Published online Apr 28, 2019. doi: 10.3748/wjg.v25.i16.1986
Peer-review started: February 6, 2019
First decision: March 5, 2019
Revised: March 14, 2019
Accepted: March 24, 2019
Article in press: March 24, 2019
Published online: April 28, 2019
Processing time: 83 Days and 13.6 Hours
Regional lymph node metastasis in patients with hepatocellular carcinoma (HCC) is not uncommon, and is often under- or misdiagnosed. Regional lymph node metastasis is associated with a negative prognosis in patients with HCC, and surgical resection of lymph node metastasis is considered feasible and efficacious in improving the survival and prognosis. It is critical to characterize lymph node preoperatively. There is currently no consensus regarding the optimal method for the assessment of regional lymph nodes in patients with HCC.
To evaluate the diagnostic value of single source dual energy computed tomography (CT) in regional lymph node assessment for HCC patients.
Forty-three patients with pathologically confirmed HCC who underwent partial hepatectomy with lymphadenectomy were retrospectively enrolled. All patients underwent dual-energy CT preoperatively. Regional lymph nodes (n = 156) were divided into either a metastatic (group P, n = 52) or a non-metastasis group (group N, n = 104), and further, according to pathology, divided into an active hepatitis (group P1, n = 34; group N1, n = 73) and a non-active hepatitis group (group P2, n = 18; group N2, n = 31). The maximal short axis diameter (MSAD), iodine concentration (IC), normalized IC (NIC), and the slope of the spectral curve (λHU) of each group in the arterial phase (AP), portal phase (PP), and delayed phase (DP) were analyzed.
Analysis of the MSAD, IC, NIC, and λHU showed statistical differences between groups P and N (P < 0.05) during all three phases. To distinguish benign from metastatic lymph nodes, the diagnostic efficacy of IC, NIC, and λHU in the PP was the best among the three phases (AP, PP, and DP), with a sensitivity up to 81.9%, 83.9%, and 81.8%, and a specificity up to 82.4%, 84.1% and 84.1%, respectively. The diagnostic value of combined analyses of MSAD with IC, NIC, or λHU in the PP was superior to the dual energy CT parameters alone, with a sensitivity up to 84.5%, 86.9%, and 86.2%, and a specificity up to 83.0%, 93.6% and 89.8%, respectively. Between groups P1 and P2 and groups N1 and N2, only IC, NIC, and λHU between groups N1 and N2 in the PP had a statistically significant difference (P < 0.05).
Dual-energy CT contributes beneficially to regional lymph node assessment in HCC patients. Combination of MSAD with IC, NIC, or λHU values in the PP is superior to using any single parameter alone. Active hepatitis does not deteriorate the capabilities for characterization of metastatic lymph nodes.
Core tip: Dual-energy computed tomography (CT) contributes beneficially to regional lymph node assessment in hepatocellular carcinoma (HCC) patients, which can differentiate metastatic and non-metastatic lymph nodes for improving regional lymph node staging of HCC. Combination of maximal short axis diameter with dual-energy CT quantifiable parameters (iodine concentration, normalized iodine concentration, or the slope of the spectral curve) in the portal phase can be more advantageous in regional lymph node assessment. Active hepatitis does not deteriorate the detection and characterization of metastatic lymph nodes in HCC.
- Citation: Zeng YR, Yang QH, Liu QY, Min J, Li HG, Liu ZF, Li JX. Dual energy computed tomography for detection of metastatic lymph nodes in patients with hepatocellular carcinoma. World J Gastroenterol 2019; 25(16): 1986-1996
- URL: https://www.wjgnet.com/1007-9327/full/v25/i16/1986.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i16.1986
Regional lymph node metastasis in patients with hepatocellular carcinoma (HCC) is not uncommon. Although it is found in only 8% of cases at the time of hepatectomy[1], in autopsies it has been reported to be as high as 26% to 37%[1], suggesting that lymph node metastases are often under- or misdiagnosed[2,3]. Regional lymph node metastasis is associated with a negative prognosis in patients with HCC[3,4], as the incidence of lymph node metastasis usually indicates an advanced tumor stage with infiltration of blood vessels[2]. Even with aggressive treatment such as radical resection, the reported median survival rate is much lower than that for patients without lymph node metastasis (28 mo vs 53 mo), and the tumor recurrence rate (a median follow-up of 43 mo) is significantly higher (82.05% vs 57.64%)[1-3,5]. Although effective treatment for metastatic lymph nodes of HCC has not yet been established[6], surgical resection of lymph node metastasis is considered feasible and efficacious in improving the survival and prognosis of patients with HCC[1,2,4,6].
There is currently no consensus regarding the optimal method for the assessment of regional lymph nodes in patients with HCC[7]. The diagnostic criteria of conventional computed tomography (CT) and magnetic resonance imaging (MRI) scans for differentiating malignant and benign lymph nodes were established according to size (maximal short axis diameter ≥ 10 mm)[7,8]. However, it may be inaccurate in detecting metastatic lymph nodes in HCC, as over 80% of HCC patients in China have chronic hepatitis B virus (HBV) infection[9,10], and it is well documented that patients with chronic hepatitis or cirrhosis also have benign enlarged lymph nodes[10,11]. Diffusion-weighted MRI is sensitive in detecting metastatic lymph nodes, but it is easily affected by physical motion of the abdomen, and the accuracy will be lower (as low as 76%) when metastatic lymph nodes show necrosis[12]. 18F-fluoro deoxyglucose-positron emission tomography/CT (18F-FDG-PET/CT) is a non-invasive method for diagnosing lymph node metastasis, but it is expensive, low in spatial resolution[13,14], and can give false positive results[13,15,16]. Additionally, for lymph node metastases in HCC, the diagnostic value of a 18F-FDG-PET/CT scan is lower than conventional imaging modalities[13]. Therefore, it is critical to explore better techniques for lymph node assessment in HCC patients.
