Published online Jun 7, 2020. doi: 10.3748/wjg.v26.i21.2864
Peer-review started: January 4, 2020
First decision: February 24, 2020
Revised: March 27, 2020
Accepted: May 28, 2020
Article in press: May 28, 2020
Published online: June 7, 2020
Processing time: 153 Days and 15.2 Hours
Assessing liver fibrosis is important for predicting the efficacy of direct-acting antivirals (DAAs) and patient prognosis. Non-invasive techniques to assess liver fibrosis are becoming important. Recently, serum Mac-2 binding protein glycosylation isomer (M2BPGi) was identified as a non-invasive marker of liver fibrosis.
To investigate the diagnostic accuracy of M2BPGi in assessing liver fibrosis in patients with chronic hepatitis C (CHC) treated with DAAs.
From December 2017 to August 2018, 80 treatment-naïve adult patients with CHC who were eligible for DAAs therapy were consecutively enrolled in this observational cohort study. For 12 weeks, 65 patients were treated with sofosbuvir/daclatasvir, and 15 patients were treated with sofosbuvir/daclatasvir and a weight-based dose of ribavirin at knowledge and technology association for hepatitis C management clinic, Cairo, Egypt. We measured serum M2BPGi levels, PAPAS index, fibrosis-4 (FIB-4) score and liver stiffness measurements (LSM) at baseline and 12 weeks after the end of treatment. Serum M2BPGi levels were measured using enzyme-linked immunosorbent assay.
All patients achieved sustained virologic response (SVR12) (100%). Serum M2BPGi levels, LSM, FIB-4 score and PAPAS index decreased significantly at SVR12 (P < 0.05). Serum M2BPGi levels correlated positively with LSM at baseline and SVR12 (P < 0.001). At baseline, compared with the FIB-4 score and PAPAS index, M2BPGi was the best marker to distinguish patients with grade F4 fibrosis (AUC = 0.801, P < 0.001), patients with grade F2 from grade F0-1 fibrosis (AUC = 0.713, P = 0.012), patients with grade F3-4 from grade F0-2 fibrosis (AUC = 0.730, P < 0.001), and patients with grade F2-4 from grade F0-1 fibrosis (AUC = 0.763, P < 0.001). At SVR12, M2BPGi had the greatest AUCs for differentiating patients with grade F4 fibrosis (AUC = 0.844, P < 0.001), patients with grade F3 from grade F0-2 fibrosis (AUC = 0.893, P = 0.002), patients with grade F3-4 from grade F0-2 fibrosis (AUC = 0.891, P < 0.001), and patients with grade F2-4 from grade F0-1 fibrosis (AUC = 0.750, P < 0.001).
M2BPGi is a reliable marker for the non-invasive assessment and prediction of liver fibrosis regression in patients with CHC who achieved an SVR with DAAs therapy.
Core tip: For 12 wk, 80 chronic hepatitis C patients received a sofosbuvir/daclatasvir/± ribavirin treatment. All patients achieved sustained virologic response (SVR12). Serum Mac-2 binding protein glycosylation isomer (M2BPGi) levels, liver stiffness measurements, fibrosis-4 (FIB-4) and PAPAS index decreased significantly at SVR12. At baseline, M2BPGi was the best marker to distinguish patients with grade F4 fibrosis, patients with grade F2 from grade F0-1 fibrosis, patients with grade F3-4 from grade F0-2 fibrosis, and patients with grade F2-4 from grade F0-1 fibrosis. At SVR12, M2BPGi had the greatest AUCs for differentiating patients with grade F4 fibrosis, patients with grade F3 from grade F0-2 fibrosis, patients with grade F3-4 from grade F0-2 fibrosis, and patients with grade F2-4 from grade F0-1 fibrosis.
- Citation: Saleh SA, Salama MM, Alhusseini MM, Mohamed GA. M2BPGi for assessing liver fibrosis in patients with hepatitis C treated with direct-acting antivirals. World J Gastroenterol 2020; 26(21): 2864-2876
- URL: https://www.wjgnet.com/1007-9327/full/v26/i21/2864.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i21.2864
Hepatitis C virus (HCV) is considered a public health problem, as approximately 3% of the global population is infected with HCV[1]. It is imperative to assess the degree of liver fibrosis in patients with chronic hepatitis C (CHC) because fibrogenesis causes all the clinical events, including decompensated liver disease and hepatocellular carcinoma (HCC), affecting the prognosis of and treatment strategies used in patients with CHC[2].
Although a liver biopsy is considered the gold standard for stratifying hepatic fibrosis, its clinical utility is substantially limited because of the invasiveness and the sampling variability[3]. Additionally, a liver biopsy is impractical particularly during follow-up due to its invasive nature[4,5].
Consequently, non-invasive methods have been previously proposed and validated for the assessment of hepatic fibrosis, such as ultrasound or magnetic resonance imaging[6], elastographic techniques[7], serum biomarkers including hyaluronic acid, type IV collagen, and type III procollagen-N-peptide[8], and surrogate markers, e.g., the aspartate aminotransferase (AST)-to-platelet ratio index[9], the fibrosis-4 (FIB-4) score[10], AST to alanine aminotransferase (ALT) ratio[11] and PAPAS [platelets/age /phosphatase/alpha fetoprotein (AFP)/AST] index[12].
Mac-2 binding protein glycosylation isomer (M2BPGi) is a glycoprotein that is produced by hepatic stellate cells (HSCs). It functions as a messenger between HSCs and Kupffer cells to promote fibrogenesis[13]. The feasibility of monitoring serum M2BPGi levels to assess hepatic fibrosis was evaluated, and some studies recommended it as an accurate method for staging hepatic fibrosis[14,15].
Subsequently, several investigators validated the usefulness of M2BPGi in various aetiologies of liver diseases, such as viral hepatitis[16-19], mortality in liver cirrhosis[20], biliary atresia[21], non-alcoholic fatty liver disease[22,23], non-alcoholic steatohepatitis[24], primary biliary cirrhosis[25], autoimmune hepatitis[26] and primary sclerosing cholangitis[27]. Furthermore, it was investigated as a marker to assess the risk of HCC development[28,29]. According to recent studies[17,18,28,30-32], M2BPGi is a useful marker for monitoring the improvement of patients with liver fibrosis who have achieved a sustained virologic response (SVR) after antiviral therapy.
Recently, interferon (IFN)-based treatment has been replaced by direct-acting antivirals (DAAs). The approval of DAAs was a revolution in HCV eradication, with SVR rates exceeding 90%, good tolerability, and increased efficacy with shorter treatment durations[33]. However, a few reports have documented the improvement in liver fibrosis in patients treated with IFN-free DAAs[31,34-37].
We aimed to investigate the diagnostic accuracy of serum M2BPGi levels in assessing the grade of liver fibrosis in patients with CHC before and after DAAs-based treatment, as well as to compare its diagnostic value with the FIB-4 score and PAPAS index.
