Published online Aug 14, 2019. doi: 10.3748/wjg.v25.i30.4181
Peer-review started: April 30, 2019
First decision: May 30, 2019
Revised: June 12, 2019
Accepted: July 5, 2019
Article in press: July 5, 2019
Published online: August 14, 2019
Processing time: 106 Days and 18.8 Hours
Acute and chronic colitis affect a huge proportion of the population world-wide. The etiology of colitis cases can be manifold, and diet can significantly affect onset and outcome of colitis. While many forms of acute colitis are easily treatable, chronic forms of colitis such as ulcerative colitis and Crohn’s disease (summarized as inflammatory bowel diseases) are multifactorial with poorly understood pathogenesis. Inflammatory bowel diseases are characterized by exacerbated immune responses causing epithelial dysfunction and bacterial translocation. There is no cure and therapies aim at reducing inflammation and restoring intestinal barrier function. Unfortunately, most drugs can have severe side effects. Changes in diet and inclusion of nutritional supplements have been extensively studied in cell culture and animal models, and some supplements have shown promising results in clinical studies. Most of these nutritional supplements including vitamins, fatty acids and phytochemicals reduce oxidative stress and inflammation and have shown beneficial effects during experimental colitis in rodents induced by dextran sulphate sodium or 2,4,6-trinitrobenzene sulfonic acid, which remain the gold standard in pre-clinical colitis research. Here, we summarize the mechanisms through which such nutritional supplements contribute to epithelial barrier stabilization.
Core tip: This review summarizes current knowledge on how nutritional supplements with anti-inflammatory and antioxidative properties such as vitamins, fatty acids and phytochemicals contribute to intestinal epithelial junction stabilization and maintenance of barrier integrity during experimental colitis.
- Citation: Vargas-Robles H, Castro-Ochoa KF, Citalán-Madrid AF, Schnoor M. Beneficial effects of nutritional supplements on intestinal epithelial barrier functions in experimental colitis models in vivo. World J Gastroenterol 2019; 25(30): 4181-4198
- URL: https://www.wjgnet.com/1007-9327/full/v25/i30/4181.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i30.4181
Most people have suffered from acute colitis during their lives. This can be due to infections, changes in diet or stress. Most cases are self-limited and resolve without specific treatment. However, more severe forms of colitis exist as inflammatory bowel diseases (IBD) including ulcerative colitis (UC) and Crohn’s disease (CD)[1]. These forms of colitis are as of today incurable and require life-long treatment to manage the symptoms. Unfortunately, IBD incidence rates are increasing. There are around 1.6 million cases in the United States and 2 million cases in Europe[2]. Unfortunately, also in the rest of the world, increasing numbers of newly diagnosed cases are being reported.
The pathogenesis is still poorly understood, but IBD is multifactorial and characterized by loss of epithelial barrier integrity due to excessive inflammatory responses. The intestinal epithelium is a critical barrier that controls the passage of water and electrolytes and inhibits the passage of bacteria and other pathogenic agents. The epithelium is a cell monolayer in which cell contacts are sealed by the apical junctional complex that controls intestinal epithelial permeability, which is increased during active phases of IBD. Treatments include anti-inflammatory drugs such as mesalazine, biologicals such as antibodies against the proinflammatory cytokine tumor necrosis factor-α (TNF-α), steroids or immunomodulators such as azathioprine that inhibit immune cell proliferation[3]. However, these drugs can only alleviate symptoms and prolong phases of remission by reducing inflammation and restoring the intestinal epithelial barrier. In more severe cases when individuals do not respond well to such treatments, then surgery is required. Thus, alternative treatment approaches are needed.
It is well known that the diet can affect vulnerability to colitis development and duration of remission phases[4,5]. While diets with high sugar levels have detrimental effects, high fiber diets have beneficial effects[6,7]. Thus, nutritional supplements have garnered attention as alternative colitis treatment approaches, and their effectiveness is usually first studied in animal models of experimental colitis in mice or rats. Disease models include administration of dextran sulphate sodium (DSS) in drinking water, which resembles UC symptoms and rectal instillation of 2,4,6-trinitrobenzene sulfonic acid (TNBS), which more closely resembles signs of CD.
In this review, we summarize recent results from such animal models in which nutritional supplements showed beneficial effects and that may have potential as co-treatments for IBD patients to prevent relapses and/or prolong phases of remission. As this is an immensely vast field of research, we focus this review on supplements with anti-inflammatory and antioxidative properties that contribute to stabilizing epithelial cell contacts and preventing loss of barrier functions. We will not cover studies analyzing pre- and probiotics as this has been excellently reviewed elsewhere recently[8,9]. To find the studies summarized here, we performed a literature search in PubMed including terms such as IBD, colitis, UC, CD, nutritional supplements, alternative medicine, epithelial barrier function, tight junction (TJ), TNBS, DSS etc. To keep this review brief and timely, we mostly focused on studies published during the last five years and included some historical perspectives regarding the animal models.
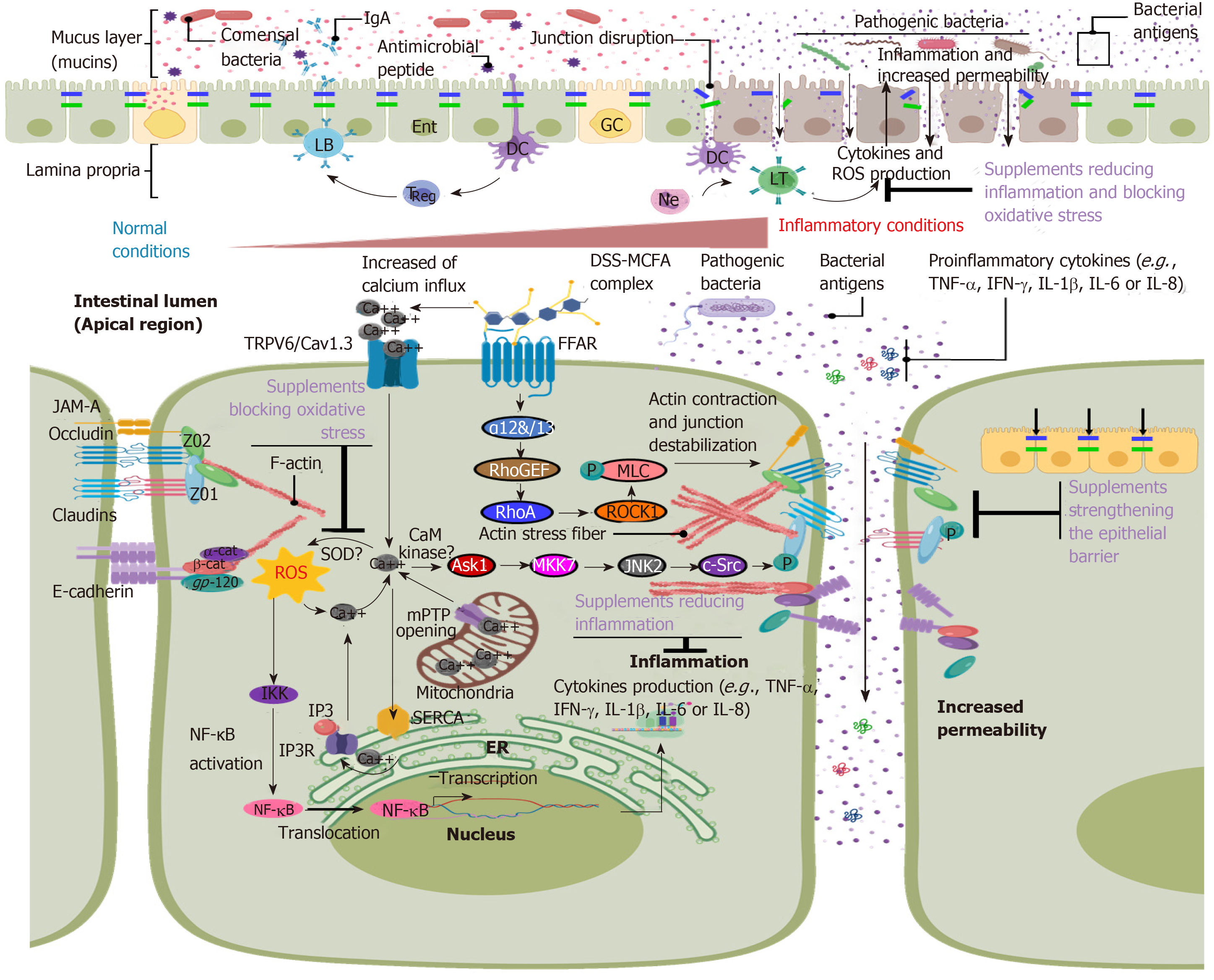
DSS is a negatively charged sulphated polysaccharide, which is routinely being used for induction of experimental UC in rodents (i.e., mouse, rat, hamster or guinea pig)[10] or zebra fish larvae[11]. Treatment of animals with DSS leads to intestinal epithelial barrier damage and increased permeability, thus causing the entrance of luminal pathogens and their antigens leading to the activation of immune cells residing in the lamina propria. Activation of immune cells then triggers an inflammatory response characterized by the production of inflammatory cytokines such as interleukin (IL)-1β, IL-6, KC, TNF-α or interferon (IFN)-γ and down-regulation of the anti-inflammatory cytokine IL-10, which in turn exacerbates epithelial barrier dysfunction[12]. Sustained DSS treatment prevents healing and regeneration of the intestinal epithelium leading to chronic inflammation and lymphocyte infiltration to the site of injury[13]. DSS in animals causes clinical symptoms similar to those seen in human patients suffering from UC including weight loss, diarrhea, bleeding and morphological changes in the colon such as epithelial erosion, edema formation and leukocyte infiltration (cryptitis)[14].
