Published online Aug 14, 2019. doi: 10.3748/wjg.v25.i30.4222
Peer-review started: April 16, 2019
First decision: June 10, 2019
Revised: June 25, 2019
Accepted: July 5, 2019
Article in press: July 5, 2019
Published online: August 14, 2019
Processing time: 120 Days and 21.9 Hours
Liver fibrosis is a refractory disease whose persistence can eventually induce cirrhosis or even liver cancer. Early liver fibrosis is reversible by intervention. As a member of the transforming growth factor-beta (TGF-β) superfamily, bone morphogenetic protein 7 (BMP7) has anti-liver fibrosis functions. However, little is known about BMP7 expression changes and its potential regulatory mechanism as well as the relationship between BMP7 and TGF-β during liver fibrosis. In addition, the mechanism underlying the anti-liver fibrosis function of BMP7 needs to be further explored.
To investigate changes in the dynamic expression of BMP7 during liver fibrosis, interactions between BMP7 and TGF-β1, and possible mechanisms underlying the anti-liver fibrosis function of BMP7.
Changes in BMP7 expression during liver fibrosis and the interaction between BMP7 and TGF-β1 in mice were observed. Exogenous BMP7 was used to treat mouse primary hepatic stellate cells (HSCs) to observe its effect on activation, migration, and proliferation of HSCs and explore the possible mechanism underlying the anti-liver fibrosis function of BMP7. Mice with liver fibrosis received exogenous BMP7 intervention to observe improvement of liver fibrosis by using Masson’s trichrome staining and detecting the expression of the HSC activation indicator alpha-smooth muscle actin (α-SMA) and the collagen formation associated protein type I collagen (Col I). Changes in the dynamic expression of BMP7 during liver fibrosis in the human body were further observed.
In the process of liver fibrosis induced by carbon tetrachloride (CCl4) in mice, BMP7 protein expression first increased, followed by a decrease; there was a similar trend in the human body. This process was accompanied by a sustained increase in TGF-β1 protein expression. In vitro experiment results showed that TGF-β1 inhibited BMP7 expression in a time- and dose-dependent manner. In contrast, high doses of exogenous BMP7 inhibited TGF-β1-induced activation, migration, and proliferation of HSCs; this inhibitory effect was associated with upregulation of pSmad1/5/8 and downregulation of phosphorylation of Smad3 and p38 by BMP7. In vivo experiment results showed that exogenous BMP7 improved liver fibrosis in mice.
During liver fibrosis, BMP7 protein expression first increases and then decreases. This changing trend is associated with inhibition of BMP7 expression by sustained upregulation of TGF-β1 in a time- and dose-dependent manner. Exogenous BMP7 could selectively regulate TGF-β/Smad pathway-associated factors to inhibit activation, migration, and proliferation of HSCs and exert anti-liver fibrosis functions. Exogenous BMP7 has the potential to be used as an anti-liver fibrosis drug.
Core tip: Liver fibrosis is the repair response of the liver to chronic injury. Its long-term persistence can eventually progress into cirrhosis or even liver cancer. Many cytokines and signaling pathways participate in the development and progression of liver fibrosis. The transforming growth factor-beta (TGF-β) superfamily member bone morphogenetic protein 7 (BMP7) is reported to have anti-fibrosis functions. This study observed changes in the dynamic expression of BMP7 during liver fibrosis, explored the relationship between BMP7 and TGF-β1, and further investigated the possible mechanism underlying the anti-liver fibrosis function of exogenous BMP7 using in vivo and in vitro experiments. This study demonstrated the prospect of exogenous BMP7 application as a potential anti-liver fibrosis drug.
- Citation: Zou GL, Zuo S, Lu S, Hu RH, Lu YY, Yang J, Deng KS, Wu YT, Mu M, Zhu JJ, Zeng JZ, Zhang BF, Wu X, Zhao XK, Li HY. Bone morphogenetic protein-7 represses hepatic stellate cell activation and liver fibrosis via regulation of TGF-β/Smad signaling pathway. World J Gastroenterol 2019; 25(30): 4222-4234
- URL: https://www.wjgnet.com/1007-9327/full/v25/i30/4222.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i30.4222
Liver fibrosis is a pathophysiological process of intrahepatic connective tissue dysplasia. In the clinic, viral hepatitis, alcoholic hepatitis, non-alcoholic fatty liver, and autoimmune diseases can all induce liver injury and further activate the repair process of liver injury to induce fibrosis in the liver. Any liver repair and healing process will induce liver fibrosis. If the long-term injury factors cannot be removed, long-term, persistent fibrosis will progress into cirrhosis and further induce liver cancer. Studies in recent years showed that although late-stage cirrhosis and liver cancer are irreversible, the early liver fibrosis process resulting from liver injury is reversible[1,2]. Using early intervention in liver fibrosis to interfere with liver disease progression is a feasible therapeutic strategy. Therefore, studies targeting liver tissue fibrosis are always a hot spot in liver disease research.
Liver repair mechanisms activated by liver injury mainly include extracellular matrix (ECM) hyperplasia and remodeling and normal hepatocyte proliferation. ECM hyperplasia and degradation are regulated by matrix metalloproteases (MMPs) and tissue inhibitors of MMPs (TIMPs) together[3]. Hepatic stellate cells (HSCs) play an important role in liver fibrosis. When liver injury factors persist, dormant HSCs are activated. HSCs inhibit MMP activity through TIMPs to cause ECM accumulation and produce large amounts of related cytokines, including vascular endothelial growth factor (VEGF), pro-inflammatory cytokine interleukin-6 (IL-6), and pro-fibrogenic factors such as transforming growth factor-β (TGF-β). Secreted TGF-β can further activate HSCs to cause its differentiation into myofibroblasts, which migrate to the injury site to further express a large amount of type I collagen (Col I) and alpha-smooth muscle actin (α-SMA); thus, ECM is replaced by newly produced collagen, eventually resulting in the formation of liver scars[4]. Inhibition of HSC activity such as promotion of their apoptosis or restoration of the dormant status of activated HSCs can exert anti-liver fibrosis functions.
