Published online Aug 14, 2019. doi: 10.3748/wjg.v25.i30.4051
Peer-review started: March 21, 2019
First decision: May 24, 2019
Revised: June 22, 2019
Accepted: July 1, 2019
Article in press: July 3, 2019
Published online: August 14, 2019
Processing time: 147 Days and 3.2 Hours
Since its original application, gastrointestinal (GI) endoscopy has undergone many innovative transformations aimed at expanding the scope, safety, accuracy, acceptability and cost-effectiveness of this area of clinical practice. One method of achieving this has been to reduce the caliber of endoscopic devices. We propose the collective term “Miniature GI Endoscopy”. In this Opinion Review, the innovations in this field are explored and discussed. The progress and clinical use of the three main areas of miniature GI endoscopy (ultrathin endoscopy, wireless endoscopy and scanning fiber endoscopy) are described. The opportunities presented by these technologies are set out in a clinical context, as are their current limitations. Many of the positive aspects of miniature endoscopy are clear, in that smaller devices provide access to potentially all of the alimentary canal, while conferring high patient acceptability. This must be balanced with the costs of new technologies and recognition of device specific challenges. Perspectives on future application are also considered and the efforts being made to bring new innovations to a clinical platform are outlined. Current devices demonstrate that miniature GI endoscopy has a valuable place in investigation of symptoms, therapeutic intervention and screening. Newer technologies give promise that the potential for enhancing the investigation and management of GI complaints is significant.
Core tip: Miniature endoscopic devices play a growing role in the practice of gastroenterology and can come in many forms. They can offer easier access to the gastrointestinal tract, are often tolerated better than standard endoscopy and have the potential to boost diagnostic accuracy. Those properties give promise to advancements in therapeutic intervention and to screening for luminal disorders. Pitfalls remain, particularly with regard to cost, but the trend towards the application of miniature gastrointestinal endoscopy is clear and justifiable.
- Citation: McGoran JJ, McAlindon ME, Iyer PG, Seibel EJ, Haidry R, Lovat LB, Sami SS. Miniature gastrointestinal endoscopy: Now and the future. World J Gastroenterol 2019; 25(30): 4051-4060
- URL: https://www.wjgnet.com/1007-9327/full/v25/i30/4051.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i30.4051
Gastrointestinal (GI) endoscopy has for many decades been an essential component in the practice of diagnosing and managing digestive diseases. Current endoscopic practice began with Schindler’s development of the flexible gastroscope in 1932[1]. Since that innovative step, approaches have been taken to expand the abilities of endoscopy, improve its safety and present exciting challenges to what further can be done. One aspect of this is the development of miniature endoscopes which have aimed to address various clinical problems. The progress of miniature endoscopic devices is largely dependent on that of optical technology and its resultant incorporation of that into clinical application.
The use of an endoscopic device with a smaller caliber has many advantages in clinical practice. Tolerance and safety of invasive GI procedures can be improved, potentially leading to greater uptake and enhanced trust in a care provider. This feature of miniature endoscopy, combined with the potential for more portable devices, could have benefits for wider access to population screening for various GI diseases. Devices that demand less sedation and carry fewer complications add to arguments for the cost-effectiveness of miniature endoscopes. These features as well as the authors’ vision for future applications are outlined in this Opinion Review.
The authors have both academic and clinical expertise in the development and use of miniature endoscopes to enhance patient care. They are aware of the present challenges to clinical practice, including rapid access to screening and diagnostics, improving early cancer detection rates and developing less invasive therapeutic interventions. Miniature endoscopy may have a place in addressing all of these challenges.
The 3 main areas explored in the review are: ultrathin endoscopy; wireless capsule endoscopy; and scanning fiber endoscopy.
Ultrathin endoscopes have many uses in gastroenterology and they are lauded as safe, cost-effective and easy-to-use tools which carry benefits that standard endoscopes do not[2]. The first recorded use of unsedated ultrathin endoscopy (UTE) was in 1994 when twenty healthy volunteers underwent esophagogastroduodenoscopy (EGD) using an Olympus GIF-N30 device[3]. Since then, its use has expanded into common practice in most endoscopy departments. In addition to diagnostic procedures, UTE has been used to varying degrees of success in therapeutic scenarios, such as self-expanding metal stent insertion, long intestinal tube placement for small bowel obstruction and some endoscopic retrograde cholangiopancreatography (ERCP) cases[4-6].
The conventional design of ultrathin endoscopes is similar to that of standard endoscopes. However, shaft diameters tend to be around 6 mm or less, allowing insertion through the nasal cavity to perform transnasal endoscopy (TNE)[2]. Portable and disposable models of ultrathin endoscopes have the potential to change the approach taken to clinical practice. The newest devices have disposable sheaths which eliminate the need for instrument decontamination and allow multiple examinations to take place using the same device in quick succession. The light source, processor and screen are integrated into a portable digital processing unit[7] (Figure 1). The employment of a portable system can obviate transfer to a hospital unit, which in itself can cause inconvenience and distress to patients and carry significant cost to time and resources. The endoscopic test can instead take place in a setting that is more acceptable to such individuals[8]. One therapeutic procedure for which this may be pertinent is percutaneous endoscopic gastrostomy (PEG) insertion. Ultrathin transnasal endoscopes can be used to insert feeding tubes using the introducer method, which inserts the tube directly into the gastric lumen and eliminates the need for passage through the mouth[9]. This technique, combined with use of a portable endoscope serves to reduce the risk of cardiorespiratory side effects in selected at-risk cases[10].
