Published online Mar 14, 2019. doi: 10.3748/wjg.v25.i10.1278
Peer-review started: January 2, 2019
First decision: January 30, 2019
Revised: February 20, 2019
Accepted: February 22, 2019
Article in press: February 23, 2019
Published online: March 14, 2019
Processing time: 73 Days and 15.1 Hours
The implementation of optical diagnosis (OD) of diminutive colorectal lesions in clinical practice has been hampered by differences in performance between community and academic settings. One possible cause is the lack of a standardized learning tool. Since the factors related to better learning are not well described, strong evidence upon which a consistent learning tool could be designed is lacking. We hypothesized that a self-designed learning program may be enough to achieve competency in OD of diminutive lesions of the colon.
To assess the accuracy of OD of diminutive lesions in real colonoscopies after application of a self-administered learning program.
This was a single-endoscopist prospective pilot study, in which an experienced endoscopist followed a self-designed, self-administered learning program in OD of colorectal lesions. An assessment phase divided in two halves with a 6-mo period in between without performance of OD was developed in a population-based colorectal cancer screening program. The accomplishment of the Preservation and Incorporation of Valuable Endoscopic Innovations criteria and performance measures were calculated overall and in the two halves of the assessment phase, assessing their response to the 6-mo stopping period. The evolution of performance through blocks of 50 lesions was also assessed.
Overall, 152 patients and 522 lesions (≤ 5 mm: 399, and 6-9 mm: 123) were included. The negative predictive value for the OD of adenoma in rectosigmoid lesions diagnosed with high confidence was 91.7% [95% confidence interval (CI): 87.3-96.6]. The proportion of agreement on surveillance interval between OD and pathological diagnosis was higher than 95%. Overall accuracy for diminutive lesions diagnosed with high confidence was 89.5% (95%CI: 86.3-92.7). The overall accuracy of OD was similar in the two halves of the assessment phase [90.1 (95%CI: 85.6-94.7) vs 88.2 (95%CI: 87.9-95.9)]. All the other performance parameters were also equivalent, except for specificity. Specificity, negative predictive value and accuracy were the parameters most affected by the stopping period between the two halves. Upon analyzing trends on blocks of 50 lesions, an improvement on sensitivity (P = 0.02) was detected only in the first half and an improvement on accuracy (P = 0.01) was detected only in the second half.
A self-administered learning program is sufficient to achieve expert-level OD. To maintain performance, continuous practice is needed, with a refresher course following any long non-practice period.
Core tip: The learning process for optical diagnosis (OD) of diminutive colorectal polyps is not standardized, and this may influence the described differences in OD performance between community and academic settings. Our study shows that an individual following a self-designed and self-administered learning program is able to reach the expert level of OD performance completely fulfilling the criteria of Preservation and Incorporation of Valuable Endoscopic Innovations. However, continuous practice is needed to maintain performance and, if a non-practice period is expected, a refresher course is needed to avoid a significant drop in performance parameters.
- Citation: Bustamante-Balén M, Satorres C, Puchades L, Navarro B, García-Morales N, Alonso N, Ponce M, Argüello L, Pons-Beltrán V. Non-guided self-learning program for high-proficiency optical diagnosis of diminutive and small colorectal lesions: A single-endoscopist pilot study. World J Gastroenterol 2019; 25(10): 1278-1288
- URL: https://www.wjgnet.com/1007-9327/full/v25/i10/1278.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i10.1278
Optical diagnosis (OD) of gastrointestinal epithelial lesions has become a reality due to the development of new image enhancing technologies. The ability to perform in situ differentiation of adenomatous and hyperplastic colorectal lesions has led to the proposal of a resect-and-discard strategy for the management of diminutive (≤ 5 mm) polyps[1]. Following this strategy, diminutive lesions would be resected and discarded after an adenoma high-confidence OD has been made, while rectal diminutive lesions with an OD of hyperplastic would be left in place. This strategy has been shown to be cost-efficient[2].
An excellent accuracy of OD is a requirement for applying such a strategy, and it has been shown to be so in many studies, most of them performed in academic centers[3]. However, this good accuracy has not been well replicated in community settings[4,5]. Learning of OD is key for its implementation in clinical practice, and the lack of standardized learning tools may explain part of the problem. A wide variety of learning tools has been described, including classroom type[6], self-directed computer-based[3] or web-based teaching programs[7]. Still pictures, videos or both have been used to explain the optical features of each type of polyp[8,9]. However, there are no head-to-head comparisons between learning tools and most of them have not been validated.