From December 2017 to August 2018, 80 treatment-naïve adult patients with CHC who were eligible for DAAs therapy were consecutively enrolled in this observational cohort study. For 12 weeks, 65 patients were treated with sofosbuvir/daclatasvir, and 15 patients were treated with sofosbuvir/daclatasvir and a weight-based dose of ribavirin at Knowledge and Technology Association for Hepatitis C Management Clinic, Cairo, Egypt. The exclusion criteria were (1) positivity for antibodies against human immunodeficiency virus or positivity for hepatitis B surface antigen; (2) other causes of liver disease (autoimmune hepatitis, primary biliary cirrhosis, haemochromatosis, sclerosing cholangitis, Wilson’s disease, or an α1-antitrypsin deficiency); (3) clinical or biochemical evidence of hepatic decompensation (ascites, bleeding varices or encephalopathy); (4) suspected HCC or other cancers; (5) excessive alcohol consumption (> 40 g/d) or intravenous drug abuse; or (6) a previous liver transplantation.
This study was approved by the Research Ethics Committee of our institution. Written informed consent was obtained from every patient, and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
We evaluated the liver stiffness measurement (LSM), serum M2BPGi levels, FIB-4 score, PAPAS index, biochemical data, haematological data, virologic data and abdominal ultrasound at baseline and 12 weeks after the end of treatment (EOT), namely, the time SVR12 was achieved, of every patient.
Plasma HCV RNA levels were measured using the Roche TaqMan real-time reverse transcriptase-PCR assay version 2.0, with lower limits of quantification and detection of 15 IU/mL. SVR12 was defined as a lack of detectable HCV RNA at week 12 after EOT.
Serum M2BPGi levels were measured using human M2BPGi enzyme-linked immunosorbent assay kits, with a detection range of 0.625 - 200 ng/mL, sensitivity 0.1 ng/mL, and intra-assay and inter-assay coefficients of variation less than 15%.
The LSM was performed using Fibroscan® (Echosens, 502 Touch, Paris, France). It was conducted by an experienced examiner after the patient had fasted for at least six hours, and 10 valid measurements were recorded. The median LS in kilopascals (kPa) was reported. Only examinations with a success rate > 60% and IQR < 25% were included and considered reliable. According to Tsochatzis et al[38], the following fibrosis staging cut-off values were used: F0-F1 < 7 kPa; F2 7 - 9.4 kPa; F3 9.5 - 11.9 kPa; and F4 > 12 kPa.
The PAPAS index and FIB-4 score were calculated using the following formulas:
PAPAS index[12] = Log (index + 1) = 0.0255 + 0.0031 × age (year) + 0.1483 × log [ALP (U/L)] + 0.004 × log [AST (U/L)] + 0.0908 × log [AFP (ng/L) + 1] - 0.028 × log [platelets count (109/L)].
FIB-4 score[39] = Age (yr) × AST (IU/L)]/{platelets count (109/L) × [ALT (IU/L)]½ }.
A FIB-4 score < 1.45 indicates no or minimal fibrosis.
A FIB-4 score > 3.25 indicates significant fibrosis.
Statistical analyses were performed using Stata® version 13.1 software (StataCorp. 2013, College Station, TX: StataCorp LP). Patients’ characteristics are presented as mean ± SD, median (IQR) or number (percentage), as appropriate. Accordingly, paired t test, Wilcoxon matched-pairs signed rank test or chi squared test was used, as appropriate. Values were compared between different grades of liver fibrosis using one-way ANOVA test. Pearson’s correlation analysis was used to study the correlation between serum M2BPGi levels and the characteristics of the study population. A receiver operating characteristic (ROC) curve analysis was used to identify the best cut-off value for the serum M2BPGi level with maximum sensitivity and specificity for the differentiation of different grades of fibrosis. A P value < 0.05 was considered significant.
The statistical methods of this study were performed by Hazem M. El-Hariri from Department of Community Medicine, National Research Centre, Cairo, Egypt.
The studied patients included 40 males (50%) and 40 females (50%), with a mean age of 52.3 ± 10.7 years and BMI (kg/m2) = 28 ± 5.4. Patients’ characteristics at baseline and SVR12 are shown in Table 1.
| Measures | Baseline | SVR12 | P value |
| Age (yr) | 52.3 ± 10.7 | ||
| Gender | 40 (50%) males and 40 (50%) females | ||
| BMI | 28 ± 5.4 | ||
| HCV RNA (log copies/mL) | 6.0 ± 0.7 | Non-detectable | 0.000b |
| Platelets count (× 103/mL) | 220.3 ± 65.3 | 245.3 ± 77.8 | 0.005b |
| ALT (IU/L) | 40.5 (29-54) | 32 (26-38) | < 0.001b |
| AST (IU/L) | 39 (29-51) | 30 (24-37) | < 0.001b |
| ALP (IU/L) | 138.5 (95.5-196) | 107 (86-141) | < 0.001b |
| Albumin (g/dL) | 3.8 ± 0.3 | 4.2 ± 0.4 | < 0.001b |
| Total bilirubin (mg/dL) | 0.7 (0.5-0.8) | 0.7 (0.5-0.95) | 0.8 |
| Creatinine (mg/dL) | 0.86 ± 0.19 | 0.75 ± 0.23 | 0.002b |
| INR | 1.06 ± 0.09 | 1.08 ± 0.15 | 0.222 |
| AFP (ng/mL) | 4.5 ± 2.1 | 3.7 ± 1.9 | 0.128 |
| LSM (kPa) | 11.4 ± 4.5 | 9.5 ± 3.3 | 0.002b |
| FIB-4 score | 1.8 ± 0.5 | 1.3 ± 0.7 | < 0.001b |
| PAPAS index | 2.2 ± 0.5 | 2.1 ± 0.3 | 0.010a |
| Serum M2BPGi (ng/mL) | 9 ± 3.8 | 6.7 ± 2.3 | < 0.001b |
All patients completed the scheduled course of treatment with follow up until 12 wk after EOT. SVR12 was achieved in all patients (100%). Overall, the treatment was well tolerated. The most commonly reported adverse events were fatigue (5%), followed by pruritus (4.2%), rash (2.3%), headache (2%), and a loss of appetite (1%), all of which were mild in severity.
Haemoglobin levels, WBC, total bilirubin levels and international normalized ratio (INR) did not change significantly after patients achieved SVR12. Platelets count and albumin levels were significantly higher at SVR12. ALT, AST, ALP and creatinine levels decreased significantly after patients achieved SVR12. AFP levels decreased after patients achieved SVR12, but the difference was not statistically significant (Table 1).
