Published online Apr 14, 2020. doi: 10.3748/wjg.v26.i14.1660
Peer-review started: December 24, 2019
First decision: January 19, 2020
Revised: March 12, 2020
Accepted: March 27, 2020
Article in press: March 27, 2020
Published online: April 14, 2020
Processing time: 112 Days and 1.4 Hours
Pancreatic cancer (PC) is a leading cause of cancer related mortality worldwide, with poor survival due to late diagnosis. Currently, biomarkers have limited use in early diagnosis of PC. Macrophage inhibitory cytokine-1 or growth differentiation factor-15 (MIC-1/GDF15) has been implicated as a potential serum biomarker in PC and other malignancies.
To determine the role of MIC-1/GDF15 in detecting pre-malignant pancreatic lesions and neoplastic tumours in an asymptomatic high-risk cohort part of Australian Pancreatic Cancer Screening Program.
A feasibility prospective single centre cohort study was performed. Participants recruited for yearly surveillance with endoscopic ultrasound (EUS) had serial fasting blood samples collected before EUS for MIC-1/GDF15, C-reactive protein and carbohydrate antigen 19-9. Patients were stratified into five groups based on EUS findings: Normal; pancreatic cysts, branch-duct intraductal papillary mucinous neoplasm; diffuse non-specific abnormalities; and neoplastic tumours. MIC-1/GDF15 serum levels were quantified using ELISA. Participants in whom EUS demonstrated abnormalities but not malignancy were closely followed up with magnetic resonance imaging (MRI) or computed tomography.
One hundred twenty participants were prospectively recruited from 2011-2018. Forty-seven participants (39.2%) had an abnormal EUS and five participants (4.2%) were diagnosed with neoplastic tumours, three by EUS (two pancreatic and one liver) and two by MRI/computed tomography (breast cancer, bladder cancer), which were performed for follow up of abnormal EUS. Baseline serum MIC-1/GDF15 was a significant predictor of neoplastic tumours on receiver operator characteristic curve analysis [area under curve (AUC) = 0.814, P = 0.023]. Baseline serum MIC-1/GDF15 had moderate predictive capacity for branch-duct intraductal papillary mucinous neoplasm (AUC = 0.644) and neoplastic tumours noted on EUS (AUC = 0.793), however this was not significant (P = 0.188 and 0.081 respectively). Serial serum MIC-1/GDF15 did not demonstrate a significant percentage change between a normal and abnormal EUS (P = 0.213). Median baseline MIC-1/GDF15 was greater in those with neoplastic tumours (Median = 1039.6, interquartile range = 727.0-1977.7) compared to those diagnosed with a benign lesion (Median = 570.1, interquartile range = 460.7-865.2) on EUS and MRI (P = 0.012).
In this pilot study MIC-1/GDF15 has predictive capacity for neoplastic tumours in asymptomatic individuals with a genetic predisposition for PC. Further imagining may be warranted in patients with abnormal EUS and raised serum MIC-1/GDF15. Larger multicentric prospective studies are required to further define the role of MIC-1/GDF15 as a serological biomarker in pre-malignant pancreatic lesions and neoplastic tumours.
Core tip: In this prospective cohort study in an asymptomatic population at high risk of developing pancreatic cancer due to a genetic predisposition serum baseline macrophage inhibitory cytokine-1 or growth differentiation factor-15 was shown to be a significant predictor of neoplastic tumours (both pancreatic and extra-pancreatic).
- Citation: O’Neill RS, Emmanuel S, Williams D, Stoita A. Macrophage inhibitory cytokine-1/growth differentiation factor-15 in premalignant and neoplastic tumours in a high-risk pancreatic cancer cohort. World J Gastroenterol 2020; 26(14): 1660-1673
- URL: https://www.wjgnet.com/1007-9327/full/v26/i14/1660.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i14.1660
Macrophage inhibitory cytokine-1 (MIC-1), also known as growth differentiation factor-15 (GDF-15) is a distant member of the transforming growth factor (TGF-b) superfamily of cytokines, with its original role being identified as a gene expressed in the context of macrophage activation[1,2]. MIC-1/GDF15 is present in the serum of all individuals with a wide normal range 150-1150 pg/mL[3]. MIC-1/GDF15 has been implicated in regulation of inflammation, metabolism and carcinogenesis, with previous literature demonstrating serum elevation in acute inflammatory conditions, congestive heart failure, renal failure and anti-inflammatory use[4-7]. More recent studies have focused on its role in malignancy, being one of the few secreted proteins induced by p53 activation and its expression was initially postulated to stimulate apoptosis in cancer cells[8-10]. More recently it was suggested that MIC-1/GDF15 directly modulates the biology of tumour progression from initial tumorigenesis to metastasis[11]. In addition to this, MIC-1/GDF15 protein and mRNA was noted to be elevated both in cancer tissue specimens along with peripheral serum samples. MIC-1/GDF15 has been implicated in colorectal cancer, with serum levels being elevated in patients with premalignant colonic polyps, and subsequently increasing with disease progression, including metastasis, along with predicting disease outcome[12-15]. In addition to this, other studies have identified a potential role of MIC-1/GDF15 in prostate[16], breast[17], pancreatic[18-20], ovarian[21], endometrial[22] and lung cancer[23]. Although the role of MIC-1/GDF15 as a biomarker in malignancy has been explored, there is still ongoing discussion regarding its precise function in malignancy, with researchers hypothesising that MIC-1/GDF15 enhances anti-tumour immunity in the early stages of malignancy, along with stimulating tumour cell spread through promoting tumour angiogenesis as demonstrated in oesophageal squamous cell carcinoma[24].
When analysing the role of MIC-1/GDF15 in pancreatic cancer (PC), at a molecular level it has been demonstrated to promote pancreatic cell invasion through its interaction with the transcription factor Twist1[25]. In the clinical domain, MIC-1/GDF15 has been demonstrated to be elevated in the serum of PC patients compared to both healthy controls and those with benign pancreatic tumours, as well as being reported to be beneficial in the diagnosis of pancreatic adenocarcinoma[18,26]. While few individual studies show that MIC-1/GDF15 is more sensitive than carbohydrate antigen 19-9 (CA19-9) in the diagnosis of PC, a meta-analysis[27] published in 2018 shows that MIC-1/GDF15 has a comparable diagnostic accuracy to CA19-9 in diagnosis of PC. Further preliminary studies have demonstrated that MIC-1/GDF15 is superior to CA19-9 in differentiating PC from chronic pancreatitis and when used in combination with CA19-9 it improves further the diagnostic accuracy of differentiating PC form chronic pancreatitis and healthy controls[27-29]. A recent meta-analysis published the diagnostic sensitivity and specificity for MIC-1/GDF15 in diagnosing PC as 80% and 85% respectively, with an area under curve (AUC) of 0.894[27]. In addition to this, MIC-1/GDF15 was found to have a positive predictive value of 78.3%, and a negative predictive value of 78.6%[30,31].
In light of the current emerging evidence that advocates for MIC-1/GDF15 as a potential serological marker of malignancy, the aim of this study was to determine the value of MIC-1/GDF15 as a serological marker of pancreatic pre-malignant lesions and neoplastic tumours in an asymptomatic high-risk population being screened for pancreatic malignancy in an established PC screening program.