Molecular pathways that play important roles in liver fibrosis include Wnt, TGF-β, and Alx[5]. As a member of the TGF-β family, TGF-β has diverse molecular functions including regulation of cell proliferation, differentiation, and wound healing, and it plays an important role in the development and progression of liver fibrosis[6]. For example, TGF-β activates Smad3, α-SMA, and Col1a1 to promote collagen fiber accumulation and further cause progression of liver fibrosis[7]. Bone morphogenetic protein-7 (BMP7) is a member of the TGF-β family. Unlike TGF-β, BMP7 inhibits fibrosis. In a carbon tetrachloride (CCl4)-induced liver fibrosis model in rats, BMP7 overexpression in the liver through tail injection inhibited liver fibrosis[8]. In a CCl4-induced liver fibrosis model in mice, oral administration of recombinant BMP7 protein could also inhibit liver fibrosis, which might be associated with affecting the TGF-β-mediated Smad signaling pathway[9]. In a Schistosoma japonicum-infected liver fibrosis model, BMP7 regulated liver fibrosis through the TGF-β-Smad signaling pathway[10]. During the development of liver fibrosis, BMP7 activated Smad1/5/8 to inhibit fibrosis[11,12]. In addition, BMP7 inhibited ColIa1 expression by increasing MMP13 and decreasing TIMP2 expression to further inhibit fibrosis[13]. A series of studies reported that TGF-β1 and BMP7 play important regulatory roles in fibrosis. During liver fibrosis in rats, the ratio of the TGF-β/BMP7 expression levels was associated with epithelial-mesenchymal transition (EMT). Elevation of TGF-β/BMP7 suggested aggravation of liver fibrosis[14]. In retinal pigmented epithelial fibrosis, there was an interaction between BMP7/Smad1/5/8 and TGF-β/Smad3, suggesting that the dynamic balance between BMP7 and TGF-β could determine the outcome of liver fibrosis[15]. In the development and progression of kidney diseases, BMP7 also plays an important role[16]. During renal fibrosis caused by kidney injury, BMP7 had an anti-fibrosis function[17], and the mechanism might be associated with inhibition of the Akt signaling pathway. Furthermore, BMP7 also inhibited the progression of renal fibrosis through inhibition of Smad3, Col1a1, and α-SMA[18]. In patients with corneal abrasion, ITF2357 attenuated corneal fibrosis through regulation of the TGF-β/BMP7 signaling pathway[19]. In patients with chronic hepatitis B, the blood BMP7 level suggested its anti-fibrosis potential[20].
The exertion of BMP7 functions has significant tissue specificity and cell spe-cificity[21,22]. In previous research, BMP7 played an important role in repressing hepatic fibrosis. However, the expression mode and potential mechanisms of BMP7 are still unknown, and whether there exists a crosstalk between BMP7 and TGF-β1 remains to be revealed. We hypothesized that the activation of BMP7-pSmad1/5/8 pathway may repress hepatic fibrosis by antagonizing TGFβ1 signaling in CCL4-induced fibrotic mice model. The goal of the current study was to investigate the role of BMP7 in liver fibrosis and its potential mechanisms based on its expression mode. We established a CCl4-induced liver fibrosis model in mice to confirm that with the progression of liver fibrosis, BMP7 expression showed a changing trend of first increasing and then decreasing; in contrast, TGF-β1 expression persistently increased. The mechanism study showed that TGF-β1 inhibited BMP7 expression in a time- and dose-dependent manner within a certain dosage range. In addition, we showed that high doses of BMP7 antagonized the TGF-β1-pSmad3 signaling pathway through the pSmad1/5/8 pathway to inhibit HSC activation and subsequent pro-liver fibrosis processes such as proliferation, migration, and collagen secretion. Our data demonstrate that BMP7 is a vital anti-fibrotic factor, and recombinant BMP7 may serve as a potential therapeutic agent for liver fibrosis.
TGF-β1 was obtained from R&D Systems (Minneapolis, MN, United States). The following antibodies were purchased: BMP7 (Biosource, Camarillo, CA, United States), procollagen alpha 1 type 1 (1A1) (Santa Cruz Biotechnology, Santa Cruz, CA), p-Smad3, p-Smad1/5/8, p-P38 (EMD Millipore, Billerica, MA, United States), and glyceraldehyde 3-phosphate dehy-drogenase (GAPDH) (Research Diagnostics, Flanders, NJ, United States). Anti-α-SMA antibody, carbon tetrachloride (CCl4), corn oil, and OptiPrep were purchased from Sigma-Aldrich (St. Louis, MO, United States). BMP7 was purchased from Selleckchem and Fisher Scientific (Waltham, MA, United States). All other chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO, United States) and Fisher Scientific (Waltham, MA, United States).
All animal interventions were approved by the Institutional Animal Care and Use Committee (IACUC) of Guizhou Medical University. The methods and experiment procedures were carried out in accordance with the relevant guidelines and regulations. Mice (C57BL6, eight to ten weeks old) were housed in standard conditions, and sex matched mice were treated with 2.0 µL/g body weight carbon tetrachloride (CCl4) [1:10 (v/v) dilution with corn oil] or corn oil as control by intraperitoneal (i.p.) injections, three times per week for 4 wk[23]. Mice were challenged with CCl4 or corn oil (Control), followed by intraperitoneal injection of BMP7 (100 ng/g, three times per week for 4 wk) or 0.9% saline (Vehicle). Mice were sacrificed at 48 h after the last CCl4 injection and tissues were harvested.
Immunoblotting was performed using 1% NP-40 whole liver tissue lysates or whole cell lysates as described previously[23]. The total proteins were extracted and quantified using Bradford protein quantification kits. Protein samples of 40 g each were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The proteins were transferred onto polyvinylidene fluoride (PVDF) membranes and incubated with primary antibodies overnight at 4 °C. On the second day, the signal was developed with an electrochemiluminescence detection kit after incubation with the appropriate secondary antibodies.
Collagen deposition in liver tissue sections (5-10 µm, paraffin embedded tissues) was localized by Masson’s trichrome staining using a commercially available staining kit according to the manufacturer’s instructions (Poly Scientific, Bay Shore, NY, United States). Fibrotic lesional density by area was measured on Masson’s trichrome stained sections and quantified by morphometric methodology.
Isolation of primary mouse HSCs was performed by enzymatic digestion and density gradient centrifugation as described previously[24]. Briefly, cells were isolated from the livers by in situ liver perfusion with pronase, liberase, and collagenase followed by density gradient centrifugation. Dispersed cell suspension was filtered and centrifuged at 50 g for 2 min to remove hepatocytes. The remaining cell fraction was washed and resuspended in 11.5% OptiPrep, then gently transferred to the tube containing a bottom of 15% OptiPrep, followed by adding the top layer of PBS. The cell fraction was then centrifuged at 1400 g for 20 min. The HSCs fraction layer was obtained at the interface between the top and intermediate layers. The purity of the HSCs fraction was estimated based on autofluorescence. Purity of HSCs was assessed and estimated by auto-florescence one day after isolation, and was always higher than 97%. Cell viability was also examined and HSCs were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and antibiotics as described previously[25]. Cells were starved or treated on day 2 after isolation and the duration of starvation or treatment is described in each figure legend. The duration of the whole experiments was within 5-7 d of isolation of HSCs and the passages were 1-2 (as some experiments were needed to passage cells from regular culture flask to experimental cell culture wells).
BrdU proliferation assay was performed per manufacturer's protocol (Millipore #2750). Briefly, HSCs were seeded at a density of 2 × 104 cells per well in a 96-well plate and treated with BMP7 (100 ng/mL). BrdU was added for the last 16 h of incubation. Cells were then fixed, washed, and incubated with anti-BrdU monoclonal antibody and secondary antibody per the manufacture’s protocol. After washing, TMB peroxidase substrate was added and incubated for 30 min in the dark, followed by addition of stop solution. Incorporated BrdU was measured with a microplate reader at 450 nm (Tecan, Invitrogen). For cell count, HSCs were seeded at a density of 1 × 104 cells per well in a 96-well plate and treated with BMP7 (100 ng/mL). Cells were trypsinized and viable cells were stained with trypan blue and counted every 24 h after treatment for 5 d.