Moreover, people learn at different rates, as has been shown by some studies monitoring the learning curve of OD. Some learners never get competency in OD, while others need long-term monitoring[4,6]. Unfortunately, since the factors related to better learning are not well described, the strong evidence upon which a consistent learning tool could be designed is lacking. Despite these challenges, we hypothesized that a self-designed learning program may be enough to achieve competency in OD of diminutive lesions of the colon.
Our study was designed according to the following aims: (1) to assess the accuracy of OD of diminutive lesions in real colonoscopies from a colorectal carcinoma (CRC) screening program using narrow band imaging (NBI) and the NBI International Colorectal Endoscopic (NICE) classification after following a non-guided self-administered learning program; and (2) to describe the OD learning curve by analyzing which parameters may be more suitable for monitoring competency.
This was a single-endoscopist prospective pilot study, in which an experienced endoscopist (> 500 colonoscopies per year and adenoma detection rate of 68%) followed a self-designed, self-administered learning program for OD of colorectal lesions. In this learning program, the NICE classification was reviewed and a published set of still pictures[8] was used to identify the main optical characteristics of hyperplastic and adenomatous polyps under NBI. Then, the NICE classification was put into practice on 50 consecutive colorectal lesions identified in CRC screening colonoscopies. The endoscopist reviewed the pathological records, when available, comparing this diagnosis with the provided OD. A detailed evaluation of inconsistencies was performed and diagnostic disagreements were reviewed with the pathologist.
After completing the learning program, an assessment phase was begun in which individuals scheduled for colonoscopy in the setting of the Valencian Government Colorectal Screening Program were consecutively included. This screening program is based on results from the immunological fecal occult blood test administered every 2 years and colonoscopy administered in cases of positivity. Exclusion criteria were poor quality preparation (Boston < 2 in any colon segment), incomplete colonoscopy, inflammatory bowel disease, coagulopathy that precluded taking samples, or unwillingness to participate in the study. This assessment phase was divided in two halves, with a predefined stopping period of 6 mo in between, in which no OD was performed. No OD refresher course was given before the beginning of the second phase.
For bowel preparation, a split-dose scheme using sodium picosulphate plus magnesium citrate (Citraflet®; Casen Recordati, S.L., Zaragosa, Spain) or 2-L polyethylene glycol (PEG) plus ascorbate (Moviprep®; Salix Pharmaceuticals, Bridgewater, NJ, United States) was administered. All colonoscopies were performed using high-resolution CF-HQ190AL or CF-H190L endoscopes (Olympus, Optical Co., Ltd., Tokyo, Japan) and a video endoscope system (EVIS EXERA III; Olympus).
Data on age, sex, and personal and familiar histories of colon polyps or CRC were recorded. For every lesion, data on size, morphology (following the Paris classification[10]), location, NICE classification[11] group, and final OD were also recorded. All data were prospectively included in a database built in Access 2003 (Microsoft Corp., Redmon, WA, United States). Pathological diagnosis was introduced in the database by a researcher involved neither in the colonoscopies nor in the OD process. Therefore, during this phase of the study, the endoscopist was blind to the pathological report and no feedback was provided. Only diminutive lesions (1-5 mm) or small lesions (6-9 mm) were considered for the analysis.
The optical and pathologic diagnostics were compared for the diagnosis of adenoma vs non-adenomatous lesions, considering pathology as the gold standard. For analysis purposes, hyperplastic polyps, sessile serrated polyps, inflammatory polyps, and biopsies informed as normal were considered as non-adenomatous lesions.
The primary end-point was the Preservation and Incorporation of Valuable Endoscopic Innovations (PIVI) criteria[1] accomplishment at the end of the study. The final surveillance recommendation when using OD was the combination of OD of diminutive lesions and the pathology report of larger lesions. The concordance between the recommended follow-up from OD and from pathology was calculated for the three main currently available guidelines (European Union[12], European Society of Gastrointestinal Endoscopy[13], and American Society for Gastrointestinal Endoscopy[14]). Patients in whom an in-situ surveillance recommendation could not be given (i.e., those with no diminutive lesions, with at least one polyp diagnosed with low confidence, or diagnosed with a CRC or a large polyp scheduled for endoscopic mucosal resection) were not included in this analysis. Secondary end-points were the evaluation of overall accuracy, sensitivity, specificity, positive and negative predictive values (PPV and NPV, respectively), and positive likelihood ratio for the diagnosis of adenoma. All performance values were calculated at the end of the first half, at the end of the study and during the assessment phase in groups of 50 lesions.
