Published online Aug 28, 2019. doi: 10.3748/wjg.v25.i32.4580
Peer-review started: May 21, 2019
First decision: June 9, 2019
Revised: July 3, 2019
Accepted: July 19, 2019
Article in press: July 19, 2019
Published online: August 28, 2019
Processing time: 99 Days and 16.8 Hours
Chronic delta hepatitis is the most severe form of viral hepatitis affecting nearly 65 million people worldwide. Individuals with this devastating illness are at higher risk for developing cirrhosis and hepatocellular carcinoma. Delta virus is a defective RNA virus that requires hepatitis B surface antigen for propagation in humans. Infection can occur in the form of a co-infection with hepatitis B, which can be self-limiting, vs superinfection in a patient with established hepatitis B infection, which often leads to chronicity in majority of cases. Current noninvasive tools to assess for advanced liver disease have limited utility in delta hepatitis. Guidelines recommend treatment with pegylated interferon, but this is limited to patients with compensated disease and is efficacious in about 30% of those treated. Due to limited treatment options, novel agents are being investigated and include entry, assembly and export inhibitors of viral particles in addition to stimulators of the host immune response. Future clinical trials should take into consideration the interaction of hepatitis B and hepatitis D as suppression of one virus can lead to the activation of the other. Also, surrogate markers of treatment efficacy have been proposed.
Core tip: Delta hepatitis is a progressive disease affecting millions worldwide. Current treatment options have limited efficacy. This review focuses on the history of interferon therapy, novel therapies that have been developed and future treatment options for a possible functional cure.
- Citation: Gilman C, Heller T, Koh C. Chronic hepatitis delta: A state-of-the-art review and new therapies. World J Gastroenterol 2019; 25(32): 4580-4597
- URL: https://www.wjgnet.com/1007-9327/full/v25/i32/4580.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i32.4580
Hepatitis D virus (HDV) infection was initially identified in 1977 in Italy by Dr. Mario Rizzetto and colleagues who noted a severe hepatitis in individuals thought to be mono-infected with hepatitis B virus (HBV). Initial immunofluorescence studies led to the finding of a novel antigen which was thought to be a new biomarker for HBV[1]. Serum analysis of these affected patients showed that they developed an antibody to a newly identified antigen which was subsequently termed the delta antigen (HDAg). In the early 1980s, collaborative studies in chimpanzees revealed more information regarding this novel finding. Hepatitis B susceptible and immune animals were inoculated with hepatitis B surface antigen (HBsAg) positive sera from patients with HDAg and only susceptible animals developed HBV and delta markers. Also, synthesis of delta markers caused the synthesis of HBV gene products to be diminished. This study importantly highlighted that HDV was transmissible, requires HBV for its own replicative cycle and interferes with HBV replication[2]. Over the past 40+ years since the initial identification of HDV, chronic HDV infection has come to be known as the most severe form of viral hepatitis[3] which leads to higher rates of cirrhosis[4] and hepatocellular carcinoma (HCC)[5] compared to HBV mono-infection[6-8] and chronic hepatitis C (HCV) infection[9]. To date, there is no treatment approved by the United States Food and Drug Administration (FDA) for chronic HDV infection. Current treatment with interferon alpha, as recommended by professional societies, has limited efficacy thereby leaving liver transplant a final viable option. However, new and promising treatment options are being investigated in clinical trials.
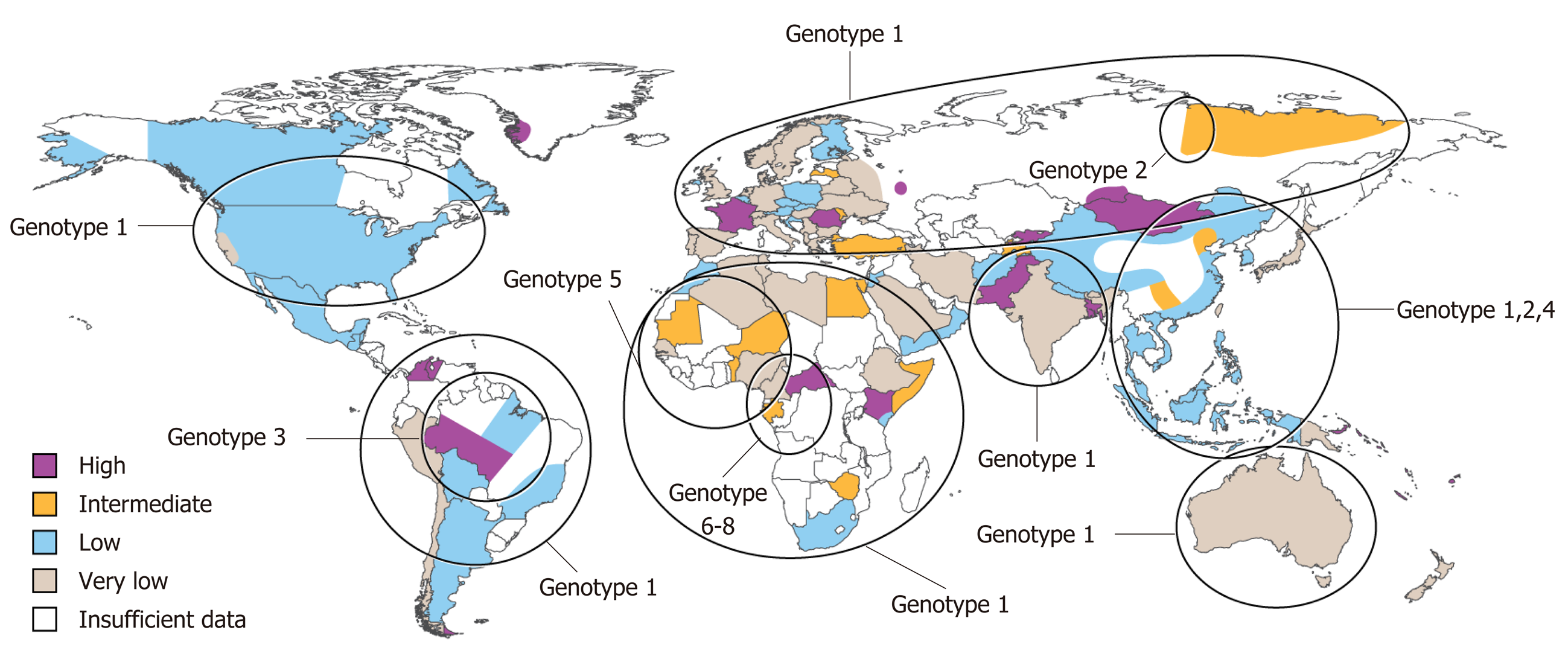
Approximately 587 million people are infected with chronic HBV infection with 62-72 million chronically infected with HDV[10]. Eight HDV genotypes have been identified to date with genotype 1 being widely distributed, 2 and 4 predominantly in Asia, 3 localized to the Amazon Basin and 5-8 of African lineage (Figure 1)[11,12]. Prevalence rates vary widely with high rates noted in Africa and South America (Table 1). Retrospective studies have noted that HDV infection is more common in men than women[13,14]. High risk populations include individuals from endemic areas, intravenous drug users, men who have sex with men, individuals with human immunodeficiency virus (HIV) or HCV and patients with multiple sexual partners[5,13,15,16]. In a retrospective analysis in Spain, there was a reduction of new cases of acute HBV and HDV infection between the time periods 1983-1995 and 1996-2008 and it is thought that policy changes during the 1990s for HBV vaccination to include all newborns and adolescents and not just high risk groups played a substantial role[13,17]. Immigration and sexual transmission are now the leading risk factors for HDV infection[17].
| Geographic area | Prevalence (%) | Ref. |
| HDV ab | HDV RNA | ||
| Africa | ||
| Gabon | 63-70.6 | X | [137] |
| Nigeria | 4.9 | 9 | [138] |
| Sub-Saharan | 1.3-50 | 21.4-100 | [139] |
| Tunisia | 2 | 33.3 | [140] |
| Asia | ||
| Japan | 21.1 | X | [141] |
| Mongolia | 74.4 | 97.9 | [9] |
| 90.7 | 91.4 | [142] | |
| Pacific | ||
| Kiribati | X | 37 | [143] |
| North America | ||
| United States | 42 | X | [144] |
| South America | ||
| Brazil | 1.8 | X | [145] |
| 34.4 | X | [146] | |
| Europe | ||
| Belgium | 3.7-5.5 | X | [147] |
| Greece | 4.2 | X | [148] |
| Italy | 67 | 93 | [149] |
| 23.4 | X | [150] | |
| London | 4.5 | 20.2 | [151] |
| Romania | 23.1 | 71.0 | [8] |
| Spain | 4 | X | [152] |
| 8.2 | X | [17] |
In the United States, testing for HDV infection has been poor[4,15,18]. In a retrospective study of 1191 patients in northern California from 1989-2007, only 499 (42%) patients were tested for HDV (HDV ab or HDAg or both)[4]. Of those tested, 42 (8%) patients were confirmed to be HDV infected [HDV ab (n = 29), HDAg (n = 6), both (n = 7)]. Interestingly, 67% of the HDV patients were diagnosed with cirrhosis compared to only 17% of HBV monoinfected patients tested for HDV and 22% of the total HBV monoinfected cohort (including patients not tested for HDV). In a retrospective study of patients in the Veterans Affairs medical system from 1999-2013, 2008 (7.8%) of 25,603 HBsAg positive patients were tested for HDV and 73 (3.6%) had a positive HDV ab[5]. In a cross-sectional study of electronic medical records from 1994-2014, 121 (12%) of 1007 HBsAg positive were tested for HDV and 4 (3.3%) had a positive HDV ab[18]. These studies highlight the need for HDV screening in all patients with HBV in congruence with the Asian Pacific Association for the Study of the Liver (APASL)[19] and European Association for the Study of the Liver (EASL)[20] guidelines for education, possible need for treatment and prevention of transmission.
