Published online Dec 14, 2018. doi: 10.3748/wjg.v24.i46.5280
Peer-review started: September 6, 2018
First decision: October 4, 2018
Revised: October 27, 2018
Accepted: November 9, 2018
Article in press: November 9, 2018
Published online: December 14, 2018
Processing time: 16 Days and 6.2 Hours
To evaluate a 3D-printed coplanar template for iodine-125 seed implantation therapy in patients with pancreatic cancer.
A retrospective analysis of our database was performed, and a total of 25 patients with pancreatic cancer who underwent iodine-125 seed implantation between January 2014 and November 2017 were analyzed. Of these, 12 implantations were assisted by a 3D-printed coplanar template (group A), and 13 implantations performed freehand were selected as a control group (group B). A 3D coplanar template was designed and printed according to a preoperative CT scan and treatment planning system. The iodine-125 seeds were then implanted using the template as a guide. Dosimetric verification was performed after implantation. Pre- and postoperative D90, V100, and V150 were calculated. The success rate of iodine-125 seed implantation, dosimetric parameters, and complications were analyzed and compared between the two groups.
Iodine-125 seed implantation was successfully performed in both groups. In group A, the median pre- and postoperative D90 values were 155.32 ± 8.05 Gy and 154.82 ± 16.43 Gy, respectively; the difference between these values was minimal and not statistically significant (P > 0.05). Postoperative V100 and V150 were 91.05% ± 4.06% and 64.54% ± 13.40%, respectively, which met the treatment requirement. A better dosimetric parameter was observed in group A than in group B, and the difference was statistically significant (V100: 91.05% ± 4.06% vs 72.91% ± 13.78%, P < 0.05). No major procedure-related complications were observed in either group. For group A, mild hemorrhage was observed in 1 patient with a peritoneal local hematoma due to mesenteric vein damage from the iodine-125 seed implantation needle. The hematoma resolved spontaneously without treatment. Postoperative blood amylase levels remained within the normal range for all patients.
A 3D-printed coplanar template appears to be a safe and effective iodine-125 seed implantation guidance tool to improve implantation accuracy and optimize dosimetric distribution.
Core tip: 3D-printed templates have been used as a new iodine-125 seed implantation guidance tool to improve implantation accuracy and radiotherapy dosimetry in superficial organs. However, few studies have sufficient information on template application in profound organs, especially the pancreas. Our retrospective study evaluates the effectiveness and safety of a 3D-printed coplanar template for iodine-125 seed implantation therapy in patients with pancreatic cancer. The template was designed and printed according to a preoperative computed tomography scan. Under 3D-printed template guidance, the postoperative dosimetric parameter was better than that obtained with traditional free-hand implantation.
- Citation: Huang W, Lu J, Chen KM, Wu ZY, Wang QB, Liu JJ, Gong J, Chen ZJ, Ding XY, Wang ZM. Preliminary application of 3D-printed coplanar template for iodine-125 seed implantation therapy in patients with advanced pancreatic cancer. World J Gastroenterol 2018; 24(46): 5280-5287
- URL: https://www.wjgnet.com/1007-9327/full/v24/i46/5280.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i46.5280
Pancreatic cancer is one of the most common and lethal malignant digestive neoplasms. The incidence and mortality of pancreatic cancer have increased in recent years[1], and surgical resection remains the only curative treatment for this disease. Unfortunately, only 10%-20% of patients present with early-stage disease at diagnosis and are candidates for surgery[2,3]. For most patients with unresectable advanced tumors or metastatic disease, gemcitabine-based chemotherapy remains the mainstay treatment. Nevertheless, the prognosis is poor, and the median survival time from diagnosis is less than one year[4,5]. During the course of the disease, patients frequently suffer from intractable pain due to tumor perineural invasion. This pain can be relieved by traditional external radiotherapy in more than 50% of patients but with significant systemic side effects[6-8]. To reduce these side effects, in previous studies, radioactive iodine-125 seeds were implanted as a radiation source in pancreatic lesions under computed tomography (CT) guidance, and this approach demonstrated efficacy for local tumor control and pain relief[9-11]. However, the deep retroperitoneal location of the pancreas, as well as the surrounding bowel loop and adjacent blood vessels, precludes equidistant insertion of parallel needles, which leads to inconsistent dosimetric parameters before and after implantation. The individual template guidance technique, which can enhance the accuracy of radioactive iodine-125 seed implantation and simplify operation procedures, has been developed for the treatment of superficial malignant tumors[12]. Nevertheless, for profound organs, the effectiveness and safety of individual template-assisted implantation are not clear. In this study, we attempted to improve the accuracy of iodine-125 seed implantation for pancreatic cancer treatment using a 3D-printed coplanar template to assist needle insertion. The dosimetric parameters and complications were assessed.
