [Reference Citation Analysis (2)] |
| 30. | Pavlov CS, Casazza G, Nikolova D, Tsochatzis E, Burroughs AK, Ivashkin VT, Gluud C. Transient elastography for diagnosis of stages of hepatic fibrosis and cirrhosis in people with alcoholic liver disease. Cochrane Database Syst Rev. 2015;1:CD010542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 31. | Wanless IR, Godwin TA, Allen F, Feder A. Nodular regenerative hyperplasia of the liver in hematologic disorders: a possible response to obliterative portal venopathy. A morphometric study of nine cases with an hypothesis on the pathogenesis. Medicine (Baltimore). 1980;59:367-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 184] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 32. | Bosch J, Abraldes JG, Berzigotti A, García-Pagan JC. The clinical use of HVPG measurements in chronic liver disease. Nat Rev Gastroenterol Hepatol. 2009;6:573-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 545] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 33. | Bissonnette J, Généreux A, Côté J, Nguyen B, Perreault P, Bouchard L, Pomier-Layrargues G. Hepatic hemodynamics in 24 patients with nodular regenerative hyperplasia and symptomatic portal hypertension. J Gastroenterol Hepatol. 2012;27:1336-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Ghabril M, Vuppalanchi R. Drug-induced nodular regenerative hyperplasia. Semin Liver Dis. 2014;34:240-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Keiding S, Solvig J, Grønbaek H, Vilstrup H. Combined liver vein and spleen pulp pressure measurements in patients with portal or splenic vein thrombosis. Scand J Gastroenterol. 2004;39:594-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | Sørensen M, Larsen LP, Villadsen GE, Aagaard NK, Grønbæk H, Keiding S, Vilstrup H. β-Blockers Improve Presinusoidal Portal Hypertension. Dig Dis Sci. 2018;63:3153-3157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Sharma P, Kumar A, Mehta V, Sharma BC, Sarin SK. Systemic and pulmonary hemodynamics in patients with non-cirrhotic portal fibrosis (NCPF) is similar to compensated cirrhosis. Hepatol Int. 2007;1:275-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 38. | Futagawa S, Fukazawa M, Musha H, Isomatsu T, Koyama K, Ito T, Horisawa M, Nakayama S, Sugiura M, Kameda H, Okuda K. Hepatic venography in noncirrhotic idiopathic portal hypertension. Comparison with cirrhosis of the liver. Radiology. 1981;141:303-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 43] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 39. | Tsuneyama K, Kouda W, Nakanuma Y. Portal and parenchymal alterations of the liver in idiopathic portal hypertension: a histological and immunochemical study. Pathol Res Pract. 2002;198:597-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 40. | Kitao A, Sato Y, Sawada-Kitamura S, Harada K, Sasaki M, Morikawa H, Shiomi S, Honda M, Matsui O, Nakanuma Y. Endothelial to mesenchymal transition via transforming growth factor-beta1/Smad activation is associated with portal venous stenosis in idiopathic portal hypertension. Am J Pathol. 2009;175:616-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 41. | Guido M, Alves VAF, Balabaud C, Bathal PS, Bioulac-Sage P, Colombari R, Crawford JM, Dhillon AP, Ferrell LD, Gill RM, Hytiroglou P, Nakanuma Y, Paradis V, Quaglia A, Rautou PE, Theise ND, Thung S, Tsui WMS, Sempoux C, Snover D, van Leeuwen DJ; International Liver Pathology Study Group. Histology of portal vascular changes associated with idiopathic non-cirrhotic portal hypertension: nomenclature and definition. Histopathology. 2019;74:219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 42. | Verheij J, Schouten JN, Komuta M, Nevens F, Hansen BE, Janssen HL, Roskams T. Histological features in western patients with idiopathic non-cirrhotic portal hypertension. Histopathology. 2013;62:1083-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 43. | Guido M, Sarcognato S, Sacchi D, Colloredo G. Pathology of idiopathic non-cirrhotic portal hypertension. Virchows Arch. 2018;473:23-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 44. | Tsuneyama K, Ohba K, Zen Y, Sato Y, Niwa H, Minato H, Nakanuma Y. A comparative histological and morphometric study of vascular changes in idiopathic portal hypertension and alcoholic fibrosis/cirrhosis. Histopathology. 2003;43:55-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 45. | Nakanuma Y, Tsuneyama K, Ohbu M, Katayanagi K. Pathology and pathogenesis of idiopathic portal hypertension with an emphasis on the liver. Pathol Res Pract. 2001;197:65-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 73] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 46. | Wanless IR, Nakashima E, Sherman M. Regression of human cirrhosis. Morphologic features and the genesis of incomplete septal cirrhosis. Arch Pathol Lab Med. 2000;124:1599-1607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 47. | Hübscher SG. Pathology of non-cirrhotic portal hypertension and incomplete septal cirrhosis. Diagnostic Histopathol. 2011;17:530-538. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 48. | de Franchis R; Baveno V Faculty. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010;53:762-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1066] [Cited by in RCA: 1047] [Article Influence: 65.4] [Reference Citation Analysis (0)] |