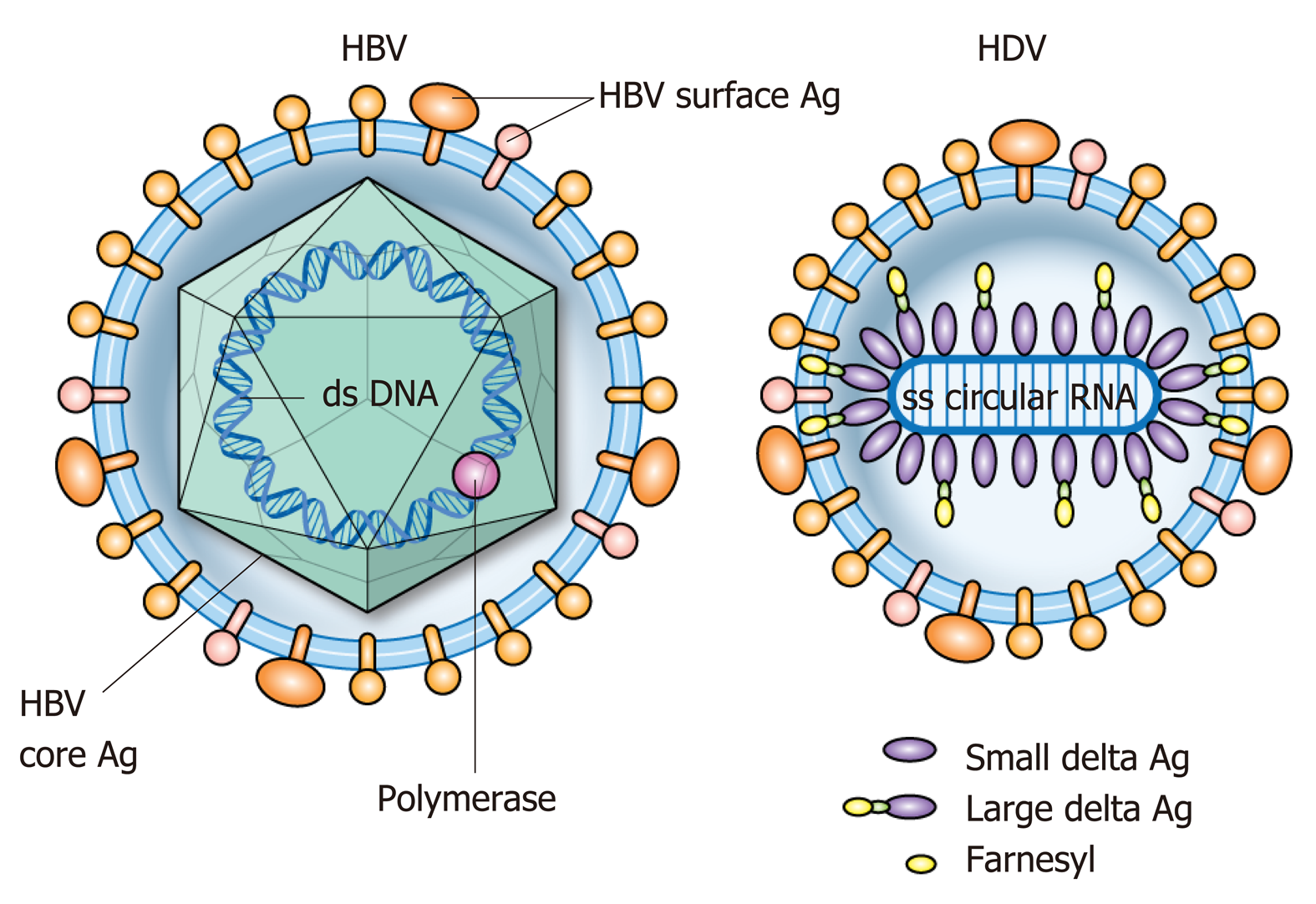
HDV is the smallest known human RNA virus and is a defective RNA virus which requires HBsAg[21]. It is about 36 nm in diameter and consists of a circular single stranded RNA (about 1700 BP)[22], that folds into a rod like structure[23] due to self-complementarity[24], and HDAg thus forming the HDV ribonucleoprotein (RNP)[25] surrounded by the HBsAg envelope (Figure 2)[21,26,27]. Entry of the HDV RNP into hepatocytes occurs through binding of the sodium taurocholate co-transporting polypeptide (NTCP) receptor[28,29] through the preS1 region of the large HBsAg. Once in the hepatocyte, transport to the nucleus is mediated by HDAg[25,30] through a nuclear localization signal[31-34] and possibly through phosphorylation[35], acetylation[36] and methylation[37] of HDAg. Replication occurs through the host RNA poly-merase[38-41] in a double-rolling cycle[22]. Rolling cycle replication allows for transcription of full-length antigenomic RNA which is used to generate genomic RNA. The antigenomic RNA contains the sequence for HDAg[37,42], which undergoes RNA editing and self-cleavage[43,44], and translation occurs in the endoplasmic reticulum. HDAg is present in two forms based on RNA editing[44] and are referred to as small (SHDAg, 195 amino acids, 24 kDa) and large (LHDAg, 214 amino acids, 27 kDa) delta antigen[42]. This editing process adds additional amino acids to the C-terminus of LHDAg[45]. Replication is promoted by SHDAg[46,47]. LHDAg suppresses SHDAg[47], contains an isoprenylation motif and nuclear export signal[48,49] and promotes assembly of the virus[46,50-52]. Genomic RNA is exported to the cytoplasm through signaling in HDV RNA[34]. LHDAg promotes prenylation and association with HBsAg[53] generating a viral particle. SHDAg alone is insufficient for virion formation and it is thought that the LHDAg acts as a bridge between HBsAg and SHDAg and HDV RNA[25,34,51,52,54,55] (Figure 3).
Studies have shown that there is an interaction between HBV and HDV though the exact mechanism has not been elucidated. In a longitudinal analysis of 33 chronic HDV patients, HDV was the predominant replicating virus in 54.5% of cases, whereas HBV was the predominant replicative virus in 30.3% of cases and both were codominant 15.2% of cases[56]. Compared to HBV mono-infection, it has been reported that HBV/HDV infection leads to more severe liver damage[8,57-59] including extensive necrosis[3,60-62] and patients develop cirrhosis at a faster rate[8]. Also, though suppressed, low level HBV replication may be capable of causing liver damage[63]. In chimpanzees, damage by HBV is immune mediated whereas in HDV it is cytopathic[64] or possibly cytotoxic as evidenced with a predominance of macrophages in areas of necrosis[62]. An in vitro model to determine the effect of HDAg expression in HeLa and HepG2 cells showed that HDAg is directly cytotoxic[65]. In a study analyzing cytokine responses in patients with chronic HDV infection before and during interferon alpha (IFN-α) treatment, it was found that interleukin (IL)-2, interferon gamma (IFN-γ), interferon-inducible protein-10 and IL-10 responses were detectable and that these responses declined during treatment with interferon[66]. In a humanized mouse model evaluating the antiviral state of human hepatocytes in the setting of HBV/HDV co-infection compared to HBV mono-infection, the JAK STAT pathway was triggered by HDV infection[67]. Natural killer cells were studied in chronic untreated HDV patients[68]. Elevated STAT1 levels were observed in chronic HDV patients and treatment with pegylated interferon alpha (peg-IFN-α) further augmented this elevation and reduced induction of STAT4 signaling with expected decrease in IFN-γ. An integrated genomic approach of liver specimens from HDV-HCC (n = 5) and non-HCC HDV cirrhosis (n = 7) at the time of liver transplantation showed that HDV-HCC tissue exhibited upregulated gene expression of cell cycle pathways important for DNA replication, damage and repair and the pattern is disease specific[69]. A more recent study suggests that the pathways to damage of hepatocytes may be due to the imbalance in type 1 and 2 immunity, activated HDV-specific CD8+ T cells and presence of escape variants[70,71].
It is important to have a high clinical suspicion for HDV especially in high risk patient populations and HBsAg positive individuals with high alanine aminotransferase (ALT) and low or undetectable HBV DNA levels[5,63,72]. The recommended screening test is anti-HDV antibodies with follow up HDV RNA testing if screening is positive[15]. The clinical presentation of HDV can be variable and patients can be asymptomatic with mild enzyme elevation vs fulminant hepatic failure. HDV can present as a co-infection vs superinfection with HBV. The main serological difference between them is positive anti-HBc IgM in co-infected individuals[64,73]. Co-infection occurs with acute infection of both viruses. A biphasic pattern of ALT and bilirubin can be observed[74]. Clinical course is typically benign, similar to acute HBV infection, spontaneous recovery occurs in up to 90% of individuals and progression to chronicity occurs in 2-8% of individuals[13,73,75,76]. Superinfection occurs in individuals with chronic HBV infection who are later infected with HDV. Spontaneous recovery occurs in 10% of individuals with progression to chronicity in 90%-100% of individuals[13,73,75]. Chronic HDV infection leads to cirrhosis in about 80% of individuals in 5-10 years[4,77].
Disease progression is striking in patients with chronic HDV infection[57] and scoring systems in addition to noninvasive markers of fibrosis have been evaluated for predicting damage and clinical outcomes. The baseline-event-anticipation score (BEA score) resulted from a 16-year study of 75 HBsAg-anti-HDV-positive patients with delta hepatitis and validated in two independent cohorts[78]. Age, sex, region of origin, bilirubin, platelets and international normalized ratio (INR) and based were included and allocated points and the summation resulted in mild, moderate and high risk groups[78].
The Delta Fibrosis Score (DFS) was developed in a cohort of 100 HDV RNA positive patients in Europe[79]. Eight non-invasive fibrosis scores were evaluated (aspartate aminotransferase (AST) to ALT ratio (AAR), AST to platelet ratio index (APRI), fibrosis-4 score (FIB-4), Non-alcoholic fatty liver disease (NAFLD) score, BARD score, European Liver Fibrosis (ELF) score, SHASTA index, Amino-terminal propeptide of type III collagen) and compared to liver biopsy using the Ishak scoring system. F3-F4 indicated advanced fibrosis and F5-F6 indicated cirrhosis. Goal AUROC was > 0.8, sensitivity > 80% and positive predictive value (PPV) > 90%. None of the scores met criteria even after adapting cut-off values to optimize for chronic HDV infection. The ELF score did meet criteria of sensitivity and PPV but was determined to not be cost effective especially for endemic regions with limited sources of funding. Thus, the DFS was developed and components included older age, elevated gamma glutamyl transferase (GGT), low albumin and low cholinesterase with an AUROC of 0.87, sensitivity of 85% and PPV of 93%.
The utility of serum fibrosis markers in HDV were also explored in a retrospective, cross-sectional study of 1003 patients with chronic hepatitis B (n = 240), C (n = 701) and D (n = 62) who underwent liver biopsy[61]. Markers included FIB-4, AAR, age-platelet index (API), APRI and Hui score. Advanced fibrosis was defined as Ishak ≥ 4 and cirrhosis as Ishak 6. FIB-4 appeared to perform the best in this comparison with an AUROC of 0.70, although the performance was substantially better in the comparative HBV and HCV cohorts. Current therapy for chronic HDV infection is limited to interferon alpha. Identifying patients who warrant treatment highlights the need for specific HDV scoring systems and fibrosis markers.
Currently there is no treatment approved by the United States FDA for chronic HDV infection. Current guidelines of the American Association for the Study of Liver Diseases (AASLD), Asian Pacific Association for the Study of the Liver (APASL) and the European Association for the Study of the Liver (EASL) recommend peg-IFN-α for 12 mo[15,19,20]. IFN-α, peg-IFN-α and combination therapy with nucleos(t)ide analogues (NA) have been studied in chronic HDV infection but therapy with IFN is largely limited to patients who are non-cirrhotic. Novel treatment options have been evaluated recently and four areas of interest include drugs that target entry, assembly and release of viral particles in addition to treatment that activates the immune host response.
As IFN was thought to be able to inhibit viral nucleic acid replication, the tolerability and efficacy of IFN-α in chronic HDV patients was studied. Sixty one patients were randomized to receive IFN-α-2b three times a week (5 MU/m2 for 4 mo, then 3 MU/m2 for 8 mo, n = 31) vs no treatment (n = 30)[80]. A complete biochemical response was defined as the normalization of ALT or decrease of 50% from baseline and was met in 42%, 26% and 3% of treated patients at 4, 12 and 24 mo, respectively vs 7%, 7% and 0% at the same timepoints in the control group. A complete virological response was defined as negative HDV RNA and was not met in either group. A decrease in histologic portal inflammation was observed though not significant.
Forty two patients with chronic HDV infection were randomly assigned to receive either 9 million (high dose, n = 14) or 3 million units (low dose, n = 14) of recombinant IFN-α-2a three times a week for 48 wk or no treatment (control, n = 13)[81]. Biochemical response was defined as normal ALT, virologic response as the absence of HDV RNA and complete response as normal ALT and absence of HDV RNA at the end of treatment. ALT became normal in 71%, 29% (P = 0.029 compared to high) and 8% (P = 0.001 compared to high) of patients in the high, low and control cohorts at 48 wk, respectively. HDV RNA was not detectable in 71%, 36% and 0% of patients in the high, low and control cohorts at 48 wk, respectively. Complete response occurred in 50%, 21% and 0% of patients in the high, low and control cohorts, respectively. Biochemical response was maintained in 50% of the patients whose ALT normalized in the high dose group and was persistent at 12 years[82]. Virological response was not sustained in any cohort but in the high dose group, there was a significant decrease in HDV RNA at the end of treatment compared with baseline (P = 0.009) and this decrease was sustained during long term follow up and two patients were able to clear HBsAg. Liver histology significantly improved in the high dose group as well in terms of interface hepatitis, lobular necrosis, portal inflammation and fibrosis stage after a mean of 11.5 years. In 4 patients in the high dose group who maintained a sustained biochemical response who had cirrhosis initially, fibrosis was absent in the last liver biopsy. Overall, long-term survival was significantly longer in the high-dose group than in the low-dose group (P = 0.019) and untreated controls (P = 0.003).
The safety and efficacy of peg-IFN-α-2b 1.5 μg/kg per week subcutaneous (SC) for 12 mo was evaluated in 14 patients with chronic HDV infection[83]. The primary endpoint was virological response with undetectable HDV RNA at the end of treatment and was met in 57% of patients and 43% of patients achieved sustained virological response (undetectable HDV RNA 6 months after end of treatment). Biochemical response was normal ALT levels at the end of treatment and was met in 36% of patients and 57% of patients achieved a sustained biochemical response (normal ALT 6 mo after end of treatment). One patient cleared HBsAg in the sustained responder group. Treatment withdrawal was not needed but dose reduction to 1 μg/kg per week occurred in 5 patients due to leukopenia. This preliminary study indicated the safety and efficacy of peg-IFN-α-2b and additional studies have been completed.
