Published online Jun 14, 2019. doi: 10.3748/wjg.v25.i22.2799
Peer-review started: March 14, 2019
First decision: March 27, 2019
Revised: April 4, 2019
Accepted: April 29, 2019
Article in press: April 29, 2019
Published online: June 14, 2019
Processing time: 93 Days and 18.5 Hours
The imbalance of Th17/Treg cells and the IL-23/IL-17 axis have been confirmed to be associated with sepsis and various inflammatory diseases. Early enteral nutrition (EEN) can modulate the inflammatory response, improve immune dysfunction, and prevent enterogenic infection in critically ill patients; however, the precise mechanisms remain unclear. Considering the important roles of Th17 and Treg lymphocytes in the development of inflammatory and infectious diseases, we hypothesized that EEN could improve the immune dysfunction in sepsis by maintaining a balanced Th17/Treg cell ratio and by regulating the IL-23/IL-17 axis.
To investigate the effects of EEN on the Th17/Treg cell ratios and the IL-23/IL-17 axis in septic patients.
In this prospective clinical trial, patients were randomly divided into an EEN or delayed enteral nutrition (DEN) group. Enteral feeding was started within 48 h in the EEN group, whereas enteral feeding was started on the 4th day in the DEN group. The Th17 and Treg cell percentages and the interleukin levels were tested on days 1, 3, and 7 after admission. The clinical severity and outcome variables were also recorded.
Fifty-three patients were enrolled in this trial from October 2017 to June 2018. The Th17 cell percentages, Th17/Treg cell ratios, IL-17, IL-23, and IL-6 levels of the EEN group were lower than those of the DEN group on the 7th day after admission (P < 0.05). The duration of mechanical ventilation and of the intensive care unit stay of the EEN group were shorter than those of the DEN group (P < 0.05). However, no difference in the 28-d mortality was found between the two groups (P = 0.728).
EEN could regulate the imbalance of Th17/Treg cell ratios and suppress the IL-23/IL-17 axis during sepsis. Moreover, EEN could reduce the clinical severity of sepsis but did not reduce the 28-d mortality of septic patients.
Core tip: The aim of this prospective clinical trial was to investigate the effects of early enteral nutrition (EEN) on Th17/Treg cell ratios and the IL-23/IL-17 axis in septic patients. Patients were randomly divided into an EEN or delayed enteral nutrition (DEN) group. As a result, the Th17 cell percentages, Th17/Treg cell ratios, IL-17, IL-23, and IL-6 levels of the EEN group were lower than those of the DEN group on the 7th day after admission. In other words, EEN could regulate the imbalance of Th17/Treg cell ratios and suppress the IL-23/IL-17 axis during sepsis. Moreover, EEN could reduce the clinical severity but did not reduce the 28-d mortality of sepsis.
- Citation: Sun JK, Zhang WH, Chen WX, Wang X, Mu XW. Effects of early enteral nutrition on Th17/Treg cells and IL-23/IL-17 in septic patients. World J Gastroenterol 2019; 25(22): 2799-2808
- URL: https://www.wjgnet.com/1007-9327/full/v25/i22/2799.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i22.2799
As a severe systemic response to infection, sepsis is often accompanied by multiple organ dysfunction syndrome (MODS). Recent studies[1-4] have reported that immune disorders might play a vital role in the development of sepsis, and these disorders (immune excess or suppression) are closely related to T lymphocytes during both the early and late phases of sepsis. In addition to Th1 and Th2 lymphocytes, the roles of Th17 and Treg lymphocytes (which are also differentiated from CD4+ T cell subsets) in sepsis have been clarified gradually[5,6].
Mature Th17 cells cause a pro-inflammatory response, mainly by secreting IL-17 and IL-6, whereas Treg cells exert anti-inflammatory effects by secreting TGF-β and IL-10. The imbalance of Th17/Treg cells has been confirmed to be associated with sepsis and various inflammatory diseases[1,3,6]. It was reported that Th17 cells could be activated by another cytokine, IL-23 (belonging to the IL-12 family), which then increased the secretion of IL-17, forming a positive feedback loop[7,8]. This positive feedback loop of the IL-23/IL-17 axis has considerable roles in the progression of infection, cancer, and inflammatory bowel disease. Therefore, improving the imbalance of the Th17/Treg cells and the IL-23/IL-17 axis might be a valuable strategy for the treatment of sepsis.
As an essential therapeutic measure for sepsis, enteral nutrition (EN), especially early EN (EEN), can modulate the inflammatory response, improve immune dysfunction, and prevent enterogenic infection in critically ill patients[9-11]; however, the precise mechanisms remain unclear. Considering the important roles of Th17 and Treg lymphocytes in the development of inflammatory and infectious diseases, we hypothesized that EN could improve the immune dysfunction in sepsis by maintaining a balanced Th17/Treg cell ratio and by regulating the IL-23/IL-17 axis. Our previous retrospective study[12] also confirmed that EEN could improve the imbalance of Th1/Th2 and Th17/Treg cell ratios during the early stage of sepsis. However, it is unclear how EN affects the IL-23/IL-17 axis in septic patients. Moreover, due to the limitations of retrospective studies, a prospective clinical trial is still needed to verify our conclusions. Therefore, we hypothesized that EEN could decrease the Th17/Treg cell ratio and regulate the IL-23/IL-17 axis in septic patients, and this prospective randomized clinical study was performed to verify our hypothesis.
