Published online May 21, 2019. doi: 10.3748/wjg.v25.i19.2327
Peer-review started: February 17, 2019
First decision: March 20, 2019
Revised: March 29, 2019
Accepted: April 19, 2019
Article in press: April 20, 2019
Published online: May 21, 2019
Processing time: 95 Days and 3 Hours
Acute exacerbation in patients with chronic hepatitis B virus (HBV) infection results in different severities of liver injury. The risk factors related to progression to hepatic decompensation (HD) and acute-on-chronic liver failure (ACLF) in patients with severe acute exacerbation (SAE) of chronic HBV infection remain unknown.
To identify risk factors related to progression to HD and ACLF in compensated patients with SAE of chronic HBV infection.
The baseline characteristics of 164 patients with SAE of chronic HBV infection were retrospectively reviewed. Independent risk factors associated with progression to HD and ACLF were identified. The predictive values of our previously established prediction model in patients with acute exacerbation (AE model) and the model for end-stage liver disease (MELD) score in predicting the development of ACLF were evaluated.
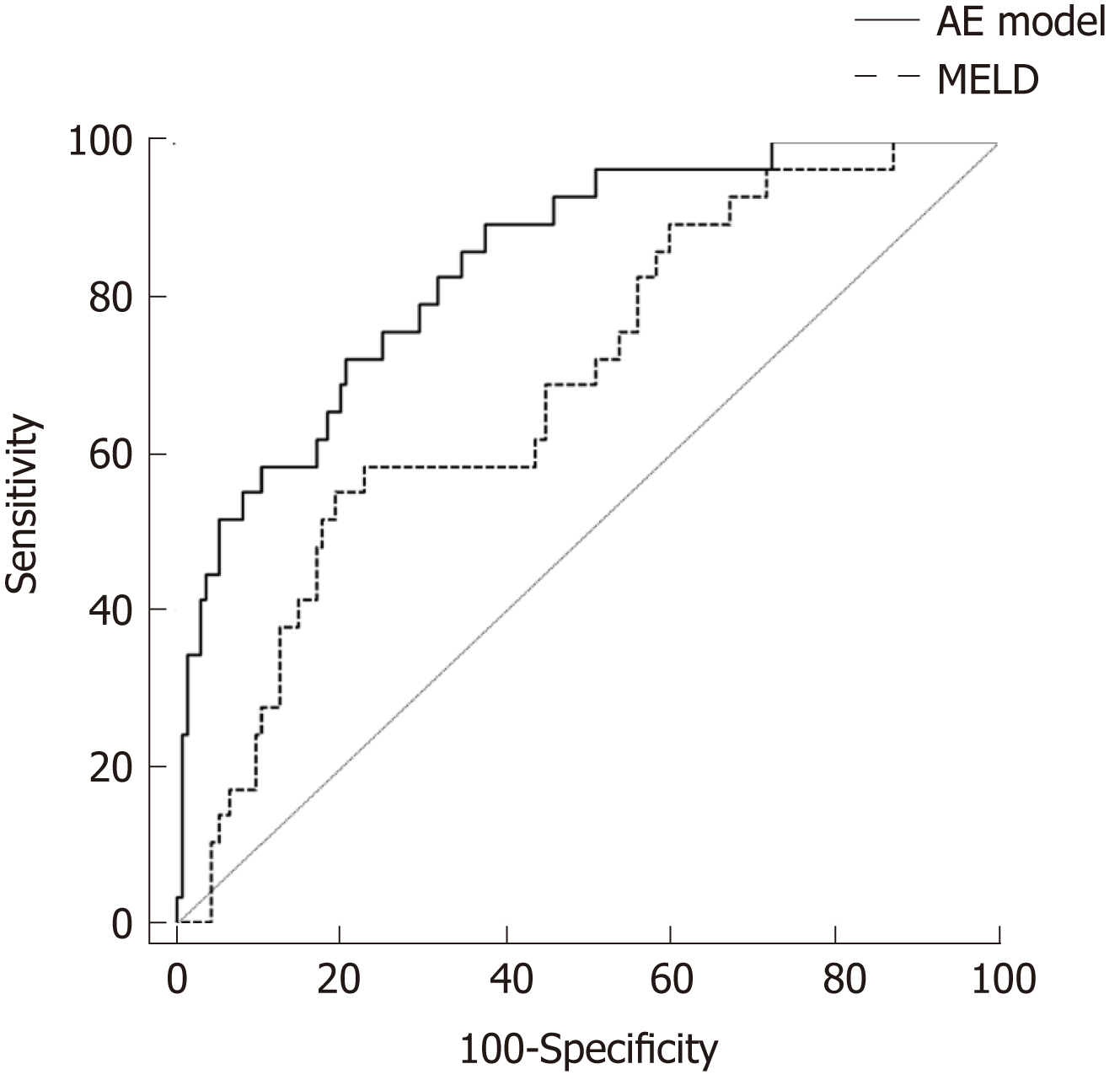
Among 164 patients with SAE, 83 (50.6%) had compensated liver cirrhosis (LC), 43 had progression to HD without ACLF, and 29 had progression to ACLF within 28 d after admission. Independent risk factors associated with progression to HD were LC and low alanine aminotransferase. Independent risk factors for progression to ACLF were LC, high MELD score, high aspartate aminotransferase (AST) levels, and low prothrombin activity (PTA). The area under the receiver operating characteristic of the AE model [0.844, 95% confidence interval (CI): 0.779-0.896] was significantly higher than that of MELD score (0.690, 95%CI: 0.613-0.760, P < 0.05) in predicting the development of ACLF.
In patients with SAE of chronic HBV infection, LC is an independent risk factor for progression to both HD and ACLF. High MELD score, high AST, and low PTA are associated with progression to ACLF. The AE model is a better predictor of ACLF development in patients with SAE than MELD score.