Eligible participants were enrolled in the Australian Pancreatic Cancer Screening study for high-risk individuals performed at St Vincent’s Hospital in Sydney, Australia which had started in 2011. The study was approved by St Vincent’s Hospital Ethics Committee (HREC/10/SVH/33) and uses annual endoscopic ultrasound (EUS) as a screening modality. Asymptomatic individuals with a hereditary predisposition to PC were recruited between May 2011-May 2018 (Inclusion criteria Supplementary file 1). Participants were referred by Australian Family Cancer Clinics, the Australian Familial Pancreatic Cancer Registry, medical practitioners or participants had self-referred. At enrolment participants completed a questionnaire detailing past medical history, smoking and alcohol intake, and basic parameters such as height and weight. Participants were excluded from the study if they had a concurrent diagnosis of active malignancy or were not medically suitable for EUS (renal failure, congestive heart failure, human immunodeficiency virus) thus controlling for conditions that could have influenced MIC-1/GDF15 level.
MIC-1/GDF15, CA19-9 and C-reactive protein (CRP) levels were determined on a fasting 10 mL blood sample collected from the participants at the time of EUS. CRP levels was used to control for inflammatory conditions that could have increased MIC-1/GDF15 level. When malignancy was detected, EUS fine need aspiration was performed. Participants in whom EUS demonstrated abnormalities but not malignancy were closely followed up with magnetic resonance imaging (MRI) or computed tomography (CT) (if claustrophobic) and repeat EUS in 3-6 mo as per study protocol. MIC-1/GDF15, CRP and CA19-9 were repeated when a follow up EUS become abnormal.
Statistical analyses were performed using IBM SPSS statistics for Windows (Version 25.0. Armonk, NY). The baseline characteristics of the study population were stratified according to EUS findings: Normal EUS, pancreatic cyst, branch-duct intraductal papillary mucinous neoplasm (BD-IPMN), diffuse non-specific abnormalities (e.g., hyperechoic foci, strands, lobularity) and solid neoplastic tumours. Further analysis was then performed on those diagnosed with neoplastic tumours on EUS and subsequent MRI/CT.
Fisher’s exact test (2-tailed) was used to compare categorical characteristics between respective groups. Continuous baseline characteristics including age, body mass index (BMI), number of cigarettes smoked daily, weekly alcohol intake and age of drinking initiation were evaluated for an association with MIC-1/GDF15 serum levels using Spearman rank correlation. An ANOVA test was used to compare normally distributed continuous variables, whereas a Kruskal-Wallis test was used to compare non-normally distributed continuous variables with two or more samples. Mann-Whitney U test was used to compare non-normally distributed continuous variables. A receiver operating characteristic curve (ROC) of MIC-1/GDF15 was generated for its ability to determine the presence or absence of pancreatic cyst, BD-IPMN, diffuse non-specific abnormality or neoplastic tumours on EUS using serum levels adjusted for variables shown to either be significantly related to MIC-1/GDF15 concentrations in this study, or have shown to correlate with MIC-1/GDF15 in previous studies. This included: Age, gender, BMI, history of colonic polyps, smoking status, alcohol use, metformin use, past history of cancer, nonsteroidal anti-inflammatory drug (NSAID), and aspirin use. All analyses performed were 2-sided and statistical significance was defined as P < 0.05.
A total of 120 asymptomatic participants based on the EUS results were stratified as follows; (1) Normal EUS (n = 74, 61.7%) as the control group; (2) Pancreatic cyst (n = 25, 20.8%); (3) BD-IPMN (n = 9, 7.5%); (4) Diffuse non-specific abnormalities (n = 9, 7.5%); and (5) Solid neoplastic tumours (n = 3, 2.5% which included pancreatic adenocarcinoma, pancreatic neuroendocrine tumour and liver cancer), outlined in Table 1. Two further neoplastic tumours: One breast cancer and a bladder cancer were identified on further imaging (MRI pancreas and CT abdomen) performed for close monitoring of a diffusely abnormal pancreas.
| Baseline characteristics | Normal EUS (n = 74) | Pancreatic Cyst (n = 25) | BD-IPMN (n = 9) | Diffuse abnormality (n = 9) | Neoplastic tumours on EUS (n = 3) | P value |
| Age (yr), mean (SD) | 55.0 (9.8) | 57.3 (7.9) | 60.1 (10.0) | 59.3 (8.8) | 57.7 (4.5) | 0.388 |