Using tolerability assessment scores, unsedated transnasal endoscopy (TNE) is reported by patients as comparable to sedated conventional EGD (C-EGD)[11]. The tolerability, safety and effectiveness of UTE lends itself well to use in endoscopic screening for esophageal disorders such as Barrett’s esophagus (BE) and esophageal varices[11-13]. BE can be reliably diagnosed using UTE and the yield for intestinal metaplasia using smaller biopsy forceps is comparable to those used in C-EGD[14]. The productivity of a screening program is enhanced by portable, disposable models, opening up the possibility that screening using UTE can be a cost-effective measure. In a United States based Barrett’s screening study of 209 patients, unsedated TNE was significantly lower in cost compared to sedated C-EGD, with mobile endoscopy costs proving less costly than TNE delivered in a hospital setting[15]. This applied to both direct and indirect medical costs. Options for the setting of this test could also expand, with office-based esophagoscopy becoming a potential reality[13].
UTE does carry drawbacks and limitations. Low-caliber endoscopes carry less capacity for constituent components (such as access channels for biopsy and therapeutic interventions); relatively lower image resolution and angle of view compared to C-EGD. There is some evidence that biopsies taken through an ultrathin channel carry comparable diagnostic yield for dysplasia to standard biopsies but larger studies are necessary[16]. The diagnostic accuracy of UTE for early superficial gastric cancers also continues to be prone to scrutiny in countries with high incidence such as Japan, albeit UTE being used for gastric cancer screening in this region[17].
The use of wireless capsule endoscopy (WCE) was first described in 2000 and has enjoyed widespread use since Food and Drug Administration approval in 2001[18]. The common application of WCE for identifying small bowel bleeding follows evidence that it is a superior diagnostic test to push enteroscopy and barium contrast studies[19]. It is a reliable test for Crohn’s disease, with a diagnostic yield as high as 71% and a high safety profile provided the risk of capsule retention is lowered by sufficient imaging or patency studies[20,21]. Improvements in diagnostic yield continue to be developed by widening field of view and increasing the number of recorded images, including the development of adaptive frame rates[22,23]. Various software tools have been developed to reduce reading time while maintaining the diagnostic yield. Their properties include omission of almost identical images, provisional selection of the most standout images and multiple-view modes[24]. The limitations of even very experienced and skilled readers in identifying pathologies are acknowledged. In response to this, the place of artificial intelligence in WCE is now recognized as a very real prospect. Applicable technology remains in the embryonic stages but over time, this, as well as patient and physician acceptance, are seen as barriers that can be overcome[25].