In a pilot study of ribavirin treatment in 7 patients with HDV, an oral dose of 15mg/kg daily of ribavirin was administered for 16 wk[84]. Complete biochemical response was defined as normal ALT and a complete virological response as negative serum HDV RNA. Biochemical response was not met. Significant virological response was not observed by the end of follow up and at 12 mo post-treatment. Peg-IFN-α-2b at a dose of 1.5 μg/kg for 48 wk in 2 out of 12 patients resulted in undetectable HDV RNA and normal of ALT by the end of follow up and at 24 wk post-treatment[85]. This same dose of peg-IFN-α-2b was assessed as monotherapy or in combination with ribavirin (800 mg oral daily) for 48 wk with maintenance therapy of peg-IFN-α-2b for 24 wk[86]. The primary and secondary endpoints were suppression or clearance of HDV RNA and normal ALT at end of treatment or at the end of 6 month follow up, respectively. Though not significant, the prolonged course of peg-IFN led to HDV RNA clearance and a sustained virological response in about 20% of patients in the monotherapy group. The addition of ribavarin to peg-IFN did not demonstrate added anti-HDV effects. Extending peg-IFN-α-2b therapy from 12 to 24 mo resulted in no significant advantage in virological and biochemical change[87]. Five-year therapy with peg-IFN-α-2a showed unsatisfactory results[88]. Based on these and other landmark studies, the consensus of current guidelines is treatment with peg-IFN-α for 12 mo (12-18 mo, APASL) in patients with compensated liver disease (EASL) with consideration of nucleos(t)ide analogue therapy with ongoing HBV DNA replication (AASLD, EASL) and referral to specialized centers for experimental therapies given limited efficacy of peg-IFN-α (AASLD)[15,19,20].
The utility of HBV nucleos(t)ide (NA) analogues as monotherapy or in combination with IFN-α therapy has been explored in chronic HDV infected patients. The efficacy of oral Lamivudine on HDV RNA, ALT, liver histology and HBsAg seroconversion was evaluated in a multicenter randomized-controlled study. Thirty one patients with chronic HDV were randomized to receive Lamivudine 100mg daily or placebo for 52 weeks then all patients received Lamividune 100mg daily for 52 weeks with 16 wk post treatment follow up. HDV RNA levels were not affected, there was a partial histological response in 26% of patients and there was no HBsAg seroconversion[89]. Combination therapy with peg-IFN-α-2a, tenofovir and emtricitabine for one year in one patient with HDV showed that after 10 months of treatment, a sustained response was obtained and seroconversion of HBsAg occurred suggesting that combination therapy was more effective than IFN monotherapy[90]. Thus, more studies using mono or combination therapies were evaluated especially with adefovir.
In a large randomized treatment trial, the Hep-Net-International-Delta-Hepatitis-Intervention-Study 1 (HIDIT-I), 90 patients were treated with adefovir (ADV, 10 mg oral daily, n = 30) vs peg-IFN-α-2a (180 μg/weekly, n = 29) and placebo vs adefovir and peg-IFN-α-2a (n = 31) for 48 wk with 24 wk follow up[91]. The primary endpoint was undetectable HDV RNA and normal ALT at the end of treatment. This was met in 0, 2 and 2 patients in the ADV, peg-IFN and combination groups, respectively. HDV RNA was negative in 0%, 24% and 23% in the ADV, peg-IFN and combination groups, respectively. Twenty eight percent of patients receiving peg-IFN based treatments had negative HDV RNA at 24 wk post treatment. Zero, 2 and 10 patients developed a decline in HBsAg levels of > 1 log10 IU/mL from baseline to week 48 in the ADV, peg-IFN (P = 0.01 compared to combination) and combination groups (P < 0.001 compared to ADV alone), respectively.
A retrospective-prospective follow-up of the HIDIT-I trial was completed in 77 patients with a median time follow-up of 4.5 years[92]. Twenty-one, 19 and 18 patients from the ADV, peg-IFN and the combination group were included, respectively. The overall posttreatment clinical-event rate (hepatic decompensation, HCC, liver transplantation and liver-related death) was 2.5% (4.9% in patients with cirrhosis). Ten percent of peg-IFN based treated patients achieved HBsAg loss by the end of follow up. HDV RNA relapses occurred during long term follow up in nearly 50% of responder patients and the authors recommended that the term “sustained” virological response be avoided in chronic HDV infection. Univariate and mul-tivariate analyses of HIDIT-I to identify factors associated with outcomes of peg-IFN treatment, with and without adefovir revealed that the level of HDV RNA at week 24 of treatment was associated more strongly with response to therapy and could help to identify patients who will test negative for HDV RNA 24 wk after the end of treatment[93].
In HIDIT-II, two parallel, multicenter, double-blind, randomized, controlled trials evaluated peg-IFN-α-2a (180 µg/weekly) combination therapy with oral Tenofovir Disoproxil Fumarate (TDF, 300 mg daily, n = 59) or placebo (n = 61) for 96 wk in 120 patients with chronic HDV infection[94]. The primary endpoint was negative HDV RNA at the end of treatment. Secondary endpoints included negative HDV RNA at treatment week 48 and post-treatment week 24, HBsAg loss or decline > 0.5 log10 from baseline to treatment weeks 48 and 96 and normal ALT at treatment weeks 48, 96 and post-treatment week 24. Addition of TDF did not provide additional benefit for treatment endpoints as there were no significant differences between both cohorts and the authors suggest that HBV nucleotide analogous should not be recommended as standard of care at this time. However, there were other important off target findings. Ninety-six weeks of peg-IFN-α-2a was tolerated by most of the patients in this study, which also included patients with cirrhosis, and that prolonged treatment was associated with improvement in histological fibrosis scores overall. Also, the presence of liver cirrhosis was associated with an increased likelihood for an HDV RNA response at post-treatment week 24. Therefore, retreatment of patients with peg-IFN can be considered if prior treatment was tolerated and progression of disease is likely. Though peg-IFN was tolerated and showed histological improvement, this did not correlate with virological or biochemical responses and extension of treatment to 96 wk did not cause significant change in HDV RNA negativity in either group and seemed to peak at week 48 and did not prevent relapse. Therefore, if suppression of HDV RNA is the main endpoint, then treatment beyond 48 wk may not yield additional benefit.
Long-term outcome of delta hepatitis after treatment with antiviral treatment regimens was evaluated in a large, single-center cohort of 136 patients for a mean follow-up of 5.2 years[95]. Thirty-nine patients had not received treatment, 52 received IFN-α based therapy and 45 patients received treatment with NA analogues only. Clinical endpoints of hepatic decompensation, HCC, liver transplantation and liver-related death were monitored and occurred in 55 patients. Regarding IFN-α based therapy, patients developed clinical endpoints significantly less frequently than the other two cohorts (NA, P < 0.01; untreated, P < 0.01), was independently associated with a more benign clinical long-term outcome (P = 0.04; OR = 0.25; 95% CI: 0.07-0.9), and hepatic decompensation (P < 0.01) and need for liver transplant (P < 0.01) occurred less often compared to the NA group. Though efficacy of IFN-α is not optimal, this study highlights the fact that IFN-α is necessary in those that require treatment until improved options are available.
As mentioned previously, the only recommended therapy for chronic HDV infection is IFN-α based treatment with limited efficacy and many side effects and identifying patients who warrant treatment highlight the need for specific HDV scoring systems and fibrosis markers. Current guidelines suggest treating individuals with elevated HDV-RNA levels and ALT elevation with peg-IFN-α for 12 months with con-sideration of NA therapy with ongoing HBV DNA replication[15,20]. In a cross-sectional study of two independent cohorts of patients including individuals from the HIDIT-II trial, anti-HDV IgM was assessed for its utility as a biomarker to determine disease activity and to predict the risk of disease progression in patients with chronic HDV infection[96]. One hundred and twenty patients were included from HIDIT-II and 78 patients from a ‘‘real-world’’ validation cohort. The validation cohort received various forms of treatment: no treatment, n = 46; NA analogues, n = 16; peg-IFN, n = 3; combination NA analogues and peg-IFN, n = 13. Liver-related endpoints of hepatic decompensation (encephalopathy, variceal bleeding, ascites), liver transplantation, HCC or liver-related death were evaluated in the validation cohort. Anti-HDV IgM serum levels were tested in 102 (85%) HIDIT-II patients and 67 (85.9%) cohort patients. Negative, low, intermediate and high anti-HDV IgM levels were defined as negative, < 0.5, 0.5-2.5 and > 2.5, respectively. Though not significant, the validation cohort had more patients with intermediate (32% vs 21%) or high anti-HDV IgM levels (5% vs 0%) and in both cohorts there was a trend with histological activity. There was significant congruency between both cohorts regarding ALT and anti-HDV IgM levels (negative vs low or intermediate). In HIDIT-II patients, HBV DNA and anti-HDV IgM were inversely correlated and significant in the negative vs low or intermediate patients. There was no association between HDV RNA or HBsAg with anti-HDV IgM. Clinical long-term outcome was investigated in the validation cohort who were followed over a period of 14 years. Clinical decompensation occurred only in one anti-HDV IgM negative patient and in 26 patients with positive IgM levels (P = 0.05). These findings suggest that anti-HDV IgM identified a subgroup of patients with a very mild course of liver disease who may not require urgent treatment.
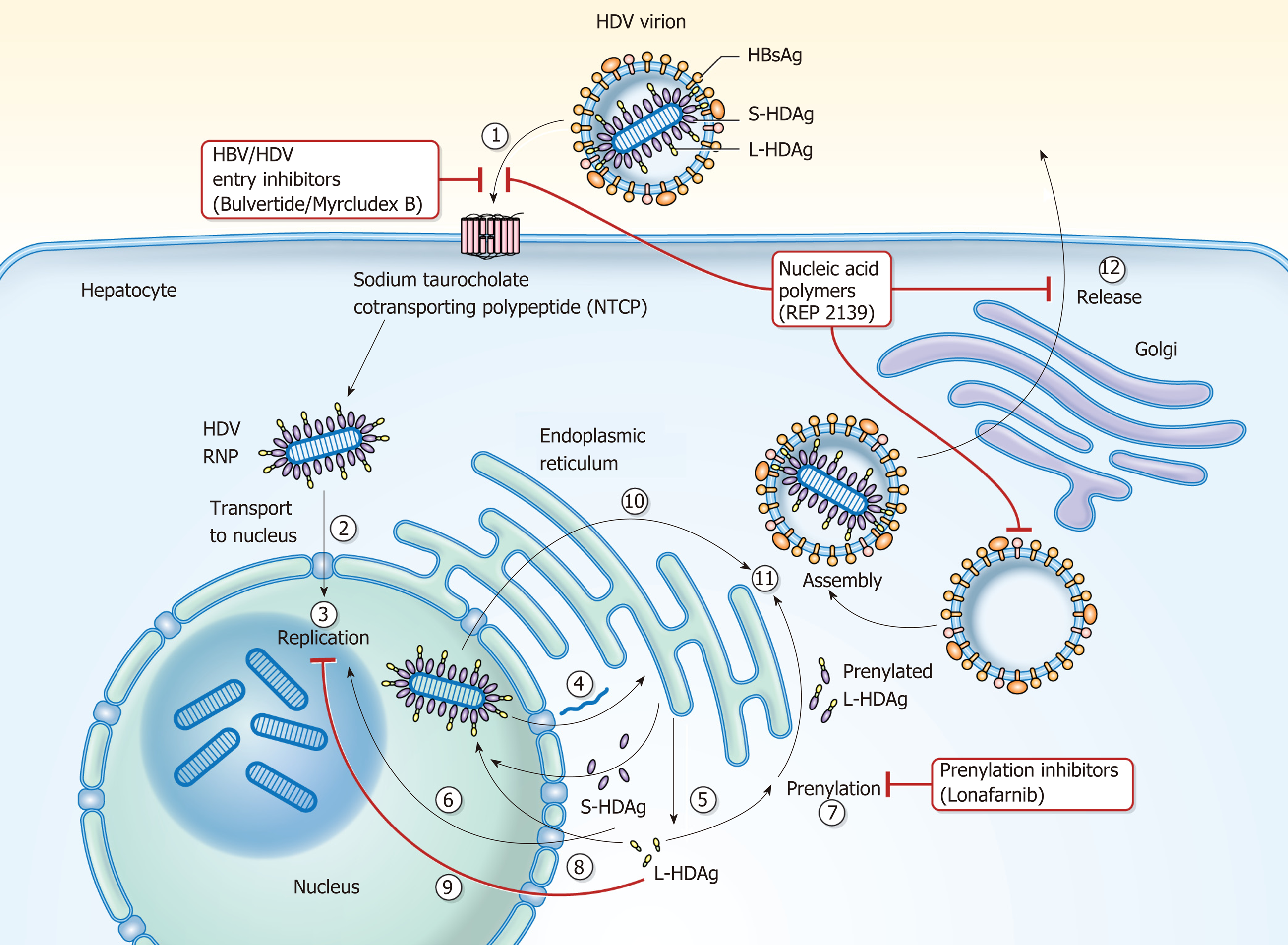
Prior studies have demonstrated that myristoylation of the preS1 domain of the L envelope protein of HBsAg is required for HBV infectivity[97-99] and that interference of infectivity occurs through specific cell receptor targeting on the hepatocyte surface[100,101]. The Sodium Taurocholate Co-Transporting Polypeptide (NTCP) receptor was identified as the specific receptor for HBV and HDV[28,29] and Myrcludex B (Bulevirtide), a synthetic preS1 derived peptide, has been identified as an NTCP receptor inhibitor[99,102-104] with little effect on bile acid transport[105].