| Age quartile, n (%) | ||||||
| Quartile 1 (35-50) | 23 (31.1) | 5 (20.0) | 1 (11.1) | 2 (22.2) | 0 (0.0) | |
| Quartile 2 (51-56) | 17 (23.0) | 7 (28.0) | 3 (33.3) | 1 (11.1) | 1 (33.3) | |
| Quartile 3 (57-63) | 20 (27.0) | 6 (24.0) | 2 (22.2) | 3 (33.3) | 2 (66.7) | |
| Quartile 4 (64-78) | 14 (18.9) | 7 (28.0) | 3 (33.3) | 3 (33.3) | 0 (0.0) | |
| BMI, mean (SD) | 27.3 (5.2) | 27.8 (5.4) | 26.8 (4.2) | 31.6 (3.4) | 24.0 (5.2) | 0.117 |
| BMI quartile, n (%) | 0.0131 | |||||
| Quartile 1 (19.5-23.8) | 18 (24.3) | 6 (24.0) | 4 (44.4) | 0 (0.0) | 2 (66.7) | |
| Quartile 2 (23.9-27.2) | 22 (29.7) | 8 (32.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Quartile 3 (27.3-30.4) | 18 (24.3) | 6 (24.0) | 2 (22.2) | 3 (33.3) | 1 (33.3) | |
| Quartile 4 (30.5-46.7) | 16 (21.6) | 5 (20.0) | 3 (33.3) | 6 (66.7) | 0 (0.0) | |
| Gender, n (%) | 0.362 | |||||
| Female | 51 (68.9) | 18 (72.0) | 5 (55.6) | 4 (44.4) | 1 (33.3) | |
| Male | 23 (31.1) | 7 (28.0) | 4 (44.4) | 5 (55.6) | 2 (66.7) | |
| BRCA2 positive, n (%) | 10 (13.5) | 7 (28.0) | 0 (0.0) | 3 (33.3) | 2 (66.7) | 0.0321 |
| First degree relatives with PC, n (%) | 0.947 | |||||
| 0 | 3 (4.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| 1 | 43 (58.1) | 16 (64.0) | 4 (44.4) | 5 (55.6) | 2 (66.7) | |
| 2 | 21 (28.4) | 5 (20.0) | 5 (55.6) | 4 (44.4) | 1 (33.3) | |
| 3 | 7 (9.5) | 4 (16.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Second degree relative with PC, n (%) | 0.432 | |||||
| 0 | 23 (31.1) | 9 (36.0) | 5 (55.6) | 1 (11.1) | 2 (66.7) | |
| 1 | 17 (23.0) | 9 (36.0) | 0 (0.0) | 7 (77.8) | 1 (33.3) | |
| 2 | 20 (27.0) | 3 (12.0) | 2 (22.2) | 1 (11.1) | 0 (0.0) | |
| 3 | 8 (10.8) | 3 (12.0) | 2 (22.2) | 0 (0.0) | 0 (0.0) | |
| 4 | 6 (8.1) | 1 (4.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Youngest PC diagnosis, median (IQR) | 50 (44-64.5) | 60 (46-66) | 65 (45.5-68.5) | 53 (38-70) | 75 (22-75) | 0.519 |
| Ethnicity, n (%) | 0.848 | |||||
| Asian | 1 (1.4) | 1 (4.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Caucasian | 70 (94.6) | 24 (96.0) | 9 (100.0) | 9 (100.0) | 3 (100.0) | |
| Other | 3 (4.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Jewish origin, n (%) | 5 (6.8) | 7 (28.0) | 1 (11.1) | 1 (11.1) | 0 (0.0) | 0.079 |
| Ashkenazi | 5 (7.4) | 6 (24.0) | 0 (0.0) | 1 (11.1) | 0 (0.0) | 0.121 |
| Medical history | ||||||
| Personal history of cancer, n (%) | 13 (17.6) | 5 (20.0) | 3 (33.3) | 4 (44.4) | 1 (33.3) | 0.350 |
| Diabetes, n (%) | 4 (5.4) | 1 (4.0) | 1 (11.1) | 2 (22.2) | 0 (0.0) | 0.434 |
| Insulin, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (14.3) | 0 (0.0) | 0.184 |
| Oral hypoglycaemic medication, n (%) | 4 (7.4) | 3 (16.7) | 1 (16.7) | 1 (14.3) | 0 (0.0) | 0.840 |
| Smoking status, n (%) | 0.188 | |||||
| Never smoked | 32 (47.8) | 17 (68.0) | 5 (55.6) | 6 (66.7) | 2 (66.7) | |
| Stopped smoking | 32 (47.8) | 7 (28.0) | 4 (44.4) | 3 (33.3) | 0 (.0) | |
| Still smoking | 3 (4.5) | 1 (4.0) | 0 (.0) | 0 (.0) | 1 (33.3) | |
| Cigarettes per day, Median (IQR) | 13.5 (6.0-20.0) | 12.5 (6.3-23.8) | 12.0 (1.0-12.0) | 10.0 (5.0-10.0) | 20.0 (20.0-20.0) | 0.929 |
| Cigarettes per day quartile, n (%) | 0.963 | |||||
| Quartile 1 (1-6) | 11 (30.6) | 2 (25.0) | 1 (33.3) | 1 (33.3) | 0 (0.0) | |
| Quartile 2 (7-12) | 7 (19.4) | 2 (25.0) | 1 (33.3) | 1 (33.3) | 0 (0.0) | |
| Quartile 3 (15-20) | 14 (38.9) | 2 (25.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) | |
| Quartile 4 (25-75) | 4 (11.1) | 2 (25.0) | 1 (33.3) | 1 (33.3) | 0 (0.0) | |
| Years smoking, n (%) | 0.629 | |||||
| < 10 | 12 (33.3) | 3 (37.5) | 2 (50.0) | 1 (33.3) | 0 (0.0) | |
| 11-20 | 11 (30.6) | 3 (37.5) | 0 (0.0) | 1 (33.3) | 0 (0.0) | |
| 21-30 | 8 (22.2) | 1 (12.5) | 2 (50.0) | 1 (33.3) | 0 (0.0) | |
| 31-40 | 4 (11.1) | 1 (12.5) | 0 (0.0) | 0 (0.0) | 1 (100.0) | |
| 41-50 | 1 (2.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| > 50 | 12 (33.3) | 3 (37.5) | 2 (50.0) | 1 (33.3) | 0 (0.0) | |
| Alcohol consumption, n (%) | 0.209 | |||||
| Daily | 19 (25.7) | 7 (28.0) | 0 (0.0) | 3 (33.3) | 2 (66.7) | |
| Weekly | 14 (18.9) | 5 (20.0) | 1 (11.1) | 2 (22.2) | 0 (0.0) | |
| Social | 5 (6.8) | 5 (20.0) | 2 (22.2) | 2 (22.2) | 1 (33.3) | |
| No history of chronic consumption | 36 (48.6) | 8 (32.0) | 6 (66.7) | 2 (22.2) | 0 (0.0) | |
| Drinks per week, Median (IQR) | 6.0 (3.0-15.0) | 4 (2.0-10.0) | 2.5 (1.0-6.0) | 6.0 (1.0-15.0) | 21.0 (1.0-21.0) | 0.331 |
| Drinks per week quartile, n (%) | 0.328 | |||||
| Quartile 1 (1 - 3) | 16 (25.8) | 6 (31.6) | 5 (62.5) | 2 (28.6) | 1 (33.3) | |