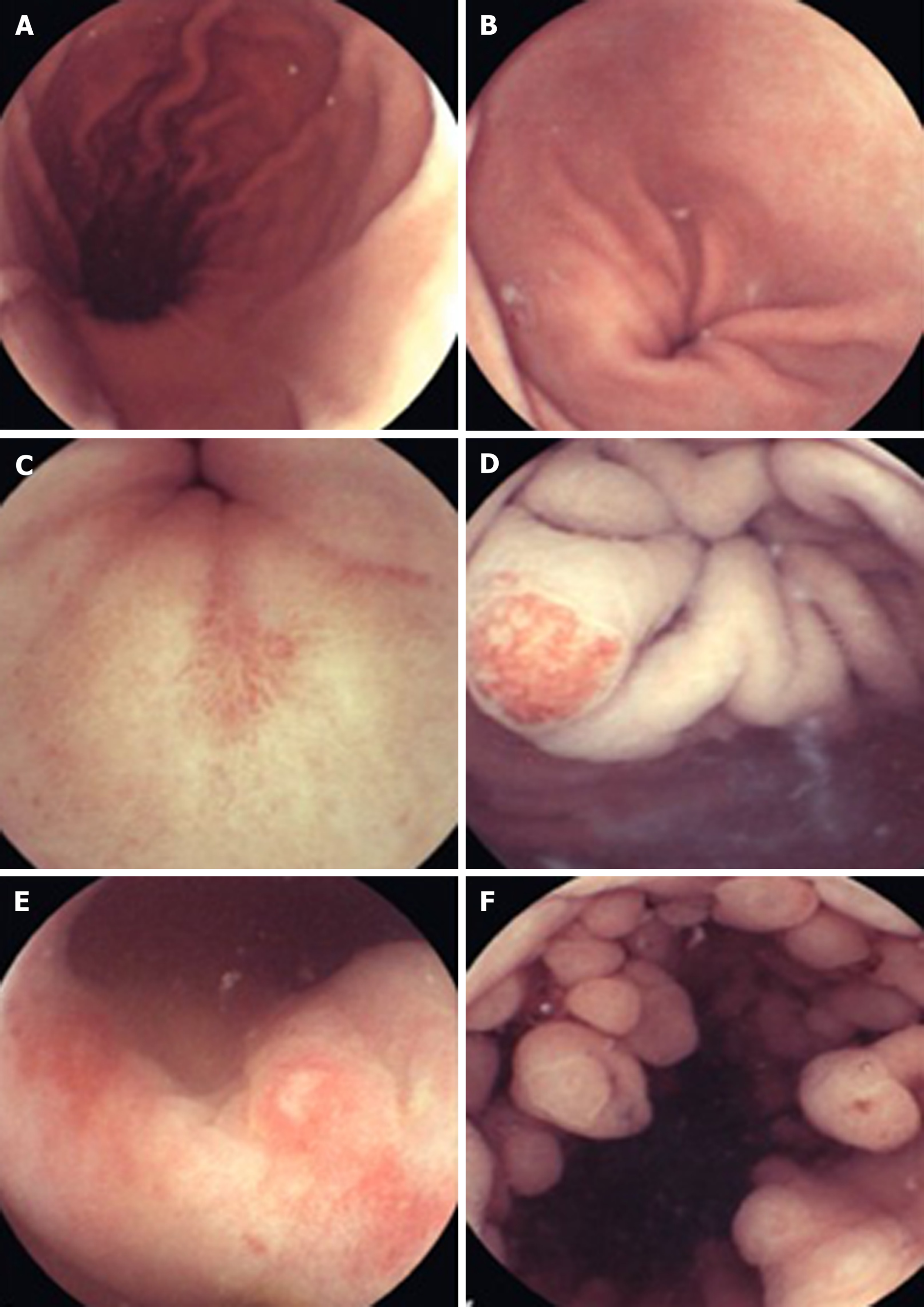
Beyond diagnosing small bowel pathology, colon capsule endoscopes (CCE) have been produced in response to concerns over the resource intensiveness driven by increased demand for colonoscopies, the chance of failure of cecal intubation and suboptimal patient uptake due to the poor tolerance of more conventional en-doscopy[26]. The second generation CCE-2 has two optical cameras at each end giving a 172° view and adaptive frame rate up to 35 frames per second. This provides bidirectional views in real time. Its dimensions are 31.5 mm by 11.6 mm and its recording capacity is ten hours. The software contains a polyp size estimation function and a flexible spectral imaging color enhancement for enhanced visualization[27]. Provided bowel preparation is excellent, detection of polyps > 6 mm and > 10 mm for the CCE-2 carries sensitivity and specificity rates of over 85%, supporting claims that this may be applicable in a screening setting[28]. In controlled settings, CCE have also been comparable to colonoscopy in assessing the colonic mucosa of those with inflammatory bowel disease[29,30].
Esophageal diseases such as BE and esophagitis may be detected using a capsule device. A blinded study comparing EGD as gold standard with the PillCam ESO 2 device (Given Imaging Ltd., Yoqneam, Israel) yielded promising detection rates for BE and esophagitis with a sensitivity of 100% and a specificity of 74%, and a sensitivity of 80% and a specificity of 87%, respectively[31]. In an attempt to overcome the impairment to diagnostic sensitivity exacerbated by rapid esophageal transit, tethered wireless capsule endoscopes have been developed. For the detection of Barrett’s esophagus, early results have been mixed and further large scale studies in relevant populations are advocated[32]. WCE has also been trialed in the emergency setting of acute upper GI bleeding. A prospective study found it to be a feasible and safe way of detecting and stratifying such cases[33]. It may also have a place in future practice for screening and surveillance of esophageal varices. The current literature, with a pooled sensitivity of 72%, does not support its use over EGD[34].
Active locomotion in capsules using mechanical actuation, in a crawling, inch-worm or swimming motion has been proposed as a way of controlling transit through the GI tract and resisting peristalsis in cases where prolonged inspection of an abnormal lumen is desired. Development of such equipment has not reached clinical trial stage primarily due to power capacity issues and mechanical complexity[35]. Future development would depend on enhanced power storage or usage te-chnology[36].
