Published online Aug 28, 2019. doi: 10.3748/wjg.v25.i32.4598
Peer-review started: May 13, 2019
First decision: May 30, 2019
Revised: July 13, 2019
Accepted: July 19, 2019
Article in press: July 19, 2019
Published online: August 28, 2019
Processing time: 110 Days and 9.2 Hours
Eosinophilic esophagitis is an immune-allergic pathology of multifactorial etiology (genetic and environmental) that affects both pediatric and adult patients. Its symptoms, which include heartburn, regurgitation, and esophageal stenosis (with dysphagia being more frequent in eosinophilic esophagitis in young adults and children), are similar to those of gastroesophageal reflux disease, causing delays in diagnosis and treatment. Although endoscopic findings such as furrows, esophageal mucosa trachealization, and whitish exudates may suggest its presence, this diagnosis should be confirmed histologically based on the presence of more than 15 eosinophils per high-power field and the exclusion of other causes of eosinophilia (parasitic infections, hypereosinophilic syndrome, inflammatory bowel disease, among others) for which treatment could be initiated. Currently, the 3 “D”s (“Drugs, Diet, and Dilation”) are considered the fundamental components of treatment. The first 2 components, which involve the use of proton pump inhibitors, corticosteroids, immunosuppressants and empirical diets or guided food elimination based on allergy tests, are more useful in the initial phases, whereas endoscopic dilation is reserved for esophageal strictures. Herein, the most important aspects of eosinophilic esophagitis pathophysiology will be reviewed, in addition to evidence for the various treatments.
Core tip: Eosinophilic esophagitis affects both pediatric and adult patients. It causes symptoms that are initially attributed to gastroesophageal reflux, for which treatment with proton pump inhibitors is prescribed, with limited response. The objective of this review is to define the fundamental aspects of the development of this disease and the complementary and beneficial role of proton pump inhibitors without neglecting the need for diets that involve spaced withdrawal of certain foods.
- Citation: Gómez-Aldana A, Jaramillo-Santos M, Delgado A, Jaramillo C, Lúquez-Mindiola A. Eosinophilic esophagitis: Current concepts in diagnosis and treatment. World J Gastroenterol 2019; 25(32): 4598-4613
- URL: https://www.wjgnet.com/1007-9327/full/v25/i32/4598.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i32.4598
Eosinophilic esophagitis (EoE) is a pathology that has emerged only recently. The first report in the literature dates from 1978[1], and as an emerging disease, EoE has gradually increased in frequency. As a pathological entity, it was recognized in the literature between 1993 and 1994 with the reports by Attwood and Straumann[2,3] that identified an exaggerated response of the immune system to contact with allergens. In the last decade, awareness of this pathology has increased, and the incidence and prevalence have increased[4]. A recent meta-analysis found that the incidence rate was 6.6/100000 person-years in children and 7.7/100000 person-years in adults and that the prevalence was 34 cases per 100000 children and 42.2 cases per 100000 adults[4]. It is more common in men, with a male to female ratio of 3:1[5]. More than 65% of cases occur during childhood and there is a peak between 30 and 44 years of age[6]. In population studies, a higher prevalence of EoE has been found in Europe and North America, whereas there is a low prevalence in Eastern countries, suggesting that it is associated with environmental and immune factors[6]. EoE is more common in rural areas with low population densities, which can be explained by vegetation, pollution, and other environmental factors[7], and it varies according to climate zone and season, with more frequent diagnoses during summer[8].
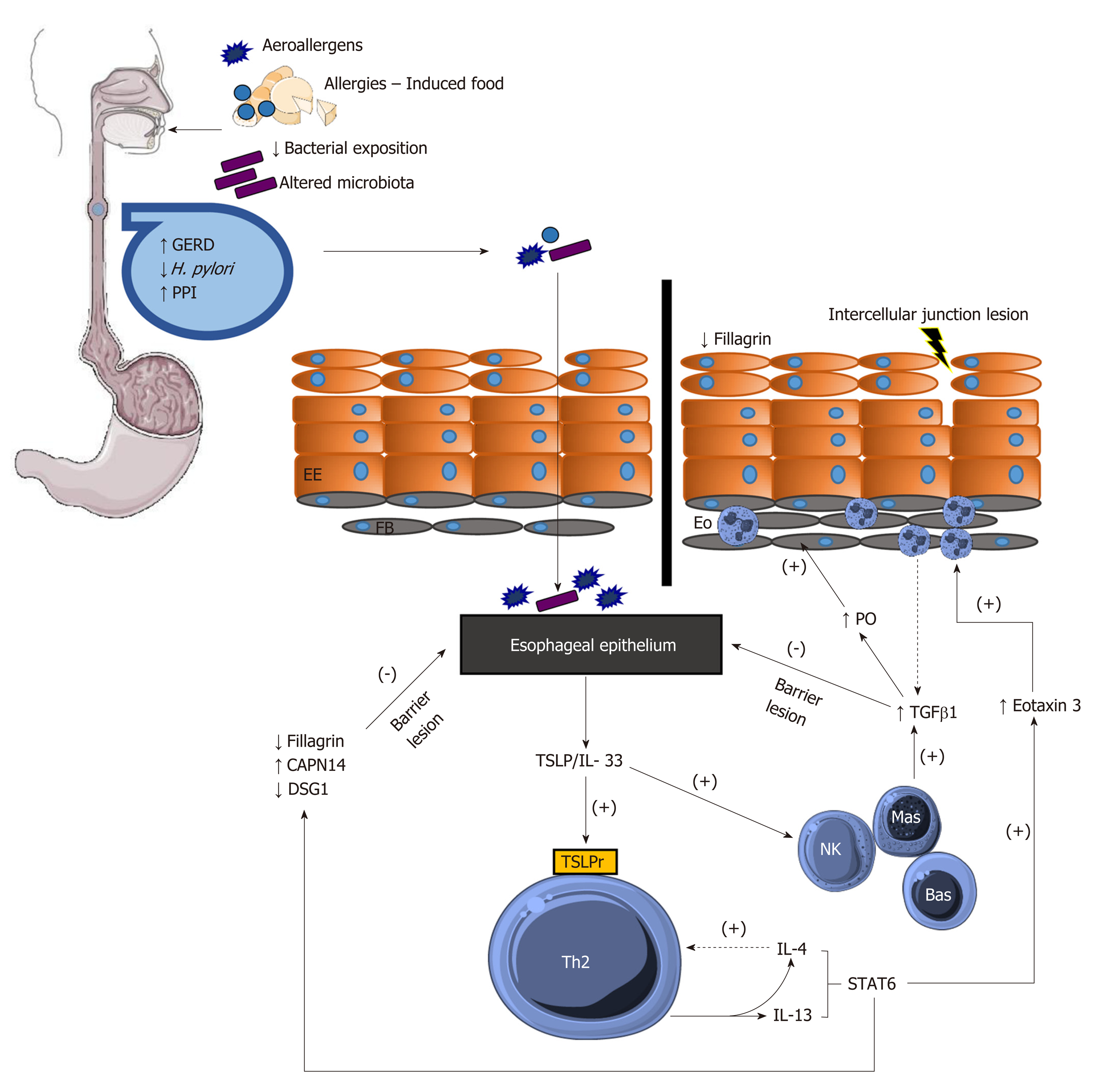
Several risk factors and mechanisms have been described by which external agents and factors inherent to each person may lead to EoE (Figure 1).
There are several factors that are thought to contribute to the increase in the incidence of EoE in recent years[9]: (1) The hypothesis of hygiene and bacterial dysbiosis, that is, that modern life and hygiene conditions have decreased the incidence of infections, resulting in less bacterial exposure and thus altering the body’s microbiota and epithelial permeability[10]; (2) Environmental factors, such as changes in aeroallergen air conditions and concentrations of airborne pollen[11,12] and/or genetic and chemical modifications used in the cultivation, processing, and packaging of food[9]; (3) Decreased frequency of infection by Helicobacter pylori (H. pylori) in certain countries (it has been found that this bacterium increases Th1 and Th17 populations, downregulating Th2)[13]; (4) Gastroesophageal reflux disease (GERD), an entity that leads to the injury of intraepithelial junctions, causing greater allergen permeability in the esophageal epithelium as measured by mucosal impedance[14,15]; and (5) The use of acid-suppressive drugs, which is paradoxical, given that proton pump inhibitors (PPIs) exert an anti-inflammatory effect by blocking eotaxin secretion[16] and inhibiting acid secretion, thereby reducing activation of digestive enzymes such that the antigens in food do not degrade and the digestive system is protected from the immune response mediated by these proteins[17].
The main foods that induce an immune response in a patient’s esophagus are milk, wheat, soy, eggs, peanuts/nuts, and fish/shellfish, a theory that is reaffirmed in studies that remove those 6 foods (SFED)[17]. Because of that observation, and because it involves an immune response mediated by Th2 lymphocytes, this disease is considered a form of food allergy[18].
Patients with EoE have genetic risk factors. Genetic variants in genes such as CCL26, which encodes eotaxin 3, thymic stromal lymphopoietin (TSLP), filaggrin (FLG), desmoglein-1 (DSG1), STAT6, calpain 14 (CAPN14) and CRLF2 have been identified in patients with EoE[19]. TSLP plays a very important role in the Th2-mediated immune response not only in EoE but also in other disease entities, such as asthma[20].
TSLP is overexpressed in the epithelium of people who have EoE; it activates Th2 lymphocytes, which in turn activate basophils[21]. Lastly, the secretion of IL-4, IL-5, IL-13, CCL3, and CCL4 activates the STAT5 and STAT6 pathways, the latter of which, in turn, regulates very important molecules such as eotaxin 3, calpain 14 and de-smoglein-1[21,22].
IL-13 induces the secretion of 2 molecules in the esophageal epithelium: First, eotaxin 3, an important chemokine that attracts eosinophils to the esophagus[23], causing remodeling and deposition of collagen in esophageal tissue[23], and second, calpain 14, a proteolytic enzyme specific to the esophagus that cleaves desmoglein-1[24], thus leading to disruption of the esophageal epithelial barrier. IL-13 then decreases the production of desmoglein-1, an important desmosomal protein, thus increasing lesions in the esophageal epithelial barrier[22].
EoE has not been shown to be influenced by immunoglobulin E (IgE)[25]; however, an increase in IgG4 has been found in tissue samples, and IgG4 specific to some SFED food allergens has been found in serum samples[26,27], which supports the theory that EoE is truly an IgG4-mediated disease. In clinical practice, immunostaining for IgG4 in esophageal biopsies has not been effective in diagnosing EoE, as it has a low sensitivity of 48%[28].
