Published online Apr 28, 2019. doi: 10.3748/wjg.v25.i16.1975
Peer-review started: December 6, 2018
First decision: January 11, 2019
Revised: January 25, 2019
Accepted: January 28, 2019
Article in press: January 28, 2019
Published online: April 28, 2019
Processing time: 142 Days and 17.4 Hours
Emergency surgical resection is a standard treatment for right-sided malignant colonic obstruction; however, the procedure is associated with high rates of mortality and morbidity. Although a bridge to surgery can be created to obviate the need for emergency surgery, its effects on long-term outcomes and the most practical management strategies for right-sided malignant colonic obstruction remain unclear.
To determine the appropriate management approach for right-sided malignant colonic obstruction.
Forty patients with right-sided malignant colonic obstruction who underwent curative resection from January 2007 to April 2017 were included in the study. We compared the perioperative and long-term outcomes of patients who received bridges to surgery established using decompression tubes and those created using self-expandable metallic stents (SEMS). The primary outcome was the overall survival duration (OS) and the secondary endpoints were the disease-free survival (DFS) duration and the preoperative and postoperative morbidity rates. Analysis was performed on an intention-to-treat basis.
There were 21 patients in the decompression tube group and 19 in the SEMS group. There were no significant differences in the perioperative morbidity rates of the two groups. The OS rate was significantly higher in the decompression tube group than in the SEMS group (5-year OS rate; decompression tube 79.5%, SEMS 32%, P = 0.043). Multivariate analysis revealed that the bridge to surgery using a decompression tube was significantly associated with the OS (hazard ratio, 17.41; P = 0.004). The 3-year DFS rate was significantly higher in the decompression tube group than in the SEMS group (68.9% vs 45.9%; log-rank test, P = 0.032). A propensity score–adjusted analysis also demonstrated that the prognosis was significantly better in the decompression tube group than in the SEMS group.
The bridge to surgery using trans-nasal and trans-anal decompression tubes for right-sided malignant colonic obstruction is safe and may improve long-term outcomes.
Core tip: Patients with malignant colonic obstructions typically undergo emergency surgery, which is associated with high rates of mortality and morbidity. To overcome this, bridges to surgery have been proposed, but their efficacy in patients with right-sided malignant colonic obstructions remains unclear, mainly because obstructions are less common in patients with right- than left-sided colon cancer. We compared two bridges to surgery: Decompression tubes and self-expandable metallic stents. The short-term outcomes of the two groups did not differ, but the overall survival and disease-free survival rates were better in the former patients, suggesting that decompression tube placement may be optimal.
- Citation: Suzuki Y, Moritani K, Seo Y, Takahashi T. Comparison of decompression tubes with metallic stents for the management of right-sided malignant colonic obstruction. World J Gastroenterol 2019; 25(16): 1975-1985
- URL: https://www.wjgnet.com/1007-9327/full/v25/i16/1975.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i16.1975
Colorectal cancer is highly prevalent, and increasing in incidence in many developed countries. Of patients with colorectal cancer, 8%-16% present to emergency departments with bowel obstruction[1-3]. A large proportion of patients with malignant colonic obstruction have left-sided colon cancer, in which the incidence of obstruction is reportedly higher than that in right-sided colon cancer[4,5]. This is likely due to the differences in diameter and fecal consistency between the right and left sides of the colon.
Although patients with malignant colonic obstruction typically undergo emergency surgery, the procedure is associated with higher rates of mortality and morbidity compared to elective surgery[6]. To overcome this, a bridge to surgery using metallic stents was proposed. However, a bridge to surgery using metallic stents for curable left-sided malignant colonic obstruction is not recommended due to the poor oncologic outcomes[7-11]. Most prior studies of the management of malignant colonic obstruction involved patients with left-sided colon cancer; thus, evidence for the management of right-sided malignant colonic obstruction (RMCO) is lacking. Patients who undergo emergency surgery for RMCO have a mortality rate of 10%-16%[6,12], but a retrospective study suggested that a bridge to surgery for RMCO improved short- and long-term outcomes[13].
As an alternative to metallic stents, a bridge to surgery using a decompression tube, first reported by Lelcuk et al[14] in 1985, can be used in patients with malignant colonic obstruction. In Asian countries, a decompression tube is widely used for malignant bowel obstruction. Because of the soft feces in the right colon, bowel decompression using a trans-nasal or trans-anal tube is effective. Although the efficacy of using a decompression tube for left-sided malignant colonic obstruction has been evaluated[15-18], whether this is also the case for RMCO is unclear. The aim of this study was to evaluate the optimum management strategy for patients with RMCO by comparing the perioperative and oncologic outcomes of bridges to surgery using decompression tubes and metallic stents.
We enrolled patients diagnosed with clinically and radiologically confirmed large bowel obstruction who subsequently underwent curative surgical resection for confirmed colonic adenocarcinoma at our hospital from January 2007 to April 2017. The inclusion criteria were as follows: (1) Clinically and radiologically confirmed malignant large bowel obstruction; (2) pathologically confirmed American Joint Committee on Cancer stage II–IV colon cancer; (3) a history of curative surgery including resection of metastatic lesions; and (4) a primary tumor located between the cecum and the proximal transverse colon. The exclusion criteria were as follows: (1) Double cancer; (2) lack of intent to perform bowel decompression preoperatively; and (3) unavailability of case data. The study was conducted with the approval of the Ethics Committee of our hospital (approval number 2018-27).
