Published online Nov 7, 2019. doi: 10.3748/wjg.v25.i41.6248
Peer-review started: May 22, 2019
First decision: June 16, 2019
Revised: July 8, 2019
Accepted: July 19, 2019
Article in press: June 16, 2019
Published online: November 7, 2019
Processing time: 169 Days and 12.4 Hours
Platelets have been reported to participate in tumor cell growth, extravasation, epithelial–mesenchymal transition, metastasis, and drug resistance. However, the importance of platelets in pancreatic neuroendocrine tumor (pNET) lacks adequate literature support. The predictive value of tumor-infiltrating platelets (TIPs) in pNET remains unclear.
To investigate the relationship between TIPs and the prognosis of patients with pNET following radical resection.
In total, 113 patients who had undergone radical surgical resection with a pathologic diagnosis of pNET were enrolled in this study. Immunohistochemical analysis of cluster of differentiation 42b (CD42b) expression in the tumor specimens was performed to determine the presence of TIPs. Univariate and multivariate analyses were used to analyze the prognostic value of TIPs.
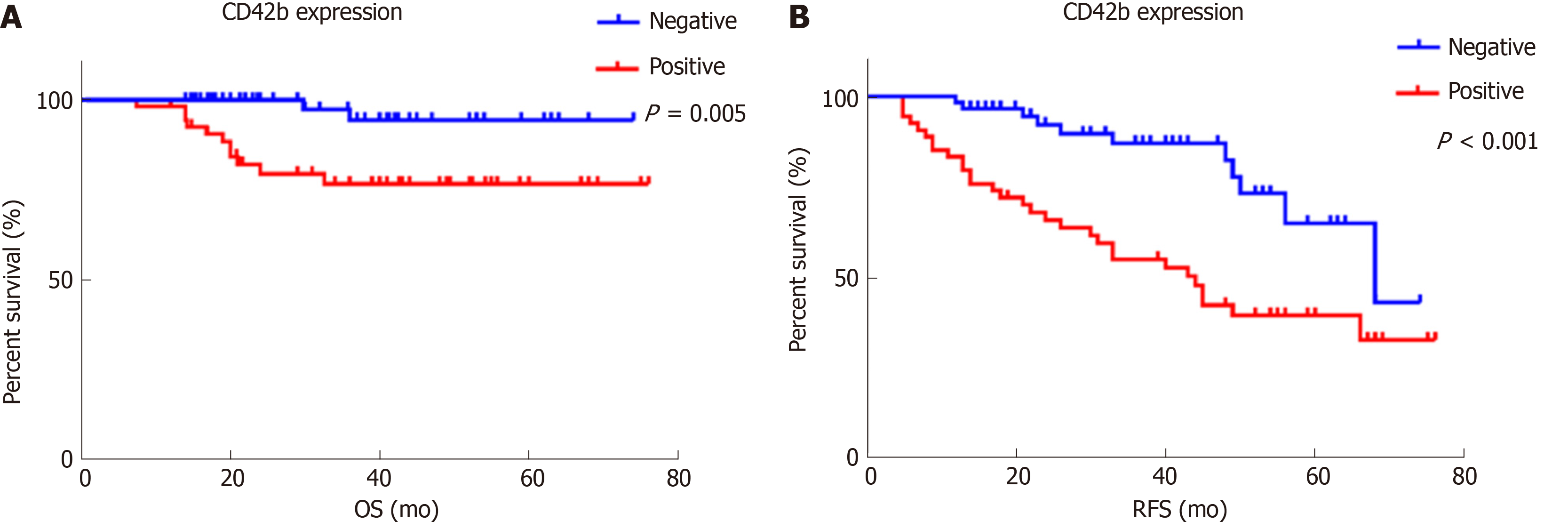
TIPs were observed in intratumoral areas in 54 patients. Neither basic characteristics nor preoperative platelet-associated indicators showed a significant relationship with the presence of TIPs (all P > 0.05). Patients with positive intratumoral CD42b expression had worse overall survival (P = 0.005) and recurrence-free survival (P < 0.001) than those with negative intratumoral CD42b expression. Multivariate analysis demonstrated that TIPs were independent prognostic factors for overall survival (P = 0.049) and recurrence-free survival (P = 0.003). Nevertheless, platelet count, mean platelet volume, and platelet-to-lymphocyte ratio were not associated with postoperative survival or recurrence in pNET patients (all P > 0.05).
TIPs are a useful prognostic biomarker for patients with resectable pNET, and their detection represents a promising tool for pNET treatment strategy decisions.