Core tip: The risk factors related to progression in patients with severe acute exacerbation (SAE) of chronic hepatitis B virus (HBV) infection remain unknown. This is the largest study to identify the risk factors related to progression to hepatic decompensation (HD) and acute-on-chronic liver failure (ACLF) in compensated patients with SAE of chronic HBV infection. We found that liver cirrhosis is an independent risk factor for progression to both HD and ACLF. High model for end-stage liver disease score, high aspartate aminotransferase, and low prothrombin activity are associated with progression to ACLF.
- Citation: Yuan L, Zeng BM, Liu LL, Ren Y, Yang YQ, Chu J, Li Y, Yang FW, He YH, Lin SD. Risk factors for progression to acute-on-chronic liver failure during severe acute exacerbation of chronic hepatitis B virus infection. World J Gastroenterol 2019; 25(19): 2327-2337
- URL: https://www.wjgnet.com/1007-9327/full/v25/i19/2327.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i19.2327
In China, the majority of patients with end-stage liver diseases and liver disease-related death are caused by chronic hepatitis B virus (HBV) infection[1]. There are five phases in the natural history of chronic HBV infection, including hepatitis B e-antigen (HBeAg)-positive chronic HBV infection, HBeAg-positive chronic hepatitis B (CHB), HBeAg-negative chronic HBV infection, HBeAg-negative CHB, and hepatitis B surface antigen (HBsAg)-negative phase[2]. Hepatitis flares with different degrees of liver injury mostly occur in the HBeAg-positive CHB and HBeAg-negative CHB phases[3-5]. Acute exacerbation (AE) and severe AE (SAE) refer to the severe liver injury occurring in patients with chronic HBV infection during hepatitis flare[6,7]. AE of chronic HBV infection occurs in 40%-50% of HBeAg–positive patients and in 15%-30% of HBeAg-negative CHB patients[8-10]. Patients with AE or SAE eventually progress to hepatic decompensation (HD) and acute-on-chronic liver failure (ACLF) if their liver injury deteriorates further.
In patients with chronic HBV infection, HD manifesting as ascites, esophagogastric variceal bleeding (EVB), or hepatic encephalopathy is a significant stage during progression from AE to liver failure[11,12]. Once progressed to HD, the liver function of patients with AE or SAE becomes more unstable and undergoes more rapid deterioration to ACLF following intrahepatic or extrahepatic insults[13,14]. Despite improved clinical management, the mortality of patients with ACLF ranges from 50%-80% without liver transplantation[15]. It has been recognized that early diagnosis and treatment play an important role in survival of patients with ACLF; in earlier stages, intensive treatment may be effective in impeding disease progression[15,16]. Therefore, it is imperative to find the risk factors related to deterioration of HD and ACLF in patients with AE and SAE.
Several studies have been conducted to determine these risk factors. However, most of these studies have included patients with diverse etiologies and degrees of liver injury, with understandably inconsistent results[8,17-18]. It is therefore challenging to identify common factors related to disease progression in patients with high heterogeneity in terms of severity and cause. In a previous study, we assessed risk factors in patients with AE of chronic HBV infection, wherein AE was defined as alanine transaminase (ALT) > 5 × upper limits of normal (ULN), total bilirubin (TBil) ≥ 5 × ULN, and prothrombin time activity (PTA) of 40%-60%. We found that baseline age, HBV DNA, and international normalized ratio (INR) levels were independent factors related to the development of ACLF[19]. Based on this study, we established an AE model to predict the progression to ACLF in patients with AE. Although patients with AE or SAE of chronic HBV infection show different degrees of liver injury, it remains to be elucidated whether the risk factors in progression to ACLF are similar among these patients. Therefore, in this study, we included patients with SAE as ALT > 10 × ULN, TBil ≥ 3 × ULN, and PTA of 40%-60% and aimed to identify the baseline risk factors associated with post-admission progression to HD or ACLF.
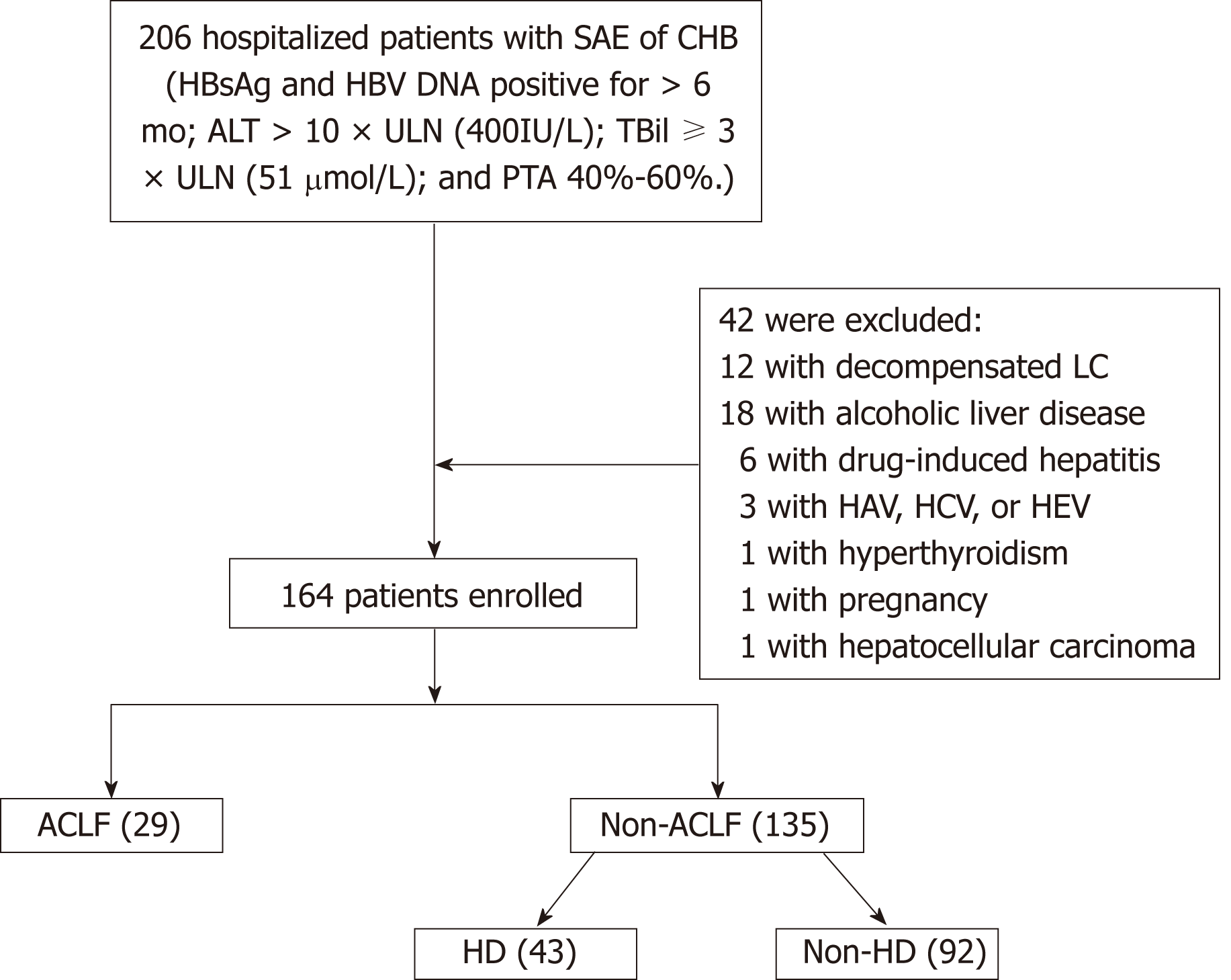