| Quartile 2 (4 - 6) | 19 (30.6) | 4 (21.1) | 2 (25.0) | 2 (28.6) | 0 (0.0) | |
| Quartile 3 (7 - 14) | 11 (17.7) | 7 (36.8) | 0 (0.0) | 1 (14.3) | 0 (0.0) | |
| Quartile 4 (15 - 35) | 16 (25.8) | 2 (10.5) | 1 (12.5) | 2 (28.6) | 2 (66.7) | |
| Age of first drink, Median (IQR) | 18.0 (17.0-18.0) | 20.0 (18.0-25.0) | 19.0 (18.0-21.0) | 17.0 (15.0-20.0) | 18.0 (15.0-18.0) | 0.0331 |
| Years drinking, n (%) | 0.129 | |||||
| < 10 | 2 (3.4) | 2 (11.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| 11-20 | 11 (18.6) | 3 (17.6) | 3 (37.5) | 0 (0.0) | 0 (0.0) | |
| 21-30 | 13 (22.0) | 6 (35.3) | 0 (0.0) | 2 (28.6) | 1 (33.3) | |
| 31-40 | 22 (37.3) | 5 (29.4) | 2 (25.0) | 1 (14.3) | 2 (66.7) | |
| 41-50 | 8 (13.6) | 1 (5.9) | 2 (25.0) | 2 (28.6) | 0 (0.0) | |
| > 50 | 2 (3.4) | 0 (0.0) | 1 (12.5) | 2 (28.6) | 0 (0.0) | |
| Biochemistry | ||||||
| CRP, Median (IQR) | 1.3 (0.6-2.5) | 1.7 (0.7-4.2) | 1.4 (0.5-1.9) | 0.8 (0.6-4.4) | 0.8 (0.3-0.8) | 0.835 |
| CA19-9, Median (IQR) | 9.0 (6.0-16.0) | 9.0 (7.0-15.8) | 9.0 (5.7-15.0) | 16.0 (8.5-19.5) | 47.0 (22.0-47.0) | 0.058 |
| MIC-1/GDF15, Median (IQR) | 558.2 (449.6-715.3) | 574.3 (448.5-830.3) | 659.3 (484.2-1077.3) | 553.2 (512.9-967.0) | 849.1 (604.9- 849.1) | 0.178 |
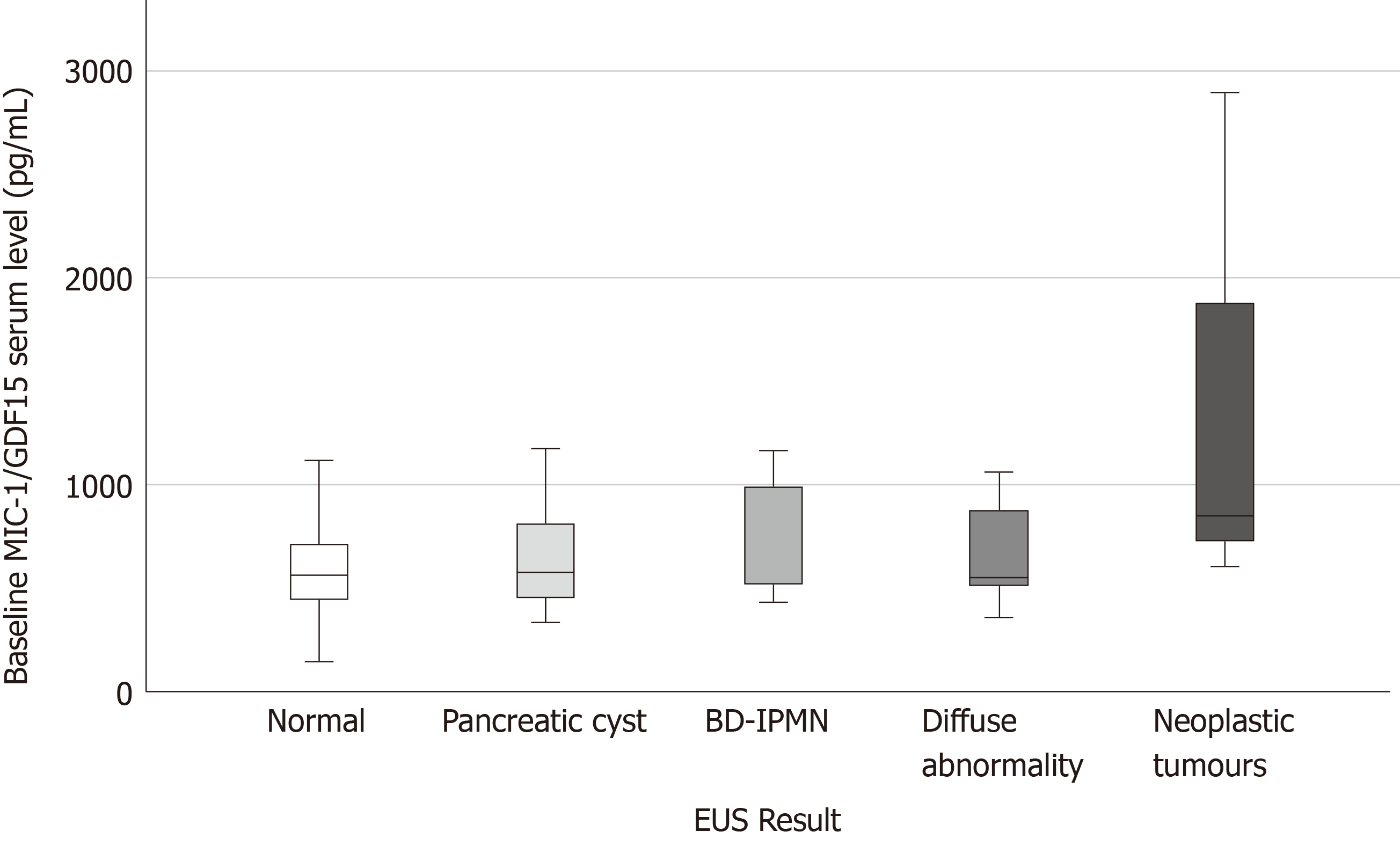
Table 1 outlines the baseline characteristics of the 120 subjects. The median age of participants diagnosed with BD-IPMN on EUS was higher compared to their counterparts, however this was not statistically significant (P = 0.388). There was no significant difference in the number of first-degree relatives (FDR) (P = 0.947) or second-degree relatives (SDR) diagnosed with PC (P = 0.432) between groups. The median age of those diagnosed with neoplastic tumours on EUS was higher compared to those with a normal EUS, however this was not statistically significant (P = 0.519). Furthermore, those with neoplastic tumours identified on EUS had a higher median number of cigarettes smoked per week (Median = 20) compared to the other groups, however this was not significant (P = 0.929). Participants diagnosed with neoplasia on EUS had a higher serum MIC-1/GDF15 [Median = 849.1, interquartile range (IQR) = 604.9-849.1] compared to the other groups however this was not significant (P = 0.178) but approached significance when compared to participants with a normal EUS (P = 0.061) (Figure 1). Percentage change between serial MIC-1/GDF15 was not significant in those participants who had a normal EUS and subsequent abnormal EUS (tumour, BD-IPMN, cyst, diffuse abnormality) (P = 0.213). Median serum CA19-9 was greatest in patients with an EUS indicative of malignancy, this approached significance (P = 0.058) when compared to the other groups included in the analysis.
Baseline MIC-1/GDF15 was significantly correlated with advancing age for the entire cohort (correlation coefficient = 0.602, P < 0.01) and age of youngest PC diagnosis (correlation coefficient = 0.223, P = 0.015). Increasing BMI did not correlate with increasing serum MIC-1/GDF15 (P = 0.548). The number of cigarettes smoked per day, and number of drinks per week did not correlate with increased baseline serum MIC-1/GDF15 values in this population (P = 0.138 and P = 0.451 respectively).
The total number of both FDR and SDR diagnosed with PC had a significant negative correlation with baseline serum MIC-1/GDF15 (correlation coefficient = -0.190, P = 0.038). The number of FDR diagnosed with PC did not correlate with baseline serum MIC-1/GDF15 (P = 0.238), however the number of SDR diagnosed with PC had a significant negative correlation with baseline serum MIC-1/GDF15 (correlation coefficient = -0.225, P = 0.014).
Baseline serum MIC-1/GDF15 did not correlate with gender (P = 0.176), BRCA2 status (P = 0.097), ethnicity (P = 0.570) or Jewish background (P = 0.606). Further analysis of dichotomous variables demonstrated that baseline serum MIC-1/GDF15 was significantly greater in those with a history of cancer (P < 0.001), history of diabetes (P = 0.001), those taking oral hypoglycaemic medication (P = 0.001) and history of coronary artery disease (P = 0.005), hypercholesterolaemia (P = 0.013) and colon polyps (P = 0.005). Serum MIC-1/GDF15 levels were elevated in those participants taking aspirin regularly (P = 0.019) and metformin (P = 0.001). Baseline serum MIC-1/GDF15 was not elevated in those with regular NSAID, folate or antidepressant use (P = 0.863, 0.928 and 0.172 respectively) in this study population.