The wound closure monolayer/scratch motility assay was performed as described previously[25]. Briefly, fibroblasts were plated in serum-free DMEM with 1% bovine serum albumin for 24 h. Mitomycin C was added to inhibit cell proliferation. The monolayer was scratched, and the wound area covered by cell migration over indicated time on digital photomicrograph images was calculated. The migration capabilities of HSCs were assessed using a scratch wound assay. Cells were seeded in 24-well plate and maintained with 5% CO2 at 37 °C overnight to form a confluent monolayer. Then, a linear wound of 0.4-0.5 mm in width was generated in the monolayer with a sterile 200 μL plastic pipette tip. Any cellular debris was removed by washing with PBS. Cells were then incubated in the presence or absence of BMP7 (100 ng/mL). Wound closure was monitored by collecting digitized images at 0, 12, and 24 h post scratch, respectively.
The normal and liver fibrosis tissue samples were obtained from patients at the Department of Hepatobiliary Surgery of the Affiliated Hospital of Guizhou Medical University (Guiyang, China). Informed consent in writing was obtained from patients. This study protocol was approved by the Review Committee of the Affiliated Hospital of Guizhou Medical University (No. 20150050).
Data were analyzed using the Student’s t-test (Sigma Plot, SPSS Inc.) for differences between two groups, and are expressed as the mean ± SE. For comparisons among multiple groups, ANOVA (3-way) was performed, followed by t-test with Bonferronni correction using SAS 9.3 (SAS Institute Inc., Cary, NC, United States). All experiments were repeated at least 3 times. Differences were considered statistically significant at P < 0.05 (aP < 0.05, bP < 0.01).
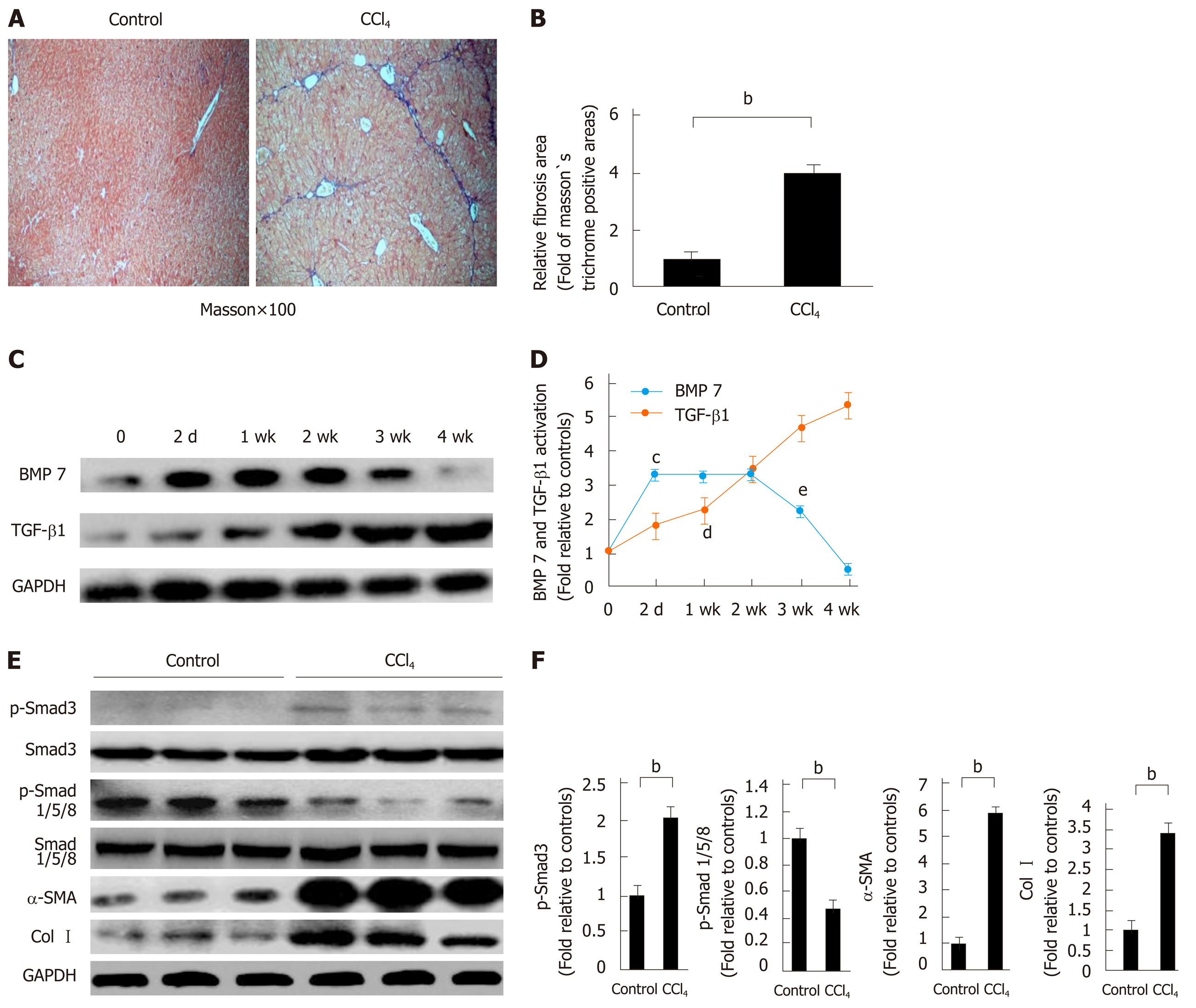
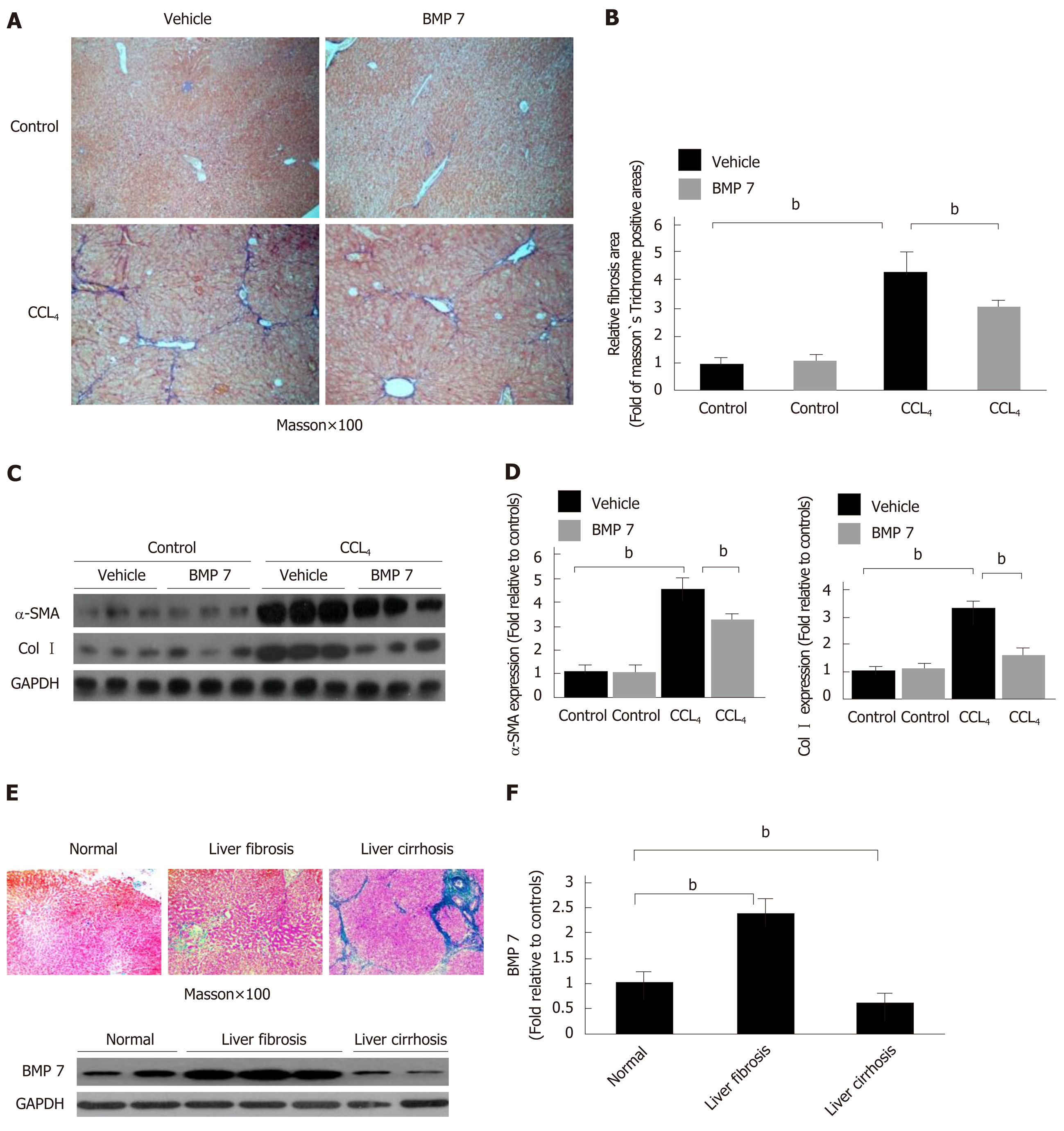
Previous studies suggested that BMP7 has anti-fibrosis functions[8]. To explore the potential molecular mechanism underlying the anti-fibrosis function of BMP7, we first established a CCl4-induced liver fibrosis model. Mice received intraperitoneal injections of CCl4 and were sacrificed after 4 wk. Livers were removed for Masson’s trichrome staining. The CCl4 group exhibited a large area of collagen deposition, whereas the control group rarely had traces of collagen formation. The quantitative analysis results showed that the collagen expression level was 4 times higher in the CCl4 group than