Published online Feb 14, 2018. doi: 10.3748/wjg.v24.i6.763
Peer-review started: November 17, 2017
First decision: December 13, 2017
Revised: December 15, 2017
Accepted: December 20, 2017
Article in press: December 20, 2017
Published online: February 14, 2018
Processing time: 81 Days and 4.2 Hours
Nowadays acute gastroenteritis infection caused by Escherichia coli (E. coli) O157:H7 is frequently associated with hemolytic uremic syndrome (HUS), which usually developed after prodromal diarrhea that is often bloody. The abdominal pain accompanied by failure kidney is a suspicious symptom to develop this disorder. Their pathological characteristic is vascular damage which manifested as arteriolar and capillary thrombosis with abnormalities in the endothelium and vessel walls. The major etiological agent of HUS is enterohemorragic (E coli) strain belonging to serotype O157:H7. The lack of papers about HUS associated to gastroenteritis lead us to report this case for explain the symptoms that are uncommon. Furthermore, this report provides some strategies to suspect and make an early diagnosis, besides treatment approach to improving outcomes and prognosis for patients with this disorder.
Core tip: Bloody diarrhea and Hemolytic uremic syndrome are frequent caused by E. coli serotype O157:H7. The most causes of gastroenteritis are diagnosed as non-infectious illness and this could be the reason that clinicians did not usually associated with the hemolytic uremic syndrome development. This case report not only represents the importance of the diagnosis and the treatment approach, but also is one of the few studies where it is emphasized the gastrointestinal role and the critical symptoms that the clinical has to recognize it.
- Citation: Chinchilla-López P, Cruz-Ramón V, Ramírez-Pérez O, Méndez-Sánchez N. Gastroenteritis in an adult female revealing hemolytic uremic syndrome: Case report. World J Gastroenterol 2018; 24(6): 763-766
- URL: https://www.wjgnet.com/1007-9327/full/v24/i6/763.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i6.763
Thrombotic thrombocytic purpura (TTP) and hemolytic-uremic syndrome (HUS) are acute fulminant disorders characterized by thrombocytopenia and microangiopathic hemolytic anemia. Gastroenteritis is recognized as a precursor of the HUS, which is often accompanied by pain abdominal, vomiting and bloody stools. Other specific symptoms to establish the diagnosis are kidney failure and cognitive impairment[1].
A 61-year-old woman with a history of hypertension, osteoarthritis and irritable bowel syndrome presented to the emergency department (ED) after 5 d of bloody diarrhea without mucus, after eating high-fat food. Her vital signs upon arrival in ED were within normal limits. Physical examination revealed diffuse abdominal tenderness on deep palpation without signs of peritonitis, spasms in the upper extremities, and muscle weakness.
The results of laboratory testing were: hemoglobin, 12.3 g/dL; platelet count, 250 × 103/µL; serum creatinine, 0.76 mg/dL; serum sodium, 133 mmol/L; serum potassium, 3.04 mmol/L; serum calcium, 6.2 mmol/L; and serum magnesium, 0.54 mmol/L. She was diagnosed with hydroelectric disequilibrium as a consequence of diarrheic syndrome and admitted for immediate treatment of hypocalcemia with fluids and electrolytes via central venous access; in addition intravenous antibiotic therapy with ertapenem was begun because culture showed the presence of extended-spectrum beta-lactamase (ESBL)-producing E. coli. Despite aggressive antibiotic therapy, she continued to clinically deteriorate with petechiae and ecchymoses appearing on her lower legs and her biochemical profile did not improve (Table 1). At that point, the results of blood tests were: hemoglobin, 9.8 g/dL; platelet count, 11 × 103; serum creatinine, 3.5 mg/dL; and lactate dehydrogenase, 3182 units/mL. She was diagnosed with microangiopathic hemolytic anemia. Her uremia required immediate hemodialysis after the insertion of a Mahurkar catheter. Overall, she required seven hemodialysis sessions and seven plasmaphereses during the 13 d after admission. Hematological tests showed a slight increase in her erythrocyte count, so she did not need erythrocyte transfusion until the sixth day of her hospital stay. Altogether, she required three transfusions of packed cells.