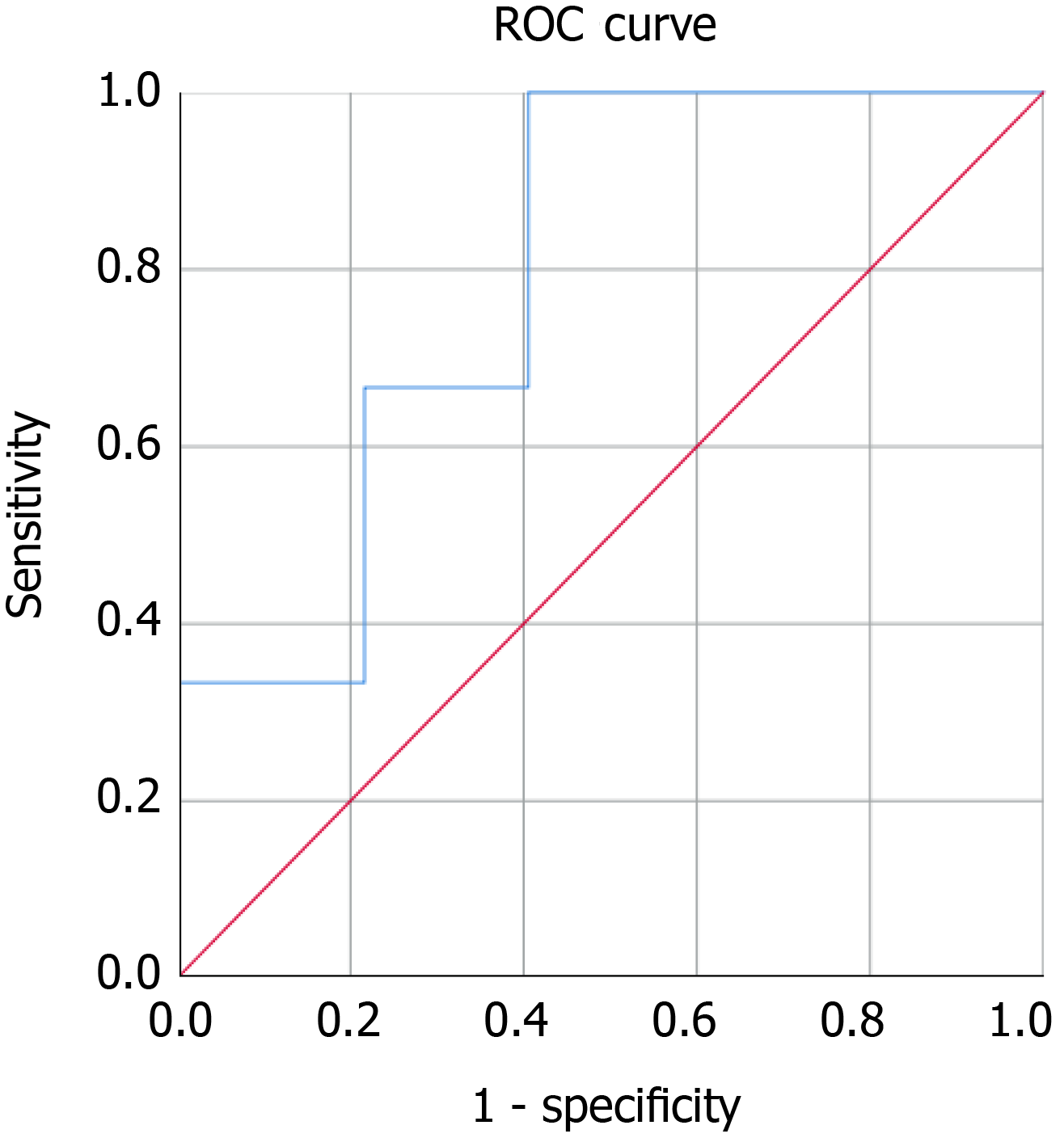
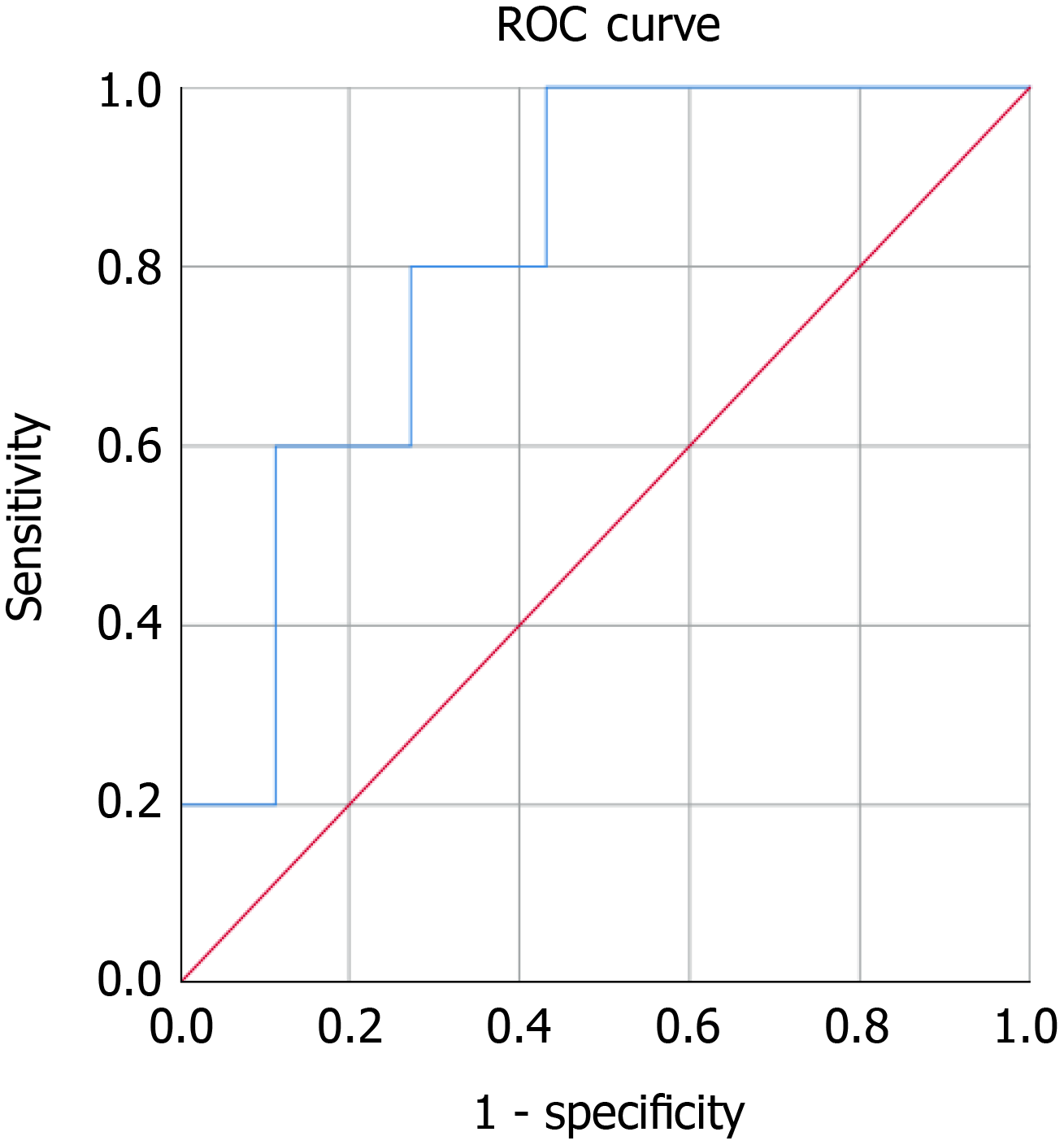
Baseline serum MIC-1/GDF15 was a poor predictor of abnormal EUS in our cohort of asymptomatic high-risk patients as determined using a ROC curve for the capacity for MIC-1/GDF15 to predict an abnormal EUS. The MIC-1/GDF15 serum level, when adjusted for aspirin use, alcohol intake per week, smoking status, BMI, NSAID use, history of colonic polyps, gender, metformin use and age had an AUC of 0.576 (95%CI: 0.454-0.698) (P = 0.234) (Figure 2A). Similarly, baseline serum MIC-1/GDF15 could not predict BD-IPMN (AUC = 0.644, 95%CI: 0.414-0.875, P = 0.223) (Figure 2B), pancreatic cyst (AUC = 0.347, 95%CI: 0.162-0.532, P = 0.131) (Figure 2C) and diffuse abnormalities (AUC = 0.510, 95%CI: 0.254-0.764, P = 0.935) (Figure 2D). In those with neoplastic tumours diagnosed on EUS and subsequent biopsy (n = 3), the AUC was 0.793, however this was not statistically significant (P = 0.081) (Figure 3).
Baseline MIC-1/GDF15 was a significant predictor of neoplastic tumours diagnosed on EUS and MRI/CT (n = 5) with an AUC=0.814 (95%CI: 0.657-0.970, P = 0.023) (Figure 4). In this asymptomatic cohort three neoplastic tumours were diagnosed on EUS and two other malignancies were diagnosed on further imaging performed to monitor the pancreas (one breast cancer on MRI pancreas and one bladder cancer on CT abdomen). In addition to this, median baseline serum MIC-1/GDF15 in asymptomatic patients found to have neoplastic tumours (Median = 1039.6, IQR = 727.0-1977.7) was significantly greater than benign lesions (Median = 570.1, IQR = 460.7-865.2) (P = 0.012) as demonstrated in Figure 5.