A single center, randomized, 3-arm, parallel, open-label clinical trial was performed in Russia to evaluate the effect of Myrcludex B alone or in combination with peg-IFN-α-2a vs peg-IFN-α-2a alone in 24 patients with HDV infection[106]. The primary endpoint was HBsAg response at week 12 of treatment (HBsAg decline of at least 0.5 log10 IU/mL at any time during the study). Secondary endpoints included the responses of HBsAg (24 wk), HDV RNA, HBV DNA and ALT at 24 and 48 wk of therapy and at 24 wk following the completion of treatment. The 3 treatment groups included the Myr cohort - 2 mg Myrcludex B SC daily for 24 wk followed by 180 μg peg-IFN-α-2a SC weekly for 48 wk; Myr-IFN cohort – 2 mg Myrcludex B SC daily plus 180 μg peg-IFN-α-2a SC weekly for 24 wk followed by peg-IFN-α-2a alone for 24 wk; IFN cohort – 180 μg peg-IFN-α-2a SC weekly for 48 wk. The results at 12 and 24 wk were reported. There was no significant change of HBsAg at 12 and 24 wk in all cohorts. At week 24, there was significant mean change from baseline in HDV RNA in 6, 7 and 6 patients and HDV RNA levels became undetectable in 2, 5 and 2 patients in the Myr, Myr-IFN and IFN cohorts, respectively. The only significant change in HBV DNA and ALT response was seen in the Myr-IFN cohort. The drug was well tolerated and there was an asymptomatic rise in plasma bile acid concentrations. At the time of publication, the trial is still ongoing as this report included data from weeks 12 and 24 of treatment.
The multicenter, open-label, randomized trial to assess the safety and efficacy of varying doses of Myrcludex B for 24 wk in combination with Tenofovir vs Tenofovir alone in patients with chronic HDV infection is completed[107]. One hundred and twenty patients were randomized to 4 treatment arms (n = 30/arm): Myrcludex B (2, 5, or 10mg SC daily) and Tenofovir (245 mg daily) for 24 wk with 24 wk follow up vs Tenofovir alone for 48 wk. Myrcludex B was generally well tolerated. The primary endpoint was HDV RNA reduction by 2 log10 or negativity at the end of treatment and was achieved in 46.4%, 46.8%, 76.6% and 3.3% in the 2, 5 and 10mg Myrcludex B cohorts and Tenofovir alone cohort, respectively. ALT normalization was achieved in 42.8%, 50%, 40% and 6.6%, respectively. At follow up 12 wk post-treatment, HDV RNA relapse occurred in 60%, 80% and 83% of treatment responder patients in the Myrcludex B arms, respectively.
Interim results for a phase 2, multicenter, open-label, randomized trial assessing the safety and efficacy of Myrcludex B in combination with peg-IFN-α-2a vs peg-IFN-α-2a alone in patients with compensated chronic HDV infection was made available[108]. Sixty patients (n = 15/arm) were treated for 48 wk with 24 wk follow up. Treatment cohorts were as follows: peg-IFN-α-2a (180 μg weekly) vs Myrcludex B (2 mg daily) vs Myrcludex B (2 mg daily) + peg-IFN-α-2a vs Myrcludex B (5 mg daily) + peg-IFN-α-2a (180 μg weekly). Myrcludex B was generally well tolerated with an asymptomatic dose dependent increase in bile salts. The primary endpoint was undetectable HDV RNA at week 72. Secondary endpoints included undetectable HDV RNA at week 48 and ALT normalization, undetectable HBsAg or decline and combination response (decline or undetectable HDV RNA and normal ALT) at weeks 48 and 72. Median HDV RNA log10 change from baseline to week 48 was -1.14, -2.84, -3.62 and -4.48, respectively. At week 48, undetectable HDV RNA was achieved in 2, 2, 9 and 6 patients, ALT normalization in 4, 10, 4 and 7 patients and combined treatment response in 2, 8, 4, 6 patients, respectively. HBsAg clearance was achieved in 0, 0, 7 and 2 patients, respectively.
In a randomized, open-label trial of daily Myrcludex B and peg-IFN-α-2a in patients with HBeAg negative chronic HDV infection, 24 patients were randomized into 3 treatment arms: Pre-treatment with Myrcludex B (2 mg daily) for 24 wk followed by peg-IFN-α-2a (180 μg weekly) for 48 wk vs combination Myrcludex B and peg-IFN-α-2a for 24 wk then peg-IFN-α-2a for 24 wk vs peg-IFN-α-2a for 48 wk and results are pending (NCT02637999). Future studies include a phase 2b study of Bulevirtide with peg-IFN-α-2a for 96 wk (NCT03852433) and a phase 3 study of Bulevirtide for 144 wk (NCT03852719) in patients with chronic HDV infection.
As mentioned previously, HDV replication is promoted by SHDAg and inhibited by LHDAg, which promotes assembly and export of HDV through prenylation of LHDAg. Prenylation of LHDAg promotes the interaction of LHDAg with HBsAg[53]. Farnesyl is the prenyl moiety added to LHDAg by farnesyltransferase and inhibitors of farnesyltransferase have been shown to prevent the production of HDV virions[109]. Lonafarnib is a farnesyl transferase inhibitor being studied in chronic HDV infection.
A proof-of-concept randomized, double-blind, placebo-controlled phase 2a trial assessed the safety, tolerability and effect of HDV RNA levels of oral Lonafarnib in chronic HDV infection[110]. Fourteen patients were randomly assigned to receive Lonafarnib 100 mg BID or placebo (Group 1) or Lonafarnib 200 mg BID or placebo (Group 2) for 28 d with follow-up for 6 months. Adverse effects including nausea, diarrhea, bloating and weight loss were notable in Group 2, but treatment discontinuation was not required. Mean log10 HDV RNA levels were significantly decreased in both groups compared to placebo (Group 2 -1.54 log10 IU/mL vs Group 1 -0.73 log10 IU/mL) and correlated with Lonafarnib concentrations. There were no significant changes noted in ALT, HBsAg or HBV DNA levels but a trend towards increased HBV DNA levels was noted in Group 2.
These findings led to a single center, phase 2, open label trial to optimize the treatment regimen of Lonafarnib with 21 patients in 7 treatment arms (Lonafarnib with and without Ritonavir in HDV-1, LOWR HDV-1)[111]. When comparing Lonafarnib 200 mg BID vs 300 mg BID monotherapy, the higher dose of Lonafarnib resulted in greater initial decreases in HDV levels though with increased gastrointestinal side effects and when combined with peg-IFN-α 180 μg weekly was intolerable resulting in discontinuation of treatment in those arms. Addition of the CYP3A4 inhibitor Ritonavir 100 mg oral daily to Lonafarnib 100 mg BID to achieve high post absorption levels of Lonafarnib without needing high doses of Lonafarnib resulted in a 2.4 and 3.2 log10 reduction in HDV RNA at 4 and 8 wk of treatment, respectively. Similar results were seen with the same dose of Lonafarnib and peg-IFN-α and both combinations resulted in normalization of ALT. Therapeutic flares were seen in 2/6 patients in the Lonafarnib monotherapy groups in posttreatment follow up.
In LOWR HDV-2, even lower doses of Lonafarnib proved to be effective. In this open-label, dose-optimization study, 55 patients were given combinations of Lonafarnib (25 or 50 mg BID vs 75 mg or more BID) with Ritonavir (100 mg BID), with or without peg-IFN-α (180 µg weekly) for 12, 24, or 48 wk[112]. As responses were comparable with the lower and higher dose Lonafarnib groups, the study was extended beyond 12 wk for only the 25 or 50 mg BID groups. Response was defined as HDV RNA decrease of 2 log10 IU/mL or more. At week 24, 50% of patients who received Lonafarnib 50mg BID and 89% of patients who received Lonafarnib (25 or 50 mg BID) and peg-IFN-α exhibited a response. Also, at week 24, ALT levels normalized in majority of patients with combination therapy (60% with Lonafarnib 25 mg BID and 88% with Lonafarnib 50 mg BID). Therapeutic flares were also seen in several patients similar to LOWR HDV-1.
In the randomized, double-blind LOWR HDV-3 study, 21 patients received a daily oral combination of Lonafarnib (50, 75, or 100 mg) and Ritonavir (100 mg) for 24 wk[113]. Twelve patients received Lonafarnib and Ritonavir for 24 wk and 9 patients received 12 wk of placebo followed by Lonafarnib. Median decrease in HDV RNA from baseline after 12 wk of treatment was 1.6, 1.33 and 0.83 log10 IU/mL in the Lonafarnib 50, 75 and 100 mg groups, respectively. Six patients achieved a decrease in serum level of HDV RNA of 2 log or more and ALT normalized in 47% of patients.
In the open-label, dose escalation LOWR HDV-4 study, 15 patients with chronic HDV infection were given Lonafarnib (50 mg BID initially, then increased to 75 mg BID after 4 wk and then increase to 100 mg BID after 1 week pending tolerability) with Ritonavir (100 mg BID) for 24 wk[114]. Sixty six percent of patients were able to tolerate dose escalation to 100 mg BID with 33% being able to maintain this dose to 24 wk. Mean decrease in HDV RNA for all patients was 1.7 log10 IU/mL and ALT normalized in 53% of patients.
Finally, a multicenter, randomized, partially double-blind, phase 3 study will evaluate the safety and efficacy of Lonafarnib 50mg BID and Ritonavir 100 mg BID with and without peg-IFN-α-2a 180 µg weekly vs peg-IFN-α-2a 180 µg weekly monotherapy vs placebo for 48 wk with 24 wk follow up in patients with chronic HDV infection (Delta Liver Improvement and Virologic Response in HDV, D-LIVR) (NCT03719313). Four hundred patients will be included and will receive Entecavir or Tenofovir for at least 12 wk prior to initiating study therapy. Endpoints include virologic response (2 log10 IU/mL reduction from baseline) and ALT normalization.
Nucleic acid polymers (NAP) are amphipathic phosphorothioate oligonucleotides that exhibit broad spectrum antiviral activity which was initially observed in HIV[115]. Studies of NAPs (REP 2006, 2031, 2055) in the duck hepatitis B virus model of HBV infection also showed exhibition of antiviral activity[116-118]. These studies revealed that there was rapid clearance of duck HBsAg in serum, though it was still present in the liver, and that duck HBV DNA was persistent in the serum highlighting the possibility that the target of NAPs is subviral particle secretion from the liver. REP 2139 is a nucleic acid polymer currently being investigated in chronic HBV and HDV infection.
Clinical studies in patients with treatment naive HBeAg positive chronic hepatitis B infection have shown that REP 2055 monotherapy (n = 8) for 40 wk or REP 2139-Ca monotherapy (n = 12) for 40 wk or combined treatment (n = 9/12) for 13 wk with immunotherapy (pegylated interferon or thymosin alpha 1) resulted in reduction in HBsAg and HBV DNA and development of serum anti-HBsAg antibodies[119]. In the REP 2055 cohort, HBsAg loss occurred in 3 patients during treatment and HBV DNA suppression was maintained at weeks 231 and 290 wk in 2 patients after end of treatment. In the REP 2139 cohort, 9/12 patients experienced reductions in HBsAg with 3 patients achieving HBsAg loss during monotherapy treatment. Of these 9 patients who were transitioned to combination treatment with immunotherapy, HBsAg loss was maintained in the same 3 patients and the remaining 6 patients experienced further HBsAg decline and eventually achieved HBsAg loss by the end of therapy.
In 2017, an open-label, non-randomized, phase 2 trial was published which assessed the safety and efficacy of REP 2139-Ca and peg-IFN-α-2a in patients with HBV and HDV co-infection[120]. Twelve treatment naïve, non-cirrhotic, HBeAg negative patients with HBsAg levels greater than 1000 IU/mL received 500 mg REP 2139 weekly infusions for 15 wk, then combined therapy with 250 mg REP 2139 weekly and 180μg SC peg-IFN-α-2a weekly for 15 wk and finally monotherapy with 180 μg peg-IFN-α-2a weekly for 33 wk. Primary endpoints included safety and tolerability of therapy. Secondary endpoints included HBsAg < 50 IU/mL and suppressed HBV DNA and maintenance of response during follow up. For the primary endpoints, 4 patients experienced a serious adverse event and all patients had treatment-emergent laboratory abnormalities. For the secondary endpoints, 6 patients had HBsAg levels < 50 IU/mL by the end of treatment and 5 patients maintained this by the end of 1 year follow up. Nine patients had suppressed HBV DNA (< 10 IU/mL) at the end of treatment and 7 patients were able to maintain this by the end of 1 year follow up. One patient established suppressed HBV DNA at the end of 1 year follow up. Eleven patients were HDV RNA negative during treatment, 9 remained that way at the end of treatment and 7 patients maintained this at 1 year follow up. Follow up at 1.5-2 years showed that 7/11 patients had persistent HDV RNA negativity[121].