The study was approved by the institutional review board, and all subjects signed an informed consent form.
From January 2014 to December 2017, 25 patients with unresectable advanced pancreatic carcinoma were treated with iodine-125 seed implantation. Of these, 12 implantations were assisted by a 3D-printed coplanar template (group A), and the remaining 13 implantations were performed without template guidance and selected as a control group (group B). Patient characteristics are listed in Table 1.
| Characteristic | Group A | Group B |
| Sex, n (%) | ||
| Male | 6 (50) | 6 (46.2) |
| Female | 6 (50) | 7 (53.8) |
| Age, yr | ||
| Median (range) | 65.5 (48-81) | 63.8 (47-84) |
| Location of lesions, n (%) | ||
| Pancreatic head | 11 (92) | 9 (69.2) |
| Pancreatic body and tail | 1 (8) | 4 (30.8) |
| Lesion size, n (%) | ||
| ≤ 3 cm | 1 (8.3) | 3 (23.1) |
| 3-5 cm | 10 (83.3) | 9 (69.2) |
| > 5 cm | 1 (8.3) | 1 (7.7) |
| Stage, n (%) | ||
| III | 7 (58.3) | 8 (61.5) |
| IV | 5 (41.6) | 5 (38.5) |
The criteria for enrollment included the following: (1) histological diagnosis of pancreatic adenocarcinoma by CT-guided fine needle aspiration before implantation; (2) contraindications to surgical resection; (3) Eastern Cooperative Oncology Group (ECOG) score ≤ 2; (4) neutrophil leukocyte ≥ 3 × 109/L, platelets ≥ 70 × 109/L, and hemoglobin ≥ 90 g/L in peripheral blood; (5) prothrombin index (PI) greater than 50% and partial thromboplastin time (PTT) less than 50 s; (6) liver and kidney functions within normal ranges; (7) absence of systemic infection or focal infection on the designed puncture route; and (8) absence of refractory ascites.
All abdominal multidetector computed tomography (MDCT) scans were performed on a 64-slice multidetector-row CT scanner (Lightspeed 64; GE Medical Systems, Milwaukee, WI, United States) using the conditions 120 kV and 250 mA. All axial CT images were obtained when the patient held their breath, and after the initial administration of contrast materials, the scan delay times were 30 to 35 s for the early arterial phase and 65 to 70 s for the portal venous phase (Omnipaque 300 or 350; Amersham, Shanghai, China) at a flow rate of 3.0 L/s. The images were acquired at 5-mm slice thicknesses and sent to the treatment planning system (TPS Astro technology Ltd. Co., Beijing, China). The iodine-125 seed (XinKe Pharmaceutical Ltd., Shanghai, China) was 0.8 mm × 4.5 mm (diameter × length) with a radioactivity of 0.6 mCi and radioactive half-life of 59.6 d. The prescription dose for the target pancreatic areas was set at 140 Gy. The puncture route and iodine-125 seed distribution were designed according to the dosimetric parameter calculated by TPS.