| Test | Initial evaluation | Middle evaluation | Final evaluation |
| Hemoglobin | 12.3 g/dL (13-17 g/dL) | 6.8 g/dL | 9.7 g/dL |
| Platelets | 250 × 103/μL (150-450 × 103/µL) | 37 × 103/μL | 213 × 103/μL |
| Leukocytes | 7.9 × 103/μL (4.5-11 × 103/µL) | 6.6 × 103/μL | 5.3 × 103/μL |
| Neutrophils | 84.3 % (40%-75%) | 75% | 88% |
| Lymphocytes | 10.7% (12%-46%) | 14% | 8.00% |
| Blood urea nitrogen | 4.9 mg/dL (8.0-20 mg/dL) | 70.4 mg/dL | 59.5 mg/dL |
| Urea | 10.5 mg/dL (17.1-42.8 mg/dL) | 150.7 mg/dL | 127.3 mg/dL |
| Creatinine | 0.76 mg/dL (0.44-1.03 md/dL) | 6.24 mg/dL | 1.97 mg/dL |
| Sodium | 133 mmol/L (136-144 mmol/L) | 129 mmol/L | 139 mmol/L |
| Potassium | 3.04 mmol/L (3.60-5.10 mmol/L) | 2.95 mmol/L | 4.27 mmol/L |
| Calcium | 6.7 mg/dL (8.9-10.3 mmol/dL) | 6.9 mg/dL | 8.5 mg/dL |
| Magnesium | 0.52 mg/dL (1.80-2.50 mg/dL) | 2.03 mg/dL | 1.41 mg/dL |
| Partial thromboplastin time | 31.9 s (24.8-31.8 s) | 29.4 s | 25.3 s |
| Fibrinogen | 249 mg/dL (177-410 mg/dL) | 223 mg/dL | 221 mg/dL |
| D-dimer | 4620 ng/mL (0-199 ng/mL) | 2520 ng/mL | 321 ng/ mL |
| Alanine aminotransferase | 48 U/L (14-54 U/L) | 37 U/L | 20 U/L |
| Aspartate aminotransferase | 251 U/L (15-41 U/L) | 121 U/L | 35 U/L |
| Dehydrogenase lactic | 3182 U/L (98-192 U/L) | 1781 U/L | 314 U/L |
| Albumin | 4.0 g/dL (3.5-4.8 g/dL) | 2.5 g/dL | 3.5 g/dL |
Finally, the patient showed slow improvement in her renal function, recovering diuresis after 3 wk. She was discharged with a platelet count of 213 × 103/µL.
TTP and HUS have been considered rare diseases with high mortality. They continue to be rare in adults, although we have seen them more frequently than in the past. The prevalence of TTP is around 30%-40%, whereas HUS rate is approximately 4%-10%; their mortality ranges around 90% and 15%, respectively[2]. The most important reason that our patient had a positive outcome was that we never excluded HUS as a possible diagnosis. The unusual features that led us to the diagnosis were the bloody diarrhea, abdominal and diffuse pain evolving over 5 d after eating high-fat food, and the abnormal blood test results, specifically the presence of hemolytic anemia and acute kidney failure[3].
Karpac et al[4] reported that the most common symptom associated with typical HUS is diarrhea; however, the majority of gastrointestinal diseases can cause this. In addition, the bloody diarrhea is often caused by Campylobacter, E. coli O157:H7, and other Shiga toxin-producing E. coli, Salmonella, Shigella, and Yersinia; however, E. coli O157:H7 is the most important pathogen, which usually causes abdominal tenderness, more than 5 watery stools in the last 24 h, and especially bloody diarrhea that frequently persists during first 8 h[5]. Incidentally, Kuehne et al[6] demonstrated that the true STEC gastroenteritis incidence in a computed estimate was 32.3-fold higher than the incidence based on notified HUS cases.