A current study for NAPs is an open-label, randomized, active controlled, parallel comparison trial of the safety and efficacy of REP 2139-Mg (250 mg weekly) or REP 2165-Mg (REP 2139 derivative, 250 mg weekly) in combination with Tenofovir (300 mg daily) and peg-IFN-α-2a (180 μg weekly) in patients with HBeAg negative chronic hepatitis B[122]. Forty patients were treated for 24 wk with Tenofovir monotherapy before randomization into experimental (48 wk of Tenofovir, peg-IFN and REP 2139 or REP 2165) and control (48 wk of Tenofovir and peg-IFN) groups. All patients in the control group crossed over to experimental therapy at week 49 as they did not achieve a 3 log10 HBsAg decline and are labeled as the adaptive control group. Interim follow up analysis of the experimental group shows that treatment was well tolerated. Eight of 10 REP 2139 and 5/10 REP 2165 patients in this group achieved functional control and normalization of liver enzymes.
In 2003, interferon lambda (IFN-λ), a type III interferon, was discovered[123] and was subsequently studied as an alternative treatment to IFN-α in HCV[124-127] and HBV[127,128]. Prior studies have shown that IFN-λ has similar downstream signaling pathways to IFN-α[129] but IFN-λ signaling is localized to the liver[129,130] and IFN-λ may have a protective role in the liver[131,132].
In a multicenter, randomized, open-label, phase 2 trial, the safety, tolerability and efficacy of IFN-λ was evaluated in 33 patients with chronic HDV infection (Lambda Interferon Monotherapy Study in HDV, LIMT HDV)[133]. Patients received IFN-λ 120 μg (n = 19) or 180 μg (n = 14) SC weekly for 48 wk with 24 wk follow up. Patients also received Tenofovir or Entecavir for HBV suppression. The primary endpoint was change in HDV RNA from baseline to weeks 48 and at week 72 and responders were defined as those exhibiting a 2 log10 decline or below limit of quantification of HDV RNA. IFN-λ was generally well tolerated and patients that received prior treatment with IFN-α reported fewer side effects with IFN-λ. A durable virologic response was achieved in 36% in the high dose IFN-λ cohort compared to 16% in the lower dose cohort and this was maintained in both groups at 24 wk follow up. By the end of follow up, ALT normalized in 36% vs 26% in the high vs low dose cohorts, respectively.
Currently, an open label, phase 2 trial investigating the safety and efficacy of combination lonafarnib, ritonavir and IFN-λ is ongoing at the National Institutes of Health. Twenty-six patients will be treated with Lonafarnib (50 mg BID), Ritonavir (100 mg BID) and peg-IFN-λ-1a (180 μg weekly) for 24 wk with 24 wk follow up. The patients will also receive Tenofovir or Entecavir for HBV suppression. The primary safety endpoint will be drug tolerability at the prescribed dosages for the duration of treatment. The primary therapeutic endpoint is a decline of HDV RNA of 2 log10 at the end of therapy. Secondary endpoints include sustained undetectable HDV RNA, loss of HBsAg, normalization of ALT, reduction in hepatic venous pressure gradient measurements and reduction in histologic inflammatory scores. To date, the study has completed enrollment and the investigators are awaiting final results.
With the increasing number of clinical trials investigating novel therapies for chronic HDV infection, it is important to determine surrogate makers to monitor treatment efficacy. A panel of experts have proposed that a ≥ 2 log10 decline in HDV RNA be used as a target for initial treatment efficacy in this patient population[134]. Additionally, nucleos(t)ide analogues suppressing hepatitis B should be considered as suppressing HDV can cause reactivation of HBV.
In addition to the novel treatments mentioned prior, research has been growing in identifying treatments to achieve a functional cure in chronic hepatitis B infection which could potentially be useful in the treatment of chronic hepatitis D infection[135,136]. Three areas of interest include capsid assembly modulators, immune system stimulators (toll-like receptor agonists and checkpoint inhibitors) and RNAi gene silencing. These studies in addition to those of NTCP receptor inhibitors, farnesyl transferase inhibitors, nucleic acid polymers in combination with interferon therapy will lead to further insight into the management of this devastating disease in the near future and hopefully one day a cure.
| 1. | Rizzetto M, Canese MG, Aricò S, Crivelli O, Trepo C, Bonino F, Verme G. Immunofluorescence detection of new antigen-antibody system (delta/anti-delta) associated to hepatitis B virus in liver and in serum of HBsAg carriers. Gut. 1977;18:997-1003. [PubMed] |
| 2. | Rizzetto M, Canese MG, Gerin JL, London WT, Sly DL, Purcell RH. Transmission of the hepatitis B virus-associated delta antigen to chimpanzees. J Infect Dis. 1980;141:590-602. [PubMed] |
| 3. | Sagnelli E, Felaco FM, Filippini P, Pasquale G, Peinetti P, Buonagurio E, Aprea L, Pulella C, Piccinino F, Giusti G. Influence of HDV infection on clinical, biochemical and histological presentation of HBsAg positive chronic hepatitis. Liver. 1989;9:229-234. [PubMed] |
| 4. | Gish RG, Yi DH, Kane S, Clark M, Mangahas M, Baqai S, Winters MA, Proudfoot J, Glenn JS. Coinfection with hepatitis B and D: epidemiology, prevalence and disease in patients in Northern California. J Gastroenterol Hepatol. 2013;28:1521-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 5. | Kushner T, Serper M, Kaplan DE. Delta hepatitis within the Veterans Affairs medical system in the United States: Prevalence, risk factors, and outcomes. J Hepatol. 2015;63:586-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 136] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 6. | Tamura I, Kurimura O, Koda T, Ichimura H, Katayama S, Kurimura T, Inaba Y. Risk of liver cirrhosis and hepatocellular carcinoma in subjects with hepatitis B and delta virus infection: a study from Kure, Japan. J Gastroenterol Hepatol. 1993;8:433-436. [PubMed] |
| 7. | Fattovich G, Giustina G, Christensen E, Pantalena M, Zagni I, Realdi G, Schalm SW. Influence of hepatitis delta virus infection on morbidity and mortality in compensated cirrhosis type B. The European Concerted Action on Viral Hepatitis (Eurohep). Gut. 2000;46:420-426. [PubMed] |
| 8. | Gheorghe L, Csiki IE, Iacob S, Gheorghe C, Trifan A, Grigorescu M, Motoc A, Suceveanu A, Curescu M, Caruntu F, Sporea I, Brisc C, Rogoveanu I, Cerban R, Tugui L, Alexandrescu A. Hepatitis Delta Virus Infection in Romania: Prevalence and Risk Factors. J Gastrointestin Liver Dis. 2015;24:413-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Oyunsuren T, Kurbanov F, Tanaka Y, Elkady A, Sanduijav R, Khajidsuren O, Dagvadorj B, Mizokami M. High frequency of hepatocellular carcinoma in Mongolia; association with mono-, or co-infection with hepatitis C, B, and delta viruses. J Med Virol. 2006;78:1688-1695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Chen HY, Shen DT, Ji DZ, Han PC, Zhang WM, Ma JF, Chen WS, Goyal H, Pan S, Xu HG. Prevalence and burden of hepatitis D virus infection in the global population: a systematic review and meta-analysis. Gut. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 279] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 11. | Radjef N, Gordien E, Ivaniushina V, Gault E, Anaïs P, Drugan T, Trinchet JC, Roulot D, Tamby M, Milinkovitch MC, Dény P. Molecular phylogenetic analyses indicate a wide and ancient radiation of African hepatitis delta virus, suggesting a deltavirus genus of at least seven major clades. J Virol. 2004;78:2537-2544. [PubMed] |
| 12. | Le Gal F, Gault E, Ripault MP, Serpaggi J, Trinchet JC, Gordien E, Dény P. Eighth major clade for hepatitis delta virus. Emerg Infect Dis. 2006;12:1447-1450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 154] [Article Influence: 7.7] [Reference Citation Analysis (1)] |
| 13. | Buti M, Homs M, Rodriguez-Frias F, Funalleras G, Jardí R, Sauleda S, Tabernero D, Schaper M, Esteban R. Clinical outcome of acute and chronic hepatitis delta over time: a long-term follow-up study. J Viral Hepat. 2011;18:434-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 125] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 14. | Wranke A, Pinheiro Borzacov LM, Parana R, Lobato C, Hamid S, Ceausu E, Dalekos GN, Rizzetto M, Turcanu A, Niro GA, Lubna F, Abbas M, Ingiliz P, Buti M, Ferenci P, Vanwolleghem T, Hayden T, Dashdorj N, Motoc A, Cornberg M, Abbas Z, Yurdaydin C, Manns MP, Wedemeyer H, Hardtke S; Hepatitis Delta International Network. Clinical and virological heterogeneity of hepatitis delta in different regions world-wide: The Hepatitis Delta International Network (HDIN). Liver Int. 2018;38:842-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 15. | Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 3090] [Article Influence: 386.3] [Reference Citation Analysis (1)] |
| 16. | Kucirka LM, Farzadegan H, Feld JJ, Mehta SH, Winters M, Glenn JS, Kirk GD, Segev DL, Nelson KE, Marks M, Heller T, Golub ET. Prevalence, correlates, and viral dynamics of hepatitis delta among injection drug users. J Infect Dis. 2010;202:845-852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 17. | Ordieres C, Navascués CA, González-Diéguez ML, Rodríguez M, Cadahía V, Varela M, Rodrigo L, Rodríguez M. Prevalence and epidemiology of hepatitis D among patients with chronic hepatitis B virus infection: a report from Northern Spain. Eur J Gastroenterol Hepatol. 2017;29:277-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Safaie P, Razeghi S, Rouster SD, Privitera I, Sherman KE. Hepatitis D diagnostics:Utilization and testing in the United States. Virus Res. 2018;250:114-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, Chen DS, Chen HL, Chen PJ, Chien RN, Dokmeci AK, Gane E, Hou JL, Jafri W, Jia J, Kim JH, Lai CL, Lee HC, Lim SG, Liu CJ, Locarnini S, Al Mahtab M, Mohamed R, Omata M, Park J, Piratvisuth T, Sharma BC, Sollano J, Wang FS, Wei L, Yuen MF, Zheng SS, Kao JH. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1985] [Cited by in RCA: 2052] [Article Influence: 205.2] [Reference Citation Analysis (12)] |
| 20. | European Association for the Study of the Liver. European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3745] [Cited by in RCA: 4009] [Article Influence: 445.4] [Reference Citation Analysis (1)] |
| 21. | Rizzetto M, Hoyer B, Canese MG, Shih JW, Purcell RH, Gerin JL. delta Agent: association of delta antigen with hepatitis B surface antigen and RNA in serum of delta-infected chimpanzees. Proc Natl Acad Sci USA. 1980;77:6124-6128. [PubMed] |
| 22. | Chen PJ, Kalpana G, Goldberg J, Mason W, Werner B, Gerin J, Taylor J. Structure and replication of the genome of the hepatitis delta virus. Proc Natl Acad Sci USA. 1986;83:8774-8778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 254] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 23. | Kos A, Dijkema R, Arnberg AC, van der Meide PH, Schellekens H. The hepatitis delta (delta) virus possesses a circular RNA. Nature. 1986;323:558-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 539] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 24. | Wang KS, Choo QL, Weiner AJ, Ou JH, Najarian RC, Thayer RM, Mullenbach GT, Denniston KJ, Gerin JL, Houghton M. Structure, sequence and expression of the hepatitis delta (delta) viral genome. Nature. 1986;323:508-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 541] [Article Influence: 13.5] [Reference Citation Analysis (1)] |
| 25. | Lin JH, Chang MF, Baker SC, Govindarajan S, Lai MM. Characterization of hepatitis delta antigen: specific binding to hepatitis delta virus RNA. J Virol. 1990;64:4051-4058. [PubMed] |
| 26. | Bonino F, Hoyer B, Shih JW, Rizzetto M, Purcell RH, Gerin JL. Delta hepatitis agent: structural and antigenic properties of the delta-associated particle. Infect Immun. 1984;43:1000-1005. [PubMed] |
| 27. | Bonino F, Heermann KH, Rizzetto M, Gerlich WH. Hepatitis delta virus: protein composition of delta antigen and its hepatitis B virus-derived envelope. J Virol. 1986;58:945-950. [PubMed] |