in the control group (Figure 1A and B). Because TGF-β1 is an important pro-fibrogenic factor[6,12], we examined the expression of BMP7 and TGF-β1 in the liver of mice in the CCl4 group and the control group. After CCl4 injection, the expression levels of BMP7 and TGF-β1 in the liver were already increased after 2 d. With the persistent increase in TGF-β1 expression, the BMP7 expression level was significantly reduced at week 3, and the reduction was even more evident at week 4 (Figure 1C and D), suggesting that TGF-β1 might inhibit BMP7 expression. Furthermore, we examined the expression of Smad family proteins and important proteins in muscle fiber formation, including α-SMA and Col1a1. Compared to the control group, pSmad3 expression in the CCl4 group was upregulated and pSmad1/5/8 expression was downregulated, which was consistent with the changing trend of TGF-β1 and BMP7. These results suggested that CCl4 treatment activated the TGF-β1-pSmad3 signaling pathway and inhibited the BMP7-pSmad1/5/8 signaling pathway. In addition, the α-SMA and Col I expression levels both significantly increased (Figure 1E and F).
In the CCl4-induced liver fibrosis model in mice, the expression of TGF-β1 and BMP7 showed opposite changing trends. With the aggravation of fibrosis, TGF-β1 expression increased and BMP7 expression was downregulated, suggesting that TGF-β1 might regulate BMP7 expression. To investigate the potential regulatory re-lationship between TGF-β1 and BMP7, in vitro cell experiments were further performed.
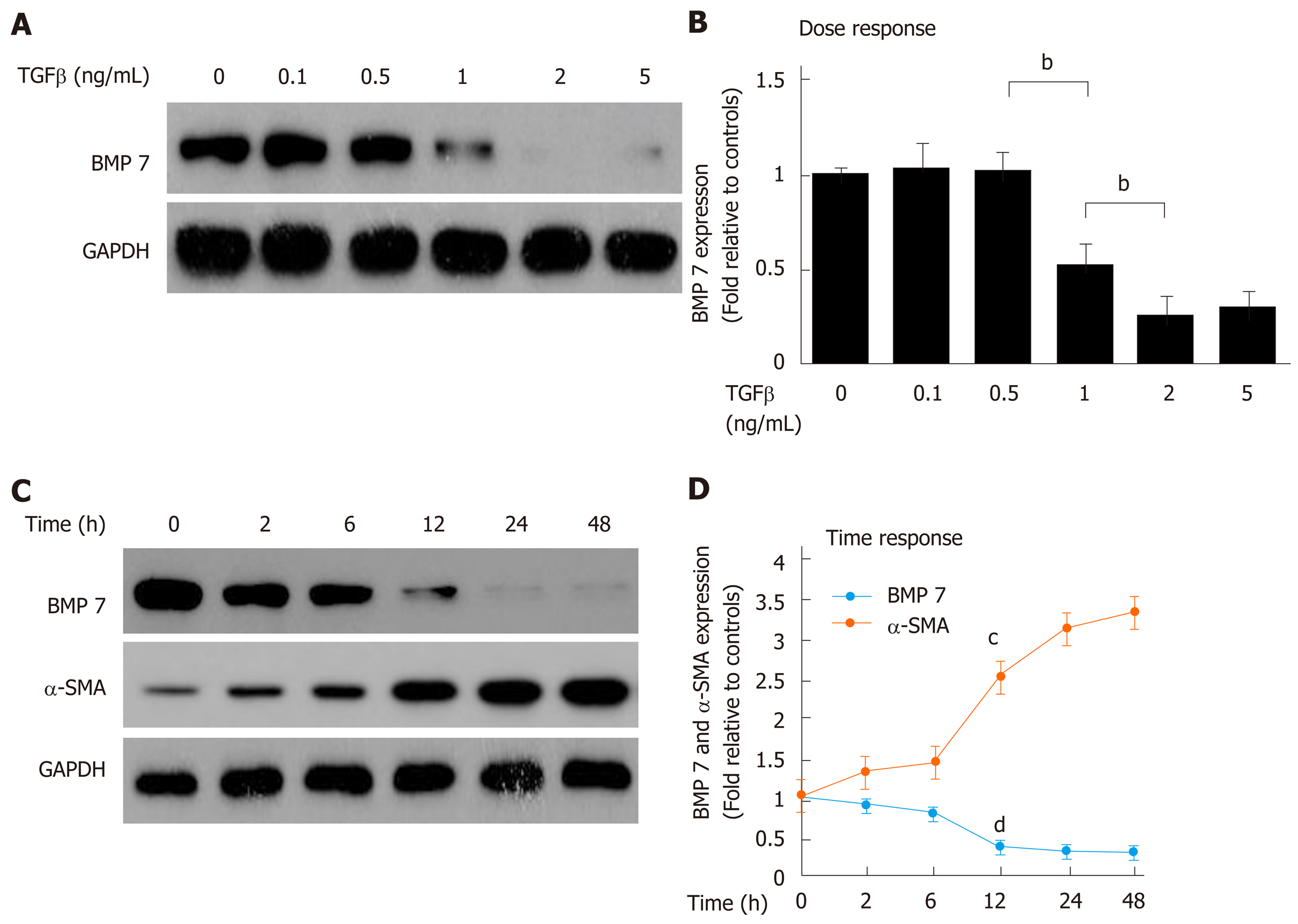
Mouse HSCs were isolated, starved, and then treated with different doses of TGF-β1. BMP7 expression was then measured. The results showed that when the TGF-β1 concentration reached 1.0 ng/mL, BMP7 expression began to decrease. Under stimulation at higher concentrations (2.0 ng/mL and 5.0 ng/mL), the BMP7 expression level significantly decreased with increasing TGFβ1 doses (Figure 2A and B), suggesting that TGF-β1 could inhibit BMP7 expression in a dose-dependent manner. Was the inhibitory effect of TGF-β1 on BMP7 also time-dependent? BMP7 expression was examined at different time points after HSCs were stimulated with 2 ng/mL TGF-β1. The results showed that inhibition of BMP7 expression by TGF-β1 indeed was time-dependent (Figure 2C and D). Our results suggested that TGF-β1 could inhibit BMP7 expression, and this effect was time- and dose-dependent.