Core tip: We uncovered the importance of platelets in pancreatic neuroendocrine tumors and investigated the association between clinical outcome and preoperative platelet-associated indicators and tumor-infiltrating platelets in pancreatic neuroendocrine tumors. Platelet count, mean platelet volume, and platelet-to-lymphocyte ratio determined by preoperative blood tests were not related to overall survival or recurrence-free survival. Thus, tumor-infiltrating platelets are potential independent indicators of survival and recurrence in patients with resectable pancreatic neuroendocrine tumors.
- Citation: Xu SS, Xu HX, Wang WQ, Li S, Li H, Li TJ, Zhang WH, Liu L, Yu XJ. Tumor-infiltrating platelets predict postoperative recurrence and survival in resectable pancreatic neuroendocrine tumor. World J Gastroenterol 2019; 25(41): 6248-6257
- URL: https://www.wjgnet.com/1007-9327/full/v25/i41/6248.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i41.6248
Pancreatic neuroendocrine tumor (pNET) is the second most common malignancy among all pancreatic tumors, and its incidence has been increasing over the years[1]. Surgery remains the preferred treatment for pNET[2,3]. However, patient prognoses after surgery differ because of the inherent heterogeneity of these tumors. Various guidelines have been modified to classify the prognosis of pNET patients. Most studies have concentrated on the significance of tumor cells, but few have focused on the importance of the tumor microenvironment in pNET.
Platelets are an important component of the tumor microenvironment. They participate in growth, extravasation, epithelial–mesenchymal transition, and metastasis of tumor cells by secreting microparticles and exosomes[4]. They also mediate the interaction between tumor cells and immune cells[5]. Moreover, platelets reportedly influence the prognosis and drug resistance of different tumors and can serve as treatment carriers[6,7].
Nevertheless, the ability of platelet-associated indicators determined by blood tests to predict clinical outcome is in dispute. Although one study identified platelet count as a risk factor for recurrence and 3-year survival probability in resectable pNET[8], another study reported that platelet count was not associated with overall survival (OS) in inoperable advanced or metastatic pNET[9]. Other platelet-associated indicators, such as mean platelet volume (MPV) and platelet-to-lymphocyte ratio (PLR), have been described as distinguishable serous indicators of pNET risk and prognosis[10,11]. However, in another study, MPV and PLR in pNET were reported not to be independent recurrence risk factors for patients with radical resection[12]. Tumor-infiltrating platelets (TIPs) are educated and activated by tumor cells, and their distribution promotes metastasis of tumor cells. A previous study revealed the prognostic significance of TIPs in resectable pancreatic cancer[13]. However, the role of TIPs in pNET remains unclear.
In this study, we evaluated the potential value of TIPs assessment as a biomarker to predict survival and recurrence outcome in patients with pNET undergoing radical resection.
In total, 113 patients who had undergone radical surgical resection from 2012 to 2017 at our institution were retrospectively enrolled. All specimens were selected via pathologic diagnosis as pNET without distant metastasis or other tumor history. None of the patients had received any preoperative chemotherapy or radiotherapy or died of postoperative complications within 30 d. All cases included complete clinical preoperative and postoperative data, and all patients received therapy at Fudan University Shanghai Cancer Center. This study was approved by the Human Research Ethics Committee of Fudan University Shanghai Cancer Center and was performed in accordance with the tenets of the World Medical Association Declaration of Helsinki.
Age referred to the time when a patient was definitively diagnosed with pNET. Tumor location was divided into pancreatic head or pancreatic body with tail. Tumor grade was classified according to the Ki-67 labeling index of the World Health Organization in 2017, and Tumor Nodes Metastases (TNM) staging was assessed as the eighth edition norm of the American Joint Committee on Cancer. Preoperative platelet-associated indicators, including platelet count, MPV, and PLR, were measured and calculated via blood tests within 3 d before surgery.
Postoperative patients were routinely evaluated according to clinical manifestations and auxiliary examinations, including tumor markers (carbohydrate antigen 19-9, cancer antigen 125, and carcinoembryonic antigen, among others) and imaging examinations (enhanced computed tomography, enhanced magnetic resonance imaging, etc). All patients were strictly followed up until the last follow-up in January 2019. OS was defined as the interval from the date of surgery to death or the last follow-up. Recurrence-free survival (RFS) was defined as the interval from the date of surgery to the date of tumor recurrence or the last follow-up.
