Published online May 21, 2020. doi: 10.3748/wjg.v26.i19.2403
Peer-review started: February 26, 2020
First decision: April 2, 2020
Revised: April 9, 2020
Accepted: May 1, 2020
Article in press: May 1, 2020
Published online: May 21, 2020
Processing time: 84 Days and 23.9 Hours
Different types of periampullary diverticulum (PAD) may differentially affect the success of endoscopic retrograde cholangiopancreatography (ERCP) cannulation, but the clinical significance of the two current PAD classifications for cannulation is limited.
To verify the clinical value of our newly proposed PAD classification.
A new PAD classification (Li-Tanaka classification) was proposed at our center. All PAD patients with native papillae who underwent ERCP from January 2012 to December 2017 were classified according to three classification systems, and the effects of various types of PAD on ERCP cannulation were compared.
A total of 3564 patients with native papillae were enrolled, including 967 (27.13%) PAD patients and 2597 (72.87%) non-PAD patients. In the Li-Tanaka classification, type I PAD patients exhibited the highest difficult cannulation rate (23.1%, P = 0.01), and type II and IV patients had the highest cannulation success rates (99.4% in type II and 99.3% in type IV, P < 0.001). In a multivariable-adjusted logistic model, the overall successful cannulation rate in PAD patients was higher than that in non-PAD patients [odds ratio (OR) = 1.87, 95% confidence interval (CI): 1.04-3037, P = 0.037]. In addition, compared to the non-PAD group, the difficulty of cannulation in the type I PAD group according to the Li-Tanaka classification was greater (OR = 2.04, 95%CI: 1.13-3.68, P = 0.004), and the successful cannulation rate was lower (OR = 0.27, 95%CI: 0.11-0.66, P < 0.001), while it was higher in the type II PAD group (OR = 4.44, 95%CI: 1.61-12.29, P < 0.01).
Among the three PAD classifications, the Li-Tanaka classification has an obvious clinical advantage for ERCP cannulation, and it is helpful for evaluating potentially difficult and successful cannulation cases among different types of PAD patients.
Core tip: Unlike previous studies conducted more than a decade ago, most current studies no longer suggest that periampullary diverticulum (PAD) significantly increases the difficulty of cannulation. However, we found that different clinical types of PAD may affect the difficulty and even success of endoscopic retrograde cholangiopancreatography (ERCP) cannulation. Furthermore, existing PAD classifications have limited clinical guidance value. We proposed a new PAD classification method (Li-Tanaka classification) in 2012 based on the number of PADs and their anatomical relationship with the major papilla, and we conducted a retrospective study to evaluate the clinical value of the Li-Tanaka PAD classification for ERCP cannulation. Our study showed that the Li-Tanaka classification has good clinical significance for ERCP cannulation.
- Citation: Yue P, Zhu KX, Wang HP, Meng WB, Liu JK, Zhang L, Zhu XL, Zhang H, Miao L, Wang ZF, Zhou WC, Suzuki A, Tanaka K, Li X. Clinical significance of different periampullary diverticulum classifications for endoscopic retrograde cholangiopancreatography cannulation. World J Gastroenterol 2020; 26(19): 2403-2415
- URL: https://www.wjgnet.com/1007-9327/full/v26/i19/2403.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i19.2403
Diverticulum is a common intestinal anatomical variation. A periampullary diverticulum (PAD) is defined as a duodenal depressed lesion of more than 5 mm with an intact mucosa within a radius of 2.5 cm of the major papilla. The clinical discovery rates of PAD vary from 6% to 31.7%[1-3,23], and the prevalence of PAD increases with age. Our previous study[4] found that PAD may increase the incidence of choledocholithiasis and could be a potential risk factor for recurrent choledocholithiasis, especially after cholecystectomy. Previous studies have mainly proposed that PAD may increase the difficulty of endoscopic retrograde cholangiopancreatography (ERCP) cannulation[5]. However, many current studies no longer suggest that PAD significantly increases the difficulty of cannulation[2,6,7]. In clinical practice, we found that the effect of PAD on ERCP cannulation was related to the characteristics of the diverticulum.
Previously, two classifications of PAD based on the characteristics of the diverticulum were used. In 1998, Lobo et al[5] first divided PAD into two types: Intradiverticular papilla (IDP) and juxtapapillary diverticulum (JPD). In 2006, Boix et al[2] identified three types of PAD, and types I and II were further divided into four subtypes. However, the clinical significance of the two current PAD classifications for cannulation is limited.
Based on the number of PADs and their anatomical relationship with the major papilla, we worked with the Kyoto Second Red Cross Hospital to propose a new PAD classification method (Li-Tanaka classification) in 2012, and conducted a retrospective study to evaluate the clinical value of the Li-Tanaka PAD classification method for ERCP cannulation.