In addition, Pedersen et al[7] showed that the incidence rate per 100000 persons of STEC infections was highest in children < 5 years of age (32.17) and in adults ≥ 65 years of age (11.64) compared to 15-64 years old population (7.89).
Regarding to the first symptoms of our patient, the appearance of ecchymoses and petechiae, besides the sudden decrease in platelets and the increase in serum creatinine confirmed our diagnosis of microangiopathy.
As indicated in the guidelines for management of microangiopathies, our first-line therapy was plasma exchange[2] combined with hemodialysis and aggressive antibiotic therapy because ESBL-producing E. coli was detected in blood cultures. However, at that time we could not differentiate between HUS and TTP. Later, the normal levels of ADAMTS 13 protease led us to the definitive diagnosis of HUS[8].
Differentiation of HUS and TTP remains complex. Acute renal failure is usually more severe in HUS[9], while TTP more frequently results in damage to the central nervous system. Moake[5,8] reported that systemic aggregation of platelets, especially in the central nervous system, usually indicates TTP because platelet aggregation in HUS is predominantly confined to the renal circulation[8]. In relation to ADMTS 13, a protein responsible for cleaving Von Willebrand factor, our patient had normal levels and this was another characteristic that assisted our diagnosis.
The key features of HUS treatment are firstly, fluid therapy with isotonic solutions to avoid the occurrence of oliguria, anuria, and the requirement for dialysis[10]. In our patient, diuresis was less than 0.5 mL/kg/h for more than 12 h, meaning that hemodialysis was necessary[11]. Secondly, the guidelines recommend blood transfusion when hemoglobin drops to 6 mg/dL, therefore, our patient required three transfusions of packed cells. Thirdly, patient needed antihypertensive therapy because patients with HUS develop arterial hypertension caused by an overexpansion of the intravascular volume and/or ischemia-induced activation of the renin–angiotensin system[12]. Finally, management with low-molecular-weight heparin was necessary to prevent a thrombotic event.