Formalin-fixed and paraffin-embedded serial pathological sections of surgical resection specimens were utilized to perform immunostaining. Immunohisto-chemistry is described in detail below. Baked slides were deparaffinized in xylene and rehydrated in an ethanol concentration gradient. Endogenous peroxidase activity was inhibited with a 3% methanol solution of hydrogen peroxide in an aqueous chamber at 37 °C, and antigen retrieval was conducted using high-pressure heated induction in citrate buffer (pH 6.0, G1201, Servicebio, Wuhan, China). Then, nonspecific binding was blocked with 10% normal goat serum, and primary monoclonal anti-CD42b antibody (diluted 1:150, anti-CD42b, EPR6995; Abcam, Cambridge, MA, United States) was applied to detect the platelet-specific marker CD42b. Slides were incubated with secondary antibody along with the 3,3’-diaminobenzidine chromogen using a Secondary Antibody Kit (G1210-2-A, Servicebio). Next, nuclei were stained with hematoxylin (G1004, Servicebio). Finally, the slices were sequentially exposed to an ethanol concentration gradient and xylene and were then sealed airtight with neutral resin and coverslips.
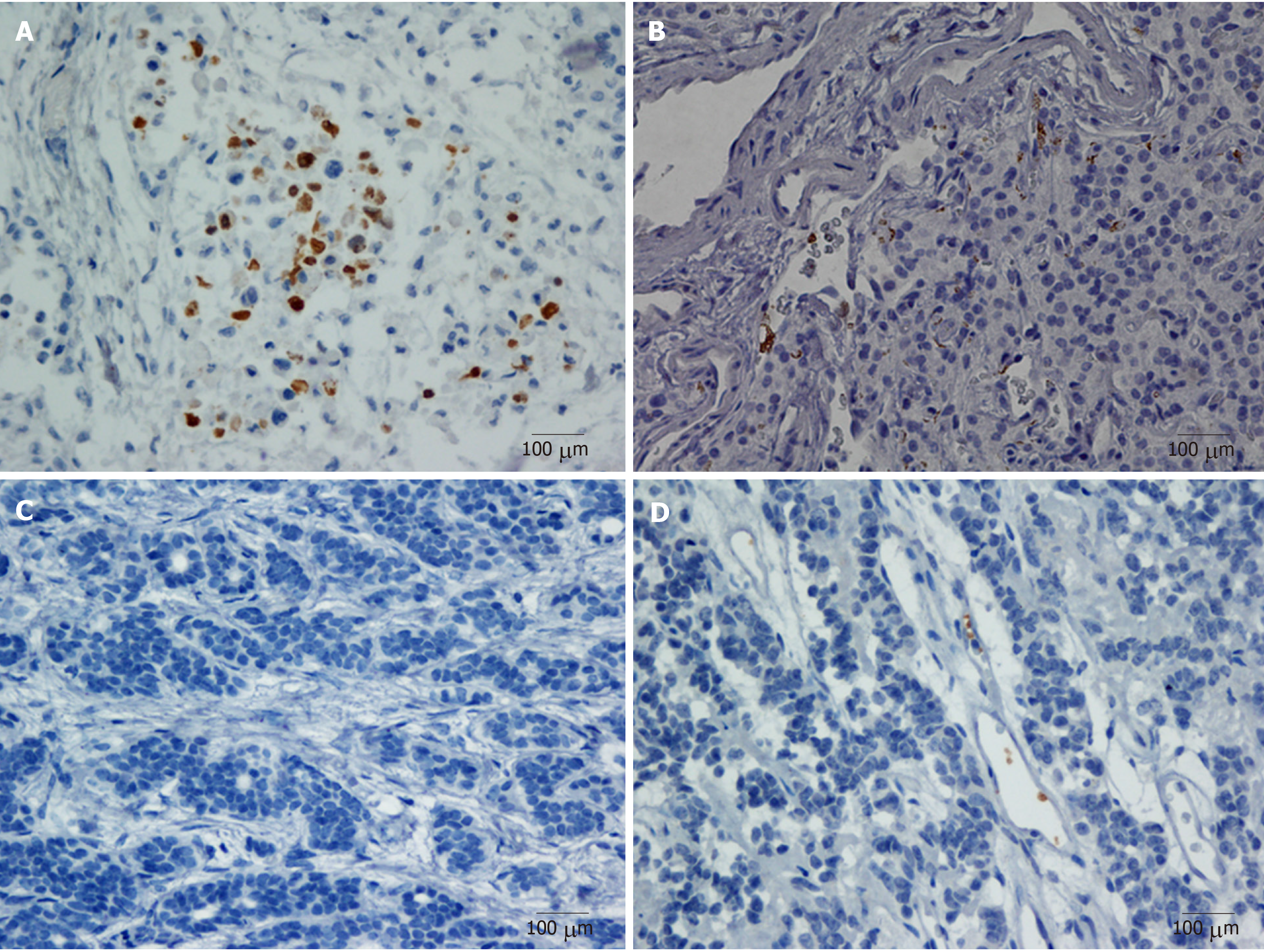
The immunostaining images of the whole slide from each case were evaluated under low-power scanning magnification (× 100). Hotspot images were defined as the areas in the tumor with the highest number of cells with immunoreactive staining. Under high-power magnification (× 200), five representative photographs of the hotspot were captured to identify the number of TIPs. The results were independently reviewed by two blinded, independent, clinically experienced pathologists. Positive tests were performed with pancreatic adenocarcinoma that definitively showed CD42b staining. Negative controls were treated identically but with the primary antibodies omitted. The evaluation criteria were that positive referred to an immunostained platelet distribution that accounted for ≥ 10% of the intratumoral region and negative referred to an immunostained platelet distribution that accounted for < 10% of intratumoral region; furthermore, samples with platelet immunostaining limited to intratumoral vessels or the peritumoral area were also regarded as negative[13-15]. In the study, we evaluated 5%, 10%, and 20% as the cut-off values for CD42b expression. The proportion of positive CD42b expression at cut-off values of 5%, 10%, and 20% was 60.18%, 47.79%, and 25.66%, respectively. The P value for survival comparison between patients with positive CD42b expression and those with negative CD42b expression were 0.042, 0.005, and 0.771, respectively, at cut-off values of 5%, 10%, and 20%. The cut-off value of 10% had the best survival discrimination and was chosen as the cut-off value.