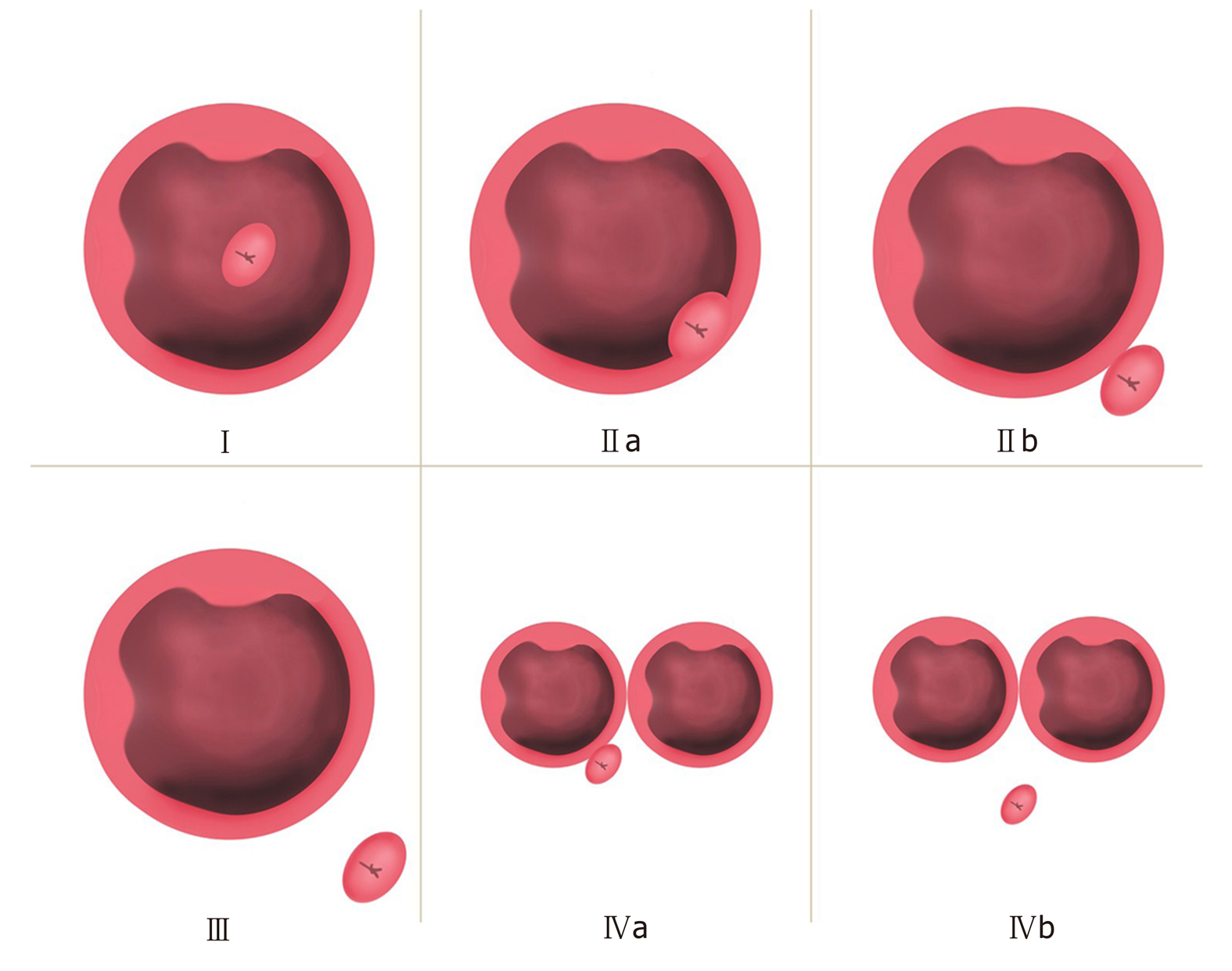
The Lobo classification[5] categorizes PAD as IDP and JPD. In IDP, the major papilla is located in a diverticulum, while in JPD, the papilla is outside the duodenal diverticulum (Figure 1A). The Boix classification[2] includes three types of PAD, of which types I and II are divided into four subtypes: Type I: The papilla is located inside the diverticulum (Ia: Up; Ib: Left; Ic: Down; Id: Right); type II: The papilla is located in the margin of the diverticulum (IIa: Apical left margin; IIb: Apical right margin; IIc: Center left or right margin; IId: Between two diverticula); and type III: The papilla is located near the diverticulum (Figure 1B). According to the number of diverticula and their anatomical relationship with the major papilla, we divided PAD into four types and then further divided types II and IV into two subtypes in terms of the distance from the major papilla to the edge of the diverticulum. In type I, the papilla is located inside the diverticulum and is not adjacent to the margin; in type II, the papilla is located in the margin of the diverticulum (type IIa, inside of the margin; type IIb, outside of the margin, < 1 cm); in type III, the papilla is located outside of the margin, ≥ 1 cm; and in type IV, the papilla is located in the margin of the diverticulum and ≥ 2 diverticula are present (type IVa: The papilla is located outside the margins of at least one diverticulum, < 1 cm; type IVb: The papilla is located outside the margins of all of the diverticula, ≥ 1 cm) (Figure 2).
According to the most current international consensus recommendations of the American Society for Gastrointestinal Endoscopy on difficult biliary access in 2017[8], procedures that satisfy one or more of the following conditions are regarded as difficult cannulation: (1) Failure of selective bile duct cannulation by the standard ERCP technique within 10 min; (2) More than 5 cannulation attempts; or (3) Failure to gain access to the major papilla.
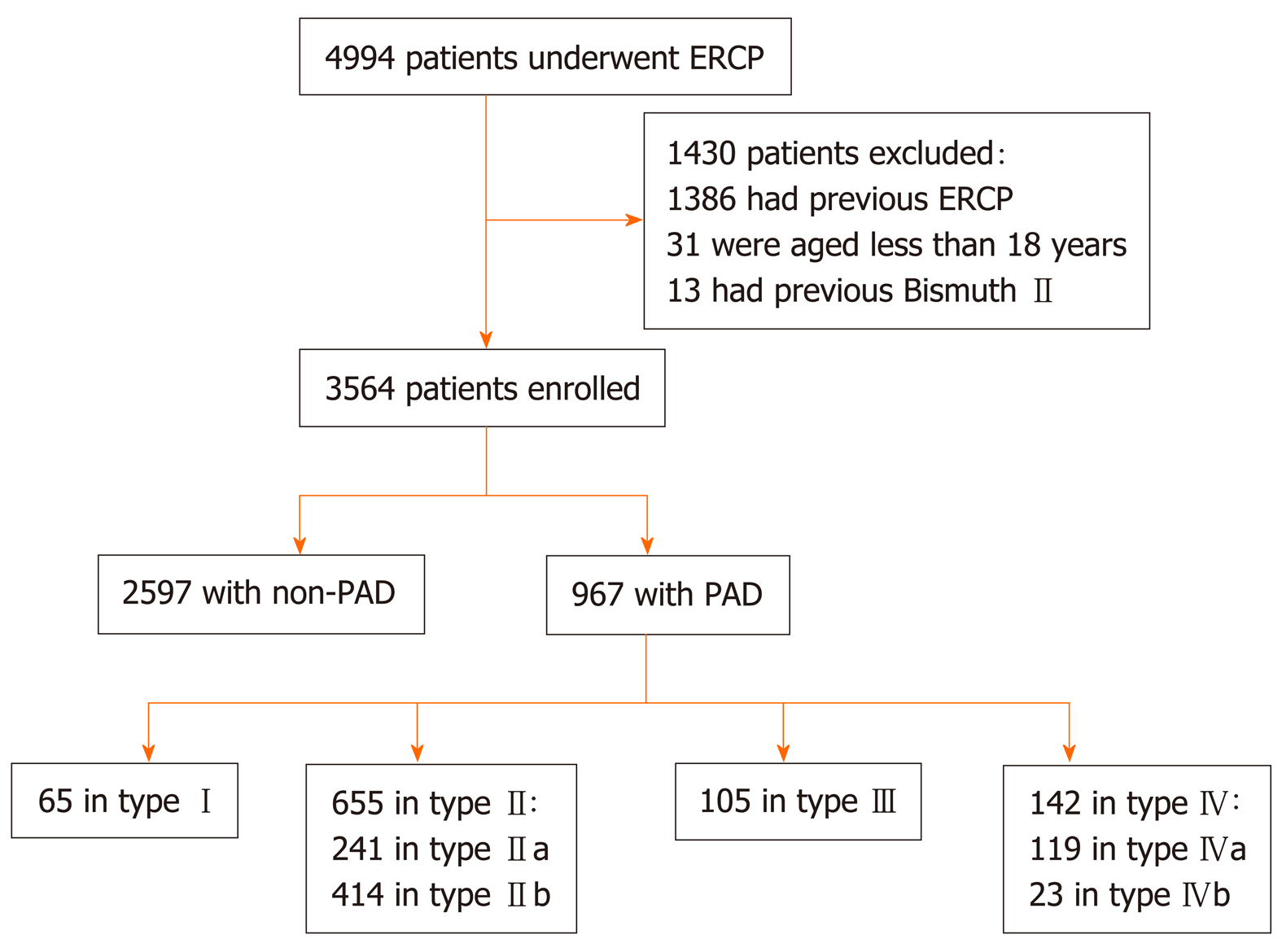
A total of 4994 consecutive patients who underwent ERCP from January 2012 to December 2017 at the First Hospital of Lanzhou University were retrospectively analyzed. Written informed consent was obtained from the patients before the procedure, and this study was approved by the institutional ethics committee at our hospital. A total of 1430 cases were excluded based on the following exclusion criteria: Patients with a history of ERCP or Billroth II anastomosis and patients under 18 years of age. These patients were excluded because these factors may directly affect the observation of diverticula and make ERCP cannulation extremely easy or difficult. Ultimately, 3564 patients aged 18 years or above with native papillae were included (Figure 3).