Serum M2BPGi levels, LSM, FIB-4 score and PAPAS index decreased significantly after patients achieved SVR12 (Table 1). The improvement in LSM was more noticeable in patients with grade F4 fibrosis (Table 2). Only serum M2BPGi levels were significantly different between patients with different grades of fibrosis at baseline and SVR12 (Table 3).
| Total baseline | Changes in the fibrosis grade at SVR12 | |||
| F0-1 (%) | F2 (%) | F3 (%) | F4 (%) | |
| F0-1 29 (36.3%) | 23 (79.3) | 5 (17.2) | 1 (3.4) | 0 (0.0) |
| F2 20 (25%) | 10 (50) | 10 (50) | 0 (0.0) | 0 (10) |
| F3 5 (6.3%) | 1 (20) | 2 (40) | 1 (20) | 1 (20) |
| F4 26 (32.5%) | 1 (3.8) | 6 (23.1) | 4 (15.4) | 15 (57.7) |
| Total SVR12 | 35 (43.7) | 23 (28.7) | 6 (7.5) | 16 (20) |
| Parameter | P value | ||||
| Baseline (Total n = 80) | F0-1 (n = 29) | F2 (n = 20) | F3 (n = 5) | F4 (n = 26) | |
| LSM (kPa) | 5.9 ± 0.6 | 7.8 ± 0.5 | 10.8 ± 1.2 | 20.6 ± 7.7 | < 0.001b |
| FIB-4 score | 1.7 ± 1.4 | 1.4 ± 0.7 | 1.8 ± 0.4 | 2.3 ± 1.5 | 0.108 |
| PAPAS index | 2.1 ± 0.4 | 2.2 ± 0.6 | 2.3 ± 0.3 | 2.3 ± 0.5 | 0.303 |
| Serum M2BPGi (ng/mL) | 4.5 ± 2.2 | 5.3 ± 2.8 | 9.4 ± 4 | 14.5 ± 6.7 | 0.001b |
| SVR 12 (Total n = 80) | F0-1 (n = 35) | F2 (n = 23) | F3 (n = 6) | F4 (n = 16) | |
| LSM (kPa) | 5.8 ± 1.2 | 7.6 ± 0.6 | 9.9 ± 0.8 | 15 ± 2.3 | < 0.001b |
| FIB-4 score | 1.1 ± 0.5 | 1.5 ± 1 | 1.3 ± 0.6 | 1.6 ± 0.5 | 0.075 |
| PAPAS index | 2 ± 0.3 | 2.1 ± 0.3 | 2.1 ± 0.3 | 2.3 ± 0.3 | 0.069 |
| Serum M2BPGi (ng/mL) | 3.4 ± 1.6 | 4.9 ± 2.1 | 12.7 ± 5.1 | 13.3 ± 5.2 | 0.001b |
At baseline, serum M2BPGi levels correlated positively with total bilirubin levels and negatively with AST levels. At SVR12, serum M2BPGi levels correlated positively with INR and negatively with platelets count (Table 4).
| Variables | Baseline | SVR12 | ||
| r | P value | r | P value | |
| Age | 0.050 | 0.662 | 0.107 | 0.346 |
| BMI | -0.007 | 0.951 | 0.057 | 0.617 |
| HCV RNA | 0.043 | 0.702 | 0.025 | 0.827 |
| Hb | 0.143 | 0.205 | 0.131 | 0.246 |
| WBC | 0.047 | 0.677 | -0.152 | 0.178 |
| Platelets count | -0.118 | 0.297 | -0.299 | 0.007b |
| ALT | -0.090 | 0.425 | -0.129 | 0.256 |
| AST | -0.227 | 0.043a | 0.006 | 0.961 |
| ALP | -0.024 | 0.831 | 0.106 | 0.350 |
| Albumin | 0.016 | 0.888 | 0.070 | 0.535 |
| Total bilirubin | 0.268 | 0.016a | 0.111 | 0.326 |
| Creatinine | 0.158 | 0.162 | 0.088 | 0.439 |
| INR | 0.063 | 0.582 | 0.220 | 0.049a |
| AFP | -0.098 | 0.388 | 0.026 | 0.822 |
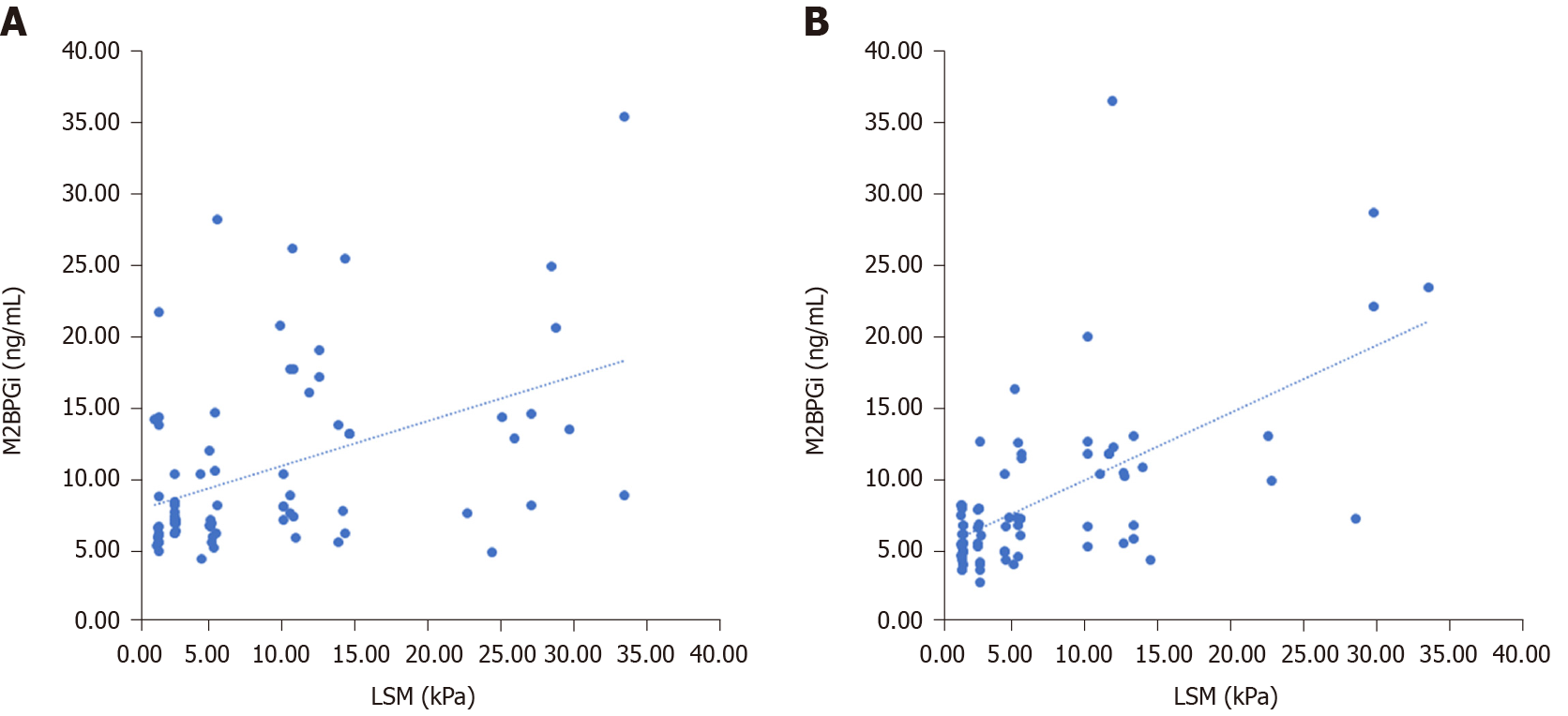
At baseline, LSM correlated with serum M2BPGi levels and FIB-4 score. In addition, a significant correlation was observed between FIB-4 score and PAPAS index. At SVR12, LSM correlated with serum M2BPGi levels, FIB-4 score and PAPAS index (Table 5 and Figure 1).