Dual-energy CT can provide quantitative information with monochromatic spectral images, material decomposition images, spectrum curves, and effective atomic number images, based on the principle that different materials have their own characteristic X-ray absorption coefficient, combined with the technology of instantaneously switching 80/140 kVp (kilovolt peak) voltage[17]. The role of dual-energy CT in the evaluation of malignant lymph nodes in colorectal cancer, gynecological malignancies, hypopharyngeal cancer, and lung cancer has been reported[18-23]. However, there have been no dual-energy CT studies regarding lymph node assessment in HCC. The objective of this study was to evaluate the diagnostic value of dual-energy CT in preoperative lymph node assessment for HCC patients.
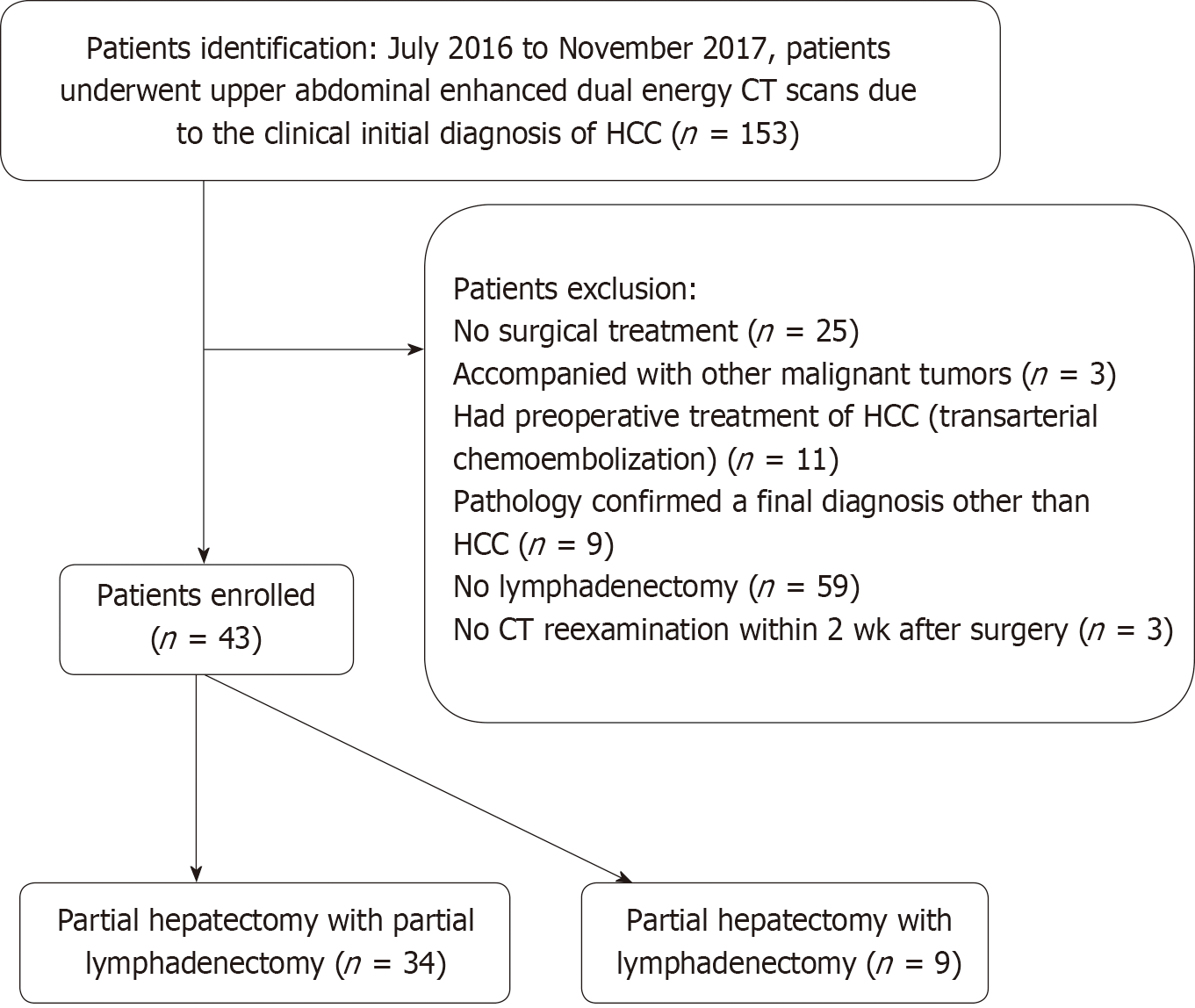
This retrospective study was approved by the institutional review board of Sun Yat-sen Memorial Hospital. In this study, we conducted searches in a Picture Archiving and Communication System for patients suspected of having HCC and who underwent contrast-enhanced upper abdomen dual energy CT scans between July 2016 and November 2017. There were 153 patients identified with this search process, and among them 110 were excluded for the following reasons (Figure 1): Patients did not receive surgical treatment (n = 25), patients also had other malignant tumors (n = 3), patients underwent preoperative treatment of HCC (transarterial chemoembolization) (n = 11), pathology results confirmed a final diagnosis of non-HCC (n = 9), patients did not undergo lymphadenectomy or partial lymphadenectomy (n = 59), and patients did not have a CT re-examination within two weeks after surgery (n = 3). Ultimately, there were 43 patients (37 males and 6 females; median age of 51 years; 34 patients with HBV and 10 patients with liver cirrhosis; average size of resected tumor: 4.6 cm) enrolled in the study.