DSS induces epithelial barrier dysfunction by destabilizing intercellular junctions (i.e., tight and adherens junctions) due to internalization and degradation of junction proteins[15] (Figure 1). However, epithelial apoptosis, necrosis and a decrease in cell proliferation have been observed after DSS treatment that contribute to epithelial barrier dysfunction and increased intestinal epithelial permeability[13,16]. Diet greatly affects the outcome of DSS colitis. For example, clinical symptoms in BALB/c mice treated with DSS were exacerbated when supplemented with a diet rich in ω-3 fatty acid, accompanied by decreased adiponectin mRNA expression in colonic myofibroblasts[17]. In agreement with its known anti-inflammatory role, induction of adiponectin expression by the agonist pioglitazone ameliorated colitis symptoms in mice. Moreover, dodecanoic acid, a medium-chain fatty acid composed of 12 carbons, facilitated the entrance of DSS into the cell and the induction of colitis by forming nanomeric DSS-dodecanoate complexes that preferentially interacted with enterocyte membranes[18]. Therefore, it is important to consider the diet when analyzing results from DSS-induced experimental colitis assays.
In addition to epithelial barrier disruption, an increase in reactive oxygen species (ROS) has been identified as an important causative mechanism of DSS colitis (Figure 1). Increased concentrations of the second messenger Ca2+ mediated by TRPV6 and Cav1.3 symporter channels have been observed after DSS treatment that induced elevated levels of ROS and thus oxidative stress within enterocytes[19,20] (Figure 1). ROS could then contribute to the release of intracellular calcium from the endoplasmic reticulum and mitochondria translating into a feedback loop that triggered oxidative stress[21]. Furthermore, increased intracellular calcium levels activated the Ask1-MKK7-JNK2-cSrc signaling pathway[19] leading to the phosphorylation and relocalization of junction molecules and consequently to increased paracellular permeability, inflammation and neutrophil infiltration. Blocking ROS activity using N-acetyl-L-cysteine and L-nitroarginine methyl ester protected against the disruption of intercellular junctions[20]. Phosphorylation of TJ proteins including claudins is a common mechanism for intercellular junction destabilization and increased permeability during DSS colitis[22]. However, the responsible kinases remain elusive.
Another pathway contributing to epithelial barrier dysfunction, which is also regulated by diet components, is activation of ROCK1- or MLCK-induced actomyosin contractility[23]. For example, dodecanoate acid activated the G-protein-coupled receptor GPR40 leading to dissociation of the G-protein Gα11/12 that in turn activated the small GTPase RhoA[24]. We previously demonstrated in vivo that DSS treatment activated RhoA and its effector ROCK1 in a manner dependent on the actin-binding protein cortactin[25] leading to phosphorylation of myosin light chain, increased actin stress fiber formation, relocalization of ZO-1 and occludin, and increased epithelial permeability.
DSS treatment also activates nuclear factor-ĸB (NF-κB) and mitogen-activated protein kinase (MAPK)-dependent signaling pathways leading to the expression of proinflammatory mediators such as TNF-α, IL-1β, IFN-γ and IL-6[26]. Initial release of TNF-α by resident immune cells further triggered PI3K/Akt-dependent NF-κB activation and nuclear translocation in enterocytes, thus promoting an acute inflammatory response[27]. Additionally, INF-γ strengthened the TNF-α response by inducing the expression of TNF-receptor 2, thus further triggering disruption of the intestinal epithelial barrier during colitis[28]. TNF-α also induced the production of cyclo-oxygenase-2[29], which in turn exacerbated the inflammatory response via production of prostaglandins[30] and nitric oxide synthase[31]. As recently demonstrated, selenium treatment reverted and ameliorated inflammation and oxidative stress in DSS colitis[32].
Therefore, blocking the induction of proinflammatory mediators and oxidative stress to prevent epithelial barrier dysfunction by using known antioxidative and anti-inflammatory diet supplements is a promising strategy used by many research groups to discover alternative treatment strategies for patients with IBD. Applying such supplements in the DSS colitis model is a common approach to examine their safety and efficacy. In the following chapter, we summarize the most recent advances in this field.
Vitamin D (VD) and its receptor (VDR) play important roles in controlling infla-mmation by regulating innate and adaptive immune responses[33]. DSS-treated wild type (WT) mice showed a remarkable improvement of UC symptoms when they were supplemented with 1,25-dihydroxy-vitamin D3 [1,25(OH)2D3] characterized by strengthening of barrier function through maintaining the expression of ZO-1, occludin and claudin-1[34]. VD and VDR deficiency were related to the onset of colorectal cancer[35] and IBD[36]. VDR−/− mice were more susceptible to DSS treatment and showed more severe ulcerations, increased permeability and relocalization of ZO-1 and occludin[37]. In DSS-treated Caco-2 cells, administration of 1,25(OH)2D3 increased transepithelial electrical resistance and expression of ZO-1, thus restoring epithelial barrier function. Interestingly, in DSS-treated VDR-deficient colonic mouse cells, 1,25(OH)2D3 treatment converted macrophages into an M2 phenotype[38]. In a similar manner, a combination of micronutrients (vitamin C, vitamin E, L-arginine, and eicosapentaenoic and docosahexaenoic acid) administered in mice during DSS treatment significantly ameliorated colitis due to reduced expression of IL-1, IL-6, IFN-γ and KC, reduced oxidative stress and neutrophil infiltration leading to better maintenance of epithelial junction architecture[39] (Table 1).
| Product | Active compound | Effects on barrier function and mechanism | Ref. |
| Long chain fatty acid | 10-hydroxy-cis-12-octadecenoic acid (HYA) | Preserves stability and expression of occludin and ZO-1 Decreases acute inflammation induced by TNF-α and increases expression of GPR40 HYA decreases expression of TNFR2 by GPR40-MEK-ERK signaling pathway | [40] |
| Short chain fatty acid | Propionate | Stabilizes intercellular junctions and avoid down-regulation of ZO-1, occludin and E-cadherin Reduces acute inflammation Inhibits production of IL-1β, IL-6 and TNF-α Reduces neutrophil recruitment Inhibits STAT3 phosphorylation | [41] |
| Terpenoid | Oleanolic acid | Stabilizes intercellular junctions and avoid down-regulation of ZO-1, occludin and E-cadherin Modulates inflammatory response suppressing Th17 cell differentiation Promotes expression of anti-inflammatory cytokine IL-10 Inhibits activation of NF-κB and IL-17 expression | [42] |
| Scavenger enzymes | NAC/L-NAME | Protects against disruption of intercellular junctions Decreases oxidative stress mediated by intracellular calcium. Decreases activation of JNK and c-Src preventing ZO-1 phosphorylation | [19,20] |
| Peptides | Chromofungin | Prevents down-regulation of claudin-1, ZO-1 and Ecadherin Decreases acute inflammation Decreases expression of IL-18 Increases expression of IL-10 Promotes IL-10, Arg1, Fizz1, and Ym1 expression, and the release of IL-10 Promotes arginase activity probably through p38 MAP kinase or STAT1 | [43] |
| Catestin | Maintains expression of claudin-1, ZO-1 and occludin Protects against inflammation induced by DSS Decreases expression of IL-8 and IL-18 Decreases activation of STAT-3 signaling pathway | [45] | |
| Substance P | Maintains expression of ZO-1 and E-cadherin Reduces inflammation and damage induces by DSS Decreased apoptosis and inflammation via Neurokinin-1 receptor | [46] | |
| Porcine β-defensin 2 | Decreases paracellular flux Preserves expression of ZO-1, ZO-2 and claudin-1. Decreases production of NO synthetase and COX-2 Decreases expression of TNF-α, IL-6 and IL-8 Reduces apoptosis and neutrophil infiltration Inhibits the activation of NF-κB | [47] | |
| 8-kDa antrum mucosal protein | Strengthens the formation of intercellular barrier by promoting the assembly of ZO-1, ZO-2 and JAM-A Ameliorates UC symptoms Promotes the phosphorylation PKCζ and recruitment of the Par6/Cdc42∙GTP/ECT2/Par3 complex | [48,49] | |
| Vitamin D | 1,25-dihydroxy-vitamin D3 [1,25(OH)2D3] | Restores epithelial barrier function and expression of ZO-1, occludin and claudin-1 Modulates inflammation turning M1 into M2 macrophage responses Reduces the expression of TNF-α, IL-12 and IL-1β | [34,37,38] |
| Corabion | Vitamin C, vitamin E, L-arginine | Preserves ZO-1 and E-cadherin expression Decreases inflammation Reduces production of IL-1, IL-6, INF-γ and KC | [39] |
| Compatible solutes | Ectoine, Homoectoine | Decreases Evans blue permeability Increases expression of ZO-1 and occludin Prevents claudin-1 to claudin-2 switch Decreases ROS production | [50] |
| Exopolysaccharides | Streptococcus thermophilus MN-BM-A01 | Increases expression of claudin-1 and occludin | [51] |
| Synechococcus sp. PCC 7002 | Increases expression of ZO-1, occludin, claudin-4 and Hsp27 | [52] | |
| Lucilia sericata larvae | Maggot protein | Upregulation of MUC2 gene Increases expression of ZO-1 and occludin | [53] |
| Periplaneta americana | Ethanol extract | Increases expression of ZO-1, occludin and claudin-1 | [54] |
| Donkey milk | Lysozyme | Increases expression of ZO-1 and occludin Decreases expression of claudin-2 | [55] |
| Naringenin | Polyphenol | Decreases FITC-Dextran permeability Decreases LPS-binding protein levels in plasma Increases expression of occludin, JAM-A and claudin-3 Normalizes the colonic localization of occludin and claudin-3 proteins | [56] |
| Naringin | Flavonoid | Increases expression of ZO-1 Decreases NF-ĸB signaling pathway Regulation of PPARγ activation Inhibition of the MAPK activation Suppression of NLRP3 inflammasome activity | [57] |
| Red raspberries | Polyphenol | Increases mRNA expression of MUC2 Reduces pore forming TJ protein claudin-2 Increases ZO-1 and claudin-3 proteins Decreases NF-ĸB p65 phosphorylation Activates AMPK | [58] |
| Phlorentin | Flavonoid | Increases the expression of ZO-1 and occludin Inhibition of serum LPS levels Inhibits oxidative stress Decreases TLR4 expression Increases PPARγ expression Decreases NLRP3 inflammasome pathway (NLRP3, ASC, Caspase-1 and IL-1β protein expression) Suppression of NF-ĸB activation | [59] |
| Formomentin | Isoflavone | Increases ZO-1, claudin-1 and occludin protein expression Inhibition of NLRP3 inflammasome pathway (NLRP3, IL-1β and ASC protein expression) | [60] |