Lesions in the esophageal epithelial barrier are a key element of the pathophysiology of EoE. IL-13 plays an important role in epithelial lesions because it induces a decrease in filaggrin, a protein that is present in the stratum corneum of the esophagus[29], thus allowing the passage of allergens. Because desmoglein-1 is decreased, the barrier function of the esophageal epithelium is also diminished[30]. TGF-B1 produced by eosinophils and mast cells[31] also contributes to epithelial barrier dysfunction by decreasing the levels of claudin 7, an intercellular tight junction protein[32].
Once acute inflammation is established, several mechanisms are induced, causing chronic damage at the esophageal level. At this stage, TGF-B1 induces periostin, facilitating remodeling at the esophageal level, as well as an increase in smooth muscle and fibrotic tissue (Figure 1)[33].
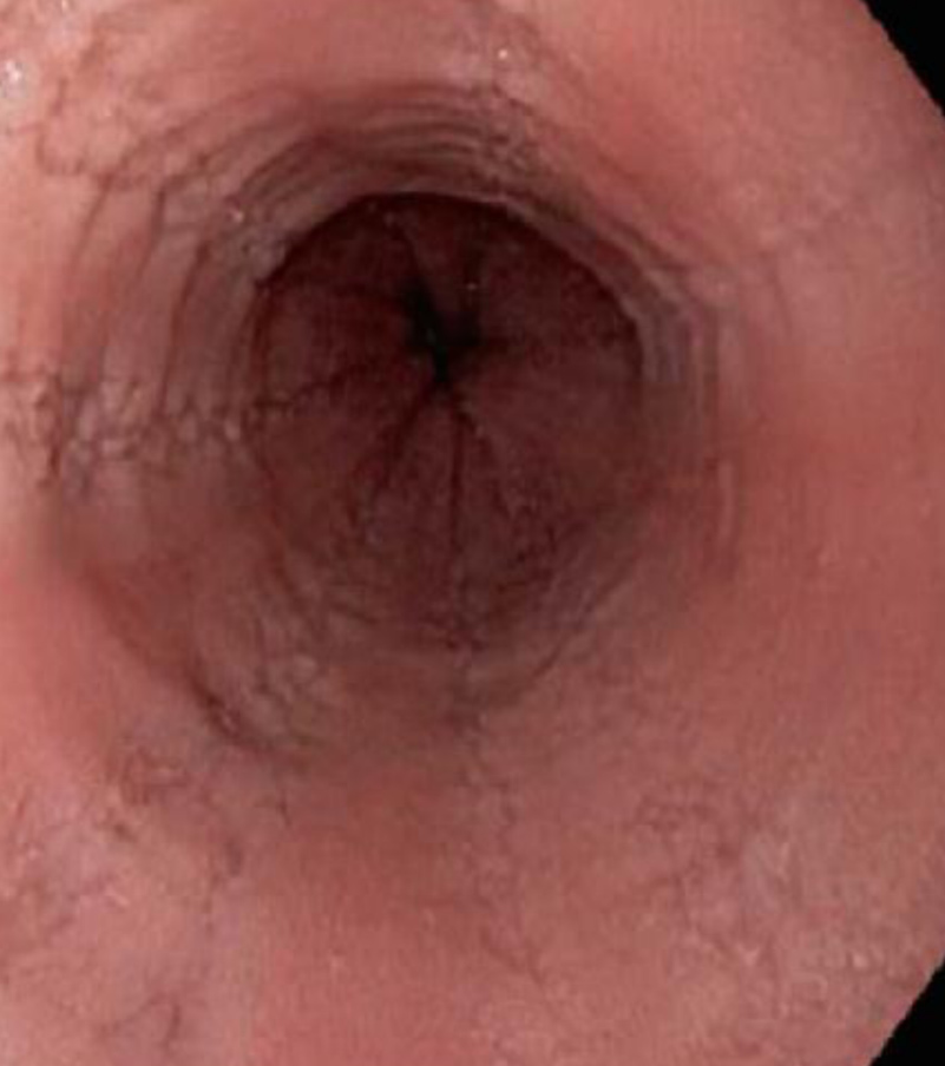
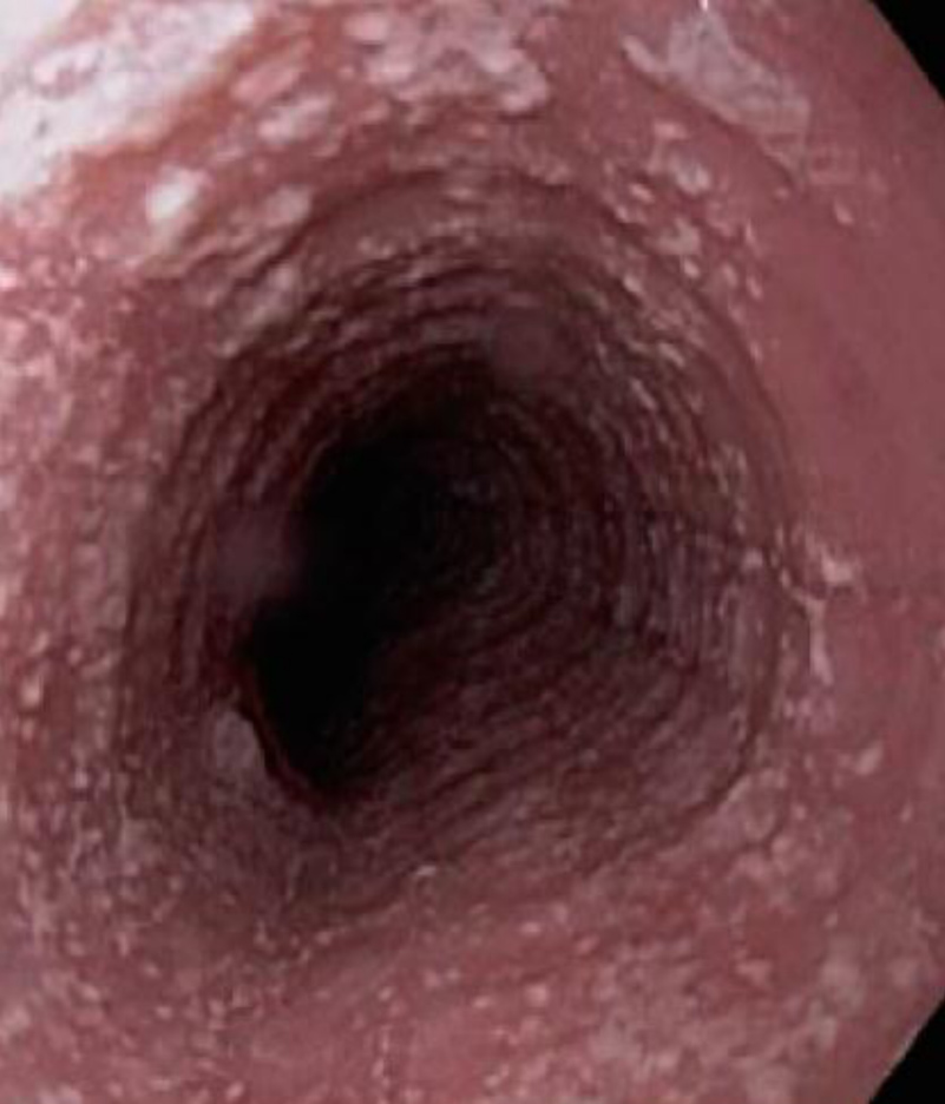
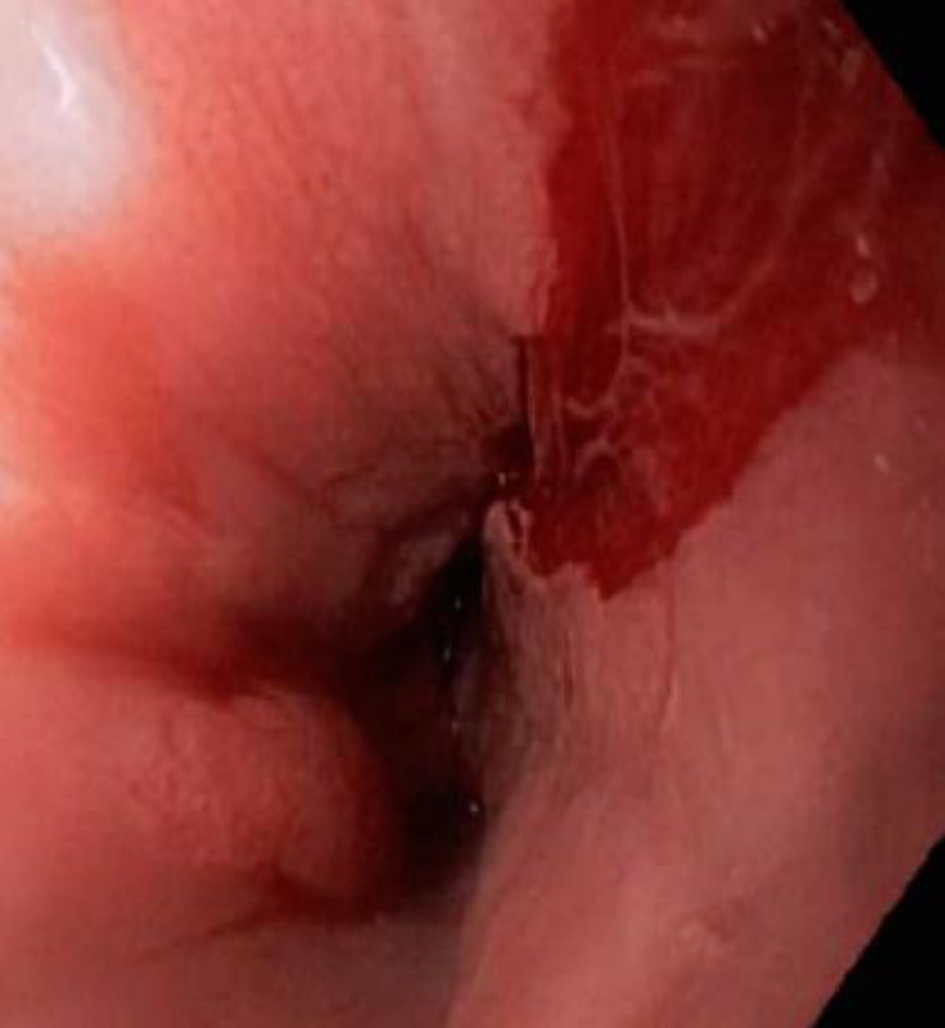
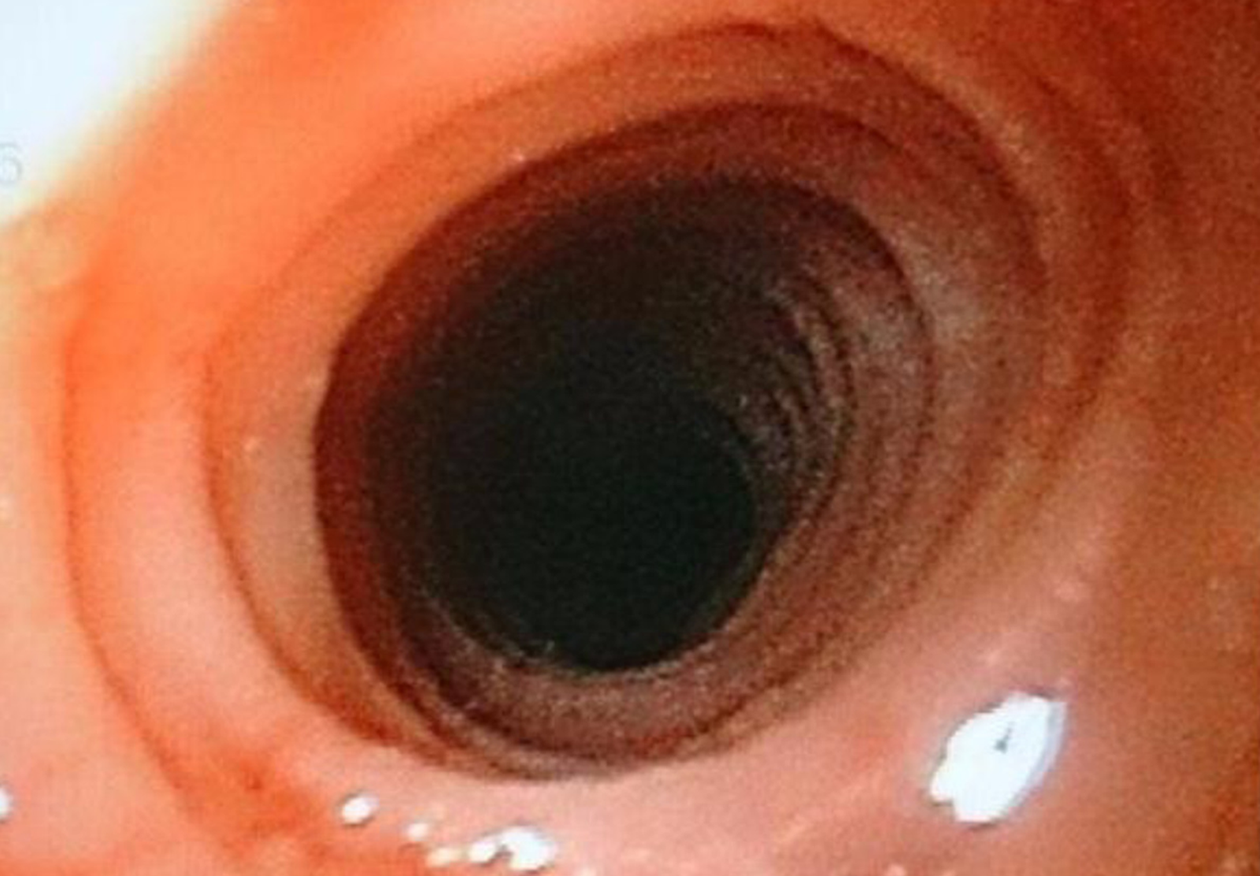
The diagnosis of EoE depends on the clinical manifestations and endoscopic and histological findings in esophageal mucosa biopsies[34-36]. Although EoE typically begins in childhood, it can present at any age. The clinical manifestations depend on the age at presentation[37,38]. In infants and small children, it presents with vomiting, food rejection, dysphagia and, less commonly, growth retardation. School-aged children and adolescents present with symptoms such as dysphagia and food impaction, especially with food that has a coarse consistency. Other symptoms at these ages are chest and abdominal pain, vomiting, and regurgitation[39]. In adults, the main symptoms are dysphagia and untreatable heartburn[38]. Endoscopic findings in most patients include linear grooves on the esophagus wall, concentric rings, whitish exudates that resemble candidal infection, Schatzki rings, a reduction in the size of the esophagus and superficial tears of the mucosa after endoscope introduction[40-41] (Figures 2-5). The EoE Endoscopic Reference Score (EREFS), the classification system for EoE endoscopic findings, has been validated and includes major esophageal features (rings, furrows, exudates, and edema) as well as additional features such as narrow esophageal caliber, feline esophagus, strictures and crepe paper esophagus[42]. A prospective study of children between 2 and 17 years of age with histological diagnosis of EoE who underwent diagnostic and post-treatment endoscopy after therapy consisting of steroids and dietary elimination found that visual detection of more than 1 abnormality during diagnostic endoscopy identified children with EoE with 89.6% sensitivity and 87.9% specificity. Children who responded to therapy had an average EREFS of 0.5 compared to 2.4 in those who did not respond. The EREFS identified children with EoE with an area under the curve (AUC) of 0.93 and identified children with active EoE despite treatment with an AUC of 0.81 before treatment and an AUC of 0.79 after treatment[43].
A meta-analysis of EoE endoscopic findings including more than 100 publications, comprising a total of 4678 patients with EoE and 2742 controls, found that the sensitivity, specificity, and predictive value of these endoscopic findings in isolation are insufficient for the diagnosis of EoE[44]. In addition, the endoscopic appearance of the esophagus may be normal in 10% to 25% of patients with EoE[45-47].
It is necessary to perform esophageal biopsies in patients in whom there is clinical suspicion of EoE, even if there are no endoscopic alterations of the esophageal mucosa. Because eosinophilic infiltrates occur in patches, biopsies should be taken from the upper, middle, and lower thirds of the esophagus[48,49]. Studies have shown that taking 6 to 9 esophageal mucosal samples increases the sensitivity to 100%[48,49-51]. A histological diagnosis is confirmed when there are ≥ 15 eosinophils per high-power field (HPF)[36]. EoE is a clinicopathological disorder in which clinical and pathological findings should be