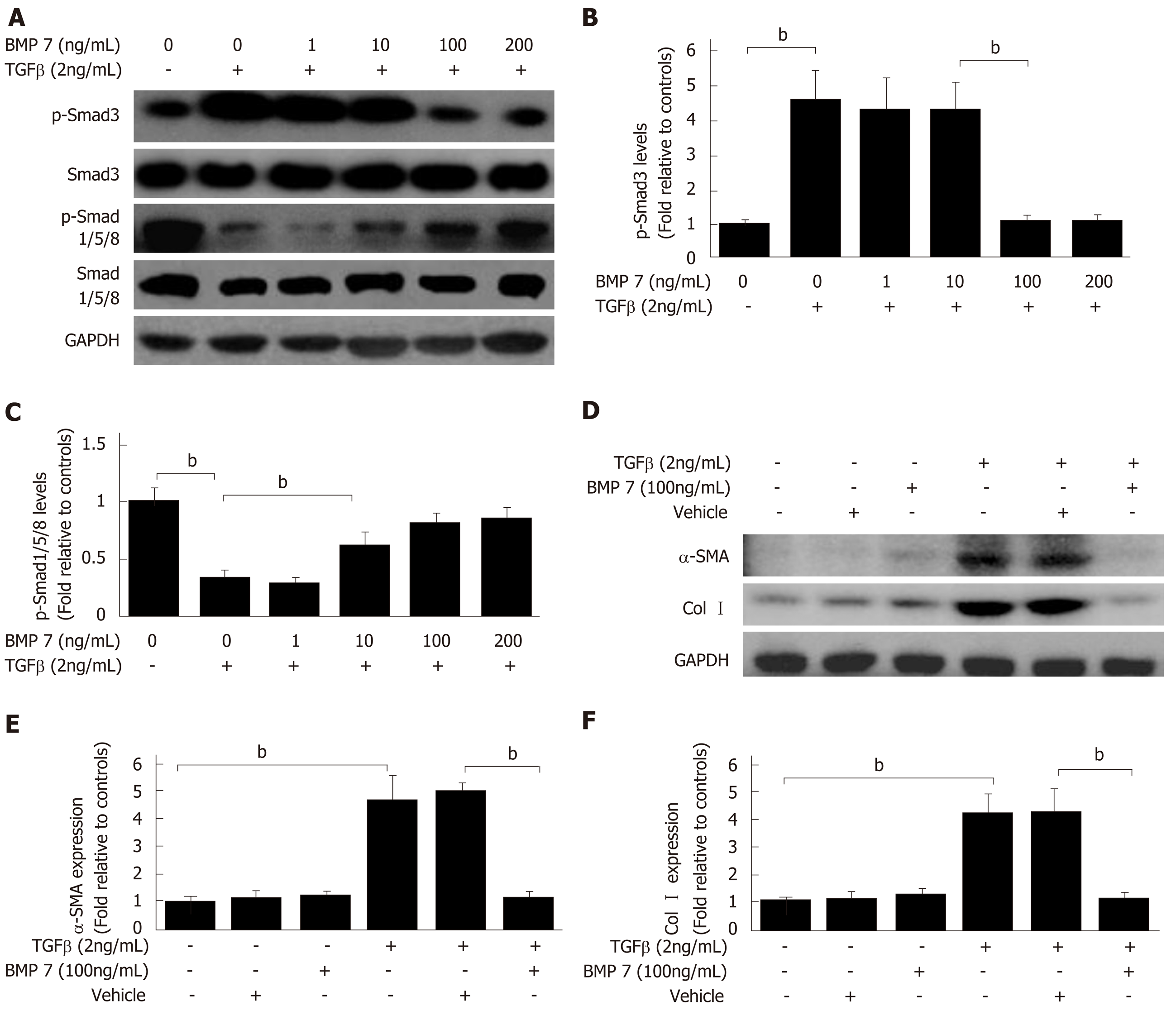
TGF-β1 can activate HSCs to differentiate into myofibroblasts, which express a large amount of Col I and α-SMA; therefore, ECM is replaced by newly produced collagen that eventually results in liver scar formation[4]. To confirm whether the anti-fibrosis function of BMP7 is associated with HSCs, BMP7-regulated HSCs activation experiments were performed. BMP7 expression was upregulated in the early stage of liver fibrosis, suggesting that BMP7 has an anti-fibrosis function. It has been reported that BMP7 could achieve its anti-fibrosis function through inhibition of α-SMA and Col I expression[8]. During treatment with 2 ng/mL TGF-β1, stimulation with different concentrations of BMP7 occurred. The results showed that TGF-β1 inhibited BMP7-pSmad1/5/8 expression through activation of pSmad3 when the BMP7 dose was low, whereas TGF-β1 activated pSmad1/5/8 and inhibited pSmad3 expression when the BMP7 dose reached 100 ng/mL. These results suggested that the anti-fibrosis function of BMP7 might be associated with feedback inhibition of TGF-β1 expression by high doses of BMP7 (Figure 3A-C). Next, HSCs were stimulated with 2 ng/mL TGF-β1 and 100 ng/mL BMP7, and the expression of α-SMA and Col I was detected to measure HSC activation and myofibroblast fiber formation ability, respectively. With BMP7 stimulation alone, the expression levels α-SMA and Col I were low. With TGF-β1 stimulation, α-SMA and Col I expression levels both increased. With stimulation by both, high doses of BMP7 reversed TGF-β1 stimulation-induced upregulation of α-SMA and Col I protein expression (Figure 3D and E). In summary, these results suggested that high doses of BMP7 could inhibit HSC activation and formation of myofibroblast fibers through the pSmad1/5/8 signaling pathway to achieve the anti-fibrosis function.