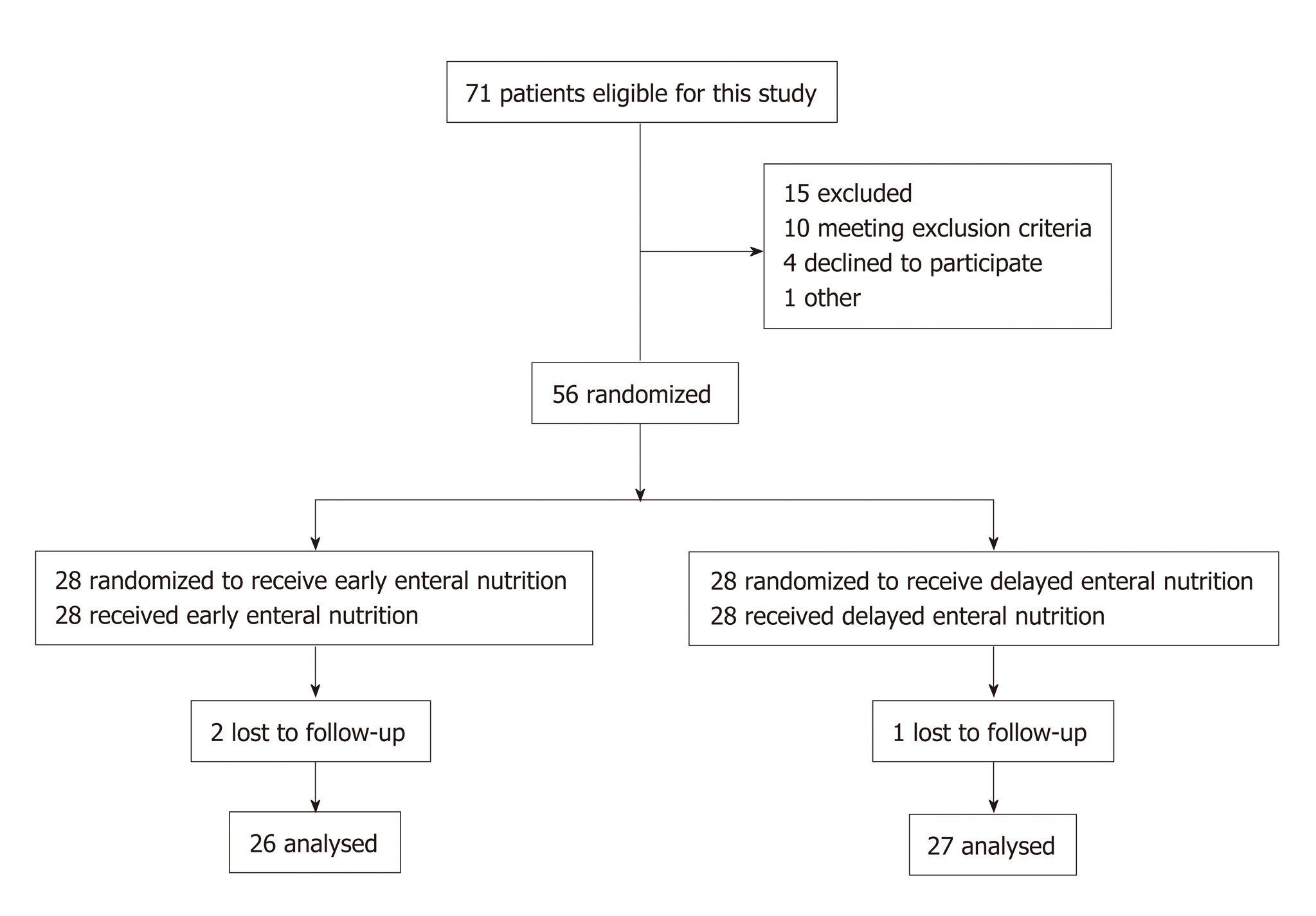
This was a prospective, single-center, and randomized clinical trial. Patients were randomly allocated to receive EEN or delayed EN (DEN) on admission. We used the simple randomization or complete randomization, which was based on the remainder grouping method of random numbers. After generating random numbers with a computer, the remainder grouping method (divided by two) was performed to decide which group (EEN or DEN) the patients would be assigned into. The treatment allocation was concealed before a patient was included in this study, and patients would not be removed from the study after treatment allocation became known. The main intervention difference of the two groups was the initial time of enteral feeding, therefore, it was not possible to blind treating physicians to the treatment allocation. The inclusive patients were blind to the treatment allocations. Therefore, this is a single-blind clinical trial. The sample size was calculated with the Power Analysis and Sample Size software (2011) before our study started; however, considering the small sample size of this study, we defined our trial as a prospective pilot study. The study protocol was approved by the Institutional Ethics Committee of Nanjing First Hospital (Approval Number: KY20170921-02), and informed consent was obtained from each patient’s first-degree relatives. This study was also registered at Clinical Trials.gov (ID: NCT03385850). Participation in the study did not necessitate any changes in treatment. Figure 1 shows the flow diagram of the participants.