considered together rather than separately. A recent cross-sectional study aimed to establish correlations between the clinical and the histological and endoscopic findings of patients with EoE based on a set of 96 esophageal transcriptional profiles known as the Eosinophilic Esophagitis diagnostic panel (EDP). Biopsies of the distal esophagus were taken and assessed using the histological scoring system (HSS) and the aforementioned EREFS. Associations between the histological, endoscopic and molecular characteristics were identified using Spearman correlation analysis. The EDP showed was significantly correlated with EoE and findings were similar between adults and children. Among the 8 HSS domains, basal zone hyperplasia was found to correlated with EDP; and of the 5 endoscopic findings included in the EREFS, distal grooves were correlated with EDP. Based on the analysis of patients with active EoE the EDP identified three groups associated with different endotypes, named EoEe1-3. The EoEe1 endotype was associated with a normal-appearing esophagus (RR = 3.27, 95%CI: 1.04-10.27; P = 0.0443), and inversely correlated with a history of esophageal dilation (0.27, 0.09-0.82; P = 0.0105) and mild molecular, endoscopic and histological changes. EoEe2 patients had an inflammatory and steroid-refractory phenotype (2.77, 95%CI: 1.11-6.95; P = 0.0376) and the highest expression of inflammatory cytokines. The EoEe3 endotype was associated with a narrow-gauge esophagus (RR 2.77, 95%CI: 1.11-6.95; P = 0.0376) beginning in adulthood (2.22, 1.19-4.12; P = 0.0155), and these patients had the highest degree of endoscopic and histological severity[51]. Patients with GERD and EoE exhibit dilation of intercellular spaces between esophageal epithelial cells. The degree of dilation of the intercellular spaces is inversely correlated with the measured mucosal impedance (MI). Direct measurement of the esophageal epithelium’s integrity by measuring MI can potentially obviate the need for endoscopies and repeated biopsies in EoE and reduce the pH monitoring time in GERD. A recent prospective study of 69 patients evaluated the performance of a balloon catheter system that measures MI in a long segment of the esophagus in the diagnosis of esophageal disorders including GERD and EoE. In this study, patients were classified into three groups: GERD, EoE and non-GERD, according to the endoscopic, histological and ambulatory pH monitoring results. The pattern of MI along the esophagus was different in the three groups. Patients without GERD had the highest MI values in all segments. In patients with GERD, the mucosal impedance (MI) values were low in the distal esophagus and normal in the proximal esophagus. In EoE patients, MI measurements were low in all segments of the esophagus. The increase in MI per distance from the squa-mocolumnar junction identified patients with GERD with an AUC of 0.67, patients with EoE with an AUC of 0.84 and non-GERD patients with an AUC of 0.83[52].
The American College of Gastroenterology guidelines for this entity have established the following diagnostic criteria[34]: (1) Symptoms of esophageal dysfunction; (2) Biopsies of the esophagus with predominant eosinophilic in-flammation, typically equal to or greater than 15 eosinophils per HPF; (3) Eosinophilia limited to the esophagus that persists despite therapeutic trials with PPIs; (4) Exclusion of secondary causes of esophageal eosinophilia; and (5) Response to treatment with dietary elimination and topical corticosteroids, which supports but is not required for diagnosis.
A recent update has established the following diagnostic criteria[36]: (1) Symptoms of esophageal dysfunction; (2) Concomitant atopic conditions; (3) Endoscopic findings of rings, grooves, exudate, stenosis, luminal narrowing, and fragility of the mucosa or crepe mucosa; (4) ≥ 15 eosinophils per HPF (60 eosinophils/mm2) in an esophageal biopsy; (5) Eosinophilic infiltration should be isolated to the esophagus; and (6) Evaluation of disorders other than EoE that potentially contribute to esophageal eosinophilia.