To obtain a precision of 3% in the estimation of the accuracy of OD for diminutive lesions, using a bilateral 95% confidence interval (CI) and expecting an accuracy of 90%, at least 385 diminutive lesions had to be included.
Each patient’s and lesion’s characteristics were summarized by median (standard deviation) for continuous variables and by number (percentage) for categorical variables. Sensitivity, specificity, PPV and NPV, and positive likelihood ratio were calculated as measures of accuracy together with their 95%CIs. True positive and negative values were defined as an agreement between OD and histology. The Cochran-Armitage test for trend was used to determine if performance improved through blocks of 50 lesions in both halves of the study. P-values were two-sided, and differences were considered significant at P < 0.05. Analysis was performed by using the Stata statistical package, version 14.2 (Stata Corp, College Station, TX, United States). The results of this study are reported in accordance with Standards for Reporting of Diagnostic Accuracy guidelines[15].
From January 2015 to January 2017, 152 individuals who underwent a CRC screening colonoscopy were selected for study inclusion. Their main characteristics are summarized in Table 1. These patients harbored 522 lesions [1-5 mm in 399 (76.4%) and 6-9 mm in 123 (23.6%)], the main characteristics of which are summarized in Table 2.
| Characteristic | Value |
| Age, yr | 61.1 ± 6.2 |
| Female sex n (%) | 56 (36.8) |
| Familiar history of CRC n (%) | 33 (21.7) |
| Number of polyps | 3.8 ± 3.0 |
| Number of adenomas | 2.7 ± 2.6 |
| Number of advanced adenomas | 0.6 ± 1.0 |
| Characteristic | 1-5 mm | 6-9 mm | |
| Number of lesions by size | 399 (76.4) | 123 (23.6) | |
| Paris classification | |||
| 0-Ip | 3 (0.7) | 22 (17.9) | |
| 0-Is | 273 (68.4) | 88 (71.5) | |
| 0-IIa | 117 (29.3) | 11 (8.9) | |
| 0-IIc | 2 (0.5) | 0 | |
| 0-IIa + IIc | 3 (0.7) | 0 | |
| 0-IIb | 1 (0.2) | 2 (1.6) | |
| Pathology | |||
| Adenoma | 255 (63.9) | 97 (78.9) | |
| Hyperplastic | 106 (26.6) | 17 (13.6) | |
| SSP | 8 (2.0) | 7 (5.6) | |
| Other | 26 (6.5) | 1 (0.8) | |
| Lost/not enough sample | 4 (1.0) | 1 (0.8) | |
| Location | |||
| Proximal | 248 (62.2) | 56 (45.5) | |
| Distal | 151 (37.8) | 67 (54.5) | |
| Optical diagnosis1 | |||
| NICE 1 | 288 (72.2) | 102 (82.9) | |
| NICE 2 | 110 (27.6) | 20 (16.3) | |
| High-confidence diagnosis | 347 (87.0) | 116 (94.3) | |
Overall, 55 (59.8%) diminutive rectosigmoid lesions were diagnosed as hyperplastic and 34 (36.9%) as adenoma. One lesion was lost for analysis and two were categorized as normal mucosa. The NPV for the OD of adenoma in rectal lesions diagnosed with high confidence was 91.7% (95%CI: 87.3-96.6). In 59 patients (38.8%), an in-situ surveillance recommendation could not be given; these patients included 40 with at least one lesion diagnosed with low confidence, 7 with no diminutive lesion, 9 with a CRC or a malignant polyp diagnosed in the same colonoscopy, and 3 with large polyps suitable for endoscopic mucosal resection. The proportion of agreement on surveillance interval between OD and pathological diagnosis following the different guidelines for the remaining 92 patients is summarized in Table 3.
| Guideline | Concordance | Too long | Too short | |||
| n | % (95%CI) | n | % (95%CI) | n | % (95%CI) | |
| EU | 89 | 95.7 (91.9-100) | 2 | 2.1 (0-21.4) | 2 | 2.1 (0-21.4) |
| ESGE | 90 | 96.8 (93.5-100) | 2 | 2.1 (0-21.4) | 1 | 1.1 (0-20.5) |
| ASGE | 89 | 95.7 (91.9-100) | 3 | 3.2 (0-22.3) | 1 | 1.1 (0-20.5) |
Regarding the OD with NBI, 520 lesions were classified as adenomas or hyperplastic polyps, with 347 (87.0%) diminutive lesions and 116 (94.3%) small lesions diagnosed with high confidence (Table 2).