Correlations between TIP expression and clinicopathologic characteristics were analyzed using a chi-square test. Kaplan–Meier survival curves were used to display OS and RFS, and differences between groups were compared with a log-rank test. Univariate and multivariate Cox regression analyses were used to identify independent prognostic factors for recurrence and survival. All tests were two sided, and P < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS 24.0 software (SPSS Inc., Armonk, NY, United States).
The clinicopathologic characteristics of all the patients are shown in Table 1. The median age was 54 years, and 50 (44.24%) patients were males. There were 66 (58.41%) patients with pancreatic head tumors, and 5 (4.42%) patients with functional pNET, all belonging to insulinoma. The majority (58.41%) of patients was stage 1 and stage 2, and the rest were stage 3. Similarly, 101 (89.38%) tumors were grade 1, and the remaining were grade 2. The median preoperative platelet count, MPV, and PLR were 225 × 109/L, 11.1 fL, and 130.7, respectively. At the last follow-up, 13 patients (11.50%) had died, and 41 patients (36.28%) had recurrence. Furthermore, the 1-year, 3-year, and 5-year mortality rates were 3.6%, 8.8%, and 14.2%, respectively, and the 1-year, 3-year, and 5-year recurrence rates were 8.0%, 15.7%, and 27.7%, respectively.
| Clinical characteristics | |
| Age in yrs, as median (range) | 54 (25, 82) |
| Gender, male/female | 50/63 |
| Tumor location, head/body, tail | 66/47 |
| Tumor diameter, as median (range) | 3.8 (1.0, 10.5) cm |
| Extend of disease, localized/nodal | 66/47 |
| Tumor grade, 1/2 | 101/12 |
| TNM stage, 1, 2/3 | 66/47 |
| Functional pNET, no/yes | 108/5 |
| MPV, as median (range) | 11.1 (9.1, 13.8) fL |
| PLR, as median (range) | 130.7 (13.6, 413.3 ) |
| Platelet count, as median (range) | 225 (79, 436) × 109/L |
| Death, no/yes | 100/13 |
| Recurrence, no/yes | 72/41 |
Positive CD42b (a platelet-specific marker) expression was seen in the intratumoral spaces surrounding cancer cells (Figure 1A and B); in contrast, negative CD42b staining referred to an absence of intratumoral CD42b expression or positive CD42b expression only in intratumoral vessels (Figure 1C and D). Positive CD42b expression was observed in 47.79% of the patients. As shown in Table 2, neither basic characteristics nor preoperative platelet count, MPV, or PLR showed a statistical relationship with CD42b expression (all P > 0.05). Kaplan-Meier survival curves revealed that patients with positive CD42b expression showed worse OS (P = 0.005) and RFS (P < 0.001) than those with negative CD42b expression (Figure 2), indicating that the presence of TIPs was associated with poorer clinical outcomes.