The following disorders/diseases are within the differential diagnosis of EoE: GERD; infections such as schistosomiasis, anisakiasis, and toxocariasis; celiac disease; hypereosinophilic syndrome; and other diseases such as inflammatory bowel disease and eosinophilic granulomatosis with polyangiitis. There are 2 clinical situations in which esophageal eosinophilia can respond to PPI therapy that deserve special mention because they pose a diagnostic challenge: (1) Patients with GERD symptoms and endoscopic findings of esophagitis or Barrett esophagus who are most likely diagnosed with GERD, but in whom esophageal biopsies show eosinophilia[52,54]; these patients will mostly respond to PPI therapy with symptomatic and histological improvement of esophageal eosinophilia; and (2) Patients with symptoms of EoE, endoscopic findings of EoE and esophageal eosinophilia that respond to PPIs[55]; the diagnosis for this group is PPI-responsive esophageal eosinophilia or PPI-REE[34]. Up to one-third of patients with esophageal eosinophilia respond to PPIs[55,58]. There is controversy as to whether this phenotype of patients with PPI-REE corresponds to a variant of EoE or to GERD. However, recent evidence, mainly in adults, has found that PPI-REE is within the same spectrum as EoE, as it shares clinical, histological, and endoscopic characteristics; moreover, there is overlap in terms of inflammation mediated by a Th2 immune response and abnormal gene expression, which are different from GERD[59,60]. Management guidelines worldwide suggest that, to make a true diagnosis of EoE, a therapeutic trial using high doses of PPIs should be performed for 8 weeks, confirming the persistence of at least 15 eosinophils per HPF in esophageal biopsies taken after completion of the therapeutic regimen[34]; however, a recent consensus has excluded therapeutic challenge with PPIs from the diagnostic criteria and considers it purely therapeutic[36].
The treatment of EoE revolves around controlling causative factors and the Th2 response, which is altered in the same way as in allergic respiratory diseases[61] for which “Drugs” are the main treatment option; in addition, “Diet” modifications are used to control interacting environmental factors[62], and endoscopic “Dilation” is used for the management of endoscopic complications[63]. The ultimate goal of treatment is to control esophageal eosinophilia and inflammation, which triggers the symptoms and fibrotic processes in the esophagus[64]. In some cases, a combination of therapies may be necessary to reduce esophageal eosinophilia (achieve < 15 eosinophils/HPF in biopsies), in order to control symptoms, prevent remodeling and reverse fibrosis[64].
There are currently no drugs approved by the Food and Drug Administration for the treatment of EoE.
GERD plays an important role in EoE because mild eosinophilia (< 5-10 eosinophils/HPF) can also be found in biopsies of GERD patients[65]. PPIs increase the pH of gastric contents, reducing esophageal damage caused by exposure to acid[61,63]. Additionally, they exert an anti-inflammatory effect by blocking the Th2 response, with a reduction in inflammatory cytokine exposure, and they repair the epithelial barrier of intercellular spaces that is altered by acid exposure[66,67]. PPIs are useful as a first-line treatment for patients with EoE[15], based on the concept that PPI-REE is within the same spectrum as EoE[59,60]. Initial PPI doses of 20-40 mg or 1 mg/kg per dose are recommended twice daily in adults and children, respectively[63]. A meta-analysis that included 33 studies of 188 children and 431 adults found that PPIs induce clinical remission and histological remission in 60.8% and 50.5% of patients, respectively[68]. In a study that included 121 patients with EoE, 33% reached complete remission (sustained clinical remission and histological remission with < 15 eosinophils/HPF) after taking 40 mg omeprazole twice a day for 8 weeks. After reducing the dose of omeprazole to 40 mg per day, 81% remained in complete remission, and of those, 83% remained in remission with 20 mg omeprazole per day[69]. That observation was supported in another study of 53 patients, in which the expression of eotaxin-3, IL-13, and IL-5 in the proximal and distal esophagus was evaluated in patients prescribed PPIs for the management of PPI-REE and EoE; similar decreases in the levels of these molecules were observed in all portions of the esophagus[66].
Corticosteroids are another effective therapy for the treatment of EoE[70,71]. the formulation of the most commonly used corticosteroids, including budesonide, fluticasone propionate, and ciclesonide, influences histological remission. In a study by Dellon et al[72], the ingestion of corticoids in a viscous vehicle such as sucralose was more effective than the deglutition of inhaled corticosteroids. This effect was also observed when comparing effervescent tablets and viscous oral budesonide[73,74]. The initial recommended treatment consists of 1 mg budesonide or 880 mg fluticasone in a viscous solution, twice a day[64]. Lucendo et al[75] conducted a recent clinical trial that included 88 adults with EoE and compared oral budesonide tablets with placebo; the study found 58% complete remission at 6 wk in patients treated with budesonide compared to no remission in any patient treated with placebo, and the proportion of complete remission increased to 85% at 12 wk. Histological remission was achieved in 93% of patients treated with budesonide vs 0% in patients treated with placebo[75].
Additionally, along with the histological response, an improvement in symptoms, mainly dysphagia, has been observed. A study using validated questionnaires to evaluate dysphagia, such as the Dysphagia Symptom Questionnaire, and EoE, identified a correlation between histological and symptomatic improvement[76]. However, EoE is a chronic disease, and treatment is not curative. A study by Eluri et al[77] that included 55 patients with EoE with an initial histological response (< 15 eosinophils/HPF) after 8 wk of topical steroid treatment found a decrease in response to treatment, to 50% at 18.5 mo and 75% at 29.6 mo, when decreasing the corticosteroid dose. A recent study that included 229 patients with a median follow-up of 5 years found that patients were taking the topical corticoid by swallowing it in 41.0% of visits; these patients had a higher frequency of clinical remission (31.0% vs 4.5%), endoscopic remission (48.8% vs 17.8%), histological remission (44.8% vs 10.1%) and complete remission (16.1% vs 1.3%) compared with patients who were not swallowing the topical corticoid[78]. In a placebo-controlled clinical trial, a maintenance dosage of 0.25 mg of deglutinized budesonide twice daily for 50 wk resulted in higher rates of histological and clinical remission, reduced noneosinophilic markers of inflammation, epithelial cell apoptosis, and remodeling events, was associated with a significant decrease in mucosal thickness, and had no serious adverse effects[79].
Long-term maintenance therapy has been proposed for patients with esophageal stenosis, symptomatic relapse upon discontinuation of treatment, recurrent food impaction, comorbidities that increase the risk of endoscopy and dilation, and previous spontaneous perforation or perforation induced by dilation, as well as for patients who travel to areas where there is greater risk of food impaction[64].
Among the adverse effects of steroid therapy, esophageal candidiasis has been described in 5%-30% of patients and oral candidiasis in 1%; (mostly incidental findings, as many patients are asymptomatic), and adrenal insufficiency was observed in 16% of patients in a systematic review of 17 studies with 596 patients[80,81].
In patients with corticosteroid dependence, there are reports of cases in which immunomodulators such as azathioprine and 6-mercaptopurine have been used, achieving induction and long-term maintenance of clinical and histological remission[82]. However, there are no controlled clinical trials of these therapies.
Leukotriene D4 is a proinflammatory molecule that serves as a chemotactic factor for eosinophils[83]. Montelukast is an antagonist of the type-1 receptor of cysteinyl leukotrienes whose therapeutic effect has been limited in EoE. In a prospective study that included 11 adults with EoE in remission treated with fluticasone propionate and montelukast 10 mg/d for 3 mo, the clinical and histological remission achieved by topical steroids could not be maintained[84]. Alexander et al[85] conducted a study in which they included 41 adults with EoE in symptomatic remission with topical steroids; montelukast 20 mg/d for 26 wk resulted in no significant difference compared to placebo for maintaining symptomatic remission. The guidelines do not recommend the use of leukotriene antagonists as induction or maintenance therapy for EoE.
EoE is a chronic inflammatory disease in which immune cells and cytokines are responsible for the inflammatory response and symptoms. For this reason, monoclonal antibodies with therapeutic targets against these pathophysiological effectors offer more powerful symptomatic and histological control while minimizing adverse effects[86]. Monoclonal antibodies against IL-5 (mepolizumab, reslizumab) have been evaluated in 5 studies in children and adults and they have shown evidence of decreased esophageal eosinophilia, symptomatic improvement, and increased quality of life, with an acceptable safety profile. The most frequent adverse events were headache, cough, nasal congestion and upper respiratory tract in-fections[87-91]. Monoclonal antibodies against IL-13 (QAX576, RPC4046) were evaluated in 2 studies in adults that showed a tendency toward improvement of symptoms (mainly dysphagia), endoscopic and histological improvement, improvement in the expression of esophageal transcripts including eotaxin-3, periostin, and mast cell markers, and improved barrier function; the most frequent adverse events were headache and upper respiratory tract infections[92,93]. In a pilot study evaluating omalizumab (antibody against immunoglobulin E) , complete remission was found in only 33% of patients[94]. Straumann et al[95] conducted a pilot study in which they evaluated 3 patients with EoE who were treated with an antibody against tumor necrosis factor-alpha (TNF-a) (infliximab) and found no resolution of esophageal eosinophilic infiltration or reduction of symptoms. Recently, dupilumab, a monoclonal antibody that acts on the IL-4 receptor, inhibiting IL-4/IL-13 signaling by negatively regulating the Th2 response, has been considered as a possible therapy for this disease[96]. Although biological therapy is safe, with limited and reversible adverse effects, well-designed clinical trials are needed to define the exact role of monoclonal antibodies in EoE[86].
Diet modification is a nonpharmacological therapy for the management of EoE in adults and children, and it has been used because of the role that dietary antigens play in the pathogenesis of this disease. Currently, there are 3 primary choices for initial dietary therapy for EoE: Elemental diets, elimination diets guided by food allergy testing, and empiric elimination diets[97].
The benefits of the elemental diet were first described more than 2 decades ago. Kelly et al[98] evaluated 10 children with GERD and eosinophilic infiltration in the esophagus while receiving Neocate or Neocate-1-Plus based on simple amino acids. For a minimum of 6 wk, patients had resolution or improvement of symptoms and a significant decrease in esophageal eosinophilia, and the symptoms recurred when specific proteins from the diet were reintroduced during open food challenges, implying that food allergies are responsible for esophageal inflammation. In a prospective study that included 18 adults with EoE, 4 wk of an elemental diet achieved a histological response in 72% of patients[99]. Subsequently, Warners et al[100] evaluated 21 patients with EoE who were administered an elemental diet for 4 wk and found a complete histological response in 71% of patients, as well as marked improvement in endoscopic signs. A meta-analysis found that elemental diets were effective in 90.8% of cases and that they were superior to both the 6-food elimination diet (SFED), which was effective in 72.1% of cases, and allergy testing-guided food elimination, which was effective in 45.5% of cases[101]. However, this dietary strategy seems unfeasible in clinical practice; the main limitations include poor palatability of the formula (requiring a nasogastric tube in the majority of children, resulting in adherence by only one-third of patients), psychological and social disturbances, alterations in quality of life due to lack of variety of foods, and high cost, as these diets are not universally covered by health systems[98]. Although the goal of the diet is to eliminate specific dietary triggers, another major defect of elemental diets is the time period and number of endoscopies required to identify specific triggers during the food reintroduction phase[102].