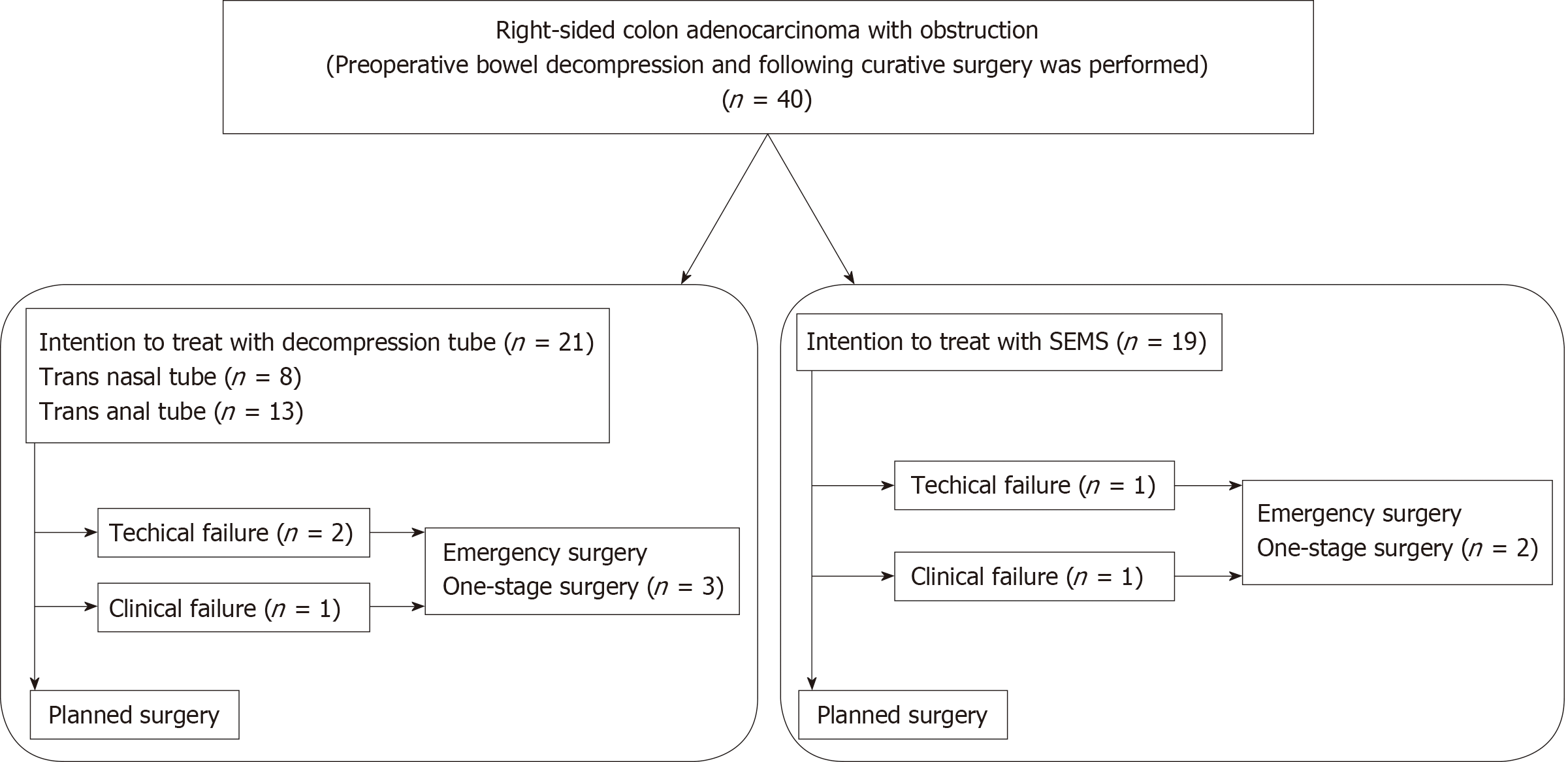
The patients were divided into a decompression tube group and a self-expandable metallic stent (SEMS) group. The decompression tube group consisted of patients who intended to be treated preoperatively with a trans-nasal or trans-anal decompression tube, and the SEMS group consisted of patients intended to be treated preoperatively with a SEMS. The feasibility of preoperative bowel decompression was evaluated by two colorectal surgeons and preoperative treatment was attempted when possible. We routinely used a decompression tube preoperatively in all patients from 2007 to 2011. Following the initiation of coverage by national health insurance in 2012, SEMS have been the standard method for preoperative bowel decompression. Patients with technical or clinical failure of preoperative bowel decompression were analyzed in the decompression tube and SEMS groups on an intention-to-treat basis.
In the decompression tube group, the selection of a trans-nasal or trans-anal tube was dependent on the surgeon’s preference. The trans-nasal tube included a nasogastric tube and a long intestinal tube. The trans-anal and long intestinal tubes were inserted under radiological guidance, with additional endoscopic guidance used during the insertion of the trans-anal tube (Supplemental Figures 1 and 2). Details of trans-anal decompression and tube insertion are provided elsewhere[18]. The tip of the long intestinal tube was placed in the distal intestine at Treiz’s ligament and the balloon was inflated with distilled water. Following decompression tube insertion, oral intake was restricted during decompression. In patients with trans-anal tubes, the intestinal tract was cleaned once or twice daily using 500-1000 mL of water for a few days until the feces content of the colon was reduced to an acceptable level.
SEMS placement was performed by two experienced endoscopists. If the bowel dilatation was relieved by the SEMS, oral intake until the day before elective surgery was permitted. Colectomy was performed according to optimal oncological principles. Colectomy was performed approximately 7 d after decompression tube insertion in the decompression tube group and approximately 14-21 d after SEMS placement in the SEMS group to avoid the increased risk of complications due to prolonged tube or SEMS patency.