In summary, our patient presented typical clinical features of HUS. This is an uncommon disease in adults, which has a relatively good prognosis with low mortality. The gastroenterologist may encounter the HUS as it presents with primarily intestinal symptoms or may assist in the management of the abdominal complications. Anticipation of the broad clinical scope of the HUS is essential for the optimal management of this serious entity.
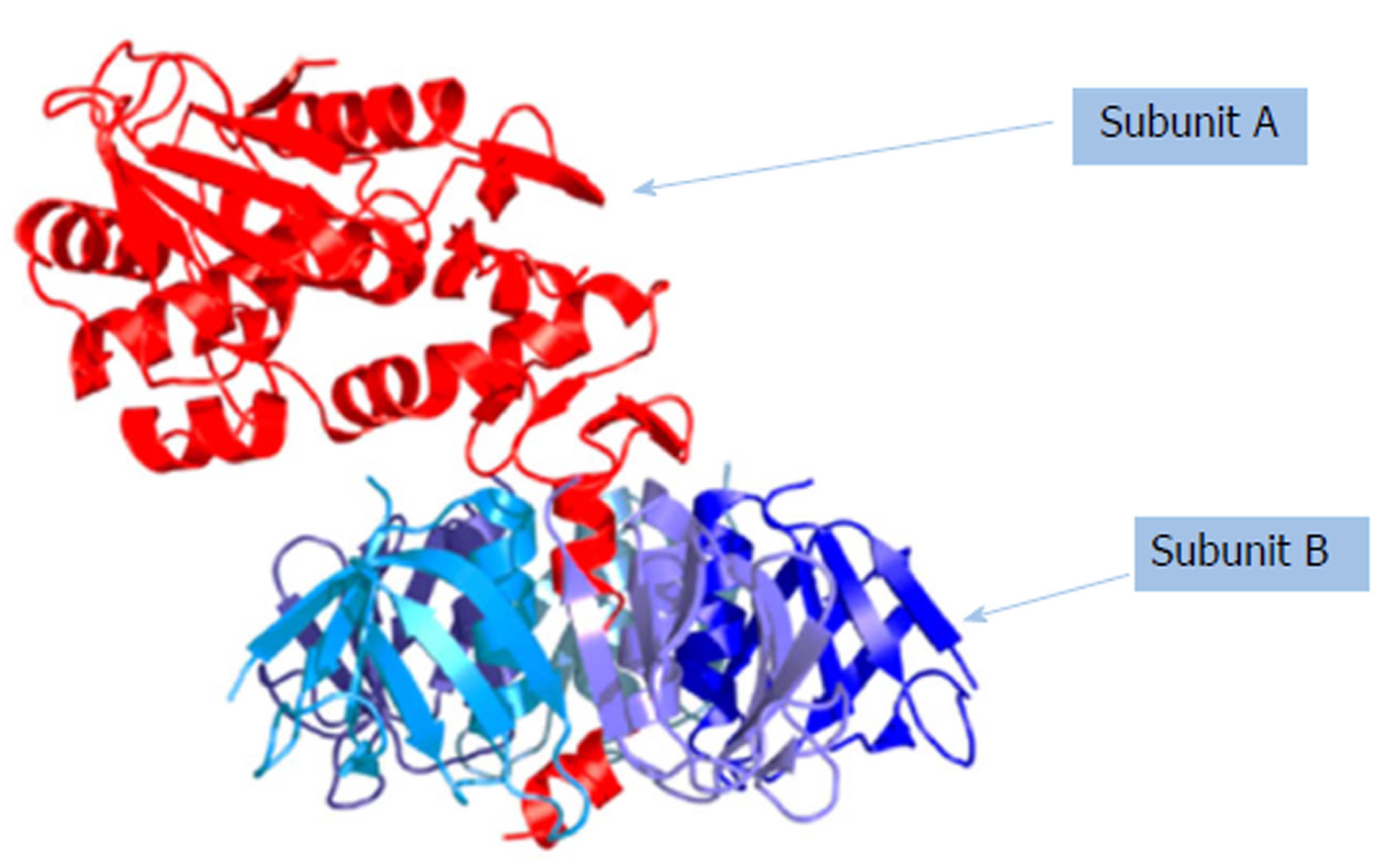
After contaminated food is ingested, the Shiga toxin (Figure 1) is released into the blood circulation, which leads to damage of the intestinal mucosa and the renal endothelium. Damaged renal endothelial cells promote a prothrombin state with an increase in platelet adhesion and formation of microthrombi[13]. Clinicians should consider E. coli O157:H7 when evaluating patients with diarrhea, especially those with a history of bloody diarrhea, and should be aware that patients with E. coli O157:H7 infection can get a wrong diagnosis with a non-infectious disease. Afterwards, that is the reason why it is necessary to perform culture stools for E coli 0157:H7 since may lead to early recognition of outbreaks and to implement public health control measures. Definitely, surveillance on the organism is needed to define more clearly the clinical illness, populations at risk of infection and risks and benefits of treatment methods.
A 61-year-old woman, with hypertension, osteoarthritis and irritable bowel syndrome, presented to emergency department after 5 d of bloody diarrhea without mucus.
The diagnosis of hemolytic uremic syndrome (HUS) was based on the presence of diarrheal prodrome, thrombocytopenia and the development of acute renal failure.
Thrombotic thrombocytic purpura and other causes of thrombocytopenia.
Moderate hydroelectrolytic disequilibrium, thrombocytopenia and microangiopathic hemolytic anemia.
The culture showed the presence of E. coli producing extended-spectrum β-lactamases.