Hepatopancreatobiliary diseases and indications for ERCP were confirmed by laboratory and imaging examinations, including abdominal ultrasound, computed tomography, and magnetic resonance cholangiopancreatography. Patients were placed in the left lateral position before nontracheal intubation and general anesthesia, and a duodenoscope (JF-240, TJF-240, TJF-260 or CV-290, Olympus, Japan) was used to complete the ERCP procedure. The size, number, shape, and specific type of PAD for each classification method were noted before cannulation.
Then, major papillary cannulation was routinely performed using a sphincterotome. If the cannulation was difficult, double-wire, precut, and other techniques were used to assist cannulation. After successful cannulation, all ERCP procedures, including cholangiography, endoscopic sphincterotomy (EST), endoscopic papillary balloon dilatation (EPBD), mechanical lithotripsy, endoscopic retrograde biliary drainage, and endoscopic retrograde pancreatic drainage, were performed selectively as required. If the cannulation failed, the patient was rescheduled for ERCP or surgery.
All clinical values are presented as the mean and standard deviation for continuous variables and counts and percentages for dichotomous variables. Global tests for quality were evaluated using analysis of variance (ANOVA), the Kruskal-Wallis test, the Pearson chi-square test, and Fisher’s exact test, as appropriate. For variables in which a significant effect of global test (P < 0.05) was identified, multiple comparisons were performed among the different types of PAD by Bonferroni correction, in which an α of 0.05 was adjusted to 0.017 and 0.008 for three and four groups, respectively. Multivariable-adjusted logistic models were used to estimate the odds ratio (OR) of PAD for difficult and successful cannulation. All models were adjusted for age and sex, and the results are presented as ORs with 95% confidence intervals (CIs). P values less than 0.05 were considered to indicate significance. All analyses were conducted using STATA 14 (Stata Corp, College Station, TX, United States).
Of the 3564 patients enrolled, 967 (27.13%) were in the PAD group, and 2597 (72.87%) were in the non-PAD group. Compared to those in the non-PAD group, the patients in the PAD group had a greater average age (65 ± 13 vs 58 ± 16, P < 0.001), comprised a greater proportion of males (53.5% vs 48.1%, P = 0.004), had a higher incidence of common bile duct stones (85.1% vs 71.1%) and acute cholangitis (17.7% vs 11.2%), and had a lower concurrency of malignant biliary stricture (5.2% vs 13.8%) and acute pancreatitis (5.6% vs 9.6%) (P < 0.001) (Table 1). The baseline clinical characteristics of different types of PAD patients among the three classifications are presented in Table 2.
| Non-PAD group (n = 2597) | PAD group (n = 967) | P value | |
| Age, mean ± SD | 58 ± 16 | 65 ± 13 | < 0.001 |
| Sex, n (%) | 0.004 | ||
| Male | 1248 (48.1) | 517 (53.5) | |
| Female | 1349 (51.9) | 450 (46.5) | |
| History of cholecystectomy | 909 (35.0) | 356 (36.8) | 0.32 |
| Gallbladder stones | 1001 (38.5) | 369 (38.2) | 0.83 |
| Indications, n (%) | |||
| Common bile duct stones | 1846 (71.1) | 823 (85.1) | < 0.001 |
| Acute cholangitis | 291 (11.2) | 171 (17.7) | < 0.001 |
| Malignant biliary stricture | 359 (13.8) | 50 (5.2) | < 0.001 |
| Benign biliary stricture | 101 (3.9) | 33 (3.4) | 0.51 |
| Acute pancreatitis | 248 (9.6) | 54 (5.6) | < 0.001 |
| Pancreatic duct stones | 18 (0.7) | 4 (0.4) | 0.35 |
| Comorbidity, n (%) | |||
| Hypertension | 536 (20.6) | 239 (24.7) | 0.009 |
| Diabetes | 212 (8.2) | 100 (10.3) | 0.04 |
| Coronary disease | 67 (2.6) | 29 (3.0) | 0.49 |
| Virus hepatitis | 140 (5.4) | 31 (3.2) | 0.007 |
| Lobo classification, 1998 | Boix classification, 2006 | Li-Tanaka classification, 2012 | |||||||
| IDP | JPD | I | II | III | I | II | III | IV | |
| Total | 65 | 902 | 306 | 556 | 105 | 65 | 655 | 105 | 142 |
| Age, mean ± SD | 65 ± 11 | 65 ± 14 | 64 ± 13 | 65 ± 13 | 65 ± 15 | 65 ± 11 | 64 ± 14 | 65 ± 15 | 66 ± 12 |
| Sex, n (%) | |||||||||
| Male | 32 (49.2) | 485 (53.8) | 167 (54.6) | 293 (52.7) | 57 (54.3) | 32 (49.2) | 358 (54.7) | 57 (54.3) | 70 (49.3) |
| Female | 33 (50.8) | 417 (46.2) | 139 (45.4) | 263 (47.3) | 48 (45.7) | 33 (50.8) | 297 (45.3) | 48 (45.7) | 72 (50.7) |
| History of cholecystectomy | 15 (23.1) | 341 (37.8)a | 111 (36.3) | 221 (39.8) | 24 (22.9)a | 15 (23.1) | 263 (40.2) | 24 (22.9) | 54 (38.0)a |
| Gallbladder stones | 28 (43.1) | 341 (37.8) | 117 (38.2) | 215 (38.7) | 37 (35.2) | 28 (43.1) | 249 (38.0) | 37 (35.2) | 55 (38.7) |
| Indications, n (%) | |||||||||