The follow-up investigation was performed according to the Japanese guidelines[19]. Data on the patients’ clinical characteristics, operative findings, and pathological findings were collected from the medical records. Follow-up data for all patients were available, and the study was terminated in July 2018.
The primary outcome was the overall survival (OS) duration on an intention-to-treat basis. OS was defined as the time from resection of the primary tumor to death from any cause, or was censored at the date of the last follow-up. The secondary endpoints were the disease-free survival (DFS) duration and the preoperative and postoperative morbidity rates. DFS was defined as the time between curative surgery and the first relapse, a second primary colon cancer, death from any cause when no evidence of relapse was recorded, or the last date at which the patient was known to be free of disease (time of censoring).
Continuous variables are expressed as medians and interquartile ranges (IQR). Correlations between categorical variables were analyzed using chi-squared tests, and continuous variables were analyzed using the Kruskal–Wallis test. The Kaplan–Meier method and log-rank test were used to compare survival curves. The Cox proportional hazards regression model was used for univariate and multivariate analyses. A forward-backward stepwise method was used to retain all of the variables with P < 0.05 in the final multivariate model. In addition, we calculated the propensity scores of the treatments and adjusted the hazard ratios (HRs) for OS and DFS by using inverse propensity scores as weights. All analyses were two-sided, and values of P < 0.05 were considered to indicate statistical significance. Statistical analyses were performed using IBM SPSS software version 25 (IBM Corp., Armonk, NY, United States) and R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria), with the “MASS”, “survival,” and “rms” packages.
A summary of the study design is shown in Figure 1. Forty patients underwent curative resection after intention to treat with bridge to surgery based on a diagnosis of RMCO. Of these, decompression tube insertion was attempted in 21 patients and SEMS placement in 19 patients. These patients comprised the decompression tube group and the SEMS group, respectively. In the decompression tube group, 8 and 13 patients received trans-nasal and trans-anal decompression tubes, respectively; 3 patients who received trans-anal tubes underwent emergency surgery due to technical or clinical failure of tube insertion. In the SEMS group, two patients received emergency surgery due to failure of SEMS placement.
Table 1 shows the clinical characteristics and pathological findings of the patients. The median age of the patients at the time of surgery was 67.5 years (IQR, 59-78.75 years). The median follow-up time was 3.02 years (IQR, 1.51-5.00 years). The TNM stage distribution was 0% stage I, 42.5% stage II, 42.5% stage III, and 15.0% stage IV. Patients with stage IV colon cancer underwent curative surgery with resection of metastatic lesions. There were no significant differences in patient characteristics among the three groups, with the exception of presence of lymphatic invasion (P = 0.042).
| All cases | Decompression tube | SEMS | P value | |
| Cases | 40 | 21 | 19 | |
| Age (median, IQR) | 67.5(59-78.75) | 68(62.5-78.5) | 66.5(56.5-81) | 0.63 |
| Sex | ||||
| Male | 19 | 11 | 8 | |
| Female | 21 | 10 | 11 | 0.545 |
| BMI (median, IQR) | 22.1 (21.1-24.9) | 23.0 (21.8-26.1) | 21.6 (20.6-23.5) | 0.098 |
| Location | ||||
| Cecum | 4 | 4 | 0 | |
| Ascending colon | 17 | 6 | 11 | |
| Transverse colon | 19 | 11 | 8 | 0.053 |
| Histological type | ||||
| W/D and M/D | 35 | 18 | 17 | |
| P/D, muc, and sig | 5 | 3 | 2 | 1 |
| Lymphatic invasion | ||||
| absent | 4 | 0 | 4 | |
| present | 36 | 21 | 15 | 0.042 |
| Vascular invasion | ||||
| absent | 5 | 1 | 4 | |
| present | 35 | 20 | 15 | 0.172 |
| TNM stage | ||||
| 2 | 17 | 8 | 9 | |
| 3 | 17 | 12 | 5 | |
| 4 | 6 | 1 | 5 | 0.063 |
| Observation period (median, IQR) | 3.02 (1.51-5.00) | 4.43 (1.88-5.83) | 2.05 (1.32-3.39) | 0.075 |
Table 2 shows the operative findings and complications for each group. The technical success rate of the decompression tube group was 90.5% (19 of 21 patients) and the overall success rate was 85.7% (18 of 21 patients). In two patients with technical failure of decompression tube insertion, the guide wire could not pass the tumor. Perforation of the colon wall occurred in one patient with clinical failure of decompression tube insertion. The median time from decompression tube insertion to surgery was 8 d. In the SEMS group, the technical success rate was 94.7% (18 of 19 patients) and the overall success rate of SEMS placement was 89.5% (17 of 19 patients). In the case of technical failure of SEMS placement, the guide wire did not pass the tumor. The one case of clinical failure was due to perforation of the colon wall. The median time from SEMS placement to surgery was 23 d.