| Variables | LSM | Serum M2BPGi | FIB-4 score | |
| At baseline | ||||
| Serum M2BPGi level | r | 0.453 | ||
| P value | < 0.001b | |||
| FIB-4 score | r | 0.328 | -0.099 | |
| P value | 0.003b | 0.384 | ||
| PAPAS index | r | 0.110 | -0.049 | 0.444 |
| P value | 0.330 | 0.664 | < 0.001b | |
| At SVR12 | ||||
| Serum M2BPGi level | r | 0.517 | ||
| P value | < 0.001b | |||
| FIB-4 score | r | 0.231 | 0.188 | |
| P value | 0.039a | 0.095 | ||
| PAPAS index | r | 0.328 | 0.185 | 0.188 |
| P value | 0.003b | 0.100 | 0.095 | |
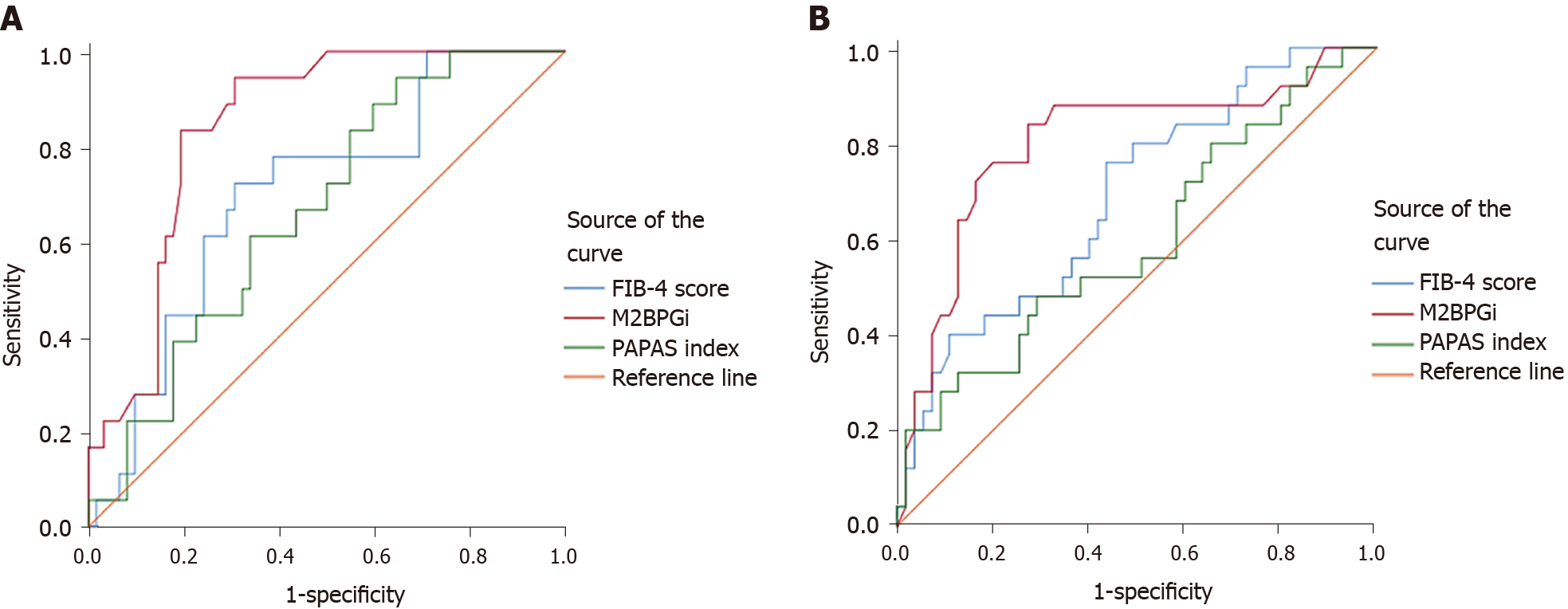
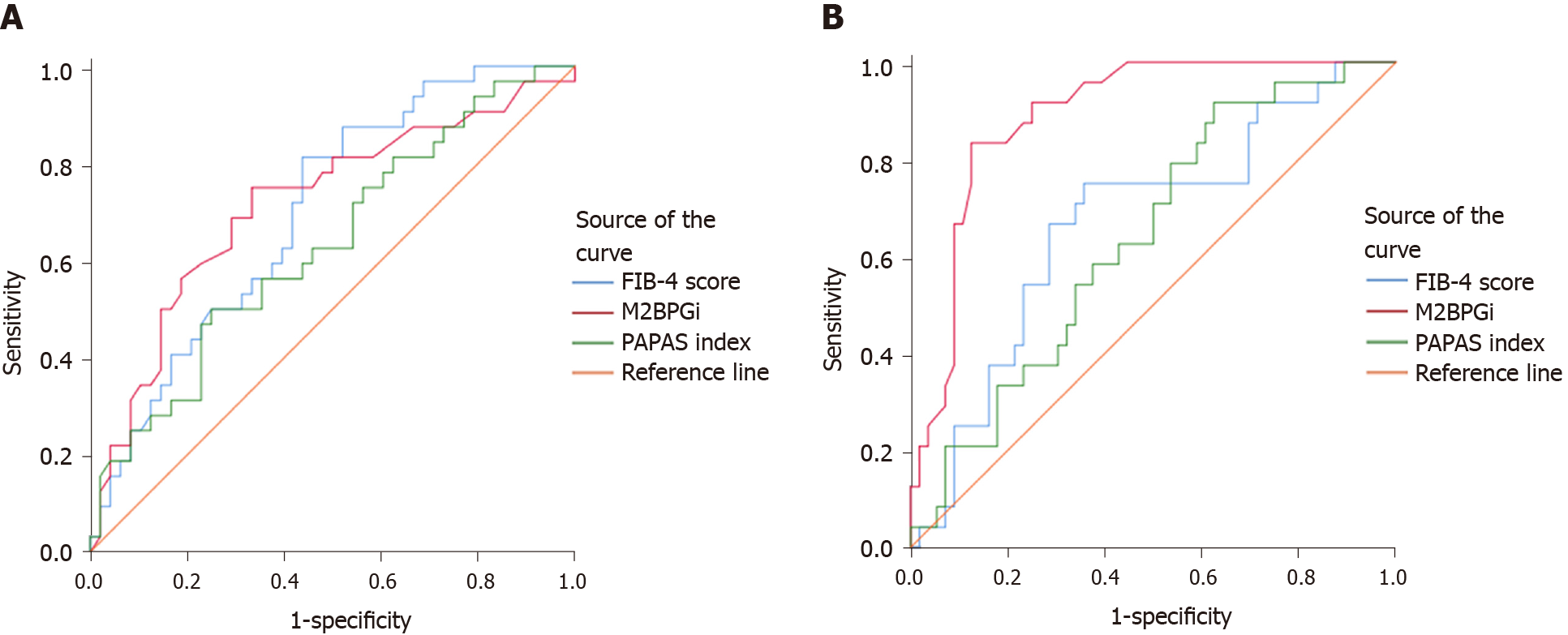
At baseline, compared with the FIB-4 score and PAPAS index, M2BPGi was the best marker to distinguish patients with grade F4 fibrosis (AUC = 0.801, P < 0.001), patients with grade F2 from grade F0-1 fibrosis (AUC = 0.713, P = 0.012), patients with grade F3-4 from grade F0-2 fibrosis (AUC = 0.730, P < 0.001), and patients with grade F2-4 from grade F0-1 fibrosis (AUC = 0.763, P < 0.001) (Supplementary Table 1 , Figures 1 and 3).