| 49. | Sun Y, Lan X, Shao C, Wang T, Yang Z. Clinical features of idiopathic portal hypertension in China: A retrospective study of 338 patients and literature review. J Gastroenterol Hepatol. 2019;34:1417-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 50. | Franchi-Abella S, Fabre M, Mselati E, De Marsillac ME, Bayari M, Pariente D, Jacquemin E, Bernard O. Obliterative portal venopathy: a study of 48 children. J Pediatr. 2014;165:190-193.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 51. | Seijo S, Lozano JJ, Alonso C, Reverter E, Miquel R, Abraldes JG, Martinez-Chantar ML, Garcia-Criado A, Berzigotti A, Castro A, Mato JM, Bosch J, Garcia-Pagan JC. Metabolomics discloses potential biomarkers for the noninvasive diagnosis of idiopathic portal hypertension. Am J Gastroenterol. 2013;108:926-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 52. | Siramolpiwat S. Transjugular intrahepatic portosystemic shunts and portal hypertension-related complications. World J Gastroenterol. 2014;20:16996-17010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 53. | Garcia-Pagán JC, Hernández-Guerra M, Bosch J. Extrahepatic portal vein thrombosis. Semin Liver Dis. 2008;28:282-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 54. | Ma J, Yan Z, Luo J, Liu Q, Wang J, Qiu S. Rational classification of portal vein thrombosis and its clinical significance. PLoS One. 2014;9:e112501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 55. | Hernández-Gea V, De Gottardi A, Leebeek FWG, Rautou PE, Salem R, Garcia-Pagan JC. Current knowledge in pathophysiology and management of Budd-Chiari syndrome and non-cirrhotic non-tumoral splanchnic vein thrombosis. J Hepatol. 2019;71:175-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 56. | Gupta S, Pottakkat B, Verma SK, Kalayarasan R, Chandrasekar A S, Pillai AA. Pathological abnormalities in splenic vasculature in non-cirrhotic portal hypertension: Its relevance in the management of portal hypertension. World J Gastrointest Surg. 2020;12:1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 57. | Vilgrain V, Condat B, Bureau C, Hakimé A, Plessier A, Cazals-Hatem D, Valla DC. Atrophy-hypertrophy complex in patients with cavernous transformation of the portal vein: CT evaluation. Radiology. 2006;241:149-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 58. | Krishnasamy VP, Hagar MJ, Chun AK, Levy E. Noncirrhotic portal hypertension: Imaging, hemodynamics, and endovascular therapy. Clin Liver Dis (Hoboken). 2015;6:67-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 59. | Nunoi H, Hirooka M, Ochi H, Koizumi Y, Tokumoto Y, Abe M, Tada F, Ikeda Y, Matsuura B, Tanaka H, Tsuda T, Mochizuki T, Hiasa Y, Onji M. Portal biliopathy diagnosed using color Doppler and contrast-enhanced ultrasound. Intern Med. 2013;52:1055-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 60. | Noronha Ferreira C, Seijo S, Plessier A, Silva-Junior G, Turon F, Rautou PE, Baiges A, Bureau C, Bosch J, Hernández-Gea V, Valla D, García-Pagan JC. Natural history and management of esophagogastric varices in chronic noncirrhotic, nontumoral portal vein thrombosis. Hepatology. 2016;63:1640-1650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 61. | Guido M, Sarcognato S, Sonzogni A, Lucà MG, Senzolo M, Fagiuoli S, Ferrarese A, Pizzi M, Giacomelli L, Colloredo G. Obliterative portal venopathy without portal hypertension: an underestimated condition. Liver Int. 2016;36:454-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 62. | DeLeve LD, Valla DC, Garcia-Tsao G; American Association for the Study Liver Diseases. Vascular disorders of the liver. Hepatology. 2009;49:1729-1764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 739] [Cited by in RCA: 670] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 63. | Rubbia-Brandt L, Audard V, Sartoretti P, Roth AD, Brezault C, Le Charpentier M, Dousset B, Morel P, Soubrane O, Chaussade S, Mentha G, Terris B. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15:460-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 752] [Cited by in RCA: 774] [Article Influence: 35.2] [Reference Citation Analysis (2)] |