| 28. | Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, Huang Y, Qi Y, Peng B, Wang H, Fu L, Song M, Chen P, Gao W, Ren B, Sun Y, Cai T, Feng X, Sui J, Li W. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife. 2012;1:e00049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1280] [Cited by in RCA: 1648] [Article Influence: 117.7] [Reference Citation Analysis (1)] |
| 29. | Ni Y, Lempp FA, Mehrle S, Nkongolo S, Kaufman C, Fälth M, Stindt J, Königer C, Nassal M, Kubitz R, Sültmann H, Urban S. Hepatitis B and D viruses exploit sodium taurocholate co-transporting polypeptide for species-specific entry into hepatocytes. Gastroenterology. 2014;146:1070-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 641] [Article Influence: 53.4] [Reference Citation Analysis (0)] |
| 30. | Chang MF, Baker SC, Soe LH, Kamahora T, Keck JG, Makino S, Govindarajan S, Lai MM. Human hepatitis delta antigen is a nuclear phosphoprotein with RNA-binding activity. J Virol. 1988;62:2403-2410. [PubMed] |
| 31. | Xia YP, Yeh CT, Ou JH, Lai MM. Characterization of nuclear targeting signal of hepatitis delta antigen: nuclear transport as a protein complex. J Virol. 1992;66:914-921. [PubMed] |
| 32. | Alves C, Freitas N, Cunha C. Characterization of the nuclear localization signal of the hepatitis delta virus antigen. Virology. 2008;370:12-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Chang MF, Chang SC, Chang CI, Wu K, Kang HY. Nuclear localization signals, but not putative leucine zipper motifs, are essential for nuclear transport of hepatitis delta antigen. J Virol. 1992;66:6019-6027. [PubMed] |
| 34. | Tavanez JP, Cunha C, Silva MC, David E, Monjardino J, Carmo-Fonseca M. Hepatitis delta virus ribonucleoproteins shuttle between the nucleus and the cytoplasm. RNA. 2002;8:637-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Hong SY, Chen PJ. Phosphorylation of serine 177 of the small hepatitis delta antigen regulates viral antigenomic RNA replication by interacting with the processive RNA polymerase II. J Virol. 2010;84:1430-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Mu JJ, Tsay YG, Juan LJ, Fu TF, Huang WH, Chen DS, Chen PJ. The small delta antigen of hepatitis delta virus is an acetylated protein and acetylation of lysine 72 may influence its cellular localization and viral RNA synthesis. Virology. 2004;319:60-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Li YJ, Stallcup MR, Lai MM. Hepatitis delta virus antigen is methylated at arginine residues, and methylation regulates subcellular localization and RNA replication. J Virol. 2004;78:13325-13334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 38. | Moraleda G, Taylor J. Host RNA polymerase requirements for transcription of the human hepatitis delta virus genome. J Virol. 2001;75:10161-10169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 39. | Fu TB, Taylor J. The RNAs of hepatitis delta virus are copied by RNA polymerase II in nuclear homogenates. J Virol. 1993;67:6965-6972. [PubMed] |
| 40. | Macnaughton TB, Shi ST, Modahl LE, Lai MM. Rolling circle replication of hepatitis delta virus RNA is carried out by two different cellular RNA polymerases. J Virol. 2002;76:3920-3927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 101] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 41. | Modahl LE, Macnaughton TB, Zhu N, Johnson DL, Lai MM. RNA-Dependent replication and transcription of hepatitis delta virus RNA involve distinct cellular RNA polymerases. Mol Cell Biol. 2000;20:6030-6039. [PubMed] |
| 42. | Weiner AJ, Choo QL, Wang KS, Govindarajan S, Redeker AG, Gerin JL, Houghton M. A single antigenomic open reading frame of the hepatitis delta virus encodes the epitope(s) of both hepatitis delta antigen polypeptides p24 delta and p27 delta. J Virol. 1988;62:594-599. [PubMed] |
| 43. | Sharmeen L, Kuo MY, Dinter-Gottlieb G, Taylor J. Antigenomic RNA of human hepatitis delta virus can undergo self-cleavage. J Virol. 1988;62:2674-2679. [PubMed] |
| 44. | Casey JL, Gerin JL. Hepatitis D virus RNA editing: specific modification of adenosine in the antigenomic RNA. J Virol. 1995;69:7593-7600. [PubMed] |
| 45. | Wang JG, Cullen J, Lemon SM. Immunoblot analysis demonstrates that the large and small forms of hepatitis delta virus antigen have different C-terminal amino acid sequences. J Gen Virol. 1992;73:183-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 46. | Glenn JS, White JM. trans-dominant inhibition of human hepatitis delta virus genome replication. J Virol. 1991;65:2357-2361. [PubMed] |
| 47. | Chao M, Hsieh SY, Taylor J. Role of two forms of hepatitis delta virus antigen: evidence for a mechanism of self-limiting genome replication. J Virol. 1990;64:5066-5069. [PubMed] |
| 48. | Lee CH, Chang SC, Wu CH, Chang MF. A novel chromosome region maintenance 1-independent nuclear export signal of the large form of hepatitis delta antigen that is required for the viral assembly. J Biol Chem. 2001;276:8142-8148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 74] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 49. | Glenn JS, Watson JA, Havel CM, White JM. Identification of a prenylation site in delta virus large antigen. Science. 1992;256:1331-1333. [PubMed] |
| 50. | Ryu WS, Bayer M, Taylor J. Assembly of hepatitis delta virus particles. J Virol. 1992;66:2310-2315. [PubMed] |
| 51. | Chang FL, Chen PJ, Tu SJ, Wang CJ, Chen DS. The large form of hepatitis delta antigen is crucial for assembly of hepatitis delta virus. Proc Natl Acad Sci USA. 1991;88:8490-8494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 265] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 52. | Lee CZ, Chen PJ, Chen DS. Large hepatitis delta antigen in packaging and replication inhibition: role of the carboxyl-terminal 19 amino acids and amino-terminal sequences. J Virol. 1995;69:5332-5336. [PubMed] |
| 53. | Hwang SB, Lai MM. Isoprenylation mediates direct protein-protein interactions between hepatitis large delta antigen and hepatitis B virus surface antigen. J Virol. 1993;67:7659-7662. [PubMed] |
| 54. | Sureau C, Guerra B, Lanford RE. Role of the large hepatitis B virus envelope protein in infectivity of the hepatitis delta virion. J Virol. 1993;67:366-372. [PubMed] |
| 55. | Sheu SY, Chen KL, Lee YW, Lo SJ. No intermolecular interaction between the large hepatitis delta antigens is required for the secretion with hepatitis B surface antigen: a model of empty HDV particle. Virology. 1996;218:275-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 56. | Schaper M, Rodriguez-Frias F, Jardi R, Tabernero D, Homs M, Ruiz G, Quer J, Esteban R, Buti M. Quantitative longitudinal evaluations of hepatitis delta virus RNA and hepatitis B virus DNA shows a dynamic, complex replicative profile in chronic hepatitis B and D. J Hepatol. 2010;52:658-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 155] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 57. | Saracco G, Rosina F, Brunetto MR, Amoroso P, Caredda F, Farci P, Piantino P, Bonino F, Rizzetto M. Rapidly progressive HBsAg-positive hepatitis in Italy. The role of hepatitis delta virus infection. J Hepatol. 1987;5:274-281. [PubMed] |
| 58. | Arico S, Aragona M, Rizzetto M, Caredda F, Zanetti A, Marinucci G, Diana S, Farci P, Arnone M, Caporaso N. Clinical significance of antibody to the hepatitis delta virus in symptomless HBsAg carriers. Lancet. 1985;2:356-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 44] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 59. | Craxì A, Raimondo G, Longo G, Giannuoli G, De Pasquale R, Caltagirone M, Patti S, Squadrito G, Pagliaro L. Delta agent infection in acute hepatitis and chronic HBsAg carriers with and without liver disease. Gut. 1984;25:1288-1290. [PubMed] |
| 60. | Liaw YF, Chen TJ, Chu CM, Lin HH. Acute hepatitis delta virus superinfection in patients with liver cirrhosis. J Hepatol. 1990;10:41-45. [PubMed] |
| 61. | Takyar V, Surana P, Kleiner DE, Wilkins K, Hoofnagle JH, Liang TJ, Heller T, Koh C. Noninvasive markers for staging fibrosis in chronic delta hepatitis. Aliment Pharmacol Ther. 2017;45:127-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 62. | Govindarajan S, Fields HA, Humphrey CD, Margolis HS. Pathologic and ultrastructural changes of acute and chronic delta hepatitis in an experimentally infected chimpanzee. Am J Pathol. 1986;122:315-322. [PubMed] |
| 63. | Colombo P, Di Blasi F, Magrin S, Fabiano C, Di Marco V, D'Amelio L, Lojacono F, Spinelli G, Craxì A. Smouldering hepatitis B virus replication in patients with chronic liver disease and hepatitis delta virus superinfection. J Hepatol. 1991;12:64-69. [PubMed] |
| 64. | Smedile A, Farci P, Verme G, Caredda F, Cargnel A, Caporaso N, Dentico P, Trepo C, Opolon P, Gimson A, Vergani D, Williams R, Rizzetto M. Influence of delta infection on severity of hepatitis B. Lancet. 1982;2:945-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 283] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 65. | Cole SM, Gowans EJ, Macnaughton TB, Hall PD, Burrell CJ. Direct evidence for cytotoxicity associated with expression of hepatitis delta virus antigen. Hepatology. 1991;13:845-851. [PubMed] |
| 66. | Grabowski J, Yurdaydìn C, Zachou K, Buggisch P, Hofmann WP, Jaroszewicz J, Schlaphoff V, Manns MP, Cornberg M, Wedemeyer H; HIDIT-1 study group. Hepatitis D virus-specific cytokine responses in patients with chronic hepatitis delta before and during interferon alfa-treatment. Liver Int. 2011;31:1395-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 67. | Giersch K, Allweiss L, Volz T, Helbig M, Bierwolf J, Lohse AW, Pollok JM, Petersen J, Dandri M, Lütgehetmann M. Hepatitis Delta co-infection in humanized mice leads to pronounced induction of innate immune responses in comparison to HBV mono-infection. J Hepatol. 2015;63:346-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 68. | Lunemann S, Malone DF, Grabowski J, Port K, Béziat V, Bremer B, Malmberg KJ, Manns MP, Sandberg JK, Cornberg M, Ljunggren HG, Wedemeyer H, Björkström NK. Effects of HDV infection and pegylated interferon α treatment on the natural killer cell compartment in chronically infected individuals. Gut. 2015;64:469-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 69. | Diaz G, Engle RE, Tice A, Melis M, Montenegro S, Rodriguez-Canales J, Hanson J, Emmert-Buck MR, Bock KW, Moore IN, Zamboni F, Govindarajan S, Kleiner DE, Farci P. Molecular Signature and Mechanisms of Hepatitis D Virus-Associated Hepatocellular Carcinoma. Mol Cancer Res. 2018;16:1406-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 70. | Townsend EC, Zhang GY, Ali R, Firke M, Moon MS, Han MAT, Fram B, Glenn JS, Kleiner DE, Koh C, Heller T. The balance of type 1 and type 2 immune responses in the contexts of hepatitis B infection and hepatitis D infection. J Gastroenterol Hepatol. 2019;34:764-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 71. | Kefalakes H, Koh C, Sidney J, Amanakis G, Sette A, Heller T, Rehermann B. Hepatitis D Virus-Specific CD8+ T Cells Have a Memory-Like Phenotype Associated With Viral Immune Escape in Patients With Chronic Hepatitis D Virus Infection. Gastroenterology. 2019;156:1805-1819.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 72. | Krogsgaard K, Kryger P, Aldershvile J, Andersson P, Sørensen TI, Nielsen JO. Delta-infection and suppression of hepatitis B virus replication in chronic HBsAg carriers. Hepatology. 1987;7:42-45. [PubMed] |
| 73. | Buti M, Estebán R, Jardi R, Allende H, Estebán JI, Genesca J, Guardia J. Clinical and serological outcome of acute delta infection. J Hepatol. 1987;5:59-64. [PubMed] |
| 74. | Moestrup T, Hansson BG, Widell A, Nordenfelt E. Clinical aspects of delta infection. Br Med J (Clin Res Ed). 1983;286:87-90. [PubMed] |
| 75. | Caredda F, Rossi E, d'Arminio Monforte A, Zampini L, Re T, Meroni B, Moroni M. Hepatitis B virus-associated coinfection and superinfection with delta agent: indistinguishable disease with different outcome. J Infect Dis. 1985;151:925-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 76. | Wu JC, Chen TZ, Huang YS, Yen FS, Ting LT, Sheng WY, Tsay SH, Lee SD. Natural history of hepatitis D viral superinfection: significance of viremia detected by polymerase chain reaction. Gastroenterology. 1995;108:796-802. [PubMed] |
| 77. | Smedile A, Dentico P, Zanetti A, Sagnelli E, Nordenfelt E, Actis GC, Rizzetto M. Infection with the delta agent in chronic HBsAg carriers. Gastroenterology. 1981;81:992-997. [PubMed] |