The puncture trajectory, which includes the entry point, angle, and direction of the implantation needles, was designed based on the location and depth of the pancreatic tumor and the structures adjacent to the tumor. According to the severity of complication outcomes using the Society of Interventional Radiology (SIR) classification[13], the peripancreatic organs and structures were divided into high- and low-risk groups. The high-risk group included the abdominal aorta and its first or second branches, the portal vein trunk, and the colon. These structures should be prioritized during puncture route design to avoid any injury during implantation and to prevent massive bleeding and abdominal infection. The low-risk group included the liver, kidney, stomach, intestine, and mesenteric vein branches. These structures were penetrated to meet the dosimetric requirement when no other alternative puncture routes existed.
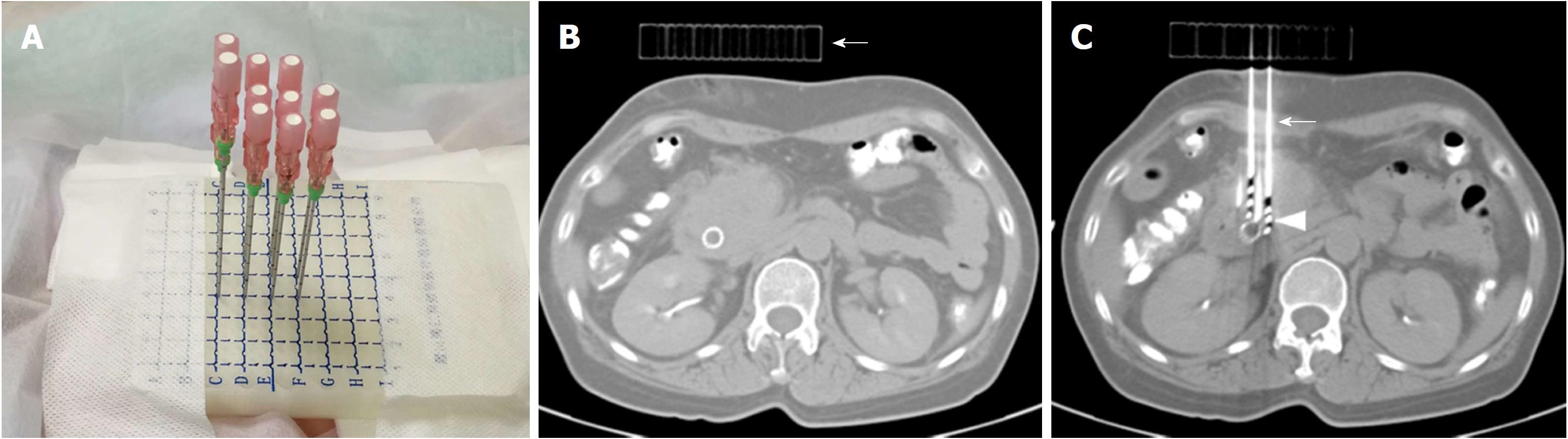
For group A, the template was made of polymethyl methacrylate (PMMA). Its shape and size were designed and printed based on the entry point and puncture angle. The preset puncture holes were 1.5 mm in diameter with 5-mm intervals. These holes were parallel to each other in 3D space, namely, the 3D-printed coplanar template.
Patients adhered to a low-residue diet for 3 d, fasted for 24 h and took oral laxatives 12 h prior to the procedure to decrease intestinal volume. Pancreatic secretion was inhibited with somatostatin 24 h before the operation.