| Factor | CD42b expression | P value | |
| Negative | Positive | ||
| Age in yr | 0.157 | ||
| < 54 | 33 | 23 | |
| ≥ 54 | 26 | 31 | |
| Gender | 0.968 | ||
| Male | 26 | 24 | |
| Female | 33 | 30 | |
| Tumor location | 0.186 | ||
| Head | 31 | 35 | |
| Body, Tail | 28 | 19 | |
| Tumor grade | 0.439 | ||
| 1 | 54 | 47 | |
| 2 | 5 | 7 | |
| TNM stage | 0.556 | ||
| 1, 2 | 36 | 30 | |
| 3 | 23 | 24 | |
| Functional pNET | 1.00 | ||
| No | 56 | 52 | |
| Yes | 3 | 2 | |
| Platelet count, as × 109/L | 0.928 | ||
| < 225 | 29 | 27 | |
| ≥ 225 | 30 | 27 | |
| MPV in fL | 0.298 | ||
| < 11.1 | 32 | 24 | |
| ≥ 11.1 | 27 | 30 | |
| PLR | 0.507 | ||
| < 130.7 | 31 | 25 | |
| ≥ 130.7 | 28 | 29 | |
As shown in Table 3, univariate analysis showed that TNM stage (OS: P = 0.005; RFS: P = 0.001), tumor grade (OS: P < 0.001; RFS: P < 0.001), and CD42b expression (OS: P = 0.015; RFS: P = 0.001) were associated with patient clinical outcomes, while platelet count, MPV, and PLR were not associated with patient OS or RFS (all P>0.05). Multivariate analysis (Table 4) showed that CD42b expression was an independent prognostic factor for both OS (P = 0.049) and RFS (P = 0.003). Tumor grade and TNM stage were also independently associated with patient prognosis. The above results demonstrate that the presence of TIPs is a predictor of poor OS and RFS.
| Factor | OS | RFS | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Age, < 54/≥ 54 | 1.790 | 0.585-5.481 | 0.308 | 0.995 | 0.538-1.840 | 0.987 |
| Gender, male/female | 2.689 | 0.740-9.770 | 0.133 | 0.972 | 0.526-1.796 | 0.929 |
| Tumor location, head/body, tail | 0.760 | 0.233-2.482 | 0.650 | 1.026 | 0.531-1.982 | 0.939 |
| Tumor grade, 1/2 | 13.403 | 4.332-41.466 | < 0.001 | 5.509 | 2.299-13.199 | < 0.001 |
| TNM stage, 1, 2/3 | 8.861 | 1.960-40.064 | 0.005 | 2.892 | 1.527-5.477 | 0.001 |
| Functional pNET, no/yes | 0.046 | 0.000-5340.899 | 0.606 | 0.412 | 0.056-3.007 | 0.382 |
| Platelet count, as × 109/L, < 225/≥ 225 | 2.497 | 0.768-8.114 | 0.128 | 1.188 | 0.642-2.201 | 0.583 |
| MPV in fL, < 11.1/≥ 11.1) | 1.442 | 0.472-4.411 | 0.521 | 1.547 | 0.827-2.893 | 0.172 |
| PLR, < 130.7/≥ 130.7 | 1.217 | 0.409-3.624 | 0.724 | 1.043 | 0.564-1.927 | 0.894 |
| CD42b expression, negative/positive | 6.432 | 1.425-29.025 | 0.015 | 3.203 | 1.603-6.400 | 0.001 |
| Factor | OS | RFS | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Tumor grade, 1/2 | 5.882 | 1.848-18.719 | 0.003 | 3.283 | 1.345-8.016 | 0.009 |
| TNM stage, 1, 2/3 | 5.136 | 1.062-24.842 | 0.042 | 2.366 | 1.219-4.591 | 0.011 |
| CD42b expression, negative/positive | 4.601 | 1.004-21.095 | 0.049 | 2.893 | 1.439-5.817 | 0.003 |
In this study, platelet count determined by a blood test had no significant value in prediction of survival and recurrence in pNET. Similar results were observed with other platelet-associated indicators determined by a blood test. Notably, we demonstrated that the presence of TIPs in the tumor microenvironment in pNET exhibited potential as a risk biomarker to predict the prognosis of pNET following radical resection. The determination of TIPs in tumors could improve prognostic classification of pNET after resection.
Several reasons were attributed to the irrelative association between preoperative platelet count, MPV, and PLR determined by blood tests and clinical outcome in resectable pNET. First, these indicators themselves might not be appropriate to predict prognosis. Platelet count, MPV, and PLR provide limited information concerning the malignant features of pNET, and their levels remained unchanged even after tumor recurrence[12]. Second, preoperative platelet count, MPV, and PLR determined by blood tests are easily affected by other diseases, such as hematopoietic disease, hypersplenism, and common cardiovascular and cerebrovascular diseases. They can also be influenced by drugs, such as aspirin[16]. Last, platelet count, MPV, and PLR detected as systemic indicators might not accurately reflect the specific regional details of the tumor microenvironment. Minor changes in the tumor microenvironment cannot be determined by systemic indicators.
