Published online Jun 14, 2019. doi: 10.3748/wjg.v25.i22.2788
Peer-review started: February 6, 2019
First decision: February 21, 2019
Revised: April 22, 2019
Accepted: April 29, 2019
Article in press: April 29, 2019
Published online: June 14, 2019
Processing time: 134 Days and 10.5 Hours
Inflammatory bowel disease (IBD), a chronic inflammatory disease of the gastrointestinal tract, could play a role in the pathophysiology of atrial fibrillation (AF).
To investigate the association between IBD and AF development.
We performed a population-based cohort study using records in the Korean National Health Insurance Services database between 2010 and 2014. A total of 37696 patients with IBD (12349 with Crohn’s disease and 25397 with ulcerative colitis) were identified. The incidence rate of newly diagnosed AF in patients with IBD was compared with that in a 3 times larger cohort of 113088 age- and sex-matched controls without IBD.
During 4.9 ± 1.3 years of follow-up, 1120 patients newly diagnosed with AF (348 in the IBD group and 772 in controls) were identified. After adjustments using multivariable Cox proportional hazards, patients with IBD were at a 36% [95% confidence interval (CI) 20%-54%] higher risk of AF than controls. The association between IBD and the development of AF was stronger in younger than in older patients. Patients without cardiovascular risk factors showed a higher risk of AF primarily. Additionally, patients receiving immun-omodulators [Hazard ration (HR) 1.46, 95%CI 1.31-1.89], systemic corticosteroids (HR 1.37, 95%CI 1.10-1.71), or biologics agents (HR 2.38, 95%CI 1.51-3.75) were at higher risk of AF than patients without them.
IBD significantly increased the risk of AF, and the impact of IBD on developing AF was in patients with moderate to severe disease.
Core tip: Inflammatory bowel disease (IBD), a chronic inflammatory disease of the gastrointestinal tract, was strongly associated with an increased risk of atrial fibrillation (AF). Both Crohn’s disease (CD) and ulcerative colitis (UC) increase the risk of AF, with a higher risk in patients with CD than UC. And the impact of IBD on developing AF was stronger in patients receiving immunomodulators, systemic corticosteroids or biologics agents, which are prescribed for moderate-to-severe disease than patients without them. Therefore, physicians need to consider screening for AF in patients with IBD, particularly those who use more potent therapies.
- Citation: Choi YJ, Choi EK, Han KD, Park J, Moon I, Lee E, Choe WS, Lee SR, Cha MJ, Lim WH, Oh S. Increased risk of atrial fibrillation in patients with inflammatory bowel disease: A nationwide population-based study. World J Gastroenterol 2019; 25(22): 2788-2798
- URL: https://www.wjgnet.com/1007-9327/full/v25/i22/2788.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i22.2788
Inflammatory bowel disease (IBD), including ulcerative colitis (UC) and Crohn’s disease (CD), is a chronic progressive inflammatory disease that can affect the mucosa in any part of the gastrointestinal tract[1]. IBD is associated with an increased risk of cardiovascular diseases such as stroke, myocardial infarction, and early athero-sclerosis with increased intimal thickness of the common carotid artery[2,3]. High levels of C-reactive protein (CRP), a major marker reflecting activity and severity of inflammation in IBD, are also associated with atherogenesis and atherosclerotic cardiovascular disease[4-6].
Atrial fibrillation (AF) is the most common arrhythmia observed in clinical practice. Recently, inflammation is being recognized as a pathogenic contributor to the development of AF[7]. Previous research has shown a significant association between serum inflammatory mediators such as CRP, tumor necrosis factor-α, interleukin (IL)-2, IL-6, and IL-8, and the development and persistence of AF[8,9]. Several cardiovascular disorders, notably coronary atherosclerosis, are associated with inflammation, and cytokines are known to affect plaque rupture and thrombus formation resulting in myocardial infarction[10]. However, it is unclear whether autoimmune-mediated inflammation contributes to the development and persistence of AF.
Considering that pathogenesis of AF is being increasingly linked to systemic inflammation, IBD may a potential risk factor for AF. However, there is limited information regarding the association between the risk of development of AF and the presence of IBD. Therefore, we performed a nationwide population-based study to investigate the association between IBD and the development of AF, including in young individuals and those without cardiovascular risk factors.
Most Koreans (97.2%, approximately 50 million individuals) are enrolled in the mandatory National Health Insurance Service (NHIS) provided by the Korean government. This database includes all healthcare utilization-related information, including in-patient and outpatient services, diagnoses, and prescriptions recorded for reimbursement by the Health Insurance and Review Agency.
Additionally, a registration program for Rare Intractable Diseases (RID), a part of the NHIS, offers special support to patients with RID including IBD, since 2006. The diagnostic accuracy of IBD based on the RID registry was 95.5%-100% and 92.5%-95.5% in terms of sensitivity and specificity, respectively, as assessed by a previous epidemiological study[11]. This study used data from the NHIS and the RID program database, which stores diagnoses in the form of the International Classification of Disease, Tenth Revision, Clinical Modification (ICD-10-CM) codes.
We identified patients diagnosed with IBD between January 1, 2010 and December 31, 2014 from the NHIS database (ICD-10-CM codes: K50.0-50.9 for CD and K51.0-51.9 for UC) and confirmed these diagnoses based on special codes (V130 for CD and V131 for UC) provided by the RID program. The special codes of the RID program are assigned to a patient with IBD who meets the following diagnostic criteria determined by certified physicians: (1) Diarrhea and abdominal pain lasting more than 16 wk; (2) Endoscopic features corresponding to IBD; and (3) Histopathological confirmation based on endoscopic biopsy. We excluded patients who had been diagnosed with AF prior to or within a month from the initial diagnosis of IBD.