CT scans were performed on a single source dual energy CT scanner (Discovery CT750 HD, GE Healthcare) with rapid KeV switching, including a pre-contrast CT plain scan and a post-contrast scan in the arterial phase (AP), portal phase (PP), and delayed phase (DP). The contrast enhanced scan began at 25 s (AP), 65 s (PP), and 180 s (DP) after the injection of Ioversol 320 (Optiray-Covidean) at a flow rate of 3 mL/s and an amount of 1.0 mL per kilogram of body weight. The scanning parameters were as follows: Tube current, 600 mA; tube voltage, 80/140 Kvp; tube rotation time, 0.8 s; pitch, 1.375:1; slice thickness, 1.25 mm; interval thickness, 1.25 mm. Further analysis of images was performed on a GE Advantage Workstation AW4.4 (GE Healthcare).
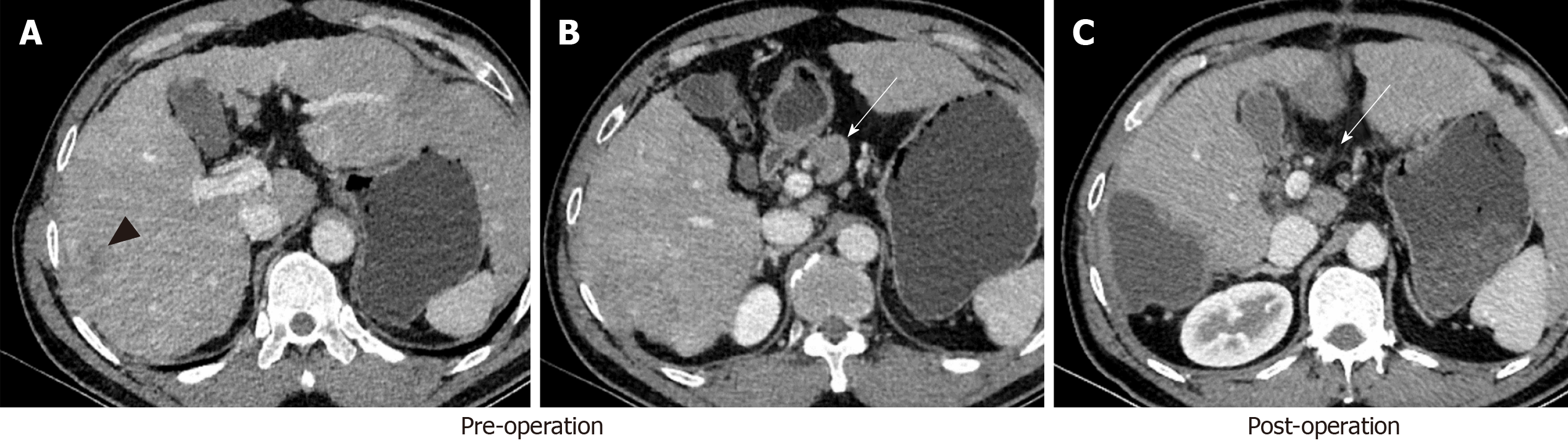
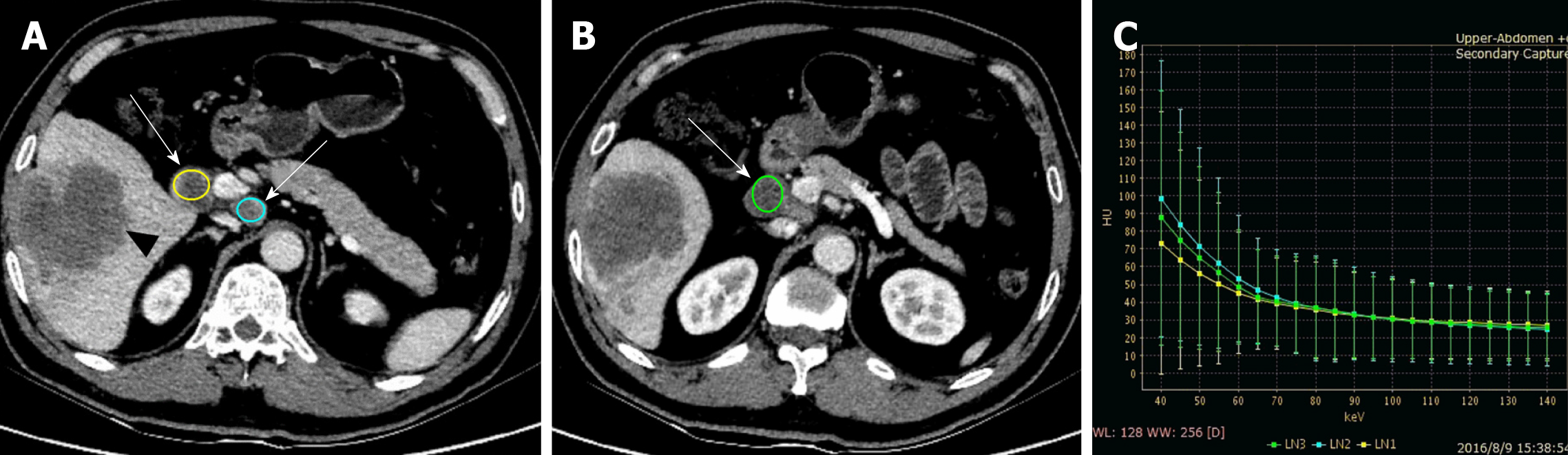
The image data of 43 patients with pathologically confirmed HCC were analyzed. Regional lymph nodes were measured independently by two radiologists with more than 10 years of experience. The radiologists were blinded to the pathological results (metastatic or non-metastatic) but were aware of the location of any removed lymph nodes. Lymph nodes with a short axis (≥ 4.0 mm), and which were confirmed in the resection range by comparing preoperative and postoperative CT images (performed within two weeks after operation), were all measured (Figure 2). A circle of region of interest (ROI) was manually drawn in the maximal axial section for lymph nodes, containing most of the lymph node volume as possible, and the average size of ROIs was larger than 12.0 mm2. Measurements were repeated three times and the average values were recorded. The iodine (water)-based material decomposition images and monochromatic images (energy ranging from 40 to 140 keV with a 10 keV interval) were reconstructed and analyzed. The following parameters were all measured in the AP, PP, and DP: (1) Iodine concentrations (IC) (mg/mL), the average value of which was calculated on the iodine (water)-based material decomposition images; (2) normalized IC (NIC), which was a calculation of the IC of lymph nodes to the IC of the thoracic aorta on the same slice, namely NIC = IC/ICaorta; and (3) slope of the spectral curve (λHU), which was divided from the HU curve by the difference between the CT values at 40 keV and 80 keV (as the curve from 80 keV to 140 keV tends to be a horizontal line): λHU = (HU40kev - HU80Kev)/40.
