Published online May 7, 2020. doi: 10.3748/wjg.v26.i17.2111
Peer-review started: February 29, 2020
First decision: April 2, 2020
Revised: April 8, 2020
Accepted: April 28, 2020
Article in press: April 28, 2020
Published online: May 7, 2020
Processing time: 67 Days and 23.1 Hours
Carcinosarcoma (spindle cell carcinoma) of the esophagus is an extremely rare event; the etiology and origins of this neoplasm have not yet been determined. Epithelial-mesenchymal transition (EMT) has been associated with invasion and metastasis, and may be related to the generation of a stem cell population within this tumor.
We present the case of a 61-year-old male with nausea and fever. Upper gastrointestinal endoscopy revealed the presence of type 1 and 0-IIc lesions located 35 cm from the incisors toward the esophago-gastric junction. Thoracoscopic esophagectomy was performed. Macroscopic analysis revealed three polypoid lesions in the abdominal esophagus that accompanied the main lesion in the lower thoracic esophagus and 0-IIc lesions that spread continuously with them. Histologically, the lesions included proliferating spindle cells. Adeno-carcinomatous components were detected in a section near the foot, and squamous cell carcinoma was identified in the mucosa at the base of the tumor. The patient was diagnosed with multiple carcinosarcomas, staged at pT1b (SM3), pN1 (#110, #7), cM0, Stage II (sarcomatous metastasis to the lymph nodes). Spindle cells did not express E-cadherin but were positive for EMT markers, including zinc finger E-box-binding homeobox 1, TWIST, and snail family transcriptional repressor 2. The patient has experienced no recurrence at 5 years and 2 mo after surgery.
This report suggests that multiple sarcomatous tumors may be generated from primary squamous cell carcinoma via mechanisms related to EMT.
Core tip: Epithelial-mesenchymal transition (EMT) has been associated with cancer invasion and metastasis as epithelial cells acquire the ability to migrate and invade; these observations have been related to the generation of a stem cell population within the neoplasm. We report here a resected case of multiple carcinosarcomas with adeno-carcinomatous components that may have developed secondary to EMT. Detailed elucidation of the relationship between the pathogenesis of carcinosarcoma and EMT are among the future challenges in this field.
- Citation: Okamoto H, Kikuchi H, Naganuma H, Kamei T. Multiple carcinosarcomas of the esophagus with adeno-carcinomatous components: A case report. World J Gastroenterol 2020; 26(17): 2111-2118
- URL: https://www.wjgnet.com/1007-9327/full/v26/i17/2111.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i17.2111
Esophageal carcinosarcoma (spindle cell carcinoma) represents only 0.2%-2.3% of all esophageal malignant tumors[1,2]; detection of multiple esophageal carcinosarcomas in a single patient is an extremely rare event. Although the origins and details of this process are not fully clear, the sarcomatous component of esophageal carcinosarcoma may result from conversion of the epithelial components to mesenchymal or spindle cell metaplasia [i.e., the epithelial-mesenchymal transition (EMT)][3,4].
EMT and mesenchymal–epithelial transition (the reverse process of EMT) play important roles in embryonic development and are critical for processes including gastrulation and neural crest formation; secondary EMT promotes differentiation of somites, palate, pancreas, liver, and reproductive tracts, and tertiary EMT is critical for development of the heart[5,6]. In these processes, epithelial cells lose their polarity and junctions and acquire motility and the capacity to dissolve the extracellular matrix. This is associated with diminished expression of E-cadherin and increased expression of the mesenchymal marker, vimentin. These events have been associated with the cancer invasion and metastasis as epithelial cells acquire the ability to migrate and to invade[7]. Furthermore, several groups have reported that EMT may relate to the generation of stem cell population within the tumor[7,8]. EMT has been associated with the development of undifferentiated cells with sarcomatous morphology[9]; in a previous study of esophageal cancer, the sarcomatous elements were reported to express specific EMT markers[10]. We report here a case of resected multiple carcinosarcomas with adeno-carcinomatous components with deve-lopmental features associated with EMT.
The patient presented with nausea and a fever of over 38 °C with weight loss of 10 kg during the previous 3 mo.
A 61-year-old male visited nearby clinic with complaint of heartburn which was treated as esophageal reflux. Three months later, he returned with nausea and a fever of over 38 °C and reported a weight loss of 10 kg. Laboratory values at this time were notable for a high white blood cell count (18800/μL) and elevated serum C-reactive protein at 15.9 mg/dL. He was referred to the Department of Gastroenterology at our hospital.