The study subjects were patients with SAE admitted to the Department of Infectious Diseases, Affiliated Hospital of Zunyi Medical University between January 2011 and August 2018. Figure 1 shows the flow chart for the patient selection process. In all, 164 patients with SAE of chronic HBV infection were included. The remaining 42 patients were excluded from the study by the exclusion criteria, which included coexistence with drug-induced hepatitis, alcoholic liver disease, hyperthyroidism, pregnancy, hepatocellular carcinoma, or acute hepatitis A, C, or E. Patients who had de-compensated liver cirrhosis (LC) or were previously diagnosed with decompensated LC were also excluded.
SAE of chronic HBV infection was diagnosed on the basis of the criteria proposed by Tsubota et al[20] and Wong et al[21]. The criteria for SAE of chronic HBV infection were as follows: (1) Presence of HBsAg and HBV DNA for > 6 mo before hospitalization; (2) ALT > 10 × ULN (400 IU/L) and TBil ≥ 3 × ULN (51 μmol/L); and (3) PTA of 40%-60%. Further, ACLF was diagnosed as the recent development of jaundice (TBil ≥ 5 × ULN) and coagulopathy (PTA < 40% or INR ≥ 1.5), complicated within four weeks by ascites and/or encephalopathy in a patient with previously diagnosed or undiagnosed chronic liver disease[12]. Cirrhosis was diagnosed based on previous liver biopsy findings or a composite of clinical signs and laboratory test, endoscopy, radiology, and FibroScan (Echosens, Paris, France) results. HD was defined as the presence of one of the following: new onset of hepatic encephalopathy, EVB, or ascites[22].
After admission, all patients received standard conservative therapy. None of them received liver transplantation. The standard conservative therapy included bed rest, liver-protective treatment (glutathione, adenosylmethionine, and branched-chain amino acids), nutritional and energy supplements, intravenous plasma and albumin (ALB) infusions, water-electrolyte and acid-base equilibrium maintenance, and prevention and treatment of complications. All patients received antiviral therapy including lamivudine, entecavir, or telbivudine within 3 days of admission according to their HBV replication levels and patient willingness. Plasma exchange was administered to patients who had progression to ACLF.
Patient demographics, clinical and laboratory parameters, and imaging findings were retrospectively collected from computerized and paper medical records. Laboratory variables including aspartate transaminase (AST), ALT, TBil, ALB, prealbumin, cholinesterase (CHE), gamma-glutamyl transpeptidase, PTA, INR, white blood cell count, platelet (PLT), serum sodium (Na+), blood urea nitrogen (BUN), creatinine (Cr), and HBV DNA levels were obtained within 24 h of the first diagnosis. In addition, the model for end-stage liver disease (MELD) score was calculated according to the following formula: MELD score = 3.78 × ln[TBil (mg/dL)] + 11.2 × ln(INR) + 9.57 × ln[Cr (mg/dL)] + 6.43 × (constant for liver disease etiology = 0, if cholestatic or alcoholic, otherwise = 1). The AE model was calculated as R = -13.323 + 0.553 × log HBV DNA (copies/mL) + 3.631 × INR + 0.053 × age (years)[19].
The protocol conformed to the provisions of the Declaration of Helsinki and was approved by the Human Ethical Committee of the Affiliated Hospital of Zunyi Medical University. All patients were informed of the use of their data in writing for clinical research purposes and accepted.
Statistical analyses were performed using SPSS version 19.0 (IBM Corp., Armonk, NY, United States) and MedCalc® 15.8 (MedCalc Software BVBA, Ostend, Belgium). Patient characteristics were compared between patients with and without HD or ACLF. χ2 tests, t-tests, and Mann–Whitney U tests were used for categorical variables, variables with normal distribution, and variables with a non-normal distribution, respectively. Univariate and multivariate analyses were performed using logistic regression analysis. The predictive values of our previously established AE model in patients with SAE and the MELD score were evaluated by the receiver operating characteristic (ROC) curve and the area under the ROC curve (AUROC). To identify the optimal cut-off point for each model, the Youden index was used. The cut-off sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated. P < 0.05 was considered statistically significant.