From October 2017 to June 2018, all adult patients (age 18-70 years) admitted into the intensive care unit (ICU) of Nanjing First Hospital with a diagnosis of sepsis were included in this prospective clinical study. The diagnostic criteria for sepsis were in accordance with the surviving sepsis guidelines[13,14]. Patients with ileus or digestive tract hemorrhage, inflammatory bowel disease, severe abdominal hypertension (intra-abdominal pressure > 25 mmHg), chronic organ dysfunction (e.g., hepatic or renal dysfunction), malnutrition, or immunodeficiency, and patients with a history of long-term use of hormones were excluded. All patients received specialized treatments for sepsis as needed, including antimicrobial therapy, fluid resuscitation, vasopressor administration, oxygen administration, mechanical ventilation (MV), glucose control, and renal replacement therapy.
Before EN began, a nasogastric or nasojejunal feeding tube (size 10F, Flocare, Nutricia Ltd) was placed as needed. The nasojejunal tube was inserted using our novel method of blind bedside post-pyloric placement[15]. In the EEN group, enteral feeding was initiated within the first 24-48 h, whereas in the DEN group, enteral feeding was initiated on the 4th day after admission. A peptide-based formula (Peptisorb, Nutricia Ltd) was used in the first 24-48 hours, and if the patients were tolerant, whole protein formula (Nutrison Fibre, Nutricia Ltd) was used subsequently[11,12]. The goal intake was defined as 20-25 kcal/kg/day, and the protein need was defined as 1.2-2.0 g/kg/day[10]. The feeding rate was started at 15-20 mL/h and increased by 15-20 mL every 6-8 h. If patients were intolerant because of abdominal distension, diarrhea, or high gastric residual volume (>500 mL), we diluted the feeding concentration, slowed down the feeding rate, or used prokinetic agents.
Parenteral nutrition (PN) was supplemented if the enteral feedings alone could not meet > 60% of the energy and protein requirements after 7 d[10]. The caloric intake of PN was defined as 20-25 kcal/kg/day, and the calorie/nitrogen ratio was defined as 120-150:1. Fifty to seventy percent of the total energy intake was provided by glucose, whereas the supply of lipids was based on serum triglyceride levels. Moreover, sufficient insulin, electrolytes, vitamins, and trace elements were also added.
On admission, the baseline data, including sex, age, body-mass index, and the etiology of sepsis, were collected. The acute physiology and chronic health evaluation II (APACHE II) scores and sequential organ failure assessment (SOFA) scores were recorded on days 1, 3, and 7 after admission. Meanwhile, white blood cell (WBC) count (109/L), hemoglobin (g/L), total bilirubin (umol/L), and albumin (g/L) levels in peripheral blood were also recorded. The Th17 and Treg lymphocyte percentages, IL-17, IL-23, IL-6 and IL-10 levels in peripheral blood were tested on days 1, 3, and 7 after admission. Th17 and Treg lymphocytes were measured by flow cytometry. After human peripheral blood mononuclear cells were isolated, the proliferation analysis of Th17 cell subpopulations was performed by using a BD PharmingenTM Human Th17 Phenotyping Kit (BD Biosciences, United States), and the Treg cell subpopulation was detected by using CD4 (antigen presenting cells), CD25 (PE), and Foxp3 (Fluorescein isothiocyanate)-labeled antibodies (e-Bioscience, United States). Serum IL-17, IL-23, IL-6, and IL-10 cytokines were detected with commercially available Human Quantikine enzyme-linked immunosorbent assays (ELISA) kits (R and D Systems, Bio-Techne Corporation, United States) according to the manufacturer’s instructions. The sensitivity of ELISA kits was 15 pg/mL for IL-17, 16.3 pg/mL for IL-23, 0.7 pg/mL for IL-6, and 3.9 pg/mL for IL-10. The assay range of ELISA kits was 31.2-2000 pg/mL for IL-17, 39.0-2500 pg/mL for IL-23, 3.1-300 pg/mL for IL-6, and 7.8-500 pg/mL for IL-10. In addition, clinical outcome variables including the 28-d mortality, days in the ICU, days of MV, and the number of patients receiving continuous renal replacement therapy (CRRT) were also recorded.
The Kolmogorov-Smirnov test was first performed to test the normal distribution of the data. Normally distributed data are expressed as the mean ± standard deviation and were compared by t-tests. Abnormally distributed data are expressed as medians (interquartile ranges) and were compared by the Mann-Whitney U test or Kruskal-Wallis test. Categorical variables are presented as absolute numbers or percentages and were analyzed using the χ2 test or Fisher’s exact test. Survival curves for up to 28 d after admission were generated using the Kaplan–Meier method and were compared by the log-rank test. IBM SPSS software (version 22.0, NY, United States) was used for statistical analyses, and P < 0.05 was considered statistically significant. To take into account the repeated nature of variables, analysis of variance (ANOVA) for repeated measurements of the general linear model was implemented. The statistical methods of this study were reviewed by Qiao Liu, a biostatistician from the Center for Disease Control and Prevention of Jiangsu Province in China.