His past medical history included glaucoma, depression, and smoking. He reported smoking 1.5 packs of cigarettes per day for 36 years until he was 55 years old. He reported daily use of alcohol.
The patient was 171.8 cm tall and weighed 58 kg. Blood pressure was 104/66 mmHg and heart rate was 120/min. He had a mild fever of over 37.3 °C. All other vital signs were stable.
Abnormal laboratory findings at time of the referral included a white blood cell count of 18800/μL with 82% neutrophils, serum C-reactive protein at 14.8 mg/dL, hemoglobin at 11.8 g/dL, a platelet count of 42.7/µL, serum albumin at 3.1 g/mL, and serum interleukin-6 (IL-6) at 138 pg/mL (normal range, ≤ 4.0 pg/mL). Other laboratory examinations including serum levels of squamous cell carcinoma antigen (SCC-Ag), carcinoembryonic antigen, and carbohydrate antigen 19-9 were within normal limits at 0.5 mg/mL, 0.7 ng/mL, < 2 ng/mL, respectively.
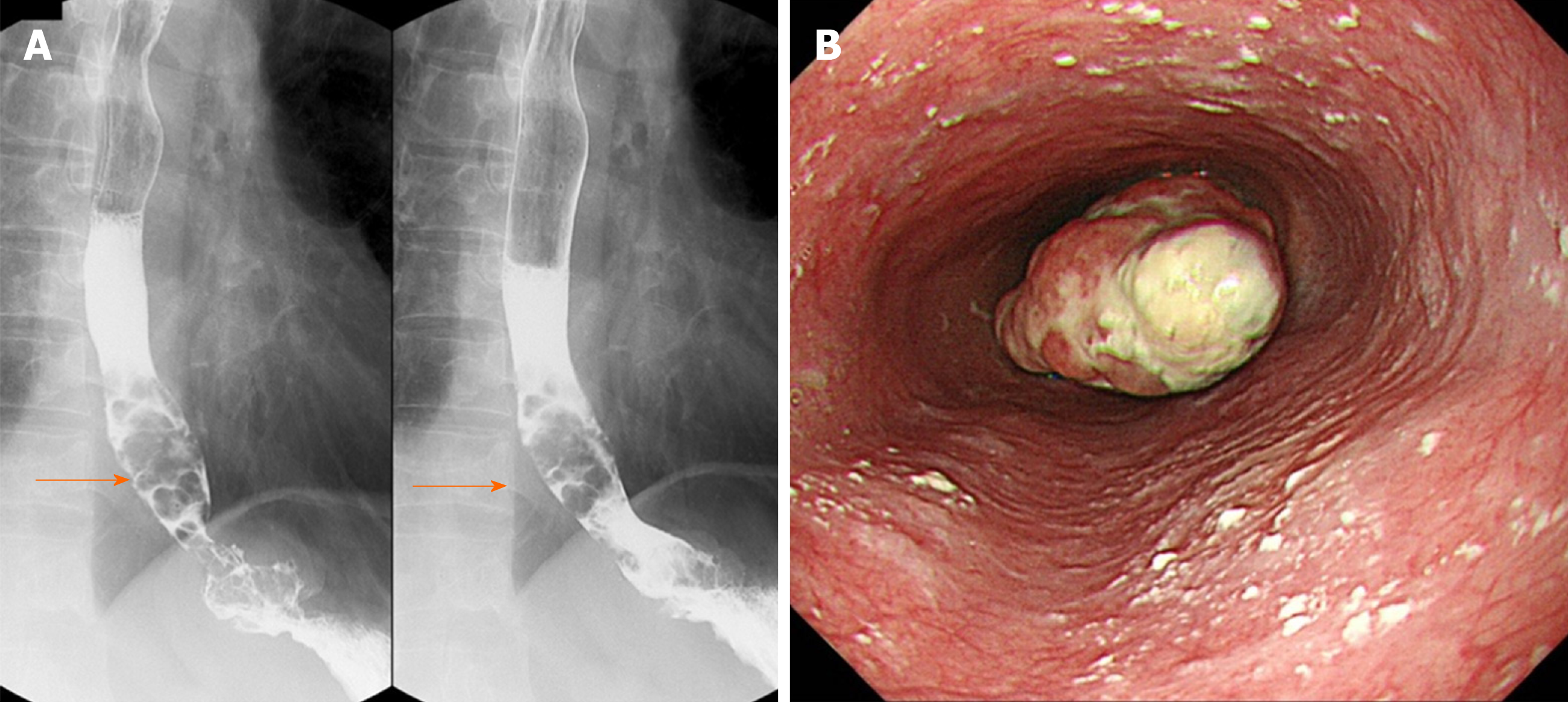
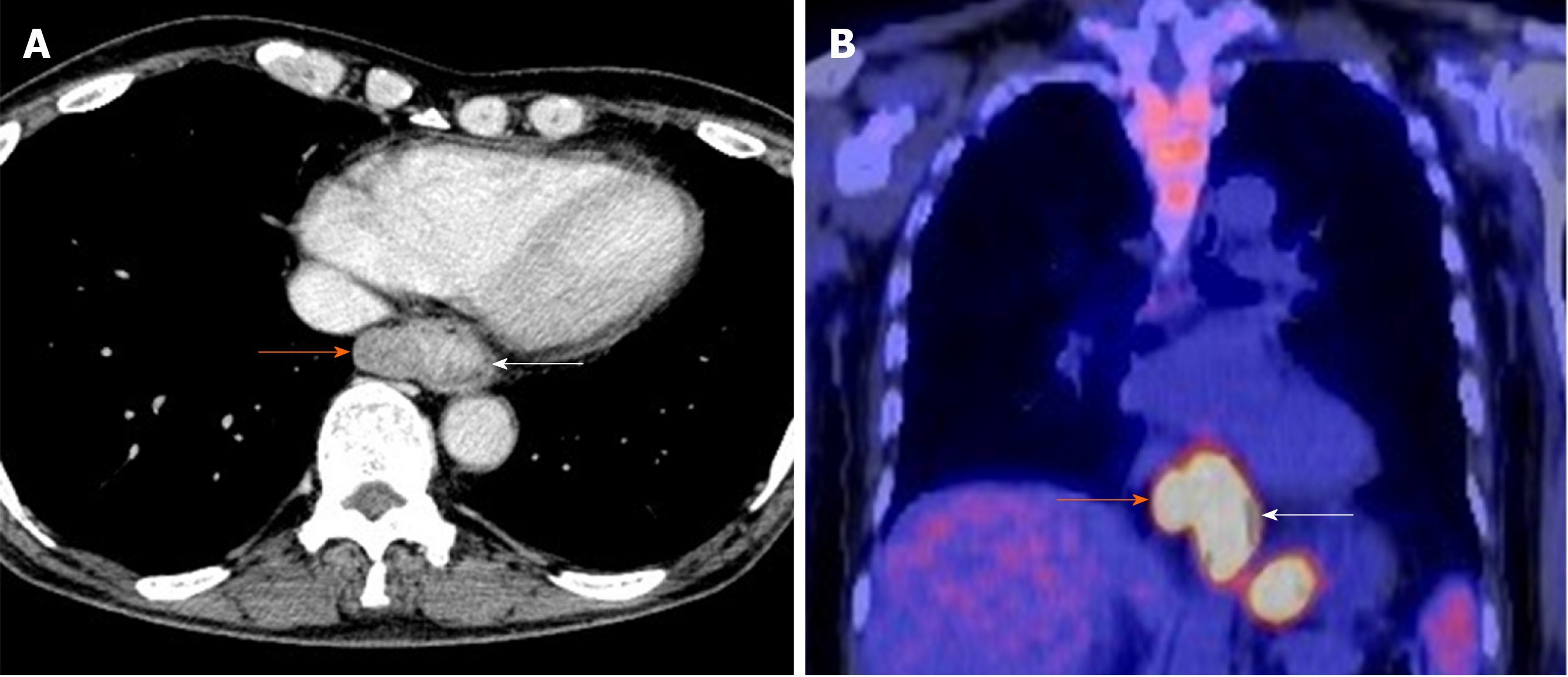
Upper gastrointestinal series revealed stenosis due to the lesion protruding from the lower esophagus (Figure 1A). Upper gastrointestinal endoscopy revealed the presence of type 1 and 0-IIc lesions at a point 35 cm from the incisors toward the esophago-gastric junction (Figure 1B). At biopsy, the type 1 lesion was diagnosed as suspicious for carcinosarcoma; the type 0-IIc lesion was diagnosed as SCC. The major axis of the main tumor was 42 mm and maximum standardized uptake value of the lesion was determined to be 13.9 by computed tomography and 18F-2-fluoro-2-deoxy-D-glucose positron-emission tomography (Figure 2A and B). While paraesophageal lymph node metastasis was suspected, there was no evidence suggesting more distant metastases.