| Phellinus igniarius fruiting body | Polyphenols and polysaccharides | Increases mRNA levels of ZO-1 protein Decreases LPS plasma levels Inhibition of NF-ĸB pathway Decreases the transcription of ASC3 and caspase-1 pathway | [63] |
| Barley and soybean mixture | Isoflavone β-glucan | Increases ZO-1, claudin-1 and occludin protein expression and improves their localization Reduces FITC-Dextran serum levels Prevents bacterial translocation into the MLNs Anti-inflammatory activity | [64] |
| Evodia rutaecarpa | Alkaloid extract (Evodiamine) | Increases ZO-1, occludin and MUC2 expression Decreases plasmatic levels of LPS Decreases NF-ĸB signaling pathway Down-regulation of NRLP3, ASC, caspase-1 and IL-1β protein expression | [65] |
| Salvia miltorrhiza Bunge | Water-soluble-phenolic acid | Increases ZO-1 and occludin expression in rat colons | [66] |
| Magnolia officianalis | Magnolol | Increases ZO-1 and occludin protein expression Decreases NF-ĸB signaling pathway and regulation of PPAR-γ expression | [67] |
| Artemisia argy and Artemisia asiatica | Ethanol extract. Flavonoid (Eupatilin) | Increases occludin and ZO-1 protein expression Decreases NOX4 protein expression Down-regulation of NF-ĸB/MAPK signaling pathway Increases AMPK activation | [68] |
| QingBai Decoction | Decreases FITC-Dextran permeability Increases MUC2, ZO-1, claudin-1 and occludin protein expression Increases the number of Ki67 positive cells Decreases active caspase-3 expression Inhibits Notch and NF-ĸB signaling pathway | [70] | |
| Zanthoxylum bungeanum | Essencial oil (Terpinen-4-ol) | Increases ZO-1 and occludin protein expression Decreases NF-ĸB pathway and NLRP3 inflammasome activation | [71] |
| Alnus japonica Steud | Ethanol extract. | Increases of ZO-1 and occludin expression Increases HO-1 expression | [75] |
| Pogostemon cablin Benth | Aqueous extract: Patchouli alcohol | Increases expression of MUC1, MUC2, claudin-1, occludin, ZO-1 and ZO-2 Down-regulation of apoptosis Suppresses tryptophan catabolism | [76] |
| Rhizoma Coptidis | Berberine Berberrubine | Upregulates gene expression of MUC1 and MUC2 Increases ZO-1, ZO-2, claudin-1 and occludin protein expression Decreases the Bax/Bcl-2 ratio | [77] |
| Moringa oleifera | Flavonoids, phenolic acids and glucosinolates | Increases expression of ZO-1 and claudin-1 Upregulates GSTP1 and Nrf2-mediated phase II detoxifying enzyme | [78] |
| Wasabia japonica | Glucosinolates, isothiocyanate. | Increases MUC2 and ZO-1 expression Regulation of ERK pathway | [79] |
| Acer palmatum Thumb | Ethanol extract (KIOM-2015E) | Increases ZO-1 and occludin expression | [80] |
| Guar gum | Galactomannan | Upregulates ZO-1, occludin, JAM-A, claudin-3, -4 and -7 protein expression Decreases plasmatic levels of LBP | [83] |
In normal conditions, fatty acids modulate intestinal homeostasis and immune responses. Fatty acids derived from linoleic acid have shown an important anti-inflammatory effect after TNF-α and DSS treatment. Administration of 10-hydroxy-cis-12-octadecanoic acid counteracted loss of junction molecules induced by TNF-α in vitro and DSS in vivo[40] (Table 1).
Propionate, a short-chain fatty acid, also had anti-inflammatory effects in DSS colitis. Pre-administration and co-treatment of propionate during DSS treatment reduced inflammation by inhibiting expression of the proinflammatory cytokines IL-1β, IL-6 and TNF-α and reduced myeloperoxidase activity and neutrophil recruitment[41]. Propionate treatment also reduced the paracellular flux of FITC-dextran and restored epithelial barrier functions by inhibiting the down-regulation of occludin, ZO-1 and E-cadherin. In a similar manner, oleanolic acid stabilized intercellular junctions during DSS treatment by preventing down-regulation of ZO-1, occludin and E-cadherin expression and promoting the expression of IL-10[42]. Moreover, oleanolic acid promoted differentiation of T-cells into Treg cells while suppressing differentiation into Th17 cells (Table 1).
Peptides derived from more complex molecules such as hormones have demonstrated promising anti-inflammatory activities during colitis. Chromofungin, a short peptide derived from Chromogranin-A (CgA) has been shown to ameliorate colitis after intracolonic injection of CgA by decreasing expression of IL-18, promoting the expression of IL-10 and preventing the down-regulation of intercellular junctions such as claudin-1, ZO-1, E-cadherin and occludin[43]. This is interesting as DSS increased the number of CgA-positive enteroendocrine cells in the mucosa. UC patients showed higher numbers of CgA-positive cells[44] suggesting that this increase could be a protective countermeasure during colitis (Table 1).
Catestin (CST) is an enterochromaffin-derived peptide that has anti-inflammatory activity. However, in UC patients presence of catestin was reduced compared to healthy controls, and intrarectal administration of catestin decreased clinical signs of DSS colitis by reducing the expression of IL-8 and IL-18 preventing the activation of STAT3 and maintaining claudin-1, ZO-1 and occludin expression[45]. In a similar way, substance P, a neuropeptide of 11 amino acids expressed in the intestines, attenuated DSS colitis by preventing decreased expression of ZO-1 and E-cadherin and colon tissue damage[46].
Porcine β-defensine-2 is an antimicrobial peptide produced in the intestines. DSS-colitis signs were significantly improved after intrarectal administration of porcine β-defensine-2 due to reduced expression of proinflammatory cytokines, reduced epithelial apoptosis and neutrophil infiltration, suppression of nitric oxide synthase and cyclo-oxygenase-2 and preservation of intercellular junctions[47]. In IL-10-deficient and T-cell adoptive transfer mouse models of colitis, treatment with antrum mucosa peptide (AMP-18) protected against weight loss and colon shortening[48]. Of note, AMP-18-deficient mice treated with DSS showed exacerbated colitis and high mortality, which could be counteracted by AMP administration[48]. In WT mice treated with DSS, AMP-18 protected against UC by strengthening PKCζ phosphorylation and recruitment of a molecular complex (Par6/Cdc42∙GTP/ECT2/Par3) that facilitated the assembly of TJs[49] (Table 1).
In search of new treatments for inflammatory diseases such as IBD, the anti-inflammatory properties of bacterial compatible solutes, metabolites, exopolysaccharides and biogenic polyphosphate nanoparticles have garnered interest. Compatible solutes are small organic molecules produced by bacteria to protect them from extreme environmental conditions such as heat and high salinity. The advantage of these solutes is that even at high concentrations they do not cause any adverse effects in vivo and in vitro. Ectoine and its synthetic derivative homoectoine have shown promising results in experimental colitis[50]. When ectoine or homoectoine were administered via oral gavage in parallel to the administration of DSS, treated mice showed significant improvement in intestinal bleeding and stool consistency. Excessive intestinal epithelial permeability was reduced to normal levels in ectoine- and homoectoine-treated mice, and expression of ZO-1 and occludin was maintained. Importantly, only homoectoine was able to prevent the claudin switch (claudin1 replacement by the leaky claudin-2) resulting in a stronger epithelial barrier. Moreover, both compatible solutes ameliorated oxidative stress in the mucosa[50] (Table 1).
Exopolysaccharides are synthesized and secreted by microorganisms into their environment and have been proposed as the mechanism that mediates the anti-inflammatory effects of probiotic bacteria. When purified exopolysaccharides from Streptococcus thermophilus MN-BM-A01 were administered orally in mice with DSS-induced colitis, these mice showed a significant amelioration of intestinal inflammation due to reversed down-regulation of claudin-1 and occludin, thus restoring barrier function[51]. Anti-inflammatory and barrier-stabilizing properties were recently also reported for biogenic polyphosphate nanoparticles from cyanobacterium Synechococcus sp. PCC 7002. Colitic mice received biogenic polyphosphate nanoparticles orally for 9 d, the first 5 d together with 3% DSS. Overall, colitis development was slower compared to untreated mice, and barrier function was maintained due to upregulation of ZO-1, occludin and claudin-4[52] (Table 1).
Besides plants, Chinese traditional medicine uses various insects to alleviate common diseases. Dried Lucilia sericata larvae, also known as "wu gu chong", were fed to DSS-colitic mice and ameliorated colitis signs very similarly to the standard drug mesalazine by improving stool consistency, intestinal bleeding and decreasing weight loss and colon shortening[53]. Interestingly, colitic mice that received the dried larvae showed a restoration of mucin-2, ZO-1 and occludin levels suggesting a protective effect on epithelial barrier functions. Periplaneta americana (American cockroach) is widely used in China as treatment for gastrointestinal disorders. Rats that received DSS in parallel to Periplaneta americana extracts developed a milder form of colitis compared to rats treated only with DSS because TJ architecture was better conserved and ZO-1, occludin and claudin-1 protein levels were similar to control mice[54]. Lysozyme has been linked to the modulation of the intestinal immune response. When lysozyme isolated from donkey milk was administered to mice with DSS-colitis, they developed a milder colitis due to lower levels of TNF-α and IL-13 and reduced oxidative stress. Epithelial barrier function was improved by preventing DSS-induced down-regulation in ZO-1 and occludin and upregulation of claudin-2[55] (Table 1).
Citrus fruits are a good source of fiber, vitamins and polyphenols and have many health benefits. Naringenin is a major polyphenol isolated from citrus fruits that protected the intestinal barrier during DSS-colitis in mice. Naringenin reverted DSS-induced reduced expression of occludin, junctional adhesion molecule-A (JAM-A) and claudin-3 and epithelial hyperpermeability[56]. Naringin, another flavonoid of citrus fruits, showed protective effects on epithelial barrier functions during DSS colitis due to activation of peroxisome proliferator-activated receptor γ and subsequent inhibition of NF-ĸB leading to reduced proinflammatory cytokine levels[57]. Interestingly, naringin significantly suppressed DSS-induced NLRP3 inflammasome activation by decreasing the expression of NLRP3, ASC, caspase-1 and IL-1β. Naringin also inhibited DSS-induced MAPK activation and epithelial barrier dysfunction through regulation of ZO-1 expression suggesting that naringin protects epithelial barrier integrity on multiple levels.