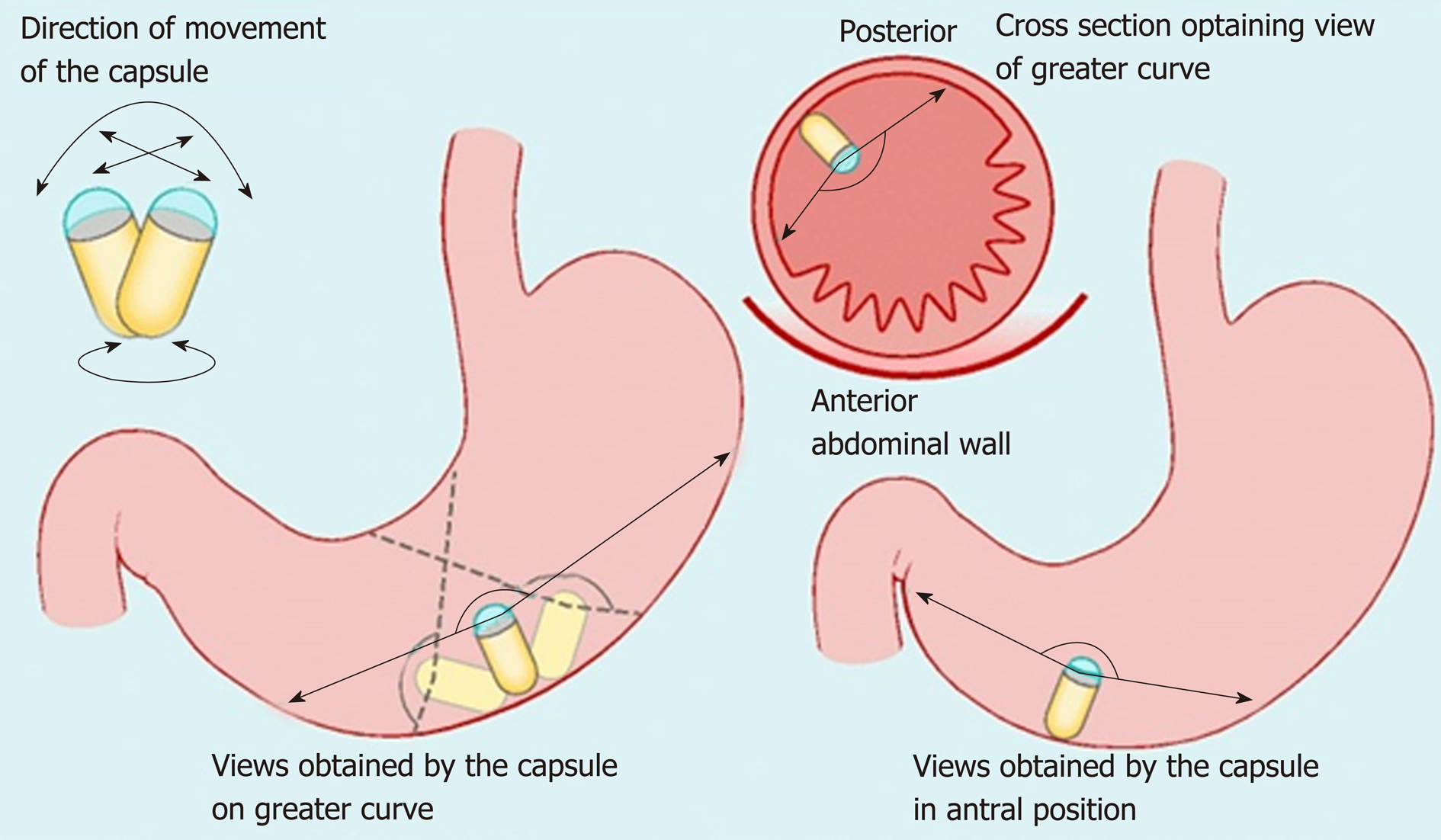
Non-actuated wireless capsules have struggled to completely examine the stomach lumen, owing to its large size impeding full visualization[37]. However, a feasibility study has suggested that with one liter of simethicone-containing swallowed water, good views of the upper GI tract can be obtained[38]. More advanced software that incorporates larger frame rates and artificial intelligence may also potentiate the diagnostic accuracy of this approach. Magnetically guided wireless capsules have been developed to be able to better navigate the device around a fluid distended stomach. There may also be a role for this test in screening for gastric cancer, with provisional feasibility studies of asymptomatic patients showing promise[39]. Magnetically driven capsules also help to lower storage needs for power thus potentially allowing space for interventional tools[35]. Progress continues on the development of biopsy models, which have shown promise in in vitro and animal models[40]. Further application in clinical trials is needed before the potential for interventional WCE in healthcare can be realized. Robotic assistance in controlling magnetic wireless capsules has been the subject of some clinical trials, showing superiority of this method of actuation over manual manipulation of magnetically guided WCE on viewing installed targets on an ex-vivo colon model[41]. A multicenter blinded study of patients with upper abdominal complaints examined robotically-assisted magnetically guided WCE with the gold standard of conventional gastroscopy and concluded that detection of focal lesions in the upper and lower stomach had comparable accuracy[42]. This device has also shown better diagnostic yield than EGD in patients presenting for investigation of iron deficiency anemia[43]. The evidence points towards WCE having a greater future role for diagnosis of GI disorders although it will require more time and research, particularly on the cost-effectiveness front, to determine which manifestations warrant widespread application[43,44] (Figures 2-4).