As shown in Figure 1, a total of 53 septic patients were enrolled in this randomized clinical trial during the study period. Of these patients, 26 received EEN and 27 received DEN. The demographic data and clinical parameters of the patients on admission are presented in Table 1. Seven (13.2%) patients received CRRT, and 10 (18.9%) patients died of MODS or infectious complications during the hospital stay (within 28 d).
| EEN group | DEN group | P-value | |
| (n = 26) | (n = 27) | ||
| Age (yr) | 58.85 ± 10.48 | 57.30 ± 14.82 | 0.663 |
| Sex (Male:Female) | 18:8 | 18:9 | 0.842 |
| Etiology (n, %) | |||
| Abdominal infection | 11 (42.3%) | 14 (51.9%) | 0.487 |
| Thoracic/Pulmonary infection | 10 (38.5%) | 9 (33.3%) | 0.697 |
| Urinary infection | 3 (11.5%) | 2 (7.4%) | 0.669 |
| Mucocutaneous infection | 2 (7.7%) | 2 (7.4%) | 1.000 |
| BMI | 25.25 ± 5.97 | 24.24 ± 4.15 | 0.476 |
| APACHE II | 21.69 ± 6.24 | 22.19 ± 5.69 | 0.765 |
| SOFA | 9.08 ± 1.94 | 9.44 ± 3.82 | 0.659 |
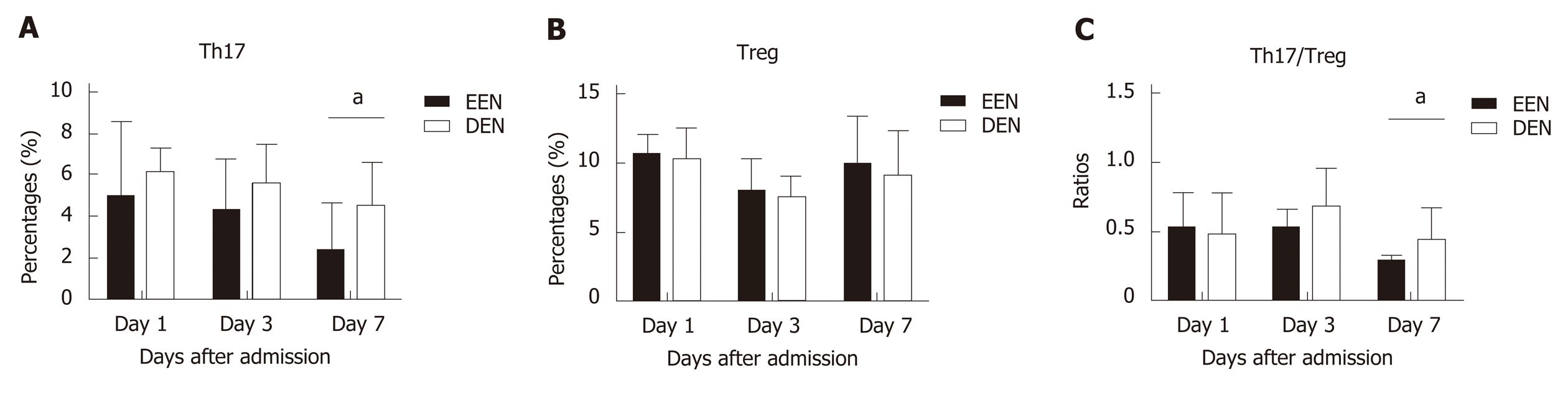
Figure 2 shows the difference in the Th17 and Treg lymphocyte percentages and the Th17/Treg cell ratios between the two groups on days 1, 3, and 7 after admission. As shown in Figure 2A, patients in the EEN group had a significantly lower Th17 cell percentage on the 7th day (P = 0.002) after admission compared to that in the DEN group. Similar results were also found for the Th17/Treg cell ratios (P = 0.01) (Figure 2C). However, no significant difference in the Treg cell percentages was found during the 7 d after admission (P > 0.05) between the two groups (Figure 2B).
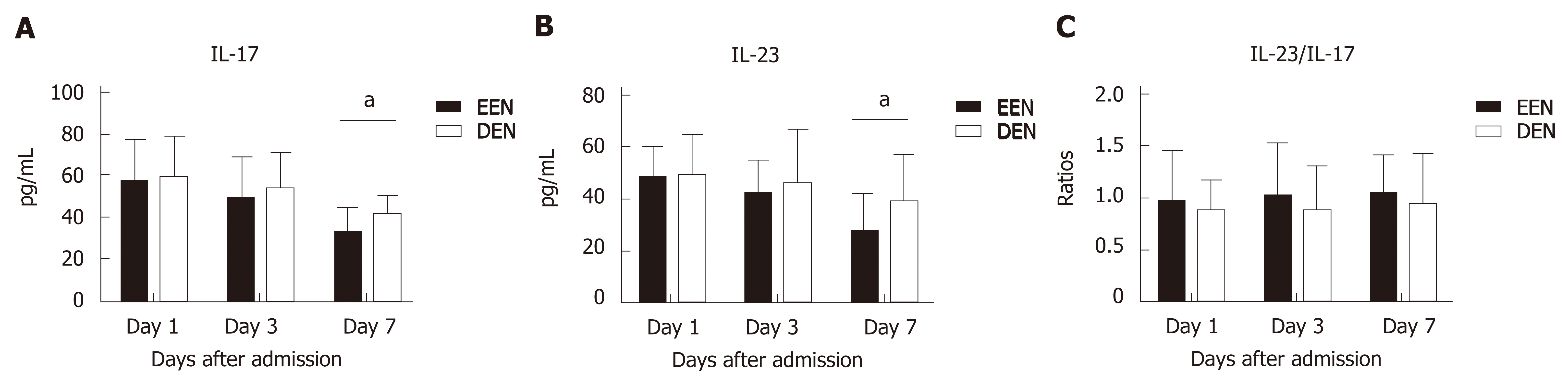
Figure 3 shows the difference in the serum IL-17 and IL-23 levels and IL-23/IL-17 ratios between the two groups on days 1, 3, and 7 after admission. As shown in Figure 3A, patients in the EEN group had a significantly lower IL-17 level on the 7th day (P = 0.01) after admission compared to that of the DEN group. Similar results were also found for the IL-23 levels (P = 0.016) (Figure 2B). No significant difference in the IL-23/IL-17 ratios was found during the 7 days after admission between the two groups (P > 0.05) (Figure 3C), and the results indicated that the expression of the members of the IL-23/IL-17 axis was simultaneously suppressed in both groups.