Red raspberry (RB) is another fruit that has numerous health benefits and contains high amounts of fiber and polyphenols with antioxidative and anti-inflammatory properties. RB supplementation in a DSS-induced colitis model prevented DSS-induced mucosal damage, reduction of mucin-2, ZO-1 and claudin-3 expression and decreased expression of the pore-forming TJ protein claudin-2. Of note, RB also reversed DSS-induced NF-ĸB activation and AMP-activated protein kinase (AMPK) inhibition[58]. Thus, RB also protects the epithelial barrier against DSS-induced colitis via its anti-inflammatory effects on multiple levels.
Apples and strawberries contain phloretin. Phloretin is a flavonoid with antioxidative and anti-inflammatory properties. Phloretin treatment improved mucosal injury caused by DSS-treatment in the colon in a dose-dependent manner. Phloretin inhibited loss of goblet cells, oxidative stress, inflammation and epithelial barrier dysfunction. Mechanistically, phloretin protected against colitis through inhibition of the NF-ĸB pathway and the NLRP3 inflammasome. Of note, phloretin decreased the expression of toll-like receptor 4 and increased peroxisome proliferator-activated receptor γ expression, which are activating and inhibiting signaling proteins upstream of NF-ĸB, respectively[59]. Thus, inhibition of NF-ĸB and the NLRP3 inflammasome seem to be common protective mechanisms shared by different flavonoids.
Formononetin is a natural isoflavone and one of the major biologically active compounds in a variety of Chinese medicinal herbs such as Astragalus membranaceus. Intraperitoneal injection of formononetin attenuated in a dose-dependent manner DSS-induced leukocyte infiltration, increase of proinflammatory cytokine expression and reduction of claudin-1, occludin and ZO-1 levels in the colon of DSS-treated mice due to inhibition of the NLRP3 inflammasome[60]. Phellinus igniarius is a medicinal mushroom that has been widely used in traditional Chinese medicine to treat stomach ache, inflammation and tumors. The aqueous extract of Phellinus igniaurius possesses antitumor, antidiabetic and immunity-modulating effects[61,62]. In a model of chronic DSS-induced colitis in mice, the aqueous extract of Phellinus igniarius significantly improved DSS-induced loss of crypts and goblets cells, epithelial barrier dysfunction, and reduced plasma lipopolysaccharide levels[63]. The Phellinus igniarius extract significantly inhibited NF-ĸB activation through inhibition of IkBα phosphorylation and the ASC3-caspase-1 pathway.
Soybeans and barley are a good source of isoflavonoids with anti-inflammatory effects. A mixture of soybeans and barley protected against DSS-colitis by reversing DSS-induced epithelial permeability, redistribution and loss of ZO-1, occludin, and claudin-1 and by preventing bacterial translocation into mesenteric lymph nodes[64]. Evodiamine is a bioactive alkaloid obtained from Evodia rutaecarpa that is used in traditional Chinese medicine because of its anti-inflammatory properties[65]. Evodiamine ameliorated DSS-induced epithelial barrier damage, increased proinflammatory cytokines levels, loss of ZO-1, occludin and mucin-2 and decreased NF-ĸB, p65 and IĸB phosphorylation and NLRP3 inflammasome activation suggesting that the protective effect of evodiamine is due to its anti-inflammatory properties.
Salvia miltiorrhiza Bunge (Danshen) also has anti-inflammatory properties and contains the active phenolic compound salvianolic acid A (SAA). SAA inhibited DSS-induced histological damage, leukocyte infiltration, ulceration and edema in the mouse colon and protected against loss of ZO-1 and occludin[66]. The cecal bacterial composition that was altered after DSS-treatment was similar to controls when mice received SAA in parallel to DSS treatment. Thus, apart from its anti-inflammatory effects, SAA protected the intestinal epithelial barrier by modulating the gut microbiota during colitis.
Magnolol is the main and active ingredient of Magnolia officinalis, which is being used in traditional Chinese medicine for the treatment of gastrointestinal disorders. Magnolol improved DSS-induced colitis signs in mice. In particular, epithelial erosion, disruption of crypt glands, loss of ZO-1 and occludin and leukocyte infiltration were reduced when colitic mice were treated with magnolol[67]. Again, the anti-inflammatory mechanism of magnolol was shown to relate to the regulation of NF-ĸB, p65 and IĸB phosphorylation and peroxisome proliferator-activated receptor γ expression.
Eupatilin (Eup) is a major flavonoid found in the leaves of Artemisia argyi and is also the principal bioactive compound of Artemisia asiatica Nakai ex Kitam. Eup is used in traditional medicine due to its antioxidative and anti-inflammatory activities. Eup treatment alleviated DSS-induced inflammation and oxidative stress in mice by decreasing nicotinamide adenine dinucleotide phosphate oxidase and increasing occludin and ZO-1 levels in the colon epithelium[68]. Eup inhibited DSS-induced NF-ĸB and MAPK activation and significantly promoted AMPK activation, thus contributing to barrier stabilization.
The Chinese herb QingBai decoction (QBD) is a mixture of six medicinal herbs that has been used in the treatment of UC[69]. QBD effectively relieved intestinal symptoms such as diarrhea and bleeding during active colitis[70]. When administered as enema in a DSS-colitis mouse model, QBD was able to diminish epithelial damage, mucosal inflammation, crypt damage and loss of goblet cells and mucin-2 in colon tissue. Hyperpermeability and loss of TJ proteins were also ameliorated by QBD, and these effects were comparable to what was observed in mesalazine-treated mice. Of note, QBD was able to inhibit epithelial apoptosis and to increase proliferation during colitis. These data suggest that QBD can contribute to wound healing and tissue recovery.
Terpinen-4-ol (TER) is a main component of Zanthoxylum bungeanum Maxim with high antibacterial, antioxidative and anti-inflammatory properties. Treatment with TEL completely blocked DSS-induced epithelial damage, crypt distortion, increase of proinflammatory cytokines and depletion of goblet cells[71]. TEL also prevented loss of ZO-1 and occludin and NF-ĸB and NLRP3 activation.
Alnus japonica Steud (Betulaceae) has been used in traditional Asian medicine to treat fever, hemorrhage and gastric disorders. Ethanol extracts from Alnus japonica bark has been reported to have antioxidative and anti-inflammatory effects due to the presence of diarylheptanoids, triterpenoids and flavonoids[72-75]. Histological analysis of colon tissues showed a protective effect on tissue damage, inflammation and leukocyte infiltration during DSS-colitis. mRNA levels of proinflammatory cytokines and cyclo-oxygenase-2 protein levels were lower in Alnus japonica bark-treated colitic mice. Of note, Alnus japonica bark treatment not only increased ZO-1, occludin and heme-oxygenase 1 protein levels but also protected against epithelial apoptosis.
Pogostemon cablin (blanco) Benth is a plant used in the treatment of gastrointestinal disorders in Asia. Patchouli alcohol (PA) is the extract containing the major active components of Pogostemon cablin with anti-inflammatory and immune-modulatory properties. PA given during DSS-colitis in mice reduced proinflammatory cytokine levels and infiltration of inflammatory cells into the mucosa in a dose-dependent manner[76]. Expression of mucin-1, mucin-2, ZO-1, ZO-2, claudin-1 and occludin were higher after PA treatment compared to the DSS group. In addition, PA inhibited DSS-induced apoptosis and improved tryptophan metabolism. Of note, the effect of PA was similar to that observed with sulfasalazine treatment.
Rhizoma coptidis is commonly used in traditional Chinese medicine as treatment for various diseases including IBD[77]. Berberine (BBR) is the most abundant and major active isoquinoline alkaloid of Rhizoma coptidis but is characterized by poor intestinal absorption. Berberrubine is a BBR metabolite with better intestinal absorption. Treatment with both berberrubine and BBR ameliorated signs of DSS-colitis although higher doses were required for BBR. Mucosal inflammation, leukocyte infiltration, increased cytokine levels and decreased mRNA levels of mucin-1 and mucin-2 were effectively counteracted by both compounds. ZO-1, ZO-2, claudin-1 and occludin levels were maintained with BBR and berberrubine treatment, and apoptosis was inhibited.
Moringa oleifera (moringa) is a tropical plant traditionally used for its nutritional value and as treatment for a number of acute and chronic conditions such as inflammation and diabetes. Moringa has anti-inflammatory, antibacterial and antioxidative properties attributed mainly to glucosinolates, which can be metabolized into the main bioactive metabolites, moringa isothiocyanates (MICs). MIC-1 [4-(α-L-rhamnosyloxy)benz-isothiocyanates] is the most abundant MIC in moringa seeds. Moringa seed extracts enriched in MIC-1 were given as treatment during acute and chronic DSS-colitis in mice and, surprisingly only protective effects were observed in the acute model. Beneficial effects of moringa seed extracts during acute colitis included reduced inflammation and proinflammatory cytokine levels and increased levels of claudin-1, ZO-1 and phase-II-detoxifying enzymes including GSTP1, NQO1 and HO1[78]. Moreover, the anti-inflammatory and antioxidative activities of moringa seed extracts were associated with Nrf2-mediated signaling.
Wasabia japonica is a popular traditional spice in Asia. Allyl isothiocynate is a bioactive molecule present in Wasabia japonica with anti-inflammatory effects. Allyl isothiocynate treatment during DSS-colitis increased mucin-2 and ZO-1 protein expression and the number of goblet cells[79]. These protective effects were related in vitro after treatment of epithelial cells with lipopolysaccharides to attenuation of phosphorylation of p65 and ERK1/2, reduced expression of IL-1β and TNF-α and increased mucin-2 expression.