In this specific dietary approach, the skin-prick test and the atopy patch test are performed to test for food allergies, with subsequent elimination of foods with positive test results[97]. Spergel et al[103] conducted a study in which they included 26 children with EoE and showed that the combination of skin-prick testing and patch testing can identify potential causative foods; the foods most commonly identified by patients were milk and eggs. In a retrospective study that included 941 children with EoE, the elimination of foods associated with positive allergy tests resulted in a histological response in 53% of patients[107]. With respect to adults, there is low concordance between the results of cutaneous tests for food allergies and the identified EoE food triggers[108]. In a prospective study that included 43 adults with EoE, a serum IgE-targeted elimination diet for 6 wk resulted in histological remission in 73% of patients, which was not significantly superior to the SFED, and effectively identified cow's milk as a food trigger in IgE-sensitized patients[109]. In a study in which 5 different food allergy tests were conducted via skin and blood (skin-prick and skin-patch tests, serum allergen-specific IgE, basophil activation test and serum food-specific IgG), no allergy test could accurately predict actual food triggers[107]. Data from recent studies support the ideas that the pathogenesis of EoE is not IgE-mediated but, rather, mainly associated with IgG4; these studies conclude that skin and serum allergy tests are not advisable in adults and of questionable utility in children[25-28,108].
Given the limitations on the use of elemental diets in clinical practice, mainly because it requires an exclusive liquid formula with poor palatability, a retrospective study was performed in 2006 that included 60 children with EoE and compared the SFED (eliminating proteins from cow milk, soy, wheat, eggs, peanuts, and shellfish) with the elemental diet; after 6 wk, clinical and histological remission was observed in 74% of patients on the SFED, a result that was not inferior to the elemental diet but had better acceptance, adherence and cost[109]. A cost analysis found that the SFED has similar effectiveness to topical corticosteroids for first-line treatment in EoE; however, the SFED is less expensive[110].
After the reintroduction phase, only 1 or 2 foods trigger symptoms in 65%-85% of patients. In a prospective study in which 52 adults with EoE were included, an elimination diet was implemented (milk, wheat, egg and legumes) for 6 weeks; 54% of patients achieved clinicopathological remission, with milk being the only causal food in 27% of patients[111].
The number of follow-up endoscopies and the numerous dietary restrictions limit the implementation of empirical elimination diets, for which a step-up approach is proposed in which one begins with the elimination of milk and gluten, and additional foods are removed according to the response[112]. Molina-Infante et al[112] conducted a prospective study that included 130 adults and children with EoE and found that 6 wk of a diet that eliminated 2 foods (milk and cereals with gluten), 4 foods (2 aforementioned foods plus eggs and legumes), or 6 foods (4 aforementioned foods plus nuts and fish/shellfish) resulted in clinicopathological remission in 43%, 60%, and 79% of patients, respectively; additionally, compared with the SFED, the step-up strategy reduced endoscopic procedures and diagnostic test times by 20%.
Lastly, a meta-analysis found no significant difference in remission after dietary interventions in adults and children (67.2% vs 63.3%, respectively)[101].
Bougie and balloon dilation is the only endoscopic treatment available for EoE[63]. It should be performed in patients with persistent dysphagia resistant to medical treatment in whom remission of inflammation has been achieved and in patients with severe dysphagia and a history of food impaction[63]. However, the main issue with esophageal dilation is that it does not control the chronic inflammation that contributes to esophageal remodeling[64].
Esophageal dilation is a safe and effective treatment for fibrostenotic Crohn’s disease[113]. In a retrospective study that included 509 patients with EoE, patients who underwent endoscopic dilation had a longer duration of symptoms before diagnosis; 58% required more than one dilation, and of these individuals, 75% required repeat dilation within one year[114]. The overall complication rate was 5%, primarily due to postprocedural pain[114]. In a meta-analysis, 468 patients were evaluated who underwent a total of 671 dilations; only one perforation (0.1%) occurred, a rate similar to those observed in other causes of esophageal stenosis[115]. In a recent meta-analysis included 845 adults and children with EoE, in whom 1820 esophageal dilations were performed; endoscopic dilations achieved clinical improvement in 95% of patients, with very low rates (< 1%) of major complications[116]. Dilation should be performed gradually, using the rule of 3, without passing more than 3 dilators with 2-mm incremental diameter increases per session if moderate or severe resistance is found. It should be performed frequently, with several sessions every 2 to 3 wk, depending on the symptoms and the initial diameter of the esophageal lumen, in order to decrease the risk of complications such as chest pain or esophageal per-foration[64].
In monitoring treatment, a system has been proposed that evaluates response along a spectrum that includes complete normalization, response, and nonresponse, based on histology, symptoms, and endoscopic findings[117] (Table 1).
| Spectrum of response | ||||
| Spectrum of outcomes | Non- response | Response | Complete normalization | |
| Histology | Persistent eosinophilia ≥ 15 eos/hpf | Reduced eosinophilia 7-14 eos/hpf, 1-6 eos/hpf | Normal biopsy < 1 eos/hpf | |
| Symptoms | Persistent symptons < 30% decrease in a symptom metric | Decrease eosinophilia 30%-90% decrease in a symptom metric | Symptom resolution > 90% decrease in a symptom metric; EEsAI score < 20 | |
| Endoscopy | Persistent endoscopic findings: < 30% decrease in EREFS | Improved findings: EREFS ≥ 2 but less than baseline | Normal esophagus EREFS < 2 | |
Additionally, noninvasive methods for follow-up are being studied. These include the cytosponge[118] and esophageal string device[119]; blood markers such as IL-3, IL-5, IL-6, IL-9, IL-13, transforming growth factor-alpha (TGF-a) and -beta (TGF-b), TNF-a, eotaxin-1, -2 and -3, thymic stromal lymphopoietin (TSLP), and major basic protein and neurotoxin derived from eosinophils[120]; urine markers such as 3-bro-motyrosine[121]; and modifications in gene expression[122].
EoE is considered an immune and allergic entity that involves the activation of a mainly Th2-driven immune response to certain allergens, coupled with genetic susceptibility. Although the use of PPIs was previously thought to facilitate diagnostic differentiation, it has since been found that PPIs can act in synergy and facilitate the resolution of EoE symptoms. Given this phenomenon, the use of PPIs should be considered in early stages of treatment, in conjunction with other therapies such as topical corticosteroids or elimination diets.
| 1. | Landres RT, Kuster GG, Strum WB. Eosinophilic esophagitis in a patient with vigorous achalasia. Gastroenterology. 1978;74:1298-1301. [PubMed] |
| 2. | Attwood SE, Smyrk TC, Demeester TR, Jones JB. Esophageal eosinophilia with dysphagia. A distinct clinicopathologic syndrome. Dig Dis Sci. 1993;38:109-116. [PubMed] |
| 3. | Straumann A, Spichtin HP, Bernoulli R, Loosli J, Vögtlin J. [Idiopathic eosinophilic esophagitis: a frequently overlooked disease with typical clinical aspects and discrete endoscopic findings]. Schweiz Med Wochenschr. 1994;124:1419-1429. [PubMed] |
| 4. | Navarro P, Arias Á, Arias-González L, Laserna-Mendieta EJ, Ruiz-Ponce M, Lucendo AJ. Systematic review with meta-analysis: the growing incidence and prevalence of eosinophilic oesophagitis in children and adults in population-based studies. Aliment Pharmacol Ther. 2019;49:1116-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 240] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 5. | Kapel RC, Miller JK, Torres C, Aksoy S, Lash R, Katzka DA. Eosinophilic esophagitis: a prevalent disease in the United States that affects all age groups. Gastroenterology. 2008;134:1316-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 225] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 6. | Dellon ES, Hirano I. Epidemiology and Natural History of Eosinophilic Esophagitis. Gastroenterology. 2018;154:319-332.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 609] [Article Influence: 76.1] [Reference Citation Analysis (0)] |
| 7. | Jensen ET, Hoffman K, Shaheen NJ, Genta RM, Dellon ES. Esophageal eosinophilia is increased in rural areas with low population density: results from a national pathology database. Am J Gastroenterol. 2014;109:668-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Jensen ET, Shah ND, Hoffman K, Sonnenberg A, Genta RM, Dellon ES. Seasonal variation in detection of oesophageal eosinophilia and eosinophilic oesophagitis. Aliment Pharmacol Ther. 2015;42:461-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Spechler SJ. Speculation as to why the Frequency of Eosinophilic Esophagitis Is Increasing. Curr Gastroenterol Rep. 2018;20:26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Lambrecht BN, Hammad H. The immunology of the allergy epidemic and the hygiene hypothesis. Nat Immunol. 2017;18:1076-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 282] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 11. | Fahey L, Robinson G, Weinberger K, Giambrone AE, Solomon AB. Correlation Between Aeroallergen Levels and New Diagnosis of Eosinophilic Esophagitis in New York City. J Pediatr Gastroenterol Nutr. 2017;64:22-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Green DJ, Cotton CC, Dellon ES. The Role of Environmental Exposures in the Etiology of Eosinophilic Esophagitis: A Systematic Review. Mayo Clin Proc. 2015;90:1400-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Dowling PJ, Neuhaus H, Polk BI. The Role of the Environment in Eosinophilic Esophagitis. Clin Rev Allergy Immunol. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Blevins CH, Iyer PG, Vela MF, Katzka DA. The Esophageal Epithelial Barrier in Health and Disease. Clin Gastroenterol Hepatol. 2018;16:608-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Spechler SJ, Genta RM, Souza RF. Thoughts on the complex relationship between gastroesophageal reflux disease and eosinophilic esophagitis. Am J Gastroenterol. 2007;102:1301-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 258] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 16. | Cheng E, Zhang X, Huo X, Yu C, Zhang Q, Wang DH, Spechler SJ, Souza RF. Omeprazole blocks eotaxin-3 expression by oesophageal squamous cells from patients with eosinophilic oesophagitis and GORD. Gut. 2013;62:824-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 267] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 17. | Lin SK, Sabharwal G, Ghaffari G. A review of the evidence linking eosinophilic esophagitis and food allergy. Allergy Asthma Proc. 2015;36:26-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | McGowan EC, Platts-Mills TAE, Wilson JM. Food allergy, eosinophilic esophagitis, and the enigma of IgG4. Ann Allergy Asthma Immunol. 