| Decompression tube | SEMS | P value | |
| Cases | 21 | 19 | |
| Operation method | |||
| Open | 8 | 0 | |
| Laparoscopy | 13 | 19 | 0.004 |
| Harvested LN (median, IQR) | 15 (9.5-27) | 28 (15-34) | 0.028 |
| Operation time (min, median, IQR) | 134 (85.5-147) | 124 (84-143) | 0.807 |
| Blood loss (mL, median, IQR) | 50 (10-81.5) | 10 (0-50) | 0.033 |
| Complications associated with bowel decompression | 3 | 2 | 1.000 |
| Technical failure | 2 | 1 | |
| Colon perforation during decompression | 1 | 1 | |
| Overall postoperative complications | 8 | 6 | 0.748 |
| sSSI | 3 | 3 | |
| dSSI | 0 | 2 | |
| Prolonged ileus | 3 | 0 | |
| Heart failure | 1 | 0 | |
| Pneumonia | 1 | 0 | |
| Pseudomembranous colitis | 1 | 0 | |
| Delirium | 0 | 1 | |
| Clavian-Dindo classification | |||
| 0 | 13 | 13 | |
| I | 2 | 4 | |
| II | 5 | 0 | |
| III | 1 | 2 | 0.116 |
Laparoscopic procedures were performed more frequently in the SEMS group than in the decompression tube group (100% vs 61.9%, P = 0.004). The SEMS group had significantly less blood loss and a larger number of dissected lymph nodes. The overall postoperative morbidity rate did not differ significantly between the two treatment groups (decompression tube, 38.1%; SEMS, 31.6%; P = 0.748), but prolonged ileus occurred only in the decompression tube group. No mortality occurred within 30 d after surgery.
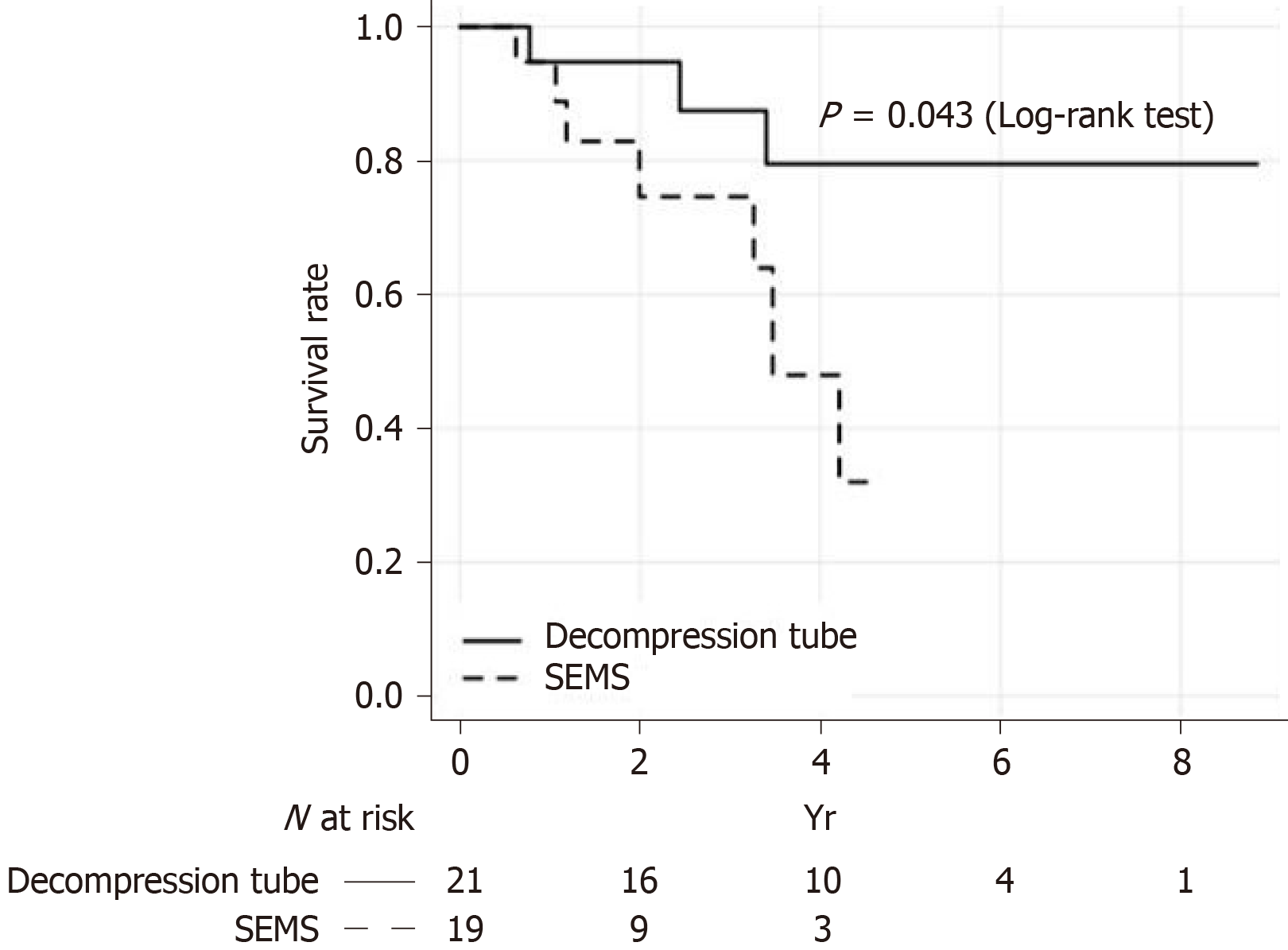
The OS of patients on an intention-to-treat basis is shown in Figure 2. The 5-year OS rate was significantly higher in the decompression tube group than in the SEMS group (79.5% vs 32%; log-rank test, P = 0.043). The relationship between management strategy and OS was analyzed using the Cox proportional hazards regression model. In a multivariate analysis using the stepwise method, the bridge to surgery using a decompression tube was an independent prognostic factor for OS along with sex, histological type, and TNM stage (Table 3). Compared with the decompression tube group, the HR of the bridge to surgery using SEMS was 17.41 [95% confidence interval (CI), 2.50-121; P = 0.004]. Propensity scores for the two treatments were calculated using a logistic analysis that included the following preoperative factors: age, sex, BMI, primary site, and TNM stage. The propensity score–adjusted OS was significantly higher in the decompression tube group than in the SEMS groups (HR = 4.51, P = 0.046).