KIOM-20I5E is an active anti-inflammatory ingredient of Acer palmatum thumb that is widely distributed in various regions of Asia and has been used in traditional medicine for its antioxidative and anti-inflammatory properties. KIOM-20I5E ameliorated DSS-induced reduction of goblet cells and ZO-1 and occludin levels[80]. However, the underlying mechanism was not determined.
Dietary fibers consumption affects the intestinal microbiota and consequently intestinal epithelial barrier functions[81]. Intestinal microorganisms metabolize dietary fibers to short-chain fatty acids that suppress the transcription of proinflammatory mediators, thus positively affecting intestinal barrier functions[82]. Like other fibers, Guar gum (GG) cannot be digested in the small intestine of mammals; however, it is quickly metabolized by colonic bacteria. Short-chain fatty acids then promote epithelial cell proliferation in the colon, mucosal blood flow and colonic motility. Fermentable GG and partially hydrolyzed GG improved the expression levels of ZO-1, ZO-2, occludin, JAM-A, claudin-3, claudin-4 and claudin-7 in a murine model of DSS-colitis[83]. Both partially hydrolyzed GG and GG inhibited lipopolysaccharide-binding protein levels in the plasma indicating that both compounds protected the colonic barrier and increased the production of fecal organic acids, which could be important mediators of the observed protective effects.
Moreover, pre-treatment of mice with psyllium fiber before colitis induction with DSS ameliorated loss of ZO-2, occludin, JAM-A, claudin-3 and claudin-7 and reduced lipopolysaccharide-binding protein plasma concentration[84]. In addition, the psyllium fiber diet increased intestinal cytoprotective heat-shock protein 25 levels and expression of extracellular matrix-associated genes including collagens and fibronectin. In summary, many phytochemicals have benefits during experimental colitis mostly due to anti-inflammatory and antioxidative effects that help to maintain junction architecture and epithelial barrier integrity. Thus, people susceptible to colitis should take great care of their diets.
The induction of colitis by the exposure of mice and rats to the reagent TNBS has been used as an animal model that closely resembles the pathophysiology of human CD. The key factor that makes TNBS colitis an indispensable model for the study of CD is the importance of nucleotide-binding oligomerization domain-containing protein 2 (NOD2) in the development of both murine TNBS-colitis and human CD[85]. CD is a chronic inflammatory disorder that affects any part of the gastrointestinal tract. It is characterized by transmural infiltration, which can result in complications such as granulomas, fistulas and strictures[86]. Genome-wide studies have associated polymorphisms in the NOD2 gene as a high genetic risk factor for the development of CD[85]. NOD2 is an intracellular pattern recognition receptor that recognizes muramyl peptides derived from bacterial peptidoglycans. NOD2 stimulation leads to the activation of NF-κB and MAPK signaling pathways[87], and mutations in NOD2 lead to irregular host-microbe interactions that can trigger a dysregulated immune response as observed during TNBS colitis[88]. TNBS haptenizes colonic proteins turning them immunogenic to the host’s immune system[89].
The induction of colitis by this method is simple and reproducible. TNBS is dissolved and instilled intrarectally in ethanol, which acts as the vehicle but also aids in breaking the mucosal barrier to induce intestinal inflammation. Depending on the desired colitis severity, concentrations of 50 to 150 mg/kg bodyweight can be administered either as a single dose for acute colitis or repetitive lower doses for chronic colitis[90]. After TNBS administration, animals will gradually develop intestinal bleeding, diarrhea and weight loss. During the development of colitis, the intestinal epithelial barrier is disrupted, and the architecture of intercellular junctions is compromised leading to the development of a leaky barrier. This allows for translocation of luminal antigens to the lamina propria and loss of tolerance to its own microbiota thus inducing Th1-Th17 cell-mediated immune responses with elevated production of IL-12, IL-17, IL-18, IL-23, IL-27 and IFN-γ[91,92]. Histologically, TNBS-treated animals develop a transmural inflammation characterized by severe infiltration of leukocytes in the mucosa and submucosa at an early stage followed by infiltration to the muscularis propria resulting in ulceration, goblet cell depletion, fibrosis and thickening of the colon wall, characteristics also found in CD patients[86,93].
Traditional medicine worldwide utilizes a variety of herbal extracts to treat diverse inflammatory disorders. Thus, there is a current interest in finding the exact phytochemicals that exert the anti-inflammatory property and the underlying mechanisms. For example, nobiletin, a flavone found in citrus fruit peels, ameliorated TNBS colitis by restoring barrier function due to reduced MLCK and NF-κB expression and Akt activation. Treatment with 20 mg/kg or 40 mg/kg of nobiletin during 7 d after the induction of colitis reversed excessive intestinal epithelial permeability as effectively as the standard drug sulfasalazine[94] (Table 2).
| Product | Active compound | Animals | Effects on barrier function | Ref. |
| Citrus fruit peels | Nobiletin | Rats | Decreases FITC-dextran permeability Reduces MLCK and NF-κB and PI3K expression Decreases Akt phospho-rylation | [94] |
| Rhododendron | Farrerol | Mice | Upregulates ZO-1, claudin-1 and occludin genes | [95] |
| Garnedia jasminoides | Geniposide | Rats | Increases expression of ZO-1 and occludin Decreases FITC-Dextran permeability Decreases MLCK activity and increases AMPK phosphorylation | [96] |
| Anemarrhena asphodeloides, Mangifera indica L | Neomangiferin | Mice | Increases expression of ZO/1, claudin-1 and occludin | [97] |
| Ziziphus jujuba | Sarcocarp polysaccharides | Rats | Decreases Evans blue permeability Increases expression of ZO-1, occludin, claudin-1 and claudin-4 Decreases AMPK activity | [98] |
| Dietary fiber | Sodium butyrate | Mice | Decreases FITC-Dextran permeability Upregulation of ZO-1, claudin-1 and occludin genes | [100] |
| Schistosoma japonicum | Ova | Mice | Increases expression of ZO-1 and occludin Upregulation of ZO-1 and occludin genes | [101] |
Moreover, the flavonoid farrerol extracted from rhododendron showed anti-inflammatory properties during TNBS-colitis and prevented the reduction of mRNA expression of the TJ molecules ZO-1, claudin-1 and occludin, suggesting that farrerol may also prevent hyperpermeability; however, this was not specifically tested[95]. Geniposide purified from Garnedia jasminoides fruits had barrier stabilizing activity during intestinal inflammation in TNBS-treated Sprague-Dawley rats. Geniposide treatment reduced excessive intestinal permeability by preventing MLCK activation and stabilizing the expression of occludin and ZO-1. Of note, the TNBS-induced decrease in AMPK phosphorylation was prevented by geniposide when administered at a high dose. This is important as AMPK is involved in the regulation of TJ integrity, and its activity is decreased during colitis. Overall, geniposide-treated rats developed a milder colitis compared to controls[96] (Table 2).
The plants Anemarrhena asphodeloides and Mangifera indica L. contain neomangiferin that possesses anti-inflammatory effects. In a recent study, purified neomangiferin was fed to TNBS-colitic C57Bl/6 mice causing a less severe form of colitis due to reduced levels of TNF-α, IL-1β and IL-6. Of note, neomangiferin prevented the typical imbalance between Th17 cells and Treg cells associated with colitis. Although barrier function in vivo was not determined, TNBS-induced down-regulation of TJ proteins ZO-1, claudin-1 and occludin was prevented by neomangiferin[97].
The barrier stabilizing effects of wild jujube [Ziziphus jujuba Mill. var. spinosa (Bunge) Hu ex H. F. Chou] sarcocarp polysaccharides (WJPs) were proven in TNBS-induced colitis in rats. WJPs attenuated weight loss, diarrhea, intestinal bleeding and inflammation and led to a better preservation of overall tissue structure. WJPs-treated colitic rats showed significantly reduced intestinal permeability compared to control rats, and down-regulation of the expression of ZO-1, occludin, claudin-1 and claudin-4 was prevented. WJPs also induced the activation of AMPK in vitro and in vivo thus contributing to correct TJ assembly and epithelial barrier function[98].
Butyrate is a short-chain fatty acids product of dietary fiber fermentation by gut microbiota and a ligand of GPR109A that is associated with inhibition of inflammation[99]. WT and GPR109A-deficient mice received sodium butyrate (SB) in drinking water 6 wk before the induction of TNBS-colitis[100] (Table 2). WT mice treated with SB developed a significantly milder colitis compared to GPR109A-deficient mice. Increased permeability and down-regulation of ZO-1, claudin-1 and occludin were prevented by SB in the WT but not in GPR109A-deficient mice. These data confirm that GPR109A activation by SB suppresses inflammatory effects during TNBS colitis.
An excess of hygiene has been correlated with a higher susceptibility to intestinal inflammatory disorders, so it is not surprising that the presence of helminths in the gastrointestinal tract has protective effects during experimental colitis. However, parasitic infections are still harmful for the host. That is why isolating and studying the components responsible for this anti-inflammatory effect in the intestines have become a priority. While it has been shown that Schistosoma japonicum ova prevented barrier dysfunction by increasing ZO-1 and occludin levels[101], the mechanistic basis for the protective effects of parasitic compounds during the development of experimental colitis remain elusive (Table 2).
Most nutritional supplements discussed here show anti-inflammatory and antioxidative properties in experimental colitis models leading to improved epithelial junction architecture and barrier stability. Many do so by inhibiting proinflammatory signaling mediated by NF-ĸB and MAPK and reducing the production of proinflammatory cytokines. Thus, preservation of epithelial barrier functions is an important goal of colitis treatments (drugs or supplements). However, while some studies have analyzed the effects of supplements on proinflammatory signaling and epithelial barrier function in detail, other studies remain rather superficial and only describe the observed effects without providing mechanistic details. Thus, it will be critical in the future to better understand the mechanistic details underlying the beneficial effects. This will be even more critical when addressing another major limitation of the described animal studies, i.e., whether findings from experimental colitis models are applicable to human disease. This remains to be seen in many cases. In some cases, clinical studies have been conducted with different results[4,7]. While some supplements with beneficial effects in rodents have shown none in humans, other supplements showed beneficial effects in both organisms. The interested reader is referred to another recent review that highlights clinical studies using supplements and active compounds from alternative medicines[102]. Clearly, humans are very different from rodents, thus limiting the usefulness of these data so that data from experimental colitis models have to be interpreted with caution. On the other hand, these models are inexpensive, easy to perform and reproducible so that they are indispensable for the search of new treatment strategies.