Figure 4 shows the difference in serum IL-6 and IL-10 levels between the two groups on days 1, 3, and 7 after admission. As shown in Figure 4A, patients in the EEN group had a significantly lower IL-6 level on the 7th day (P < 0.001) after admission compared to that in the DEN group. However, no significant difference in the IL-10 levels was found during the 7 d after admission (P > 0.05) between the two groups (Figure 4B).
Table 2 shows the difference in the clinical severity variables between the two groups during the ICU stay. The APACHE II and SOFA scores of the EEN group were significantly lower than those of the DEN group on the 7th day (P < 0.05). The duration (days) of MV and ICU stay of the EEN group were also significantly shorter than those of the DEN group (P < 0.05). However, no significant difference in the number of patients receiving CRRT was found between the two groups (4/26 vs 3/27, P = 0.704).
| EEN group | DEN group | P-value | ||
| (n = 26) | (n = 27) | |||
| APACHE II | Day 1 | 21.69 ± 6.24 | 22.19 ± 5.69 | 0.765 |
| Day 3 | 17.54 ± 26 | 19.04 ± 4.92 | 0.199 | |
| Day 7 | 11.73 ± 4.82 | 15.11 ± 3.90 | <0.001 | |
| SOFA | Day 1 | 9.08 ± 1.94 | 9.44 ± 3.82 | 0.659 |
| Day 3 | 6.31 ± 1.52 | 7.30 ± 3.30 | 0.132 | |
| Day 7 | 3.58 ± 0.99 | 5.22 ± 2.33 | <0.001 | |
| MV days | 4.50 ± 2.58 | 7.15 ± 3.95 | 0.006 | |
| Number of CRRT (n, %) | 4 (15.4%) | 3 (11.1%) | 0.704 | |
| ICU days | 8.31 ± 4.26 | 11.22 ± 5.43 | 0.035 | |
The effects of EEN on WBC counts, hemoglobin, total bilirubin, and albumin levels were also investigated, and we found that EEN had a tendency of decreasing WBC count (9.57 ± 3.12 vs 12.03 ± 5.53, P = 0.051) and total bilirubin (14.04 ± 11.06 vs 20.14 ± 18.21, P = 0.146) on the 7th day after admission. Moreover, EEN also had a tendency of increasing albumin level (33.51 ± 3.75 vs 31.47 ± 3.82, P = 0.055) on the 7th day after admission. No similar tendency on hemoglobin (106.73 ± 16.53 vs 105.56 ± 23.60, P = 0.835) was found during the 7 d after admission between the two groups. It may be part of the reasons why EEN can decrease APACH II and SOFA scores on 7th day after admission.
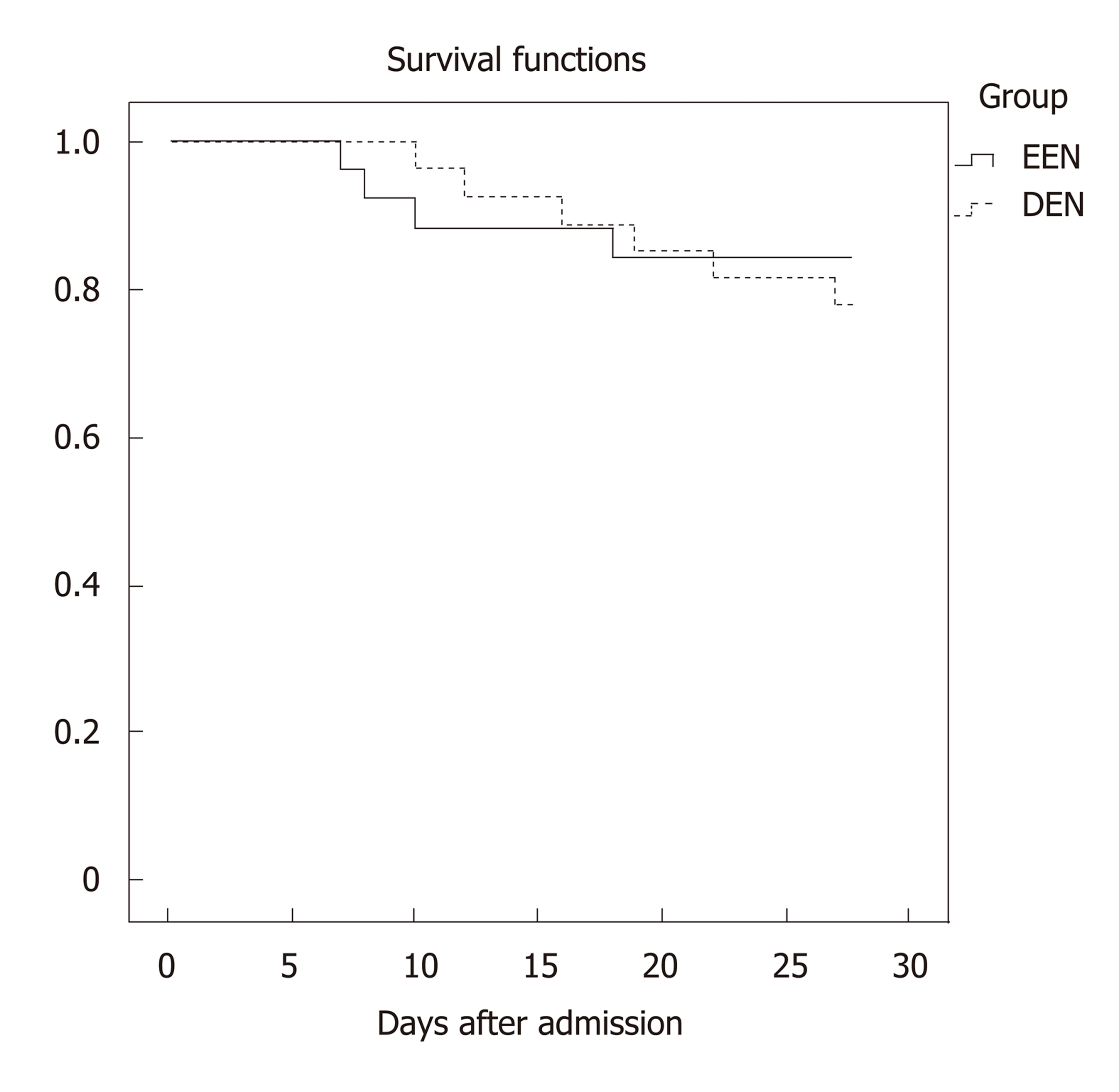
During the 28 d of admission, 4 (15.4%) of 26 patients in the EEN group and 6 (22.2%) of 27 patients in the DEN group died of MODS or infectious complications. As shown in Figure 5, no difference in the 28-d mortality was found between the EEN and DEN groups (P = 0.728).
This prospective clinical pilot study investigated the effects of EEN on the Th17/Treg cell ratios and the IL-23/IL-17 axis in septic patients. We found that EEN could decrease the Th17 lymphocyte percentages, Th17/Treg cell ratios, serum IL-17, IL-23, and IL-6 levels and could thus suppress the immune overactivation during the early stage of sepsis. Moreover, EEN could improve the clinical disease severity but could not reduce the 28-d mortality of septic patients.
Despite the fact that the surviving sepsis guidelines recommended a series of therapeutic methods to treat sepsis, its mortality is still approximately 20%-50% in adults[13,14]. Previous studies have shown that immune dysregulation plays an important role in the development of sepsis, and T lymphocytes, especially Th17 and Treg cells, are the main mediators of this dysregulation[2,5,6]. Li et al[2] found that the Th17/Treg cell ratios were reduced in septic patients, and Wu et al[4] reported that higher Th17 differentiation was associated with lower mortality in sepsis. Therefore, the imbalance of Th17/Treg cells is considered a crucial pathogeny of sepsis[1,3,6]. An animal study[5] showed that Xuebijing injection (a Chinese herbal medicine) regulated the balance of Treg and Th17 cells and improved survival in septic shock. Another clinical study[6] reported that the Th17/Treg cell imbalance in sepsis patients could be attenuated by high-volume hemofiltration. However, no consensus has been reached on how to modulate the imbalance of Th17/Treg cells. Our previous retrospective study[12] revealed that EEN could improve the imbalance of Th17/Treg cell ratios during the early stage of sepsis, and this prospective study also supported this conclusion. These findings provide a new and easier strategy for treating the imbalance of Th17/Treg cells.
However, the percentage of T lymphocytes is not representative of their function. T cells trigger an inflammatory response mainly by secreting relevant cytokines. Th17 cells mainly express the pro-inflammatory factors IL-17 and IL-6, whereas Treg cells mainly express anti-inflammatory factors such as TGF-β and IL-10. Accordingly, we compared the difference in the serum IL-17, IL-6, and IL-10 levels between the EEN and DEN groups, and we found that EEN could decrease the IL-17 and IL-6 levels but not the IL-10 levels during sepsis compared to those of the DEN group. The results indicated that EEN ameliorated immune dysfunction by preventing the release of pro-inflammatory cytokines, but it did not increase the release of anti-inflammatory cytokines during the early stage of sepsis. This conclusion is in accordance with our previous study[11].