TIPs exhibit the activated and agminated status of intratumoral platelets, which are enticed by tumor cells into sustaining stability. They are induced by tumor cells via direct and indirect mechanisms through a series of indispensable processes that can be divided into penetration, activation, and aggregation[17]. TIPs travel easily through tumor-associated vessels due to the fragility of these vessels, which are characterized by overexpressed, abnormal, and leaky tumor vasculature[18]. They are subsequently activated by cancer-released factors, cancer-surface molecules, or cancer-induced aggregation[19]. TIPs gather around tumor cells and further motivate other platelets through the process of platelet coagulation[20]. TIPs are seldom affected by changes in blood contents because TIPs are formed by activated platelets that are primarily attracted from the bloodstream into the tumor region by tumor cells. Thus, no statistical relationship was observed between TIPs and platelet count, MPV, or PLR (Table 2).
The presence of TIPs is considered a potential biomarker for predicting prognosis outcome in resectable pNET, which is attributed to the interaction between platelets and tumor cells. Not only can tumor cells stimulate platelets, but platelets can also communicate with tumor cells by releasing numerous proteins, growth factors, microparticles, and microRNAs to promote tumor progression[17]. Platelets enhance tumor proliferation by secreting transforming growth factor-β[21], promote tumor invasion via secretion of microRNA-223[22], and facilitate tumor metastasis through activation of YAP1 signaling in tumor cells[23]. Activated platelets rely on paracrine activation of the epidermal growth factor receptor and downstream DNA-dependent protein kinase to motivate the anti-apoptosis response of tumor cells[24]. Platelets produce the proangiogenic proteins vascular endothelial growth factors, which contribute to the neovascularization process that assists tumor cells in obtaining sufficient nutrition and aid in further extravasation[17,25]. The antitumor reactivity of natural killer cells can also be impaired by platelets through platelet-derived transforming growth factor-β[26]. In addition, platelets induce cisplatin resistance through the Akt/Bad/Bcl-2 signaling pathway under endoplasmic reticulum stress[7]. The interaction between platelets and tumor cells forms a mutually reinforcing cycle that promotes tumor metastasis. Thus, a reduction in platelet infiltration in the tumor microenvironment might be a potential treatment strategy for pNET. In addition, one of the mechanisms underlying the antitumor effect of aspirin in pNET with multiple endocrine neoplasia type 1 was attributed to targeted therapy of platelets[27].
Our study has some limitations. First, the nature of a retrospective study with a limited sample size restricts the level of evidence. Another prospective study with a larger sample size needs be conducted with multicenter cooperation. Next, the mechanism by which platelets influence the recurrence of patients with resectable pNET was not thoroughly investigated, and thus, additional experiments to examine the mechanism need to be implemented.
In conclusion, we found that TIPs were potential pNET prognosis indicators. In addition, therapy targeting platelets in pNET could improve the long-term survival of patients.
Pancreatic neuroendocrine tumor (pNET) is the second most common malignancy among pancreatic tumors. Most studies have primarily concentrated on the significance of tumor cells, but few have focused on the importance of the tumor microenvironment in pNET. Platelets are an important component of the tumor microenvironment. However, the importance of platelets in pNET lacks adequate literature support.
To assess the potential clinical meaning of platelets in pNET and offer evidence concerning a potential anti-platelet therapeutic strategy for pNET.
Tumor-infiltrating platelets are potential independent indicators of survival and recurrence outcome for patients with resectable pNET.
In total, 113 patients who had undergone radical surgical resection were retrospectively enrolled. All the specimens were selected via pathologic diagnosis of pNET without distant metastasis or other tumor history. None of the patients had received any preoperative chemotherapy or radiotherapy or died of postoperative complications within 30 d. Immunohistochemical analysis of CD42b expression in tumor specimens was performed to determine the presence of TIPs. Univariate and multivariate analyses were applied to analyze the prognostic value of tumor-infiltrating platelets.
Tumor-infiltrating platelets were observed in intratumoral areas in 54 patients. Neither basic characteristics nor preoperative platelet count, mean platelet volume, or platelet lymphoid ratio showed a statistical relationship with CD42b expression. Platelet count, mean platelet volume, and platelet-to-lymphocyte ratio determined by preoperative blood tests were not related to overall survival or recurrence-free survival. Tumor-infiltrating platelets were found to be potential independent indicators of survival and recurrence outcome for patients with resectable pNET. The major limitations were that the nature of a retrospective study with a limited sample size restricts the level of evidence and that the mechanism by which platelets influence the recurrence of patients was not thoroughly investigated.