The primary endpoint was new-onset non-valvular AF, that occurred during the follow-up period. AF was defined using ICD-10-CM codes (I480-I484, I489), which were registered by the physicians responsible for diagnosis. To ensure diagnostic accuracy and exclude patients with transient AF, patients with AF were defined as those with AF, which have been diagnosed with discharge, or had been confirmed more than twice in an outpatient clinic. In addition, to include only non-valvular AF, patients diagnosed with mitral stenosis (I050, I052, I059) or those with mechanical heart valves (Z952-Z954) were and excluded from the analysis. The definition of AF was validated in previous nationwide studies[12-14].
Baseline comorbidities and medication history were obtained from the medical claims data in the NHIS database by ICD-10-CM and prescription codes. We investigated the comorbidities of patients when they were first enrolled in the RID registry due to IBD. Baseline comorbidities included hypertension, diabetes mellitus (DM), dyslipidemia, ischemic heart disease, venous thromboembolism, end-stage renal disease (ESRD) and hyperthyroidism. Definitions of covariates were verified from previous papers and have been summarized in S1 Table[12,13]. Low income was defined as participation in the medical aid program or a monthly income corresponding to the lowest 25% of the total population. Cities with a population > 1 million were considered urban areas.
Of note, 5-aminosalicylic acid (5-ASA) is the preferred primary drug used to treat mild-to-moderate IBD in Asians, while drugs such as immunomodulators (aza-thioprine, methotrexate, cyclosporine, and tacrolimus) or biological agents (infliximab, adalimumab, and golimumab) are recommended as first- or second-line therapy for moderate-to severe IBD[15]. Therefore, we estimated the severity of IBD based on a previous report of describing treatment of IBD; those receiving systemic corticosteroids in combination with 5-ASA, immunomodulators (azathioprine, methotrexate, cyclosporine, and tacrolimus) or biological agents (infliximab, adalimumab, and golimumab) were classified as having moderate-to-severe IBD in this study[16].
Demographic and baseline clinical characteristics are reported as means with standard deviation (SD) for normally distributed continuous variables and as numbers with percentages for categorical variables. The independent samples t-test was used for continuous variables and the chi-square test for categorical variables for intergroup comparisons.
The incidence rates (IR) of AF are presented per 1000 person-years and were calculated as the number of patients with AF divided by the total population for each year because the total population changes every year owing to death and emigration. The cumulative IR of AF in patients with IBD and matched control group were compared using Kaplan–Meier censoring estimates and the log-rank test. Univariate and multivariate Cox proportional hazards regression models were used to evaluate the association between IBD and the risk of development of AF. Adjusted hazard ratios (HR) have been presented with a 95% confidence interval (CI) and adjusted for known risk factors of AF and IBD, including age, sex, place, income, DM, hypertension, dyslipidemia, stroke, ischemic heart disease, venous thromboembolism, and ESRD. A two-sided P value < 0.05 was considered statistically significant. All statistical analyses were performed using the SAS software version 9.3 (SAS Institute, Cary, NC, United States) and the SPSS software version 23.0 (SPSS Inc., Chicago, IL, United States).
The NHIS database is an open database with patient information anonymized to protect privacy. This information is accessible to all researchers whose study protocols have been approved by the official review committee. Our study was performed in accordance with the Declaration of Helsinki.
We enrolled 37696 patients with IBD (12349 patients with CD and 25347 patients with UC). We selected a 3-fold larger group of age and sex-matched individuals without IBD (total 113088 patients: 37047 matched with patients with CD and 76041 matched with patients with UC) as the control group.
Baseline clinical characteristics of patients have been summarized in Table 1. The percentage of patients with IBD was greater in urban than in rural areas (50.4 vs 46.8%, respectively, P < 0.001). Additionally, the percentage of patients with IBD showed a higher income level (23.3 vs 19.8% respectively, P < 0.001). Prevalence of DM, hypertension, and dyslipidemia was lower in patients with IBD than in the control group, whereas prevalence of ischemic heart disease, venous thrombo-embolism, ESRD, and hyperthyroidism was higher in those with IBD than in the control group (P < 0.001 for all comparisons except dyslipidemia). Among patients with IBD, those with CD were younger than those with UC (30.4 ± 14.2 years vs 43.8 ± 15.6 years), and the former group members more frequently received immunomodulators (55.8 vs 13.0%), systemic corticosteroid (58.64 vs 56.67%) and biological agents (13.3 vs 1.7%).