PC is a leading cause of cancer mortality worldwide, with a very poor survival rate due to late diagnosis, primarily due to symptoms presenting at advanced stages of the disease. The prognosis correlates strongly with pathological stage at the time of diagnosis, and despite advances in medicine in the last forty years, the 5-year survival has increased only from 4% to 7%[32]. As a result, efforts are made in detecting PC early at asymptomatic stage and multiple PC screening programs in high risk individuals have been established around the world. These screening programs target individuals with a genetic predisposition for developing PC (people with hereditary cancer syndromes due to known mutations and familial PC). Current screening modalities rely on pancreatic imaging (EUS and MRI) and biomarkers are at research level. Ideally, we need an early sensitive and specific serological marker that can be used as a first line screening tool in a high-risk population and help select cases that need further investigations, such as EUS or MRI. CA19-9 is not sensitive enough to be a marker for early detection of PC, having a specificity of 77%, sensitivity 75%, a positive predictive value of 0.5%-0.9%[33,34] and can be increased in other conditions such as biliary obstruction. Similarly, carcinoembryonic antigen (CEA) has no utility in early detection of PC with a sensitivity and specificity of 65%[35].
MIC-1/GDF15 has been explored as a novel candidate tumour marker for PC with initial results proving to be elevated in the serum of patients with PC compared to healthy controls and those with benign lesions[18]. As MIC-1/GDF15 can be increased in other malignancies, studies report an increase in its diagnostic specificity if CA19-9 is used in combination with MIC-1/GDF15[28,30]. In addition to this, serum MIC-1/GDF15 has been proven to be more sensitive than CA19-9 in detecting early-stage PC. Importantly, MIC-1/GDF15 had a sensitivity of 63.1% in detecting patients with CA19-9-negative PC[26].
In this feasibility prospective cohort study in an asymptomatic population at high risk of developing PC undertaking yearly screening with EUS, serum baseline MIC-1/GDF15 was shown to be a significant predictor of neoplastic tumours (both pancreatic and extra-pancreatic) after ROC curve analysis, with an AUC of 0.814 (P = 0.023). In addition, those diagnosed with neoplastic tumours on EUS or MRI/CT had a higher median baseline MIC-1/GDF15 compared to those diagnosed with benign lesions on EUS. Baseline serum MIC-1/GDF15 had a significant positive correlation with advancing age and age of PC diagnosis in family members. Further analysis of the screening cohort demonstrated that serum MIC-1/GDF15 was elevated in those with a family history of cancer, history of diabetes, current metformin use and those with previous colonic polyps.
When evaluating the utility of serum baseline MIC-1/GDF15 comparing to EUS results only, using ROC curve analysis, we found that it was best utilised when used in participants who were diagnosed with solid neoplastic tumours or BD-IPMN on EUS, with AUCs of 0.793 and 0.644 respectively, with solid tumours diagnosed on EUS approaching significance despite having only 3 cases. These results demonstrated that MIC-1/GDF15 is elevated in participants with pre-malignant and neoplastic tumours, and seems to bear similar predictive value to prostate-specific antigen testing for prostate cancer and the faecal occult blood test for colonic adenoma[36-39]. Previously Koopmann et al[18] were able to demonstrate an AUC for MIC-1/GDF15 of 0.81 for the detection of pancreatic adenocarcinoma, and when used in combination with CA19-9, this increased to 0.87.
Compared with previous studies that evaluate the role of MIC- 1/GDF15 in patients with known PC or other malignancies our study design is unique. This is a pilot study, the first to the authors knowledge, to evaluate serum MIC-1/GDF15 in an asymptomatic population at high risk of malignancy in an established PC screening program. Based on the inclusion criteria (patients with a genetic predisposition for PC) these participants are at risk of developing other malignancies not just pancreatic, as shown in our cohort where three non-pancreatic malignancies were found at an asymptomatic stage (liver, breast and bladder cancer). This study shows that baseline MIC-1/GDF15 is elevated in patients with neoplastic tumours and could be potentially used to guide further investigations such as MRI or CT if EUS is negative for PC.
The authors acknowledge that due to the nature of the screening program, the recruitment of asymptomatic high-risk participants is time intensive and the subsequent low incidence of abnormal EUS results and malignant lesions are two limitations of this prospective study. Further larger prospective multi-centre cohort studies are required to further assess the value of MIC-1/GDF15 in screening for malignancy in this type of cohort.
The authors echo the findings of Wang et al[40] who stated that serum MIC-1/GDF15 should be interpreted cautiously due to the potential for a broad range of values in the general population and the need to control for multiple confounding factors, particularly inflammation promoting an elevated MIC-1/GDF15 serum level. We controlled for conditions that influence MIC-1/GDF15 levels by using CRP as marker of active inflammation and excluding patients with congestive heart failure, renal failure, human immunodeficiency virus and known malignancy.
Although this study was not able to detect a significant change in serum MIC-1/GDF15 in participants who had a normal then subsequent abnormal EUS , further studies should endeavour to explore whether percentage change in MIC-1/GDF15 is indicative of tumorigenesis in populations at high risk for developing cancer.
A limitation of the use of MIC-1/GDF15 as a biomarker is a wide normal serum range. Serial monitoring of an individual’s MIC-1/GDF15 serum level would identify those with increasing levels, even those that were within the normal range. It is the aim of this screening program to implement serial serum MIC-1/GDF15 to assess if with a large enough sample size and long-term follow-up, a statistically significant result can be achieved.
Future studies should aim to further evaluate and analyse MIC-1/GDF15 in both the general population and in patients at risk of malignancy due to a genetic predisposition to determine how this serum biomarker can be better applied in the clinical setting with intention to facilitate its progressive implementation regularly in the clinical domain, along with being further assessed in the academic setting[40].
In conclusion, this pilot study, the first of its kind to implement MIC-1/GDF15 as a screening tool in an asymptomatic population with a genetic predisposition of developing PC, provides moderate support to the previous findings that MIC-1/GDF15 is elevated in patients with neoplastic tumours, however the sample size used to assess this was small. In addition, this study highlights that an elevated MIC-1/GDF15 in the context of a negative pancreatic EUS in a high risk of malignancy cohort may warrant further investigation to determine whether an occult malignancy exists.
While population based screening is difficult to implement due to wide range of normal values and its elevation in select disease processes, MIC-1/GDF15 might be better suited for screening for malignancy in patients with hereditary cancer syndromes where baseline and serial measurement can be used in combination with other validated serological markers to overcome many of these limitations and potentially select patients who require further investigations.
Larger multicentric prospective studies are required to further define the role of MIC-1/GDF15 as a serological biomarker in pre-malignant pancreatic lesions and neoplastic tumours.
Early detection of pancreatic cancer (PC) is a key priority in order to improve survival. Macrophage inhibitory cytokine-1 or growth differentiation factor-15 (MIC-1/GDF15) is a novel candidate tumour marker for PC with initial results proving to be elevated in the serum of patients with PC compared to healthy controls and those with benign lesions.
We need an early sensitive and specific serological marker that can be used as a first line screening tool in patients at risk of PC and help select cases that need further investigations, such as endoscopic ultrasound (EUS) or magnetic resonance imaging. This study evaluates the role of MIC-1/GDF15 in patients at high risk of developing PC.
This is a pilot study to determine the role of MIC-1/GDF15 in detecting pre-malignant pancreatic lesions and neoplastic tumours in an asymptomatic high-risk cohort part of Australian Pancreatic Cancer Screening Program and correlate with imaging finding.
Participants recruited for yearly surveillance with EUS had serial fasting blood samples collected for MIC-1/GDF15, C-reactive protein and carbohydrate antigen 19-9. Patients were stratified into five groups based on EUS findings. MIC-1/GDF15 serum levels were quantified using ELISA and correlations of MIC-1/GDF15 with population variables and imaging findings were performed. A receiver operating characteristic curve of MIC-1/GDF15 was generated for its ability to determine the presence or absence of neoplastic tumours , pancreatic cysts, branch-duct intraductal papillary mucinous neoplasm and diffuse non-specific abnormality using serum levels adjusted for variables shown to either be significantly related to MIC-1/GDF15 concentrations in this study, or have shown to correlate with MIC-1/GDF15 in previous studies.