The performance values for the OD of small and diminutive lesions are summarized in Table 4. Overall accuracy for diminutive and small lesions diagnosed with high confidence was 89.5% (95%CI: 86.3-92.7) and 99.1% (95%CI: 97.4-100.0) respectively. Values were, as expected, much lower for lesions diagnosed with low confidence (Table 4). These values did not differ significantly when comparing location (distal vs proximal) and morphology (sessile vs flat) (data not shown).
| Parameter | High-confidence | Low-confidence | ||
| 1-5 mm, n = 347 | 6-9 mm, n = 115 | 1-5 mm, n = 51 | 6-9 mm1, n = 7 | |
| Sensitivity | 97.0 (95.2-98.8) | 100.0 | 76.0 (64.3-87.7) | N/A |
| Specificity | 74.3 (69.4-78.6) | 94.4 (90.2-98.6) | 46.1 (32.4-59.8) | 57.1 (20.4-57.1) |
| PPV | 88.5 (84.6-91.4) | 99.0 (97.2-100.0) | 57.6 (44.0-71.2) | 0 |
| NPV | 92.3(89.1-94.8) | 100.0 | 66.7 (53.8-79.6) | 100.0 |
| LR+ | 3.8 (1.2-4.8) | 18.0 (10.1-23.9) | 1.4 (-1.8-4.6) | N/A |
| Accuracy | 89.5 (85.7-92.3) | 99.1 (97.4-100.0) | 60.7 (47.3-74.1) | 57.1 (20.4-57.1) |
The overall accuracy of OD was similar in the two halves of the study [90.1% (95%CI: 85.6-94.7) vs 88.2 (95%CI: 87.9-95.9)]. All the other performance parameters were also equivalent, except for specificity (Table 5). The NPV for adenoma in rectosigmoid lesions and agreement on surveillance intervals were also similar between both halves of the study (Table 5).
| Parameter | 1st half, n = 1651 | 2nd half, n = 1821 | |
| Sensitivity | 96.1 (93.1-99.0) | 97.6 (95.4-99.8) | |
| Specificity | 82.0 (76.1-87.9) | 65.4 (58.5-72.3) | |
| PPV | 90.1 (85.5-94.7) | 87.2 (82.3-92.0) | |
| NPV | 92.6 (88.6-96.6) | 91.9 (87.9-95.9) | |
| Accuracy | 90.1 (85.6-94.7) | 88.2 (87.9-95.9) | |
| NPV rectosigmoid lesions | 92.3 (87.3-96.6) | 90.5 (86.2-94.8) | |
| Surveillance interval agreement | |||
| UE | 100.0 | 93.3 (89.7-96.9) | |
| ESGE | 100.0 | 95.1 (92.0-98.2) | |
| ASGE | 100.0 | 93.3 (91.6-98.2) | |
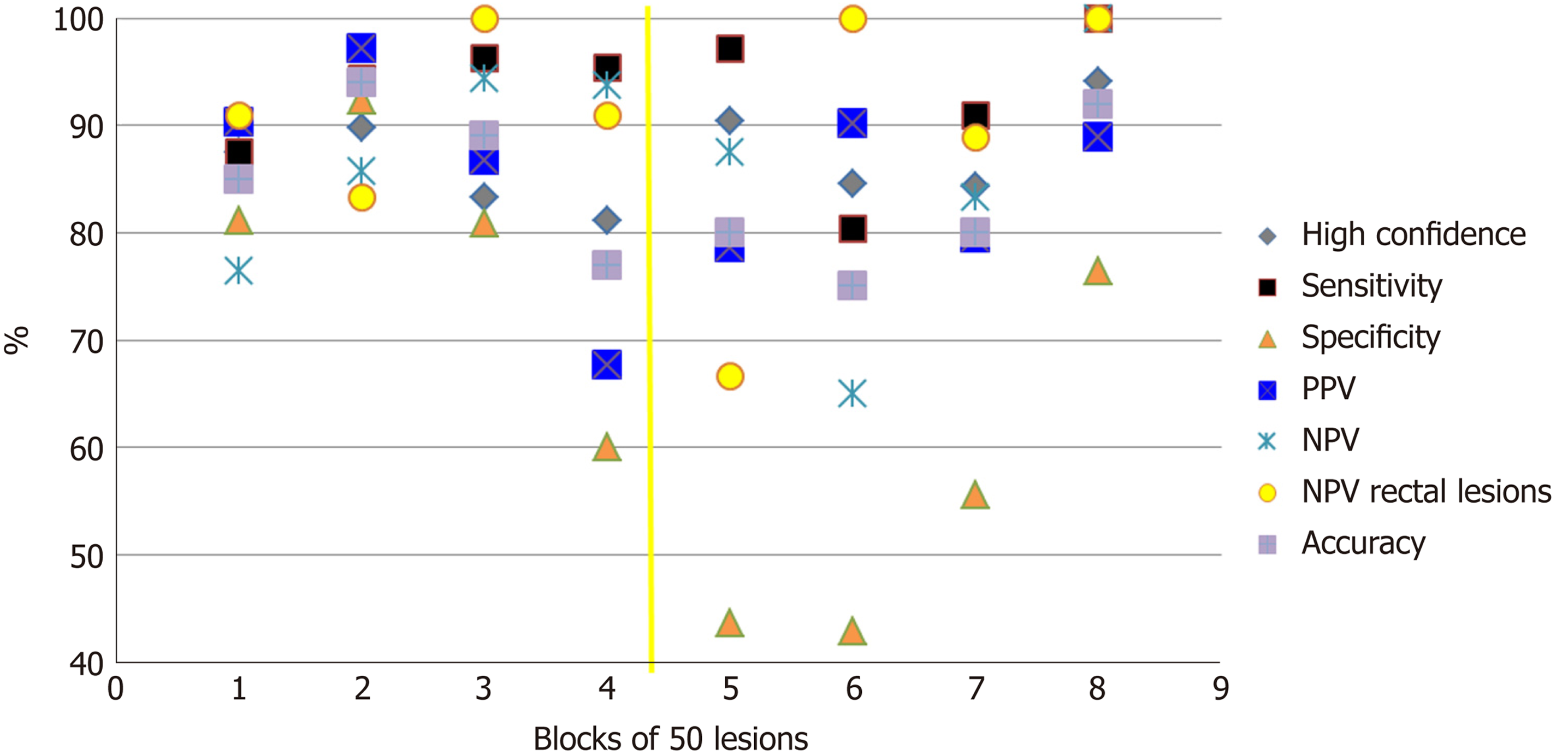
Figure 1 depicts the evolution during time of OD performance of diminutive lesions. Specificity, NPV and accuracy were the parameters most affected by the stopping period between the two halves. However, sensitivity and the percentage of lesions diagnosed with high confidence are more robust parameters. Analyzing trends on blocks of 50 lesions showed an improvement in specificity (P = 0.0001) and