The final preoperative diagnosis was esophageal carcinosarcoma classified as T2N1M0, Stage IIB according to the seventh edition of the Union for International Cancer Control system[11] and T2N1M0, Stage III according to the 11th edition of Japanese classification for esophageal cancer[12,13].
The patient was referred to our department for surgical treatment. Thoracoscopic esophagectomy and gastric tube reconstruction was performed via posterior mediastinal route.
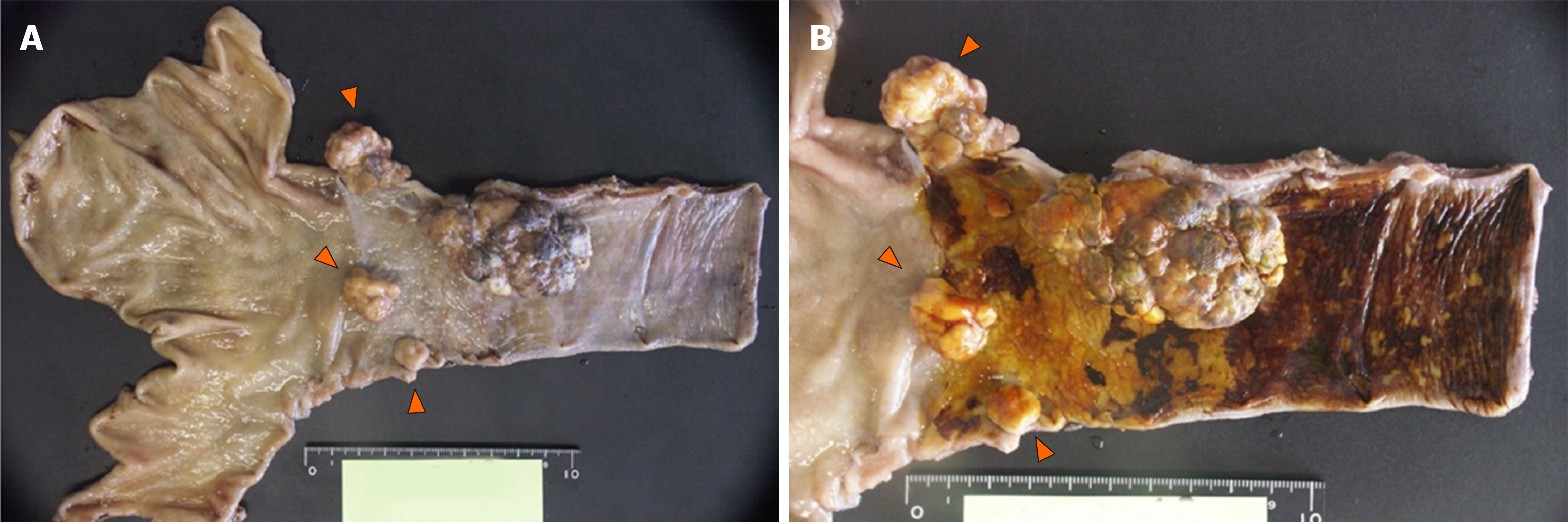
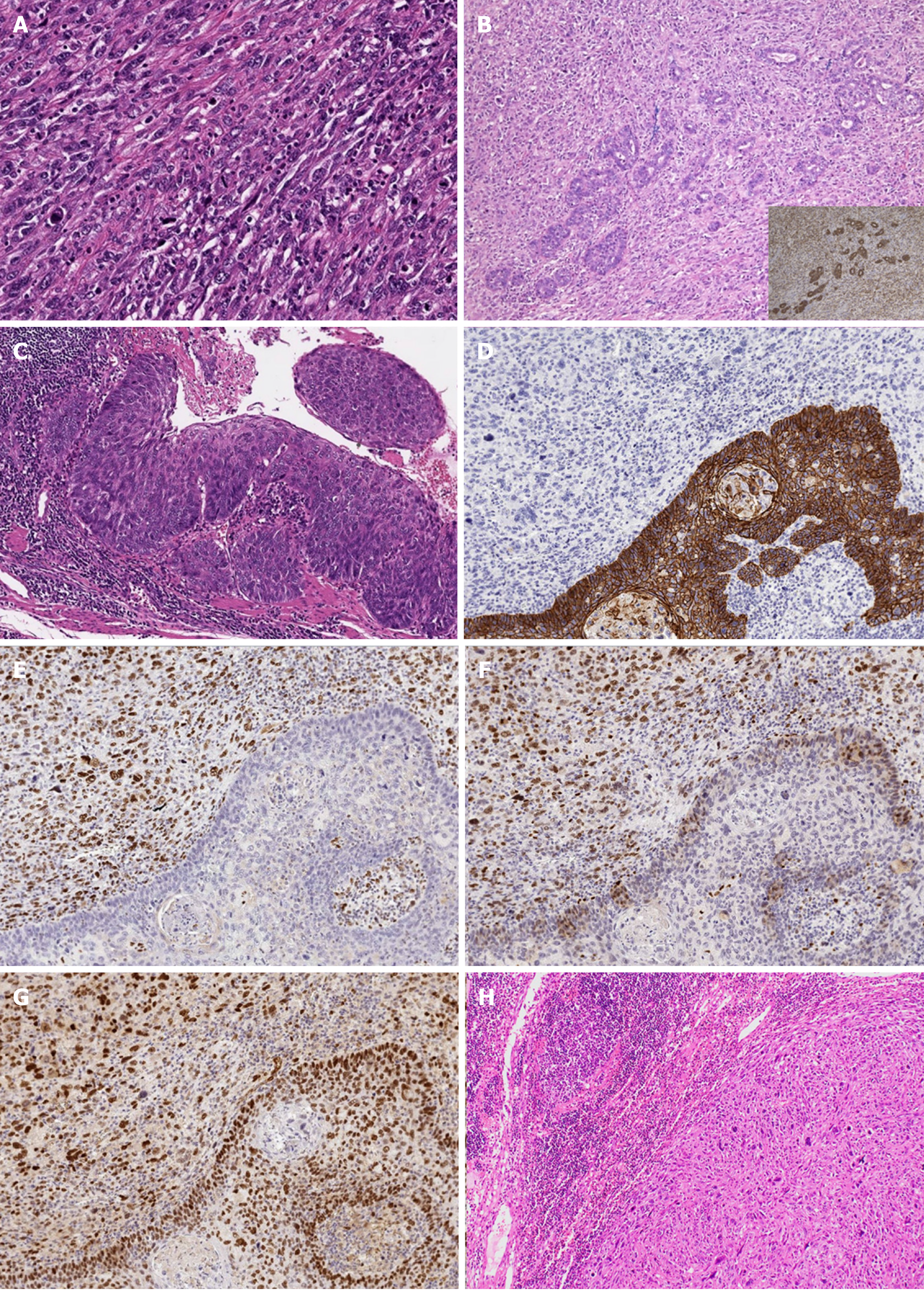
Macroscopic findings included three polypoid lesions located in the abdominal esophagus in addition to the main lesion in the lower esophagus (Figure 3A). Iodine staining revealed a large unstained zone spreading out from the base of the main tumor (Figure 3B). Histologically, dense proliferation and infiltration of spindle cells mixed with giant cells with large atypical nuclei and polynuclear cells were observed (Figure 4A). Histology consistent with adenocarcinoma was also detected in section near the root of the tumor (Figure 4B). SCC in situ was identified in the mucosa at the base of the tumor (Figure 4C). Immunostaining revealed the following antigen profile: cytokeratin AE1/AE3, partial positive; CAM 5.2, partial positive; vimentin, positive; α-smooth muscle actin, negative; muscle actin (HHF35), weak positive; c-kit, negative; discovered on gastrointestinal stromal tumors protein (DOG)-1, weak positive; CD34, negative; S100, partial weak positive for the spindle cells. Taken together, these findings supported the final diagnosis of multiple carcinosarcoma. Spindle cells did not express E-cadherin and but were positive for EMT-related markers, including zinc finger E-box-binding homeobox 1 (ZEB-1), TWIST, and snail family transcriptional repressor 2 (SNAIL 2; Figure 4D-G). We also observed relatively mild intravenous and lymphatic invasion. The final classification was pT1b (SM3), pN1 (one paraesophageal and one along left gastric artery), cM0, Stage IIB as per the Union for International Cancer Control criteria, and pT1b, pN2, cM0, Stage II as per the Japanese classification system. All lymph node metastases were sarcomatous in nature (Figure 4H).