| Common bile duct stones | 53 (81.5) | 770 (85.4) | 265 (86.6) | 471 (84.7) | 87 (82.9) | 53 (81.5) | 561 (85.7) | 87 (82.9) | 122 (85.9) |
| Acute cholangitis | 11 (16.9) | 160 (17.7) | 61 (19.9) | 96 (17.3) | 14 (13.3) | 11 (16.9) | 119 (18.2) | 14 (13.3) | 27 (19.0) |
| Malignant biliary stricture | 2 (3.1) | 48 (5.3) | 13 (4.3) | 28 (5.0) | 9 (8.6) | 2 (3.1) | 30 (4.6) | 9 (8.6) | 9 (6.3) |
| Benign biliary stricture | 1 (1.5) | 32 (3.6) | 8 (2.6) | 22 (4.0) | 3 (2.9) | 1 (1.5) | 26 (4.0) | 3 (2.9) | 3 (2.1) |
| Acute pancreatitis | 5 (7.7) | 49 (5.4) | 25 (8.2) | 26 (4.7) | 3 (2.9)a | 5 (7.7) | 42 (6.4) | 3 (2.9) | 4 (2.8) |
| Pancreatic duct stones | 0 | 4 (0.4) | 2 (0.7) | 2 (0.4) | 0 | 0 | 4 (0.6) | 0 | 0 |
| Comorbidity, n (%) | |||||||||
| Hypertension | 19 (29.2) | 220 (24.4) | 77 (25.2) | 134 (24.1) | 28 (26.7) | 19 (29.2) | 159 (24.3) | 28 (26.7) | 33 (23.2) |
| Diabetes | 11 (16.9) | 89 (9.9) | 34 (11.1) | 54 (9.7) | 12 (11.4) | 11 (16.9) | 66 (10.1) | 12 (11.4) | 11 (7.8) |
| Coronary disease | 5 (7.7) | 24 (2.7)a | 10 (3.3) | 18 (3.2) | 1 (1.0) | 5 (7.7) | 16 (2.4) | 1 (1.0) | 7 (4.9)a |
| Virus hepatitis | 2 (3.1) | 29 (3.2) | 9 (2.9) | 17 (3.1) | 5 (4.8) | 2 (3.1) | 17 (2.6) | 5 (4.8) | 7 (4.9) |
For the Lobo classification, the difficult cannulation rate for IDP was higher than that for JPD (23.1% vs 10.3%, P = 0.002), and the success rate of cannulation was lower for IDP than for JPD (90.8% vs 99.1%, P < 0.001) (Table 3).
| Lobo classification, 1998 | Boix classification, 2006 | Li-Tanaka classification, 2012 | ||||||||||
| IDP | JPD | P value | I | II | III | P value | I | II | III | IV | P value | |
| Total | 65 (6.7) | 902 (93.3) | 306 (31.6) | 556 (57.5) | 105 (10.9) | 65 (6.7) | 655 (67.7) | 105 (10.9) | 142 (14.7) | |||
| Diameter of CBD (mm) | 14 ± 4 | 14 ± 5 | 0.33 | 14 ± 5 | 15 ± 5 | 14 ± 6 | 0.37 | 14 ± 4 | 14 ± 5 | 14 ± 6 | 15 ± 5 | 0.56 |
| Max. size of PAD (mm) | 19 ± 8 | 13 ± 7 | < 0.001 | 15 ± 8 | 13 ± 7 | 11 ± 7 | < 0.001 | 19 ± 8 | 13 ± 7 | 11 ± 7 | 14 ± 9 | < 0.001 |
| ≤ 10 | 11 (16.9) | 531 (58.9) | 146 (47.7) | 325 (58.5) | 71 (67.6) | 11 (16.9) | 384 (58.6) | 71 (67.6) | 76 (53.5) | |||
| 10-20 | 38 (58.5) | 287 (31.8) | 118 (38.6) | 182 (32.7) | 25 (23.8) | 38 (58.5) | 217 (33.1) | 25 (23.8) | 45 (31.7) | |||
| > 20 | 16 (24.6) | 84 (9.3) | 42 (13.7) | 49 (8.8) | 9 (8.6) | 16 (24.6) | 54 (8.2) | 9 (8.6) | 21 (14.8) | |||
| CBD stones | 51 (86.4) | 765 (85.6) | 0.85 | 262 (87.9) | 470 (85.0) | 84 (82.4) | 0.31 | 51 (86.4) | 559 (85.9) | 84 (82.4) | 122 (86.5) | 0.79 |
| Max diameter (mm) | 13 ± 5 | 12 ± 7 | 0.23 | 12 ± 6 | 13 ± 7 | 12 ± 6 | 0.26 | 13 ± 5 | 12 ± 6 | 12 ± 6 | 13 ± 8 | 0.58 |
| Multiple stone | 19 (37.3) | 353 (46.1) | 0.22 | 115 (43.9) | 224 (47.7) | 33 (39.3) | 0.29 | 19 (37.3) | 258 (46.2) | 33 (39.3) | 62 (50.8) | 0.24 |
| Difficult stone removal | 16 (31.4) | 273 (35.7) | 0.53 | 96 (36.6) | 159 (33.8) | 34 (40.5) | 0.44 | 16 (31.4) | 195 (34.9) | 34 (40.5) | 44 (36.1) | 0.71 |
| Mechanical lithotripsy | 4 (6.2) | 35 (3.9) | 0.33 | 21 (6.9) | 16 (2.9) | 2 (1.9) | 0.008 | 4 (6.2) | 31 (4.7) | 2 (1.9) | 2 (1.4) | 0.13 |
| Retained stones | 3 (5.9) | 59 (7.7) | 0.79 | 20 (7.6) | 34 (7.2) | 8 (9.5) | 0.77 | 3 (5.9) | 38 (6.8) | 8 (9.5) | 13 (10.7) | 0.42 |
| Guide wire in PD | 6 (9.2) | 86 (9.5) | 0.94 | 30 (9.8) | 48 (8.6) | 14 (13.3) | 0.32 | 6 (9.2) | 66 (10.1) | 14 (13.3) | 6 (4.2) | 0.08 |
| ERPD | 1 (1.5) | 45 (5.0) | 0.36 | 11 (3.6) | 31 (5.6) | 4 (3.8) | 0.38 | 1 (1.5) | 36 (5.5) | 4 (3.8) | 5 (3.5) | 0.50 |
| Operative time (min) | 44 ± 21 | 41 ± 18 | 0.35 | 41 ± 18 | 41 ± 17 | 43 ± 21 | 0.81 | 44 ± 21 | 41 ± 17 | 43 ± 21 | 39 ± 17 | 0.48 |
| Difficult cannulation | 15 (23.1) | 93 (10.3) | 0.002 | 38 (12.4) | 57 (10.3) | 13 (12.4) | 0.57 | 15 (23.1) | 68 (10.4) | 13 (12.4) | 12 (8.5) | 0.01 |
| Successful cannulation | 59 (90.8) | 894 (99.1) | < 0.001 | 298 (97.4) | 553 (99.5)a | 102 (97.1) | 0.02 | 59 (90.8) | 651 (99.4)b | 102 (97.1) | 141 (99.3)b | < 0.001 |
| Adverse events | 8 (12.3) | 79 (8.8) | 0.33 | 26 (8.5) | 48 (8.6) | 13 (12.4) | 0.44 | 8 (12.3) | 57 (8.7) | 13 (12.4) | 9 (6.3) | 0.30 |
| Post-ERCP pancreatitis | 5 (7.7) | 53 (5.9) | 0.58 | 19 (6.2) | 29 (5.2) | 10 (9.5) | 0.23 | 5 (7.7) | 38 (5.8) | 10 (9.5) | 5 (3.5) | 0.24 |
| Acute cholangitis | 2 (3.1) | 25 (2.8) | 0.70 | 6 (2.0) | 18 (3.2) | 3 (2.9) | 0.55 | 2 (3.1) | 18 (2.8) | 3 (2.9) | 4 (2.8) | 0.98 |
| Perforation | 1 (1.5) | 1 (0.1) | 0.13 | 1 (0.3) | 1 (0.2) | 0 (0.0) | 1.00 | 1 (1.5) | 1 (0.2) | 0 (0.0) | 0 (0.0) | 0.20 |