One hundred twenty participants were recruited over 8 years. Baseline serum MIC-1/GDF15 was a significant predictor of neoplastic tumours on receiver operating characteristic curve analysis. Baseline serum MIC-1/GDF15 had moderate predictive capacity for branch-duct intraductal papillary mucinous neoplasm (AUC = 0.644) and neoplastic tumours noted on EUS (AUC = 0.793), however this was not significant (P = 0.188 and 0.081 respectively). Serial serum MIC-1/GDF15 did not demonstrate a significant percentage change between a normal and abnormal EUS. Median baseline MIC-1/GDF15 was greater in those with neoplastic tumours compared to those diagnosed with a benign lesion.
MIC-1/GDF15 has predictive capacity for neoplastic tumours in asymptomatic individuals with a genetic predisposition for PC. Further imagining may be warranted in patients with raised serum MIC-1/GDF15 and abnormal EUS.
This pilot study is the first of its kind to implement MIC-1/GDF15 as a screening tool in an asymptomatic population with a genetic predisposition of developing PC. Our study is a feasibility study and we hope our results will start a new wave of research (larger, multicentric, prospective trials) into investigating the role of this biomarker in early detection of neoplastic tumours to validate our finding and provide further characterisation of this biomarker.
We would like to acknowledge Professor Sam Breit, Professor David Brown and Michelle Ng from the Inflammation and Cytokine Biology Research Program, St Vincent’s Centre of Applied Medical Research, St Vincent’s Hospital, Sydney, NSW, Australia who helped with performing the MIC-1/GDF15 assay and provided guidance in assessing its role in screening for PC. We thank Pancare Foundation for their ongoing support and providing funding for the coordinator position. We also acknowledge Ms Skye Mackay, who was the trial coordinator until 2017 and Ms Tanya Dwarte who is the current trial coordinator. A special thanks to Professor Anthony Gill and Ms Amber Jones, Australian Pancreatic Cancer Genome Initiative, Garvan Institute of Medical Research for their support and ongoing collaboration.
| 1. | Bootcov MR, Bauskin AR, Valenzuela SM, Moore AG, Bansal M, He XY, Zhang HP, Donnellan M, Mahler S, Pryor K, Walsh BJ, Nicholson RC, Fairlie WD, Por SB, Robbins JM, Breit SN. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc Natl Acad Sci USA. 1997;94:11514-11519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 810] [Cited by in RCA: 975] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 2. | Fairlie WD, Zhang HP, Wu WM, Pankhurst SL, Bauskin AR, Russell PK, Brown PK, Breit SN. The propeptide of the transforming growth factor-beta superfamily member, macrophage inhibitory cytokine-1 (MIC-1), is a multifunctional domain that can facilitate protein folding and secretion. J Biol Chem. 2001;276:16911-16918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Brown DA, Ward RL, Buckhaults P, Liu T, Romans KE, Hawkins NJ, Bauskin AR, Kinzler KW, Vogelstein B, Breit SN. MIC-1 serum level and genotype: associations with progress and prognosis of colorectal carcinoma. Clin Cancer Res. 2003;9:2642-2650. [PubMed] |
| 4. | Skipworth RJ, Deans DA, Tan BH, Sangster K, Paterson-Brown S, Brown DA, Hunter M, Breit SN, Ross JA, Fearon KC. Plasma MIC-1 correlates with systemic inflammation but is not an independent determinant of nutritional status or survival in oesophago-gastric cancer. Br J Cancer. 2010;102:665-672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Kempf T, von Haehling S, Peter T, Allhoff T, Cicoira M, Doehner W, Ponikowski P, Filippatos GS, Rozentryt P, Drexler H, Anker SD, Wollert KC. Prognostic utility of growth differentiation factor-15 in patients with chronic heart failure. J Am Coll Cardiol. 2007;50:1054-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 374] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 6. | Breit SN, Carrero JJ, Tsai VW, Yagoutifam N, Luo W, Kuffner T, Bauskin AR, Wu L, Jiang L, Barany P, Heimburger O, Murikami MA, Apple FS, Marquis CP, Macia L, Lin S, Sainsbury A, Herzog H, Law M, Stenvinkel P, Brown DA. Macrophage inhibitory cytokine-1 (MIC-1/GDF15) and mortality in end-stage renal disease. Nephrol Dial Transplant. 2012;27:70-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (1)] |
| 7. | Brown DA, Hance KW, Rogers CJ, Sansbury LB, Albert PS, Murphy G, Laiyemo AO, Wang Z, Cross AJ, Schatzkin A, Danta M, Srasuebkul P, Amin J, Law M, Breit SN, Lanza E. Serum macrophage inhibitory cytokine-1 (MIC-1/GDF15): a potential screening tool for the prevention of colon cancer? Cancer Epidemiol Biomarkers Prev. 2012;21:337-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Kannan K, Amariglio N, Rechavi G, Givol D. Profile of gene expression regulated by induced p53: connection to the TGF-beta family. FEBS Lett. 2000;470:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 85] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Li PX, Wong J, Ayed A, Ngo D, Brade AM, Arrowsmith C, Austin RC, Klamut HJ. Placental transforming growth factor-beta is a downstream mediator of the growth arrest and apoptotic response of tumor cells to DNA damage and p53 overexpression. J Biol Chem. 2000;275:20127-20135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 219] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 10. | Sun Y. Identification and characterization of genes responsive to apoptosis: application of DNA chip technology and mRNA differential display. Histol Histopathol. 2000;15:1271-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 11. | Tsai VWW, Husaini Y, Sainsbury A, Brown DA, Breit SN. The MIC-1/GDF15-GFRAL Pathway in Energy Homeostasis: Implications for Obesity, Cachexia, and Other Associated Diseases. Cell Metab. 2018;28:353-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 317] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 12. | Danta M, Barber DA, Zhang HP, Lee-Ng M, Baumgart SWL, Tsai VWW, Husaini Y, Saxena M, Marquis CP, Errington W, Kerr S, Breit SN, Brown DA. Macrophage inhibitory cytokine-1/growth differentiation factor-15 as a predictor of colonic neoplasia. Aliment Pharmacol Ther. 2017;46:347-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Song M, Mehta RS, Wu K, Fuchs CS, Ogino S, Giovannucci EL, Chan AT. Plasma Inflammatory Markers and Risk of Advanced Colorectal Adenoma in Women. Cancer Prev Res (Phila). 2016;9:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Chen H, Qian J, Werner S, Cuk K, Knebel P, Brenner H. Development and validation of a panel of five proteins as blood biomarkers for early detection of colorectal cancer. Clin Epidemiol. 2017;9:517-526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Mehta RS, Chong DQ, Song M, Meyerhardt JA, Ng K, Nishihara R, Qian Z, Morikawa T, Wu K, Giovannucci EL, Fuchs CS, Ogino S, Chan AT. Association Between Plasma Levels of Macrophage Inhibitory Cytokine-1 Before Diagnosis of Colorectal Cancer and Mortality. Gastroenterology. 