The initial recovery period was uneventful. He was able to take food and drink orally on postoperative day (POD) 5 and was discharged on POD 18. Inflammatory responses declined and serum levels of IL-6 gradually decreased to 16.4 pg/mL, 7.1 pg/mL and 3.0 pg/mL on POD 10, 1 mo after the procedure, and 2 mo after the procedure, respectively. He received one course of combination chemotherapy consisting of continuous infusion of 5-fluorouracil; cisplatin was administered as adjuvant therapy, but it was stopped as per patient request. There has been no evidence of recurrence of disease for 5 years and 2 mo since the surgery.
Multiple esophageal carcinosarcomas in a single patient is an extremely rare event. Lymph node metastases are typically in evidence in SCC, but in this case the lesions were clearly sarcomatous. To the best of our knowledge, this is the first published report of multiple carcinosarcomas with adeno-carcinomatous components in a single patient. Although collision cancer can be considered as a differential diagnosis, we do not consider the condition to be collision cancer owing to its morphological findings.
Elements of the fibrotic and tumor-related microenvironment, including hypoxia and inflammation, can activate EMT-promoting transcription factors by via induction of transforming growth factor β and hypoxia inducible factor. Among these, members of the TWIST, SNAIL, and ZEB transcription factor families have characterized activities and can promote EMT. As a group, they inhibit tumor suppressive programs (p53/RB pathways) and allow the tumor cells to escape cell cycle arrest, apoptosis and/or senescence. They also can have a profound impact on the differentiation of tumor cells by promoting the acquisition of stem-cell-like properties[14]. Expression of ZEB1, TWIST and SNAIL2 within the sarcomatous tissue suggested that EMT may be a factor underlying the generation of multiple sarcomas (spindle cell carcinoma), which may have all been derived from the original SCC. Although the limited amount of sample available precluded a detailed evaluation of this possibility, the adenomatous tissue may also be subjected to this mechanism. Considering the diversified differentiation pattern and the unusual nature of the lymph node metastases, the tumor may have high potential for generating stem cells. Presuming that this is in fact the case, it is also not clear why the cells underwent this specific differentiation pathway. The emergence of stem cells is frequently related to drug resistance and this population represents an important therapeutic target. As such, a focus on cases such as these may also lead to the treatment of carcinosarcoma.
Surgical treatment for esophageal cancer is based on results from cases of SCC. Although esophageal cancer patients with lymph node metastases at the time of surgery have a poor prognosis[1], this patient responded to surgery and chemotherapy with prolonged survival. Notably, postoperative adjuvant chemotherapy in addition to esophagectomy with lymph node dissection, was successful in this one patient; it is not clear whether this reflects the typical course for this disease.
The laboratory data at the time of diagnosis revealed an elevated inflammatory response. Of interest, carcinoma of the esophagus has been associated with the production of both granulocyte colony-stimulating factor (G-CSF) and IL-6, although these findings are also rare[15,16]. Serum IL-6 levels decreased gradually after surgery; unfortunately, we did not evaluate serum G-CSF levels in this case. We now recognize that it will be important to consider both G-CSF and IL-6 in the patient with high fever and elevated white blood cell count. As mentioned above, inflammation is one of the factors that activates EMT-promoting transcription factors. However, whether such inflammation serves to activate EMT requires further study.
We report a rare case of mixed esophageal carcinosarcoma with adenocarcinoma in multiple lesions and sarcomatous lymph node metastases. We suggest that the sarcomatous lesions may have arisen from SCC via EMT. Elucidation of the pathogenesis of carcinosarcoma and the potential role of EMT in this process will be addressed in future studies.