For the Boix classification, no significant difference in the rate of difficult cannulation was observed (P = 0.57), while the successful cannulation rate of type II PAD was highest (99.5% vs 97.4% in type I and 97.1% in type III, P = 0.02) (Table 3).
For the Li-Tanaka classification, type I PAD had the highest difficult cannulation rate (23.1% vs 10.4% in type II, 12.4% in type III, and 8.5% in type IV, P = 0.01), and type II PAD had the highest successful cannulation rate (99.4% vs 90.8% in type I, 97.1% in type III, and 99.3% in type IV, P < 0.001). No significant difference in terms of procedure-related complications such as post-ERCP pancreatitis (PEP), acute cholangitis, and perforation were observed among the groups for all three classifications (Table 3).
Overall, compared to the non-PAD group, all the patients in the PAD group did not exhibit increased cannulation difficulty (OR = 0.86, 95%CI: 0.68-1.09, P = 0.21), and the success rate of cannulation was higher (OR = 1.87, 95%CI: 1.04-3.37, P = 0.037). For the Li-Tanaka classification, the cannulation difficulty for type I PAD was higher than that in the non-PAD group (OR = 2.04, 95%CI: 1.13-3.68, P = 0.004), and the successful cannulation rate for type I PAD was lower than that in the non-PAD group (OR = 0.27, 95%CI: 0.11-0.66, P < 0.001). Moreover, the successful cannulation rate for type II PAD was higher than that of the non-PAD group (OR = 4.44, 95%CI: 1.61-12.29, P < 0.01) (Tables 4 and 5).
| Variable | n/N (%) | Difficult cannulation | |
| OR (95%CI) | P value | ||
| Total | |||
| Non-PAD | 317/2597 (12.2) | Ref | |
| PAD | 108/967 (11.2) | 0.86 (0.68-1.09) | 0.21 |
| Lobo classification | |||
| Non-PAD | 317/2597 (12.2) | Ref | |
| IDP | 15/65 (23.1) | 2.04 (1.13-3.68) | 0.006 |
| JPD | 93/902 (10.3) | 0.79 (0.61-1.01) | 0.001 |
| Boix classification | |||
| Non-PAD | 317/2597 (12.2) | Ref | |
| I | 38/306 (12.4) | 0.98 (0.68-1.40) | 0.73 |
| II | 57/556 (10.3) | 0.78 (0.58-1.05) | 0.21 |
| III | 13/105 (12.4) | 0.97 (0.53-1.75) | 0.85 |
| Li-Tanaka classification | |||
| Non-PAD | 317/2597 (12.2) | Ref | |
| I | 15/65 (23.1) | 2.04 (1.13-3.68) | 0.004 |
| II | 68/655 (10.4) | 0.80 (0.60-1.06) | 0.13 |
| III | 13/105 (12.4) | 0.97 (0.53-1.75) | 0.91 |
| IV | 12/142 (8.5) | 0.62 (0.34-1.13) | 0.06 |
| Variable | n/N (%) | Successful cannulation | |
| OR (95%CI) | P value | ||
| Total | |||
| Non-PAD | 2531/2597 (97.5) | Ref | |
| PAD | 953/967 (98.6) | 1.87 (1.04-3.37) | 0.037 |
| Lobo classification | |||
| Non-PAD | 2531/2597 (97.5) | Ref | |
| IDP | 59/65 (90.8) | 0.27 (0.11-0.66) | < 0.001 |
| JPD | 894/902 (99.1) | 3.07 (1.46-6.46) | < 0.001 |
| Boix classification | |||
| Non-PAD | 2531/2597 (97.5) | Ref | |
| I | 298/306 (97.4) | 1.02 (0.48-2.15) | 0.27 |
| II | 553/556 (99.5) | 5.09 (1.59-16.30) | 0.009 |
| III | 102/105 (97.1) | 0.94 (0.29-3.04) | 0.33 |
| Li-Tanaka classification | |||
| Non-PAD | 2531/2597 (97.5) | Ref | |
| I | 59/65 (90.8) | 0.27 (0.11-0.66) | < 0.001 |
| II | 651/655 (99.4) | 4.44 (1.61-12.29) | 0.01 |
| III | 102/105 (97.1) | 0.94 (0.29-3.04) | 0.49 |
| IV | 141/142 (99.3) | 3.97 (0.55-28.95) | 0.19 |
Currently, diverticulum is no longer considered an obstacle to the success of ERCP cannulation in many studies[4,9,10]. However, PAD with different features may exert different influences on the difficulty of ERCP cannulation[11,12]. Using the Li-Tanaka classification, we observed differences in the difficult cannulation rate and success rate of cannulation among the four types of PAD. The difficult cannulation rate in the type I PAD patients reached 23.08%, but the success rate of cannulation (90.8%) was significantly lower in this type than in types II, III, and IV. Notably, the successful cannulation rate of type II PAD patients was highest (99.4%). Compared to the non-PAD group, the PAD group showed no significant difference in the difficult cannulation rate (P = 0.21), but the difficult cannulation rate of patients in the type I PAD group was higher than that of patients in the non-PAD group. The reason is that type I PAD accounts for the lowest percentage (6.7%) among the four types; therefore, it does not affect the difficult cannulation rate of the entire PAD group. The cannulation success rate of the entire PAD group was higher than that of the non-PAD group (P = 0.037), but it was lower in the type I PAD group than in the non-PAD group (P < 0.001) and higher in the type II PAD group (P = 0.01). No significant difference was observed between the type III and IV PAD groups and the non-PAD group. The Li-Tanaka classification can be used to evaluate the cannulation success rate of different types of PAD compared to non-PAD. Therefore, it has good clinical significance for ERCP cannulation.