The regional lymphadenectomy included the lymph nodes in the hepatic pedicle (including the cystic duct, peri-choledochal, hilar, periportal, and peri proper hepatic artery), retro-pancreatic space, common hepatic artery, and celiac trunk[2,5]. Partial lymphadenectomy restricted the extent of lymphadenectomy only to the suspected lymph nodes which were enlarged on CT images. Removed regional lymph nodes were categorized into groups according to the gastric cancer treatment guidelines of the Japanese Gastric Cancer Association[24] for pathological examination. The status of lymph nodes larger than 4.0 mm was reported pathologically. When the removed lymph nodes were pathologically confirmed to be metastatic, we included them in a metastatic group (group P), and when the removed lymph nodes were confirmed to be non-metastatic, they were classified into a non-metastatic group (group N).
Clinical data and laboratory results of biochemical blood examinations of all patients were collected. Active hepatitis was diagnosed according to the Chinese guidelines of prevention and treatment of viral hepatitis (2000)[25]. Patients who met one of the following conditions were considered to have active hepatitis: (1) Positive findings of epidemiological contact history, clinical presentation, and viral hepatitis pathogenic examination, with an elevated serum alanine aminotransferase (ALT) level; (2) patients with chronic hepatitis and who had symptoms of hepatitis again, with positive biochemical tests results (elevated serum ALT); or (3) a histopathological examination of the liver confirmed hepatitis. Patients who did not meet the above requirements were considered as having no active hepatitis.
Statistical analyses were performed with SPSS 17.0 (SPSS Inc.). Quantitative data are presented as median (P25, P75). Independent samples t-test was used for comparing normally distributed quantitative data in the two groups and a nonparametric test was applied when the data were not normally distributed or there was heterogeneity of variances. The diagnostic efficacies of the axial size, IC, NIC, and λHU for lymph nodes in the AP, PP, and DP were determined by the area under the curve (AUC) from the corresponding receiver operative characteristics (ROC) curves. A P-value ≤ 0.05 was considered statistically significant. Cut-off values, AUC, sensitivity, and specificity of DECT parameters (axial size, IC, NIC, and λHU) for metastatic vs non-metastatic lymph nodes in HCC were measured.
A total of 156 lymph nodes (104 non-metastatic and 52 metastatic) from 43 patients were finally included in this study. Among them, ten patients had a total of 52 lymph nodes which were pathologically proven to be metastatic and therefore divided into group P, while the other 33 patients with 104 total lymph nodes that were non-metastatic and thus divided into group N. According to the active hepatitis diagnosis standard described above, the lymph nodes in groups P and N were further divided into an active hepatitis group (group P1, n = 34; group N1, n = 73) and a non-active hepatitis group (group P2, n = 18; group N2, n = 31), respectively.
The mean maximal short axis diameter of group P (metastasis group) was significantly longer than that of group N (non-metastasis group) [1.25 (0.90, 1.80) vs 0.60 (0.50, 0.80), P < 0.001)] (Table 1). There was no significant difference between group P1 (metastasis with active hepatitis) and group P2 (metastasis with non-active hepatitis), or group N1 (non-metastasis with active hepatitis) and group N2 (non-metastasis with non-active hepatitis) (Table 2).