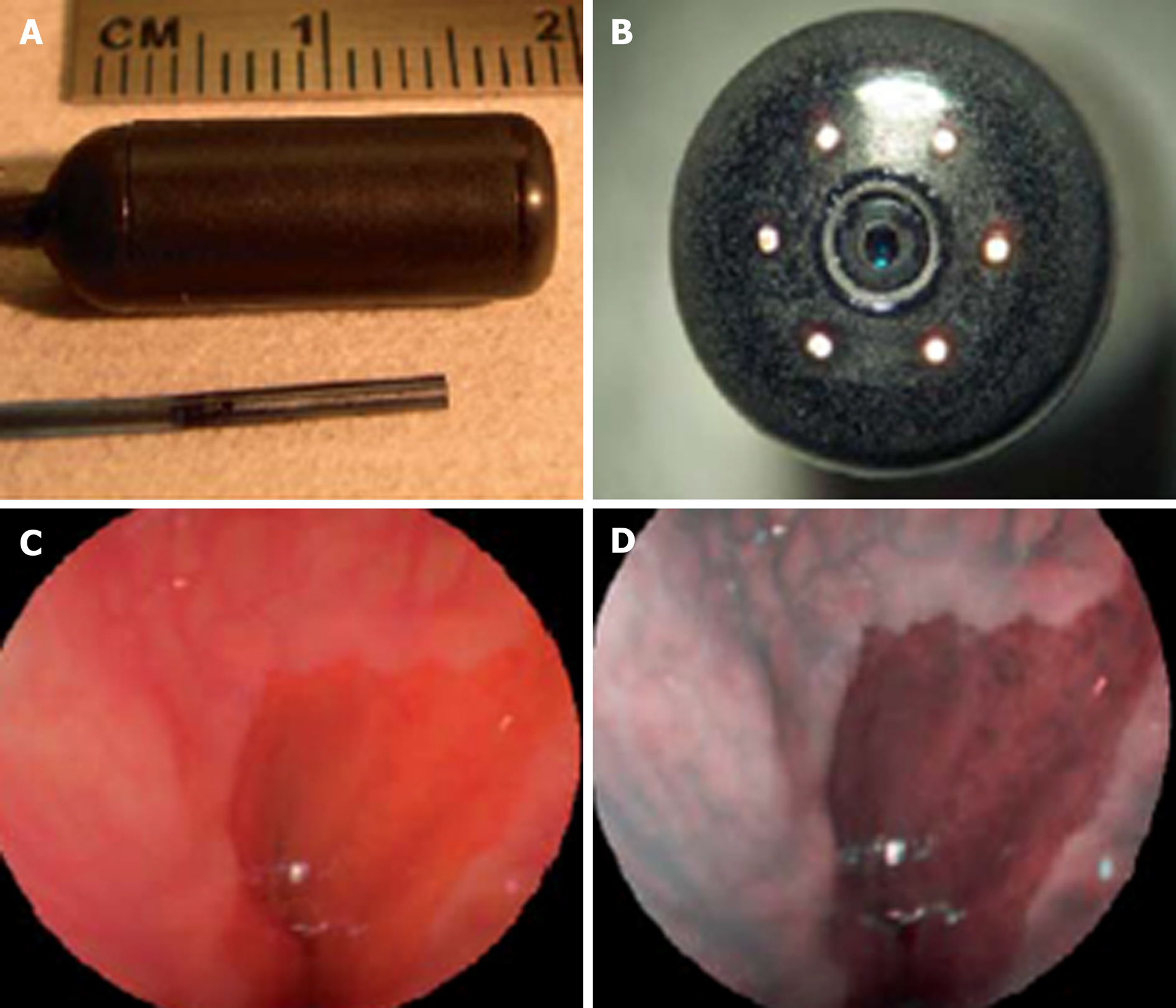
Newer forms of optical technology have been developed to meet the demands for endoscopic imaging that is of higher resolution than UTE can provide[46] (Figure 5). Scanning single fiber endoscopy (SFE) involves narrow bands of light being projected onto tissue and reflecting back onto the fiber, before an image is created one pixel at a time. The resultant image is of a superior quality to those from an ultrathin endoscope of a similar caliber[47]. In gastrointestinal endoscopy, as well as permitting access to poorly accessible areas like the upper biliary tree and pancreas, SFE may have a place as an adjunct to more conventional endoscopes. One example of this could be more complete visualization of a lesion whenever full views are not obtained on a single plane.
Spectrally-encoded scanning fiber endoscopy uses polychromatic light emissions from the endoscopic probe, encoded by wavelength which is then reflected from the surface and decoded outside the body to produce a one-dimensional impression. Rotation of the instrument builds information for a two-dimensional image of the visualized surface[46]. The endoscope can be as thin as 80-250 micrometers in diameter, limited only by the size of the light-emitting fiber and any accessory instruments[48,49]. Spectral encoded endoscopy using a single fiber can perform three-dimensional topological analysis and real-time subsurface imaging[50]. This multispectral SFE may be used in combination with white light endoscopy to collect wide field fluorescence images which can permit early detection of dysplasia and cancer[51]. Although research has shown progress in animal models, development of this technology for analyzing human tissue is required.
SFE has been undertaken in limited cases to perform cholangioscopy in patients with pancreaticobiliary strictures. It is a feasible technology to directly view such areas with better resolution than current cholangioscopic tools[52]. A tethered SFE “capsule” for conducting esophagoscopy has been developed, in what could represent an evolution of the tethered wireless capsule endoscope[53]. The patient swallows the device and images are transmitted live up the single fiber into a processor, in contrast with the tethered WCE which stores images for viewing at a later time. With the SFE capsule, real time images mean that pathologies and potential problems are identified at an immediate stage. Research into the application of SFE in real clinical scenarios is required but this has the potential for gastrointestinal endoscopy to be safer, more cost-effective, better tolerated and more advanced than current technology allows[47]. The progress of this technology continues at a rapid pace, with prototype devices as thin as a human hair that carry better resolution, being developed[54].
Miniature GI endoscopy has many forms and is in many ways a relative term. Through the recognition that endoscopy is an invasive procedure to which patients are prone to experiencing significant discomfort, and that accessibility to areas of the gastrointestinal tract requires development of existing equipment, endoscopes with narrower calibers have been produced. The three main domains in miniature endoscopy currently are ultrathin devices, scanning fiber endoscopes and wireless capsules. Within these domains many products are being developed at a rapid pace.