NPV (P = 0.00001) in both halves. However, an improvement in sensitivity (P = 0.02) was detected only in the first half and an improvement in accuracy (P = 0.01) was detected only in the second half. There was no significant improvement in the percentage of lesions diagnosed with high confidence in either of the two halves as the trainee progressed through lesion batches.
Our study shows that a good accuracy, reaching an expert level, and complete fulfillment of the PIVI criteria can be accomplished by self-learning. At the end of the study, the NPV for the OD of adenoma in rectal lesions was 91.7% and the proportion of agreement in the surveillance intervals between OD and pathology was higher than 95%.
Previous studies on OD learning have shown conflicting results. When it comes to performing OD in real colonoscopies, several studies and a meta-analysis have shown lower levels of performance (i.e., not fulfilling the PIVI criteria) in community hospitals than in academic centers, despite a structured learning program having been followed[4,5,16]. On the contrary, other authors have shown that trainees without previous experience in NBI can meet PIVI thresholds after following a standardized learning program[17]. One of the possible explanations for this discrepancy may be the different design of the learning tool.
We used a validated set of still pictures followed by a practice on real colonoscopies with auto-administered feedback, hypothesizing that the latter would ease the transition from still pictures to real practice and shorten the learning curve. For the initial learning steps, several training modules have been used in the literature, including classroom-type[8,18], computer training[19] and web-based[20] modules. All systems may have similar efficacy as it has been suggested in a recent report showing that self-learning using a computer-based program with pictures and videos is as efficient as a classroom-type teaching session for learning OD[21]. Therefore, the key to efficacy of the learning program may be more in other adds-on or modifications.
Other authors have also shown a good efficacy of learning when introducing an in vivo phase during the learning program, with a pre-defined number of colonoscopies[22] or lesions[6]. In our study, 50 lesions were sufficient to meet the PIVI criteria at the end of the assessment phase.