A total of 164 patients (mean age: 39.8 ± 11.1 years, 143 men and 21 women) were enrolled in the study. In all, 101 (61.6%) patients were HBeAg-positive and 83 (50.6%) had LC. Further, 43 (26.2%) patients had progression to HD without developing ACLF, and 29 (17.7%) patients had progression to ACLF within 28 d of admission. After admission of patients with SAE, the mean duration for development of HD and ACLF was 9.6 d (1-18 d) and 10.5 d (2-21 d), respectively. Thirteen (44.8%) patients with ACLF died during the 3 mo of follow-up. The other 92 patients (mean age: 36.6 ± 9.4 years, 82 men and 10 women) did not progress to HD or ACLF.
By comparing the baseline clinical characteristics and laboratory findings in patients with and without post-admission progression to HD, we found that among 43 patients who had progression to HD, 42 (97.7%) had LC on admission. However, among the 92 patients without progression to HD, only 20 (21.7%) had LC on admission. Further details on comparisons between baseline characteristics of patients with and without post-admission progression to HD and ACLF can be found in Tables 1 and 2, respectively.
| Variable | Total (n = 135) | Patients | P-value | |
| Without progression to HD (n = 92) | With progression to HD (n = 43) | |||
| Males | 120 (88.9) | 82 (88.2) | 38 (90.5) | 0.7771 |
| Age (yr) | 39.1 ± 10.6 | 36.6 ± 9.4 | 44.6 ± 11.2 | 0.0002 |
| LC | 62 (45.9) | 20 (21.7) | 42 (97.7) | 0.0001 |
| HBeAg–positive | 53 (39.3) | 38 (40.9) | 15 (35.7) | 0.3551 |
| log HBV DNA (copies/mL) | 6.7 (5.6, 7.5) | 6.9 (5.9, 7.5) | 6.3 (4.2, 7.2) | 0.0443 |
| Na+ (mmol/L) | 137.0 ± 2.9 | 136.9 ± 3.0 | 136.9 ± 2.8 | 0.9272 |
| ALT (IU/L) | 680.0 (421.0, 1191.0) | 838.0 (495.5, 1333.5) | 469.0 (265.5, 725.5) | 0.0003 |
| AST (IU/L) | 592.0 (313.0, 980.0) | 636.0 (369.0, 1212.5) | 502.5 (268.5, 760.0) | 0.0223 |
| GGT (IU/L) | 145.0 (108.0, 223.0) | 145.0 (110.5, 221.5) | 145.5 (94.5, 225.0) | 0.8453 |
| CHE (IU/L) | 3.9 ± 1.5 | 4.3 ± 1.4 | 3.2 ± 1.4 | 0.0002 |
| TBil (μmol/L) | 174.1 (96.1, 288.7) | 153.7 (93.9, 283.5) | 160.7 (88.8, 250.5) | 0.9323 |
| ALB (g/L) | 33.6 ± 4.9 | 34.4 ± 4.2 | 31.6 ± 5.2 | 0.0012 |
| PA (mg/L) | 48.0 (35.0, 69.0) | 54.4 ± 25.4 | 49.4 ± 30.5 | 0.3293 |
| BUN (mmol/L) | 3.9 (3.0, 4.6) | 3.8 (2.9, 4.4) | 4.5(3.3, 4.4) | 0.0103 |
| Cr (μmol/L) | 74.0 (67.0, 83.0) | 74.0 (67.0, 84.0) | 73.0 (66.3, 82.0) | 0.5573 |
| INR | 1.46 ± 0.18 | 1.45 ± 0.18 | 1.47 ± 0.16 | 0.5102 |
| PTA (%) | 51.9 ± 5.5 | 51.6 ± 5.5 | 52.9 ± 5.5 | 0.2082 |
| WBC (109/L) | 5.2 (3.18, 6.7) | 5.4 (3.9, 6.5) | 4.7 (3.8, 7.5) | 0.6133 |
| PLT (109/L) | 120.2 ± 49.1 | 128.9 ± 49.5 | 100.9 ± 42.8 | 0.0022 |
| MELD | 17.5 ± 3.8 | 17.5 ± 3.9 | 17.3 ± 3.7 | 0.8302 |
| Variable | Total (n = 164) | Patients | P-value | |
| Without progression to ACLF (n = 135) | With progression to ACLF (n = 29) | |||
| Males | 143 (87.2) | 120 (88.9) | 23 (79.3) | 0.2161 |
| Age (yr) | 39.8 ± 10.1 | 39.1 ± 10.6 | 43.1 ± 11.1 | 0.0662 |
| LC | 83 (50.6) | 62 (45.9) | 21 (72.4) | 0.0131 |
| HBeAg–positive | 101 (61.6) | 82 (60.7) | 19 (65.5) | 0.6791 |
| log HBV DNA (copies/mL) | 6.7 (5.6, 7.5) | 6.7 (5.5, 7.5) | 7.1 (6.4, 7.7) | 0.0383 |
| Na+ (mmol/L) | 137.0 ± 2.9 | 137.1 ± 2.7 | 137.7 ± 2.9 | 0.8282 |
| ALT (IU/L) | 724.5 (441.5, 1227.6) | 680.0 (421.0, 1191.0) | 833.0 (532.0, 1770.0) | 0.0563 |
| AST (IU/L) | 628.0 (330.3, 1101.0) | 592.0 (313.0, 980.0) | 779.0 (426.5, 1411.0) | 0.0413 |
| GGT (IU/L) | 139.5 (99.0, 210.8) | 145.0 (108.0, 223.0) | 111.0 (84.5, 162.5) | 0.0493 |
| CHE (IU/L) | 4.0 ± 1.5 | 3.9 ± 1.5 | 4.1 ± 1.5 | 0.5332 |
| TBil (μmol/L) | 174.1 (96.1, 288.7) | 155.1 (93.6, 272.9) | 211.7 (147.3, 340.9) | 0.0383 |
| ALB (g/L) | 33.5 ± 4.8 | 33.6 ± 4.8 | 33.3 ± 5.5 | 0.7852 |
| PA (mg/L) | 46.0 (31.3, 67.0) | 48.0 (35.0, 69.0) | 42.0 (26.5, 51.5) | 0.0413 |
| BUN (mmol/) | 3.9 (3.0, 4.6) | 3.9 (3.0, 4.6) | 3.8 (3.2, 4.4) | 0.9233 |
| Cr (μmol/L) | 73.0 (66.0, 82.0) | 74.0 (67.0, 83.0) | 71.0 (63.0, 77.5) | 0.0773 |
| INR | 1.50 ± 0.21 | 1.46 ± 0.18 | 1.72 ± 0.24 | 0.0002 |
| PTA (%) | 51.1 (45.8, 55.8) | 52.5 (48.1, 57.0) | 44.0 (41.0, 47.8) | 0.0003 |
| WBC (109/L) | 5.4 (3.9, 6.8) | 5.2 (3.18, 6.7) | 5.7 (4.4, 6.8) | 0.2073 |
| PLT (109/L) | 118.9 ± 47.8 | 120.2 ± 49.1 | 112.5 ± 41.5 | 0.4332 |
| MELD | 17.9 ± 3.8 | 17.5 ± 3.8 | 19.8 ± 3.1 | 0.0032 |
Among 135 patients without progression to ACLF, 43 had progression to HD. Forty-two patients developed ascites and one patient developed EVB. As shown in Table 3, older age of patients; LC; lower serum levels of ALB, ALT, AST, CHE, PLT; and a higher serum level of BUN were found as the risk factors associated with post-admission HD in univariate logistic regression analysis. The independent risk factors associated with progressing to HD were LC and lower ALT level.