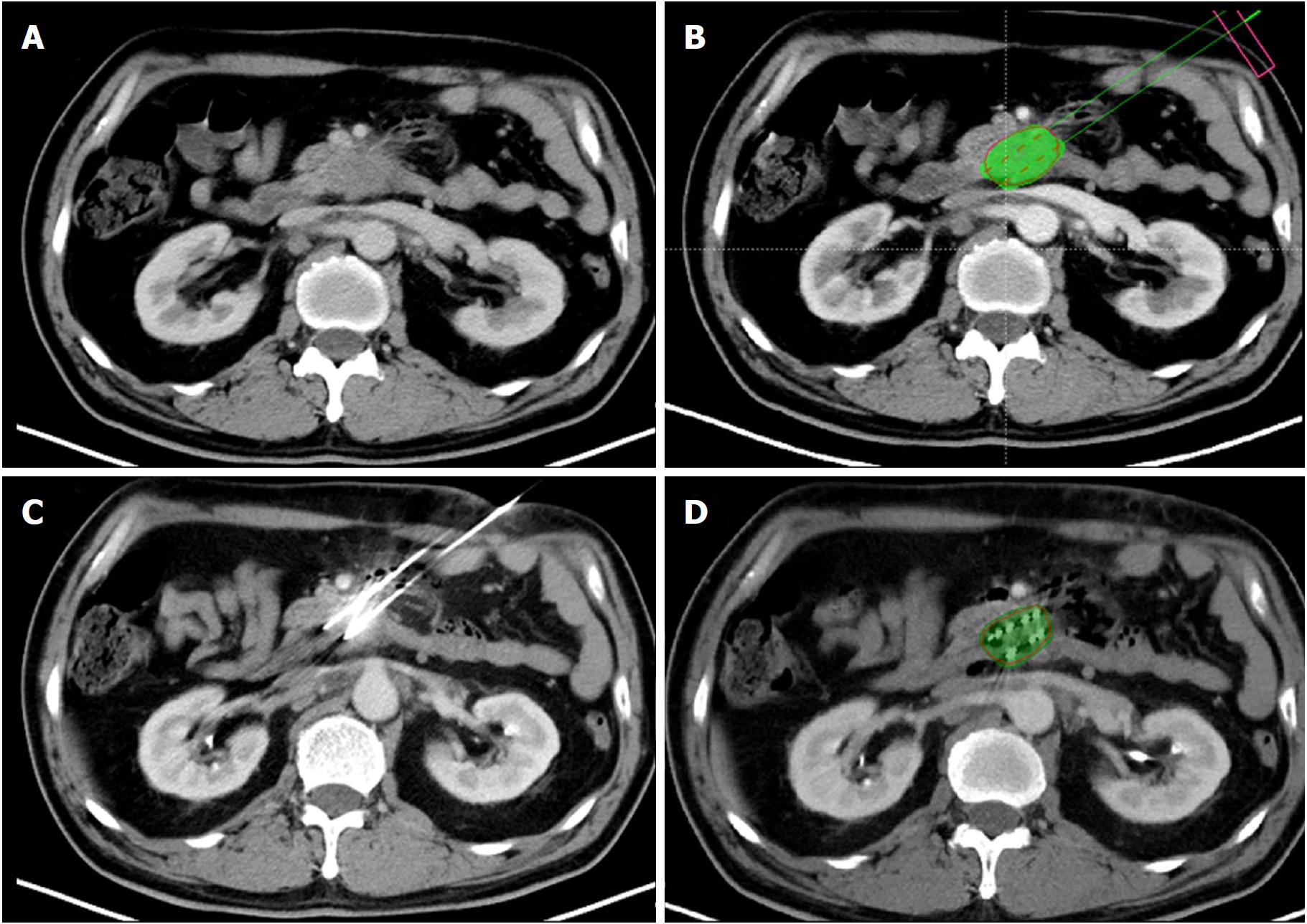
All iodine-125 seed implantations were performed under CT guidance by three interventional radiologists with 14-30 years of interventional experience. The CT image acquisition protocol was the same as that described above. The scan area included the whole pancreatic tumor. The patient was positioned based on the planned access trajectory. For group A, after skin disinfection and local anesthesia with 2% lidocaine, a 3D-printed coplanar template was placed on the patient skin entry point using a supporting structure that could be adjusted to different angles. The 18-G implantation needles (HAKKO Co., Ltd. Nagano, Japan) were inserted into the lesions through the puncture holes on the coplanar template (Figure 1). The procedure was discontinuous, and the needle direction and parallelism were controlled under CT guidance every 2-3 cm of insertion depth. The CT images were sent to TPS after all needles were placed. The dosimetry was calculated according to the needle position (Figure 2). The iodine-125 seeds were then implanted if the dosimeter results matched the preoperative plan. Additional needles were inserted, preferably through the 3D-printed coplanar template, if a cold spot was detected. The needle position was adjusted freehand if traversing high-risk organs was inevitable using the template. For group B, the implantation procedure was performed freehand, i.e., without a template. For all patients, a CT scan was carried out after iodine-125 seed implantation to evaluate the iodine-125 seed position and complications.