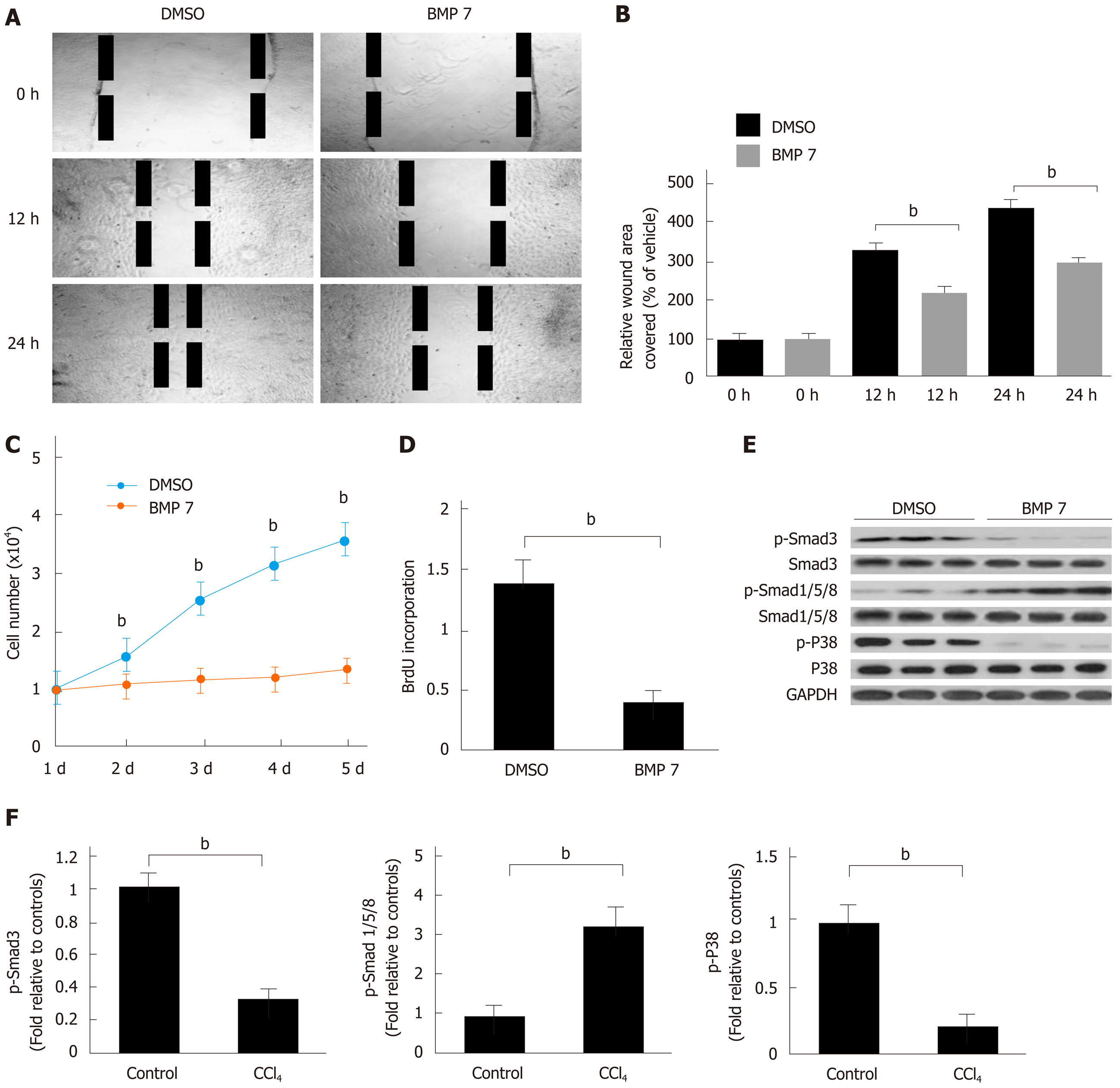
After activation, HSCs differentiate into myofibroblasts and migrate to the injury site to promote collagen formation and generate fibrotic tissues. Because BMP7 inhibits HSC activation and collagen formation, BMP7 might inhibit migration and proliferation of HSCs. To test this hypothesis, we performed wound healing experiments and cell proliferation experiments to explore the regulatory function of BMP7 on the migration and proliferation of HSCs. Compared to the control group, the BMP7 treatment group exhibited significant inhibition of HSC migration (Figure 4A and B) and proliferation (Figure 4C and D). Next, we further detected changes in related molecular pathways. BMP7 treatment upregulated pSmad1/5/8 expression and inhibited pSmad3 expression. Previous studies reported that p38 plays a positive regulatory role in HSC activation and proliferation[22]. Our results showed that p38 expression was significantly downregulated in the BMP7 treatment group (Figure 4E and F), suggesting that BMP7 might regulate HSC migration and proliferation through inhibition of the p38 signaling pathway.
In vitro experiment results suggested that BMP7 has anti-fibrosis function. To clarify whether this function is still present in mice, we treated CCl4-induced liver fibrosis mice with BMP7. Mice in the corn oil treatment and the CCl4 treatment groups were stimulated with Vehicle and BMP7. Compared to the mice in the model group, the degree of liver fibrosis was significantly attenuated in mice after BMP7 treatment (Figure 5A and B), and α-SMA and Col I expression in liver tissues was significantly decreased (Figure 5C and D), suggesting that BMP7 has the functions of anti-collagen fiber deposition and inhibition of liver fibrosis.
In the CCl4-induced liver fibrosis model in mice, BMP7 showed a changing trend of rising first, followed by decreasing, suggesting that BMP7 has an anti-liver fibrosis function (Figure 1C). Does BMP7 expression in patients with liver fibrosis also present this trend? BMP7 expression levels were detected in liver tissues of patients with liver fibrosis and cirrhosis. Compared to normal individuals, BMP7 expression was upregulated in patients with liver fibrosis and downregulated in patients with cirrhosis (Figure 5E and F). This trend is consistent with that in the mouse model, suggesting that BMP7 in the liver exhibits a compensatory increase to exert its anti-liver fibrosis function. When the disease further developed into cirrhosis, BMP7 expression was basically in a state of exhaustion, suggesting that the compensatory anti-fibrosis ability of the body is significantly weakened.
In this study, we found that negatively regulated by TGFβ1, the specific over-expression of BMP7 in fibrotic liver functions as an anti-fibrotic factor by repressing the expression of Col I and α-SMA, thus reducing the formation of collagens.