In 1998, Lobo et al[5] investigated 100 (8.26%) PAD cases among a total of 1211 cases and found that the cannulation failure rate in the IDP group was higher than that in the JPD group. Similar results were observed for the Lobo classification in our study. IDP in the Lobo classification is equivalent to type I PAD in the Li-Tanaka classification. The difference is that after further subdividing JPD into types II, III, and IV, we found that the success rate was significantly increased only in type II PAD rather than types III and IV PAD compared to non-PAD. In 2006, Boix et al[2] analyzed 131 (32.75%) of 400 PAD patients and found that diverticula did not increase the difficulty of ERCP cannulation. Boix et al[2] proposed a new PAD classification system, but only the proportion of each type was listed, and the influence of the PAD type on cannulation was not examined. Katsinelos et al[13] analyzed 107 PAD cases using this classification method and found no significant difference in the success rates among the three PAD types. Using the Boix classification, in our study, the cannulation success rate in the type II PAD group was higher than that in the non-PAD group. However, it cannot reflect the low success rate of cannulation in IDP. The potential reason is that type I PAD of the Boix classification includes the IDP type and part of the JPD type. As a result, the low successful cannulation rates of IDP were masked.
Does the distance between the diverticulum and papilla affect ERCP cannulation? Some studies have found that when the papilla is located in the diverticulum or is less than 1 cm from the margin of the diverticulum, the diverticulum will cause partial or complete loss of the papillary sphincter[14-16], which makes it relatively easy for the guidewire to pass through. However, the main reason that the diverticulum increases the difficulty of cannulation is due to the following two aspects[17,18]: The blockage of papillary openings by the diverticulum wall, such as in type I PAD, and the axial deviation of the papilla due to diverticulum compression, such as in types II, III, and IV. Therefore, when the diverticulum covers the papilla and causes difficult cannulation, air should be continuously inflated to fill the diverticulum cavity and expose the opening of the papilla. Moreover, having the diverticulum close to the papilla is beneficial for the success of cannulation. Thus, for cases of types II, III, and IV PAD, attention should be paid to the adjustment of the axial direction. If necessary, specific methods such as double-wire, precut, and pancreatic duct stent placement should be used for cannulation[19-22]. Cappell et al[23] reported a case in which the diverticulum caused the papilla to be skewed, which led to difficulty in cannulation. Tissue clips were used between the papilla and the intestinal wall, which corrected the direction of the papillary opening, resulting in successful cannulation. Kim et al[24] reported successful cannulation using biopsy forceps to correct the direction of the papillary opening. The major papilla of type II PAD is located in the margin of the diverticulum (< 1 cm), and a variety of methods are available to correct the axial direction. These may be the main reasons for the high cannulation success rate in type II PAD according to the Li-Tanaka classification. In addition, 655 (67.7%) of the 967 PAD patients were type II patients; therefore, the overall success rate of cannulation in the PAD group was also higher than that in the non-PAD group.
For the Li-Tanaka classification, the EST rate (75.38%) of type I PAD was lowest, and the EPBD and EST with large balloon dilation (ESLBD) rates were approximately 43.1% and 41.5%, respectively. Some studies[25-27] showed that a small EST followed by EPBD and EPBD alone are both safe and effective methods for PAD patients with cholelithiasis. These techniques do not increase the incidence of adverse events regardless of the PAD subtype. Kim et al[28] found no significant difference between EPBD and EST. Vaira et al[29] found that EST was a safe method in both the PAD and non-PAD groups but that the success rate of EST in the PAD group was lower than that in the non-PAD group. In our study, no significant difference in complications was found among the different PAD types for the 967 PAD cases. Perforation was noted at 72 h after ERCP in one patient with type I PAD, and fully covered self-expandable metal stents were placed. However, the condition could not be improved until surgery was performed. Another case of ERCP-related perforation was found in a type II PAD patient after 24 h; the patient was cured after double biliary stents and one pancreatic duct stent were placed. Because the diameter of type I PAD is larger than that of the other three types and the incision ranges of types I, II, and IV are relatively small, the length and direction of the incision should be strictly controlled to avoid serious adverse events such as perforation[30]. While types III and IVb PADs involve a larger distance between the papilla and diverticulum, the ERCP procedures are similar to those used for non-PAD cases. Some studies have suggested that needle-knife precut fistulotomy is an effective and safe method for difficult cannulation in PAD cases[19]. In addition, most papillae are tilted outwards, which does not increase the risk of EST-related complications[31-33]. Some studies[34,35] have reported that difficult cannulation is a risk factor for PEP. Although type I PAD exhibited the highest rate of difficult cannulation, the incidence of PEP was not significantly different for this group, and PAD is likely a protective factor against PEP. At present, there is no clear theory to explain this phenomenon, which may be due to the existence of PAD resulting in asymmetric relaxation of the sphincter of Oddi. Therefore, cannulation, EPBD, mechanical lithotripsy, and other ERCP procedures have little impact on the pancreatic sphincter.