2019;122:563-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Clayton F, Peterson K. Eosinophilic Esophagitis: Pathophysiology and Definition. Gastrointest Endosc Clin N Am. 2018;28:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Kottyan LC, Rothenberg ME. Genetics of eosinophilic esophagitis. Mucosal Immunol. 2017;10:580-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 21. | Cianferoni A, Spergel JM. From genetics to treatment of eosinophilic esophagitis. Curr Opin Allergy Clin Immunol. 2015;15:417-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | O'Shea KM, Aceves SS, Dellon ES, Gupta SK, Spergel JM, Furuta GT, Rothenberg ME. Pathophysiology of Eosinophilic Esophagitis. Gastroenterology. 2018;154:333-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 382] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 23. | Davis BP. Pathophysiology of Eosinophilic Esophagitis. Clin Rev Allergy Immunol. 2018;55:19-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Davis BP, Stucke EM, Khorki ME, Litosh VA, Rymer JK, Rochman M, Travers J, Kottyan LC, Rothenberg ME. Eosinophilic esophagitis-linked calpain 14 is an IL-13-induced protease that mediates esophageal epithelial barrier impairment. JCI Insight. 2016;1:e86355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 133] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 25. | Simon D, Cianferoni A, Spergel JM, Aceves S, Holbreich M, Venter C, Rothenberg ME, Terreehorst I, Muraro A, Lucendo AJ, Schoepfer A, Straumann A, Simon HU. Eosinophilic esophagitis is characterized by a non-IgE-mediated food hypersensitivity. Allergy. 2016;71:611-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 176] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 26. | Clayton F, Fang JC, Gleich GJ, Lucendo AJ, Olalla JM, Vinson LA, Lowichik A, Chen X, Emerson L, Cox K, O'Gorman MA, Peterson KA. Eosinophilic esophagitis in adults is associated with IgG4 and not mediated by IgE. Gastroenterology. 2014;147:602-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 361] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 27. | Weidlich S, Nennstiel S, Jesinghaus M, Brockow K, Slotta-Huspenina J, Bajbouj M, Schmid RM, Schlag C. IgG4 is Elevated in Eosinophilic Esophagitis but Not in Gastroesophageal Reflux Disease Patients. J Clin Gastroenterol. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Pope AE, Stanzione N, Naini BV, Garcia-Lloret M, Ghassemi KA, Marcus EA, Martin MG, Wozniak LJ. Esophageal IgG4: Clinical, Endoscopic, and Histologic Correlations in Eosinophilic Esophagitis. J Pediatr Gastroenterol Nutr. 2019;68:689-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Wu L, Oshima T, Li M, Tomita T, Fukui H, Watari J, Miwa H. Filaggrin and tight junction proteins are crucial for IL-13-mediated esophageal barrier dysfunction. Am J Physiol Gastrointest Liver Physiol. 2018;315:G341-G350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 30. | Sherrill JD, Kc K, Wu D, Djukic Z, Caldwell JM, Stucke EM, Kemme KA, Costello MS, Mingler MK, Blanchard C, Collins MH, Abonia JP, Putnam PE, Dellon ES, Orlando RC, Hogan SP, Rothenberg ME. Desmoglein-1 regulates esophageal epithelial barrier function and immune responses in eosinophilic esophagitis. Mucosal Immunol. 2014;7:718-729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 248] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 31. | Abonia JP, Franciosi JP, Rothenberg ME. TGF-β1: Mediator of a feedback loop in eosinophilic esophagitis--or should we really say mastocytic esophagitis? J Allergy Clin Immunol. 2010;126:1205-1207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Nguyen N, Fernando SD, Biette KA, Hammer JA, Capocelli KE, Kitzenberg DA, Glover LE, Colgan SP, Furuta GT, Masterson JC. TGF-β1 alters esophageal epithelial barrier function by attenuation of claudin-7 in eosinophilic esophagitis. Mucosal Immunol. 2018;11:415-426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 33. | Politi E, Angelakopoulou A, Grapsa D, Zande M, Stefanaki K, Panagiotou I, Roma E, Syrigou E. Filaggrin and Periostin Expression Is Altered in Eosinophilic Esophagitis and Normalized With Treatment. J Pediatr Gastroenterol Nutr. 2017;65:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 34. | Dellon ES, Gonsalves N, Hirano I, Furuta GT, Liacouras CA, Katzka DA; American College of Gastroenterology. ACG clinical guideline: Evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol. 2013;108:679-92; quiz 693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 784] [Cited by in RCA: 862] [Article Influence: 66.3] [Reference Citation Analysis (0)] |
| 35. | Lucendo AJ, Molina-Infante J, Arias Á, von Arnim U, Bredenoord AJ, Bussmann C, Amil Dias J, Bove M, González-Cervera J, Larsson H, Miehlke S, Papadopoulou A, Rodríguez-Sánchez J, Ravelli A, Ronkainen J, Santander C, Schoepfer AM, Storr MA, Terreehorst I, Straumann A, Attwood SE. Guidelines on eosinophilic esophagitis: evidence-based statements and recommendations for diagnosis and management in children and adults. United European Gastroenterol J. 2017;5:335-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 769] [Article Influence: 85.4] [Reference Citation Analysis (1)] |
| 36. | Dellon ES, Liacouras CA, Molina-Infante J, Furuta GT, Spergel JM, Zevit N, Spechler SJ, Attwood SE, Straumann A, Aceves SS, Alexander JA, Atkins D, Arva NC, Blanchard C, Bonis PA, Book WM, Capocelli KE, Chehade M, Cheng E, Collins MH, Davis CM, Dias JA, Di Lorenzo C, Dohil R, Dupont C, Falk GW, Ferreira CT, Fox A, Gonsalves NP, Gupta SK, Katzka DA, Kinoshita Y, Menard-Katcher C, Kodroff E, Metz DC, Miehlke S, Muir AB, Mukkada VA, Murch S, Nurko S, Ohtsuka Y, Orel R, Papadopoulou A, Peterson KA, Philpott H, Putnam PE, Richter JE, Rosen R, Rothenberg ME, Schoepfer A, Scott MM, Shah N, Sheikh J, Souza RF, Strobel MJ, Talley NJ, Vaezi MF, Vandenplas Y, Vieira MC, Walker MM, Wechsler JB, Wershil BK, Wen T, Yang GY, Hirano I, Bredenoord AJ. Updated International Consensus Diagnostic Criteria for Eosinophilic Esophagitis: Proceedings of the AGREE Conference. Gastroenterology. 2018;155:1022-1033.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 886] [Article Influence: 110.8] [Reference Citation Analysis (0)] |
| 37. | Franciosi JP, Liacouras CA. Eosinophilic esophagitis. Immunol Allergy Clin North Am. 2009;29:19-27, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Miehlke S. Clinical features of Eosinophilic esophagitis in children and adults. Best Pract Res Clin Gastroenterol. 2015;29:739-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 39. | Putnam PE. Eosinophilic esophagitis in children: clinical manifestations. Gastrointest Endosc Clin N Am. 2008;18:11-23; vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 40. | Straumann A, Rossi L, Simon HU, Heer P, Spichtin HP, Beglinger C. Fragility of the esophageal mucosa: a pathognomonic endoscopic sign of primary eosinophilic esophagitis? Gastrointest Endosc. 2003;57:407-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 113] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 41. | Straumann A, Spichtin HP, Bucher KA, Heer P, Simon HU. Eosinophilic esophagitis: red on microscopy, white on endoscopy. Digestion. 2004;70:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 97] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 42. | Hirano I, Moy N, Heckman MG, Thomas CS, Gonsalves N, Achem SR. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut. 2013;62:489-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 692] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 43. | Wechsler JB, Bolton SM, Amsden K, Wershil BK, Hirano I, Kagalwalla AF. Eosinophilic Esophagitis Reference Score Accurately Identifies Disease Activity and Treatment Effects in Children. Clin Gastroenterol Hepatol. 2018;16:1056-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 116] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 44. | Kim HP, Vance RB, Shaheen NJ, Dellon ES. The prevalence and diagnostic utility of endoscopic features of eosinophilic esophagitis: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:988-96.e5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 264] [Cited by in RCA: 263] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 45. | Prasad GA, Alexander JA, Schleck CD, Zinsmeister AR, Smyrk TC, Elias RM, Locke GR, Talley NJ. Epidemiology of eosinophilic esophagitis over three decades in Olmsted County, Minnesota. Clin Gastroenterol Hepatol. 2009;7:1055-1061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 412] [Cited by in RCA: 381] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 46. | Lucendo AJ, Pascual-Turrión JM, Navarro M, Comas C, Castillo P, Letrán A, Caballero MT, Larrauri J. Endoscopic, bioptic, and manometric findings in eosinophilic esophagitis before and after steroid therapy: a case series. Endoscopy. 2007;39:765-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 47. | Müller S, Pühl S, Vieth M, Stolte M. Analysis of symptoms and endoscopic findings in 117 patients with histological diagnoses of eosinophilic esophagitis. Endoscopy. 2007;39:339-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 111] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 48. | Saffari H, Peterson KA, Fang JC, Teman C, Gleich GJ, Pease LF. Patchy eosinophil distributions in an esophagectomy specimen from a patient with eosinophilic esophagitis: Implications for endoscopic biopsy. J Allergy Clin Immunol. 2012;130:798-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 49. | Collins MH. Histopathologic features of eosinophilic esophagitis. Gastrointest Endosc Clin N Am. 2008;18:59-71; viii-ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 50. | Gonsalves N, Policarpio-Nicolas M, Zhang Q, Rao MS, Hirano I. Histopathologic variability and endoscopic correlates in adults with eosinophilic esophagitis. Gastrointest Endosc. 2006;64:313-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 336] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 51. | Shah A, Kagalwalla AF, Gonsalves N, Melin-Aldana H, Li BU, Hirano I. Histopathologic variability in children with eosinophilic esophagitis. Am J Gastroenterol. 2009;104:716-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 52. | Ngo P, Furuta GT, Antonioli DA, Fox VL. Eosinophils in the esophagus--peptic or allergic eosinophilic esophagitis? Case series of three patients with esophageal eosinophilia. Am J Gastroenterol. 2006;101:1666-1670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 214] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 53. | Patel DA, Higginbotham T, Slaughter JC, Aslam M, Yuksel E, Katzka D, Gyawali CP, Mashi M, Pandolfino J, Vaezi MF. Development and Validation of a Mucosal Impedance Contour Analysis System to Distinguish Esophageal Disorders. Gastroenterology. 2019;156:1617-1626.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (1)] |