| Variables | HR | 95%CI | P value |
| Sex | |||
| Male | Reference | ||
| Female | 0.08 | 0.01-0.49 | 0.006 |
| Histological type | |||
| W/D and M/D | Reference | ||
| P/D, muc, and sig | 6.61 | 0.80-54.36 | 0.079 |
| TNM Stage | |||
| 2 | Reference | ||
| 3 | 14.64 | 1.98-108 | 0.009 |
| 4 | 18.90 | 1.48-242 | 0.024 |
| Bowel decompression | |||
| Decompression tube | Reference | ||
| SEMS | 17.41 | 2.50-121 | 0.004 |
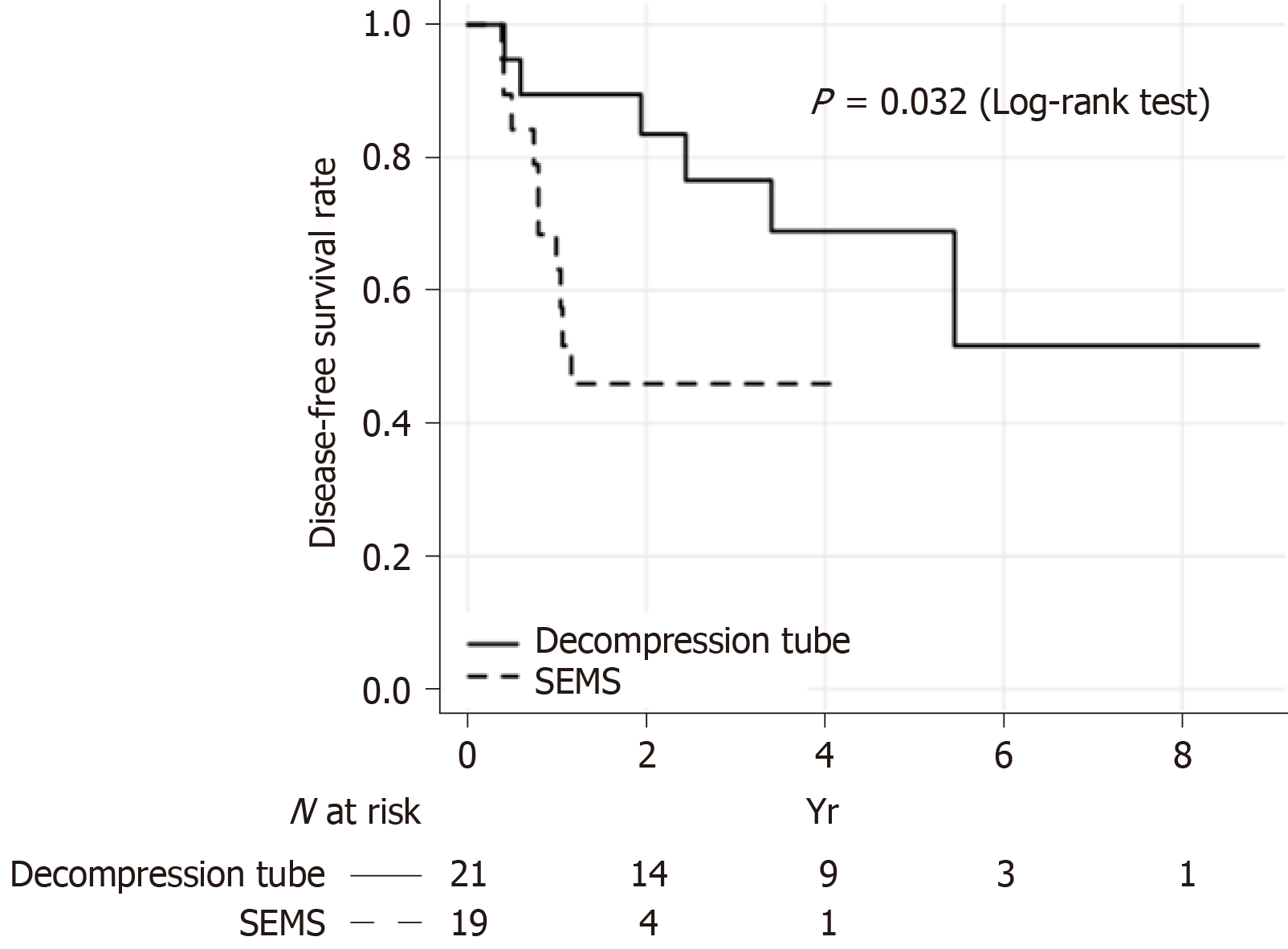
The results of the Kaplan–Meier analysis of DFS on an intention-to-treat basis are shown in Figure 3. The 3-year DFS rate was significantly higher in the decompression tube group than in the SEMS group (68.9% vs 45.9%; log-rank test, P = 0.032). In the multivariate analysis, the bowel decompression method was a significant prognostic factor along with age, histological type, number of lymph nodes dissected, and TNM stage (Table 4). Bridge to surgery using a decompression tube significantly improved the DFS compared with bridge to surgery using SEMS (HR = 14.56, P = 0.003). Analysis of the propensity score–adjusted DFS rate in the decompression tube group yielded the same results (decompression tube vs SEMS: HR = 3.83, P = 0.021).