The intestinal microvasculature and endothelial barrier functions are also of importance during experimental colitis[103]. However, only few studies have addressed direct effects of DSS or TNBS on endothelial junction proteins regulating vascular permeability and neutrophil recruitment. For example, in mice with endothelial-specific JAM-A deletion, the outcome of DSS-induced colitis was similar to WT mice[104]. On the other hand, the endothelial TRPV4 channel regulating vascular permeability was overexpressed in endothelial cells of DSS-treated mice, which was accompanied by reduced VE-cadherin expression, and TRPV4 KO mice showed less severe colitis[105]. Given the importance of leukocyte recruitment from the blood stream to the colitic mucosa, it will be also important to study in more detail the mechanistic background of endothelial barrier regulation during experimental colitis. Certainly, many exciting discoveries are still ahead of us regarding the protective effects of nutritional supplements during colitis.
Work in the lab of MS is funded by the fund SEP-Cinvestav (Project 108), by Conacyt (284292) and the Royal Society (NAF/R1/180017). As this review summarizes parts of an extremely vast field of research, we had to focus on recent discoveries describing effects on junction stability and barrier function. We apologize to all scientists whose important work we could not cite due to space restrictions.
| 1. | Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2894] [Cited by in RCA: 3412] [Article Influence: 179.6] [Reference Citation Analysis (12)] |
| 2. | Ramos GP, Papadakis KA. Mechanisms of Disease: Inflammatory Bowel Diseases. Mayo Clin Proc. 2019;94:155-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 705] [Article Influence: 100.7] [Reference Citation Analysis (2)] |
| 3. | Triantafillidis JK, Merikas E, Georgopoulos F. Current and emerging drugs for the treatment of inflammatory bowel disease. Drug Des Devel Ther. 2011;5:185-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 210] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 4. | Neuman MG, Nanau RM. Inflammatory bowel disease: Role of diet, microbiota, life style. Transl Res. 2012;160:29-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (1)] |
| 5. | Shen W, Gaskins HR, McIntosh MK. Influence of dietary fat on intestinal microbes, inflammation, barrier function and metabolic outcomes. J Nutr Biochem. 2014;25:270-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 129] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 6. | Yamamoto T. Nutrition and diet in inflammatory bowel disease. Curr Opin Gastroenterol. 2013;29:216-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Spooren CE, Pierik MJ, Zeegers MP, Feskens EJ, Masclee AA, Jonkers DM. Review article: The association of diet with onset and relapse in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2013;38:1172-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | Laurell A, Sjöberg K. Prebiotics and synbiotics in ulcerative colitis. Scand J Gastroenterol. 2017;52:477-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Derwa Y, Gracie DJ, Hamlin PJ, Ford AC. Systematic review with meta-analysis: The efficacy of probiotics in inflammatory bowel disease. Aliment Pharmacol Ther. 2017;46:389-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 268] [Article Influence: 29.8] [Reference Citation Analysis (1)] |
| 10. | Eichele DD, Kharbanda KK. Dextran sodium sulfate colitis murine model: An indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J Gastroenterol. 2017;23:6016-6029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 621] [Cited by in RCA: 630] [Article Influence: 70.0] [Reference Citation Analysis (20)] |
| 11. | Oehlers SH, Flores MV, Hall CJ, Okuda KS, Sison JO, Crosier KE, Crosier PS. Chemically induced intestinal damage models in zebrafish larvae. Zebrafish. 2013;10:184-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 12. | Lu N, Wang L, Cao H, Liu L, Van Kaer L, Washington MK, Rosen MJ, Dubé PE, Wilson KT, Ren X, Hao X, Polk DB, Yan F. Activation of the epidermal growth factor receptor in macrophages regulates cytokine production and experimental colitis. J Immunol. 2014;192:1013-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 13. | Oh SY, Cho KA, Kang JL, Kim KH, Woo SY. Comparison of experimental mouse models of inflammatory bowel disease. Int J Mol Med. 2014;33:333-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 14. | Chassaing B, Aitken JD, Malleshappa M, Vijay-Kumar M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol. 2014;104:Unit 15.25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 1426] [Article Influence: 118.8] [Reference Citation Analysis (1)] |
| 15. | Poritz LS, Garver KI, Green C, Fitzpatrick L, Ruggiero F, Koltun WA. Loss of the tight junction protein ZO-1 in dextran sulfate sodium induced colitis. J Surg Res. 2007;140:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 320] [Article Influence: 16.8] [Reference Citation Analysis (2)] |
| 16. | Araki Y, Mukaisyo K, Sugihara H, Fujiyama Y, Hattori T. Increased apoptosis and decreased proliferation of colonic epithelium in dextran sulfate sodium-induced colitis in mice. Oncol Rep. 2010;24:869-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 17. | Matsunaga H, Hokari R, Kurihara C, Okada Y, Takebayashi K, Okudaira K, Watanabe C, Komoto S, Nakamura M, Tsuzuki Y, Kawaguchi A, Nagao S, Itoh K, Miura S. Omega-3 fatty acids exacerbate DSS-induced colitis through decreased adiponectin in colonic subepithelial myofibroblasts. Inflamm Bowel Dis. 2008;14:1348-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Laroui H, Ingersoll SA, Liu HC, Baker MT, Ayyadurai S, Charania MA, Laroui F, Yan Y, Sitaraman SV, Merlin D. Dextran sodium sulfate (DSS) induces colitis in mice by forming nano-lipocomplexes with medium-chain-length fatty acids in the colon. PLoS One. 2012;7:e32084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 263] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 19. | Samak G, Chaudhry KK, Gangwar R, Narayanan D, Jaggar JH, Rao R. Calcium/Ask1/MKK7/JNK2/c-Src signalling cascade mediates disruption of intestinal epithelial tight junctions by dextran sulfate sodium. Biochem J. 2015;465:503-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 20. | Gangwar R, Meena AS, Shukla PK, Nagaraja AS, Dorniak PL, Pallikuth S, Waters CM, Sood A, Rao R. Calcium-mediated oxidative stress: A common mechanism in tight junction disruption by different types of cellular stress. Biochem J. 2017;474:731-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 21. | Görlach A, Bertram K, Hudecova S, Krizanova O. Calcium and ROS: A mutual interplay. Redox Biol. 2015;6:260-271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 733] [Cited by in RCA: 1178] [Article Influence: 107.1] [Reference Citation Analysis (0)] |
| 22. | Li J, Li YX, Chen MH, Li J, Du J, Shen B, Xia XM. Changes in the phosphorylation of claudins during the course of experimental colitis. Int J Clin Exp Pathol. 2015;8:12225-12233. [PubMed] |
| 23. | Russo JM, Florian P, Shen L, Graham WV, Tretiakova MS, Gitter AH, Mrsny RJ, Turner JR. Distinct temporal-spatial roles for rho kinase and myosin light chain kinase in epithelial purse-string wound closure. Gastroenterology. 2005;128:987-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 104] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 24. | Sit ST, Manser E. Rho GTPases and their role in organizing the actin cytoskeleton. J Cell Sci. 2011;124:679-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 387] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 25. | Citalán-Madrid AF, Vargas-Robles H, García-Ponce A, Shibayama M, Betanzos A, Nava P, Salinas-Lara C, Rottner K, Mennigen R, Schnoor M. Cortactin deficiency causes increased RhoA/ROCK1-dependent actomyosin contractility, intestinal epithelial barrier dysfunction, and disproportionately severe DSS-induced colitis. Mucosal Immunol. 2017;10:1237-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | Liu W, Liu Y, Wang Z, Yu T, Lu Q, Chen H. Suppression of MAPK and NF-κ B pathways by schisandrin B contributes to attenuation of DSS-induced mice model of inflammatory bowel disease. Pharmazie. 2015;70:598-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Huang XL, Xu J, Zhang XH, Qiu BY, Peng L, Zhang M, Gan HT. PI3K/Akt signaling pathway is involved in the pathogenesis of ulcerative colitis. Inflamm Res. 2011;60:727-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 120] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 28. | Wang F, Schwarz BT, Graham WV, Wang Y, Su L, Clayburgh DR, Abraham C, Turner JR. IFN-gamma-induced TNFR2 expression is required for TNF-dependent intestinal epithelial barrier dysfunction. Gastroenterology. 2006;131:1153-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 251] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 29. | Fitzpatrick FA. Cyclooxygenase enzymes: Regulation and function. Curr Pharm Des. 2004;10:577-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 243] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 30. | Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:986-1000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2847] [Cited by in RCA: 2836] [Article Influence: 189.1] [Reference Citation Analysis (12)] |
| 31. | Singer II, Kawka DW, Schloemann S, Tessner T, Riehl T, Stenson WF. Cyclooxygenase 2 is induced in colonic epithelial cells in inflammatory bowel disease. Gastroenterology. 1998;115:297-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 363] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 32. | Kaur R, Thakur S, Rastogi P, Kaushal N. Resolution of Cox mediated inflammation by Se supplementation in mouse experimental model of colitis. PLoS One. 2018;13:e0201356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Tabatabaeizadeh SA, Tafazoli N, Ferns GA, Avan A, Ghayour-Mobarhan M. Vitamin D, the gut microbiome and inflammatory bowel disease. J Res Med Sci. 2018;23:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 34. | Zhao H, Zhang H, Wu H, Li H, Liu L, Guo J, Li C, Shih DQ, Zhang X. Protective role of 1,25(OH)2 vitamin D3 in the mucosal injury and epithelial barrier disruption in DSS-induced acute colitis in mice. BMC Gastroenterol. 2012;12:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 230] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 35. | Sun J. The Role of Vitamin D and Vitamin D Receptors in Colon Cancer. Clin Transl Gastroenterol. 2017;8:e103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | Li YC, Chen Y, Du J. Critical roles of intestinal epithelial vitamin D receptor signaling in controlling gut mucosal inflammation. J Steroid Biochem Mol Biol. 2015;148:179-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 37. | Kong J, Zhang Z, Musch MW, Ning G, Sun J, Hart J, Bissonnette M, Li YC. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol. 2008;294:G208-G216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 515] [Article Influence: 28.6] [Reference Citation Analysis (1)] |