| Variable | Univariate analysis | Multivariate analysis | ||||||
| Β | OR | 95%CI | P-value | β | OR | 95%CI | P-value | |
| Age | 0.077 | 1.080 | 1.038-1.123 | 0.000 | ||||
| HBV DNA | -0.276 | 0.759 | 0.587-0.981 | 0.035 | ||||
| ALT | -0.002 | 0.998 | 0.997-0.999 | 0.000 | -0.003 | 0.997 | 0.995-1.000 | 0.038 |
| AST | -0.001 | 0.999 | 0.998-1.000 | 0.015 | ||||
| CHE | -0.599 | 0.549 | 0.405-0.744 | 0.000 | ||||
| ALB | -0.140 | 0.869 | 0.796-0.948 | 0.002 | ||||
| BUN | 0.238 | 1.268 | 1.029-1.563 | 0.026 | ||||
| PLT | -0.014 | 0.986 | 0.977-0.995 | 0.003 | ||||
| LC | 5.019 | 151.2 | 19.6-1167.6 | 0.000 | 5.040 | 154.5 | 16.2-1469.0 | 0.000 |
In the 29 patients who had progression to ACLF within 28 d after admission, 21 had LC on admission. As shown in Table 4, the risk factors associated with post-admission progression to ACLF were being complicated with LC, higher MELD score, higher serum levels of HBV DNA and AST, and lower serum level of PTA. The independent risk factors associated with progression to ACLF included higher MELD score, higher AST level, and lower PTA level.
| Variable | Univariate analysis | Multivariate analysis | ||||||
| Β | OR | 95%CI | P-value | β | OR | 95%CI | P-value | |
| MELD | 0.161 | 1.175 | 1.049-1.315 | 0.005 | 0.184 | 1.202 | 1.033-1.398 | 0.017 |
| HBV DNA | 0.380 | 1.463 | 1.018-2.101 | 0.040 | ||||
| AST | 0.001 | 1.001 | 1.000-1.002 | 0.013 | 0.001 | 1.001 | 1.000-1.002 | 0.021 |
| PTA | -0.252 | 0.777 | 0.704-0.858 | 0.000 | -0.257 | 0.758 | 0.672-855 | 0.000 |
| LC | 1.128 | 3.091 | 1.280-7.465 | 0.012 | 2.125 | 8.369 | 2.389-29.322 | 0.001 |
To test the predictive value of the AE model in patients with SAE, we compared the ROC curve and AUROC of the AE model with those of the MELD score. As shown in Figure 2, the performance of the AE model [AUROC = 0.844, 95% confidence interval (CI): 0.779-0.896] was significantly better than that of the MELD score (AUROC = 0.690, 95%CI: 0.613-0.760, P < 0.05) in predicting the post-admission progression to ACLF. With a cut-off of -2.085, the AE model had a higher sensitivity (89.6%) and NPV (99.6%) than the MELD score at the cut-off value of 19.92 (55.2% and 89.3%, respectively). However, the specificity (62.2%) and PPV (33.8%) of the AE model were lower than those of the MELD score (80.2% and 38.1%, respectively).
The precipitating factors and pathogenesis of AE or SAE are different in patients with compensated liver diseases and decompensated LC[23,24]. Worsening of underlying chronic liver disease is the most common precipitating factor for AE or SAE in patients with compensated liver diseases[13]. However, in decompensated LC, the occurrence of AE or SAE and progression to liver failure are mostly triggered by complications including bacterial infection, renal function impairment, and gastrointestinal bleeding[25,26]. In this study, we excluded patients with SAE induced by other hepadnavirus infection and bacterial infection. We also excluded patients with decompensated LC; therefore, SAE in patients of this study mostly resulted from HBV reactivation. This group of patients exhibited similar clinical characteristics and pathogenesis of SAE[3], thus enabling the identification of common risk factors associated with progression to HD and ACLF. As far as we know, this is the largest study cohort in an investigation of this condition.
We included patients with SAE of chronic HBV infection with ALT > 10 × ULN, TBil ≥ 3 × ULN, and PTA of 40%-60%%. Forty-three (26.2%) patients had progression to HD and 29 (17.7%) patients had progression to ACLF within 28 d of admission. Therefore, the patients in this study had more serious liver injury than patients with AE as previously reported (only 7.59% of patients had progression to ACLF)[19]. We found that LC was an independent risk factor associated with progression to both HD and ACLF, while high MELD score, high AST, and low PTA were independent risk factors associated with progression to ACLF.