| Variables | HR | 95%CI | P value |
| Age | 0.93 | 0.88-0.99 | 0.013 |
| Histological type | |||
| W/D and M/D | Reference | ||
| P/D, muc, and sig | 3.63 | 0.80-16.31 | 0.092 |
| Harvested LN | 0.93 | 0.87-1.00 | 0.047 |
| TNM Stage | |||
| 2 | Reference | ||
| 3 | 5.68 | 1.42-22.75 | 0.014 |
| 4 | 2.00 | 0.37-10.55 | 0.429 |
| Bowel decompression | |||
| Decompression tube | Reference | ||
| SEMS | 14.56 | 2.50-85.57 | 0.003 |
Five patients who were scheduled for treatment with a decompression tube or SEMS received emergency surgery due to technical or clinical failure. The 5-year survival rates of patients actually treated with the decompression tube and SEMS were 84.4% and 38.8%, respectively (P = 0.110, log-rank test), and the 3-year DFS rates were 71.3% and 51.8%, respectively (P = 0.113, log-rank test).
Among patients with RMCO, those who received bridge to surgery using a trans-nasal or trans-nasal decompression tube had better outcomes in this study. Moreover, the perioperative morbidity rate of patients treated with decompression tubes was similar to that of patients receiving SEMS. Patients treated with decompression tubes also had a better OS and DFS. These results were confirmed statistically in a multivariate analysis using the stepwise method and propensity score adjustment. Although evidence of the optimum treatment for RMCO is lacking[6,12], preoperative use of a bowel decompression tube may improve the prognosis.
We report here the efficacy of trans-nasal and trans-anal decompression tubes for RMCO. A trans-nasal long intestinal tube reportedly improves bowel expansion in patients with RMCO[20] and a trans-anal tube facilitates preoperative colonic lavage for one-stage surgery for left-sided malignant colorectal obstruction[16,17,21]. Indeed, patients with malignant colorectal obstruction treated with trans-anal decompression tubes reportedly have an improved prognosis[18], possibly due to the high morbidity and mortality rates of emergency surgery for RMCO[22] or the conservative colectomy typically performed during emergency surgery for obstructive colon cancer. Thus, preoperative decompression tube placement may be effective for RMCO, but this requires external validation.
We also investigated the effects of a bridge to surgery using SEMS. SEMS placement is not recommended for left-sided malignant colonic obstruction as a bridge to surgery, and evidence of its suitability for RMCO is lacking[10]. Although SEMS can be successfully placed in the right colon[23,24], this did not significantly improve the long-term outcomes in a multicenter retrospective study. Our findings are in agreement with these previous reports. The reason for the superiority of the decompression tube may be the risk of tumor compression (resulting in disease progression) associated with SEMS placement. The oncological risk of SEMS may counteract the advantage of avoiding emergency surgery.
Whereas left-sided malignant colonic obstruction has been researched extensively, few studies have addressed RMCO because the larger diameter and softer feces of the right colon result in a lower prevalence rate. The softer feces may explain the good results of bowel decompression. The successful SEMS insertion rate for RMCO is reportedly 96%-100%, higher than that for left-sided malignant colonic obstruction[17,24]. A decompression tube facilitates bowel decompression and lavage to a greater extent in the right than the left colon. In this study, both SEMS and decompression tubes showed highly successful decompression rates. Because trans-anal and trans-nasal decompression tubes enable colonic lavage and are not tumor-invasive, they can be recommended for the management of RMCO.
Regarding operative findings and short-term outcomes, the bridge to surgery using the SEMS was associated with a higher frequency of laparoscopic surgery and less blood loss during the operation. Previous reports suggested that bowel decompression with SEMS for malignant colonic obstruction increased the need for laparoscopic surgery[13,16,25]. Moreover, in a meta-analysis, laparoscopic surgery for malignant colonic obstruction was not found to result in significantly different morbidity and mortality rates compared with open laparotomy and was more likely to enable minimally invasive surgery[26]. In the present study, the reason for the higher rate of laparoscopic surgery in patients in the SEMS group might be historical, as we routinely used a decompression tube preoperatively in all patients with RMCO from 2007 to 2011, and have preferentially used SEMS since 2012. However, we demonstrated that laparoscopic surgery after good intestinal decompression can lead to safer and less invasive surgery. As few studies with high evidence levels regarding laparoscopic surgery for malignant obstruction are available, further investigations, including assessments of long-term outcomes, are needed.
To reduce the possibility of selection bias in this retrospective study, we applied inverse propensity scores as weights, which yielded appropriate estimates with less mean squared error, increasing the reliability of the results[27]. In addition, to increase the robustness of the study, we performed an intention-to-treat analysis. Patients in the decompression tube and SEMS groups were analyzed as if they had received only those treatments, even if they actually underwent emergency surgery due to treatment failure.
There were several limitations to our study. First, as it involved a single center, the findings require external validation. Second, relatively few patients were analyzed; this is unavoidable due to the low incidence of RMCO. Third, the treatments applied for malignant colonic obstruction differ among Europe, the United States, and Asia. In Japan, long trans-nasal and trans-anal tubes are routinely used for bowel obstruction. However, in Europe and the US, long tubes are not routinely used for bowel obstruction, based on the results of an older trial[28], and trans-anal decompression tubes are not available. In addition, the preoperative quality of life of RMCO patients who are treated with a decompression tube is obviously worse than that of those treated with SEMS. The fecal odor from the tube and the presence of the tube itself make patients feel extremely uncomfortable[16]. Nevertheless, as recent studies have demonstrated the satisfactory performance of trans-nasal and trans-anal decompression tubes[18,29], their efficacy for the management of RMCO should be evaluated in a multi-center randomized controlled study.