During renal fibrosis, BMP7 inhibits collagen fiber formation through inhibition of pSmad3, Col1a1, and α-SMA[18]. In liver fibrosis, focal adhesion kinase (FAK) could activate HSCs to promote the liver fibrosis process[23]. Are changes in BMP7/TGF-β also present in liver fibrosis? Our study showed that exogenous BMP7 inhibited HSC proliferation and migration through the pSmad1/5/8 signaling pathway to resist liver fibrosis, which is consistent with the results of previous studies showing inhibition of liver fibrosis by adenovirus-mediated BMP7 overexpression in the liver[8,9]. This study showed that exogenous BMP7 antagonized the TGF-β1-pSmad3-mediated pro-fibrosis function through activation of the pSmad1/5/8 signaling pathway. p38-AMPK participated in the processes of cell proliferation and differentiation[22]. Our results suggested that BMP7 inhibited HSC proliferation and differentiation into myofibroblasts through inhibition of p38 expression. Downregulation of the fibrosis markers α-SMA and Col I suggested that fibrosis was inhibited. Our data revealed that BMP7 inhibited myofibroblast secretion of the pro-fibrogenic factors α-SMA and Col I to resist liver fibrosis. In summary, our experiments confirmed that BMP7 antagonized the TGF-β-pSmad3 signaling pathway through activation of pSmad1/5/8 to inhibit the pro-liver fibrosis function caused by HSC activation.
This study not only used in vivo and in vitro experiments to confirm that the increased BMP7 expression in the liver in the early stage of liver fibrosis lesions mainly played an anti-fibrosis role but also discovered evidence of anti-liver fibrosis by BMP7 in an animal model and patients with liver fibrosis. Our in vitro experiments confirmed that increased BMP7 exerted its anti-fibrosis function through inhibition of HSC activation and collagen formation. In the mouse model, the BMP7 expression level first increased, followed by a decrease with the progression of liver fibrosis. The results of detection of BMP7 expression in samples from patients with liver fibrosis and cirrhosis also showed that its expression exhibited a similar change of increasing first, followed by decreasing. The reduced BMP7 expression level in the late stage was closely associated with the increase in TGF-β1 expression. In a lung fibrosis model, BMP7 inhibited TGF-β1-Smad3-ColI expression[19]. Our data suggested that TGF-β1 could inhibit BMP7 expression and that this regulatory function was time- and dose-dependent. When high doses of BMP7 were provided, activated BMP7-pSmad1/5/8 signals could in turn antagonize the pro-collagen formation signals of TGF-β1-pSmad3. The antagonizing effect of BMP7 on TGF-β1 was only present with high concentrations of BMP stimulation. In other words, TGF-β1 inhibited the anti-fibrosis function of low doses of BMP7. Increased doses of BMP7 overcame the inhibitory effect of TGF-β1 and antagonized the pro-fibrosis function of TGF-β1 through activation of downstream pathways.
In summary, we speculate that TGF-β1 negatively regulates the expression of BMP7, however, the inhibition exists within a certain dosage range. In other words, high doses of BMP7 might antagonize TGF signaling in turn. Although we tried to systemically investigate the anti-fibrosis function of BMP7, this study still has limitations. For example, we elucidated the role of TGF-β1 in the regulation of BMP7 and the function of high doses of BMP7 in antagonizing TGF-β1; however, the molecular mechanism underlying the regulation of BMP7 by TGF-β1 was not further studied. To elucidate the regulatory mode between these two factors, we need to further study the regulatory mechanism between TGF-β1 and BMP7, which is our future study direction.
This study showed that increased BMP7 expression levels had an anti-fibrosis function during liver fibrosis. With the progression of fibrosis, upregulated TGF-β1 expression inhibited the anti-fibrosis function of BMP7 to cause persistent aggravation of fibrosis into cirrhosis. Our study suggested that BMP7 has an anti-fibrosis function and that recombinant BMP7 could be used as a potential anti-fibrosis drug.
Liver fibrosis is a refractory disease. With disease progression, it can eventually progress into liver cirrhosis or even liver cancer, which severely threatens human health and brings heavy economic burdens. It has been confirmed that early liver fibrosis is reversible after intervention and that bone morphogenetic protein 7 (BMP7) has anti-fibrosis functions. However, the relationship between BMP7 expression and liver fibrosis and the specific mechanism underlying the anti-liver fibrosis function are still not very clear. It is necessary to perform further studies and provide theoretical support for application of exogenous BMP7 in anti-liver fibrosis.
The imbalance of the BMP7/transforming growth factor-beta (TGF-β1) ratio plays a very important role in the development and progression of organ fibrosis. It has been reported that BMP7 has an anti-liver fibrosis function. However, the mutual regulatory relationship between BMP7 and TGF-β1 and the mechanism underlying the anti-fibrosis function of BMP7 still require further elucidation. Therefore, observation of the dynamic changes in BMP7 and TGF-β1 during liver fibrosis and investigation of the possible mechanism underlying the anti-liver fibrosis function of BMP7 have positive significance in the further understanding and treatment of liver fibrosis.
Changes in BMP7 and TGF-β1 expression in the liver fibrosis process were observed to explore the interaction between these two and further study the possible mechanism underlying the anti-liver fibrosis function of BMP7.
Dynamic changes in BMP7 in the liver fibrosis process in mice and the interactive relationship between BMP7 and TGF-β1 were observed. Mouse primary HSCs were treated with exogenous BMP7 to observe the effect on activation, migration, and proliferation of HSCs and explore the possible mechanism underlying the anti-liver fibrosis function of BMP7. Mice with liver fibrosis were treated with exogenous BMP7 to observe the improvement of liver fibrosis by using Masson’s trichrome staining and detecting the expression of the HSC activation indicator α-SMA and the collagen formation associated protein Col I. Changes in the dynamic expression of BMP7 in liver fibrosis in the human body were further observed.
During liver fibrosis in mice, BMP7 expression first increased, followed by a decrease; the same trend was observed in the human body. This change was accompanied by sustained upregulation of TGF-β1 expression. In vitro experiment results showed that TGF-β1 could inhibit BMP7 expression in a time- and dose-dependent manner. In contrast, high doses of exogenous BMP7 could inhibit TGF-β1-induced activation, migration, and proliferation of HSCs; in addition, this inhibitory effect was associated with upregulation of pSmad1/5/8 and downregulation of pSmad3 and p-P38 protein expression by BMP7. In vivo experiment results showed that exogenous BMP7 improved liver fibrosis in mice.
Our study indicated that BMP7 expression first increased, followed by a decrease, during the liver fibrosis process. This changing trend was associated with inhibition of BMP7 expression by sustained upregulation of TGF-β1 in a time- and dose-dependent manner. High doses of BMP7 exhibited anti-liver fibrosis functions. This function was achieved through inhibition of TGF-β1-induced activation, migration, and proliferation of HSCs. Exogenous BMP7 played an anti-liver fibrosis role that was associated with activating BMP7-pSmad1/5/8 and antagonizing TGF-β1-pSmad3 and p-P38.