| Variable, n (%) | IBD group | Control group | P value | CD group | Control group | P value | UC group | Control group | P value |
| Number | 37696 | 113088 | 12349 | 37047 | 25347 | 76041 | |||
| Men | 22985 (61.0) | 68955 (61.0) | 1 | 8679 (70.3) | 26037 (70.3) | 1 | 14306 (56.4) | 42918 (56.4) | 1 |
| Age (mean ± SD, yr) | 39.4 ± 16.4 | 39.42 ± 16.4 | 1 | 30.4 ± 14.2 | 30.36 ± 14.23 | 1 | 43.8 ± 15.6 | 43.8 ± 15.6 | 1 |
| < 30 | 12184 (32.3) | 36552 (32.3) | 1 | 7080 (57.3) | 21240 (57.3) | 1 | 5104 (20.1) | 15312 (20.1) | 1 |
| 30-44 | 11459 (30.4) | 34377 (30.4) | 3354 (27.2) | 10062 (27.2) | 8105 (32.0) | 24315 (32.0) | |||
| 45-59 | 9158 (24.3) | 27474 (24.3) | 1292 (10.5) | 3876 (10.5) | 7866 (31.0) | 23598 (31.0) | |||
| ≥ 60 | 4895 (13.0) | 14685 (13.0) | 623 (5.0) | 1869 (5.0) | 4272 (16.9) | 12816 (16.9) | |||
| Urban residence | 19006 (50.4) | 52913 (46.8) | < 0.001 | 6317 (51.2) | 17416 (47.0) | < 0.001 | 12689 (50.1) | 35497 (46.7) | < 0.001 |
| Low income | 7444 (19.8) | 26338 (23.3) | < 0.001 | 2609 (21.1) | 8535 (23.0) | < 0.001 | 4835 (19.1) | 17803 (23.4) | < 0.001 |
| Comorbidities | |||||||||
| Diabetes mellitus | 1483 (3.9) | 5306 (4.7) | < 0.001 | 260 (2.1) | 782 (2.1) | 0.97 | 1223 (4.8) | 4524 (6.0) | < 0.001 |
| Hypertension | 4098 (10.9) | 13732 (12.1) | < 0.001 | 595 (4.8) | 2097 (5.7) | 0.000 | 3503 (13.8) | 11635 (15.3) | < 0.001 |
| Dyslipidemia | 2527 (6.7) | 7929 (7.0) | 0.042 | 361 (2.9) | 1250 (3.4) | 0.015 | 2166 (8.6) | 6679 (8.8) | 0.245 |
| IHD | 1557 (4.1) | 3559 (3.2) | < 0.001 | 348 (2.8) | 552 (1.5) | < 0.001 | 1209 (4.8) | 3007 (4.0) | < 0.001 |