Several risk factors for post-admission development of ACLF in patients with SAE were different from those in patients with AE. In our previous study, LC was not an independent factor associated with the development of ACLF in patients with AE[19]. In this study, however, LC was an independent risk factor for post-admission progression of both HD and ACLF in patients with SAE. It is generally considered that in patients with chronic HBV infection, total liver injury consists of acute and chronic liver injury during AE or SAE[6,15]. Therefore, when the degree of acute liver injury reaches a certain limit, LC may be the determining factor for the outcomes during hepatitis flare. However, it is still unclear at what degree of acute liver injury the compensated LC may have a significant effect on the outcome of patients with chronic HBV infection. Contradictory results have been reported in previous studies, likely attributable to high heterogeneity in the degrees of liver injury and etiologies of study subjects[20,27-29]. Our results are the first to demonstrate that in patients with SAE of chronic HBV infection, compensated LC plays a determining role in the development of both HD and ACLF.
Another differing result from our previous study in patients with AE was that HBV DNA was not an independent risk factor for progression to ACLF in patients with SAE of chronic HBV infection, although high HBV DNA level was one of the risk factors for progression to ACLF in the univariate analysis. Numerous studies have found that the occurrence of HCC and LC is closely correlated with high HBV DNA levels in patients with chronic HBV infection[30]. However, whether the short-term outcomes in patients with AE of chronic HBV infection are also influenced by HBV DNA levels remains unclear[20,21,31]. In patients with AE, it has been suggested that high HBV DNA levels might indicate an active immune attack of hepatocytes and ineffective inhibition of HBV DNA replication, resulting in prolonged liver injury, and patients may eventually progress to ACLF[3,32,33]. In a previous study, we found that HBV DNA was an independent risk factor for post-admission progression to ACLF in patients with AE[19]. Jeng et al[34] also found that in patients with AE, while TBil, PTA, and HBV DNA levels were risk factors, HBV DNA level was the only independent risk factor for HD. A high HBV DNA level of 1.55 × 109 copies/mL was predictive of HD. However, other studies found no relationship between baseline HBV DNA and AE severity or mortality of patients with AE[20,32,35,36]. Our results showing that HBV DNA played differing roles in the outcomes of patients with AE and SAE suggest that the influence of HBV DNA is dependent on the degree or stage of hepatitis flare. Patients with more severe liver injury are usually at a later stage during AE or SAE, as at this stage the deleterious role of HBV DNA may be masked by other risk factors. Hsu et al also found that in patients with SAE (defined as TBil level > 2 mg/dL and PT prolongation by more than 3 s, or with ascites/hepatic encephalopathy) of chronic HBV infection, the mortality of patients was mainly determined by the pronounced coagulopathy; only in patients with INR ≤ 1.7, HBV DNA level was the risk factor for mortality[17].
Contrary to a higher HBV DNA level in patients who showed progression to ACLF, patients with progression of HD had a lower baseline HBV DNA level than those without progression to HD. Given that almost all patients with progression to HD had LC at baseline and previous studies have found that patients with LC had a lower HBV DNA level than those without[22], we considered that the high proportion of LC in patients with progression to HD resulted in a lower HBV DNA level than in those without progression to HD. Another interesting finding was that low ALT level was an independent risk factor for progression to HD. It is difficult to explain this result. ALT level reflects the degree of hepatocyte necrosis resulting from acute injury; further, we defined patients with SAE as having PTA between 40% and 60%, which reflects the total degree of liver injury. Therefore, low ALT levels in a patient may indicate a high degree of liver fibrosis. Our results suggest that the degree of liver fibrosis determines the development of HD in patients with compensated LC.
There is currently no predictive model for predicting the post-admission progression of ACLF in patients with SAE of chronic HBV infection. The MELD score is commonly used to assess liver disease severity[37]. It remains unknown whether the MELD score and AE model can predict the post-admission progression of ACLF in patients with SAE. In our study, although MELD score was a risk factor for progression to ACLF, the AUROC of MELD was < 0.7, indicating that the predictive value of MELD was low in patients with SAE. On the other hand, the AE model which did not include MELD score performed satisfactorily in predicting the occurrence of ACLF in patients with SAE of CHB. This result is difficult to explain. Because the AE model was established from patients with AE of chronic HBV infection, our result suggested that patients with AE and SAE had similar pathogenesis of progression to ACLF.
Our study was a retrospective, single-center study and has several limitations. First, we only studied patients’ baseline clinical characteristics. We were not able to evaluate the predictive role of dynamic changes in HBV DNA as the short term changes in HBV DNA were not routinely measured in clinical practice. Although a few studies found that viral kinetics can predict the severity of AE in patients with CHB[32], a recent study showed that in patients with spontaneous SAE of CHB, either lamivudine or entecavir could induce a rapid decline of HBV viral load[38]. Second, the patients in this study were admitted from January 2011 to August 2018; although all patients received standard conservative therapy upon admission, the treatment methods may be different among the patients with SAE before admission. In addition, we did not detect HBV genotypes and HBV DNA mutation in most patients; therefore, we did not include these indicators in our study. To further elucidate the risk factors related to the development of ACLF in patients with SAE of chronic HBV infection, a prospective study involving more patients is needed.
The prognosis of patients with acute-on-chronic liver failure (ACLF) largely depends on early diagnosis and treatment. Patients with acute exacerbation (AE) or severe AE (SAE) of chronic hepatitis B virus (HBV) infection have a high tendency to further progress to hepatic decompensation (HD) and ACLF. Therefore, it is important to identify the risk factors for progression to HD and ACLF in patients with SAE of chronic HBV infection.
In a previous study, we have found that baseline age, HBV DNA, and international normalized ratio levels were independent factors associated with the development of ACLF in patients with AE of chronic HBV infection. Patients with AE or SAE have different degrees of liver injury, and it remains to be elucidated whether the risk factors for progression to ACLF are similar among these patients.
To identify risk factors related to progression to HD and ACLF in compensated patients with SAE of chronic HBV infection.
The baseline characteristics of 164 patients with SAE of chronic HBV infection were retrospectively reviewed. Independent risk factors associated with progression to HD and ACLF were identified.