The role of gastrointestinal endoscopy can be generally categorized into two aspects- diagnostic and therapeutic. From a healthcare perspective, it is clear that a suitably accurate means of diagnosing GI diseases, which is better tolerated and eventually more cost-effective than standard endoscopy warrants major consideration for future practice. Screening for various luminal GI diseases, in particular, malignant and pre-malignant conditions is a topical issue. We believe that miniature devices such as ultrathin endoscopes and capsules can bring a high quality screening service that satisfies the needs outlined by Wilson et al[55]. As evidenced above, the diagnostic capabilities of miniature endoscopic devices such as SFE and magnetically guided WCEs enhance today’s practice. Through the enhanced access provided by miniature endoscopy, therapeutic interventions like hemostasis and delivery of medication may be achievable in the future by incorporating robotics and remote controlling systems.
| 1. | Schäfer PK, Sauerbruch T. [Rudolf Schindler (1888--1968)--"father" of gastroscopy]. Z Gastroenterol. 2004;42:550-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | ASGE Technology Committee. Rodriguez SA, Banerjee S, Desilets D, Diehl DL, Farraye FA, Kaul V, Kwon RS, Mamula P, Pedrosa MC, Varadarajulu S, Song LM, Tierney WM. Ultrathin endoscopes. Gastrointest Endosc. 2010;71:893-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Shaker R. Unsedated trans-nasal pharyngoesophagogastroduodenoscopy (T-EGD): technique. Gastrointest Endosc. 1994;40:346-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 108] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Li S, Yuan C, Xu MD. Two different endoscopic long intestinal tube placements for small bowel obstruction: Transnasal ultrathin endoscopy versus conventional endoscopy. J Cancer Res Ther. 2015;11 Suppl:C248-C252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Kawakami H, Kuwatani M, Kawahata S. Peroral ultra-slim endoscopy-guided biliary drainage and stone extraction for postoperative upper gastrointestinal stenosis with a naïve papilla (with videos). J Hepatobiliary Pancreat Sci. 2015;22:571-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Park SW, Lee H, Park JC, Shin SK, Lee SK, Lee YC. Ultrathin endoscope-assisted self-expandable metallic stent placement following initial unsuccessful attempt in malignant upper gastrointestinal obstruction. Dig Endosc. 2014;26:200-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Shariff MK, Varghese S, O'Donovan M, Abdullahi Z, Liu X, Fitzgerald RC, di Pietro M. Pilot randomized crossover study comparing the efficacy of transnasal disposable endosheath with standard endoscopy to detect Barrett's esophagus. Endoscopy. 2016;48:110-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Baeg MK, Lim CH, Kim JS, Cho YK, Park JM, Lee BI, Lee IS, Choi MG. Portable disposable ultrathin endoscopy tested through percutaneous endoscopic gastrostomy. Medicine (Baltimore). 2016;95:e5423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Yoon EWT, Sakomoto M. Percutaneous endoscopic gastrostomy (PEG) using the modified introducer technique: clinical experience and description of an innovative new kit. Gastroenterol, Hepatol Endosc. 2016;1:97-99. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Sato Y, Goshi S, Kawauchi Y, Nishigaki Y, Mizuno KI, Hashimoto S, Takeuchi M, Kobayashi M, Ozawa T, Nishizawa M, Aoyagi Y. Safety of unsedated PEG placement using transoral ultrathin endoscopy in patients with amyotrophic lateral sclerosis. Nutr Neurosci. 2017;20:71-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Blevins CH, Egginton JS, Shah ND, Johnson ML, Iyer PG. Comparative Assessment of Patient Preferences and Tolerability in Barrett Esophagus Screening: Results From a Randomized Trial. J Clin Gastroenterol. 2018;52:880-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Castro Filho EC, Perazzo H, Guimaraes RAP, Machado L, Fernandes FF, Perez RM. Reliability and safety of transnasal compared to conventional endoscopy for detecting oesophageal varices in cirrhotic patients. Liver Int. 2018;38:1418-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Sami SS, Iyer PG, Pophali P, Halland M, di Pietro M, Ortiz-Fernandez-Sordo J, White JR, Johnson M, Guha IN, Fitzgerald RC, Ragunath K. Acceptability, Accuracy, and Safety of Disposable Transnasal Capsule Endoscopy for Barrett's Esophagus Screening. Clin Gastroenterol Hepatol. 2019;17:638-646.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Shariff MK, Bird-Lieberman EL, O'Donovan M, Abdullahi Z, Liu X, Blazeby J, Fitzgerald R. Randomized crossover study comparing efficacy of transnasal endoscopy with that of standard endoscopy to detect Barrett's esophagus. Gastrointest Endosc. 2012;75:954-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 15. | Moriarty JP, Shah ND, Rubenstein JH, Blevins CH, Johnson M, Katzka DA, Wang KK, Wongkeesong LM, Ahlquist DA, Iyer PG. Costs associated with Barrett's esophagus screening in the community: an economic analysis of a prospective randomized controlled trial of sedated versus hospital unsedated versus mobile community unsedated endoscopy. Gastrointest Endosc. 2018;87:88-94.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Saeian K, Staff DM, Vasilopoulos S, Townsend WF, Almagro UA, Komorowski RA, Choi H, Shaker R. Unsedated transnasal endoscopy accurately detects Barrett's metaplasia and dysplasia. Gastrointest Endosc. 2002;56:472-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Toyoizumi H, Kaise M, Arakawa H, Yonezawa J, Yoshida Y, Kato M, Yoshimura N, Goda K, Tajiri H. Ultrathin endoscopy versus high-resolution endoscopy for diagnosing superficial gastric neoplasia. Gastrointest Endosc. 2009;70:240-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Iddan G, Meron G, Glukhovsky A, Swain P. Wireless capsule endoscopy. Nature. 2000;405:417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1994] [Cited by in RCA: 1410] [Article Influence: 54.2] [Reference Citation Analysis (2)] |
| 19. | Triester SL, Leighton JA, Leontiadis GI, Fleischer DE, Hara AK, Heigh RI, Shiff AD, Sharma VK. A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with obscure gastrointestinal bleeding. Am J Gastroenterol. 2005;100:2407-2418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 421] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 20. | Luján-Sanchis M, Sanchis-Artero L, Larrey-Ruiz L, Peño-Muñoz L, Núñez-Martínez P, Castillo-López G, González-González L, Clemente CB, Albert Antequera C, Durá-Ayet A, Sempere-Garcia-Argüelles J. Current role of capsule endoscopy in Crohn's disease. World J Gastrointest Endosc. 2016;8:572-583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Doherty GA, Moss AC, Cheifetz AS. Capsule endoscopy for small-bowel evaluation in Crohn's disease. Gastrointest Endosc. 2011;74:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Zwinger LL, Siegmund B, Stroux A, Adler A, Veltzke-Schlieker W, Wentrup R, Jürgensen C, Wiedenmann B, Wiedbrauck F, Hollerbach S, Liceni T, Bojarski C. CapsoCam SV-1 Versus PillCam SB 3 in the Detection of Obscure Gastrointestinal Bleeding: Results of a Prospective Randomized Comparative Multicenter Study. J Clin Gastroenterol. 2019;53:e101-e106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Monteiro S, de Castro FD, Carvalho PB, Moreira MJ, Rosa B, Cotter J. PillCam® SB3 capsule: Does the increased frame rate eliminate the risk of missing lesions? World J Gastroenterol. 2016;22:3066-3068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | McAlindon ME, Ching HL, Yung D, Sidhu R, Koulaouzidis A. Capsule endoscopy of the small bowel. Ann Transl Med. 2016;4:369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Byrne MF, Donnellan F. Artificial intelligence and capsule endoscopy: Is the truly "smart" capsule nearly here? Gastrointest Endosc. 2019;89:195-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Li J, Leung WK. Colon capsule endoscopy for inflammatory bowel disease. J Dig Dis. 2018;19:386-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Pasha SF. Applications of Colon Capsule Endoscopy. Curr Gastroenterol Rep. 2018;20:22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Spada C, Pasha SF, Gross SA, Leighton JA, Schnoll-Sussman F, Correale L, González Suárez B, Costamagna G, Hassan C. Accuracy of First- and Second-Generation Colon Capsules in Endoscopic Detection of Colorectal Polyps: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2016;14:1533-1543.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 29. | Hall B, Holleran G, McNamara D. PillCam COLON 2(©) as a pan-enteroscopic test in Crohn's disease. World J Gastrointest Endosc. 2015;7:1230-1232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Ye CA, Gao YJ, Ge ZZ, Dai J, Li XB, Xue HB, Ran ZH, Zhao YJ. PillCam colon capsule endoscopy versus conventional colonoscopy for the detection of severity and extent of ulcerative colitis. J Dig Dis. 2013;14:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Gralnek IM, Adler SN, Yassin K, Koslowsky B, Metzger Y, Eliakim R. Detecting esophageal disease with second-generation capsule endoscopy: initial evaluation of the PillCam ESO 2. Endoscopy. 2008;40:275-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | di Pietro M, Canto MI, Fitzgerald RC. Endoscopic Management of Early Adenocarcinoma and Squamous Cell Carcinoma of the Esophagus: Screening, Diagnosis, and Therapy. Gastroenterology. 2018;154:421-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 181] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 33. | Gralnek IM, Ching JY, Maza I, Wu JC, Rainer TH, Israelit S, Klein A, Chan FK, Ephrath H, Eliakim R, Peled R, Sung JJ. Capsule endoscopy in acute upper gastrointestinal hemorrhage: a prospective cohort study. Endoscopy. 2013;45:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | McCarty TR, Afinogenova Y, Njei B. Use of Wireless Capsule Endoscopy for the Diagnosis and Grading of Esophageal Varices in Patients With Portal Hypertension: A Systematic Review and Meta-Analysis. J Clin Gastroenterol. 2017;51:174-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 35. | Slawinski PR, Obstein KL, Valdastri P. Capsule endoscopy of the future: What's on the horizon? World J Gastroenterol. 2015;21:10528-10541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 44] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 36. | Mahoney AW, Abbott JJ. Five-degree-of-freedom manipulation of an untethered magnetic device in fluid using a single permanent magnet with application in stomach capsule endoscopy. Int J Rob Res. 2016;35:129-147. [DOI] [Full Text] |