Dosimetric verification was performed after implantation, and the postoperative CT images were sent to TPS to calculate the D90, V90, V100, and V150.
Chemotherapy was performed 1 wk after the implantation in all patients in groups A and B. Patients received an intravenous infusion of gemcitabine at 1000 mg/m2 on days 1, 8, and 15. The chemotherapy was repeated every 4 wk for up to six cycles.
Using SPSS 19.0 software, quantitative indicators before and after the operation were compared using the paired t-test, and dosimeter differences between groups A and B were compared using the independent sample t-test. A P-value of less than 0.05 was defined as statistically significant.
For all patients in groups A and B, iodine-125 seeds were successfully inserted into all pancreatic tumors. The preoperative and postoperative dosimetric data are listed in Table 2. The differences in dosimetric data between groups A and B are listed in Table 3.
| Group A | Group B | |||||
| Dosimeter | Preoperative | Postoperative | P value | Preoperative | Postoperative | P value |
| D90 (Gy) | 155.32 ± 8.05 | 154.82 ± 16.43 | 0.91 | 154.2 ± 9.08 | 103.27 ± 25.03 | 0.00 |
| V90 (%) | 97.14 ± 0.93 | 94.64 ± 2.35 | 0.01 | 97.17 ± 0.95 | 78.91 ± 12.71 | 0.00 |
| V100 (%) | 94.51 ± 1.61 | 91.05 ± 4.06 | 0.02 | 94.94 ± 1.05 | 72.91 ± 13.78 | 0.00 |
| V150 (%) | 59.26 ± 15.42 | 64.54 ± 13.40 | 0.36 | 62.44 ± 8.66 | 48.16 ± 15.97 | 0.02 |
| Postoperative dosimeter | Group A | Group B | t | P value |
| D90 (Gy) | 154.82 ± 16.43 | 103.27 ± 25.03 | 6.132 | 0.13 |
| V90 (%) | 94.64 ± 2.35 | 78.91 ± 12.71 | 4.38 | 0.00 |
| V100 (%) | 91.05 ± 4.06 | 72.91 ± 13.78 | 4.54 | 0.00 |
| V150 (%) | 64.54 ± 13.40 | 48.16 ± 15.97 | 2.765 | 0.44 |
For group A, the median pre- and postoperative D90 values were 155.32 ± 8.05 Gy and 154.82 ± 16.43 Gy, respectively; the difference between the values was minimal and not statistically significant (P = 0.91). The postoperative V90, V100, and V150 values were 94.64% ± 2.35%, 91.05% ± 4.06%, and 64.54% ± 13.40%, respectively. Among these, V90 and V100 were slightly lower than planned but higher than those of group B, and the difference was statistically significant (P = 0.00).