The proportion of patients with indications for ERCP including acute cholangitis (11.2% vs 17.7%) and common bile duct stones (71.1% vs 85.1%) in the non-PAD group was lower than that of the patients in PAD group. This finding may be related to PAD leading to partial loss of sphincter of Oddi muscle function and an increase in bile duct reflux. The sphincter of Oddi in type I PAD patients may be completely absent after repeated ERCP. In addition, the papilla is located in the diverticulum, which is more likely to cause Lemmel syndrome. Therefore, if acute cholangitis or choledocholithiasis recurs frequently after ERCP is performed more than twice in type I PAD patients, choledochojejunostomy should be considered to reduce the related complications caused by regurgitation. Of course, these findings and recom-mendations require further clinical validation.
Although our study had a much larger sample size than other published studies, some limitations should be noted. First, some PAD patients with biliary and pancreatic diseases could not be included in the study because they chose to undergo surgery rather than receive endoscopic treatment. Second, this study was a retrospective study, and some patients were followed for a short period of time; therefore, comparisons of long-term follow-up data for various types of PAD were not performed.
In conclusion, the Li-Tanaka PAD classification method has obvious clinical significance for ERCP cannulation and is helpful for evaluating difficult and successful cannulation for different types of PAD.
Currently, periampullary diverticulum (PAD) is no longer considered an obstacle to the success of ERCP cannulation in many studies. Different types of PAD may differentially affect the difficulty and success of endoscopic retrograde cholangiopancreatography (ERCP) cannulation; however, the clinical significance of the two existing PAD classifications for cannulation is limited.
In clinical practice, we found that the effect of PAD on ERCP cannulation was related to the characteristics of the diverticulum. A new PAD classification (Li-Tanaka classification) was proposed, and a retrospective study was conducted to evaluate its clinical guidance value for ERCP cannulation.
The objective of this study was to verify the clinical value of our newly proposed PAD classification.
A novel PAD classification (Li-Tanaka classification) was proposed at our center. All PAD patients with native papillae who underwent ERCP from January 2012 to December 2017 were classified according to three classification systems, and the effects of various types of PAD on ERCP cannulation were compared.
Unlike Lobo and Boix classifications, Li-Tanaka classification, which is based on the number of PADs and their anatomical relationship with the major papilla, showed different types of PAD with distinguishing difficulty and success rates of ERCP cannulation. In the Li-Tanaka classification, type I PAD patients exhibited the highest difficult cannulation rate, and types II and IV patients had the highest cannulation success rates. In a multivariable-adjusted logistic model, the overall successful cannulation rate in PAD patients was higher than that in non-PAD patients. In addition, compared to the non-PAD group, the difficulty of cannulation in the type I PAD group according to the Li-Tanaka classification was greater, and the success rate of cannulation was lower, while it was higher in the type II PAD group.
Among the three PAD classifications, the Li-Tanaka classification has an obvious clinical advantage for ERCP cannulation, and it is helpful for evaluating potentially difficult and successful cannulation cases among different types of PAD patients.
Long-term effects of different types of PAD on biliary diseases after ERCP are worthy studying at the subsequent follow-up. What’s more, further prospective studies are needed to determine the clinical guidance value of PAD classification in ERCP cannulation and complications, and what appropriate techniques can be used for different types of PAD with difficult cannulation.
The authors express their gratitude to Ula Nur (Department of Public Health, College of Health Sciences, Qatar University) and Elisabete Weiderpass (International Agency for Research on Cancer) for their scientific analysis and reviewing the statistical analyses performed in this study.
| 1. | Egawa N, Anjiki H, Takuma K, Kamisawa T. Juxtapapillary duodenal diverticula and pancreatobiliary disease. Dig Surg. 2010;27:105-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Boix J, Lorenzo-Zúñiga V, Añaños F, Domènech E, Morillas RM, Gassull MA. Impact of periampullary duodenal diverticula at endoscopic retrograde cholangiopancreatography: a proposed classification of periampullary duodenal diverticula. Surg Laparosc Endosc Percutan Tech. 2006;16:208-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 3. | Wu SD, Su Y, Fan Y, Zhang ZH, Wang HL, Kong J, Tian Y. Relationship between intraduodenal peri-ampullary diverticulum and biliary disease in 178 patients undergoing ERCP. Hepatobiliary Pancreat Dis Int. 2007;6:299-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Li X, Zhu K, Zhang L, Meng W, Zhou W, Zhu X, Li B. Periampullary diverticulum may be an important factor for the occurrence and recurrence of bile duct stones. World J Surg. 2012;36:2666-2669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Lobo DN, Balfour TW, Iftikhar SY. Periampullary diverticula: consequences of failed ERCP. Ann R Coll Surg Engl. 1998;80:326-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (1)] |
| 6. | Balik E, Eren T, Keskin M, Ziyade S, Bulut T, Buyukuncu Y, Yamaner S. Parameters That May Be Used for Predicting Failure during Endoscopic Retrograde Cholangiopancreatography. J Oncol. 2013;2013:201681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Panteris V, Vezakis A, Filippou G, Filippou D, Karamanolis D, Rizos S. Influence of juxtapapillary diverticula on the success or difficulty of cannulation and complication rate. Gastrointest Endosc. 2008;68:903-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Liao WC, Angsuwatcharakon P, Isayama H, Dhir V, Devereaux B, Khor CJ, Ponnudurai R, Lakhtakia S, Lee DK, Ratanachu-Ek T, Yasuda I, Dy FT, Ho SH, Makmun D, Liang HL, Draganov PV, Rerknimitr R, Wang HP. International consensus recommendations for difficult biliary access. Gastrointest Endosc. 2017;85:295-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 97] [Article Influence: 10.8] [Reference Citation Analysis (1)] |