| 38. | Wang F, Johnson RL, DeSmet ML, Snyder PW, Fairfax KC, Fleet JC. Vitamin D Receptor-Dependent Signaling Protects Mice From Dextran Sulfate Sodium-Induced Colitis. Endocrinology. 2017;158:1951-1963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 39. | Vargas Robles H, Citalán Madrid AF, García Ponce A, Silva Olivares A, Shibayama M, Betanzos A, Del Valle Mondragón L, Nava P, Schnoor M. Experimental Colitis Is Attenuated by Cardioprotective Diet Supplementation That Reduces Oxidative Stress, Inflammation, and Mucosal Damage. Oxid Med Cell Longev. 2016;2016:8473242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 40. | Miyamoto J, Mizukure T, Park SB, Kishino S, Kimura I, Hirano K, Bergamo P, Rossi M, Suzuki T, Arita M, Ogawa J, Tanabe S. A gut microbial metabolite of linoleic acid, 10-hydroxy-cis-12-octadecenoic acid, ameliorates intestinal epithelial barrier impairment partially via GPR40-MEK-ERK pathway. J Biol Chem. 2015;290:2902-2918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 205] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 41. | Tong LC, Wang Y, Wang ZB, Liu WY, Sun S, Li L, Su DF, Zhang LC. Propionate Ameliorates Dextran Sodium Sulfate-Induced Colitis by Improving Intestinal Barrier Function and Reducing Inflammation and Oxidative Stress. Front Pharmacol. 2016;7:253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 250] [Article Influence: 25.0] [Reference Citation Analysis (1)] |
| 42. | Kang GD, Lim S, Kim DH. Oleanolic acid ameliorates dextran sodium sulfate-induced colitis in mice by restoring the balance of Th17/Treg cells and inhibiting NF-κB signaling pathway. Int Immunopharmacol. 2015;29:393-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 43. | Eissa N, Hussein H, Kermarrec L, Grover J, Metz-Boutigue ME, Bernstein CN, Ghia JE. Chromofungin Ameliorates the Progression of Colitis by Regulating Alternatively Activated Macrophages. Front Immunol. 2017;8:1131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 44. | Hernández-Trejo JA, Suárez-Pérez D, Gutiérrez-Martínez IZ, Fernandez-Vargas OE, Serrano C, Candelario-Martínez AA, Meraz-Ríos MA, Citalán-Madrid AF, Hernández-Ruíz M, Reyes-Maldonado E, Valle-Rios R, Feintuch-Unger JH, Schnoor M, Villegas-Sepúlveda N, Medina-Contreras O, Nava P. The pro-inflammatory cytokines IFNγ/TNFα increase chromogranin A-positive neuroendocrine cells in the colonic epithelium. Biochem J. 2016;473:3805-3818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 45. | Eissa N, Hussein H, Mesgna R, Bonin S, Hendy GN, Metz-Boutigue MH, Bernstein CN, Ghia JE. Catestatin Regulates Epithelial Cell Dynamics to Improve Intestinal Inflammation. Vaccines (Basel). 2018;6:pii: E67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 46. | Hwang DY, Kim S, Hong HS. Substance-P Ameliorates Dextran Sodium Sulfate-Induced Intestinal Damage by Preserving Tissue Barrier Function. Tissue Eng Regen Med. 2017;15:63-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 47. | Han F, Zhang H, Xia X, Xiong H, Song D, Zong X, Wang Y. Porcine β-defensin 2 attenuates inflammation and mucosal lesions in dextran sodium sulfate-induced colitis. J Immunol. 2015;194:1882-1893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 137] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 48. | Chen P, Bakke D, Kolodziej L, Lodolce J, Weber CR, Boone DL, Toback FG. Antrum Mucosal Protein-18 Peptide Targets Tight Junctions to Protect and Heal Barrier Structure and Function in Models of Inflammatory Bowel Disease. Inflamm Bowel Dis. 2015;21:2393-2402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 49. | Chen P, Kartha S, Bissonnette M, Hart J, Toback FG. AMP-18 facilitates assembly and stabilization of tight junctions to protect the colonic mucosal barrier. Inflamm Bowel Dis. 2012;18:1749-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 50. | Castro-Ochoa KF, Vargas-Robles H, Chánez-Paredes S, Felipe-López A, Cabrera-Silva RI, Shibayama M, Betanzos A, Nava P, Galinski EA, Schnoor M. Homoectoine Protects Against Colitis by Preventing a Claudin Switch in Epithelial Tight Junctions. Dig Dis Sci. 2019;64:409-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 51. | Chen Y, Zhang M, Ren F. A Role of Exopolysaccharide Produced by Streptococcus thermophilus in the Intestinal Inflammation and Mucosal Barrier in Caco-2 Monolayer and Dextran Sulphate Sodium-Induced Experimental Murine Colitis. Molecules. 2019;24:pii: E513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 52. | Feng G, Zeng M, Huang M, Zhu S, Guo W, Wu H. Protective effect of biogenic polyphosphate nanoparticles from Synechococcus sp. PCC 7002 on dextran sodium sulphate-induced colitis in mice. Food Funct. 2019;10:1007-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 53. | Wang R, Wang L, Luo Y, Wang D, Du R, Du J, Wang Y. Maggot protein ameliorates dextran sulphate sodium-induced ulcerative colitis in mice. Biosci Rep. 2018;38:pii: BSR20181799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 54. | Ma X, Hu Y, Li X, Zheng X, Wang Y, Zhang J, Fu C, Geng F. Periplaneta americana Ameliorates Dextran Sulfate Sodium-Induced Ulcerative Colitis in Rats by Keap1/Nrf-2 Activation, Intestinal Barrier Function, and Gut Microbiota Regulation. Front Pharmacol. 2018;9:944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 55. | Jiang L, Lv J, Liu J, Hao X, Ren F, Guo H. Donkey milk lysozyme ameliorates dextran sulfate sodium-induced colitis by improving intestinal barrier function and gut microbiota composition. J Funct Foods. 2018;48:144-152. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 56. | Azuma T, Shigeshiro M, Kodama M, Tanabe S, Suzuki T. Supplemental naringenin prevents intestinal barrier defects and inflammation in colitic mice. J Nutr. 2013;143:827-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 57. | Cao H, Liu J, Shen P, Cai J, Han Y, Zhu K, Fu Y, Zhang N, Zhang Z, Cao Y. Protective Effect of Naringin on DSS-Induced Ulcerative Colitis in Mice. J Agric Food Chem. 2018;66:13133-13140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 154] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 58. | Bibi S, Kang Y, Du M, Zhu MJ. Dietary red raspberries attenuate dextran sulfate sodium-induced acute colitis. J Nutr Biochem. 2018;51:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 59. | Zhang Z, Li S, Cao H, Shen P, Liu J, Fu Y, Cao Y, Zhang N. The protective role of phloretin against dextran sulfate sodium-induced ulcerative colitis in mice. Food Funct. 2019;10:422-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 113] [Article Influence: 16.1] [Reference Citation Analysis (1)] |
| 60. | Wu D, Wu K, Zhu Q, Xiao W, Shan Q, Yan Z, Wu J, Deng B, Xue Y, Gong W, Lu G, Ding Y. Formononetin Administration Ameliorates Dextran Sulfate Sodium-Induced Acute Colitis by Inhibiting NLRP3 Inflammasome Signaling Pathway. Mediators Inflamm. 2018;2018:3048532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 61. | Zheng S, Deng S, Huang Y, Huang M, Zhao P, Ma X, Wen Y, Wang Q, Yang X. Anti-diabetic activity of a polyphenol-rich extract from Phellinus igniarius in KK-Ay mice with spontaneous type 2 diabetes mellitus. Food Funct. 2018;9:614-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 62. | Gao W, Wang W, Sun W, Wang M, Zhang N, Yu S. Antitumor and immunomodulating activities of six Phellinus igniarius polysaccharides of different origins. Exp Ther Med. 2017;14:4627-4632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 63. | Sun Y, Zhong S, Yu J, Zhu J, Ji D, Hu G, Wu C, Li Y. The aqueous extract of Phellinus igniarius (SH) ameliorates dextran sodium sulfate-induced colitis in C57BL/6 mice. PLoS One. 2018;13:e0205007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 64. | Woo JK, Choi S, Kang JH, Kim DE, Hurh BS, Jeon JE, Kim SY, Oh SH. Fermented barley and soybean (BS) mixture enhances intestinal barrier function in dextran sulfate sodium (DSS)-induced colitis mouse model. BMC Complement Altern Med. 2016;16:498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 65. | Shen P, Zhang Z, Zhu K, Cao H, Liu J, Lu X, Li Y, Jing Y, Yuan X, Fu Y, Cao Y, Zhang N. Evodiamine prevents dextran sulfate sodium-induced murine experimental colitis via the regulation of NF-κB and NLRP3 inflammasome. Biomed Pharmacother. 2019;110:786-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 66. | Wang K, Yang Q, Ma Q, Wang B, Wan Z, Chen M, Wu L. Protective Effects of Salvianolic Acid A against Dextran Sodium Sulfate-Induced Acute Colitis in Rats. Nutrients. 2018;10:pii: E791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 67. | Shen P, Zhang Z, He Y, Gu C, Zhu K, Li S, Li Y, Lu X, Liu J, Zhang N, Cao Y. Magnolol treatment attenuates dextran sulphate sodium-induced murine experimental colitis by regulating inflammation and mucosal damage. Life Sci. 2018;196:69-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 68. | Zhou K, Cheng R, Liu B, Wang L, Xie H, Zhang C. Eupatilin ameliorates dextran sulphate sodium-induced colitis in mice partly through promoting AMPK activation. Phytomedicine. 2018;46:46-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 69. | Dai YC, Zheng L, Zhang YL, Chen X, Chen DL, Tang ZP. Effects of Jianpi Qingchang decoction on the quality of life of patients with ulcerative colitis: A randomized controlled trial. Medicine (Baltimore). 