| 54. | Ravi K, Katzka DA, Smyrk TC, Prasad GA, Romero Y, Francis DL, Lutzke L, Tian J, Wang KK. Prevalence of esophageal eosinophils in patients with Barrett's esophagus. Am J Gastroenterol. 2011;106:851-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 55. | Molina-Infante J, Ferrando-Lamana L, Ripoll C, Hernandez-Alonso M, Mateos JM, Fernandez-Bermejo M, Dueñas C, Fernandez-Gonzalez N, Quintana EM, Gonzalez-Nuñez MA. Esophageal eosinophilic infiltration responds to proton pump inhibition in most adults. Clin Gastroenterol Hepatol. 2011;9:110-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 286] [Article Influence: 19.1] [Reference Citation Analysis (1)] |
| 56. | Dranove JE, Horn DS, Davis MA, Kernek KM, Gupta SK. Predictors of response to proton pump inhibitor therapy among children with significant esophageal eosinophilia. J Pediatr. 2009;154:96-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 57. | Sayej WN, Patel R, Baker RD, Tron E, Baker SS. Treatment with high-dose proton pump inhibitors helps distinguish eosinophilic esophagitis from noneosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2009;49:393-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 107] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 58. | Dohil R, Newbury RO, Aceves S. Transient PPI responsive esophageal eosinophilia may be a clinical sub-phenotype of pediatric eosinophilic esophagitis. Dig Dis Sci. 2012;57:1413-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 59. | Moawad FJ, Schoepfer AM, Safroneeva E, Ally MR, Chen YJ, Maydonovitch CL, Wong RK. Eosinophilic oesophagitis and proton pump inhibitor-responsive oesophageal eosinophilia have similar clinical, endoscopic and histological findings. Aliment Pharmacol Ther. 2014;39:603-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 60. | Molina-Infante J, Bredenoord AJ, Cheng E, Dellon ES, Furuta GT, Gupta SK, Hirano I, Katzka DA, Moawad FJ, Rothenberg ME, Schoepfer A, Spechler SJ, Wen T, Straumann A, Lucendo AJ; PPI-REE Task Force of the European Society of Eosinophilic Oesophagitis (EUREOS). Proton pump inhibitor-responsive oesophageal eosinophilia: an entity challenging current diagnostic criteria for eosinophilic oesophagitis. Gut. 2016;65:524-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 227] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 61. | Arora AS, Yamazaki K. Eosinophilic esophagitis: asthma of the esophagus? Clin Gastroenterol Hepatol. 2004;2:523-530. [PubMed] |
| 62. | Furuta GT, Katzka DA. Eosinophilic Esophagitis. N Engl J Med. 2015;373:1640-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 420] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 63. | Richter JE. Endoscopic Treatment of Eosinophilic Esophagitis. Gastrointest Endosc Clin N Am. 2018;28:97-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 64. | Straumann A, Katzka DA. Diagnosis and Treatment of Eosinophilic Esophagitis. Gastroenterology. 2018;154:346-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 128] [Article Influence: 16.0] [Reference Citation Analysis (1)] |
| 65. | Pesek RD, Gupta SK. Emerging drugs for eosinophilic esophagitis. Expert Opin Emerg Drugs. 2018;23:173-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 66. | Molina-Infante J, Rivas MD, Hernandez-Alonso M, Vinagre-Rodríguez G, Mateos-Rodríguez JM, Dueñas-Sadornil C, Perez-Gallardo B, Ferrando-Lamana L, Fernandez-Gonzalez N, Bañares R, Zamorano J. Proton pump inhibitor-responsive oesophageal eosinophilia correlates with downregulation of eotaxin-3 and Th2 cytokines overexpression. Aliment Pharmacol Ther. 2014;40:955-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 67. | Zhang X, Cheng E, Huo X, Yu C, Zhang Q, Pham TH, Wang DH, Spechler SJ, Souza RF. Omeprazole blocks STAT6 binding to the eotaxin-3 promoter in eosinophilic esophagitis cells. PLoS One. 2012;7:e50037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 195] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 68. | Lucendo AJ, Arias Á, Molina-Infante J. Efficacy of Proton Pump Inhibitor Drugs for Inducing Clinical and Histologic Remission in Patients With Symptomatic Esophageal Eosinophilia: A Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol. 2016;14:13-22.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 258] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 69. | Gómez-Torrijos E, García-Rodríguez R, Castro-Jiménez A, Rodríguez-Sanchez J, Méndez Díaz Y, Molina-Infante J. The efficacy of step-down therapy in adult patients with proton pump inhibitor-responsive oesophageal eosinophilia. Aliment Pharmacol Ther. 2016;43:534-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 70. | Straumann A, Conus S, Degen L, Felder S, Kummer M, Engel H, Bussmann C, Beglinger C, Schoepfer A, Simon HU. Budesonide is effective in adolescent and adult patients with active eosinophilic esophagitis. Gastroenterology. 2010;139:1526-1537, 1537.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 407] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 71. | Alexander JA, Jung KW, Arora AS, Enders F, Katzka DA, Kephardt GM, Kita H, Kryzer LA, Romero Y, Smyrk TC, Talley NJ. Swallowed fluticasone improves histologic but not symptomatic response of adults with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2012;10:742-749.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 250] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 72. | Dellon ES, Sheikh A, Speck O, Woodward K, Whitlow AB, Hores JM, Ivanovic M, Chau A, Woosley JT, Madanick RD, Orlando RC, Shaheen NJ. Viscous topical is more effective than nebulized steroid therapy for patients with eosinophilic esophagitis. Gastroenterology. 2012;143:321-4.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 259] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 73. | Aceves SS, Dohil R, Newbury RO, Bastian JF. Topical viscous budesonide suspension for treatment of eosinophilic esophagitis. J Allergy Clin Immunol. 2005;116:705-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 95] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 74. | Chuang MY, Chinnaratha MA, Hancock DG, Woodman R, Wong GR, Cock C, Fraser RJ. Topical Steroid Therapy for the Treatment of Eosinophilic Esophagitis (EoE): A Systematic Review and Meta-Analysis. Clin Transl Gastroenterol. 2015;6:e82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 75. | Lucendo AJ, Miehlke S, Schlag C, Vieth M, von Arnim U, Molina-Infante J, Hartmann D, Bredenoord AJ, Ciriza de Los Rios C, Schubert S, Brückner S, Madisch A, Hayat J, Tack J, Attwood S, Mueller R, Greinwald R, Schoepfer A, Straumann A; International EOS-1 Study Group. Efficacy of Budesonide Orodispersible Tablets as Induction Therapy for Eosinophilic Esophagitis in a Randomized Placebo-Controlled Trial. Gastroenterology. 2019;157:74-86.e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 227] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 76. | Dellon ES, Katzka DA, Collins MH, Hamdani M, Gupta SK, Hirano I; MP-101-06 Investigators. Budesonide Oral Suspension Improves Symptomatic, Endoscopic, and Histologic Parameters Compared With Placebo in Patients With Eosinophilic Esophagitis. Gastroenterology. 2017;152:776-786.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 192] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 77. | Eluri S, Runge TM, Hansen J, Kochar B, Reed CC, Robey BS, Woosley JT, Shaheen NJ, Dellon ES. Diminishing Effectiveness of Long-Term Maintenance Topical Steroid Therapy in PPI Non-Responsive Eosinophilic Esophagitis. Clin Transl Gastroenterol. 2017;8:e97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 78. | Greuter T, Safroneeva E, Bussmann C, Biedermann L, Vavricka SR, Katzka DA, Schoepfer AM, Straumann A. Maintenance Treatment Of Eosinophilic Esophagitis With Swallowed Topical Steroids Alters Disease Course Over A 5-Year Follow-up Period In Adult Patients. Clin Gastroenterol Hepatol. 2019;17:419-428.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 79. | Straumann A, Conus S, Degen L, Frei C, Bussmann C, Beglinger C, Schoepfer A, Simon HU. Long-term budesonide maintenance treatment is partially effective for patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2011;9:400-9.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 311] [Article Influence: 20.7] [Reference Citation Analysis (1)] |
| 80. | Hsu S, Wood C, Pan Z, Rahat H, Zeitler P, Fleischer D, Menard-Katcher C, Furuta GT, Atkins D. Adrenal Insufficiency in Pediatric Eosinophilic Esophagitis Patients Treated with Swallowed Topical Steroids. Pediatr Allergy Immunol Pulmonol. 2017;30:135-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 81. | Philpott H, Dougherty MK, Reed CC, Caldwell M, Kirk D, Torpy DJ, Dellon ES. Systematic review: adrenal insufficiency secondary to swallowed topical corticosteroids in eosinophilic oesophagitis. Aliment Pharmacol Ther. 2018;47:1071-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 82. | Netzer P, Gschossmann JM, Straumann A, Sendensky A, Weimann R, Schoepfer AM. Corticosteroid-dependent eosinophilic oesophagitis: azathioprine and 6-mercaptopurine can induce and maintain long-term remission. Eur J Gastroenterol Hepatol. 2007;19:865-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 134] [Article Influence: 7.1] [Reference Citation Analysis (1)] |
| 83. | Markham A, Faulds D. Montelukast. Drugs. 1998;56:251-6; discussion 257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 84. | Lucendo AJ, De Rezende LC, Jiménez-Contreras S, Yagüe-Compadre JL, González-Cervera J, Mota-Huertas T, Guagnozzi D, Angueira T, González-Castillo S, Arias A. Montelukast was inefficient in maintaining steroid-induced remission in adult eosinophilic esophagitis. Dig Dis Sci. 2011;56:3551-3558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 85. | Alexander JA, Ravi K, Enders FT, Geno DM, Kryzer LA, Mara KC, Smyrk TC, Katzka DA. Montelukast Does not Maintain Symptom Remission After Topical Steroid Therapy for Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2017;15:214-221.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 86. | Eskian M, Khorasanizadeh M, Assa'ad AH, Rezaei N. Monoclonal Antibodies for Treatment of Eosinophilic Esophagitis. Clin Rev Allergy Immunol. 2018;55:88-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 87. | Stein ML, Collins MH, Villanueva JM, Kushner JP, Putnam PE, Buckmeier BK, Filipovich AH, Assa'ad AH, Rothenberg ME. Anti-IL-5 (mepolizumab) therapy for eosinophilic esophagitis. J Allergy Clin Immunol. 2006;118:1312-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 307] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 88. | Straumann A, Conus S, Grzonka P, Kita H, Kephart G, Bussmann C, Beglinger C, Smith DA, Patel J, Byrne M, Simon HU. Anti-interleukin-5 antibody treatment (mepolizumab) in active eosinophilic oesophagitis: a randomised, placebo-controlled, double-blind trial. Gut. 2010;59:21-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 443] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 89. | Assa'ad AH, Gupta SK, Collins MH, Thomson M, Heath AT, Smith DA, Perschy TL, Jurgensen CH, Ortega HG, Aceves SS. An antibody against IL-5 reduces numbers of esophageal intraepithelial eosinophils in children with eosinophilic esophagitis. Gastroenterology. 2011;141:1593-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 333] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 90. | Spergel JM, Rothenberg ME, Collins MH, Furuta GT, Markowitz JE, Fuchs G, O'Gorman MA, Abonia JP, Young J, Henkel T, Wilkins HJ, Liacouras CA. Reslizumab in children and adolescents with eosinophilic esophagitis: results of a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2012;129:456-463, 463.e1-463.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 419] [Article Influence: 27.9] [Reference Citation Analysis (1)] |