We uncovered a relationship between tumor-infiltrating platelets and the prognosis of patients with pNET following radical resection. We found that tumor-infiltrating platelets were potential pNET prognosis indicators. In addition, therapy targeting platelets in pNET could improve long-term survival. We further provide the clinical meaning of platelets in the tumor microenvironment in pNET.
A further prospective study with a large sample size should be conducted with multicenter cooperation, and additional experiments to assess the mechanism need to be implemented. If possible, we recommend a randomized double-blind controlled clinical trial for platelet-targeted therapy for pNET.
We appreciate the support and help from Dr. Ya-Fei Chen, Dr. Houpu Xu, Dr. Dan Huang, and Dr. Cong Tan.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Neri V, Tsolakis AV, Vagholkar KR S-Editor: Ma RY L-Editor: Filipodia E-Editor: Ma YJ
| 1. | Lee MR, Harris C, Baeg KJ, Aronson A, Wisnivesky JP, Kim MK. Incidence Trends of Gastroenteropancreatic Neuroendocrine Tumors in the United States. Clin Gastroenterol Hepatol. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 2. | Öberg K, Knigge U, Kwekkeboom D, Perren A; ESMO Guidelines Working Group. Neuroendocrine gastro-entero-pancreatic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23 Suppl 7:vii124-vii130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 341] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 3. | Kaderli RM, Spanjol M, Kollár A, Bütikofer L, Gloy V, Dumont RA, Seiler CA, Christ ER, Radojewski P, Briel M, Walter MA. Therapeutic Options for Neuroendocrine Tumors: A Systematic Review and Network Meta-analysis. JAMA Oncol. 2019; [Epub ahead of print]. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 4. | Haemmerle M, Stone RL, Menter DG, Afshar-Kharghan V, Sood AK. The Platelet Lifeline to Cancer: Challenges and Opportunities. Cancer Cell. 2018;33:965-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 475] [Article Influence: 59.4] [Reference Citation Analysis (0)] |
| 5. | Placke T, Örgel M, Schaller M, Jung G, Rammensee HG, Kopp HG, Salih HR. Platelet-derived MHC class I confers a pseudonormal phenotype to cancer cells that subverts the antitumor reactivity of natural killer immune cells. Cancer Res. 2012;72:440-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 349] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 6. | Rao L, Bu LL, Ma L, Wang W, Liu H, Wan D, Liu JF, Li A, Guo SS, Zhang L, Zhang WF, Zhao XZ, Sun ZJ, Liu W. Platelet-Facilitated Photothermal Therapy of Head and Neck Squamous Cell Carcinoma. Angew Chem Int Ed Engl. 2018;57:986-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 137] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 7. | Wang Z, Fang M, Li J, Yang R, Du J, Luo Y. High Platelet Levels Attenuate the Efficacy of Platinum-Based Treatment in Non-Small Cell Lung Cancer. Cell Physiol Biochem. 2018;48:2456-2469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Kaltenborn A, Matzke S, Kleine M, Krech T, Ramackers W, Vondran FW, Klempnauer J, Bektas H, Schrem H. Prediction of survival and tumor recurrence in patients undergoing surgery for pancreatic neuroendocrine neoplasms. J Surg Oncol. 2016;113:194-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Zou J, Li Q, Kou F, Zhu Y, Lu M, Li J, Lu Z, Shen L. Prognostic value of inflammation-based markers in advanced or metastatic neuroendocrine tumours. Curr Oncol. 2019;26:e30-e38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Karaman K, Bostanci EB, Aksoy E, Kurt M, Celep B, Ulas M, Dalgic T, Surmelioglu A, Hayran M, Akoglu M. The predictive value of mean platelet volume in differential diagnosis of non-functional pancreatic neuroendocrine tumors from pancreatic adenocarcinomas. Eur J Intern Med. 2011;22:e95-e98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Salman T, Kazaz SN, Varol U, Oflazoglu U, Unek IT, Kucukzeybek Y, Alacacioglu A, Atag E, Semiz HS, Cengiz H, Oztop I, Tarhan MO. Prognostic Value of the Pretreatment Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio for Patients with Neuroendocrine Tumors: An Izmir Oncology Group Study. Chemotherapy. 2016;61:281-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 12. | Gaitanidis A, Patel D, Nilubol N, Tirosh A, Sadowski S, Kebebew E. Markers of Systemic Inflammatory Response are Prognostic Factors in Patients with Pancreatic Neuroendocrine Tumors (PNETs): A Prospective Analysis. Ann Surg Oncol. 2018;25:122-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Zhang SR, Yao L, Wang WQ, Xu JZ, Xu HX, Jin W, Gao HL, Wu CT, Qi ZH, Li H, Li S, Ni QX, Yu XJ, Fu DL, Liu L. Tumor-Infiltrating Platelets Predict Postsurgical Survival in Patients with Pancreatic Ductal Adenocarcinoma. Ann Surg Oncol. 