| 78. | Calle Serrano B, Großhennig A, Homs M, Heidrich B, Erhardt A, Deterding K, Jaroszewicz J, Bremer B, Koch A, Cornberg M, Manns MP, Buti M, Wedemeyer H. Development and evaluation of a baseline-event-anticipation score for hepatitis delta. J Viral Hepat. 2014;21:e154-e163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 79. | Lutterkort GL, Wranke A, Yurdaydin C, Budde E, Westphal M, Lichtinghagen R, Stift J, Bremer B, Hardtke S, Keskin O, Idilman R, Koch A, Manns MP, Dienes HP, Wedemeyer H, Heidrich B. Non-invasive fibrosis score for hepatitis delta. Liver Int. 2017;37:196-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 80. | Rosina F, Pintus C, Meschievitz C, Rizzetto M. A randomized controlled trial of a 12-month course of recombinant human interferon-alpha in chronic delta (type D) hepatitis: a multicenter Italian study. Hepatology. 1991;13:1052-1056. [PubMed] |
| 81. | Farci P, Mandas A, Coiana A, Lai ME, Desmet V, Van Eyken P, Gibo Y, Caruso L, Scaccabarozzi S, Criscuolo D. Treatment of chronic hepatitis D with interferon alfa-2a. N Engl J Med. 1994;330:88-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 219] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 82. | Farci P, Roskams T, Chessa L, Peddis G, Mazzoleni AP, Scioscia R, Serra G, Lai ME, Loy M, Caruso L, Desmet V, Purcell RH, Balestrieri A. Long-term benefit of interferon alpha therapy of chronic hepatitis D: regression of advanced hepatic fibrosis. Gastroenterology. 2004;126:1740-1749. [PubMed] |
| 83. | Castelnau C, Le Gal F, Ripault MP, Gordien E, Martinot-Peignoux M, Boyer N, Pham BN, Maylin S, Bedossa P, Dény P, Marcellin P, Gault E. Efficacy of peginterferon alpha-2b in chronic hepatitis delta: relevance of quantitative RT-PCR for follow-up. Hepatology. 2006;44:728-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 176] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 84. | Garripoli A, Di Marco V, Cozzolongo R, Costa C, Smedile A, Fabiano A, Bonino F, Rizzetto M, Verme G, Craxi A. Ribavirin treatment for chronic hepatitis D: a pilot study. Liver. 1994;14:154-157. [PubMed] |
| 85. | Erhardt A, Gerlich W, Starke C, Wend U, Donner A, Sagir A, Heintges T, Häussinger D. Treatment of chronic hepatitis delta with pegylated interferon-alpha2b. Liver Int. 2006;26:805-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 103] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 86. | Niro GA, Ciancio A, Gaeta GB, Smedile A, Marrone A, Olivero A, Stanzione M, David E, Brancaccio G, Fontana R, Perri F, Andriulli A, Rizzetto M. Pegylated interferon alpha-2b as monotherapy or in combination with ribavirin in chronic hepatitis delta. Hepatology. 2006;44:713-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 175] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 87. | Ormeci N, Bölükbaş F, Erden E, Coban S, Ekiz F, Erdem H, Palabıyıkoğlu M, Beyler AR, Balık I, Bölükbaş C, Nazlıgül Y, Köklü S. Pegylated interferon alfa-2B for chronic delta hepatitis: 12 versus 24 months. Hepatogastroenterology. 2011;58:1648-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 88. | Heller T, Rotman Y, Koh C, Clark S, Haynes-Williams V, Chang R, McBurney R, Schmid P, Albrecht J, Kleiner DE, Ghany MG, Liang TJ, Hoofnagle JH. Long-term therapy of chronic delta hepatitis with peginterferon alfa. Aliment Pharmacol Ther. 2014;40:93-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 89. | Niro GA, Ciancio A, Tillman HL, Lagget M, Olivero A, Perri F, Fontana R, Little N, Campbell F, Smedile A, Manns MP, Andriulli A, Rizzetto M. Lamivudine therapy in chronic delta hepatitis: a multicentre randomized-controlled pilot study. Aliment Pharmacol Ther. 2005;22:227-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 90] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 90. | Mansour W, Ducancelle A, Le Gal F, Le Guillou-Guillemette H, Abgueguen P, Pivert A, Calès P, Gordien E, Lunel F. Resolution of chronic hepatitis Delta after 1 year of combined therapy with pegylated interferon, tenofovir and emtricitabine. J Clin Virol. 2010;47:97-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 91. | Wedemeyer H, Yurdaydìn C, Dalekos GN, Erhardt A, Çakaloğlu Y, Değertekin H, Gürel S, Zeuzem S, Zachou K, Bozkaya H, Koch A, Bock T, Dienes HP, Manns MP; HIDIT Study Group. Peginterferon plus adefovir versus either drug alone for hepatitis delta. N Engl J Med. 2011;364:322-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 369] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 92. | Heidrich B, Yurdaydın C, Kabaçam G, Ratsch BA, Zachou K, Bremer B, Dalekos GN, Erhardt A, Tabak F, Yalcin K, Gürel S, Zeuzem S, Cornberg M, Bock CT, Manns MP, Wedemeyer H; HIDIT-1 Study Group. Late HDV RNA relapse after peginterferon alpha-based therapy of chronic hepatitis delta. Hepatology. 2014;60:87-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 230] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 93. | Keskin O, Wedemeyer H, Tüzün A, Zachou K, Deda X, Dalekos GN, Heidrich B, Pehlivan S, Zeuzem S, Yalçın K, Gürel S, Tabak F, Idilman R, Bozkaya H, Manns M, Yurdaydin C. Association Between Level of Hepatitis D Virus RNA at Week 24 of Pegylated Interferon Therapy and Outcome. Clin Gastroenterol Hepatol. 2015;13:2342-49.e1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 94. | Wedemeyer H, Yurdaydin C, Hardtke S, Caruntu FA, Curescu MG, Yalcin K, Akarca US, Gürel S, Zeuzem S, Erhardt A, Lüth S, Papatheodoridis GV, Keskin O, Port K, Radu M, Celen MK, Idilman R, Weber K, Stift J, Wittkop U, Heidrich B, Mederacke I, von der Leyen H, Dienes HP, Cornberg M, Koch A, Manns MP; HIDIT-II study team. Peginterferon alfa-2a plus tenofovir disoproxil fumarate for hepatitis D (HIDIT-II): a randomised, placebo controlled, phase 2 trial. Lancet Infect Dis. 2019;19:275-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 154] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 95. | Wranke A, Serrano BC, Heidrich B, Kirschner J, Bremer B, Lehmann P, Hardtke S, Deterding K, Port K, Westphal M, Manns MP, Cornberg M, Wedemeyer H. Antiviral treatment and liver-related complications in hepatitis delta. Hepatology. 2017;65:414-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 98] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 96. | Wranke A, Heidrich B, Ernst S, Calle Serrano B, Caruntu FA, Curescu MG, Yalcin K, Gürel S, Zeuzem S, Erhardt A, Lüth S, Papatheodoridis GV, Bremer B, Stift J, Grabowski J, Kirschner J, Port K, Cornberg M, Falk CS, Dienes HP, Hardtke S, Manns MP, Yurdaydin C, Wedemeyer H; HIDIT-2 Study Group. Anti-HDV IgM as a marker of disease activity in hepatitis delta. PLoS One. 2014;9:e101002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 97. | Gripon P, Le Seyec J, Rumin S, Guguen-Guillouzo C. Myristylation of the hepatitis B virus large surface protein is essential for viral infectivity. Virology. 1995;213:292-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 153] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 98. | Blanchet M, Sureau C. Infectivity determinants of the hepatitis B virus pre-S domain are confined to the N-terminal 75 amino acid residues. J Virol. 2007;81:5841-5849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 112] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 99. | Schulze A, Schieck A, Ni Y, Mier W, Urban S. Fine mapping of pre-S sequence requirements for hepatitis B virus large envelope protein-mediated receptor interaction. J Virol. 2010;84:1989-2000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 198] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 100. | Gripon P, Cannie I, Urban S. Efficient inhibition of hepatitis B virus infection by acylated peptides derived from the large viral surface protein. J Virol. 2005;79:1613-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 287] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 101. | Schieck A, Schulze A, Gähler C, Müller T, Haberkorn U, Alexandrov A, Urban S, Mier W. Hepatitis B virus hepatotropism is mediated by specific receptor recognition in the liver and not restricted to susceptible hosts. Hepatology. 2013;58:43-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 102. | Petersen J, Dandri M, Mier W, Lütgehetmann M, Volz T, von Weizsäcker F, Haberkorn U, Fischer L, Pollok JM, Erbes B, Seitz S, Urban S. Prevention of hepatitis B virus infection in vivo by entry inhibitors derived from the large envelope protein. Nat Biotechnol. 2008;26:335-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 338] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 103. | Lütgehetmann M, Mancke LV, Volz T, Helbig M, Allweiss L, Bornscheuer T, Pollok JM, Lohse AW, Petersen J, Urban S, Dandri M. Humanized chimeric uPA mouse model for the study of hepatitis B and D virus interactions and preclinical drug evaluation. Hepatology. 2012;55:685-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 184] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 104. | Volz T, Allweiss L, Ben MBarek M, Warlich M, Lohse AW, Pollok JM, Alexandrov A, Urban S, Petersen J, Lütgehetmann M, Dandri M. The entry inhibitor Myrcludex-B efficiently blocks intrahepatic virus spreading in humanized mice previously infected with hepatitis B virus. J Hepatol. 2013;58:861-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 272] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 105. | Donkers JM, Zehnder B, van Westen GJP, Kwakkenbos MJ, IJzerman AP, Oude Elferink RPJ, Beuers U, Urban S, van de Graaf SFJ. Reduced hepatitis B and D viral entry using clinically applied drugs as novel inhibitors of the bile acid transporter NTCP. Sci Rep. 2017;7:15307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 106. | Bogomolov P, Alexandrov A, Voronkova N, Macievich M, Kokina K, Petrachenkova M, Lehr T, Lempp FA, Wedemeyer H, Haag M, Schwab M, Haefeli WE, Blank A, Urban S. Treatment of chronic hepatitis D with the entry inhibitor myrcludex B: First results of a phase Ib/IIa study. J Hepatol. 2016;65:490-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 305] [Article Influence: 30.5] [Reference Citation Analysis (1)] |
| 107. | Wedemeyer H, Bogomolov P, Blank A. Final results of a multicenter, open-label phase 2b clinical trial to assess safety and efficacy of Myrcludex B in combination with tenofovir in patients with chronic HBV/HDV co-infection. J Hepatol. 2018;. |
| 108. | Wedemeyer H, Schöneweis K, Bogomolov P. Interim results of a multicentre, open-label phase 2 clinical trial (MYR203) to assess safety and efficacy of Myrcludex B in combination with peg-Interferon alpha 2a in patients with chronic HBV/HDV co-infection. Hepatology. 2018;. |
| 109. | Bordier BB, Marion PL, Ohashi K, Kay MA, Greenberg HB, Casey JL, Glenn JS. A prenylation inhibitor prevents production of infectious hepatitis delta virus particles. J Virol. 2002;76:10465-10472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 109] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 110. | Koh C, Canini L, Dahari H, Zhao X, Uprichard SL, Haynes-Williams V, Winters MA, Subramanya G, Cooper SL, Pinto P, Wolff EF, Bishop R, Ai Thanda Han M, Cotler SJ, Kleiner DE, Keskin O, Idilman R, Yurdaydin C, Glenn JS, Heller T. Oral prenylation inhibition with lonafarnib in chronic hepatitis D infection: a proof-of-concept randomised, double-blind, placebo-controlled phase 2A trial. Lancet Infect Dis. 2015;15:1167-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 212] [Article Influence: 19.3] [Reference Citation Analysis (3)] |
| 111. | Yurdaydin C, Keskin O, Kalkan Ç, Karakaya F, Çalişkan A, Karatayli E, Karatayli S, Bozdayi AM, Koh C, Heller T, Idilman R, Glenn JS. Optimizing lonafarnib treatment for the management of chronic delta hepatitis: The LOWR HDV-1 study. Hepatology. 2018;67:1224-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 110] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 112. | Yurdaydin C, ldilman R, Keskin. A phase 2 dose optimization study of lonafarnib with ritonavir for the treatment of chronic delta hepatitis-end of treatment results from the LOWR HDV-2 study. J Hepatol. 2017;. |