The authors thank Dr. Kazuyuki Ishida, Department of Diagnostic Pathology, Dokkyo Medical University Hospital for providing some of the immunohistochemical findings from the resected specimen.
| 1. | Tachimori Y, Ozawa S, Numasaki H, Ishihara R, Matsubara H, Muro K, Oyama T, Toh Y, Udagawa H, Uno T; Registration Committee for Esophageal Cancer of the Japan Esophageal Society. Comprehensive registry of esophageal cancer in Japan, 2012. Esophagus. 2019;16:221-245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 110] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 2. | Takubo K. Carcinosarcoma and pseudosarcoma. 2nd ed. In: Pathology of the Esophagus: An Atlas and Textbook. Springer, 2007. |
| 3. | Handra-Luca A, Terris B, Couvelard A, Molas G, Degott C, Flejou JF. Spindle cell squamous carcinoma of the oesophagus: an analysis of 17 cases, with new immunohistochemical evidence for a clonal origin. Histopathology. 2001;39:125-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Matsumoto T, Fujii H, Arakawa A, Yamasaki S, Sonoue H, Hattori K, Kajiyama Y, Hirose S, Tsurumaru M. Loss of heterozygosity analysis shows monoclonal evolution with frequent genetic progression and divergence in esophageal carcinosarcoma. Hum Pathol. 2004;35:322-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6805] [Cited by in RCA: 7900] [Article Influence: 464.7] [Reference Citation Analysis (1)] |
| 6. | Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4715] [Cited by in RCA: 6473] [Article Influence: 539.4] [Reference Citation Analysis (0)] |
| 7. | Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3059] [Cited by in RCA: 3719] [Article Influence: 247.9] [Reference Citation Analysis (0)] |
| 8. | Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704-715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6972] [Cited by in RCA: 6910] [Article Influence: 383.9] [Reference Citation Analysis (0)] |
| 9. | Ishida K, Yamashita R, Osakabe M, Uesugi N, Yamada N, Nitta H, Fujishima F, Motoi F, Suzuki H, Shimamura H, Noda Y, Sawai T, Unno M, Sasano H, Sasaki A, Sugai T. Expression of Epithelial-Mesenchymal Transition Proteins in Pancreatic Anaplastic (Undifferentiated) Carcinoma. Pancreas. 2019;48:36-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Nakazawa T, Nobusawa S, Ikota H, Kuwano H, Takeyoshi I, Yokoo H. Wide expression of ZEB1 in sarcomatous component of spindle cell carcinoma of the esophagus. Pathol Int. 2015;65:635-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Wittekind C, Yamasaki S, Sobin LH, Gospodarowicz MK, Wittekind C. Sobin LH, Gospodarowicz MK, Wittekind C. Oesophagus including Oesophagogastric Junction. 7th ed. In: Sobin LH, Gospodarowicz MK, Wittekind C, editors. International Union Against Cancer TNM Classification of Malignant Tumours. New York: Wiley-Blackwell, 2009: 66-72. |
| 12. | Japan Esophageal Society. Japanese Classification of Esophageal Cancer, 11th Edition: part II and III. Esophagus. 2017;14:37-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 355] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 13. | Japan Esophageal Society. Japanese Classification of Esophageal Cancer, 11th Edition: part I. Esophagus. 2017;14:1-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 479] [Cited by in RCA: 739] [Article Influence: 82.1] [Reference Citation Analysis (2)] |
| 14. | Puisieux A, Brabletz T, Caramel J. Oncogenic roles of EMT-inducing transcription factors. Nat Cell Biol. 2014;16:488-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 836] [Article Influence: 69.7] [Reference Citation Analysis (0)] |
| 15. | Shioga T, Matsushima S, Yamada E, Uchiyama T, Noto H, Suzuki D, Nonaka T, Miyazawa S, Komatsu T, Yamamoto Y, Sekido H, Niino H. Esophageal Carcinosarcoma that Was Diagnosed as a Granulocyte-colony Stimulating Factor and Interleukin-6-producing Tumor with a Tumor Fever. Intern Med. 2018;57:2819-2825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Tamura K, Nakashima H, Makihara K, Ishikawa N, Cyaen T, Hachiya Y, Fukuyama T, Hamada T, Hirano Y. Granulocyte colony-stimulating factor and IL-6 producing carcinosarcoma of the esophagus manifesting as leukocytosis and pyrexia: a case report. Esophagus. 2011;8:295-301. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: the Japanese Society of Gastroenterology (No. 60394); the Japan Surgical Society (No. 559904); the Japanese Society of Gastroenterological Surgery (No. G0324826); the Japan Esophageal Society (No. 3768); and the Japan Society for Endoscopic Surgery (No. 5405215867).
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tuo BG, Yang JL S-Editor: Yan JP L-Editor: A E-Editor: Ma YJ