Other modifications that have been tested in the literature are refresher teaching sessions and periodic feedback. We did not introduce any refresher session, not even before the beginning of the second period of the assessment phase after the 6-mo stopping period, and it did not affect the final results on efficacy. Any feedback was allowed during the assessment phase and the endoscopist was blinded to the pathology results. Regarding these two modifications, there is some controversy in the literature. Paggi et al[23] introduced refresher teaching sessions every 2 mo and monthly feedback on individual performance, achieving an overall NPV for adenoma in rectosigmoid lesions of 91.3% and more than 90% of agreement on surveillance intervals. Patel et al[17] delivered periodic feedback to all the participants in a prospective study and were able to show an overall NPV for high-confidence diagnosis of rectosigmoid lesions of 94.7% and a surveillance interval agreement of 91.2%. However, a randomized trial was not able to show any influence of feedback on final performance[6].
We planned a stopping period at the middle of the study to investigate if a non-practice period could influence performance and to detect which parameters were affected the most. Following the stopping period (which was not followed by a refresher course), almost all performance parameters dropped significantly. Specificity was the most affected parameter, and it took 200 lesions to reach previous levels. On the other hand, sensitivity was very resistant to inactivity. Accuracy dropped from 0.89 to 0.77, and it took 150 lesions to reach 0.90. NPV for adenoma in rectal lesions also dropped significantly, from 0.90 to 0.67.
Regarding trends for improvement through blocks of 50 lesions, a significant improvement was detected in both halves for specificity and NPV, suggesting that the number of false positives and false negatives are only significantly reduced after ongoing practice. The significant trend for improvement of accuracy only in the second period suggests that if a long non-practice period has occurred, a refresher course in OD is needed. A previous study[24] of 12 endoscopists evaluating 80 videos at 12 wk apart found a significant improvement in accuracy in both periods; however, that study did not include real colonoscopies.
The strength of the current study described herein is its design as a single-endoscopist study, which allowed for detailed analysis of the learning process. Another strength is that the PIVI criteria on surveillance agreement has been calculated for the most widely applied international guidelines, showing that learning is consistently strong under different circumstances. However, some issues may limit generalizability. First, the single-endoscopist study design carries the risk of the results being dependent on the trainee´s characteristics. Studies including several endoscopists have shown that despite an overall good performance, many individuals do not reach the PIVI thresholds[4,22] and that in many cases a continuous monitoring is needed. Nonetheless, the statement that an efficient self-learning program is possible when the trainee is highly motivated seems conclusive.
Another limitation is that all patients belong to a FIT-positive population. In this situation, the probability of finding polyps is higher and this may enhance the learning process. The diagnosis of sessile serrated polyp was not considered, and these polyps were included in the non-adenomatous group. However, this only comprised 2% of samples and none of the hyperplastic lesions were more than 10 mm, having little relevance to the final results.
In conclusion, a self-administered learning program including real colonoscopies is sufficient to learn OD at an expert level. However, continuous practice is needed to maintain performance and a refresher course is needed if a long non-practice period occurs. Performance values behave differently after a stopping period, and this should be taken into account when planning a monitoring program.
The resect-and-discard strategy for the management of diminutive colon polyps is a paradigm shift based on an accurate optical diagnosis (OD). Such a high accuracy has only been achieved by experts, while the performance in community hospitals does not reach thresholds that would allow its universal implementation. The lack of a standardized learning tool for OD of colon lesions may contribute to this problem.
Although several learning tools have been described, most of them are not validated and there is a great variability in their components and designs. We hypothesized that self-learning of OD is feasible and that accuracy thresholds can be achieved with a self-administered program. A detailed description of the learning process can provide valuable information for the design of an OD learning system.
We aimed to assess the accuracy of OD of diminutive lesions in real colonoscopies using the International Colorectal Endoscopic classification system for narrow band imaging after following a non-guided self-administered learning program. We also aimed to describe in detail the learning process by analyzing which parameters may be more suitable for monitoring competency.
An experienced endoscopist followed a self-designed, self-administered learning program in OD of colorectal lesions. Then, OD was applied to lesions detected in colorectal cancer screening colonoscopies. The study period was divided in two halves, with a 6-mo period in between with no performance of OD. Sensitivity, specificity, predictive values and accuracy of the OD compared to the pathological report were calculated for overall results and for the two halves of the study. The accomplishment of the Preservation and Incorporation of Valuable Endoscopic Innovations (PIVI) criteria and the evolution of performance parameters through blocks of 50 lesions were also assessed.