At SVR12, M2BPGi had the greatest AUCs for differentiating patients with grade F4 fibrosis (AUC = 0.844, P < 0.001), patients with grade F3 from grade F0-2 fibrosis (AUC = 0.893, P = 0.002), patients with grade F3-4 from grade F0-2 fibrosis (AUC = 0.891, P < 0.001), and patients with grade F2-4 from grade F0-1 fibrosis (AUC = 0.750, P < 0.001) (Supplementary Table 1 , Figures 2 and 3).
Currently, non-invasive methods for detecting liver fibrosis are used much more frequently than liver biopsies[40]. M2BPGi has been shown to be a useful predictor of liver fibrosis[14,17,41]. Previous reports have documented a substantial improvement in liver fibrosis after patients achieve SVR12 through treatment with DAAs for HCV[42-44]. Here, we aimed to investigate the diagnostic accuracy of serum M2BPGi levels for assessing the grade of liver fibrosis in patients with CHC before and after DAAs-based treatment, as well as to compare its diagnostic value with the FIB-4 score and PAPAS index.
The present study confirms the efficacy and safety of sofosbuvir + daclatasvir ± ribavirin in real-world situations. The SVR12 is 100%.
Similar to previous studies[33,45-48], we observed an improvement in liver functions after patients achieved SVR12, as indicated by a significant decrease in the levels of ALT, AST and ALP, and a significant increase in the serum albumin levels and platelets count. Additionally, in consistency with previous literature[37,45,47,49-52], serum M2BPGi levels, LSM, FIB-4 score and PAPAS index decreased significantly after patients achieved SVR12. These findings suggest the possibility of liver fibrosis regression after viral eradication was achieved.
In agreement with previous reports[3,16,31,30,53], we observed a significantly increasing trend of serum M2BPGi levels with the progression of liver fibrosis both at baseline and SVR12 (P < 0.001). Moreover, in accordance with Akahane et al[46] and Ishikawa et al[36], serum M2BPGi levels decreased significantly at SVR12 (P < 0.001). In addition, in a study by Miyaki et al[31], serum M2BPGi levels did not change in the non-SVR group (P = 0.715), but decreased significantly in the SVR group (P < 0.0001). These results suggest that serum M2BPGi would be a good surrogate marker for predicting and differentiating liver fibrosis stages.
In the present study, pre-treatment serum M2BPGi levels correlated with bilirubin and AST levels, while serum M2BPGi levels correlated with platelets count and INR at SVR12. These findings suggest that M2BPGi reflects not only the severity of liver fibrosis but also the severity of liver inflammation in CHC patients[18]. This may be attributed to the role of M2BPGi as a messenger between HSCs and Kupffer cells and its accompanying inflammation[13].
Similar to our results, Ura et al[30] reported a significant negative correlation between serum M2BPGi levels and platelets count (r = -0.47, P < 0.0001). Additionally, Yamasaki et al[54] observed a significant positive correlation between serum M2BPGi and bilirubin levels (r = 0.091, P = 0.001) and a significant negative correlation with platelets count (r = -0.147, P < 0.001). However, in contrast to our results, Yasui et al[55] observed a positive correlation between serum M2BPGi and AFP levels (r = 0.428, P < 0.001), and a negative correlation with albumin levels (r = -0.471, P < 0.001).
In agreement with the present study, Tawara et al[53] reported a correlation coefficient between serum M2BPGi levels and FIB-4 score of less than 0.4, suggesting that the correlation between serum M2BPGi levels and FIB-4 score was weak. In contrast, Ura et al[30] and Yasui et al[55] detected a significant positive correlation between serum M2BPGi levels and FIB-4 score (r = 0.66, P < 0.0001 and r = 0.546, P < 0.001, respectively). This discrepancy can be attributed to the different sample size.
In terms of differentiation of liver fibrosis grades, consistent with the present study, Xu et al[16] reported that the AUC values of M2BPGi for predicting fibrosis grade ≥ F2 and F4 were significantly superior to the values of FIB-4 score (0.774 vs 0.702, P < 0.001 and 0.892 vs 0.818, P < 0.05), respectively. In contrast, Tawara et al[53] reported that the FIB-4 score had a greater AUC value for differentiating of fibrosis grades than M2BPGi (AUC values were 0.768, 0.827 and 0.876 for fibrosis grade F ≥ 2, F ≥ 3 and F4, respectively), while the AUC values of M2BPGi were 0.747, 0.733 and 0.796 for fibrosis grade F ≥ 2, F ≥ 3 and F4, respectively.
The limitations of the present study are the absence of a paired histological evaluation due to the invasiveness of liver biopsy, and the short duration of follow up after completion of treatment. Further large-scale studies with a longer follow-up period should be performed.
In conclusion, M2BPGi is a reliable marker for the non-invasive assessment and prediction of liver fibrosis regression in patients with CHC who achieved an SVR with DAAs therapy.
Assessing liver fibrosis is important for predicting the efficacy of direct-acting antivirals (DAAs) and patient prognosis. Non-invasive techniques to assess liver fibrosis are becoming important. Recently, serum Mac-2 binding protein glycosylation isomer (M2BPGi) was identified as a non-invasive marker of liver fibrosis.
The approval of DAAs was a revolution in hepatitis C virus eradication, with sustained virologic response (SVR) rates exceeding 90%. However, a few reports have documented the improvement in liver fibrosis in patients treated with DAAs. Although liver biopsy is considered the gold standard for stratifying hepatic fibrosis, its clinical utility is substantially limited because of the invasiveness and the sampling variability. Accordingly, serum M2BPGi was evaluated as a non-invasive marker for assessing the grade of hepatic fibrosis in patients who have achieved SVR after antiviral therapy.
We aimed to investigate the diagnostic accuracy of serum M2BPGi levels in assessing the grade of liver fibrosis in patients with chronic hepatitis C (CHC) before and after DAAs-based treatment, as well as to compare its diagnostic value with the FIB-4 score and PAPAS index.
Eighty treatment-naïve adult patients with CHC who were eligible for DAAs therapy were consecutively enrolled in this observational cohort study. For 12 weeks, 65 patients were treated with sofosbuvir/daclatasvir, and 15 patients were treated with sofosbuvir/daclatasvir and a weight-based dose of ribavirin. We measured serum M2BPGi levels, PAPAS index, FIB-4 score and liver stiffness measurements (LSM) at baseline and 12 weeks after the end of treatment. Serum M2BPGi levels were measured using enzyme-linked immunosorbent assay.