| 64. | Lawal TO, Farris AB, El-Rayes BF, Subramanian RM, Kim HS. Oxaliplatin-induced hepatoportal sclerosis, portal hypertension, and variceal bleeding successfully treated with transjugular intrahepatic portosystemic shunt. Clin Colorectal Cancer. 2012;11:224-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 65. | Rubbia-Brandt L, Lauwers GY, Wang H, Majno PE, Tanabe K, Zhu AX, Brezault C, Soubrane O, Abdalla EK, Vauthey JN, Mentha G, Terris B. Sinusoidal obstruction syndrome and nodular regenerative hyperplasia are frequent oxaliplatin-associated liver lesions and partially prevented by bevacizumab in patients with hepatic colorectal metastasis. Histopathology. 2010;56:430-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 228] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 66. | Wicherts DA, de Haas RJ, Sebagh M, Ciacio O, Lévi F, Paule B, Giacchetti S, Guettier C, Azoulay D, Castaing D, Adam R. Regenerative nodular hyperplasia of the liver related to chemotherapy: impact on outcome of liver surgery for colorectal metastases. Ann Surg Oncol. 2011;18:659-669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 67. | van den Broek MA, Olde Damink SW, Driessen A, Dejong CH, Bemelmans MH. Nodular regenerative hyperplasia secondary to neoadjuvant chemotherapy for colorectal liver metastases. Case Rep Med. 2009;2009:457975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 68. | Fan CQ, Crawford JM. Sinusoidal obstruction syndrome (hepatic veno-occlusive disease). J Clin Exp Hepatol. 2014;4:332-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 197] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 69. | Ryan P, Nanji S, Pollett A, Moore M, Moulton CA, Gallinger S, Guindi M. Chemotherapy-induced liver injury in metastatic colorectal cancer: semiquantitative histologic analysis of 334 resected liver specimens shows that vascular injury but not steatohepatitis is associated with preoperative chemotherapy. Am J Surg Pathol. 2010;34:784-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 70. | Hubert C, Sempoux C, Horsmans Y, Rahier J, Humblet Y, Machiels JP, Ceratti A, Canon JL, Gigot JF. Nodular regenerative hyperplasia: a deleterious consequence of chemotherapy for colorectal liver metastases? Liver Int. 2007;27:938-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 71. | Ferlitsch A, Teml A, Reinisch W, Ulbrich G, Wrba F, Homoncik M, Gangl A, Peck-Radosavljevic M, Vogelsang H. 6-thioguanine associated nodular regenerative hyperplasia in patients with inflammatory bowel disease may induce portal hypertension. Am J Gastroenterol. 2007;102:2495-2503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 72. | Slade JH, Alattar ML, Fogelman DR, Overman MJ, Agarwal A, Maru DM, Coulson RL, Charnsangavej C, Vauthey JN, Wolff RA, Kopetz S. Portal hypertension associated with oxaliplatin administration: clinical manifestations of hepatic sinusoidal injury. Clin Colorectal Cancer. 2009;8:225-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 73. | Grimaldi C, de Ville de Goyet J, Nobili V. Portal hypertension in children. Clin Res Hepatol Gastroenterol. 2012;36:260-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 74. | Sood V, Lal BB, Khanna R, Rawat D, Bihari C, Alam S. Noncirrhotic Portal Fibrosis in Pediatric Population. J Pediatr Gastroenterol Nutr. 2017;64:748-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 75. | Trenschel GM, Schubert A, Dries V, Benz-Bohm G. Nodular regenerative hyperplasia of the liver: case report of a 13-year-old girl and review of the literature. Pediatr Radiol. 2000;30:64-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 76. | Wu H, Vu M, Dhingra S, Ackah R, Goss JA, Rana A, Quintanilla N, Patel K, Leung DH. Obliterative Portal Venopathy Without Cirrhosis Is Prevalent in Pediatric Cystic Fibrosis Liver Disease With Portal Hypertension. Clin Gastroenterol Hepatol. 2019;17:2134-2136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 77. | Besmond C, Valla D, Hubert L, Poirier K, Grosse B, Guettier C, Bernard O, Gonzales E, Jacquemin E. Mutations in the novel gene FOPV are associated with familial autosomal dominant and non-familial obliterative portal venopathy. Liver Int. 2018;38:358-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 78. | Khanna R, Sarin SK. Non-cirrhotic portal hypertension - diagnosis and management. J Hepatol. 2014;60:421-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 281] [Article Influence: 23.4] [Reference Citation Analysis (3)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Romania
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
P-Reviewer: El-Bendary M, Gioia S, Gronbaek H, Sarma MS S-Editor: Wang JL L-Editor: A E-Editor: Zhang YL