| Parameter | Metastatic (group P) | Non-metastatic (group N) | P-value | |
| Maximal short axis diameter (cm) | 1.25 (0.90, 1.80) | 0.60 (0.50, 0.80) | < 0.001 | |
| IC (mg/mL) | AP | 10.46 (9.41, 11.36) | 8.64 (7.62, 9.82) | < 0.001 |
| PP | 22.70 (19.08, 26.35) | 13.26 (9.91, 16.26) | < 0.001 | |
| DP | 19.03 (16.18, 21.34) | 14.09 (12.14, 15.71) | < 0.001 | |
| NIC | AP | 0.12 (0.10, 0.13) | 0.08 (0.06, 0.11) | < 0.001 |
| PP | 0.54 (0.37, 0.82) | 0.10 (0.07, 0.18) | < 0.001 | |
| DP | 0.57 (0.49, 0.77) | 0.34 (0.23, 0.44) | < 0.001 | |
| λHU | AP | 0.64 (0.57, 0.73) | 0.49 (0.41, 0.58) | < 0.001 |
| PP | 1.53 (1.26, 1.90) | 0.87 (0.60, 0.94) | < 0.001 | |
| DP | 1.30 (1.15, 1.74) | 0.85 (0.71, 1.40) | < 0.001 | |
| Metastatic | P-value | Non-metastatic | P-value | ||||
| Active hepatitis (group P1) | Non-active hepatitis (group P2) | Active hepatitis (group N1) | Non-active hepatitis (group N2) | ||||
| Maximal short axis diameter (cm) | 1.50 (1.12, 2.00) | 1.40 (0.90, 1.70) | 0.058 | 0.70 (0.58, 0.90) | 0.60 (0.50, 0.78) | 0.083 | |
| IC (mg/mL) | AP | 10.12 (7.63, 12.87) | 11.02 (8.40, 13.68) | 0.899 | 9.07 (6.35, 11.53) | 7.73 (5.46, 9.58) | 0.099 |
| PP | 20.75 (18.40, 24.28) | 18.83 (14.95, 21.31) | 0.647 | 15.03(10.21, 18.02) | 13.82 (10.40, 19.05) | 0.001 | |
| DP | 14.19 (11.59, 17.47) | 13.49 (10.53, 18.77) | 0.793 | 19.65 (14.47, 22.62) | 18.03 (13.78, 21.13) | 0.908 | |
| NIC | AP | 0.12 (0.08, 0.15) | 0.11 (0.08, 0.14) | 0.269 | 0.10 (0.07, 0.12) | 0.10 (0.07, 0.13) | 0.598 |
| PP | 0.39 (0.27, 0.51) | 0.34 (0.26, 0.43) | 0.165 | 0.31 (0.23, 0.39) | 0.19 (0.16, 0.26) | 0.013 | |
| DP | 0.55 (0.42, 0.68) | 0.51 (0.43, 0.65) | 0.785 | 0.40 (0.35, 0.56) | 0.35 (0.25, 0.44) | 0.647 | |
| λHU | AP | 0.62 (0.48, 0.82) | 0.70 (0.53, 0.84) | 0.195 | 0.49 (0.35, 0.64) | 0.57 (0.40, 0.73) | 0.161 |
| PP | 1.45 (1.15, 1.67) | 1.38 (0.89, 1.43) | 0.73 | 0.95 (0.65, 1.16) | 0.80 (0.68, 1.19) | 0.002 | |
| DP | 1.23 (0.90, 1.44) | 1.17 (1.01, 1.33) | 0.812 | 0.91 (0.75, 1.11) | 0.87 (0.66, 1.12) | 0.726 | |
IC, NIC, and λHU values were significantly higher in group P than in group N in the AP, PP, and DP (P < 0.05) (Table 1), and they were also significantly higher in group N1 than in group N2 in the PP (Table 2). There were no significant differences in IC, NIC, or λHU values between groups N1 and N2 in the AP and DP, nor between groups P1 and P2 in the AP, PP, or DP (Table 2).
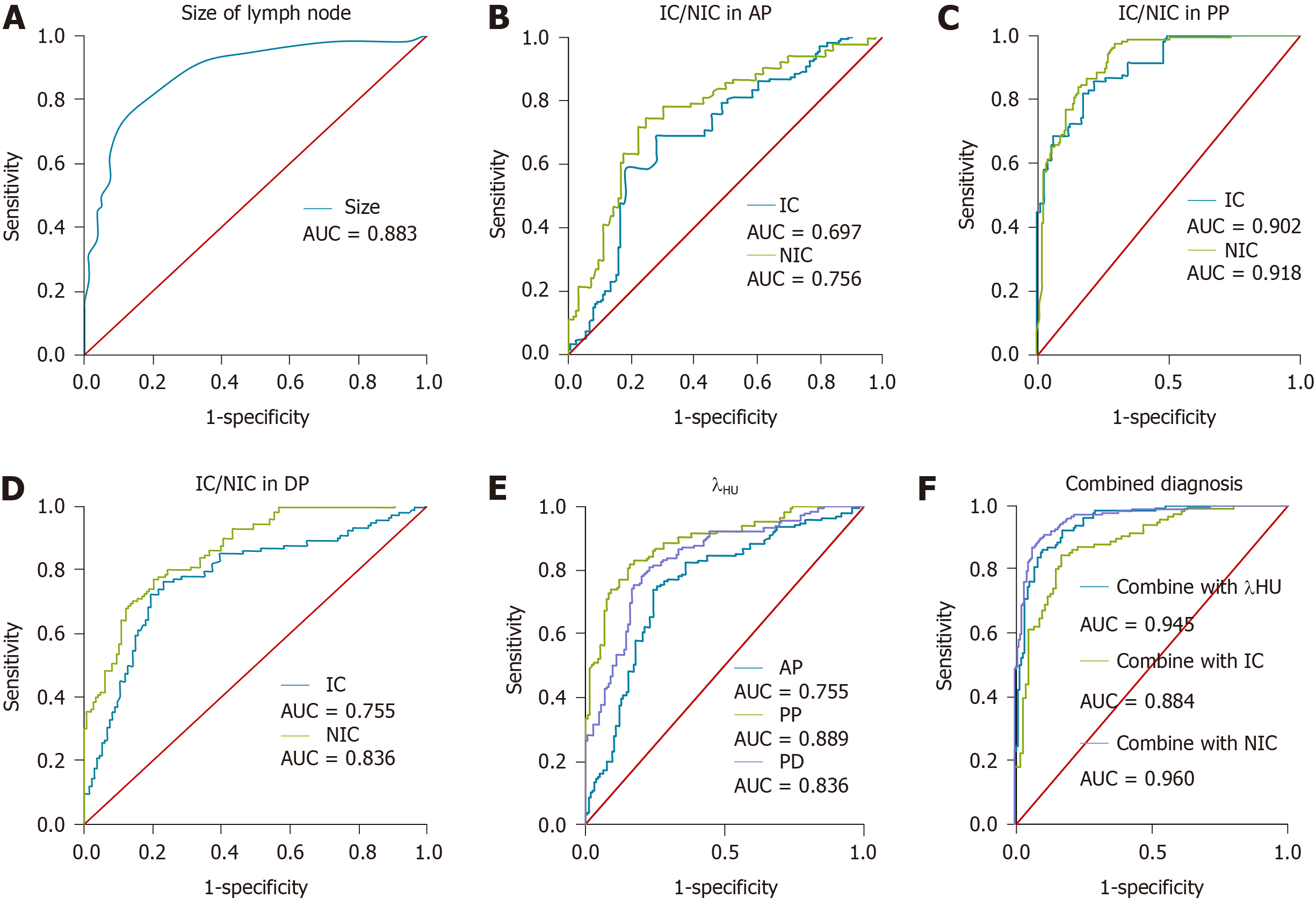
The ROC curves of maximal short axis diameter, IC, NIC, and λHU in the AP, PP, and DP for diagnosing metastatic lymph nodes were drawn. The ROC curves of combination diagnoses using the four parameters (maximal short axis diameter, IC, NIC, and λHU) were also determined (Figure 3). The AUC, optimal cut-off value, sensitivity, and specificity of each single parameter and combined parameters for differentiating group P from group N are shown in Table 3. The optimal threshold of the maximal short axis diameter was 0.950 cm, with a sensitivity and specificity of 73.3% and 88.7%, respectively. The AUCs of IC, NIC, and λHU in the PP were the largest at an optimal threshold of 18.060 mg/mL, 0.272, and 1.098, respectively, with a sensitivity and specificity up to 81.9% and 82.4%, 83.9% and 84.1%, and 81.8% and 84.1%, respectively. The diagnostic efficacy of combination of the maximal short axis diameter with IC, NIC, or λHU in the PP (combined diagnosis in parallel) was superior to using a single parameter, with a sensitivity up to 84.5%, 86.9%, and 86.2%, and a specificity up to 83.0%, 93.6%, and 89.8%, respectively. The diagnostic efficacy of the maximal short axis diameter with NIC (PP) was the best (Figures 4 and 5).