Fluid and electrolyte replacement, plasma exchange, hemodialysis and intravenous antibiotic therapy with ertapenem.
HUS associated to gastroenteritis is a rare event in adults due to very few cases had been detected. However, this disorder has high mortality but good prognosis when it is diagnosed in early stages. The treatment should be based on syndromic approach according to guidelines.
ADAMTS13 - a disintegrin-like metalloproteinase with thrombospondin motif type 1 member 13 - cleaves Von Willebrand factor anchored on the endothelial surface, in circulation, and at the sites of vascular injury.
The gastroenterologist may encounter the HUS as it presents with primarily intestinal symptoms or may assist in the management of the abdominal complications. Anticipation of the broad clinical scope of the HUS is essential for the optimal management of this serious entity.
| 1. | Furlan M, Lämmle B. Haemolytic-uraemic syndrome and thrombotic thrombocytopenic purpura--new insights into underlying biochemical mechanisms. Nephrol Dial Transplant. 2000;15:1112-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Igarashi T, Ito S, Sako M, Saitoh A, Hataya H, Mizuguchi M, Morishima T, Ohnishi K, Kawamura N, Kitayama H. Guidelines for the management and investigation of hemolytic uremic syndrome. Clin Exp Nephrol. 2014;18:525-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Blombery P, Scully M. Management of thrombotic thrombocytopenic purpura: current perspectives. J Blood Med. 2014;5:15-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Karpac CA, Li X, Terrell DR, Kremer Hovinga JA, Lämmle B, Vesely SK, George JN. Sporadic bloody diarrhoea-associated thrombotic thrombocytopenic purpura-haemolytic uraemic syndrome: an adult and paediatric comparison. Br J Haematol. 2008;141:696-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Holtz LR, Neill MA, Tarr PI. Acute bloody diarrhea: a medical emergency for patients of all ages. Gastroenterology. 2009;136:1887-1898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Kuehne A, Bouwknegt M, Havelaar A, Gilsdorf A, Hoyer P, Stark K, Werber D; HUS active surveillance network Germany. Estimating true incidence of O157 and non-O157 Shiga toxin-producing Escherichia coli illness in Germany based on notification data of haemolytic uraemic syndrome. Epidemiol Infect. 2016;144:3305-3315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Pedersen RM, Nielsen MTK, Möller S, Ethelberg S, Skov MN, Kolmos HJ, Scheutz F, Holt HM, Rosenvinge FS. Shiga toxin-producing Escherichia coli: incidence and clinical features in a setting with complete screening of patients with suspected infective diarrhoea. Clin Microbiol Infect. 2017;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Moake JL. Thrombotic microangiopathies. N Engl J Med. 2002;347:589-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 968] [Cited by in RCA: 916] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 9. | George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014;371:654-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 817] [Article Influence: 68.1] [Reference Citation Analysis (0)] |
| 10. | Ake JA, Jelacic S, Ciol MA, Watkins SL, Murray KF, Christie DL, Klein EJ, Tarr PI. Relative nephroprotection during Escherichia coli O157:H7 infections: association with intravenous volume expansion. Pediatrics. 2005;115:e673-e680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 146] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Bagshaw SM, Peets AD, Hameed M, Boiteau PJ, Laupland KB, Doig CJ. Dialysis Disequilibrium Syndrome: brain death following hemodialysis for metabolic acidosis and acute renal failure--a case report. BMC Nephrol. 2004;5:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Powell HR, Rotenberg E, Williams AL, McCredie DA. Plasma renin activity in acute poststreptococcal glomerulonephritis and the haemolytic-uraemic syndrome. Arch Dis Child. 1974;49:802-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 21] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Yau JW, Teoh H, Verma S. Endothelial cell control of thrombosis. BMC Cardiovasc Disord. 2015;15:130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 492] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Mexico
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Krishnan T S- Editor: Gong ZM L- Editor: A E- Editor: Ma YJ