All patients achieved SVR12 (100%). Serum M2BPGi levels, LSM, FIB-4 score and PAPAS index decreased significantly at SVR12 (P < 0.05). Serum M2BPGi levels correlated positively with LSM at baseline and SVR12 (P < 0.001). At baseline, compared with the FIB-4 score and PAPAS index, M2BPGi was the best marker to distinguish patients with grade F4 fibrosis (AUC = 0.801, P < 0.001), patients with grade F2 from grade F0-1 fibrosis (AUC = 0.713, P = 0.012), patients with grade F3-4 from grade F0-2 fibrosis (AUC = 0.730, P < 0.001), and patients with grade F2-4 from grade F0-1 fibrosis (AUC = 0.763, P < 0.001). At SVR12, M2BPGi had the greatest AUCs for differentiating patients with grade F4 fibrosis (AUC = 0.844, P < 0.001), patients with grade F3 from grade F0-2 fibrosis (AUC = 0.893, P = 0.002), patients with grade F3-4 from grade F0-2 fibrosis (AUC = 0.891, P < 0.001), and patients with grade F2-4 from grade F0-1 fibrosis (AUC = 0.750, P < 0.001).
M2BPGi is a reliable marker for the non-invasive assessment and prediction of liver fibrosis regression in patients with CHC who achieved an SVR with DAAs therapy.
Non-invasive methods have been previously proposed and validated for the assessment of hepatic fibrosis. In this study, we confirm that serum M2BPGi is a reliable marker for liver fibrosis. Further studies are needed to investigate its therapeutic potential and ultimate clinical utility.
| 1. | Morozov VA, Lagaye S. Hepatitis C virus: Morphogenesis, infection and therapy. World J Hepatol. 2018;10:186-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (14)] |
| 2. | Karanjia RN, Crossey MM, Cox IJ, Fye HK, Njie R, Goldin RD, Taylor-Robinson SD. Hepatic steatosis and fibrosis: Non-invasive assessment. World J Gastroenterol. 2016;22:9880-9897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 3. | Huang CI, Huang CF, Yeh ML, Lin YH, Liang PC, Hsieh MH, Dai CY, Hsieh MY, Lin ZY, Chen SC, Huang JF, Yu ML, Chuang WL. Serum Wisteria floribunda agglutinin-positive Mac-2-binding protein expression predicts disease severity in chronic hepatitis C patients. Kaohsiung J Med Sci. 2017;33:394-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Mokdad AA, Lopez AD, Shahraz S, Lozano R, Mokdad AH, Stanaway J, Murray CJ, Naghavi M. Liver cirrhosis mortality in 187 countries between 1980 and 2010: a systematic analysis. BMC Med. 2014;12:145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 720] [Cited by in RCA: 760] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 5. | Lurie Y, Webb M, Cytter-Kuint R, Shteingart S, Lederkremer GZ. Non-invasive diagnosis of liver fibrosis and cirrhosis. World J Gastroenterol. 2015;21:11567-11583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 189] [Cited by in RCA: 267] [Article Influence: 24.3] [Reference Citation Analysis (2)] |
| 6. | Wang QB, Zhu H, Liu HL, Zhang B. Performance of magnetic resonance elastography and diffusion-weighted imaging for the staging of hepatic fibrosis: A meta-analysis. Hepatology. 2012;56:239-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 198] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 7. | Cui J, Heba E, Hernandez C, Haufe W, Hooker J, Andre MP, Valasek MA, Aryafar H, Sirlin CB, Loomba R. Magnetic resonance elastography is superior to acoustic radiation force impulse for the Diagnosis of fibrosis in patients with biopsy-proven nonalcoholic fatty liver disease: A prospective study. Hepatology. 2016;63:453-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 158] [Article Influence: 15.8] [Reference Citation Analysis (1)] |
| 8. | Tatsumi C, Kudo M, Ueshima K, Kitai S, Takahashi S, Inoue T, Minami Y, Chung H, Maekawa K, Fujimoto K, Akiko T, Takeshi M. Noninvasive evaluation of hepatic fibrosis using serum fibrotic markers, transient elastography (FibroScan) and real-time tissue elastography. Intervirology. 2008;51 Suppl 1:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2762] [Cited by in RCA: 3337] [Article Influence: 145.1] [Reference Citation Analysis (0)] |
| 10. | Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, Fontaine H, Pol S. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1288] [Cited by in RCA: 1660] [Article Influence: 87.4] [Reference Citation Analysis (1)] |
| 11. | Lin ZH, Xin YN, Dong QJ, Wang Q, Jiang XJ, Zhan SH, Sun Y, Xuan SY. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011;53:726-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 821] [Cited by in RCA: 813] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 12. | Ozel BD, Poyrazoğlu OK, Karaman A, Karaman H, Altinkaya E, Sevinç E, Zararsiz G. The PAPAS index: a novel index for the prediction of hepatitis C-related fibrosis. Eur J Gastroenterol Hepatol. 2015;27:895-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Shirabe K, Bekki Y, Gantumur D, Araki K, Ishii N, Kuno A, Narimatsu H, Mizokami M. Mac-2 binding protein glycan isomer (M2BPGi) is a new serum biomarker for assessing liver fibrosis: more than a biomarker of liver fibrosis. J Gastroenterol. 2018;53:819-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 143] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 14. | Toshima T, Shirabe K, Ikegami T, Yoshizumi T, Kuno A, Togayachi A, Gotoh M, Narimatsu H, Korenaga M, Mizokami M, Nishie A, Aishima S, Maehara Y. A novel serum marker, glycosylated Wisteria floribunda agglutinin-positive Mac-2 binding protein (WFA(+)-M2BP), for assessing liver fibrosis. J Gastroenterol. 2015;50:76-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 148] [Article Influence: 13.5] [Reference Citation Analysis (1)] |
| 15. | Kuno A, Ikehara Y, Tanaka Y, Ito K, Matsuda A, Sekiya S, Hige S, Sakamoto M, Kage M, Mizokami M, Narimatsu H. A serum "sweet-doughnut" protein facilitates fibrosis evaluation and therapy assessment in patients with viral hepatitis. Sci Rep. 2013;3:1065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 292] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 16. | Xu H, Kong W, Liu L, Chi X, Wang X, Wu R, Gao X, Wang H, Qu L, Qi Y, Pan Y, Niu J. Accuracy of M2BPGi, compared with Fibro Scan®, in analysis of liver fibrosis in patients with hepatitis C. BMC Gastroenterol. 