| Parameter | AUC | Cut-off value | Sensitivity, % | Specificity, % | |
| Maximal short axis diameter(cm) | 0.883 | 0.950 | 73.3 | 88.7 | |
| IC (mg/mL) | AP | 0.697 | 9.845 | 68.6 | 72.0 |
| PP | 0.902 | 18.060 | 81.9 | 82.4 | |
| DP | 0.755 | 16.005 | 73.6 | 76.9 | |
| NIC | AP | 0.756 | 0.106 | 72.8 | 73.9 |
| PP | 0.918 | 0.272 | 83.9 | 84.1 | |
| DP | 0.836 | 0.467 | 74.5 | 79.2 | |
| λHU | AP | 0.755 | 0.582 | 73.8 | 75.6 |
| PP | 0.889 | 1.098 | 81.8 | 84.1 | |
| DP | 0.836 | 1.077 | 78.1 | 79.0 | |
| Diameter + λHU (PP) | 0.945 | Combined diagnosis in parallel | 86.2 | 89.8 | |
| Diameter + IC (PP) | 0.884 | 84.5 | 83.0 | ||
| Diameter + NIC (PP) | 0.960 | 86.9 | 93.6 | ||
In this study, we found that the parameters including maximal short axis diameter, IC, NIC, and λHU were significantly different between metastatic and non-metastatic lymph nodes in HCC patients. These parameters had favorable diagnostic value for identifying metastatic lymph nodes in the PP. The diagnostic efficacy was notably higher when using a combination of analysis of maximal short axis diameter with IC, NIC, or λHU values. The maximal short axis diameter of metastatic lymph nodes was longer than that of non-metastatic lymph nodes in the present study, with a diagnostic sensitivity of 73.3%, and a specificity of 88.7%. This finding is different from that of Grobmyer et al[7], who evaluated preoperative CT images of 236 lymph nodes in 100 patients with primary or secondary hepatic malignancies and found that the positive predictive value of diameter of lymph nodes was as low as 39%. Thus, the diagnostic efficacy of short axis diameter analysis of lymph nodes still needs to be explored further.
Dual-energy CT can evaluate lymph nodes quantitatively. IC and NIC can reflect the difference in iodine contents of lymph nodes, and indirectly, their blood supply. λHU describes the dynamic change of CT values, and each tissue type has its characteristic HU slope of the curve[17,26]. Our study found that the IC and NIC values measured in the AP, PP, and DP were significantly higher in metastatic lymph nodes than in non-metastatic lymph nodes, and one explanation is that tumor cell invasion increases blood supply to lymph nodes[27]. We achieved a higher sensitivity with both IC and NIC in the PP, which is superior to conventional CT and PET/CT for HCC patients[16].
Both metastatic and non-metastatic lymph nodes followed descending spectrum curve patterns, but the curve pattern of the metastatic nodes was much steeper, suggesting that λHU could reflect different statuses of lymph nodes (metastatic or non-metastatic). The sensitivity of detecting malignant lymph nodes with λHU in the PP in our study is comparable to spectral CT in lung cancer[19,20] and colon cancer[20].
There was no statistically significant difference in the maximal short axis diameter of lymph nodes between patients with active hepatitis and non-active hepatitis in our study. Our result is different from that of Shu et al[10], and the possible reason may be due to different statuses of hepatitis and hepatic fibrosis[4,10,11]. The IC, NIC, and λHU values of non-metastatic lymph nodes in patients with active hepatitis were statistically higher than those in patients with non-active hepatitis in the PP, indicating that active hepatitis has an influence on regional lymph nodes, such as causing inflammation, resulting in increased blood flow and metabolism compared to normal lymph nodes[13,23]. There was no statistical difference in IC, NIC, or λHU values of metastatic lymph nodes between the active hepatitis group and non-active hepatitis group, suggesting that active hepatitis does not deteriorate the capabilities for detection and characterization of metastatic lymph nodes in HCC patients.
In our study, the parameters of dual energy CT (such as IC, NIC, and λHU) reliably identified the metastatic lymph nodes. The sensitivity and specificity of maximal short axis diameter combined with IC, NIC, or λHU values were higher than those of any single parameter (maximal short axis diameter, IC, NIC, or λHU) alone (IC: 84.5% and 83.0%, NIC: 86.9% and 93.6%, λHU: 86.2% and 89.8%, respectively). This indicates that the presence of metastatic lymph nodes should be considered in patients with an enlarged lymph node (≥ 0.950 cm), especially when suspected spectral quantitative parameters were noted in a dual energy CT scan. Dual energy CT can provide more reliable information for the identification of benign and malignant lymph nodes than conventional CT.
There were some limitations in this study. First, this was a retrospective study and the population size of this study was relatively small. Second, lymph nodes with a maximal short axis size of less than 4.0 mm were excluded from the study due to the uncertainty of ROI measurements. Third, the absence of a comparison with MRI presents another limitation. Finally, the distinction between normal lymph nodes, metastatic lymph nodes, and inflammatory lymph nodes was not analyzed in our study, since it was not our main aim.
In conclusion, dual-energy CT contributes beneficially to regional lymph node assessment for HCC patients. A diagnosis combining analysis of maximal short axis diameter with IC, NIC, or λHU values is superior to using any single parameter alone. Active hepatitis does not deteriorate the capabilities for detection and characterization of metastatic lymph nodes.
Regional lymph node metastasis in patients with hepatocellular carcinoma (HCC) is not uncommon, and is often under- or misdiagnosed. Regional lymph node metastasis is associated with a negative prognosis in patients with HCC, and surgical resection of lymph node metastasis is considered feasible and efficacious in improving the survival and prognosis. It is critical to characterize lymph node preoperatively. There is currently no consensus regarding the optimal method for the assessment of regional lymph nodes in patients with HCC.
Dual-energy computed tomography (CT) can provide quantitative information with monochromatic spectral images, material decomposition images, spectrum curves, and effective atomic number images. The role of dual-energy CT parameters in the evaluation of malignant lymph nodes has been reported with excellent results. However, there have been no dual-energy CT studies regarding lymph node assessment in HCC.
The main objective of our study was to evaluate the diagnostic value of dual energy CT parameters [such as iodine concentrations (IC), normalized IC (NIC), and slope of the spectral curve (λHU)] in regional lymph node assessment for HCC patients.
In this retrospective study, a total of 156 lymph nodes (33 patients with 104 non-metastatic and 10 patients with 52 metastatic) from 43 patients were finally included. According to the lymph node status, the lymph nodes were divided into group P (52 metastatic) and group N (104 non-metastatic). According to the active hepatitis diagnosis standard, the lymph nodes in groups P and N were further divided into an active hepatitis group (group P1, n = 34; group N1, n = 73) and a non-active hepatitis group (group P2, n = 18; group N2, n = 31), respectively. All patients underwent three-phase dual-energy CT scan preoperatively [arterial phase (AP), portal phase (PP), and delayed phase (DP)]. The maximal short axis diameter (MSAD), IC, NIC, and λHU of each group in the AP, PP, and DP were analyzed.
The MSAD, IC, NIC, and λHU showed statistical differences between groups P and N (P < 0.05 for all) in dual-energy CT scans in all the three phases. The diagnostic value of IC, NIC, and λHU in the PP to distinguish benign from metastatic lymph nodes was the best among the three phases (AP, PP, and DP), with a sensitivity up to 81.9%, 83.9%, and 81.8%, and specificity up to 82.4%, 84.1% and 84.1%, respectively. To distinguish benign from metastatic lymph nodes, the diagnostic value of combined analyses of MSAD with IC, NIC, or λHU in PP was superior to the dual energy CT parameters alone, with a sensitivity up to 84.5%, 86.9%, and 86.2%, and a specificity up to 83.0%, 93.6% and 89.8%, respectively. Between groups P1 and P2 and groups N1 and N2, only IC, NIC, and λHU between groups N1 and N2 in the PP had a statistically significant difference (P < 0.05).
Dual-energy CT parameters (IC, NIC, and λHU) are sensitive and specific, and can help to differentiate benign from metastatic lymph nodes in patients with HCC, especially in PP CT scans. The diagnostic efficacy of combined analysis of MSAD with IC, NIC, or λHU values is superior to using any single parameter alone. Active hepatitis does not deteriorate the capabilities for characterization of metastatic lymph nodes.
The future direction in this field will probably focus on the comparison of diagnostic efficacy of different imaging methods to differentiate benign from metastatic lymph nodes in patients with HCC, e.g., between dual-energy CT and magnetic resonance imaging.
| 1. | Tomimaru Y, Wada H, Eguchi H, Tomokuni A, Hama N, Kawamoto K, Marubashi S, Umeshita K, Doki Y, Mori M, Wakasa K, Nagano H. Clinical significance of surgical resection of metastatic lymph nodes from hepatocellular carcinoma. Surg Today. 2015;45:1112-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Xiaohong S, Huikai L, Feng W, Ti Z, Yunlong C, Qiang L. Clinical significance of lymph node metastasis in patients undergoing partial hepatectomy for hepatocellular carcinoma. World J Surg. 2010;34:1028-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Xia F, Wu L, Lau WY, Li G, Huan H, Qian C, Ma K, Bie P. Positive lymph node metastasis has a marked impact on the long-term survival of patients with hepatocellular carcinoma with extrahepatic metastasis. PLoS One. 2014;9:e95889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Kobayashi S, Takahashi S, Kato Y, Gotohda N, Nakagohri T, Konishi M, Kinoshita T. Surgical treatment of lymph node metastases from hepatocellular carcinoma. J Hepatobiliary Pancreat Sci. 2011;18:559-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Wu X, Li B, Qiu J, Shen J, Zheng Y, Li Q, Liao Y, He W, Zou R, Yuan Y. Hepatectomy Versus Hepatectomy With Lymphadenectomy in Hepatocellular Carcinoma: A Prospective, Randomized Controlled Clinical Trial. J Clin Gastroenterol. 2015;49:520-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Hashimoto M, Matsuda M, Watanabe G. Metachronous resection of metastatic lymph nodes in patients with hepatocellular carcinoma. Hepatogastroenterology. 2009;56:788-792. [PubMed] |
| 7. | Grobmyer SR, Wang L, Gonen M, Fong Y, Klimstra D, D'Angelica M, DeMatteo RP, Schwartz L, Blumgart LH, Jarnagin WR. Perihepatic lymph node assessment in patients undergoing partial hepatectomy for malignancy. Ann Surg. 2006;244:260-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 91] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Kim HJ, Cho A, Yun M, Kim YT, Kang WJ. Comparison of FDG PET/CT and MRI in lymph node staging of endometrial cancer. Ann Nucl Med. 2016;30:104-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Kim RD, Reed AI, Fujita S, Foley DP, Mekeel KL, Hemming AW. Consensus and controversy in the management of hepatocellular carcinoma. J Am Coll Surg. 2007;205:108-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Shu J, Zhao JN, Han FG, Tang GC, Luo YD, Luo L, Chen X. Chronic hepatitis B: Enlarged perihepatic lymph nodes correlated with hepatic histopathology. World J Radiol. 2013;5:208-214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Schreiber-Dietrich D, Pohl M, Cui XW, Braden B, Dietrich CF, Chiorean L. Perihepatic lymphadenopathy in children with chronic viral hepatitis. J Ultrason. 2015;15:137-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Seber T, Caglar E, Uylar T, Karaman N, Aktas E, Aribas BK. Diagnostic value of diffusion-weighted magnetic resonance imaging: Differentiation of benign and malignant lymph nodes in different regions of the body. Clin Imaging. 2015;39:856-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Lee JE, Jang JY, Jeong SW, Lee SH, Kim SG, Cha SW, Kim YS, Cho YD, Kim HS, Kim BS, Jin SY, Choi DL. Diagnostic value for extrahepatic metastases of hepatocellular carcinoma in positron emission tomography/computed tomography scan. World J Gastroenterol. 2012;18:2979-2987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Choi EK, Yoo IeR, Park HL, Choi HS, Han EJ, Kim SH, Chung SH, O JH. Value of Surveillance (18)F-FDG PET/CT in Colorectal Cancer: Comparison with Conventional Imaging Studies. Nucl Med Mol Imaging. 2012;46:189-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Nakagawa T, Yamada M, Suzuki Y. 18F-FDG uptake in reactive neck lymph nodes of oral cancer: Relationship to lymphoid follicles. J Nucl Med. 2008;49:1053-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Ho CL, Chen S, Yeung DW, Cheng TK. Dual-tracer PET/CT imaging in evaluation of metastatic hepatocellular carcinoma. J Nucl Med. 2007;48:902-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 155] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 17. | Marin D, Boll DT, Mileto A, Nelson RC. State of the art: Dual-energy CT of the abdomen. Radiology. 2014;271:327-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 287] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 18. | Rizzo S, Radice D, Femia M, De Marco P, Origgi D, Preda L, Barberis M, Vigorito R, Mauri G, Mauro A, Bellomi M. Metastatic and non-metastatic lymph nodes: Quantification and different distribution of iodine uptake assessed by dual-energy CT. Eur Radiol. 2018;28:760-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 19. | Baxa J, Vondráková A, Matoušková T, Růžičková O, Schmidt B, Flohr T, Sedlmair M, Ferda J. Dual-phase dual-energy CT in patients with lung cancer: Assessment of the additional value of iodine quantification in lymph node therapy response. Eur Radiol. 2014;24:1981-1988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 20. | Al-Najami I, Lahaye MJ, Beets-Tan RGH, Baatrup G. Dual-energy CT can detect malignant lymph nodes in rectal cancer. Eur J Radiol. 2017;90:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Al-Najami I, Beets-Tan RG, Madsen G, Baatrup G. Dual-Energy CT of Rectal Cancer Specimens: A CT-based Method for Mesorectal Lymph Node Characterization. Dis Colon Rectum. 2016;59:640-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Liang H, Li A, Li Y, Cheng H, Zhao Q, Li J, Wang Q. A retrospective study of dual-energy CT for clinical detecting of metastatic cervical lymph nodes in laryngeal and hypopharyngeal squamous cell carcinoma. Acta Otolaryngol. 2015;135:722-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Tawfik AM, Razek AA, Kerl JM, Nour-Eldin NE, Bauer R, Vogl TJ. Comparison of dual-energy CT-derived iodine content and iodine overlay of normal, inflammatory and metastatic squamous cell carcinoma cervical lymph nodes. Eur Radiol. 2014;24:574-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 139] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 24. | Sano T. Evaluation of the gastric cancer treatment guidelines of the Japanese Gastric Cancer Association. Gan To Kagaku Ryoho. 2010;37:582-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1911] [Article Influence: 127.4] [Reference Citation Analysis (0)] |
| 25. | Chinese Society of Contagious Disease and Parasitic Disease, Chinese Society of Hepatology. Chinese Medical Association (2001) Guideline: prevention and treatment of viral hepatitis. Zhonghua Chuanranbing Zazhi. 2001;19:56-62. [DOI] [Full Text] |
| 26. | Kim S, Shuman WP. Clinical Applications of Dual-Energy Computed Tomography in the Liver. Semin Roentgenol. 2016;51:284-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Pant V, Sen IB, Soin AS. Role of 18F-FDG PET CT as an independent prognostic indicator in patients with hepatocellular carcinoma. Nucl Med Commun. 2013;34:749-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aghakhani A, Bramhall S, Köksal AS S-Editor: Yan JP L-Editor: Wang TQ E-Editor: Song H