2017;96:e6651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 70. | Lin JC, Wu JQ, Wang F, Tang FY, Sun J, Xu B, Jiang M, Chu Y, Chen D, Li X, Su S, Zhang Y, Wu N, Yang S, Wu K, Liang J. QingBai decoction regulates intestinal permeability of dextran sulphate sodium-induced colitis through the modulation of notch and NF-κB signalling. Cell Prolif. 2019;52:e12547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 71. | Zhang Z, Shen P, Lu X, Li Y, Liu J, Liu B, Fu Y, Cao Y, Zhang N. In Vivo and In Vitro Study on the Efficacy of Terpinen-4-ol in Dextran Sulfate Sodium-Induced Mice Experimental Colitis. Front Immunol. 2017;8:558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 72. | Tung NH, Kim SK, Ra JC, Zhao YZ, Sohn DH, Kim YH. Antioxidative and hepatoprotective diarylheptanoids from the bark of Alnus japonica. Planta Med. 2010;76:626-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 73. | Tung NH, Kwon HJ, Kim JH, Ra JC, Kim JA, Kim YH. An anti-influenza component of the bark of Alnus japonica. Arch Pharm Res. 2010;33:363-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 74. | Tung NH, Sun K, Fan JY, Shoyama Y, Han JY. Oregonin from the Bark of Alnus japonica restrained ischemia-reperfusion-induced mesentery oxidative stress by inhibiting NADPH oxidase activation. Microcirculation. 2014;21:688-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 75. | Chi JH, Kim YH, Sohn DH, Seo GS, Lee SH. Ameliorative effect of Alnus japonica ethanol extract on colitis through the inhibition of inflammatory responses and attenuation of intestinal barrier disruption in vivo and in vitro. Biomed Pharmacother. 2018;108:1767-1774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 76. | Qu C, Yuan ZW, Yu XT, Huang YF, Yang GH, Chen JN, Lai XP, Su ZR, Zeng HF, Xie Y, Zhang XJ. Patchouli alcohol ameliorates dextran sodium sulfate-induced experimental colitis and suppresses tryptophan catabolism. Pharmacol Res. 2017;121:70-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 77. | Yu XT, Xu YF, Huang YF, Qu C, Xu LQ, Su ZR, Zeng HF, Zheng L, Yi TG, Li HL, Chen JP, Zhang XJ. Berberrubine attenuates mucosal lesions and inflammation in dextran sodium sulfate-induced colitis in mice. PLoS One. 2018;13:e0194069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 78. | Kim Y, Wu AG, Jaja-Chimedza A, Graf BL, Waterman C, Verzi MP, Raskin I. Isothiocyanate-enriched moringa seed extract alleviates ulcerative colitis symptoms in mice. PLoS One. 2017;12:e0184709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 79. | Kim MW, Choi S, Kim SY, Yoon YS, Kang JH, Oh SH. Allyl Isothiocyanate Ameliorates Dextran Sodium Sulfate-Induced Colitis in Mouse by Enhancing Tight Junction and Mucin Expression. Int J Mol Sci. 2018;19:pii: E2025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 80. | Kim KY, Oh TW, Do HJ, Yang JH, Yang IJ, Jeon YH, Go YH, Ahn SC, Ma JY, Park KI. Acer palmatum thumb. Ethanol Extract Alleviates Interleukin-6-Induced Barrier Dysfunction and Dextran Sodium Sulfate-Induced Colitis by Improving Intestinal Barrier Function and Reducing Inflammation. J Immunol Res. 2018;2018:5718396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 81. | Bach Knudsen KE, Lærke HN, Hedemann MS, Nielsen TS, Ingerslev AK, Gundelund Nielsen DS, Theil PK, Purup S, Hald S, Schioldan AG, Marco ML, Gregersen S, Hermansen K. Impact of Diet-Modulated Butyrate Production on Intestinal Barrier Function and Inflammation. Nutrients. 2018;10:pii: E1499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 408] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 82. | Suzuki T, Yoshida S, Hara H. Physiological concentrations of short-chain fatty acids immediately suppress colonic epithelial permeability. Br J Nutr. 2008;100:297-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 269] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 83. | Hung TV, Suzuki T. Dietary Fermentable Fiber Reduces Intestinal Barrier Defects and Inflammation in Colitic Mice. J Nutr. 2016;146:1970-1979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 84. | Ogata M, Ogita T, Tari H, Arakawa T, Suzuki T. Supplemental psyllium fibre regulates the intestinal barrier and inflammation in normal and colitic mice. Br J Nutr. 2017;118:661-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 85. | Yamamoto S, Ma X. Role of Nod2 in the development of Crohn's disease. Microbes Infect. 2009;11:912-918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 86. | Gajendran M, Loganathan P, Catinella AP, Hashash JG. A comprehensive review and update on Crohn's disease. Dis Mon. 2018;64:20-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 353] [Article Influence: 39.2] [Reference Citation Analysis (1)] |
| 87. | Walsh MC, Kim GK, Maurizio PL, Molnar EE, Choi Y. TRAF6 autoubiquitination-independent activation of the NFkappaB and MAPK pathways in response to IL-1 and RANKL. PLoS One. 2008;3:e4064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 150] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 88. | Amendola A, Butera A, Sanchez M, Strober W, Boirivant M. Nod2 deficiency is associated with an increased mucosal immunoregulatory response to commensal microorganisms. Mucosal Immunol. 2014;7:391-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 89. | Elson CO, Beagley KW, Sharmanov AT, Fujihashi K, Kiyono H, Tennyson GS, Cong Y, Black CA, Ridwan BW, McGhee JR. Hapten-induced model of murine inflammatory bowel disease: Mucosa immune responses and protection by tolerance. J Immunol. 1996;157:2174-2185. [PubMed] |
| 90. | Liu Y, Wang X, Hu CA. Therapeutic Potential of Amino Acids in Inflammatory Bowel Disease. Nutrients. 2017;9:pii: E920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 130] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 91. | Sartor RB. Mechanisms of disease: Pathogenesis of Crohn's disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:390-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1158] [Cited by in RCA: 1307] [Article Influence: 65.4] [Reference Citation Analysis (2)] |
| 92. | Antoniou E, Margonis GA, Angelou A, Pikouli A, Argiri P, Karavokyros I, Papalois A, Pikoulis E. The TNBS-induced colitis animal model: An overview. Ann Med Surg (Lond). 2016;11:9-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 258] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 93. | Cheon GJ, Cui Y, Yeon DS, Kwon SC, Park BG. Mechanisms of motility change on trinitrobenzenesulfonic Acid-induced colonic inflammation in mice. Korean J Physiol Pharmacol. 2012;16:437-446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 94. | Xiong Y, Chen D, Yu C, Lv B, Peng J, Wang J, Lin Y. Citrus nobiletin ameliorates experimental colitis by reducing inflammation and restoring impaired intestinal barrier function. Mol Nutr Food Res. 2015;59:829-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 95. | Ran X, Li Y, Chen G, Fu S, He D, Huang B, Wei L, Lin Y, Guo Y, Hu G. Farrerol Ameliorates TNBS-Induced Colonic Inflammation by Inhibiting ERK1/2, JNK1/2, and NF-κB Signaling Pathway. Int J Mol Sci. 2018;19:pii: E2037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 96. | Xu B, Li YL, Xu M, Yu CC, Lian MQ, Tang ZY, Li CX, Lin Y. Geniposide ameliorates TNBS-induced experimental colitis in rats via reducing inflammatory cytokine release and restoring impaired intestinal barrier function. Acta Pharmacol Sin. 2017;38:688-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 97. | Lim SM, Kang GD, Jeong JJ, Choi HS, Kim DH. Neomangiferin modulates the Th17/Treg balance and ameliorates colitis in mice. Phytomedicine. 2016;23:131-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 98. | Yue Y, Wu S, Li Z, Li J, Li X, Xiang J, Ding H. Wild jujube polysaccharides protect against experimental inflammatory bowel disease by enabling enhanced intestinal barrier function. Food Funct. 2015;6:2568-2577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 99. | Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, Thangaraju M, Prasad PD, Manicassamy S, Munn DH, Lee JR, Offermanns S, Ganapathy V. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40:128-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1198] [Cited by in RCA: 1806] [Article Influence: 150.5] [Reference Citation Analysis (0)] |
| 100. | Chen G, Ran X, Li B, Li Y, He D, Huang B, Fu S, Liu J, Wang W. Sodium Butyrate Inhibits Inflammation and Maintains Epithelium Barrier Integrity in a TNBS-induced Inflammatory Bowel Disease Mice Model. EBioMedicine. 2018;30:317-325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 432] [Article Influence: 54.0] [Reference Citation Analysis (1)] |
| 101. | Xia CM, Zhao Y, Jiang L, Jiang J, Zhang SC. Schistosoma japonicum ova maintains epithelial barrier function during experimental colitis. World J Gastroenterol. 2011;17:4810-4816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 102. | Lin SC, Cheifetz AS. The Use of Complementary and Alternative Medicine in Patients With Inflammatory Bowel Disease. Gastroenterol Hepatol (N Y). 2018;14:415-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 103. | Deban L, Correale C, Vetrano S, Malesci A, Danese S. Multiple Pathogenic Roles of Microvasculature in Inflammatory Bowel Disease: A Jack of All Trades. Am J Pathol. 2008;172:1457-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 123] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 104. | Vetrano S, Rescigno M, Cera MR, Correale C, Rumio C, Doni A, Fantini M, Sturm A, Borroni E, Repici A, Locati M, Malesci A, Dejana E, Danese S. Unique role of junctional adhesion molecule-a in maintaining mucosal homeostasis in inflammatory bowel disease. Gastroenterology. 2008;135:173-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 207] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 105. | Matsumoto K, Yamaba R, Inoue K, Utsumi D, Tsukahara T, Amagase K, Tominaga M, Kato S. Transient receptor potential vanilloid 4 channel regulates vascular endothelial permeability during colonic inflammation in dextran sulphate sodium‐induced murine colitis. Br J Pharmacol. 2018;175:84-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Mexico
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Decorti G, Jin B, Tarnawski AS, Vasudevan A S-Editor: Yan JP L-Editor: Filipodia E-Editor: Wu YXJ