In conclusion, preoperative bowel decompression using trans-nasal and trans-anal decompression tubes for RMCO is safe and may improve the long-term outcomes.
As the incidence of obstruction with left-sided colon cancer is reportedly higher than that in right-sided colon cancer, there is a lack of data regarding the management of right-sided malignant colonic obstruction (RMCO).
Although emergency surgery is a standard treatment for malignant colonic obstruction, the efficacy of bridges to surgery using self-expandable metallic stents (SEMS) or decompression tubes has only recently been evaluated.
To evaluate the optimum management strategy for patients with RMCO by comparing the perioperative and oncologic outcomes of bridges to surgery using decompression tubes and metallic stents.
This was a single-center, retrospective observational study. The subjects were patients diagnosed with RMCO who underwent curative surgical resection. We compared patients who were preoperatively treated with SEMS to those treated with decompression tubes. The primary endpoint was the overall survival (OS) duration on an intention-to-treat basis and the secondary endpoints were the disease-free survival (DFS) duration and the preoperative and postoperative morbidity rates. In addition, to reduce the likelihood of selection bias, we applied inverse propensity scores as weights.
There was no significant difference in perioperative morbidity rate between the two groups. The OS rate was significantly higher in the decompression tube group than the SEMS group (5-year OS rates of 79.5 and 32%, respectively, P = 0.043). Multivariate analysis revealed that the bridge to surgery using a decompression tube was significantly associated with the OS (hazard ratio, 17.41; P = 0.004). The 3-year DFS rate was significantly higher in the decompression tube group than the SEMS group (68.9% vs 45.9%; log-rank test, P = 0.032). A propensity score–adjusted analysis also demonstrated that the prognosis was significantly better in the decompression tube group than in the SEMS group.
The results of this study suggest that patients with RMCO who received a bridge to surgery using a trans-nasal or trans-nasal decompression tube had better outcomes; these results were confirmed statistically in a multivariate analysis using the stepwise method and propensity score adjustment.
Because this study used a single-center retrospective design and included relatively few patients, further investigations, such as a multi-center randomized controlled study, are needed. In addition, as the decompression tubes can make patients uncomfortable, a study including quality of life measures is desirable.
| 1. | Cheynel N, Cortet M, Lepage C, Benoit L, Faivre J, Bouvier AM. Trends in frequency and management of obstructing colorectal cancers in a well-defined population. Dis Colon Rectum. 2007;50:1568-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 2. | Jullumstrø E, Wibe A, Lydersen S, Edna TH. Colon cancer incidence, presentation, treatment and outcomes over 25 years. Colorectal Dis. 2011;13:512-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 3. | Winner M, Mooney SJ, Hershman DL, Feingold DL, Allendorf JD, Wright JD, Neugut AI. Incidence and predictors of bowel obstruction in elderly patients with stage IV colon cancer: A population-based cohort study. JAMA Surg. 2013;148:715-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 4. | Phillips RK, Hittinger R, Fry JS, Fielding LP. Malignant large bowel obstruction. Br J Surg. 1985;72:296-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 270] [Article Influence: 6.6] [Reference Citation Analysis (1)] |
| 5. | Yang Z, Wang L, Kang L, Xiang J, Peng J, Cui J, Huang Y, Wang J. Clinicopathologic characteristics and outcomes of patients with obstructive colorectal cancer. J Gastrointest Surg. 2011;15:1213-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Tekkis PP, Kinsman R, Thompson MR, Stamatakis JD; Association of Coloproctology of Great Britain, Ireland. The Association of Coloproctology of Great Britain and Ireland study of large bowel obstruction caused by colorectal cancer. Ann Surg. 2004;240:76-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 220] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 7. | Gorissen KJ, Tuynman JB, Fryer E, Wang L, Uberoi R, Jones OM, Cunningham C, Lindsey I. Local recurrence after stenting for obstructing left-sided colonic cancer. Br J Surg. 2013;100:1805-1809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 8. | Sabbagh C, Browet F, Diouf M, Cosse C, Brehant O, Bartoli E, Mauvais F, Chauffert B, Dupas JL, Nguyen-Khac E, Regimbeau JM. Is stenting as "a bridge to surgery" an oncologically safe strategy for the management of acute, left-sided, malignant, colonic obstruction? A comparative study with a propensity score analysis. Ann Surg. 2013;258:107-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 206] [Article Influence: 15.8] [Reference Citation Analysis (2)] |