Moreover, previous studies showed that Th17 lymphocytes could be activated by IL-23 and increase the secretion of IL-17 in a positive feedback loop[7,8]. This positive feedback loop of the IL-23/IL-17 axis could promote the acute inflammatory reaction and the progression of infectious disease. Cauvi et al[7] reported that the increased activation of the IL-23/IL-17 pathway had detrimental effects on sepsis-induced lung inflammation. Another study[8] suggested that the determination of the preoperative Th17 cytokine (IL-23 and IL-17) mRNA levels might be useful for predicting the development of sepsis after radical cystectomy. Therefore, monoclonal antibodies that target IL-23 or IL-17 have been tested for the treatment of sepsis[16,17]; however, no consensus has been reached because of the conflicting results of the experiments and clinical studies. In this study, we found that EEN could decrease the serum IL-17 and IL-23 levels during the early stage of sepsis. Our findings indicated that EEN might improve the immune dysfunction in sepsis by regulating the IL-23/IL-17 axis.
The intestinal tract is considered to be an important immune organ[18]. Recent studies have reported that the gut immune function was closely associated with enteral feeding, and a lack of enteral stimulation could lead to immune imbalance[19,20]. Our previous trials also found that EEN could improve the immune imbalance of patients with sepsis or severe acute pancreatitis[11,12], but the underlying mechanisms were still not clear. In this study, we explored the potential mechanisms of the regulation of immune function by EEN by examining T lymphocytes and the IL-23/IL-17 axis in sepsis. We found that EEN improved the imbalance of the Th17/Treg cell ratios and suppressed the IL-23/IL-17 axis in sepsis compared to those of the DEN group. To the best of our knowledge, this is the first randomized clinical study to investigate the relationship between EEN and the IL-23/IL-17 axis in septic patients, and our results might provide a new mechanism by which EEN improves immune imbalance, as well as provide a new and easier strategy for treating the immune imbalance in sepsis.
In addition, the present study also compared the difference in the clinical outcome variables between the EEN and DEN groups. We found that EEN could reduce the duration of MV and ICU stay, as well as the APACHE II and SOFA scores, but not the 28-d mortality of septic patients compared to those of the DEN group. The results indicated that EEN can suppress the immune overactivation and improve the clinical disease severity during the early stage of sepsis. However, the main intervention difference of the two groups was the initial time of enteral feeding in this study, thus patients would receive same treatments (especially enteral nutrition) for sepsis after the acute stage. Therefore, no difference about the mortality was observed on the 28th day after admission. Moreover, this discrepancy occurred probably because this small sample trail was not powered to detect a difference in mortality. The conclusions are in accordance with previous studies[12,20].
Several limitations of this study should be discussed. Due to the single-center design and small sample size, our results might be unable to provide reliable conclusions, and the accuracy of these results should be examined with large-scale clinical studies. Moreover, because this study was not based on pathophysiological models, the precise mechanisms of EEN on the Th17/Treg cell ratios and IL-23/IL-17 axis should be examined by future basic science investigations. Finally, because our immune variables were only recorded for one week, the later effects of EEN on sepsis should be studied with further clinical trials.
In conclusion, our clinical pilot study found that EEN could improve the imbalance of the Th17/Treg cell ratios and suppress the IL-23/IL-17 axis during the early stage of sepsis. Moreover, EEN could reduce the clinical severity but not the 28-day mortality of septic patients. Further basic experiments or large-scale randomized controlled trials are needed to validate our results.
The imbalance of Th17/Treg cells and IL-23/IL-17 axis have been confirmed to be associated with sepsis and various inflammatory diseases. Early enteral nutrition (EEN) can modulate the inflammatory response, improve immune dysfunction, and prevent enterogenic infection in critically ill patients; however, the precise mechanisms remain unclear. Therefore, we hypothesized that EEN could improve the immune dysfunction in sepsis by maintaining a balanced Th17/Treg cell ratio and by regulating the IL-23/IL-17 axis.
The aim of this study was to test the hypothesis that EEN could improve the immune dysfunction in sepsis by maintaining a balanced Th17/Treg cell ratio and by regulating the IL-23/IL-17 axis.
The main objective of this prospective clinical trial was to investigate the effects of EEN on Th17/Treg cell ratios and IL-23/IL-17 axis in septic patients.
In this prospective clinical trial, patients were randomly divided into an EEN or delayed enteral nutrition (DEN) group. Enteral feeding was started within 48 h in the EEN group, whereas enteral feeding was started on the 4th day in the DEN group. The Th17 and Treg cell percentages and the interleukin levels were tested on days 1, 3, and 7 after admission. The clinical severity and outcome variables were also recorded.
Fifty-three patients were enrolled in this trial from October 2017 to June 2018. The Th17 cell percentages, Th17/Treg cell ratios, IL-17, IL-23, and IL-6 levels of the EEN group were significantly lower than those of the DEN group on the 7th day after admission (P < 0.05). The duration of mechanical ventilation and of the intensive care unit stay of the EEN group were significantly shorter than those of the DEN group (P < 0.05). However, no significant difference in the 28-d mortality was found between the two groups (P = 0.728).
EEN could regulate the imbalance of Th17/Treg cell ratios and suppress the IL-23/IL-17 axis during sepsis. Moreover, EEN could reduce the clinical severity of sepsis but did not reduce the 28-d mortality of septic patients.
More large-scale clinical studies and basic science investigations should be performed to test the accuracy of our results in future.