| Thromboembolism | 115 (0.3) | 236 (0.2) | < 0.001 | 37 (0.3) | 34 (0.1) | < 0.001 | 78 (0.3) | 202 (0.3) | 0.269 |
| ESRD | 107 (0.3) | 180 (0.2) | < 0.001 | 48 (0.4) | 40 (0.1) | < 0.001 | 59 (0.2) | 140 (0.2) | 0.130 |
| Hyperthyroidism | 750 (2.0) | 1248 (1.1) | < 0.001 | 224 (1.8) | 264 (0.7) | < 0.001 | 526 (2.1) | 984 (1.3) | < 0.001 |
| Medications | |||||||||
| Immunomodulators | 10199 (27.1) | 0 | < 0.001 | 6895 (55.8) | 0 | < 0.001 | 3304 (13.0) | 0 | < 0.001 |
| Steroids | 21606 (57.3) | 0 | < 0.001 | 7241 (58.6) | 0 | < 0.001 | 14365 (56.7) | 0 | < 0.001 |
| Biological agents | 2075 (5.5) | 0 | < 0.001 | 1641 (13.3) | 0 | < 0.001 | 434 (1.7) | 0 | < 0.001 |
| Follow-up duration (yr) | 4.9 ± 1.3 | 4.9 ± 1.3 | 4.9 ± 1.3 | 4.8 ± 1.3 | 4.9 ± 1.3 | 4.9 ± 1.3 |
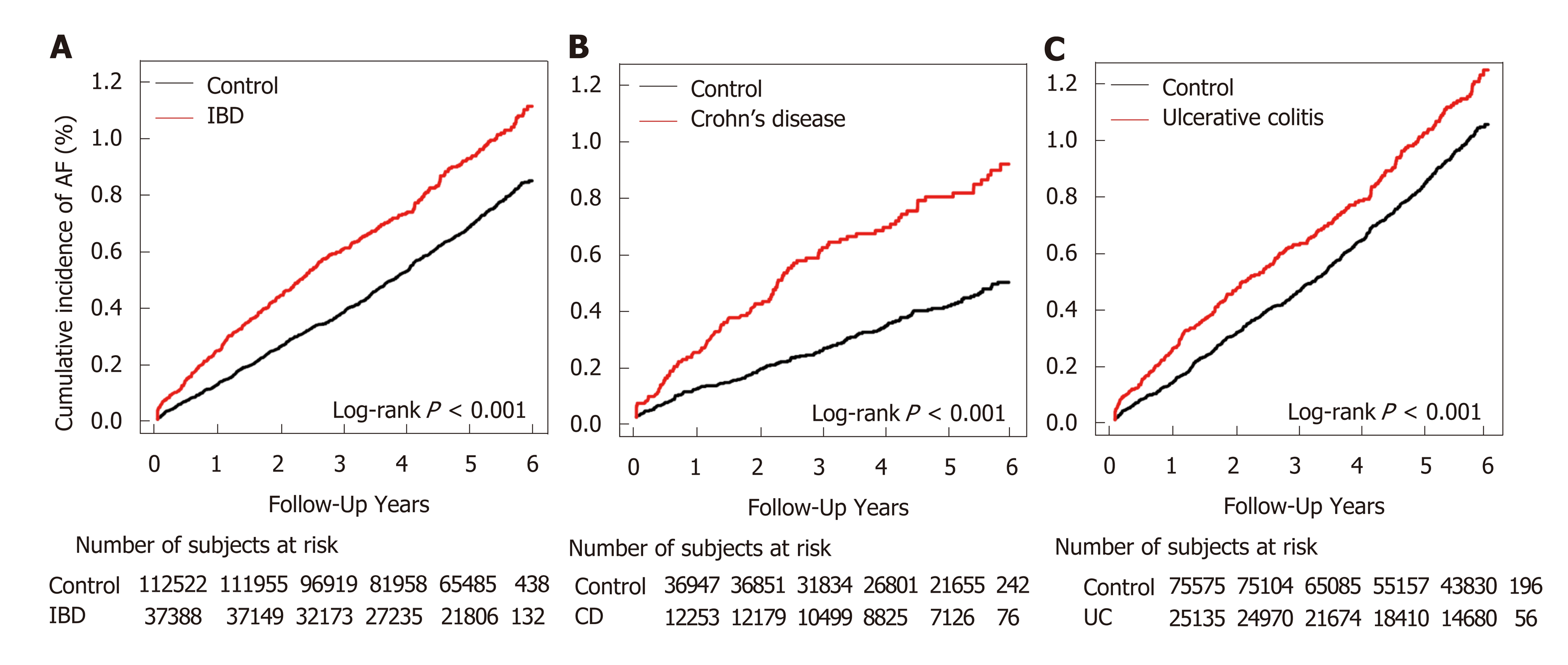
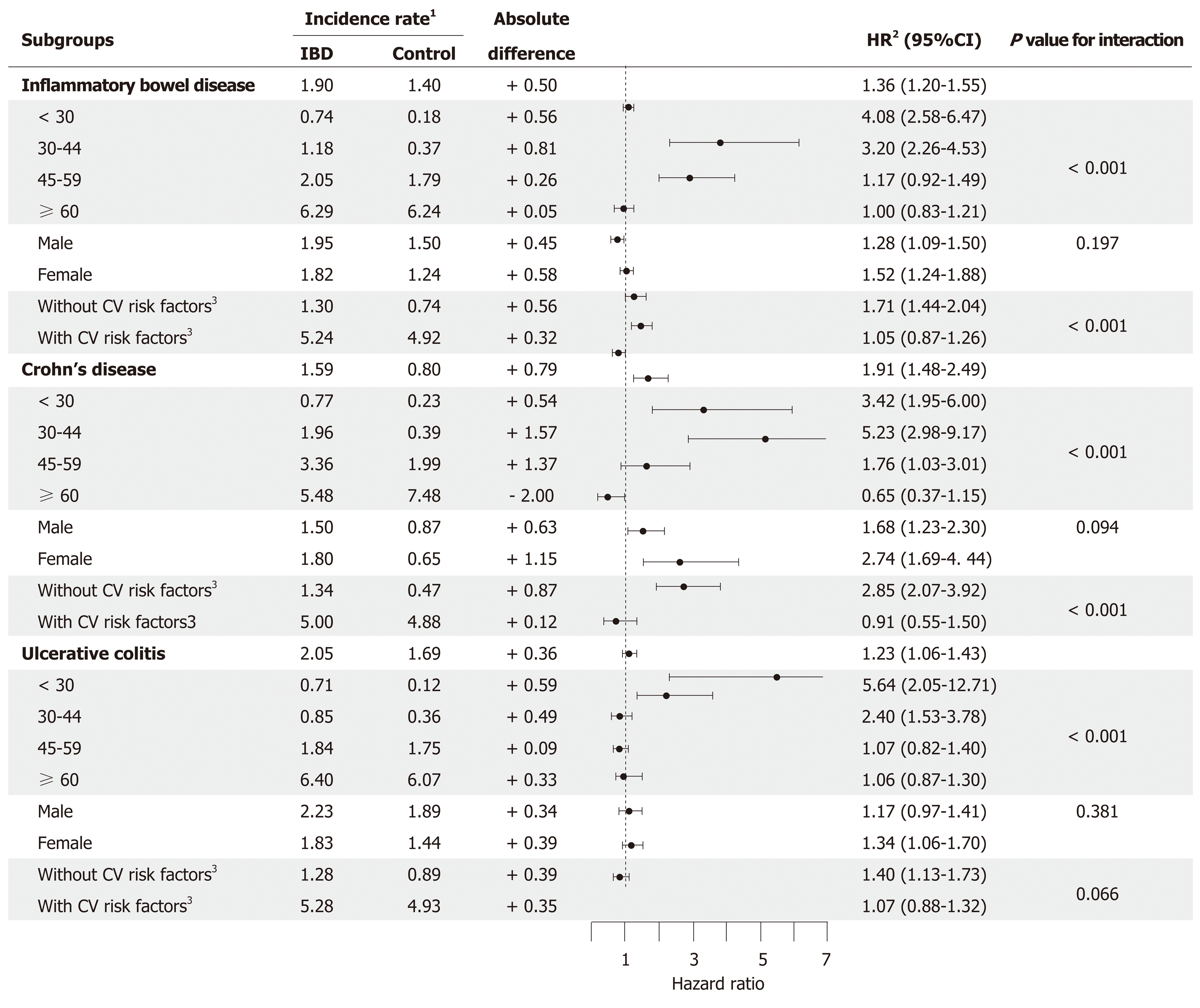
During the mean follow-up of 4.87 ± 1.28 years, we identified 1120 cases of incident AF (IBD group: 348; control group: 772), with a crude IR of 1.90 per 1000 person-years in patients with IBD and 1.40 in matched controls. The incidence of AF was higher in the IBD group than in the control group (P < 0.001 by the log-rank test, Figure 1). After adjustment for potential confounding factors, IBD was significantly associated with the incidence of AF (HR 1.36, 95%CI 1.20-1.55, Table 2). Within the IBD group, AF was significantly associated with both CD and UC compared to matched non-IBD subgroups (CD: HR 1.91, 95%CI 1.47-2.49; UC: HR 1.24, 95%CI 1.07-1.43, Table 2).