2018;25:3984-3993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Saito H, Fushida S, Miyashita T, Oyama K, Yamaguchi T, Tsukada T, Kinoshita J, Tajima H, Ninomiya I, Ohta T. Potential of extravasated platelet aggregation as a surrogate marker for overall survival in patients with advanced gastric cancer treated with preoperative docetaxel, cisplatin and S-1: a retrospective observational study. BMC Cancer. 2017;17:294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Ishikawa S, Miyashita T, Inokuchi M, Hayashi H, Oyama K, Tajima H, Takamura H, Ninomiya I, Ahmed AK, Harman JW, Fushida S, Ohta T. Platelets surrounding primary tumor cells are related to chemoresistance. Oncol Rep. 2016;36:787-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Yang J, Zhou X, Fan X, Xiao M, Yang D, Liang B, Dai M, Shan L, Lu J, Lin Z, Liu R, Liu J, Wang L, Zhong M, Jiang Y, Bai X. mTORC1 promotes aging-related venous thrombosis in mice via elevation of platelet volume and activation. Blood. 2016;128:615-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 17. | Wojtukiewicz MZ, Sierko E, Hempel D, Tucker SC, Honn KV. Platelets and cancer angiogenesis nexus. Cancer Metastasis Rev. 2017;36:249-262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 188] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 18. | Ronca R, Benkheil M, Mitola S, Struyf S, Liekens S. Tumor angiogenesis revisited: Regulators and clinical implications. Med Res Rev. 2017;37:1231-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 141] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 19. | Miao S, Shu D, Zhu Y, Lu M, Zhang Q, Pei Y, He AD, Ma R, Zhang B, Ming ZY. Cancer cell-derived immunoglobulin G activates platelets by binding to platelet FcγRIIa. Cell Death Dis. 2019;10:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Zhang Y, Qiu Y, Blanchard AT, Chang Y, Brockman JM, Ma VP, Lam WA, Salaita K. Platelet integrins exhibit anisotropic mechanosensing and harness piconewton forces to mediate platelet aggregation. Proc Natl Acad Sci USA. 2018;115:325-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 108] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 21. | Cho MS, Bottsford-Miller J, Vasquez HG, Stone R, Zand B, Kroll MH, Sood AK, Afshar-Kharghan V. Platelets increase the proliferation of ovarian cancer cells. Blood. 2012;120:4869-4872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 195] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 22. | Liang H, Yan X, Pan Y, Wang Y, Wang N, Li L, Liu Y, Chen X, Zhang CY, Gu H, Zen K. MicroRNA-223 delivered by platelet-derived microvesicles promotes lung cancer cell invasion via targeting tumor suppressor EPB41L3. Mol Cancer. 2015;14:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 157] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 23. | Haemmerle M, Taylor ML, Gutschner T, Pradeep S, Cho MS, Sheng J, Lyons YM, Nagaraja AS, Dood RL, Wen Y, Mangala LS, Hansen JM, Rupaimoole R, Gharpure KM, Rodriguez-Aguayo C, Yim SY, Lee JS, Ivan C, Hu W, Lopez-Berestein G, Wong ST, Karlan BY, Levine DA, Liu J, Afshar-Kharghan V, Sood AK. Platelets reduce anoikis and promote metastasis by activating YAP1 signaling. Nat Commun. 2017;8:310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 210] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 24. | Au AE, Sashindranath M, Borg RJ, Kleifeld O, Andrews RK, Gardiner EE, Medcalf RL, Samson AL. Activated platelets rescue apoptotic cells via paracrine activation of EGFR and DNA-dependent protein kinase. Cell Death Dis. 2014;5:e1410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Jiang L, Luan Y, Miao X, Sun C, Li K, Huang Z, Xu D, Zhang M, Kong F, Li N. Platelet releasate promotes breast cancer growth and angiogenesis via VEGF-integrin cooperative signalling. Br J Cancer. 2017;117:695-703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 26. | Kopp HG, Placke T, Salih HR. Platelet-derived transforming growth factor-beta down-regulates NKG2D thereby inhibiting natural killer cell antitumor reactivity. Cancer Res. 2009;69:7775-7783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 371] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 27. | Manoharan J, Fendrich V, Di Fazio P, Bollmann C, Roth S, Joos B, Mintziras I, Albers MB, Ramaswamy A, Bertolino P, Zhang CX, Slater EP, Bartsch DK, Lopez-Lopez CL. Chemoprevention with Enalapril and Aspirin in Men1(+/T) Knockout Mouse Model. Neuroendocrinology. 2018;107:257-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |