Published online Nov 7, 2019. doi: 10.3748/wjg.v25.i41.6258
Peer-review started: July 12, 2019
First decision: August 18, 2019
Revised: September 6, 2019
Accepted: September 11, 2019
Article in press: September 11, 2019
Published online: November 7, 2019
Processing time: 118 Days and 12.8 Hours
Increasing numbers of laboratory blood parameters (BPM) have been reported to greatly affect the long-term outcomes of gastric cancer (GC) patients. However, the existing prognostic models do not comprehensively analyze these predictors.
To construct a new prognostic tool, based on all the prognostic BPM, to achieve more accurate prognosis prediction for GC.
We retrospectively assessed 850 consecutive patients who underwent curative resection for stage II-III GC from January 2010 to April 2013. The patients were classified into developing (n = 567) and validation (n = 283) cohorts using computer-generated random numbers. A scoring system, namely BPM score, was then constructed using least absolute shrinkage and selection operator (LASSO) Cox regression model in the developing cohort, and validated in the validation cohort. A nomogram consisting of BPM score and tumor-lymph node-metastasis (TNM) stage was further created. The discrimination and calibration of the nomogram were evaluated via Harrell’s C-statistic and the Hosmer-Lemeshow test.
Using the LASSO model, we established the BPM score based on five BPM: Albumin, lymphocyte-to-monocyte ratio, neutrophil-to-lymphocyte ratio, carcinoembryonic antigen, and carbohydrate antigen 19-9. The BPM scores were divided into high- and low-BPM groups based on a cut-off value of -0.93. High-BPM patients were significantly older and had more advanced, larger tumors. In the developing cohort, significant differences were found in 5-year overall survival (OS) and 5-year disease-specific survival between the high-BPM and low-BPM patients. Similar results were found in the validation group. Multivariable analysis showed that the BPM score was an independent predictor of OS. High-BPM patients had a poorer 5-year OS for each subgroup. Furthermore, a nomogram that combined the BPM score and TNM stage had significantly better prognostic value compared with TNM stage alone.
The BPM score provides more accurate prognosis prediction in stage II-III GC patients and is an effective complement to the TNM staging system.
Core tip: The study aimed to select the optimal combination of blood parameters (BPM) and to establish a novel prognostic classifier. Using the least absolute shrinkage and selection operator model, we established the BPM score based on five BPM: Albumin, lymphocyte-to-monocyte ratio, neutrophil-to-lymphocyte ratio, carcinoembryonic antigen, and carbohydrate antigen 19-9. The BPM score provides more accurate prognosis prediction in stage II-III gastric cancer patients and is an effective complement to the tumor-lymph node-metastasis staging system.
- Citation: Lin JX, Tang YH, Wang JB, Lu J, Chen QY, Cao LL, Lin M, Tu RH, Huang CM, Li P, Zheng CH, Xie JW. Blood parameters score predicts long-term outcomes in stage II-III gastric cancer patients. World J Gastroenterol 2019; 25(41): 6258-6272
- URL: https://www.wjgnet.com/1007-9327/full/v25/i41/6258.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i41.6258
Gastric cancer (GC) remains an important malignancy worldwide; there were over 1000000 new cases of GC and an estimated 783000 deaths caused by GC (equating to 1 in every 12 deaths globally) in 2018, making it the fifth most frequently diagnosed cancer and the third leading cause of cancer death[1]. The tumor-lymph node-metastasis (TNM) staging system is the most commonly used criteria to predict GC patients’ long-term outcomes[2]. However, clinical outcomes can vary in patients with GC who have the same TNM stage and similar treatment regimens[3-5], indicating that this anatomy-based system provides incomplete prognostic information. Hence, the identification of potential predictors of risk stratification and treatment selection has become a hot topic in recent years.
There is growing evidence that the patient’s immune and nutritional statuses are closely related to long-term survival in patients with various malignancies[5-14]. Over the past decades, the lymphocyte count and serum albumin have been the most widely used biomarkers for defining the immune and nutritional statuses. As a result, several scoring systems, including the prognostic nutritional index (PNI), the modified Glasgow prognostic score (mGPS), and the controlling nutritional status (CONUT) score, have been constructed and commonly used to predict the outcomes in patients with GC[9-11]. However, these scores contain limited blood parameters (BPM) and may provide inadequate prognostic information[11,15-16]. Ongoing work is seeking other prognostic biomarkers. To date, several ratios based on circulating blood cell counts, such as the neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), and platelet-to-lymphocyte ratio (PLR), have been developed to predict the outcome of GC[12-14]. In addition, tumor markers, including carcinoembryonic antigen (CEA) and carbohydrate antigen (CA) 19-9, are routinely applied in the early detection and postoperative follow-up of GC. Some studies have proved their prognostic value in GC[17-18]. Therefore, we hypothesized that a new scoring system, based on all available laboratory BPM, would provide more accurate prognostic information in patients with GC.
In the present study, we used the least absolute shrinkage and selection operator (LASSO) Cox regression model to select the optimal combination of BPM and to establish a novel prognostic classifier, namely, the BPM score, in order to predict outcomes in patients with stage II-III GC.
We retrospectively assessed patients undergoing curative resection for GC at the Fujian Medical University Union Hospital (FMUUH) between January 2010 and April 2013. Patients who met the following criteria were included: (1) Aged ≥ 18 years; (2) Stage II-III gastric adenocarcinoma confirmed by histopathology; and (3) No pre-operative chemoradiotherapy. The following patients were excluded: (1) Distant metastasis; (2) Malignant disease of other organs; (3) R1 resection; (4) Acute infections or other inflammatory conditions within 7 days before surgery; and (5) Incomplete medical records or follow-up data. Finally, 850 patients were enrolled in this study. All surviving patients had a follow-up period of at least 5 years. The patients were classified into developing (n = 567) and validation (n = 283) cohorts using computer-generated random numbers. All surgical procedures, including lymph node dissection, were performed according to the guidelines of the Japanese Research Society for the Study of GC[19], while staging was performed according to the TNM classification (AJCC, 8th edition). All patients were routinely recommend to receive 5-fluorouracil (5-FU) based adjuvant chemotherapy after surgery[4].
Data on patient demographics and pathological results were obtained from a large-scale prospective database. Routine blood tests were carried out during 1 wk before surgery, including hemoglobin, serum albumin, total cholesterol level, total peripheral neutrophils, lymphocyte count, monocyte count, platelet count, CEA, CA 19-9, and fibrinogen. LMR was calculated by dividing lymphocyte count by monocyte count. NLR was calculated by dividing neutrophil count by lymphocyte count. PLR was calculated by dividing platelet count by lymphocyte count. We used widely accepted thresholds to define the dichotomous forms of these BPM: hemoglobin (males 120 g/L; females 110 g/L), albumin (35 g/L), cholesterol (4.6 mmol/L or 180 mg/dL), CEA (5 ng/mL), CA 19-9 (37 U/mL), and fibrinogen (400 mg/dL)[8,17,21-22]. According to the X-tile program (3.6.1 software 20, http://medicine.yale. edu/lab/rimm/research/software.aspx), NLR, LMR, and PLR cut-off values for overall survival (OS) were 3.9, 3.2, and 161, respectively[23]. The utility of clinical data was approved by the FMUUH Institutional Review Board.
All patients were followed postoperatively by physical examination and laboratory tests (including CEA, CA 19-9, and CA 72-4), every 3 mo for 2 years, every 6 mo during years 2-5, and annually thereafter. In addition, examinations, including chest radiography, abdominal computed tomography, and endoscopy, were performed at least once per year. The follow-up period was completed in April 2018 or to the date of death of patients. OS was defined as the time interval from surgery to death from any cause or to the last follow-up. Disease-specific survival (DSS) was defined as the time interval from surgery to death from GC or to the follow-up.
As assays may vary between hospitals, all the BPM are expressed as ratio of lower limit of normal (including hemoglobin, albumin, and cholesterol), upper limit of normal (including fibrinogen, CEA, and CA 19-9), or cut-off values (including NLR, LMR, and PLR). The relation between the BPM (as continuous variables) and OS was investigated in univariate Cox models with restricted cubic splines (RCS)[24]. Additional variable transformation was performed if strong nonlinear effects were shown. Next, we calculated the Akaike information criterion (AIC) scores of the continuous form and the dichotomous form of each blood parameter to determine the form in which the blood parameter was analyzed[25]. Finally, we used the LASSO Cox regression model to select the most useful predictors among the candidate BPM in the developing cohort[26,27]. A risk score based on multiple BPM, the BPM score, was then constructed to predict the prognosis of GC. Details of methods are described in the Supplementary Materials.
Continuous variables were compared using the Student’s t-test, and categorical variables were assessed using the χ2 test or the Fisher exact test. The Kaplan-Meier method was used to analyze OS and DSS, and the differences were assessed by log-rank tests. The time-dependent receiver operating characteristic (ROC) curve was generated to assess the discriminatory power of indicators for time-dependent disease outcomes[28]. Univariate and multivariate analyses were performed using the Cox proportional hazards model. A nomogram consisting of the BPM score and TNM stage was created to translate model parameter estimates into a visual scoring system to calculate the estimated survival probability. The discriminative power of the nomogram was assessed via Harrell’s C-statistic[29]. The calibration of the nomogram was evaluated by the Hosmer-Lemeshow test to assess the goodness of fit[30]. Decision curve analysis was used to examine the usefulness and benefit of the nomogram[31]. Two-tailed P values < 0.05 were considered statistically significant. All statistical analyses were conducted with SPSS software, version 18.0 (SPSS Inc., Chicago, IL, United States) and R software, version 3.1.2 (R Foundation for Statistical Computing, Vienna, Austria).
Overall, 850 patients were included in the study. There were 656 (77.2%) males and 194 (22.8%) females. The median age at the time of surgery was 62 years (range, 20-91 years). According to the TNM classification, 285 (33.5%) were classified as having stage II and 565 (66.5%) as having stage III, respectively. The majority of the patients (n = 645, 75.8%) received adjuvant chemotherapy. The median follow-up time was 61 mo (range, 1-102 mo). Patient characteristics in the developing and validation cohorts are detailed in Table 1.
| Variable | Developing cohort (n = 567) | Validation cohort (n = 283) | ||||||
| Total | Low-BPM (%) | High-BPM (%) | P value | Total | Low-BPM (%) | High-BPM (%) | P value | |
| Age (yr, mean ± SD) | 58.8 (± 11.3) | 64.6 (± 10.1) | < 0.001 | 59.5 (± 11.5) | 65.5 (± 9.5) | < 0.001 | ||
| Sex | 0.409 | 0.193 | ||||||
| Male | 430 | 218 (74.4) | 212 (77.4) | 226 | 113 (76.9) | 113 (83.1) | ||
| Female | 137 | 75 (25.6) | 62 (22.6) | 57 | 34 (23.1) | 23 (16.9) | ||
| Body mass index (mean ± SD) | 22.3 (± 2.8) | 21.8 (± 3.0) | 0.038 | 22.1 (± 2.6) | 21.7 (± 3.1) | 0.285 | ||
| Tumor stage | < 0.001 | 0.004 | ||||||
| II a | 86 | 58 (19.8) | 79 (28.8) | 61 | 39 (26.5) | 22 (16.2) | ||
| II b | 93 | 42 (14.3) | 51 (18.6) | 45 | 25 (17.0) | 20 (14.7) | ||
| III a | 142 | 89 (30.4) | 53 (19.3) | 63 | 33 (22.4) | 30 (22.1) | ||
| III b | 148 | 71 (24.2) | 77 (28.1) | 66 | 33 (22.4) | 30 (22.1) | ||
| III c | 98 | 33 (11.3) | 65 (23.7) | 48 | 17(11.6) | 31 (22.8) | ||
| Tumor location | 0.363 | 0.553 | ||||||
| Lower | 222 | 121 (41.3) | 101 (36.9) | 109 | 62 (42.2) | 47 (34.6) | ||
| Middle | 112 | 50 (17.1) | 62 (22.6) | 50 | 26 (17.7) | 24 (17.6) | ||
| Upper | 156 | 80 (27.3) | 76 (27.7) | 74 | 36 (24.5) | 38 (27.9) | ||
| Mixed | 77 | 42 (14.3) | 35 (12.8) | 50 | 23 (15.6) | 27 (19.9) | ||
| Tumor size (mm, mean ± SD) | 45.9 (± 22.3) | 56.7 (± 26.6) | < 0.001 | 47.2 (± 22.9) | 54.2 (24.8) | 0.014 | ||
| Histologic type | 0.902 | 0.613 | ||||||
| Differentiated | 92 | 47 (16.0) | 45 (16.4) | 52 | 25 (17.0) | 27 (19.9) | ||
| Undifferentiated | 475 | 246 (84.0) | 229 (83.6) | 231 | 122 (83.0) | 109 (80.1) | ||
| Lymphovascular invasion | 0.662 | 0.910 | ||||||
| No | 394 | 206 (70.3) | 188 (68.6) | 178 | 159 (65.4) | 33 (53.2) | ||
| Yes | 173 | 87 (29.7) | 86 (31.4) | 113 | 84 (34.6) | 29 (46.8) | ||
| Adjuvant chemotherapy | 0.346 | 0.355 | ||||||
| No | 137 | 66 (22.5) | 71 (25.9) | 68 | 32 (21.8) | 36 (26.5) | ||
| Yes | 430 | 227 (77.5) | 203 (74.1) | 215 | 115 (78.2) | 100 (73.5) | ||
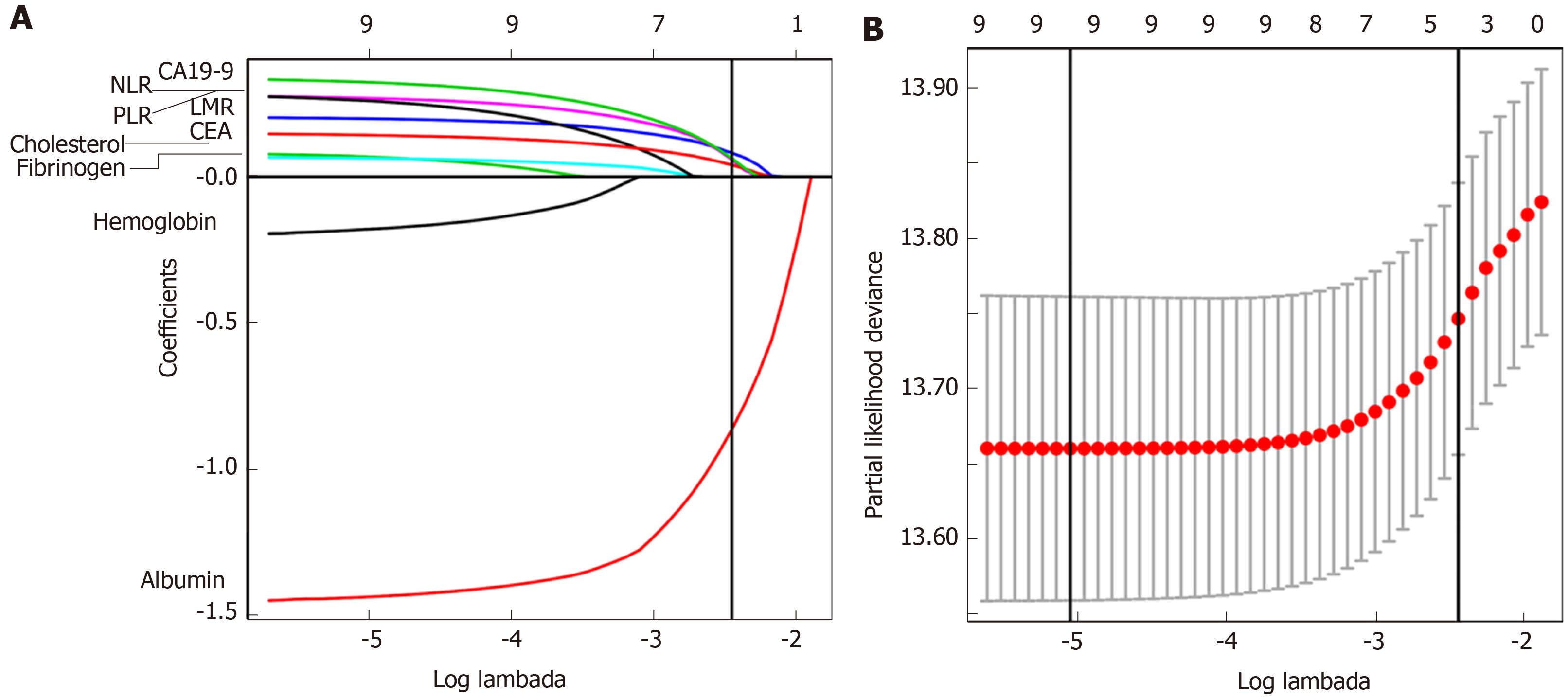
It was observed that the relationship between total cholesterol level and OS followed a U-shaped pattern, with a turning point approximately 1.5 × LLN. Therefore, the values for this variable were transformed by taking the absolute distance between cholesterol (in unit × LLN) and the turning point. Similarly, CEA and CA 19-9 were transformed to the form of ln CEA and ln CA 19-9. After transforming, no strong nonlinear effect was observed in all BPM (Supplementary Figure 1). Then, we calculated AIC scores and Harrell’s C-statistics of 9 BPM as continuous variables (Supplementary Table 1) and as dichotomous variables. It was found that only hemoglobin, albumin, ln CEA, and fibrinogen had smaller AIC scores as well as significantly higher Harrell’s C-statistics in their continuous forms (Supplementary Table 2). So, they were further analyzed as continuous variables, with the others analyzed as dichotomous variables.
Using LASSO Cox regression analysis, we identified five out of the nine BPM in the developing cohort: Albumin, LMR, NLR, CEA, and CA 19-9, and derived a formula to calculate the BPM score for each patient: BPM = -0.86259331 × Albumin (× LLN) + 0.08074780 × Low_LMR + 0.06002645 × High_NLR + 0.05939068 × High_CA19-9 + 0.04007563 × ln CEA (× ULN) (Figure 1A and B).
In this formula, LMR, NLR, and CA 19-9 were valued as 0 or 1; LMR ≤ 3.2, NLR ≥ 3.9, and CA 19-9 ≥ 37 U/mL were assigned a score of 1, and a value of 0 otherwise. The optimum cutoff value for BPM score was selected on the basis of the association with the patients’ OS by using X-tile plots (-0.93). We then assigned patients to a high- (BPM score ≥ -0.93) or low-BPM (BPM score < -0.93) group by this value.
Table 1 shows the relationship between clinicopathological factors and the BPM score. In both the developing and validation cohorts, high-BPM status was related to older age (P < 0.001 for both), higher tumor stage (P < 0.001 and P = 0.004, respectively), and larger tumor size (P < 0.001 and P = 0.014, respectively), compared with low-BPM status. In addition, although the high-BPM patients had a smaller body mass index (BMI) (P = 0.038) than the low-BPM patients in the developing cohort, it did not reach statistical significance in the validation cohort (P = 0.285).
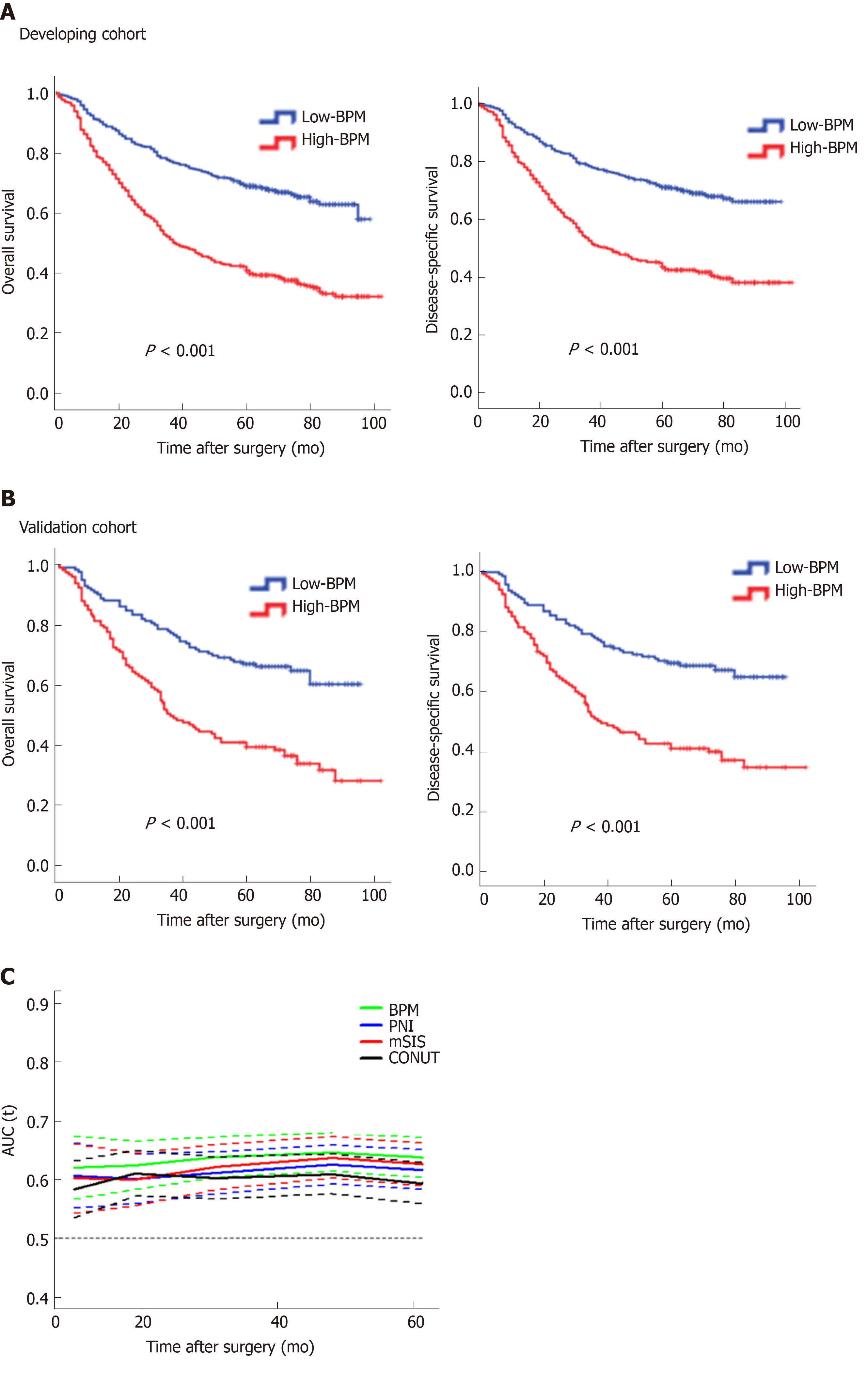
In the developing cohort, the 5-year OS rates in the low-BPM and high-BPM groups were 70.0% and 41.6%, and the 5-year DSS rates were 72.4% and 46.4% (log-rank P < 0.001, Figure 2A). We performed the same analyses in the validation cohort. The 5-year OS rates in the low-BPM and high-BPM groups were 67.3% and 39.7%, and the 5-year DSS rates were 70.1% and 41.9% (log-rank P < 0.001, Figure 2B). In the multivariate analysis, BPM score remained an independent prognostic factor for OS (P < 0.001 and P = 0.004, respectively) in both the developing and validation cohorts (Table 2).
| Variables | Developing cohort (n = 567) | Validation cohort (n = 283) | ||||||
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | |
| Age (yr) | 0.005 | 0.144 | 0.001 | 0.008 | ||||
| < 60 | Reference | Reference | Reference | Reference | ||||
| ≥ 60 | 1.432 (1.115-1.838) | 1.215 (0.936-1.579) | 1.758 (1.262-2.449) | 1.617 (1.132-2.311) | ||||
| Gender | 0.502 | 0.900 | ||||||
| Male | Reference | Not included | Reference | Not included | ||||
| Female | 1.098 (0.835-1.444) | 0.974 (0.644-1.473) | ||||||
| Body mass index | 0.058 | 0.025 | 0.241 | |||||
| < 18.5 | Reference | Not included | Reference | Reference | ||||
| 18.5-23.9 | 0.734 (0.509-1.060) | 0.551 (0.325-0.934) | 0.720 (0.415-1.247) | |||||
| > 23.9 | 0.597 (0.390-0.913) | 0.413 (0.217-0.788) | 0.645 (0.328-1.270) | |||||
| BPM score | < 0.001 | < 0.001 | < 0.001 | 0.004 | ||||
| Low | Reference | Reference | Reference | Reference | ||||
| High | 2.429 (1.893-3.117) | 1.993 (1.534-2.589) | 2.363 (1.677-3.330) | 1.717 (1.913-2.470) | ||||
| Tumor stage | < 0.001 | < 0.001 | < 0.001 | < 0.001 | ||||
| IIa | Reference | Reference | Reference | Reference | ||||
| IIb | 1.422 (0.772-2.621) | 1.220 (0.659-2.260) | 0.942 (0.453-1.957) | 0.778 (0.373-1.624) | ||||
| IIIa | 2.007 (1.161-3.471) | 1.988 (1.143-3.458) | 1.725 (0.953-3.121) | 1.609 (0.876-2.956) | ||||
| IIIb | 4.776 (2.851-8.000) | 3.977 (2.348-6.737) | 3.526 (2.030-6.125) | 3.116 (1.749-5.553) | ||||
| IIIc | 7.713 (4.550-13.074) | 5.709 (3.299-9.882) | 6.234 (3.535-10.993) | 4.251 (2.294-7.878) | ||||
| Tumor size | < 0.001 | 0.034 | 0.004 | 0.638 | ||||
| ≤40 mm | Reference | Reference | Reference | Reference | ||||
| >40 mm | 1.987 (1.542-2.560) | 1.332 (1.022-1.735) | 1.647 (1.176-2.308) | 0.913 (0.624-1.336) | ||||
| Tumor location | 0.002 | 0.596 | 0.001 | 0.043 | ||||
| Lower | Reference | Reference | Reference | Reference | ||||
| Middle | 1.307 (0.935-1.827) | 1.103 (0.786-1.547) | 1.319 (0.795-2.189) | 1.313 (0.776-2.221) | ||||
| Upper | 1.209 (0.888-1.647) | 1.125 (0.822-1.539) | 1.710 (1.117-2.618) | 1.819 (1.518-2.857) | ||||
| Mixed | 1.963 (1.396-2.762) | 1.279 (0.896-1.825) | 2.448 (1.554-3.857) | 1.805 (1.052-3.096) | ||||
| Histologic type | 0.344 | 0.871 | ||||||
| Differentiated | Reference | Not included | Reference | Not included | ||||
| Undifferentiated | 1.176 (0.841-1.646) | 0.966 (0.635-1.469) | ||||||
| Lymphovascular invasion | 0.006 | 0.832 | 0.102 | |||||
| No | Reference | Reference | Reference | Not included | ||||
| Yes | 1.422 (1.108-1.825) | 1.029 (0.793-1.334) | 1.324 (0.945-1.854) | |||||
| Adjuvant chemotherapy | < 0.001 | 0.003 | 0.001 | 0.022 | ||||
| No | Reference | Reference | Reference | Reference | ||||
| Yes | 0.626 (0.481-0.814) | 0.664 (0.508-0.868) | 0.551 (0.385-0.787) | 0.650 (0.450-0.939) | ||||
To confirm whether the BPM score has more benefits than using only one marker, we compared their areas under the curves (AUC) using ROC analysis in the entire cohort. The BPM score exhibited a higher prognostic accuracy (0.680) than each individual marker, including albumin (0.640, P < 0.001), LMR (0.580, P < 0.001), NLR (0.546, P < 0.001), CEA (0.578, P < 0.001), and CA 19-9 (0.565, P < 0.001). Furthermore, the time-dependent ROC curves calculated at different time points for BPM score (as a dichotomous variable) and other scoring systems [including PNI, CONUT score, and modified systemic inflammation score (mSIS)[32]] clearly showed that the BPM score was continuously superior to the other scoring systems at each time point (Figure 2C).
Next, we used multivariate Cox models by means of RCS to further analyze the relationship between BPM score and OS with adjustment for the clinicopathologic factors (including age, BMI, tumor stage, tumor size, lymphovascular invasion, and adjuvant chemotherapy, which reached statistical significance in the univariate analysis). A continuous linear association between BPM score and OS was observed in the analysis of the developing, validation, and entire cohorts (Supplementary Figure 2).
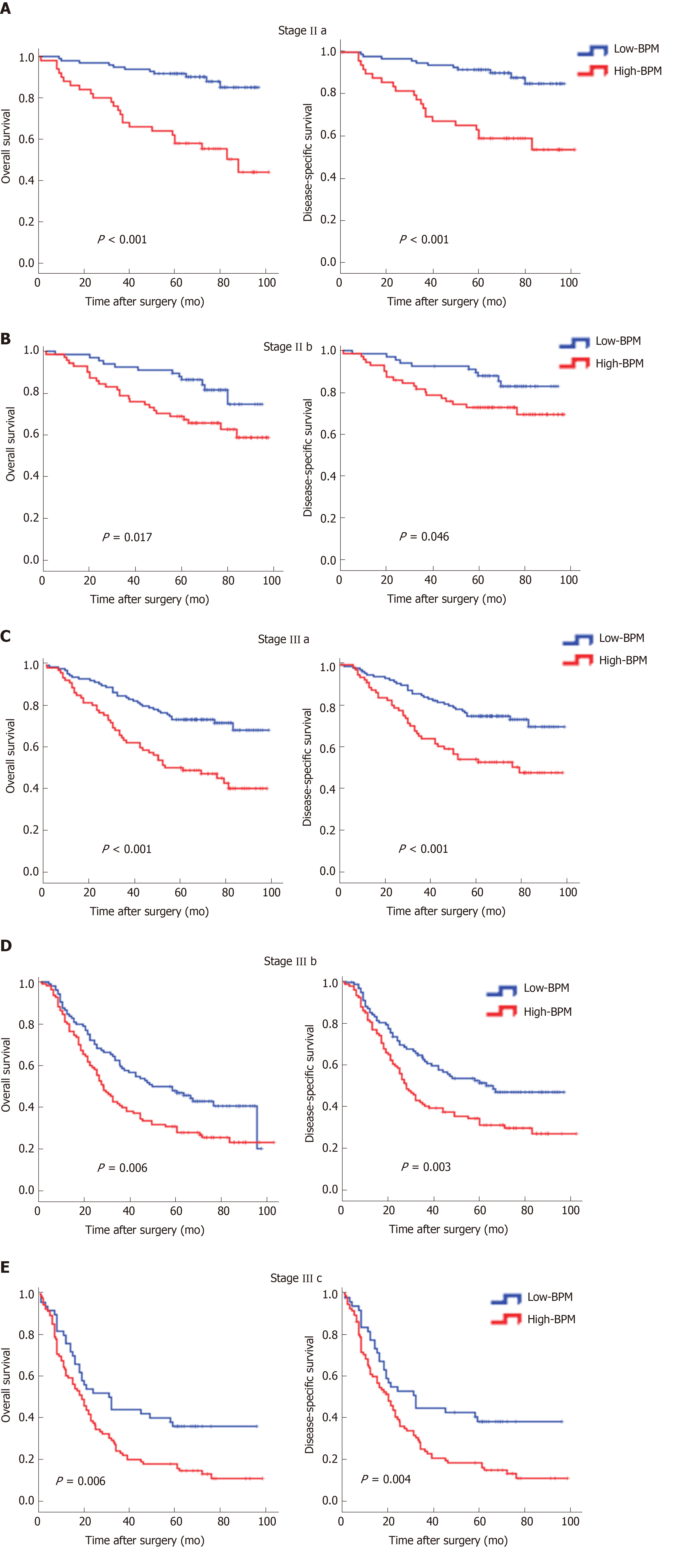
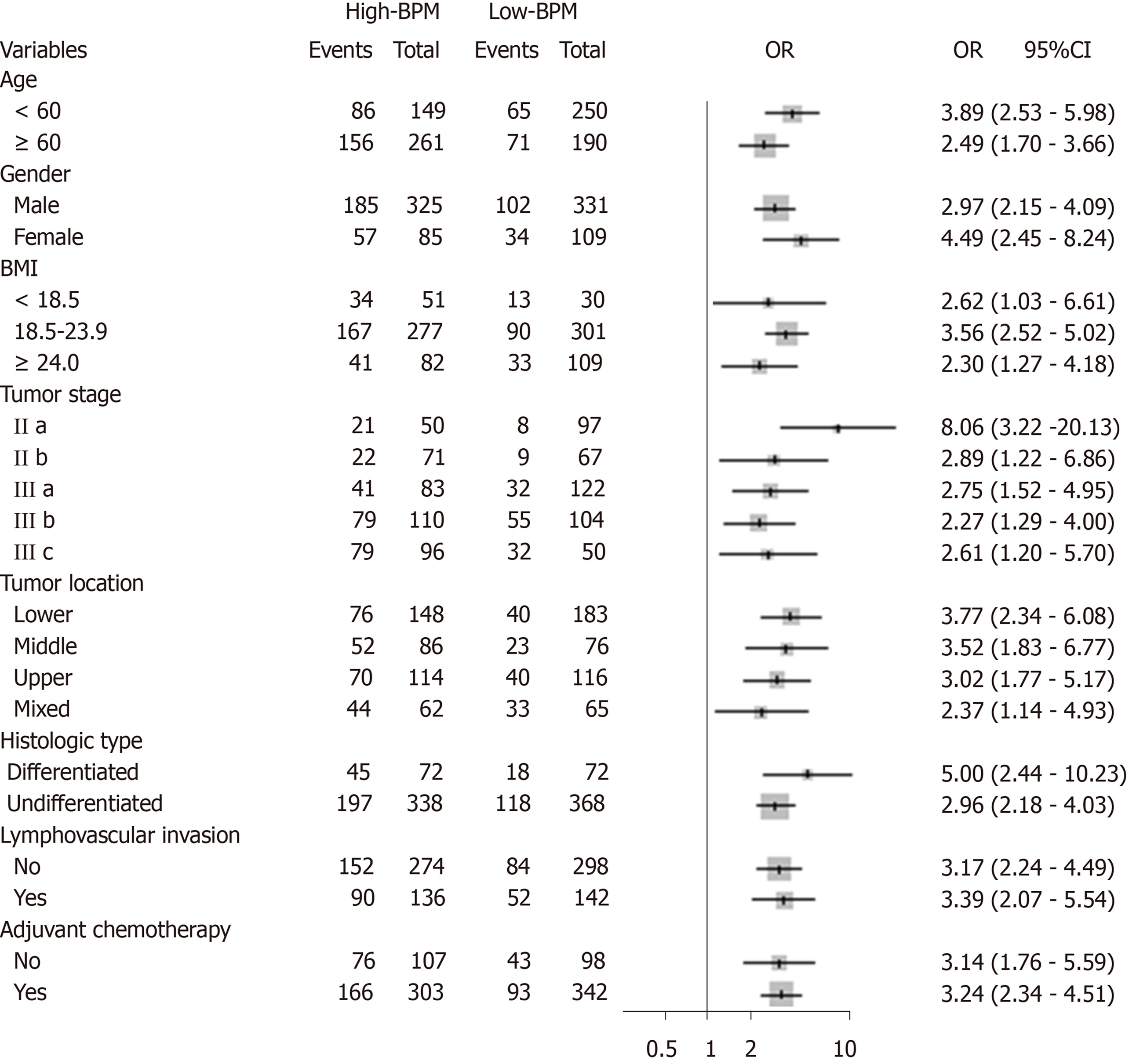
We performed stratified analyses of GC patients in the entire cohort. High-BPM patients with stages IIa, IIb, IIIa, IIIb, and IIIc disease had a shorter OS and DSS compared with low-BPM patients (log-rank P < 0.05 for all, Figure 3). A forest plot based on 5-year OS was further established, and the results confirmed that high-BPM patients had a poor prognosis among each subgroup (Figure 4). Furthermore, we assigned patients to an adjuvant chemotherapy (AC) or a non-AC group based on the receipt of postoperative adjuvant chemotherapy and then performed the Kaplan-Meier survival analysis. The results revealed that high-BPM patients had a poorer prognosis compared with low-BPM patients in both the AC and non-AC groups (log-rank P < 0.001 for all, Supplementary Figure 3).
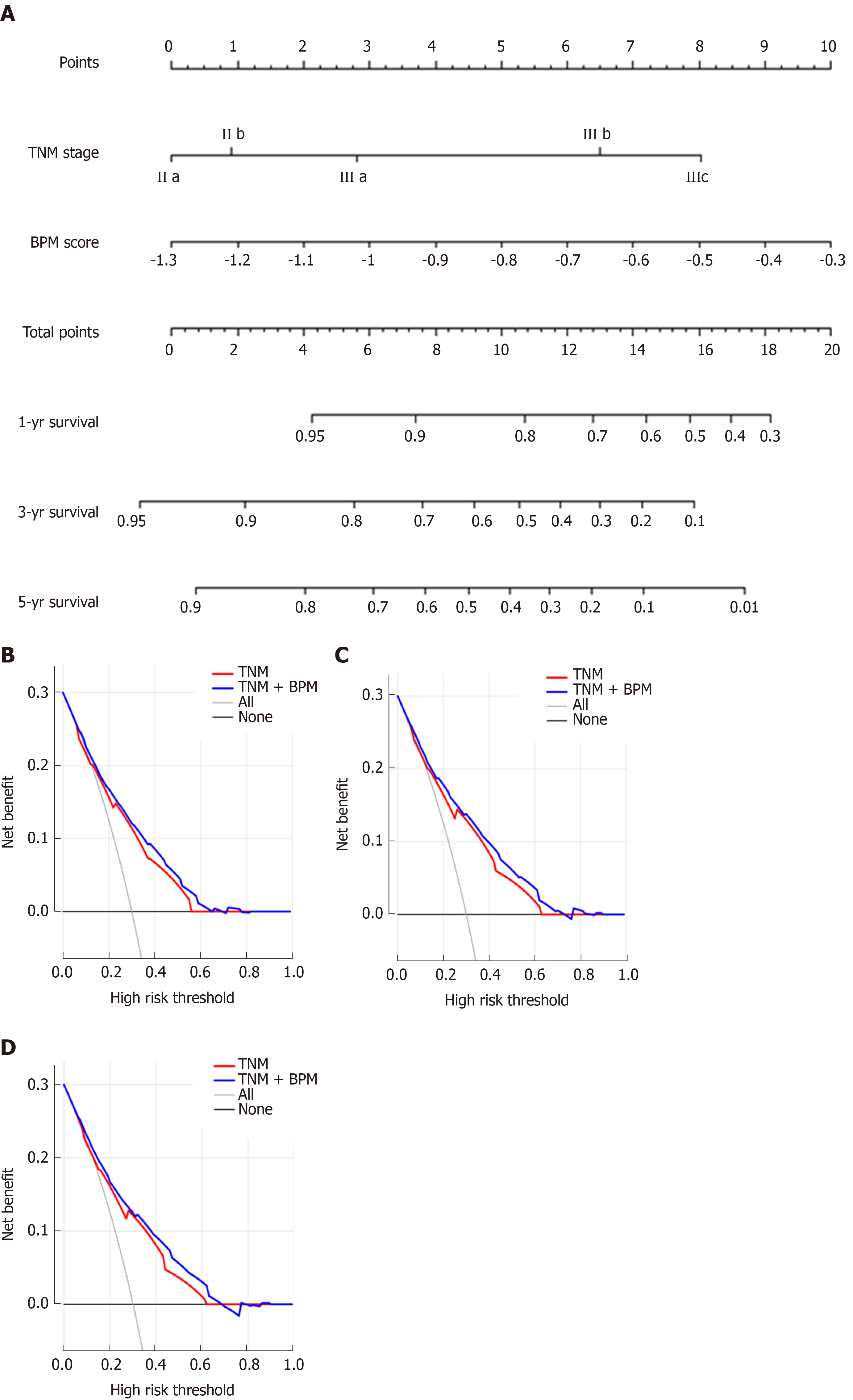
To make individualized prediction of the survival probability in stages II and III GC patients, we established a nomogram that combined the BPM score and TNM stage in the entire cohort (Figure 5A). The Harrell’s C-statistic of this nomogram was significantly higher than that of TNM stage alone (0.727 vs 0.697, P < 0.001). The Hosmer-Lemeshow test showed that the model had good fit for 1-, 3-, and 5-year OS (P = 0.979, P = 0.853, and P = 0.655, respectively). The decision curve analysis revealed that TNM + BPM had better clinical utility than TNM stage alone at 1, 3, and 5 years (Figure 5B-D).
A growing body of evidence has demonstrated the potential of some laboratory BPM as prognostic markers to predict the oncologic outcomes in human mali-gnancies[5-14,17-18,21-22]. However, how to comprehensively use these predictors to predict the long-term prognosis of patients with GC remains unclear. So far, researchers have established PNI, mGPS, CONUT, and mSIS as scoring systems that reflect immunological and nutritional statuses to more accurately predict clinical outcomes and to administer individualized treatment[9-11,32]. However, the clinical usefulness of these scoring systems is still controversial. Liu et al[15] found that low PNI was marginally associated with 5-year OS in patients with stage III GC, but the prognostic value was not significant in stages I and II GC. A retrospective study of 416 patients reported that a high CONUT score was strongly associated with 5-year OS in both pStage I and pStage II patients but not in pStage III patients[11]. Similarly, previous studies showed that the utility of mGPS and mSIS in patients with GC was also controversial[16,32]. A possible explanation was that these scores did not include all valuable BPM for comprehensive analysis, resulting in their limited prognostic value.
In addition, in the pursuit of simplicity, some of the current scoring systems handle the BPM in a categorical manner when they are, in fact, generated as continuous variables. It was reported that dichotomisation of continuous variables in a multiple regression procedure may be associated with considerable loss of statistical power and introduction of bias[33,34]. However, we should acknowledge that considering an indicator as a continuous variable does not necessarily improve the prediction accuracy. At the same time, it may not be easy to use in the clinical decision-making process[35]. Therefore, in the present study, we separately calculated and compared the AIC score and Harrell’s C-statistic of each blood parameter in the dichotomous and continuous forms to determine which form was better for this indicator. It was found that only the continuous form of hemoglobin, albumin, fibrinogen, and CEA had smaller AIC scores than their dichotomous form, with significantly stronger discriminative power. So, we further analyzed them as continuous variables, while the remaining indicators (including cholesterol, LMR, NLR, PLR, and CA 19-9) were valued as 0 or 1 according to their cut-off values.
The prognostic influence of perioperative laboratory BPM may be difficult to discern in patients with stage I GC, who experience fewer cases of DSS, and in patients with stage IV GC, who experience extremely lower survival rates[5]. Therefore, the present study enrolled stage II-III GC patients based on a prospectively collected database of a high-volume center in order to provide a novel prognostic model for these patients with resectable locally advanced GC. Using LASSO Cox regression analysis, we established the BPM score based on five objectively measured, easily obtained BPM covering inflammatory, nutritional, and tumor markers: Albumin, LMR, NLR, CEA, and CA 19-9. To our knowledge, the link of malnutrition and systemic inflammation with carcinogenesis, tumor growth, and cancer progression has been demonstrated in several cancers, including GC[5-16,36-39]. In addition, tumor markers, as circulating substances that can be measured quantitatively, have a causal relationship with malignant diseases and are currently applied in prognosis prediction of GC patients[17,18,40]. A comparison of the clinicopathological variables between high-BPM and low-BPM patients revealed that a high BPM score was associated with older age, higher tumor stage, and larger tumor size. Multivariate analysis showed that the BPM score was an independent risk factor, had a stable prognostic ability among patients in each subgroup, and had better predictive accuracy than the traditional scoring systems. More importantly, the BPM score proved to be a supplement to the TNM staging system. Therefore, we recommend the use of the TNM + BPM scoring system incorporating preoperative albumin, LMR, NLR, CEA, and CA 19-9 levels for multimodality treatment planning and risk stratification in prospective studies.
In this study, we found that the BPM score, a novel scoring system based on five BPM, was predictive of long-term outcomes in stage II-III GC patients. This tool may have important uses in prognostic stratification, therapeutic intervention, and postoperative surveillance strategies, especially after incorporating TNM stage. For example, patients with a high BPM score will be recommended to receive postoperative multimodality treatment, such as chemotherapy, immunotherapy, and targeted therapy. And more regular follow-up schedule will be offered to early detect recurrence, which may provide survival benefit[41]. A patient example of how the model can be used in clinical practice is provided in the Supplementary Materials.
This study has some limitations. First, as a retrospective, single-institution study, it may have been subject to selection bias. However, all data were derived from a prospectively collected database of a high-volume center, which can reduce this bias. Second, because C-reactive protein and prealbumin were not routinely tested before surgery in our institution, we did not include these potentially valuable biomarkers. Third, the validity and generalizability of the BPM score need to be established by testing it in other locations and groups of patients[42].
Increasing numbers of laboratory blood parameters (BPM) have been reported to greatly affect the long-term outcomes of gastric cancer (GC) patients. However, the existing prognostic models, including the prognostic nutritional index, the modified Glasgow prognostic score, and the controlling nutritional status score, contain limited BPM and provide inadequate prognostic information.
We hypothesized that a new scoring system, based on all available BPM, would provide more accurate prognostic information in patients with GC.
The present study aimed to construct a new prognostic tool, based on all the prognostic BPM, to achieve more accurate prognosis prediction for GC.
We retrospectively assessed 850 consecutive patients who underwent curative resection for stage II-III GC from January 2010 to April 2013. The patients were classified into developing (n = 567) and validation (n = 283) cohorts using computer-generated random numbers. A scoring system, namely BPM score, was then constructed using the least absolute shrinkage and selection operator (LASSO) Cox regression model. A nomogram consisting of the BPM score and tumor-lymph node-metastasis (TNM) stage was created. The discrimination and calibration of the nomogram were evaluated via Harrell’s C-statistic and the Hosmer-Lemeshow test.
Using the LASSO model, we established the BPM score based on five BPM: Albumin, lymphocyte-to-monocyte ratio, neutrophil-to-lymphocyte ratio, carcinoembryonic antigen, and carbohydrate antigen 19-9. The BPM scores were divided into high-BPM and low-BPM groups based on a cut-off value of -0.93. High-BPM patients were significantly older and had more advanced, larger tumors. In the developing cohort, significant differences were found in 5-year overall survival (OS) and 5-year disease-specific survival between the high-BPM and low-BPM patients. Similar results were found in the validation group. Multivariable analysis showed that the BPM score was an independent predictor of OS. High-BPM patients had a poorer 5-year OS for each subgroup. Furthermore, a nomogram that combined the BPM score and TNM stage had significantly better prognostic value compared with TNM stage alone.
The BPM score provides more accurate prognosis prediction in stage II-III GC patients and is an effective complement to the TNM staging system. Therefore, we recommend the use of the BPM score for multimodality treatment planning and risk stratification in the future clinical work.
Although we confirmed that the BPM score can accurately predict the outcomes in stage II-III GC patients, the efficiency of this novel scoring system need to be investigated in a prospective multicenter trial.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Merrett ND, Silsirivanit A, Young CJ S-Editor: Tang JZ L-Editor: Wang TQ E-Editor: Ma YJ
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56585] [Article Influence: 7073.1] [Reference Citation Analysis (134)] |
| 2. | Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388:2654-2664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1282] [Cited by in RCA: 1503] [Article Influence: 150.3] [Reference Citation Analysis (0)] |
| 3. | Sasako M, Inoue M, Lin JT, Khor C, Yang HK, Ohtsu A. Gastric Cancer Working Group report. Jpn J Clin Oncol. 2010;40:i28-i37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 4. | Noh SH, Park SR, Yang HK, Chung HC, Chung IJ, Kim SW, Kim HH, Choi JH, Kim HK, Yu W, Lee JI, Shin DB, Ji J, Chen JS, Lim Y, Ha S, Bang YJ; CLASSIC trial investigators. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:1389-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 783] [Cited by in RCA: 794] [Article Influence: 66.2] [Reference Citation Analysis (1)] |
| 5. | Shen Q, Liu W, Quan H, Pan S, Li S, Zhou T, Ouyang Y, Xiao H. Prealbumin and lymphocyte-based prognostic score, a new tool for predicting long-term survival after curative resection of stage II/III gastric cancer. Br J Nutr. 2018;120:1359-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Dolan RD, McSorley ST, Park JH, Watt DG, Roxburgh CS, Horgan PG, McMillan DC. The prognostic value of systemic inflammation in patients undergoing surgery for colon cancer: comparison of composite ratios and cumulative scores. Br J Cancer. 2018;119:40-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 113] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 7. | Hirahara N, Tajima Y, Fujii Y, Kaji S, Yamamoto T, Hyakudomi R, Taniura T, Miyazaki Y, Kishi T, Kawabata Y. Preoperative Prognostic Nutritional Index Predicts Long-Term Surgical Outcomes in Patients with Esophageal Squamous Cell Carcinoma. World J Surg. 2018;42:2199-2208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 8. | Harimoto N, Yoshizumi T, Inokuchi S, Itoh S, Adachi E, Ikeda Y, Uchiyama H, Utsunomiya T, Kajiyama K, Kimura K, Kishihara F, Sugimachi K, Tsujita E, Ninomiya M, Fukuzawa K, Maeda T, Shirabe K, Maehara Y. Prognostic Significance of Preoperative Controlling Nutritional Status (CONUT) Score in Patients Undergoing Hepatic Resection for Hepatocellular Carcinoma: A Multi-institutional Study. Ann Surg Oncol. 2018;25:3316-3323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 9. | Yang Y, Gao P, Song Y, Sun J, Chen X, Zhao J, Ma B, Wang Z. The prognostic nutritional index is a predictive indicator of prognosis and postoperative complications in gastric cancer: A meta-analysis. Eur J Surg Oncol. 2016;42:1176-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 198] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 10. | Nozoe T, Iguchi T, Egashira A, Adachi E, Matsukuma A, Ezaki T. Significance of modified Glasgow prognostic score as a useful indicator for prognosis of patients with gastric carcinoma. Am J Surg. 2011;201:186-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Kuroda D, Sawayama H, Kurashige J, Iwatsuki M, Eto T, Tokunaga R, Kitano Y, Yamamura K, Ouchi M, Nakamura K, Baba Y, Sakamoto Y, Yamashita Y, Yoshida N, Chikamoto A, Baba H. Controlling Nutritional Status (CONUT) score is a prognostic marker for gastric cancer patients after curative resection. Gastric Cancer. 2018;21:204-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 237] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 12. | Wang SC, Chou JF, Strong VE, Brennan MF, Capanu M, Coit DG. Pretreatment Neutrophil to Lymphocyte Ratio Independently Predicts Disease-specific Survival in Resectable Gastroesophageal Junction and Gastric Adenocarcinoma. Ann Surg. 2016;263:292-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 125] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 13. | Hsu JT, Wang CC, Le PH, Chen TH, Kuo CJ, Lin CJ, Chou WC, Yeh TS. Lymphocyte-to-monocyte ratios predict gastric cancer surgical outcomes. J Surg Res. 2016;202:284-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Gu X, Gao XS, Cui M, Xie M, Peng C, Bai Y, Guo W, Han L, Gu X, Xiong W. Clinicopathological and prognostic significance of platelet to lymphocyte ratio in patients with gastric cancer. Oncotarget. 2016;7:49878-49887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | Liu X, Qiu H, Kong P, Zhou Z, Sun X. Gastric cancer, nutritional status, and outcome. Onco Targets Ther. 2017;10:2107-2114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Wen J, Bedford M, Begum R, Mitchell H, Hodson J, Whiting J, Griffiths E. The value of inflammation based prognostic scores in patients undergoing surgical resection for oesophageal and gastric carcinoma. J Surg Oncol. 2018;117:1697-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 17. | Kochi M, Fujii M, Kanamori N, Kaiga T, Kawakami T, Aizaki K, Kasahara M, Mochizuki F, Kasakura Y, Yamagata M. Evaluation of serum CEA and CA19-9 levels as prognostic factors in patients with gastric cancer. Gastric Cancer. 2000;3:177-186. [PubMed] |
| 18. | Zhou YC, Zhao HJ, Shen LZ. Preoperative serum CEA and CA19-9 in gastric cancer--a single tertiary hospital study of 1,075 cases. Asian Pac J Cancer Prev. 2015;16:2685-2691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1575] [Cited by in RCA: 1950] [Article Influence: 216.7] [Reference Citation Analysis (1)] |
| 20. | Ajani JA, In H, Sano T, Gaspar LE, Erasmus JJ, Tang LH, Washington MK, Gerdes H, Wittekind CW, Mansfield PF, Rimmer C, Hofstetter WL, Kelsen D, Amin MB, Edge SB, Greene FL, Brierley JD. Stomach. Amin MB, Edge SB, Greene FL, Brierley JD. AJCC cancer staging manual. New York: Springer 2017; 203-350. |
| 21. | Liu X, Qiu H, Huang Y, Xu D, Li W, Li Y, Chen Y, Zhou Z, Sun X. Impact of preoperative anemia on outcomes in patients undergoing curative resection for gastric cancer: a single-institution retrospective analysis of 2163 Chinese patients. Cancer Med. 2018;7:360-369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Yu W, Wang Y, Shen B. An elevated preoperative plasma fibrinogen level is associated with poor overall survival in Chinese gastric cancer patients. Cancer Epidemiol. 2016;42:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252-7259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1947] [Cited by in RCA: 3072] [Article Influence: 146.3] [Reference Citation Analysis (8)] |
| 24. | Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1706] [Cited by in RCA: 2144] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 25. | Akaike H. A new look at the statistical model identification. IEEE Trans Autom Contr. CA. 1974;19:716-723. [DOI] [Full Text] |
| 26. | Jiang Y, Zhang Q, Hu Y, Li T, Yu J, Zhao L, Ye G, Deng H, Mou T, Cai S, Zhou Z, Liu H, Chen G, Li G, Qi X. ImmunoScore Signature: A Prognostic and Predictive Tool in Gastric Cancer. Ann Surg. 2018;267:504-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 356] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 27. | de Vries EM, Wang J, Williamson KD, Leeflang MM, Boonstra K, Weersma RK, Beuers U, Chapman RW, Geskus RB, Ponsioen CY. A novel prognostic model for transplant-free survival in primary sclerosing cholangitis. Gut. 2018;67:1864-1869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 28. | Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000;56:337-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1735] [Cited by in RCA: 2111] [Article Influence: 81.2] [Reference Citation Analysis (0)] |
| 29. | Harrell FE, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 99] [Reference Citation Analysis (0)] |
| 30. | Lemeshow S, Hosmer DW. A review of goodness of fit statistics for use in the development of logistic regression models. Am J Epidemiol. 1982;115:92-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1381] [Cited by in RCA: 1399] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 31. | Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26:565-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3515] [Cited by in RCA: 3797] [Article Influence: 189.9] [Reference Citation Analysis (2)] |
| 32. | Lin JX, Lin JP, Xie JW, Wang JB, Lu J, Chen QY, Cao LL, Lin M, Tu R, Zheng CH, Huang CM, Li P. Prognostic importance of the preoperative modified systemic inflammation score for patients with gastric cancer. Gastric Cancer. 2019;22:403-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 33. | Del Priore G, Zandieh P, Lee MJ. Treatment of continuous data as categoric variables in Obstetrics and Gynecology. Obstet Gynecol. 1997;89:351-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med. 2006;25:127-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1661] [Cited by in RCA: 1538] [Article Influence: 76.9] [Reference Citation Analysis (0)] |
| 35. | Amri R, Bordeianou LG, Sylla P, Berger DL. Preoperative carcinoembryonic antigen as an outcome predictor in colon cancer. J Surg Oncol. 2013;108:14-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Candido J, Hagemann T. Cancer-related inflammation. J Clin Immunol. 2013;33 Suppl 1:S79-S84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 534] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 37. | Mei Z, Liu Y, Liu C, Cui A, Liang Z, Wang G, Peng H, Cui L, Li C. Tumour-infiltrating inflammation and prognosis in colorectal cancer: systematic review and meta-analysis. Br J Cancer. 2014;110:1595-1605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 252] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 38. | Galdiero MR, Bonavita E, Barajon I, Garlanda C, Mantovani A, Jaillon S. Tumor associated macrophages and neutrophils in cancer. Immunobiology. 2013;218:1402-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 454] [Article Influence: 34.9] [Reference Citation Analysis (4)] |
| 39. | Van Cutsem E, Arends J. The causes and consequences of cancer-associated malnutrition. Eur J Oncol Nurs. 2005;9 Suppl 2:S51-S63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 404] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 40. | Lin JX, Wang W, Lin JP, Xie JW, Wang JB, Lu J, Chen QY, Cao LL, Lin M, Tu R, Zheng CH, Huang CM, Zhou ZW, Li P. Preoperative Tumor Markers Independently Predict Survival in Stage III Gastric Cancer Patients: Should We Include Tumor Markers in AJCC Staging? Ann Surg Oncol. 2018;25:2703-2712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 41. | Fujiya K, Tokunaga M, Makuuchi R, Nishiwaki N, Omori H, Takagi W, Hirata F, Hikage M, Tanizawa Y, Bando E, Kawamura T, Terashima M. Early detection of nonperitoneal recurrence may contribute to survival benefit after curative gastrectomy for gastric cancer. Gastric Cancer. 2017;20:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 42. | Justice AC, Covinsky KE, Berlin JA. Assessing the generalizability of prognostic information. Ann Intern Med. 1999;130:515-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 793] [Cited by in RCA: 824] [Article Influence: 30.5] [Reference Citation Analysis (0)] |