The authors thank Qiao Liu for her assistance in the statistical analysis of this study. The authors also thank Shen X, Zou L, Liu Y, Liu H, Meng C, Xu QL, Chen YM, and Song XC at the Department of Intensive Care Unit of Nanjing First Hospital for their contributions to this study.
| 1. | Gupta DL, Bhoi S, Mohan T, Galwnkar S, Rao DN. Coexistence of Th1/Th2 and Th17/Treg imbalances in patients with post traumatic sepsis. Cytokine. 2016;88:214-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 2. | Li J, Li M, Su L, Wang H, Xiao K, Deng J, Jia Y, Han G, Xie L. Alterations of T helper lymphocyte subpopulations in sepsis, severe sepsis, and septic shock: A prospective observational study. Inflammation. 2015;38:995-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 3. | Feng P, Yan R, Dai X, Xie X, Wen H, Yang S. The alteration and clinical significance of Th1/Th2/Th17/Treg cells in patients with multiple myeloma. Inflammation. 2015;38:705-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Wu HP, Chung K, Lin CY, Jiang BY, Chuang DY, Liu YC. Associations of T helper 1, 2, 17 and regulatory T lymphocytes with mortality in severe sepsis. Inflamm Res. 2013;62:751-763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | Chen X, Feng Y, Shen X, Pan G, Fan G, Gao X, Han J, Zhu Y. Anti-sepsis protection of Xuebijing injection is mediated by differential regulation of pro- and anti-inflammatory Th17 and T regulatory cells in a murine model of polymicrobial sepsis. J Ethnopharmacol. 2018;211:358-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 6. | Guo J, Tao W, Tang D, Zhang J. Th17/regulatory T cell imbalance in sepsis patients with multiple organ dysfunction syndrome: Attenuated by high-volume hemofiltration. Int J Artif Organs. 2017;40:607-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Cauvi DM, Williams MR, Bermudez JA, Armijo G, De Maio A. Elevated expression of IL-23/IL-17 pathway-related mediators correlates with exacerbation of pulmonary inflammation during polymicrobial sepsis. Shock. 2014;42:246-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Tulic C, Lazic M, Savic E, Popadic D, Djukic J, Spasic D, Markovic M, Ramic Z, Mostarica-Stojkovic M, Trajkovic V. The preoperative activity of Th1 and Th17 cytokine axes in prediction of sepsis after radical cystectomy. Eur Cytokine Netw. 2011;22:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Reintam Blaser A, Starkopf J, Alhazzani W, Berger MM, Casaer MP, Deane AM, Fruhwald S, Hiesmayr M, Ichai C, Jakob SM, Loudet CI, Malbrain ML, Montejo González JC, Paugam-Burtz C, Poeze M, Preiser JC, Singer P, van Zanten AR, De Waele J, Wendon J, Wernerman J, Whitehouse T, Wilmer A, Oudemans-van Straaten HM; ESICM Working Group on Gastrointestinal Function. Early enteral nutrition in critically ill patients: ESICM clinical practice guidelines. Intensive Care Med. 2017;43:380-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 346] [Cited by in RCA: 497] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 10. | Taylor BE, McClave SA, Martindale RG, Warren MM, Johnson DR, Braunschweig C, McCarthy MS, Davanos E, Rice TW, Cresci GA, Gervasio JM, Sacks GS, Roberts PR, Compher C; Society of Critical Care Medicine; American Society of Parenteral and Enteral Nutrition. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). Crit Care Med. 2016;44:390-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 454] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 11. | Sun JK, Mu XW, Li WQ, Tong ZH, Li J, Zheng SY. Effects of early enteral nutrition on immune function of severe acute pancreatitis patients. World J Gastroenterol. 2013;19:917-922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 90] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 12. | Sun JK, Yuan ST, Mu XW, Zhang WH, Liu Y, Zou L, Wang X, Zheng SY. Effects of early enteral nutrition on T helper lymphocytes of surgical septic patients: A retrospective observational study. Medicine (Baltimore). 2017;96:e7702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit Care Med. 2017;45:486-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1784] [Cited by in RCA: 2028] [Article Influence: 225.3] [Reference Citation Analysis (1)] |
| 14. | Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:801-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15803] [Cited by in RCA: 18775] [Article Influence: 1877.5] [Reference Citation Analysis (4)] |
| 15. | Sun JK, Wang X, Yuan ST. A novel method of blind bedside placement of postpyloric tubes. Crit Care. 2018;22:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23-IL-17 immune axis: From mechanisms to therapeutic testing. Nat Rev Immunol. 2014;14:585-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 972] [Cited by in RCA: 1264] [Article Influence: 105.3] [Reference Citation Analysis (0)] |
| 17. | Bosmann M, Ward PA. Therapeutic potential of targeting IL-17 and IL-23 in sepsis. Clin Transl Med. 2012;1:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Kamada N, Núñez G. Regulation of the immune system by the resident intestinal bacteria. Gastroenterology. 2014;146:1477-1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 192] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 19. | Hegazi RA, DeWitt T. Enteral nutrition and immune modulation of acute pancreatitis. World J Gastroenterol. 2014;20:16101-16105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Nguyen NQ, Besanko LK, Burgstad C, Bellon M, Holloway RH, Chapman M, Horowitz M, Fraser RJ. Delayed enteral feeding impairs intestinal carbohydrate absorption in critically ill patients. Crit Care Med. 2012;40:50-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
P-Reviewer: Cheungpasitporn W, Cheungpasitporn T S-Editor: Yan JP L-Editor: Wang TQ E-Editor: Zhang YL