| 9. | Sloothaak DA, van den Berg MW, Dijkgraaf MG, Fockens P, Tanis PJ, van Hooft JE, Bemelman WA; collaborative Dutch Stent-In study group. Oncological outcome of malignant colonic obstruction in the Dutch Stent-In 2 trial. Br J Surg. 2014;101:1751-1757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 170] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 10. | van Hooft JE, Bemelman WA, Oldenburg B, Marinelli AW, Lutke Holzik MF, Grubben MJ, Sprangers MA, Dijkgraaf MG, Fockens P; collaborative Dutch Stent-In study group. Colonic stenting versus emergency surgery for acute left-sided malignant colonic obstruction: A multicentre randomised trial. Lancet Oncol. 2011;12:344-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 318] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 11. | van Hooft JE, van Halsema EE, Vanbiervliet G, Beets-Tan RG, DeWitt JM, Donnellan F, Dumonceau JM, Glynne-Jones RG, Hassan C, Jiménez-Perez J, Meisner S, Muthusamy VR, Parker MC, Regimbeau JM, Sabbagh C, Sagar J, Tanis PJ, Vandervoort J, Webster GJ, Manes G, Barthet MA, Repici A; European Society of Gastrointestinal Endoscopy (ESGE). Self-expandable metal stents for obstructing colonic and extracolonic cancer: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Gastrointest Endosc. 2014;80:747-761.e1-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 12. | Hsu TC. Comparison of one-stage resection and anastomosis of acute complete obstruction of left and right colon. Am J Surg. 2005;189:384-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Kye BH, Lee YS, Cho HM, Kim JG, Oh ST, Lee IK, Kang WK, Ahn CH, Lee SC, Park JK, Kim HJ. Comparison of Long-Term Outcomes Between Emergency Surgery and Bridge to Surgery for Malignant Obstruction in Right-Sided Colon Cancer: A Multicenter Retrospective Study. Ann Surg Oncol. 2016;23:1867-1874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Lelcuk S, Ratan J, Klausner JM, Skornick Y, Merhav A, Rozin RR. Endoscopic decompression of acute colonic obstruction. Avoiding staged surgery. Ann Surg. 1986;203:292-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Chung TS, Lim SB, Sohn DK, Hong CW, Han KS, Choi HS, Jeong SY. Feasibility of single-stage laparoscopic resection after placement of a self-expandable metallic stent for obstructive left colorectal cancer. World J Surg. 2008;32:2275-2280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Matsuda A, Miyashita M, Matsumoto S, Sakurazawa N, Takahashi G, Matsutani T, Yamada M, Uchida E. Comparison between metallic stent and transanal decompression tube for malignant large-bowel obstruction. J Surg Res. 2016;205:474-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Moroi R, Endo K, Ichikawa R, Nagai H, Shinkai H, Kimura T, Ishiyama F, Yaguchi K, Kayaba S, Shimosegawa T. The Effectiveness of Self-Expandable Metallic Stent Insertion in Treating Right-Sided Colonic Obstruction: A Comparison between SEMS and Decompression Tube Placement and an Investigation of the Safety and Difficulties of SEMS Insertion in Right Colons. Gastroenterol Res Pract. 2014;2014:372918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Shigeta K, Baba H, Yamafuji K, Kaneda H, Katsura H, Kubochi K. Outcomes for patients with obstructing colorectal cancers treated with one-stage surgery using transanal drainage tubes. J Gastrointest Surg. 2014;18:1507-1513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Watanabe T, Itabashi M, Shimada Y, Tanaka S, Ito Y, Ajioka Y, Hamaguchi T, Hyodo I, Igarashi M, Ishida H, Ishihara S, Ishiguro M, Kanemitsu Y, Kokudo N, Muro K, Ochiai A, Oguchi M, Ohkura Y, Saito Y, Sakai Y, Ueno H, Yoshino T, Boku N, Fujimori T, Koinuma N, Morita T, Nishimura G, Sakata Y, Takahashi K, Tsuruta O, Yamaguchi T, Yoshida M, Yamaguchi N, Kotake K, Sugihara K; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol. 2015;20:207-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 451] [Cited by in RCA: 499] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 20. | Tomita R, Fujisaki S, Azuhata T, Takamoto Y, Sakurai K. Usefulness of Transnasal Decompression Tubes in Patients with Right-Sided Obstructive Colorectal Cancer. Gan To Kagaku Ryoho. 2016;43:1647-1649. [PubMed] |
| 21. | Li CY, Guo SB, Wang NF. Decompression of acute left-sided malignant colorectal obstruction: Comparing transanal drainage tube with metallic stent. J Clin Gastroenterol. 2014;48:e37-e42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Shimura T, Joh T. Evidence-based Clinical Management of Acute Malignant Colorectal Obstruction. J Clin Gastroenterol. 2016;50:273-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 23. | Geraghty J, Sarkar S, Cox T, Lal S, Willert R, Ramesh J, Bodger K, Carlson GL. Management of large bowel obstruction with self-expanding metal stents. A multicentre retrospective study of factors determining outcome. Colorectal Dis. 2014;16:476-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Repici A, Adler DG, Gibbs CM, Malesci A, Preatoni P, Baron TH. Stenting of the proximal colon in patients with malignant large bowel obstruction: Techniques and outcomes. Gastrointest Endosc. 2007;66:940-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Ji WB, Kwak JM, Kang DW, Kwak HD, Um JW, Lee SI, Min BW, Sung NS, Kim J, Kim SH. Clinical benefits and oncologic equivalence of self-expandable metallic stent insertion for right-sided malignant colonic obstruction. Surg Endosc. 2017;31:153-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Cirocchi R, Cesare Campanile F, Di Saverio S, Popivanov G, Carlini L, Pironi D, Tabola R, Vettoretto N. Laparoscopic versus open colectomy for obstructing right colon cancer: A systematic review and meta-analysis. J Visc Surg. 2017;154:387-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Austin PC. The performance of different propensity score methods for estimating marginal hazard ratios. Stat Med. 2013;32:2837-2849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 504] [Cited by in RCA: 682] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 28. | Fleshner PR, Siegman MG, Slater GI, Brolin RE, Chandler JC, Aufses AH. A prospective, randomized trial of short versus long tubes in adhesive small-bowel obstruction. Am J Surg. 1995;170:366-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Chen XL, Ji F, Lin Q, Chen YP, Lin JJ, Ye F, Yu JR, Wu YJ. A prospective randomized trial of transnasal ileus tube vs nasogastric tube for adhesive small bowel obstruction. World J Gastroenterol. 2012;18:1968-1974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Braticevici CF, Cremers I, Sica G, Xie Q S-Editor: Yan JP L-Editor: A E-Editor: Song H