2017;17:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 17. | Zou X, Zhu MY, Yu DM, Li W, Zhang DH, Lu FJ, Gong QM, Liu F, Jiang JH, Zheng MH, Kuno A, Narimatsu H, Zhang Y, Zhang XX. Serum WFA+ -M2BP levels for evaluation of early stages of liver fibrosis in patients with chronic hepatitis B virus infection. Liver Int. 2017;37:35-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 18. | Ishii A, Nishikawa H, Enomoto H, Iwata Y, Kishino K, Shimono Y, Hasegawa K, Nakano C, Takata R, Nishimura T, Yoh K, Aizawa N, Sakai Y, Ikeda N, Takashima T, Iijima H, Nishiguchi S. Clinical implications of serum Wisteria floribunda agglutinin-positive Mac-2-binding protein in treatment-naïve chronic hepatitis B. Hepatol Res. 2017;47:204-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Nakamura M, Kanda T, Jiang X, Haga Y, Takahashi K, Wu S, Yasui S, Nakamoto S, Yokosuka O. Serum microRNA-122 and Wisteria floribunda agglutinin-positive Mac-2 binding protein are useful tools for liquid biopsy of the patients with hepatitis B virus and advanced liver fibrosis. PLoS One. 2017;12:e0177302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Hanai T, Shiraki M, Ohnishi S, Miyazaki T, Ideta T, Kochi T, Imai K, Suetsugu A, Takai K, Shimizu M, Moriwaki H. Impact of serum glycosylated Wisteria floribunda agglutinin positive Mac-2 binding protein levels on liver functional reserves and mortality in patients with liver cirrhosis. Hepatol Res. 2015;45:1083-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Yamada N, Sanada Y, Tashiro M, Hirata Y, Okada N, Ihara Y, Urahashi T, Mizuta K. Serum Mac-2 binding protein glycosylation isomer predicts grade F4 liver fibrosis in patients with biliary atresia. J Gastroenterol. 2017;52:245-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 22. | Lai LL, Chan WK, Sthaneshwar P, Nik Mustapha NR, Goh KL, Mahadeva S. Serum Wisteria floribunda agglutinin-positive Mac-2 binding protein in non-alcoholic fatty liver disease. PLoS One. 2017;12:e0174982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Mizuno M, Shima T, Oya H, Mitsumoto Y, Mizuno C, Isoda S, Kuramoto M, Taniguchi M, Noda M, Sakai K, Koyama N, Okanoue T. Classification of patients with non-alcoholic fatty liver disease using rapid immunoassay of serum type IV collagen compared with liver histology and other fibrosis markers. Hepatol Res. 2017;47:216-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Nishikawa H, Enomoto H, Iwata Y, Kishino K, Shimono Y, Hasegawa K, Nakano C, Takata R, Yoh K, Nishimura T, Aizawa N, Sakai Y, Ikeda N, Takashima T, Ishii A, Iijima H, Nakamura H, Nishiguchi S. Clinical significance of serum Wisteria floribunda agglutinin positive Mac-2-binding protein level in non-alcoholic steatohepatitis. Hepatol Res. 2016;46:1194-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Nishikawa H, Enomoto H, Iwata Y, Hasegawa K, Nakano C, Takata R, Nishimura T, Yoh K, Aizawa N, Sakai Y, Ikeda N, Takashima T, Ishii A, Iijima H, Nishiguchi S. Impact of serum Wisteria floribunda agglutinin positive Mac-2-binding protein and serum interferon-γ-inducible protein-10 in primary biliary cirrhosis. Hepatol Res. 2016;46:575-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Nishikawa H, Enomoto H, Iwata Y, Hasegawa K, Nakano C, Takata R, Nishimura T, Yoh K, Aizawa N, Sakai Y, Ikeda N, Takashima T, Iijima H, Nishiguchi S. Clinical significance of serum Wisteria floribunda agglutinin positive Mac-2-binding protein level and high-sensitivity C-reactive protein concentration in autoimmune hepatitis. Hepatol Res. 2016;46:613-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 27. | Umetsu S, Inui A, Sogo T, Komatsu H, Fujisawa T. Usefulness of serum Wisteria floribunda agglutinin-positive Mac-2 binding protein in children with primary sclerosing cholangitis. Hepatol Res. 2018;48:355-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Nagata H, Nakagawa M, Asahina Y, Sato A, Asano Y, Tsunoda T, Miyoshi M, Kaneko S, Otani S, Kawai-Kitahata F, Murakawa M, Nitta S, Itsui Y, Azuma S, Kakinuma S, Nouchi T, Sakai H, Tomita M, Watanabe M; Ochanomizu Liver Conference Study Group. Effect of interferon-based and -free therapy on early occurrence and recurrence of hepatocellular carcinoma in chronic hepatitis C. J Hepatol. 2017;67:933-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 173] [Article Influence: 19.2] [Reference Citation Analysis (1)] |
| 29. | Shinkai N, Nojima M, Iio E, Matsunami K, Toyoda H, Murakami S, Inoue T, Ogawa S, Kumada T, Tanaka Y. High levels of serum Mac-2-binding protein glycosylation isomer (M2BPGi) predict the development of hepatocellular carcinoma in hepatitis B patients treated with nucleot(s)ide analogues. J Gastroenterol. 2018;53:883-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Ura K, Furusyo N, Ogawa E, Hayashi T, Mukae H, Shimizu M, Toyoda K, Murata M, Hayashi J. Serum WFA(+) -M2BP is a non-invasive liver fibrosis marker that can predict the efficacy of direct-acting anti-viral-based triple therapy for chronic hepatitis C. Aliment Pharmacol Ther. 2016;43:114-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 31. | Miyaki E, Imamura M, Hiraga N, Murakami E, Kawaoka T, Tsuge M, Hiramatsu A, Kawakami Y, Aikata H, Hayes CN, Chayama K. Daclatasvir and asunaprevir treatment improves liver function parameters and reduces liver fibrosis markers in chronic hepatitis C patients. Hepatol Res. 2016;46:758-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 32. | Suda T, Okawa O, Masaoka R, Gyotoku Y, Tokutomi N, Katayama Y, Tamano M. Shear wave elastography in hepatitis C patients before and after antiviral therapy. World J Hepatol. 2017;9:64-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Kusakabe A, Kurosaki M, Itakura J, Joko K, Akahane T, Tsuji K, Kobashi H, Sohda T, Kimura H, Narita R, Furuta K, Izumi N. Efficacy and safety of glecaprevir/pibrentasvir as retreatment therapy for patients with genotype 2 chronic hepatitis C who failed prior sofosbuvir plus ribavirin regimen. Hepatol Res. 2019;49:1121-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Tamori A, Hai H, Uchida-Kobayashi S, Enomoto M, Kozuka R, Motoyama H, Kawamura E, Hagihara A, Teranishi Y, Yoshida K, Morikawa H, Murakami Y, Kawada N. Outcomes for Cirrhotic Patients with Hepatitis C Virus 1b Treated with Asunaprevir and Daclatasvir Combination. Ann Hepatol. 2017;16:734-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Lee HW, Oh SR, Kim DY, Jeong Y, Kim S, Kim BK, Kim SU, Kim DY, Ahn SH, Han KH, Park JY. Daclatasvir Plus Asunaprevir for the Treatment of Patients with Hepatitis C Virus Genotype 1b Infection: Real-World Efficacy, Changes in Liver Stiffness and Fibrosis Markers, and Safety. Gut Liver. 2018;12:324-330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Ishikawa T, Imai M, Owaki T, Sato H, Nozawa Y, Sano T, Iwanaga A, Seki K, Honma T, Toshiaki Yoshida T. Serum Wisteria floribunda Agglutinin Positive Mac-2 Binding Protein and Fib-4 Index on the Clinical Course of Patients with Chronic Hepatitis C Receiving Daclatasvir/Asunaprevir Therapy. Ann Digest Liver Dis. 2017;1:1001. |
| 37. | Elsharkawy A, Alem SA, Fouad R, El Raziky M, El Akel W, Abdo M, Tantawi O, AbdAllah M, Bourliere M, Esmat G. Changes in liver stiffness measurements and fibrosis scores following sofosbuvir based treatment regimens without interferon. J Gastroenterol Hepatol. 2017;32:1624-1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 38. | Tsochatzis EA, Gurusamy KS, Ntaoula S, Cholongitas E, Davidson BR, Burroughs AK. Elastography for the diagnosis of severity of fibrosis in chronic liver disease: a meta-analysis of diagnostic accuracy. J Hepatol. 2011;54:650-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 539] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 39. | Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL, Messinger D, Nelson M; APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2633] [Cited by in RCA: 3799] [Article Influence: 190.0] [Reference Citation Analysis (0)] |