Overall, 152 patients and 522 lesions were included in the analysis. Regarding the accomplishment of the PIVI criteria, the negative predictive value for the OD of adenoma in rectal lesions diagnosed with high confidence was 92.6% (95% confidence interval: 86.4-97.6) and the proportion of agreement on surveillance interval between OD and pathological diagnosis following the different guidelines was over 95%. Overall accuracy for diminutive lesions diagnosed with high confidence was 89.5% (95% confidence interval: 85.7-92.3). Specificity, negative predictive value and accuracy were the parameters most affected by the stopping period between the two halves. Analyzing trends on blocks of 50 lesions showed an improvement in sensitivity (P = 0.02) only in the first half of the study and an improvement on accuracy (P = 0.01) only in the second half.
This study shows that a self-administered learning program based on still pictures plus an in vivo phase with auto-feedback is feasible to reach quality standards on OD of colorectal lesions. It also shows that a non-practice period deteriorates performance, and in that case a refresher course seems advisable. These results have practical implications in the design of OD learning tools and in the development of a quality monitoring system.
These data have become the base for the design and validation of a self-administered learning tool that are currently in process. The efficacy of this kind of tool should be tested with endoscopists having different levels of experience and being from different backgrounds.
| 1. | Rex DK, Kahi C, O'Brien M, Levin TR, Pohl H, Rastogi A, Burgart L, Imperiale T, Ladabaum U, Cohen J, Lieberman DA. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. 2011;73:419-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 483] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 2. | Vleugels JLA, Greuter MJE, Hazewinkel Y, Coupé VMH, Dekker E. Implementation of an optical diagnosis strategy saves costs and does not impair clinical outcomes of a fecal immunochemical test-based colorectal cancer screening program. Endosc Int Open. 2017;5:E1197-E1207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Ignjatovic A, Thomas-Gibson S, East JE, Haycock A, Bassett P, Bhandari P, Man R, Suzuki N, Saunders BP. Development and validation of a training module on the use of narrow-band imaging in differentiation of small adenomas from hyperplastic colorectal polyps. Gastrointest Endosc. 2011;73:128-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (1)] |
| 4. | Ladabaum U, Fioritto A, Mitani A, Desai M, Kim JP, Rex DK, Imperiale T, Gunaratnam N. Real-time optical biopsy of colon polyps with narrow band imaging in community practice does not yet meet key thresholds for clinical decisions. Gastroenterology. 2013;144:81-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 188] [Article Influence: 14.5] [Reference Citation Analysis (1)] |
| 5. | ASGE Technology Committee; Abu Dayyeh BK, Thosani N, Konda V, Wallace MB, Rex DK, Chauhan SS, Hwang JH, Komanduri S, Manfredi M, Maple JT, Murad FM, Siddiqui UD, Banerjee S. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. 2015;81:502.e1-502.e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 259] [Article Influence: 23.5] [Reference Citation Analysis (1)] |
| 6. | Vleugels JLA, Dijkgraaf MGW, Hazewinkel Y, Wanders LK, Fockens P, Dekker E; DISCOUNT study group. Effects of Training and Feedback on Accuracy of Predicting Rectosigmoid Neoplastic Lesions and Selection of Surveillance Intervals by Endoscopists Performing Optical Diagnosis of Diminutive Polyps. Gastroenterology. 2018;154:1682-1693.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Dias-Silva D, Pimentel-Nunes P, Magalhães J, Magalhães R, Veloso N, Ferreira C, Figueiredo P, Moutinho P, Dinis-Ribeiro M. The learning curve for narrow-band imaging in the diagnosis of precancerous gastric lesions by using Web-based video. Gastrointest Endosc. 2014;79:910-20; quiz 983-e1, 983.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 8. | Raghavendra M, Hewett DG, Rex DK. Differentiating adenomas from hyperplastic colorectal polyps: narrow-band imaging can be learned in 20 minutes. Gastrointest Endosc. 2010;72:572-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (1)] |
| 9. | Singh R, Bhat YM, Thurairajah PH, Shetti MP, Jayanna M, Nind G, Tam W, Walmsey R, Bourke M, Moss A, Chen R, Bampton P, Roberts-Thomson I, Schoeman M, Tucker G. Is narrow band imaging superior to high-definition white light endoscopy in the assessment of diminutive colorectal polyps? J Gastroenterol Hepatol. 2013;28:472-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3-43. [PubMed] |