Additionally, patients who received medications (immunomodulators, systemic corticosteroid, and biological agents) for moderate-to-severe IBD showed a higher risk of AF than those who did not receive such medications (Table 3). The highest risk of AF was observed in the subgroup receiving biological agents (HR 2.37, 95%CI 1.50-3.74) compared with those receiving immunomodulators (HR 1.45, 95%CI 1.13-1.88) and systemic corticosteroids (HR 1.37, 95%CI 1.10-1.71).
| Outcome | Immunomodulators | Systemic steroid | Biologic agents | |||
| Yes (n = 10199) | No (n = 27497) | Yes (n = 21606) | No (n = 16090) | Yes (n = 2075) | No (n = 35621) | |
| Atrial fibrillation | 85 | 263 | 227 | 121 | 21 | 327 |
| Incidence rate1 | 1.72 | 1.97 | 2.21 | 1.50 | 2.16 | 1.89 |
| Adjusted HR2 (95%CI) | 1.46 (1.13-1.89) | 1 (reference) | 1.37 (1.10-1.71) | 1 (reference) | 2.38 (1.51-3.75) | 1 (reference) |
Both patients with UC and CD subgroups showed an increased risk of AF (Figure 2). Patients with IBD aged below 45 years showed a higher risk of AF than those belonging to the control group, and both UC and CD groups showed similar trend in age subgroups. In contrast, no significant difference was observed in those aged more than or equal to 45 years. Also, the risk of AF development was not significantly different between men and women. The association between IBD and AF was weaker in subgroups with well-known cardiovascular risk factors (hypertension, DM, and dyslipidemia) than in subgroups without such risk factors (P < 0.001). These differences were statistically more significant in patients with CD (P < 0.001) than in those with UC (P = 0.07).
This study is the first large population-based cohort study to investigate the association between IBD and the incidence of AF in Asians. The primary findings of our large population-based cohort study are: (1) IBD was associated with an increased risk of AF development; (2) both CD and UC increase the risk of AF, with a higher risk in patients with CD than UC; (3) patients receiving immunomodulators, systemic corticosteroids, and/or biological agents showed a higher risk of AF; and (4) the relative risk of IBD for the development of AF was particularly high in younger patients (aged below 45 years) and in those without cardiovascular risk factors.
Several previous studies have demonstrated the relationship between IBD and AF[17,18]. A small-scale study comprising 141 IBD cases showed that the prevalence of AF was higher across all age groups in patients with IBD than in the control group[17]. In a Danish nationwide study, 24499 patients with new-onset IBD were identified and compared with age- and sex-matched controls[18]. Overall IBD-associated risk of AF was increased by 26% (95%CI, 16%-36%). We observed that the risk of AF was higher in patients with IBD, which was in agreement with previous studies. Interestingly, the absolute risk of AF in patients with CD was higher than that in patients with UC (HR for CD and UC being 1.92 and 1.23, respectively).
There is a reasonable body of evidence to support the pathophysiological features between IBD and the development of AF. Systemic inflammation is known to be a significant contributor to the development of AF[7-9,19]. Recent studies performed in patients with IBD have demonstrated that atrial electromechanical conduction time is prolonged and that structural and electrophysiological changes occurring in atrial tissues affect left atrial electromechanical function[20,21]. Several previous studies have suggested that systemic inflammation is linked to various pathological processes such as oxidative stress, apoptosis, and fibrosis of cardiomyocytes, all of which lead to structural and electrical remodeling of the atria, promoting the development and persistence of AF[19,22,23]. Moreover, increases in the level of serum inflammatory markers, such as CRP and IL-6, were observed in patient with AF and IBD, especially CD[8,24]. Chronic autoimmune and inflammatory diseases such as rheumatoid arthritis, ankylosing spondylitis, and psoriasis are known to increase the risk of development of AF[25-27].
The positive relationship between the severity of IBD and the incidence of AF accounts for the association between the two diseases. In a Danish nationwide study, IBD was classified as flare-up, persistent disease, and remission based on disease activity, and the association between disease activity of IBD and the development of AF were evaluated[18]. Incidence of AF increased during IBD flare-ups and periods of persistent disease, but not during periods of remission[18]. Particular medication such as immunomodulators, systemic corticosteroid, and biological agents are recommended as first or second-line therapy for moderate-to-severe IBD, and 5-ASA is commonly used for the initial treatment of mild IBD[28-30]. Therefore, in large population-based studies with difficulty in obtaining clinical symptoms and sign, it is preferable to assess the severity of IBD using medical history instead of a clinical scoring system. In this study, patients with IBD who received these aforementioned agents may have a moderate-to-severe disease, and have shown a higher risk of AF. This suggests that patients with moderate-to-severe IBD have a higher risk of AF than those with mild IBD.
Advancing age is a well-known risk factor for AF[31]. Surprisingly, however, in our study, the association between IBD to the development of AF gradually weakened with increasing age. Additionally, the association between IBD and AF was more significant in patients without cardiovascular risk factors including DM, hyper-tension, and dyslipidemia.
Several studies performed in Western population cohorts have reported a bimodal distribution of the incidence of IBD with a peak at a younger age (20-30 years) for CD, a later peak (30-40 years) for UC, and a lower second peak at an older age (60–70 years) for both diseases[32]. In contrast, in Asians including Koreans, the incidence of CD showed a peak in patients aged below 20 years and steadily decreased after that, whereas the incidence of UC was observed to plateau in patients aged 25-69 years[11]. In the present study, the prevalence of AF showed a peak at a younger age, and the association between IBD and the development of AF gradually became weaker with increasing age. These findings suggested that systemic inflammatory disease is one of the mechanisms contributing to the development of AF different from classic risk factors.
The incidence of IBD in Korea (2.9-3.6 per 100000 for CD and 4.2-5.1 per 100000 for UC) was the highest in East Asia, followed by that in Japan, China, and Hong Kong from 2006 to 2012[33,34]. The prevalence of IBD in Korea is steadily rising, and IBD-related healthcare costs are showing a significant increase[35]. This study is the first to demonstrate that IBD is an independent risk factor for the development of AF in Asians.
As well as having the increased IR with age, Asians have a generally higher incidence of AF-related risks of stroke and intracranial bleeding, compared with non-Asians[36,37]. The incidence rate ratio (IRR) of AF in patients with IBD in this Korean population-based cohort was not significantly different to that in patients with IBD in the previously described Danish population-based cohort (Korea: IRR 1.36, 95%CI 1.20-1.55; Denmark: IRR 1.26, 95%CI 1.16-1.36) after fully adjusting for risk factors of AF[18]. The lower prevalence of AF in Asians (approximately 1%) than that observed in non-Asians (approximately 2%) suggests that the risk of incident AF in patients with IBD is similar in Asians and non-Asians[12,38]. Our findings add new evidence that IBD, a systemic inflammatory disease, is associated with an increased prevalence of AF in Asians.
The primary limitation of this study is the observational population-based retrospective design, which does not permit specific determination of the severity of IBD. Therefore, detailed information regarding the severity, extent, location, and activity of the IBD, laboratory data, and duration of treatment modalities were not available in our study. Although we used certain prescriptions as a surrogate measure of moderate-to-severe IBD, there is still the limitation in discriminating between those with moderate-to-severe disease and those with remission.
Additionally, the role of confounders including general condition, presence of malnutrition, smoking, and other significant comorbidities such as cardiomyopathy, heart failure, and cancer could not be accurately evaluated in patients. However, this study used the certified database to identify patients diagnosed with IBD and to retrieve their medication history. The database of the RID program includes the complete medical history of patients, which is required to provide special financial support, thereby minimizing the possibility of inaccuracies in prescription data.
Secondly, the definitions of diseases were determined using ICD-10-CM codes of claims data from the NHIS database. Misclassification is a potential bias when using diagnostic codes. Also, when determining cases of IBD, the ascertainment bias could be induced depending on the patient’s residences. Additionally, the detail information on AF subtypes such as paroxysmal, persistent, permanent, and perioperative AF could not be accurately identified in our database.
Moreover, we could not obtain accurate information regarding the time interval between the diagnosis of IBD and the onset of AF. However, patients were followed-up for more than 4 years, which is sufficient to detect AF caused by IBD. We excluded patients demonstrating new-onset AF within a year of being diagnosed with IBD to reduce the chance of coincidental occurrence of the disease.
In conclusions, the key finding of our study is that IBD was associated with an increased risk of AF. Furthermore, IBD-associated AF was more common in younger patients with less comorbidities. Therefore, although IBD is a common disease in young people, we need to consider screening for AF, as timely intervention can reduce both morbidity and mortality secondary to the complications of AF.
Systemic inflammatory disease is known to increase the risk of cardiovascular diseases such as stroke, myocardial infarction, and atherosclerosis. Also, it has recently emerged as a risk factor for atrial fibrillation (AF). Inflammatory bowel disease (IBD) is a chronic progressive inflammatory disease, which can affect the gastrointestinal tract. Considering that pathogenesis of AF is linked to systemic inflammation, IBD might be a potential risk factor for AF de-velopment.
Recently, the incidence and prevalence of IBD have increased in the young Asian population. The impact of IBD on cardiovascular disease is an important issue. Therefore, we need to clarify the relationship between IBD and AF development in the Asian population and young patients
We aimed to investigate the association between IBD and AF development in Asians.
We performed a population-based cohort study using the certified claim database form the Koran National Health Insurance Services between 2010 and 2014. The special codes of the rare and intractable disease program were used to define the patient, which include the complete medical history of patients. A total of 37696 patients with IBD (12349 with Crohn’s disease and 25397 with ulcerative colitis) were identified. The primary endpoint was new-onset AF, which occurred among IBD patients without a previous history of AF during the follow-up period. AF was defined using the International Classification of Disease, Tenth Revision, Clinical Modification codes (I480-I484, I489).
During 4.9 ± 1.3 years of follow-up, 1120 patients newly diagnosed with AF (348 in the IBD group and 772 in controls). IBD patients had a 36% (95% confidence interval 20%-54%) higher risk of AF than controls. The association between IBD and the development of AF was stronger in younger than in older patients, and in patients without cardiovascular risk factors. Additionally, moderate-severe IBD patients, who received immunomodulators, systemic corticosteroids, or biologics agents were at higher risk of AF. These findings supported that systemic inflammatory disease could be an independent risk factor for AF development. However, further studies on the impact of other systemic inflammation on AF need to be performed to provide conclusive evidence.
IBD, chronic systemic disease, significantly increase the risk of AF development in Asians, as similar to the Western population. Moreover, the impact of IBD on AF incident was higher in young patients and those without cardiovascular risk factors. Therefore, our findings supported that systemic inflammatory disease is an independent risk factor for AF, even in patients without classic cardiovascular risk factors.
It is necessary to pay attention to the occurrence of cardiovascular diseases such as AF in patients with IBD, even in young age. In particular, a patient receiving immunomodulators, systemic corticosteroids, or biologics agents for moderate-severe disease, had a higher risk of AF development than those without.
| 1. | Thia KT, Loftus EV, Sandborn WJ, Yang SK. An update on the epidemiology of inflammatory bowel disease in Asia. Am J Gastroenterol. 2008;103:3167-3182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 410] [Article Influence: 22.8] [Reference Citation Analysis (1)] |
| 2. | Papa A, Danese S, Urgesi R, Grillo A, Guglielmo S, Roberto I, Bonizzi M, Guidi L, De Vitis I, Santoliquido A, Fedeli G, Gasbarrini G, Gasbarrini A. Early atherosclerosis in patients with inflammatory bowel disease. Eur Rev Med Pharmacol Sci. 2006;10:7-11. [PubMed] |
| 3. | Rungoe C, Basit S, Ranthe MF, Wohlfahrt J, Langholz E, Jess T. Risk of ischaemic heart disease in patients with inflammatory bowel disease: A nationwide Danish cohort study. Gut. 2013;62:689-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 177] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 4. | Poullis AP, Zar S, Sundaram KK, Moodie SJ, Risley P, Theodossi A, Mendall MA. A new, highly sensitive assay for C-reactive protein can aid the differentiation of inflammatory bowel disorders from constipation- and diarrhoea-predominant functional bowel disorders. Eur J Gastroenterol Hepatol. 2002;14:409-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 76] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Vermeire S, Van Assche G, Rutgeerts P. C-reactive protein as a marker for inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:661-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 355] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 6. | Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Taubert K, Tracy RP, Vinicor F; Centers for Disease Control and Prevention; American Heart Association. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4411] [Cited by in RCA: 4758] [Article Influence: 206.9] [Reference Citation Analysis (0)] |
| 7. | Aviles RJ, Martin DO, Apperson-Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, Tracy RP, Van Wagoner DR, Psaty BM, Lauer MS, Chung MK. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006-3010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 972] [Cited by in RCA: 1122] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 8. | Psychari SN, Apostolou TS, Sinos L, Hamodraka E, Liakos G, Kremastinos DT. Relation of elevated C-reactive protein and interleukin-6 levels to left atrial size and duration of episodes in patients with atrial fibrillation. Am J Cardiol. 2005;95:764-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 245] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 9. | Patel P, Dokainish H, Tsai P, Lakkis N. Update on the association of inflammation and atrial fibrillation. J Cardiovasc Electrophysiol. 2010;21:1064-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 146] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 10. | Willerson JT, Ridker PM. Inflammation as a cardiovascular risk factor. Circulation. 2004;109:II2-I10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 552] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 11. | Kim HJ, Hann HJ, Hong SN, Kim KH, Ahn IM, Song JY, Lee SH, Ahn HS. Incidence and natural course of inflammatory bowel disease in Korea, 2006-2012: A nationwide population-based study. Inflamm Bowel Dis. 2015;21:623-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 150] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 12. | Lee SR, Choi EK, Han KD, Cha MJ, Oh S. Trends in the incidence and prevalence of atrial fibrillation and estimated thromboembolic risk using the CHA2DS2-VASc score in the entire Korean population. Int J Cardiol. 2017;236:226-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 147] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 13. | Kang SH, Choi EK, Han KD, Lee SR, Lim WH, Cha MJ, Cho Y, Oh IY, Oh S. Underweight is a risk factor for atrial fibrillation: A nationwide population-based study. Int J Cardiol. 2016;215:449-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 14. | Lee SR, Choi EK, Rhee TM, Lee HJ, Lim WH, Kang SH, Han KD, Cha MJ, Cho Y, Oh IY, Oh S. Evaluation of the association between diabetic retinopathy and the incidence of atrial fibrillation: A nationwide population-based study. Int J Cardiol. 2016;223:953-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Sung JJ, Kamm MA, Marteau P. Asian perspectives in the management of inflammatory bowel disease: Findings from a recent survey. J Gastroenterol Hepatol. 2010;25:183-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Nakase H, Keum B, Ye BD, Park SJ, Koo HS, Eun CS. Treatment of inflammatory bowel disease in Asia: The results of a multinational web-based survey in the 2(nd) Asian Organization of Crohn's and Colitis (AOCC) meeting in Seoul. Intest Res. 2016;14:231-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Pattanshetty DJ, Anna K, Gajulapalli RD, Sappati-Biyyani RR. Inflammatory bowel "Cardiac" disease: Point prevalence of atrial fibrillation in inflammatory bowel disease population. Saudi J Gastroenterol. 2015;21:325-329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Kristensen SL, Lindhardsen J, Ahlehoff O, Erichsen R, Lamberts M, Khalid U, Torp-Pedersen C, Nielsen OH, Gislason GH, Hansen PR. Increased risk of atrial fibrillation and stroke during active stages of inflammatory bowel disease: A nationwide study. Europace. 