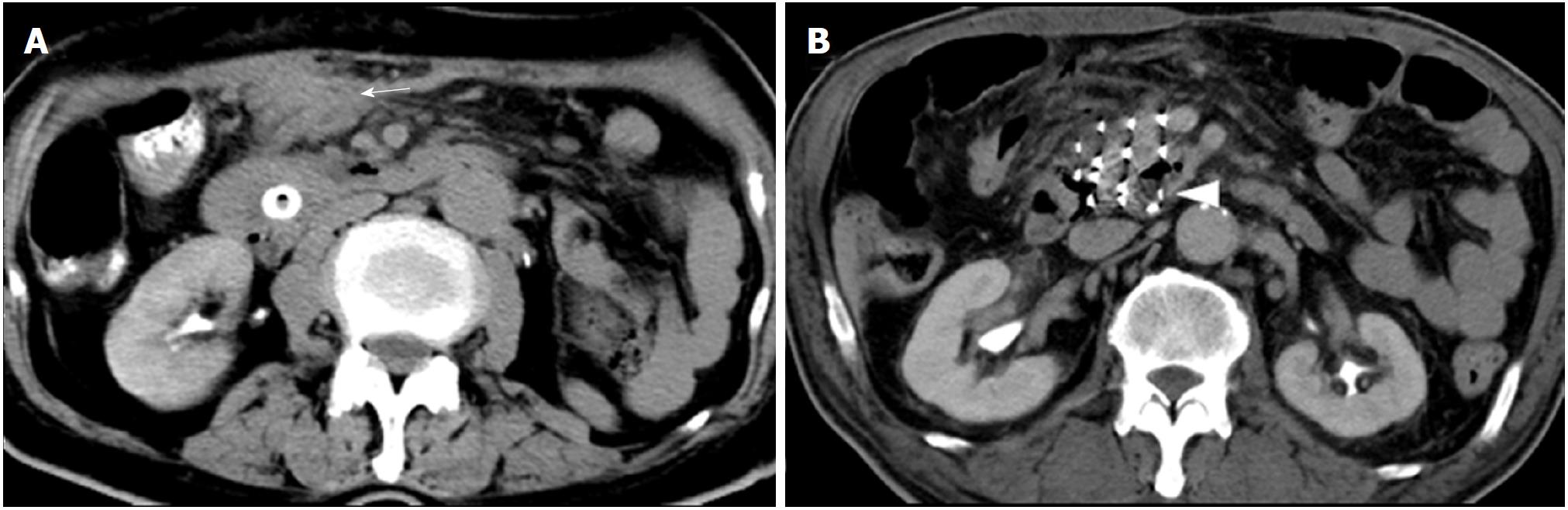
No major procedure-related complications (pancreatitis, pancreatic leakage, digestive perforation, or severe hemorrhage) were observed. For group A, mild hemorrhage was observed in 1 patient with a peritoneal local hematoma duo to mesenteric vein injury (Figure 3A). This patient did not present any hemorrhage symptoms, and blood erythrocytes and hemoglobin levels remained normal. The hematoma resolved spontaneously without treatment. No complications occurred in group B. The postoperative blood amylase level remained within the normal range in all patients and slightly decreased compared with the preoperative level (41.3 ± 17.8 U/L vs 48.0 ± 25.0 U/L).
For group A, iodine-125 seed migration in the lesion was observed in 1 patient due to tumor liquefactive necrosis (Figure 3B). Although the seed showed a 5-mm shift from its initial position, the movement did not influence the radioactive dose in the target area.
Iodine-125 seed implantation has been proven to be a safe alternative treatment for advanced pancreatic cancer[10,14,15]. Pancreatic cancer cells have been shown to be sensitive to continuous, low-energy iodine-125 seed irradiation in ex-vivo studies[16,17]. In our previous study, tumor growth and patient pain were significantly suppressed by this treatment[9]. As a type of brachytherapy, the effectiveness of iodine-125 seed implantation depends on the dose in the target area, which is influenced by seed distribution[18]. However, the pancreas is a retroperitoneal organ adjacent to many vulnerable organs, and freehand placement of pancreatic implantation needles in a parallel and equidistant arrangement is difficult. Therefore, maintaining seed distribution according to preoperative planning to prevent radiation cold spots is difficult.
In the clinic, a template is used to assist implantation and ensure the iodine-125 seed distribution accuracy. With the aid of a template, parallel and equidistant implantation needles can be maintained during insertion. However, the traditional template has limitations: the puncture angle is vertical relative to the patient’s skin, and changing the angle during the procedure is difficult. Thus, this method is more suitable for tumors in superficial organs, such as prostate and cervical tumors, than profound organs. For pancreatic cancer, the direction and distance between the puncture holes on the template should be designed according to individual anatomic characteristics to create a variable puncture angle. Therefore, in our study, we attempted to use 3D-printing technology to make a template that is suitable for pancreatic tumor iodine-125 seed implantation.