| 40. | Mauss S, Pol S, Buti M, Duffell E, Gore C, Lazarus JV, der Grient HL, Lundgren J, Mozalevskis A, Raben D, Schatz E, Wiktor S, Rockstroh JK; European consensus working group on late presentation for Viral Hepatitis Care. Late presentation of chronic viral hepatitis for medical care: a consensus definition. BMC Med. 2017;15:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 41. | Chen CC, Hsu HT, Chen YL, Chen RC, Wu WP, Chou CT. Diagnostic Accuracy of Acoustic Radiation Force Impulse (ARFI) and Wisteria floribunda Agglutinin-Positive Mac-2-Binding Protein (WFA⁺-M2BP) in Patients with Chronic Liver Disease. Med Sci Monit. 2019;25:7169-7174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 42. | Singh S, Facciorusso A, Loomba R, Falck-Ytter YT. Magnitude and Kinetics of Decrease in Liver Stiffness After Antiviral Therapy in Patients With Chronic Hepatitis C: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2018;16:27-38.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 154] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 43. | Bachofner JA, Valli PV, Kröger A, Bergamin I, Künzler P, Baserga A, Braun D, Seifert B, Moncsek A, Fehr J, Semela D, Magenta L, Müllhaupt B, Terziroli Beretta-Piccoli B, Mertens JC. Direct antiviral agent treatment of chronic hepatitis C results in rapid regression of transient elastography and fibrosis markers fibrosis-4 score and aspartate aminotransferase-platelet ratio index. Liver Int. 2017;37:369-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 201] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 44. | Dolmazashvili E, Abutidze A, Chkhartishvili N, Karchava M, Sharvadze L, Tsertsvadze T. Regression of liver fibrosis over a 24-week period after completing direct-acting antiviral therapy in patients with chronic hepatitis C receiving care within the national hepatitis C elimination program in Georgia: results of hepatology clinic HEPA experience. Eur J Gastroenterol Hepatol. 2017;29:1223-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 45. | Kobayashi N, Iijima H, Tada T, Kumada T, Yoshida M, Aoki T, Nishimura T, Nakano C, Takata R, Yoh K, Ishii A, Takashima T, Sakai Y, Aizawa N, Nishikawa H, Ikeda N, Iwata Y, Enomoto H, Hirota S, Fujimoto J, Nishiguchi S. Changes in liver stiffness and steatosis among patients with hepatitis C virus infection who received direct-acting antiviral therapy and achieved sustained virological response. Eur J Gastroenterol Hepatol. 2018;30:546-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 46. | Akahane T, Kurosaki M, Itakura J, Tsuji K, Joko K, Kimura H, Nasu A, Ogawa C, Kojima Y, Hasebe C, Wada S, Uchida Y, Sohda T, Suzuki H, Yoshida H, Kusakabe A, Tamada T, Kobashi H, Mitsuda A, Kondo M, Shigeno M, Ide Y, Morita A, Kitamura T, Abe T, Izumi N. Real-world efficacy and safety of sofosbuvir + ribavirin for hepatitis C genotype 2: A nationwide multicenter study by the Japanese Red Cross Liver Study Group. Hepatol Res. 2019;49:264-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 47. | Pan JJ, Bao F, Du E, Skillin C, Frenette CT, Waalen J, Alaparthi L, Goodman ZD, Pockros PJ. Morphometry Confirms Fibrosis Regression From Sustained Virologic Response to Direct-Acting Antivirals for Hepatitis C. Hepatol Commun. 2018;2:1320-1330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 48. | Deterding K, Höner Zu Siederdissen C, Port K, Solbach P, Sollik L, Kirschner J, Mix C, Cornberg J, Worzala D, Mix H, Manns MP, Cornberg M, Wedemeyer H. Improvement of liver function parameters in advanced HCV-associated liver cirrhosis by IFN-free antiviral therapies. Aliment Pharmacol Ther. 2015;42:889-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 49. | Iacob S, Cerban R, Pietrareanu C, Ester C, Iacob R, Gheorghe C, Popescu I, Gheorghe L. 100% sustained virological response and fibrosis improvement in real-life use of direct acting antivirals in genotype-1b recurrent hepatitis C following liver transplantation. J Gastrointestin Liver Dis. 2018;27:139-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 50. | Martini S, Sacco M, Strona S, Arese D, Tandoi F, Dell Olio D, Stradella D, Cocchis D, Mirabella S, Rizza G, Magistroni P, Moschini P, Ottobrelli A, Amoroso A, Rizzetto M, Salizzoni M, Saracco GM, Romagnoli R. Impact of viral eradication with sofosbuvir-based therapy on the outcome of post-transplant hepatitis C with severe fibrosis. Liver Int. 2017;37:62-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 51. | Korda D, Lenard ZM, Gerlei Z, Jakab Z, Haboub-Sandil A, Wagner L, Varga M, Cseprekal O, Marton A, Horvathy D, Takacs S, Doros A, Mathe Z. Shear-wave elastography for the assessment of liver fibrosis in liver transplant recipients treated for hepatitis C virus recurrence. Eur J Gastroenterol Hepatol. 2018;30:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 52. | Chan J, Gogela N, Zheng H, Lammert S, Ajayi T, Fricker Z, Kim AY, Robbins GK, Chung RT. Direct-Acting Antiviral Therapy for Chronic HCV Infection Results in Liver Stiffness Regression Over 12 Months Post-treatment. Dig Dis Sci. 2018;63:486-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 53. | Tawara S, Tatsumi T, Iio S, Kobayashi I, Shigekawa M, Hikita H, Sakamori R, Hiramatsu N, Miyoshi E, Takehara T. Evaluation of Fucosylated Haptoglobin and Mac-2 Binding Protein as Serum Biomarkers to Estimate Liver Fibrosis in Patients with Chronic Hepatitis C. PLoS One. 2016;11:e0151828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 54. | Yamasaki K, Tateyama M, Abiru S, Komori A, Nagaoka S, Saeki A, Hashimoto S, Sasaki R, Bekki S, Kugiyama Y, Miyazoe Y, Kuno A, Korenaga M, Togayachi A, Ocho M, Mizokami M, Narimatsu H, Yatsuhashi H. Elevated serum levels of Wisteria floribunda agglutinin-positive human Mac-2 binding protein predict the development of hepatocellular carcinoma in hepatitis C patients. Hepatology. 2014;60:1563-1570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 212] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 55. | Yasui Y, Kurosaki M, Komiyama Y, Takada H, Tamaki N, Watakabe K, Okada M, Wang W, Shimizu T, Kubota Y, Higuchi M, Takaura K, Tsuchiya K, Nakanishi H, Takahashi Y, Itakura J, Enomoto N, Izumi N. Wisteria floribunda agglutinin-positive Mac-2 binding protein predicts early occurrence of hepatocellular carcinoma after sustained virologic response by direct-acting antivirals for hepatitis C virus. Hepatol Res. 2018;48:1131-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lei YC, Tai DI S-Editor: Dou Y L-Editor: A E-Editor: Ma YJ