| 11. | Hewett DG, Kaltenbach T, Sano Y, Tanaka S, Saunders BP, Ponchon T, Soetikno R, Rex DK. Validation of a simple classification system for endoscopic diagnosis of small colorectal polyps using narrow-band imaging. Gastroenterology. 2012;143:599-607.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 429] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 12. | Atkin WS, Valori R, Kuipers EJ, Hoff G, Senore C, Segnan N, Jover R, Schmiegel W, Lambert R, Pox C; International Agency for Research on Cancer. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First Edition--Colonoscopic surveillance following adenoma removal. Endoscopy. 2012;44 Suppl 3:SE151-SE163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 13. | Hassan C, Quintero E, Dumonceau JM, Regula J, Brandão C, Chaussade S, Dekker E, Dinis-Ribeiro M, Ferlitsch M, Gimeno-García A, Hazewinkel Y, Jover R, Kalager M, Loberg M, Pox C, Rembacken B, Lieberman D; European Society of Gastrointestinal Endoscopy. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2013;45:842-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 411] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 14. | Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1367] [Cited by in RCA: 1470] [Article Influence: 105.0] [Reference Citation Analysis (0)] |
| 15. | Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, Lijmer JG, Moher D, Rennie D, de Vet HC; Standards for Reporting of Diagnostic Accuracy. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. BMJ. 2003;326:41-44. [PubMed] |
| 16. | Vu HT, Sayuk GS, Hollander TG, Clebanoff J, Edmundowicz SA, Gyawali CP, Thyssen EP, Weinstock LB, Early DS. Resect and discard approach to colon polyps: real-world applicability among academic and community gastroenterologists. Dig Dis Sci. 2015;60:502-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Patel SG, Schoenfeld P, Kim HM, Ward EK, Bansal A, Kim Y, Hosford L, Myers A, Foster S, Craft J, Shopinski S, Wilson RH, Ahnen DJ, Rastogi A, Wani S. Real-Time Characterization of Diminutive Colorectal Polyp Histology Using Narrow-Band Imaging: Implications for the Resect and Discard Strategy. Gastroenterology. 2016;150:406-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | Dai J, Shen YF, Sano Y, Li XB, Xue HB, Zhao YJ, Gao YJ, Song Y, Ge ZZ. Evaluation of narrow-band imaging in the diagnosis of colorectal lesions: is a learning curve involved? Dig Endosc. 2013;25:180-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Rastogi A, Rao DS, Gupta N, Grisolano SW, Buckles DC, Sidorenko E, Bonino J, Matsuda T, Dekker E, Kaltenbach T, Singh R, Wani S, Sharma P, Olyaee MS, Bansal A, East JE. Impact of a computer-based teaching module on characterization of diminutive colon polyps by using narrow-band imaging by non-experts in academic and community practice: a video-based study. Gastrointest Endosc. 2014;79:390-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | Dinis-Ribeiro M, Correia R, Santos C, Fernandes S, Palhares E, Silva RA, Amaro P, Areia M, Costa-Pereira A, Moreira-Dias L. Web-based system for training and dissemination of a magnification chromoendoscopy classification. World J Gastroenterol. 2008;14:7086-7092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Khan T, Cinnor B, Gupta N, Hosford L, Bansal A, Olyaee MS, Wani S, Rastogi A. Didactic training vs. computer-based self-learning in the prediction of diminutive colon polyp histology by trainees: a randomized controlled study. Endoscopy. 2017;49:1243-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | McGill SK, Soetikno R, Rastogi A, Rouse RV, Sato T, Bansal A, McQuaid K, Kaltenbach T. Endoscopists can sustain high performance for the optical diagnosis of colorectal polyps following standardized and continued training. Endoscopy. 2015;47:200-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Paggi S, Rondonotti E, Amato A, Fuccio L, Andrealli A, Spinzi G, Radaelli F. Narrow-band imaging in the prediction of surveillance intervals after polypectomy in community practice. Endoscopy. 2015;47:808-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Patel SG, Rastogi A, Austin G, Hall M, Siller BA, Berman K, Yen R, Bansal A, Ahnen DJ, Wani S. Gastroenterology trainees can easily learn histologic characterization of diminutive colorectal polyps with narrow band imaging. Clin Gastroenterol Hepatol. 2013;11:997-1003.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
P- Reviewer: Saligram S S- Editor: Ma RY L- Editor: A E- Editor: Yin SY