| 113. | Koh C, Surana P, Han T, Fryzek N, Kapuria D, Etzion O. A phase 2 study exploring once daily dosing of ritonavir boosted lonafarnib for the treatment of chronic delta hepatitis - end of study results from the LOWR HDV-3 study. J Hepatol. 2017;. |
| 114. | Wedemeyer H, Port K, Deterding K. A phase 2 dose-escalation study of lonafarnib plus ritonavir in patients with chronic hepatitis D: final results from the lonafamib with ritonavir in HDV-4 (LOWR HDV-4) study. J Hepatol. 2017;. |
| 115. | Vaillant A, Juteau JM, Lu H, Liu S, Lackman-Smith C, Ptak R, Jiang S. Phosphorothioate oligonucleotides inhibit human immunodeficiency virus type 1 fusion by blocking gp41 core formation. Antimicrob Agents Chemother. 2006;50:1393-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 116. | Noordeen F, Vaillant A, Jilbert AR. Nucleic acid polymers inhibit duck hepatitis B virus infection in vitro. Antimicrob Agents Chemother. 2013;57:5291-5298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 117. | Noordeen F, Vaillant A, Jilbert AR. Nucleic acid polymers prevent the establishment of duck hepatitis B virus infection in vivo. Antimicrob Agents Chemother. 2013;57:5299-5306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 118. | Noordeen F, Scougall CA, Grosse A, Qiao Q, Ajilian BB, Reaiche-Miller G, Finnie J, Werner M, Broering R, Schlaak JF, Vaillant A, Jilbert AR. Therapeutic Antiviral Effect of the Nucleic Acid Polymer REP 2055 against Persistent Duck Hepatitis B Virus Infection. PLoS One. 2015;10:e0140909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 119. | Al-Mahtab M, Bazinet M, Vaillant A. Safety and Efficacy of Nucleic Acid Polymers in Monotherapy and Combined with Immunotherapy in Treatment-Naive Bangladeshi Patients with HBeAg+ Chronic Hepatitis B Infection. PLoS One. 2016;11:e0156667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 156] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 120. | Bazinet M, Pântea V, Cebotarescu V, Cojuhari L, Jimbei P, Albrecht J, Schmid P, Le Gal F, Gordien E, Krawczyk A, Mijočević H, Karimzadeh H, Roggendorf M, Vaillant A. Safety and efficacy of REP 2139 and pegylated interferon alfa-2a for treatment-naive patients with chronic hepatitis B virus and hepatitis D virus co-infection (REP 301 and REP 301-LTF): a non-randomised, open-label, phase 2 trial. Lancet Gastroenterol Hepatol. 2017;2:877-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 204] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 121. | Bazinet N, Pantea V, Cebotarescu V, Cojuhari L, Jimbei P, Vaillant A. Establishment of persistent functional remission of HBV and HDV infection following REP 2139 and pegylated interferon alpha 2a therapy in patients with chronic HBV/HDV co-infection: 18 month follow-up results from the REP 301-LTF study. J Hepatol. 2018;. |
| 122. | Bazinet M, Pantea V, Moscalu I, Cebotarescu V, Cojuhari L, Jimbei P. Interim follow-up analysis in the REP 401 protocol: functional control of HBeAg negative chronic HBV infection persists after withdrawal of combined therapy with REP 2139 or REP 2165, tenofovir disoproxil fumarate and pegylated interferon α-2a. Hepatology. 2018;. |
| 123. | Kotenko SV, Gallagher G, Baurin VV, Lewis-Antes A, Shen M, Shah NK, Langer JA, Sheikh F, Dickensheets H, Donnelly RP. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol. 2003;4:69-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1416] [Cited by in RCA: 1500] [Article Influence: 65.2] [Reference Citation Analysis (10)] |
| 124. | Muir AJ, Shiffman ML, Zaman A, Yoffe B, de la Torre A, Flamm S, Gordon SC, Marotta P, Vierling JM, Lopez-Talavera JC, Byrnes-Blake K, Fontana D, Freeman J, Gray T, Hausman D, Hunder NN, Lawitz E. Phase 1b study of pegylated interferon lambda 1 with or without ribavirin in patients with chronic genotype 1 hepatitis C virus infection. Hepatology. 2010;52:822-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 187] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 125. | Muir AJ, Arora S, Everson G, Flisiak R, George J, Ghalib R, Gordon SC, Gray T, Greenbloom S, Hassanein T, Hillson J, Horga MA, Jacobson IM, Jeffers L, Kowdley KV, Lawitz E, Lueth S, Rodriguez-Torres M, Rustgi V, Shemanski L, Shiffman ML, Srinivasan S, Vargas HE, Vierling JM, Xu D, Lopez-Talavera JC, Zeuzem S; EMERGE study group. A randomized phase 2b study of peginterferon lambda-1a for the treatment of chronic HCV infection. J Hepatol. 2014;61:1238-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 126. | Wang X, Hruska M, Chan P, Ahmad A, Freeman J, Horga MA, Hillson J, Kansra V, Lopez-Talavera JC. Derivation of Phase 3 dosing for peginterferon lambda-1a in chronic hepatitis C, Part 1: Modeling optimal treatment duration and sustained virologic response rates. J Clin Pharmacol. 2015;55:63-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 127. | Robek MD, Boyd BS, Chisari FV. Lambda interferon inhibits hepatitis B and C virus replication. J Virol. 2005;79:3851-3854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 354] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 128. | Chan HLY, Ahn SH, Chang TT, Peng CY, Wong D, Coffin CS, Lim SG, Chen PJ, Janssen HLA, Marcellin P, Serfaty L, Zeuzem S, Cohen D, Critelli L, Xu D, Wind-Rotolo M, Cooney E; LIRA-B Study Team. Peginterferon lambda for the treatment of HBeAg-positive chronic hepatitis B: A randomized phase 2b study (LIRA-B). J Hepatol. 2016;64:1011-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 129. | Dickensheets H, Sheikh F, Park O, Gao B, Donnelly RP. Interferon-lambda (IFN-λ) induces signal transduction and gene expression in human hepatocytes, but not in lymphocytes or monocytes. J Leukoc Biol. 2013;93:377-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 130. | Pagliaccetti NE, Chu EN, Bolen CR, Kleinstein SH, Robek MD. Lambda and alpha interferons inhibit hepatitis B virus replication through a common molecular mechanism but with different in vivo activities. Virology. 2010;401:197-206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 131. | Radaeva S, Sun R, Pan HN, Hong F, Gao B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology. 2004;39:1332-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 507] [Article Influence: 23.0] [Reference Citation Analysis (7)] |
| 132. | Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Flavell RA. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 2007;27:647-659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 527] [Cited by in RCA: 550] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 133. | Etzion O, Hamid S, Yurie L. End of Study Results from LIMT HDV Study: 36% Durable Virologic Response at 24 Weeks Post-Treatment with Pegylated Interferon Lambda Monotherapy in Patients with Chronic hepatitis Delta Virus Infection. J Hepatology. 2019;70:e32. |
| 134. | Yurdaydin C, Abbas Z, Buti M, Cornberg M, Esteban R, Etzion O, Gane EJ, Gish RG, Glenn JS, Hamid S, Heller T, Koh C, Lampertico P, Lurie Y, Manns M, Parana R, Rizzetto M, Urban S, Wedemeyer H; Hepatitis Delta International Network (HDIN). Treating chronic hepatitis delta: The need for surrogate markers of treatment efficacy. J Hepatol. 2019;70:1008-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 135. | Durantel D. New treatments to reach functional cure: Virological approaches. Best Pract Res Clin Gastroenterol. 2017;31:329-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 136. | Yurdaydin C. New treatment options for delta virus: Is a cure in sight? J Viral Hepat. 2019;26:618-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 137. | Makuwa M, Mintsa-Ndong A, Souquière S, Nkoghé D, Leroy EM, Kazanji M. Prevalence and molecular diversity of hepatitis B virus and hepatitis delta virus in urban and rural populations in northern Gabon in central Africa. J Clin Microbiol. 2009;47:2265-2268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 138. | Opaleye OO, Japhet OM, Adewumi OM, Omoruyi EC, Akanbi OA, Oluremi AS, Wang B, Tong Hv, Velavan TP, Bock CT. Molecular epidemiology of hepatitis D virus circulating in Southwestern Nigeria. Virol J. 2016;13:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 139. | Andernach IE, Leiss LV, Tarnagda ZS, Tahita MC, Otegbayo JA, Forbi JC, Omilabu S, Gouandjika-Vasilache I, Komas NP, Mbah OP, Muller CP. Characterization of hepatitis delta virus in sub-Saharan Africa. J Clin Microbiol. 2014;52:1629-1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 140. | Yacoubi L, Brichler S, Mansour W, Le Gal F, Hammami W, Sadraoui A, Ben Mami N, Msaddek A, Cheikh I, Triki H, Gordien E. Molecular epidemiology of hepatitis B and Delta virus strains that spread in the Mediterranean North East Coast of Tunisia. J Clin Virol. 2015;72:126-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 141. | Nakasone H, Sakugawa H, Shokita H, Nakayoshi T, Kawakami Y, Kinjo F, Saito A, Shinjo M, Adaniya H, Mizushima T, Taira M. Prevalence and clinical features of hepatitis delta virus infection in the Miyako Islands, Okinawa, Japan. J Gastroenterol. 1998;33:850-854. [PubMed] |
| 142. | Tsatsralt-Od B, Takahashi M, Nishizawa T, Endo K, Inoue J, Okamoto H. High prevalence of dual or triple infection of hepatitis B, C, and delta viruses among patients with chronic liver disease in Mongolia. J Med Virol. 2005;77:491-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 143. | Han M, Littlejohn M, Yuen L, Edwards R, Devi U, Bowden S, Ning Q, Locarnini S, Jackson K. Molecular epidemiology of hepatitis delta virus in the Western Pacific region. J Clin Virol. 2014;61:34-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 144. | Patel EU, Thio CL, Boon D, Thomas DL, Tobian AAR. Prevalence of Hepatitis B and Hepatitis D Virus Infections in the United States, 2011-2016. Clin Infect Dis. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 145. | Viana S, Paraná R, Moreira RC, Compri AP, Macedo V. High prevalence of hepatitis B virus and hepatitis D virus in the western Brazilian Amazon. Am J Trop Med Hyg. 2005;73:808-814. [PubMed] |
| 146. | Fonseca JCF, Simonetti SRR, Schatzmayr HG, Castejon MJ, Cesario ALO, Simonetti JP. Prevalence of Infection with hepatitis Delta Virus (Hdv) among Carriers of Hepatitis-B Surface-Antigen in Amazonas State, Brazil. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1988;82:469-471. [RCA] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 147. | Ho E, Deltenre P, Nkuize M, Delwaide J, Colle I, Michielsen P; Belgian Association for the Study of the Liver. Coinfection of hepatitis B and hepatitis delta virus in Belgium: a multicenter BASL study. Prospective epidemiology and comparison with HBV mono-infection. J Med Virol. 2013;85:1513-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 148. | Manesis EK, Vourli G, Dalekos G, Vasiliadis T, Manolaki N, Hounta A, Koutsounas S, Vafiadis I, Nikolopoulou G, Giannoulis G, Germanidis G, Papatheodoridis G, Touloumi G. Prevalence and clinical course of hepatitis delta infection in Greece: a 13-year prospective study. J Hepatol. 2013;59:949-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 149. | Niro GA, Smedile A, Ippolito AM, Ciancio A, Fontana R, Olivero A, Valvano MR, Abate ML, Gioffreda D, Caviglia GP, Rizzetto M, Andriulli A. Outcome of chronic delta hepatitis in Italy: a long-term cohort study. J Hepatol. 2010;53:834-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 125] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 150. | Sagnelli E, Stroffolini T, Ascione A, Bonino F, Chiaramonte M, Colombo M, Craxi A, Giusti G, Manghisi OG, Pastore G. The epidemiology of hepatitis delta infection in Italy. Promoting Group. J Hepatol. 1992;15:211-215. [PubMed] |
| 151. | El Bouzidi K, Elamin W, Kranzer K, Irish DN, Ferns B, Kennedy P, Rosenberg W, Dusheiko G, Sabin CA, Smith BC, Nastouli E. Hepatitis delta virus testing, epidemiology and management: a multicentre cross-sectional study of patients in London. J Clin Virol. 2015;66:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 152. | Aguilera A, Trastoy R, Rodríguez-Calviño J, Manso T, de Mendoza C, Soriano V. Prevalence and incidence of hepatitis delta in patients with chronic hepatitis B in Spain. Eur J Gastroenterol Hepatol. 2018;30:1060-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
P-Reviewer: Andreone P, Lee HC, MizuguchiT, Wang L S-Editor: Ma RY L-Editor: A E-Editor: Zhang YL