Independent risk factors associated with progression to HD were liver cirrhosis (LC) and low alanine aminotransferase (ALT). Independent risk factors for progression to ACLF were LC, high MELD score, high aspartate aminotransferase (AST) levels, and low prothrombin activity (PTA).
Our results are the first to demonstrate that in patients with SAE of chronic HBV infection, compensated LC plays a determining role in the development of both HD and ACLF. High MELD score, high AST, and low PTA are associated with progression to ACLF.
We found that liver cirrhosis is an independent risk factor for progression to both HD and ACLF. High model for end-stage liver disease score, high aspartate aminotransferase, and low prothrombin activity are associated with progression to ACLF.
| 1. | Zhang S, Wang F, Zhang Z. Current advances in the elimination of hepatitis B in China by 2030. Front Med. 2017;11:490-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 2. | European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu.; European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3745] [Cited by in RCA: 4008] [Article Influence: 445.3] [Reference Citation Analysis (1)] |
| 3. | Chang ML, Liaw YF. Hepatitis B flares in chronic hepatitis B: Pathogenesis, natural course, and management. J Hepatol. 2014;61:1407-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 243] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 4. | Hoofnagle JH. Reactivation of hepatitis B. Hepatology. 2009;49:S156-S165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 438] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 5. | Kumar M, Chauhan R, Gupta N, Hissar S, Sakhuja P, Sarin SK. Spontaneous increases in alanine aminotransferase levels in asymptomatic chronic hepatitis B virus-infected patients. Gastroenterology. 2009;136:1272-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Wong VW, Chan HL. Severe acute exacerbation of chronic hepatitis B: A unique presentation of a common disease. J Gastroenterol Hepatol. 2009;24:1179-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Tsai WL, Sun WC, Cheng JS. Chronic Hepatitis B with Spontaneous Severe Acute Exacerbation. Int J Mol Sci. 2015;16:28126-28145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Liaw YF, Tai DI, Chu CM, Pao CC, Chen TJ. Acute exacerbation in chronic type B hepatitis: Comparison between HBeAg and antibody-positive patients. Hepatology. 1987;7:20-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 114] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Liaw YF, Chen JJ, Chen TJ. Acute exacerbation in patients with liver cirrhosis: A clinicopathological study. Liver. 1990;10:177-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Fattovich G, Brollo L, Alberti A, Realdi G, Pontisso P, Giustina G, Ruol A. Spontaneous reactivation of hepatitis B virus infection in patients with chronic type B hepatitis. Liver. 1990;10:141-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, Gerbes A, Wendon J, Alessandria C, Laleman W, Zeuzem S, Trebicka J, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL–CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426-1437, 1437.e1-1437.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1720] [Cited by in RCA: 2273] [Article Influence: 174.8] [Reference Citation Analysis (6)] |
| 12. | Sarin SK, Kedarisetty CK, Abbas Z, Amarapurkar D, Bihari C, Chan AC, Chawla YK, Dokmeci AK, Garg H, Ghazinyan H, Hamid S, Kim DJ, Komolmit P, Lata S, Lee GH, Lesmana LA, Mahtab M, Maiwall R, Moreau R, Ning Q, Pamecha V, Payawal DA, Rastogi A, Rahman S, Rela M, Saraya A, Samuel D, Saraswat V, Shah S, Shiha G, Sharma BC, Sharma MK, Sharma K, Butt AS, Tan SS, Vashishtha C, Wani ZA, Yuen MF, Yokosuka O; APASL ACLF Working Party. Acute-on-chronic liver failure: Consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL) 2014. Hepatol Int. 2014;8:453-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 511] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 13. | Wu T, Li J, Shao L, Xin J, Jiang L, Zhou Q, Shi D, Jiang J, Sun S, Jin L, Ye P, Yang L, Lu Y, Li T, Huang J, Xu X, Chen J, Hao S, Chen Y, Xin S, Gao Z, Duan Z, Han T, Wang Y, Gan J, Feng T, Pan C, Chen Y, Li H, Huang Y, Xie Q, Lin S, Li L, Li J; Chinese Group on the Study of Severe Hepatitis B (COSSH). Development of diagnostic criteria and a prognostic score for hepatitis B virus-related acute-on-chronic liver failure. Gut. 2018;67:2181-2191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 323] [Article Influence: 40.4] [Reference Citation Analysis (2)] |
| 14. | Gustot T, Moreau R. Acute-on-chronic liver failure vs. traditional acute decompensation of cirrhosis. J Hepatol. 2018;69:1384-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 15. | Nakayama N, Uemura H, Uchida Y, Tomiya T, Ido A, Inoue K, Genda T, Takikawa Y, Sakaida I, Terai S, Yokosuka O, Shimizu M, Takikawa H, Mochida S. A multicenter pilot survey to clarify the clinical features of patients with acute-on-chronic liver failure in Japan. Hepatol Res. 2018;48:303-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Chen EQ, Zeng F, Zhou LY, Tang H. Early warning and clinical outcome prediction of acute-on-chronic hepatitis B liver failure. World J Gastroenterol. 2015;21:11964-11973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Hsu YC, Wu CY, Chang CY, Tai CM, Tseng CH, Perng DS, Mo LR, Lin JT. Pretreatment viral DNA stratifies mortality risk in patients receiving antiviral therapy for severe acute exacerbation of chronic hepatitis B. Antivir Ther. 2013;18:221-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Tsang SW, Chan HL, Leung NW, Chau TN, Lai ST, Chan FK, Sung JJ. Lamivudine treatment for fulminant hepatic failure due to acute exacerbation of chronic hepatitis B infection. Aliment Pharmacol Ther. 2001;15:1737-1744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Ren Y, Liu L, Li Y, Yang F, He Y, Zhu Y, Hu X, Lin S. Development and validation of a scoring system to predict progression to acute-on-chronic liver failure in patients with acute exacerbation of chronic hepatitis B. Hepatol Res. 2018;48:692-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Tsubota A, Arase Y, Suzuki Y, Suzuki F, Sezaki H, Hosaka T, Akuta N, Someya T, Kobayashi M, Saitoh S, Ikeda K, Kumada H. Lamivudine monotherapy for spontaneous severe acute exacerbation of chronic hepatitis B. J Gastroenterol Hepatol. 2005;20:426-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Wong VW, Wong GL, Yiu KK, Chim AM, Chu SH, Chan HY, Sung JJ, Chan HL. Entecavir treatment in patients with severe acute exacerbation of chronic hepatitis B. J Hepatol. 2011;54:236-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 22. | European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu.; European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1777] [Cited by in RCA: 1984] [Article Influence: 248.0] [Reference Citation Analysis (2)] |
| 23. | Mochida S, Nakayama N, Ido A, Inoue K, Genda T, Takikawa Y, Sakaida I, Terai S, Yokosuka O, Shimizu M, Takikawa H. Proposed diagnostic criteria for acute-on-chronic liver failure in Japan. Hepatol Res. 2018;48:219-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Shi Y, Yang Y, Hu Y, Wu W, Yang Q, Zheng M, Zhang S, Xu Z, Wu Y, Yan H, Chen Z. Acute-on-chronic liver failure precipitated by hepatic injury is distinct from that precipitated by extrahepatic insults. Hepatology. 2015;62:232-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 248] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 25. | Fukui H, Saito H, Ueno Y, Uto H, Obara K, Sakaida I, Shibuya A, Seike M, Nagoshi S, Segawa M, Tsubouchi H, Moriwaki H, Kato A, Hashimoto E, Michitaka K, Murawaki T, Sugano K, Watanabe M, Shimosegawa T. Evidence-based clinical practice guidelines for liver cirrhosis 2015. J Gastroenterol. 2016;51:629-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 233] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 26. | Clària J, Stauber RE, Coenraad MJ, Moreau R, Jalan R, Pavesi M, Amorós À, Titos E, Alcaraz-Quiles J, Oettl K, Morales-Ruiz M, Angeli P, Domenicali M, Alessandria C, Gerbes A, Wendon J, Nevens F, Trebicka J, Laleman W, Saliba F, Welzel TM, Albillos A, Gustot T, Benten D, Durand F, Ginès P, Bernardi M, Arroyo V, Arroyo V. CANONIC Study Investigators of the EASL-CLIF Consortium and the European Foundation for the Study of Chronic Liver Failure (EF-CLIF). Systemic inflammation in decompensated cirrhosis: Characterization and role in acute-on-chronic liver failure. Hepatology. 2016;64:1249-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 583] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 27. | Zhang Q, Han T, Li Y, Nie C, Liu H. Predictors of progression into acute-on-chronic liver failure from acute deterioration of pre-existing chronic liver disease. Hepatol Res. 2016;46:320-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Gao FY, Liu Y, Li XS, Ye XQ, Sun L, Geng MF, Wang R, Liu HM, Zhou XB, Gu LL, Liu YM, Wan G, Wang XB. Score model for predicting acute-on-chronic liver failure risk in chronic hepatitis B. World J Gastroenterol. 2015;21:8373-8381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Yuen MF, Sablon E, Hui CK, Li TM, Yuan HJ, Wong DK, Doutreloigne J, Bogaerts V, Wong BC, Fan ST, Lai CL. Prognostic factors in severe exacerbation of chronic hepatitis B. Clin Infect Dis. 2003;36:979-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 3089] [Article Influence: 386.1] [Reference Citation Analysis (1)] |
| 31. | Chen CH, Lin CL, Hu TH, Hung CH, Tseng PL, Wang JH, Chang JY, Lu SN, Chien RN, Lee CM. Entecavir vs. lamivudine in chronic hepatitis B patients with severe acute exacerbation and hepatic decompensation. J Hepatol. 2014;60:1127-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 32. | Mori N, Suzuki F, Kawamura Y, Sezaki H, Hosaka T, Akuta N, Kobayashi M, Saito S, Suzuki Y, Arase Y, Ikeda K, Kobayashi M, Kumada H. Determinants of the clinical outcome of patients with severe acute exacerbation of chronic hepatitis B virus infection. J Gastroenterol. 2012;47:1022-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Tsai SL, Chen PJ, Lai MY, Yang PM, Sung JL, Huang JH, Hwang LH, Chang TH, Chen DS. Acute exacerbations of chronic type B hepatitis are accompanied by increased T cell responses to hepatitis B core and e antigens. Implications for hepatitis B e antigen seroconversion. J Clin Invest. 1992;89:87-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 227] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 34. | Jeng WJ, Sheen IS, Liaw YF. Hepatitis B virus DNA level predicts hepatic decompensation in patients with acute exacerbation of chronic hepatitis B. Clin Gastroenterol Hepatol. 2010;8:541-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 35. | Tsai WL, Lo GH, Hsu PI, Lai KH, Lin CK, Chan HH, Chen WC, Cheng JS, Liu YC, Huang TS, Ger LP, Lin HH. Role of genotype and precore/basal core promoter mutations of hepatitis B virus in patients with chronic hepatitis B with acute exacerbation. Scand J Gastroenterol. 2008;43:196-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Chien RN, Lin CH, Liaw YF. The effect of lamivudine therapy in hepatic decompensation during acute exacerbation of chronic hepatitis B. J Hepatol. 2003;38:322-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 37. | Singal AK, Kamath PS. Model for End-stage Liver Disease. J Clin Exp Hepatol. 2013;3:50-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 38. | Lee TY, Chen CY, Lia HC, Hsu YC, Yang SS. The ultra-short virological dynamics in response to entecavir or lamivudine during chronic hepatitis B with spontaneous severe acute exacerbation. Antivir Ther. 2018;23:77-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
STROBE Statement: The authors have read the STROBE Statement—checklist of items, and the manuscript was prepared and revised according to the STROBE Statement—checklist of items.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bouare N, Farshadpour F, Sagnelli C S-Editor: Yan JP L-Editor: Wang TQ E-Editor: Ma YJ