2014;16:477-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 19. | Guo Y, Lip GY, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol. 2012;60:2263-2270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 488] [Cited by in RCA: 668] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 20. | Efe TH, Cimen T, Ertem AG, Coskun Y, Bilgin M, Sahan HF, Pamukcu HE, Yayla C, Sunman H, Yuksel I, Yeter E. Atrial Electromechanical Properties in Inflammatory Bowel Disease. Echocardiography. 2016;33:1309-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Nar G, Ergul B, Aksan G, Inci S. Assessment of Atrial Electromechanical Delay and Left Atrial Mechanical Functions in Patients with Ulcerative Colitis. Echocardiography. 2016;33:970-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Harada M, Van Wagoner DR, Nattel S. Role of inflammation in atrial fibrillation pathophysiology and management. Circ J. 2015;79:495-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 378] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 23. | Kourliouros A, Savelieva I, Kiotsekoglou A, Jahangiri M, Camm J. Current concepts in the pathogenesis of atrial fibrillation. Am Heart J. 2009;157:243-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 129] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 24. | Saverymuttu SH, Hodgson HJ, Chadwick VS, Pepys MB. Differing acute phase responses in Crohn's disease and ulcerative colitis. Gut. 1986;27:809-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 104] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Lindhardsen J, Ahlehoff O, Gislason GH, Madsen OR, Olesen JB, Svendsen JH, Torp-Pedersen C, Hansen PR. Risk of atrial fibrillation and stroke in rheumatoid arthritis: Danish nationwide cohort study. BMJ. 2012;344:e1257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 143] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 26. | Rhee TM, Lee JH, Choi EK, Han KD, Lee H, Park CS, Hwang D, Lee SR, Lim WH, Kang SH, Cha MJ, Cho Y, Oh IY, Oh S. Increased Risk of Atrial Fibrillation and Thromboembolism in Patients with Severe Psoriasis: A Nationwide Population-based Study. Sci Rep. 2017;7:9973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 27. | Moon I, Choi EK, Jung JH, Han KD, Choi YJ, Park J, Cho JH, Lee E, Choe W, Lee SR, Cha MJ, Lim WH, Oh S. Ankylosing spondylitis: A novel risk factor for atrial fibrillation - A nationwide population-based study. Int J Cardiol. 2019;275:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 28. | Ye BD, Yang SK, Shin SJ, Lee KM, Jang BI, Cheon JH, Choi CH, Kim YH, Lee H; IBD Study Group of the Korean Association for the Study of the Intestinal Diseases. [Guidelines for the management of Crohn's disease]. Korean J Gastroenterol. 2012;59:141-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 29. | Choi CH, Kim YH, Kim YS, Ye BD, Lee KM, Lee BI, Jung SA, Kim WH, Lee H; IBD Study Group of the Korean Association for the Study of Intestinal Diseases. [Guidelines for the management of ulcerative colitis]. Korean J Gastroenterol. 2012;59:118-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Mowat C, Cole A, Windsor A, Ahmad T, Arnott I, Driscoll R, Mitton S, Orchard T, Rutter M, Younge L, Lees C, Ho GT, Satsangi J, Bloom S; IBD Section of the British Society of Gastroenterology. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2011;60:571-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1045] [Cited by in RCA: 966] [Article Influence: 64.4] [Reference Citation Analysis (0)] |
| 31. | Feinberg WM, Blackshear JL, Laupacis A, Kronmal R, Hart RG. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch Intern Med. 1995;155:469-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1296] [Cited by in RCA: 1254] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 32. | Loftus EV, Sandborn WJ. Epidemiology of inflammatory bowel disease. Gastroenterol Clin North Am. 2002;31:1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 307] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 33. | Ng WK, Wong SH, Ng SC. Changing epidemiological trends of inflammatory bowel disease in Asia. Intest Res. 2016;14:111-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 243] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 34. | Yang SK, Yun S, Kim JH, Park JY, Kim HY, Kim YH, Chang DK, Kim JS, Song IS, Park JB, Park ER, Kim KJ, Moon G, Yang SH. Epidemiology of inflammatory bowel disease in the Songpa-Kangdong district, Seoul, Korea, 1986-2005: A KASID study. Inflamm Bowel Dis. 2008;14:542-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 383] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 35. | Kim JW, Lee CK, Rhee SY, Oh CH, Shim JJ, Kim HJ. Trends in health-care costs and utilization for inflammatory bowel disease from 2010 to 2014 in Korea: A nationwide population-based study. J Gastroenterol Hepatol. 2018;33:847-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 36. | Li YG, Lee SR, Choi EK, Lip GY. Stroke Prevention in Atrial Fibrillation: Focus on Asian Patients. Korean Circ J. 2018;48:665-684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 37. | Son MK, Lim NK, Cho MC, Park HY. Incidence and Risk Factors for Atrial Fibrillation in Korea: The National Health Insurance Service Database (2002-2010). Korean Circ J. 2016;46:515-521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 38. | Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, Newton-Cheh C, Lubitz SA, Magnani JW, Ellinor PT, Seshadri S, Wolf PA, Vasan RS, Benjamin EJ, Levy D. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: A cohort study. Lancet. 2015;386:154-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 890] [Cited by in RCA: 1306] [Article Influence: 118.7] [Reference Citation Analysis (1)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
P-Reviewer: Cardoso CRL, Ksel IY, Wittmann T S-Editor: Yan JP L-Editor: A E-Editor: Zhang YL