| 9. | Tham TC, Kelly M. Association of periampullary duodenal diverticula with bile duct stones and with technical success of endoscopic retrograde cholangiopancreatography. Endoscopy. 2004;36:1050-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 64] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Sun Z, Bo W, Jiang P, Sun Q. Different Types of Periampullary Duodenal Diverticula Are Associated with Occurrence and Recurrence of Bile Duct Stones: A Case-Control Study from a Chinese Center. Gastroenterol Res Pract. 2016;2016:9381759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Ketwaroo G, Qureshi W. ERCP Success Rate and Periampullary Diverticula: The Pocket Makes No Difference. Dig Dis Sci. 2019;64:1072-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Jayaraj M, Mohan BP, Dhindsa BS, Mashiana HS, Radhakrishnan G, Dhir V, Trindade AJ, Adler DG. Periampullary Diverticula and ERCP Outcomes: A Systematic Review and Meta-Analysis. Dig Dis Sci. 2019;64:1364-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Katsinelos P, Chatzimavroudis G, Tziomalos K, Zavos C, Beltsis A, Lazaraki G, Terzoudis S, Kountouras J. Impact of periampullary diverticula on the outcome and fluoroscopy time in endoscopic retrograde cholangiopancreatography. Hepatobiliary Pancreat Dis Int. 2013;12:408-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Suda K, Mizuguchi K, Matsumoto M. A histopathological study on the etiology of duodenal diverticulum related to the fusion of the pancreatic anlage. Am J Gastroenterol. 1983;78:335-338. [PubMed] |
| 15. | Sfarti VC, Bălan G Jr, Chiriac AŞ, Stanciu C, Bălan G, Gafencu-Şavlovschi D, Trifan AV. Endoscopic retrograde cholangiopancreatography (ERCP) in patients with periampullary diverticula. Rom J Morphol Embryol. 2018;59:833-837. [PubMed] |
| 16. | Mohammad Alizadeh AH, Afzali ES, Shahnazi A, Mousavi M, Doagoo SZ, Mirsattari D, Zali MR. ERCP features and outcome in patients with periampullary duodenal diverticulum. ISRN Gastroenterol. 2013;2013:217261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 17. | Chen L, Xia L, Lu Y, Bie L, Gong B. Influence of periampullary diverticulum on the occurrence of pancreaticobiliary diseases and outcomes of endoscopic retrograde cholangiopancreatography. Eur J Gastroenterol Hepatol. 2017;29:105-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Corral JE, Mousa OY, Kröner PT, Gomez V, Lukens FJ. Impact of Periampullary Diverticulum on ERCP Performance: A Matched Case-Control Study. Clin Endosc. 2019;52:65-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Park CS, Park CH, Koh HR, Jun CH, Ki HS, Park SY, Kim HS, Choi SK, Rew JS. Needle-knife fistulotomy in patients with periampullary diverticula and difficult bile duct cannulation. J Gastroenterol Hepatol. 2012;27:1480-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 20. | Han J. Presence of Periampullary Diverticulum is Not a Hurdle to Successful Endoscopic Retrograde Cholangiopancreatography. Clin Endosc. 2019;52:7-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Kim J, Lee JS, Kim EJ, Kim YS, Cho JH. The Usefulness of Cap-assisted Endoscopic Retrograde Cholangiopancreatography for Cannulation Complicated by a Periampullary Diverticulum. Korean J Gastroenterol. 2018;71:168-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Myung DS, Park CH, Koh HR, Lim SU, Jun CH, Ki HS, Park SY, Rew JS. Cap-assisted ERCP in patients with difficult cannulation due to periampullary diverticulum. Endoscopy. 2014;46:352-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Cappell MS, Mogrovejo E, Manickam P, Batke M. Endoclips to facilitate cannulation and sphincterotomy during ERCP in a patient with an ampulla within a large duodenal diverticulum: case report and literature review. Dig Dis Sci. 2015;60:168-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Kim KH, Kim TN. A new technique for difficult biliary cannulation using endobiliary forceps in a patient with a periampullary diverticulum. Endoscopy. 2017;49:824-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Kim KH, Kim TN. Endoscopic papillary large balloon dilation in patients with periampullary diverticula. World J Gastroenterol. 2013;19:7168-7176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 26. | Zulli C, Grande G, Tontini GE, Labianca O, Geraci G, Sciumè C, Antypas P, Fiocca F, Manes G, Devani M, Manta R, Maurano A. Endoscopic papillary large balloon dilation in patients with large biliary stones and periampullary diverticula: Results of a multicentric series. Dig Liver Dis. 2018;50:828-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Inoue R, Kawakami H, Kubota Y, Ban T. Endoscopic Biliary Intervention Using Traction Devices for Periampullary Diverticulum. Intern Med. 2019;58:2797-2801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Kim KY, Han J, Kim HG, Kim BS, Jung JT, Kwon JG, Kim EY, Lee CH. Late Complications and Stone Recurrence Rates after Bile Duct Stone Removal by Endoscopic Sphincterotomy and Large Balloon Dilation are Similar to Those after Endoscopic Sphincterotomy Alone. Clin Endosc. 2013;46:637-642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Vaira D, Dowsett JF, Hatfield AR, Cairns SR, Polydorou AA, Cotton PB, Salmon PR, Russell RC. Is duodenal diverticulum a risk factor for sphincterotomy? Gut. 1989;30:939-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Nishiwaki M, Mizuno C, Yano K, Oya H, Amano I, Matsumoto J, Tanaka I, Sawai N, Mizuno M, Shima T, Miyamoto Y, Okanoue T. Retroperitoneal Perforation Caused by Migration of a Pancreatic Spontaneous Dislodgement Stent into Periampullary Diverticula. Intern Med. 2018;57:351-355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Tyagi P, Sharma P, Sharma BC, Puri AS. Periampullary diverticula and technical success of endoscopic retrograde cholangiopancreatography. Surg Endosc. 2009;23:1342-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 32. | Karaahmet F, Kekilli M. The presence of periampullary diverticulum increased the complications of endoscopic retrograde cholangiopancreatography. Eur J Gastroenterol Hepatol. 2018;30:1009-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Tohda G, Ohtani M, Dochin M. Efficacy and safety of emergency endoscopic retrograde cholangiopancreatography for acute cholangitis in the elderly. World J Gastroenterol. 2016;22:8382-8388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 34. | Dumonceau JM, Andriulli A, Elmunzer BJ, Mariani A, Meister T, Deviere J, Marek T, Baron TH, Hassan C, Testoni PA, Kapral C; European Society of Gastrointestinal Endoscopy. Prophylaxis of post-ERCP pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - updated June 2014. Endoscopy. 2014;46:799-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 420] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 35. | Chandrasekhara V, Khashab MA, Muthusamy VR, Acosta RD, Agrawal D, Bruining DH, Eloubeidi MA, Fanelli RD, Faulx AL, Gurudu SR, Kothari S, Lightdale JR, Qumseya BJ, Shaukat A, Wang A, Wani SB, Yang J, DeWitt JM. Adverse events associated with ERCP. Gastrointest Endosc. 2017;85:32-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 591] [Article Influence: 65.7] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: Member of Chinese Medical Association.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
P-Reviewer: Deepak P, Pizzirusso F, Raff E, Thomopoulos K S-Editor: Yan JP L-Editor: Wang TQ E-Editor: Zhang YL