| 37. | Shamsudhin N, Zverev VI, Keller H, Pane S, Egolf PW, Nelson BJ, Tishin AM. Magnetically guided capsule endoscopy. Med Phys. 2017;44:e91-e111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 38. | Ching HL, Healy A, Thurston V, Hale MF, Sidhu R, McAlindon ME. Upper gastrointestinal tract capsule endoscopy using a nurse-led protocol: First reported experience. World J Gastroenterol. 2018;24:2893-2901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Zhao AJ, Qian YY, Sun H, Hou X, Pan J, Liu X, Zhou W, Chen YZ, Jiang X, Li ZS, Liao Z. Screening for gastric cancer with magnetically controlled capsule gastroscopy in asymptomatic individuals. Gastrointest Endosc. 2018;88:466-474.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 40. | Kong K, Yim S, Choi S, Jeon D. A robotic biopsy device for capsule endoscopy. J Med Device. 2012;6:31004. [RCA] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 41. | Ciuti G, Donlin R, Valdastri P, Arezzo A, Menciassi A, Morino M, Dario P. Robotic versus manual control in magnetic steering of an endoscopic capsule. Endoscopy. 2010;42:148-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 42. | Liao Z, Hou X, Lin-Hu EQ, Sheng JQ, Ge ZZ, Jiang B, Hou XH, Liu JY, Li Z, Huang QY, Zhao XJ, Li N, Gao YJ, Zhang Y, Zhou JQ, Wang XY, Liu J, Xie XP, Yang CM, Liu HL, Sun XT, Zou WB, Li ZS. Accuracy of Magnetically Controlled Capsule Endoscopy, Compared With Conventional Gastroscopy, in Detection of Gastric Diseases. Clin Gastroenterol Hepatol. 2016;14:1266-1273.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 161] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 43. | Ching HL, Hale MF, Kurien M, Campbell JA, Chetcuti Zammit S, Healy A, Thurston V, Hebden JM, Sidhu R, McAlindon ME. Diagnostic yield of magnetically assisted capsule endoscopy versus gastroscopy in recurrent and refractory iron deficiency anemia. Endoscopy. 2019;51:409-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 44. | Li Z, Chiu PW. Robotic Endoscopy. Visc Med. 2018;34:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 45. | Keller H, Juloski A, Kawano H, Bechtold M, Kimura A, Takizawa H, Kuth R. Method for navigation and control of a magnetically guided capsule endoscope in the human stomach. In: Biomedical Robotics and Biomechatronics (BioRob), 2012 4th IEEE RAS & EMBS International Conference on. IEEE. 2012;859–865. [DOI] [Full Text] |
| 46. | Lee CM, Engelbrecht CJ, Soper TD, Helmchen F, Seibel EJ. Scanning fiber endoscopy with highly flexible, 1 mm catheterscopes for wide-field, full-color imaging. J Biophotonics. 2010;3:385-407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 213] [Cited by in RCA: 134] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 47. | Seibel EJ, Brown CM, Dominitz JA, Kimmey MB. Scanning single fiber endoscopy: a new platform technology for integrated laser imaging, diagnosis, and future therapies. Gastrointest Endosc Clin N Am. 2008;18:467-478, viii. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 48. | Yelin D, White WM, Motz JT, Yun SH, Bouma BE, Tearney GJ. Spectral-domain spectrally-encoded endoscopy. Opt Express. 2007;15:2432-2444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 49. | Tearney GJ, Shishkov M, Bouma BE. Spectrally encoded miniature endoscopy. Opt Lett. 2002;27:412-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 50. | Yelin D, Bouma BE, Tearney GJ. Volumetric sub-surface imaging using spectrally encoded endoscopy. Opt Express. 2008;16:1748-1757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 51. | Miller SJ, Lee CM, Joshi BP, Gaustad A, Seibel EJ, Wang TD. Targeted detection of murine colonic dysplasia in vivo with flexible multispectral scanning fiber endoscopy. J Biomed Opt. 2012;17:021103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 52. | Templeton AW, Webb K, Hwang JH, Seibel EJ, Saunders M. Scanning fiber endoscopy: a novel platform for cholangioscopy. Gastrointest Endosc. 2014;79:1000-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 53. | Seibel EJ, Carroll RE, Dominitz JA, Johnston RS, Melville CD, Lee CM, Seitz SM, Kimmey MB. Tethered capsule endoscopy, a low-cost and high-performance alternative technology for the screening of esophageal cancer and Barrett's esophagus. IEEE Trans Biomed Eng. 2008;55:1032-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (2)] |
| 54. | Mahalati RN, Gu RY, Kahn JM. Resolution limits for imaging through multi-mode fiber. Opt Express. 2013;21:1656-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 55. | Wilson JMG, Jungner G. Principles and Practice of Screening for Disease. Public Health Paper No. 34 World Health Organization. 1968;. |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
P-Reviewer: Cerwenka H, Farkas DT S-Editor: Ma RY L-Editor: A E-Editor: Zhang YL