Our results further emphasized the anti-liver fibrosis function of BMP7 and exhibited the relationship between dynamic changes of BMP7 and TGF-β1 in the liver fibrosis process, which is helpful for deepening our understanding of the mechanism of liver fibrosis and has guiding significance in the selection of anti-liver fibrosis targets. The investigation of the anti-liver fibrosis mechanism of BMP7 suggested the prospect of application of exogenous BMP7 as an anti-liver fibrosis drug.
| 1. | Sun M, Kisseleva T. Reversibility of liver fibrosis. Clin Res Hepatol Gastroenterol. 2015;39:S60-S63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 204] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 2. | Shen X, Cheng S, Peng Y, Song H, Li H. Attenuation of early liver fibrosis by herbal compound "Diwu Yanggan" through modulating the balance between epithelial-to-mesenchymal transition and mesenchymal-to-epithelial transition. BMC Complement Altern Med. 2014;14:418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe SW. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657-667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1625] [Cited by in RCA: 1597] [Article Influence: 88.7] [Reference Citation Analysis (0)] |
| 4. | Campana L, Iredale JP. Regression of Liver Fibrosis. Semin Liver Dis. 2017;37:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 296] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 5. | Zhang CY, Yuan WG, He P, Lei JH, Wang CX. Liver fibrosis and hepatic stellate cells: Etiology, pathological hallmarks and therapeutic targets. World J Gastroenterol. 2016;22:10512-10522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 358] [Cited by in RCA: 485] [Article Influence: 48.5] [Reference Citation Analysis (12)] |
| 6. | Morikawa M, Derynck R, Miyazono K. TGF-β and the TGF-β Family: Context-Dependent Roles in Cell and Tissue Physiology. Cold Spring Harb Perspect Biol. 2016;8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 1034] [Article Influence: 103.4] [Reference Citation Analysis (0)] |
| 7. | Inagaki Y, Okazaki I. Emerging insights into Transforming growth factor beta Smad signal in hepatic fibrogenesis. Gut. 2007;56:284-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 410] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 8. | Kinoshita K, Iimuro Y, Otogawa K, Saika S, Inagaki Y, Nakajima Y, Kawada N, Fujimoto J, Friedman SL, Ikeda K. Adenovirus-mediated expression of BMP-7 suppresses the development of liver fibrosis in rats. Gut. 2007;56:706-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 130] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 9. | Hao ZM, Cai M, Lv YF, Huang YH, Li HH. Oral administration of recombinant adeno-associated virus-mediated bone morphogenetic protein-7 suppresses CCl(4)-induced hepatic fibrosis in mice. Mol Ther. 2012;20:2043-2051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Chen BL, Peng J, Li QF, Yang M, Wang Y, Chen W. Exogenous bone morphogenetic protein-7 reduces hepatic fibrosis in Schistosoma japonicum-infected mice via transforming growth factor-β/Smad signaling. World J Gastroenterol. 2013;19:1405-1415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Guo J, Lin Q, Shao Y, Rong L, Zhang D. BMP7 suppresses excessive scar formation by activating the BMP7/Smad1/5/8 signaling pathway. Mol Med Rep. 2017;16:1957-1963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Xu F, Liu C, Zhou D, Zhang L. TGF-β/SMAD Pathway and Its Regulation in Hepatic Fibrosis. J Histochem Cytochem. 2016;64:157-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 614] [Article Influence: 61.4] [Reference Citation Analysis (0)] |
| 13. | Cervantes-Garcia D, Cuellar-Juarez AG, Borrego-Soto G, Rojas-Martinez A, Aldaba-Muruato LR, Salinas E, Ventura-Juarez J, Muñoz-Ortega MH. Adenoviralbone morphogenetic protein7 and/or doxazosin therapies promote the reversion of fibrosis/cirrhosis in a cirrhotic hamster model. Mol Med Rep. 2017;16:9431-9440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Bi WR, Xu GT, Lv LX, Yang CQ. The ratio of transforming growth factor-β1/bone morphogenetic protein-7 in the progression of the epithelial-mesenchymal transition contributes to rat liver fibrosis. Genet Mol Res. 2014;13:1005-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Yao H, Ge T, Zhang Y, Li M, Yang S, Li H, Wang F. BMP7 antagonizes proliferative vitreoretinopathy through retinal pigment epithelial fibrosis in vivo and in vitro. FASEB J. 2019;33:3212-3224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Patel SR, Dressler GR. BMP7 signaling in renal development and disease. Trends Mol Med. 2005;11:512-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Morrissey J, Hruska K, Guo G, Wang S, Chen Q, Klahr S. Bone morphogenetic protein-7 improves renal fibrosis and accelerates the return of renal function. J Am Soc Nephrol. 2002;13:S14-S21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Higgins DF, Ewart LM, Masterson E, Tennant S, Grebnev G, Prunotto M, Pomposiello S, Conde-Knape K, Martin FM, Godson C. BMP7-induced-Pten inhibits Akt and prevents renal fibrosis. Biochim Biophys Acta Mol Basis Dis. 2017;1863:3095-3104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 19. | Lim RR, Tan A, Liu YC, Barathi VA, Mohan RR, Mehta JS, Chaurasia SS. ITF2357 transactivates Id3 and regulate TGFβ/BMP7 signaling pathways to attenuate corneal fibrosis. Sci Rep. 2016;6:20841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Aktug Demir N, Kolgelier S, Inkaya AC, Sumer S, Demir LS, Pehlivan FS, Arslan M, Arpaci A. Are bone morphogenetic protein-7 (BMP-7) serum levels correlated with development of hepatic fibrosis? J Infect Dev Ctries. 2014;8:605-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Pegorier S, Campbell GA, Kay AB, Lloyd CM. Bone morphogenetic protein (BMP)-4 and BMP-7 regulate differentially transforming growth factor (TGF)-beta1 in normal human lung fibroblasts (NHLF). Respir Res. 2010;11:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 22. | An JN, Yang SH, Kim YC, Hwang JH, Park JY, Kim DK, Kim JH, Kim DW, Hur DG, Oh YK, Lim CS, Kim YS, Lee JP. Periostin induces kidney fibrosis after acute kidney injury via the p38 MAPK pathway. Am J Physiol Renal Physiol. 2019;316:F426-F437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 23. | Zhao XK, Yu L, Cheng ML, Che P, Lu YY, Zhang Q, Mu M, Li H, Zhu LL, Zhu JJ, Hu M, Li P, Liang YD, Luo XH, Cheng YJ, Xu ZX, Ding Q. Focal Adhesion Kinase Regulates Hepatic Stellate Cell Activation and Liver Fibrosis. Sci Rep. 2017;7:4032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 24. | Chen L, Li J, Zhang J, Dai C, Liu X, Wang J, Gao Z, Guo H, Wang R, Lu S, Wang F, Zhang H, Chen H, Fan X, Wang S, Qin Z. S100A4 promotes liver fibrosis via activation of hepatic stellate cells. J Hepatol. 2015;62:156-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 125] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 25. | Cai GQ, Zheng A, Tang Q, White ES, Chou CF, Gladson CL, Olman MA, Ding Q. Downregulation of FAK-related non-kinase mediates the migratory phenotype of human fibrotic lung fibroblasts. Exp Cell Res. 2010;316:1600-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
P-Reviewer: Gumerova A, Gong YW S-Editor: Ma RY L-Editor: Wang TQ E-Editor: Zhang YL