2015;149:614-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Brown DA, Stephan C, Ward RL, Law M, Hunter M, Bauskin AR, Amin J, Jung K, Diamandis EP, Hampton GM, Russell PJ, Giles GG, Breit SN. Measurement of serum levels of macrophage inhibitory cytokine 1 combined with prostate-specific antigen improves prostate cancer diagnosis. Clin Cancer Res. 2006;12:89-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Welsh JB, Sapinoso LM, Kern SG, Brown DA, Liu T, Bauskin AR, Ward RL, Hawkins NJ, Quinn DI, Russell PJ, Sutherland RL, Breit SN, Moskaluk CA, Frierson HF, Hampton GM. Large-scale delineation of secreted protein biomarkers overexpressed in cancer tissue and serum. Proc Natl Acad Sci USA. 2003;100:3410-3415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 390] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 18. | Koopmann J, Buckhaults P, Brown DA, Zahurak ML, Sato N, Fukushima N, Sokoll LJ, Chan DW, Yeo CJ, Hruban RH, Breit SN, Kinzler KW, Vogelstein B, Goggins M. Serum macrophage inhibitory cytokine 1 as a marker of pancreatic and other periampullary cancers. Clin Cancer Res. 2004;10:2386-2392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 219] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 19. | Grote T, Logsdon CD. Progress on molecular markers of pancreatic cancer. Curr Opin Gastroenterol. 2007;23:508-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Ozkan H, Demirbaş S, Ibiş M, Akbal E, Köklü S. Diagnostic validity of serum macrophage inhibitor cytokine and tissue polypeptide-specific antigen in pancreatobiliary diseases. Pancreatology. 2011;11:295-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Bock AJ, Stavnes HT, Kempf T, Tropè CG, Berner A, Davidson B, Staff AC. Expression and clinical role of growth differentiation factor-15 in ovarian carcinoma effusions. Int J Gynecol Cancer. 2010;20:1448-1455. [PubMed] |
| 22. | Staff AC, Trovik J, Eriksson AG, Wik E, Wollert KC, Kempf T, Salvesen HB. Elevated plasma growth differentiation factor-15 correlates with lymph node metastases and poor survival in endometrial cancer. Clin Cancer Res. 2011;17:4825-4833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Lerner L, Gyuris J, Nicoletti R, Gifford J, Krieger B, Jatoi A. Growth differentiating factor-15 (GDF-15): A potential biomarker and therapeutic target for cancer-associated weight loss. Oncol Lett. 2016;12:4219-4223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Wang XB, Jiang XR, Yu XY, Wang L, He S, Feng FY, Guo LP, Jiang W, Lu SH. Macrophage inhibitory factor 1 acts as a potential biomarker in patients with esophageal squamous cell carcinoma and is a target for antibody-based therapy. Cancer Sci. 2014;105:176-185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Ji H, Lu HW, Li YM, Lu L, Wang JL, Zhang YF, Shang H. Twist promotes invasion and cisplatin resistance in pancreatic cancer cells through growth differentiation factor 15. Mol Med Rep. 2015;12:3841-3848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Wang X, Li Y, Tian H, Qi J, Li M, Fu C, Wu F, Wang Y, Cheng D, Zhao W, Zhang C, Wang T, Rao J, Zhang W. Macrophage inhibitory cytokine 1 (MIC-1/GDF15) as a novel diagnostic serum biomarker in pancreatic ductal adenocarcinoma. BMC Cancer. 2014;14:578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 27. | Yang Y, Yan S, Tian H, Bao Y. Macrophage inhibitory cytokine-1 versus carbohydrate antigen 19-9 as a biomarker for diagnosis of pancreatic cancer: A PRISMA-compliant meta-analysis of diagnostic accuracy studies. Medicine (Baltimore). 2018;97:e9994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Kaur S, Chakraborty S, Baine MJ, Mallya K, Smith LM, Sasson A, Brand R, Guha S, Jain M, Wittel U, Singh SK, Batra SK. Potentials of plasma NGAL and MIC-1 as biomarker(s) in the diagnosis of lethal pancreatic cancer. PLoS One. 2013;8:e55171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 29. | Hogendorf P, Durczyński A, Skulimowski A, Kumor A, Poznańska G, Strzelczyk J. Growth differentiation factor (GDF-15) concentration combined with Ca125 levels in serum is superior to commonly used cancer biomarkers in differentiation of pancreatic mass. Cancer Biomark. 2018;21:505-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 30. | Mohamed AA, Soliman H, Ismail M, Ziada D, Farid TM, Aref AM, Al Daly ME, Abd Elmageed ZY. Evaluation of circulating ADH and MIC-1 as diagnostic markers in Egyptian patients with pancreatic cancer. Pancreatology. 2015;15:34-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Jelski W, Mroczko B. Biochemical diagnostics of pancreatic cancer - Present and future. Clin Chim Acta. 2019;498:47-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | Yabar CS, Winter JM. Pancreatic Cancer: A Review. Gastroenterol Clin North Am. 2016;45:429-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 33. | Huang Z, Liu F. Diagnostic value of serum carbohydrate antigen 19-9 in pancreatic cancer: a meta-analysis. Tumour Biol. 2014;35:7459-7465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 34. | Zhang Y, Yang J, Li H, Wu Y, Zhang H, Chen W. Tumor markers CA19-9, CA242 and CEA in the diagnosis of pancreatic cancer: a meta-analysis. Int J Clin Exp Med. 2015;8:11683-11691. [PubMed] |
| 35. | Ehmann M, Felix K, Hartmann D, Schnölzer M, Nees M, Vorderwülbecke S, Bogumil R, Büchler MW, Friess H. Identification of potential markers for the detection of pancreatic cancer through comparative serum protein expression profiling. Pancreas. 2007;34:205-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 115] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 36. | Hoffman RM, Gilliland FD, Adams-Cameron M, Hunt WC, Key CR. Prostate-specific antigen testing accuracy in community practice. BMC Fam Pract. 2002;3:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Quintero E, Castells A, Bujanda L, Cubiella J, Salas D, Lanas Á, Andreu M, Carballo F, Morillas JD, Hernández C, Jover R, Montalvo I, Arenas J, Laredo E, Hernández V, Iglesias F, Cid E, Zubizarreta R, Sala T, Ponce M, Andrés M, Teruel G, Peris A, Roncales MP, Polo-Tomás M, Bessa X, Ferrer-Armengou O, Grau J, Serradesanferm A, Ono A, Cruzado J, Pérez-Riquelme F, Alonso-Abreu I, de la Vega-Prieto M, Reyes-Melian JM, Cacho G, Díaz-Tasende J, Herreros-de-Tejada A, Poves C, Santander C, González-Navarro A; COLONPREV Study Investigators. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med. 2012;366:697-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 703] [Cited by in RCA: 675] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 38. | Imperiale TF, Ransohoff DF, Itzkowitz SH, Levin TR, Lavin P, Lidgard GP, Ahlquist DA, Berger BM. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;370:1287-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1015] [Cited by in RCA: 1317] [Article Influence: 109.8] [Reference Citation Analysis (1)] |
| 39. | Haug U, Kuntz KM, Knudsen AB, Hundt S, Brenner H. Sensitivity of immunochemical faecal occult blood testing for detecting left- vs right-sided colorectal neoplasia. Br J Cancer. 2011;104:1779-1785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 40. | Wang X, Yang Z, Tian H, Li Y, Li M, Zhao W, Zhang C, Wang T, Liu J, Zhang A, Shen D, Zheng C, Qi J, Zhao D, Shi J, Jin L, Rao J, Zhang W. Circulating MIC-1/GDF15 is a complementary screening biomarker with CEA and correlates with liver metastasis and poor survival in colorectal cancer. Oncotarget. 2017;8:24892-24901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: American Society for Gastrointestinal Endoscopy, Gastroenterological Society of Australia.
Specialty type: Gastroenterology and Hepatology
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fernandez-Perez L, Miyoshi E S-Editor: Dou Y L-Editor: A E-Editor: Liu MY