| 91. | Markowitz JE, Jobe L, Miller M, Frost C, Laney Z, Eke R. Safety and Efficacy of Reslizumab for Children and Adolescents With Eosinophilic Esophagitis Treated for 9 Years. J Pediatr Gastroenterol Nutr. 2018;66:893-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 92. | Rothenberg ME, Wen T, Greenberg A, Alpan O, Enav B, Hirano I, Nadeau K, Kaiser S, Peters T, Perez A, Jones I, Arm JP, Strieter RM, Sabo R, Gunawardena KA. Intravenous anti-IL-13 mAb QAX576 for the treatment of eosinophilic esophagitis. J Allergy Clin Immunol. 2015;135:500-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 240] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 93. | Hirano I, Collins MH, Assouline-Dayan Y, Evans L, Gupta S, Schoepfer AM, Straumann A, Safroneeva E, Grimm M, Smith H, Tompkins CA, Woo A, Peach R, Frohna P, Gujrathi S, Penenberg DN, Li C, Opiteck GJ, Olson A, Aranda R, Rothenberg ME, Dellon ES; HEROES Study Group. RPC4046, a Monoclonal Antibody Against IL13, Reduces Histologic and Endoscopic Activity in Patients With Eosinophilic Esophagitis. Gastroenterology. 2019;156:592-603.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 208] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 94. | Loizou D, Enav B, Komlodi-Pasztor E, Hider P, Kim-Chang J, Noonan L, Taber T, Kaushal S, Limgala R, Brown M, Gupta R, Balba N, Goker-Alpan O, Khojah A, Alpan O. A pilot study of omalizumab in eosinophilic esophagitis. PLoS One. 2015;10:e0113483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 95. | Straumann A, Bussmann C, Conus S, Beglinger C, Simon HU. Anti-TNF-alpha (infliximab) therapy for severe adult eosinophilic esophagitis. J Allergy Clin Immunol. 2008;122:425-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 124] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 96. | Sastre J, Dávila I. Dupilumab: A New Paradigm for the Treatment of Allergic Diseases. J Investig Allergol Clin Immunol. 2018;28:139-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 97. | Molina-Infante J, Lucendo AJ. Dietary therapy for eosinophilic esophagitis. J Allergy Clin Immunol. 2018;142:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 98. | Kelly KJ, Lazenby AJ, Rowe PC, Yardley JH, Perman JA, Sampson HA. Eosinophilic esophagitis attributed to gastroesophageal reflux: improvement with an amino acid-based formula. Gastroenterology. 1995;109:1503-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 790] [Cited by in RCA: 751] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 99. | Peterson KA, Byrne KR, Vinson LA, Ying J, Boynton KK, Fang JC, Gleich GJ, Adler DG, Clayton F. Elemental diet induces histologic response in adult eosinophilic esophagitis. Am J Gastroenterol. 2013;108:759-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 199] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 100. | Warners MJ, Vlieg-Boerstra BJ, Verheij J, van Rhijn BD, Van Ampting MT, Harthoorn LF, de Jonge WJ, Smout AJ, Bredenoord AJ. Elemental diet decreases inflammation and improves symptoms in adult eosinophilic oesophagitis patients. Aliment Pharmacol Ther. 2017;45:777-787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 101. | Arias A, González-Cervera J, Tenias JM, Lucendo AJ. Efficacy of dietary interventions for inducing histologic remission in patients with eosinophilic esophagitis: a systematic review and meta-analysis. Gastroenterology. 2014;146:1639-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 395] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 102. | Gonsalves N. Clinical Presentation and Approach to Dietary Management of Eosinophilic Esophagitis. Gastroenterol Hepatol (NY). 2018;14:706-712. [PubMed] |
| 103. | Spergel JM, Beausoleil JL, Mascarenhas M, Liacouras CA. The use of skin prick tests and patch tests to identify causative foods in eosinophilic esophagitis. J Allergy Clin Immunol. 2002;109:363-368. [PubMed] |
| 104. | Spergel JM, Brown-Whitehorn TF, Cianferoni A, Shuker M, Wang ML, Verma R, Liacouras CA. Identification of causative foods in children with eosinophilic esophagitis treated with an elimination diet. J Allergy Clin Immunol. 2012;130:461-467.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 284] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 105. | Molina-Infante J, Martin-Noguerol E, Alvarado-Arenas M, Porcel-Carreño SL, Jimenez-Timon S, Hernandez-Arbeiza FJ. Selective elimination diet based on skin testing has suboptimal efficacy for adult eosinophilic esophagitis. J Allergy Clin Immunol. 2012;130:1200-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 106. | Rodríguez-Sánchez J, Gómez Torrijos E, López Viedma B, de la Santa Belda E, Martín Dávila F, García Rodríguez C, Feo Brito F, Olmedo Camacho J, Reales Figueroa P, Molina-Infante J. Efficacy of IgE-targeted vs empiric six-food elimination diets for adult eosinophilic oesophagitis. Allergy. 2014;69:936-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 107. | Philpott H, Nandurkar S, Royce SG, Thien F, Gibson PR. Allergy tests do not predict food triggers in adult patients with eosinophilic oesophagitis. A comprehensive prospective study using five modalities. Aliment Pharmacol Ther. 2016;44:223-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 108. | Wright BL, Kulis M, Guo R, Orgel KA, Wolf WA, Burks AW, Vickery BP, Dellon ES. Food-specific IgG4 is associated with eosinophilic esophagitis. J Allergy Clin Immunol. 2016;138:1190-1192.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 109. | Kagalwalla AF, Sentongo TA, Ritz S, Hess T, Nelson SP, Emerick KM, Melin-Aldana H, Li BU. Effect of six-food elimination diet on clinical and histologic outcomes in eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2006;4:1097-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 504] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 110. | Cotton CC, Erim D, Eluri S, Palmer SH, Green DJ, Wolf WA, Runge TM, Wheeler S, Shaheen NJ, Dellon ES. Cost Utility Analysis of Topical Steroids Compared With Dietary Elimination for Treatment of Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2017;15:841-849.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 111. | Molina-Infante J, Arias A, Barrio J, Rodríguez-Sánchez J, Sanchez-Cazalilla M, Lucendo AJ. Four-food group elimination diet for adult eosinophilic esophagitis: A prospective multicenter study. J Allergy Clin Immunol. 2014;134:1093-1099.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 178] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 112. | Molina-Infante J, Arias Á, Alcedo J, Garcia-Romero R, Casabona-Frances S, Prieto-Garcia A, Modolell I, Gonzalez-Cordero PL, Perez-Martinez I, Martin-Lorente JL, Guarner-Argente C, Masiques ML, Vila-Miravet V, Garcia-Puig R, Savarino E, Sanchez-Vegazo CT, Santander C, Lucendo AJ. Step-up empiric elimination diet for pediatric and adult eosinophilic esophagitis: The 2-4-6 study. J Allergy Clin Immunol. 2018;141:1365-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 217] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 113. | Schoepfer AM, Gschossmann J, Scheurer U, Seibold F, Straumann A. Esophageal strictures in adult eosinophilic esophagitis: dilation is an effective and safe alternative after failure of topical corticosteroids. Endoscopy. 2008;40:161-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 114. | Runge TM, Eluri S, Cotton CC, Burk CM, Woosley JT, Shaheen NJ, Dellon ES. Outcomes of Esophageal Dilation in Eosinophilic Esophagitis: Safety, Efficacy, and Persistence of the Fibrostenotic Phenotype. Am J Gastroenterol. 2016;111:206-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 107] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 115. | Jacobs JW, Spechler SJ. A systematic review of the risk of perforation during esophageal dilation for patients with eosinophilic esophagitis. Dig Dis Sci. 2010;55:1512-1515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 116. | Moawad FJ, Molina-Infante J, Lucendo AJ, Cantrell SE, Tmanova L, Douglas KM. Systematic review with meta-analysis: endoscopic dilation is highly effective and safe in children and adults with eosinophilic oesophagitis. Aliment Pharmacol Ther. 2017;46:96-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 117. | Dellon ES, Gupta SK. A Conceptual Approach to Understanding Treatment Response in Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (1)] |
| 118. | Katzka DA, Geno DM, Ravi A, Smyrk TC, Lao-Sirieix P, Miremadi A, Debiram I, O'Donovan M, Kita H, Kephart GM, Kryzer LA, Camilleri M, Alexander JA, Fitzgerald RC. Accuracy, safety, and tolerability of tissue collection by Cytosponge vs endoscopy for evaluation of eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2015;13:77-83.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 119. | Furuta GT, Kagalwalla AF, Lee JJ, Alumkal P, Maybruck BT, Fillon S, Masterson JC, Ochkur S, Protheroe C, Moore W, Pan Z, Amsden K, Robinson Z, Capocelli K, Mukkada V, Atkins D, Fleischer D, Hosford L, Kwatia MA, Schroeder S, Kelly C, Lovell M, Melin-Aldana H, Ackerman SJ. The oesophageal string test: a novel, minimally invasive method measures mucosal inflammation in eosinophilic oesophagitis. Gut. 2013;62:1395-1405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 210] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 120. | Dellon ES, Rusin S, Gebhart JH, Covey S, Higgins LL, Beitia R, Speck O, Woodward K, Woosley JT, Shaheen NJ. Utility of a Noninvasive Serum Biomarker Panel for Diagnosis and Monitoring of Eosinophilic Esophagitis: A Prospective Study. Am J Gastroenterol. 2015;110:821-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 121. | Cunnion KM, Willis LK, Minto HB, Burch TC, Werner AL, Shah TA, Krishna NK, Nyalwidhe JO, Maples KM. Eosinophil Quantitated Urine Kinetic: A novel assay for assessment of eosinophilic esophagitis. Ann Allergy Asthma Immunol. 2016;116:435-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 122. | Wen T, Stucke EM, Grotjan TM, Kemme KA, Abonia JP, Putnam PE, Franciosi JP, Garza JM, Kaul A, King EC, Collins MH, Kushner JP, Rothenberg ME. Molecular diagnosis of eosinophilic esophagitis by gene expression profiling. Gastroenterology. 2013;145:1289-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 211] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Colombia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
P-Reviewer: Imaeda H, Sherman PM S-Editor: Ma RY L-Editor: A E-Editor: Zhang YL