In recent years, 3D-printing technology has increasingly been applied in medicine, especially in the domains of orthopedics and maxillofacial and vascular surgery[19-22]. According to preoperative CT data, a high-precision 3D model can be printed and used to make the morphology of an implantation material conform to individual anatomy[20,23-26]. This technology has also been preliminarily applied in template preparations for neck, pulmonary, abdominal, and pelvic tumor iodine-125 seed implantation, and the postoperative dosimetry was consistent with treatment planning[12,27,28].
There are two types of 3D-printed templates: noncoplanar and coplanar. Noncoplanar templates closely adhere to the patient’s skin, and the entry route and angle are fixed. Thus, deviation is limited, and the puncture accuracy is high. Nevertheless, the entry direction is difficult to change during the procedure, so this template is more suitable for immobile organs[12]. A coplanar template is located 2-3 cm above a patient’s skin; its accuracy is lower than that of a noncoplanar template, but the angle of the puncture direction can be changed so that the entry direction can be adjusted according to the real-time position of the organ. This method is convenient for mobile organs.
The pancreas is adjacent to the digestive tract and mesenteric vessels, and the morphology and position of which can easily change. The actual puncture route might not be completely consistent with the preprocedure plan. Therefore, in our study, we used a coplanar template to guide the implantation needle because the direction can be slightly changed during the procedure to reduce vessel and digestive tract damage.
Before iodine-125 seed implantation, a low-residue diet, fasting, and oral laxatives were applied before the intervention to decrease digestive tract volume and enlarge the interspace for the puncture target. Furthermore, slow and gradual needle advancement may keep the digestive tract from the needle tip and avoid perforation.
However, the deep location of pancreatic tumors, as well as the surrounding intestinal loop, sometimes precludes direct needle insertion. Traversing the gastrointestinal tract is inevitable for iodine-125 seed distribution according to the preprocedure plan, and preoperative bowel preparation helps reduce traversing complications. According to previous pancreatic biopsy studies, transgastrointestinal tract or solid visceral approaches are suitable and safe[29,30]; thus, in our study, these methods were considered for low-risk organs and as options when no other alternative puncture route existed. The abdominal aorta and its first or second branches, the portal vein trunk, and the colon were considered high-risk organs, and trans-high-risk organ needle implantation was avoided in our study to prevent severe hemorrhage and bacterial peritonitis. However, radiation cold spots might be present when trans-high-risk organ targets are abandoned. This issue can be detected by intraprocedure dosimetry measurements and correlated by adding an additional implantation needle that is not parallel to the needles passing through the template.
In our study, iodine-125 seed implantation for all 12 patients with pancreatic cancer was successfully performed under the guidance of a 3D coplanar template. The majority of implantation needles were parallel and equidistant. The difference in pre- and postoperative D90 values was minimal and not statistically significant. The levels of V90 and V100 were slightly lower than planned, and the reasons for this might include the following: (1) altered location and morphology of the pancreatic lesion due to the presence of implantation needles; and (2) the presence of an intratumor necrosis area into which the iodine-125 seeds might migrate due to gravity. Nevertheless, the position of the iodine-125 seeds changed only slightly, and the average postoperative V100 was still greater than 90% and likely did not influence prognosis. The dose of the 3D coplanar template guide group exceeded that of the control group.
No major complications occurred in our study. Although the number of implantation needles was more than that used for a pancreatic biopsy, the risk did not seem to be higher. The complication rate for core-needle pancreatic biopsy ranges from 8.7%-21.8%[30-32]. One patient presented with a local hematoma due to mesentery vessel injury, which was self-limiting and not clinically significant. The serum amylase level decreased after implantation in most patients according to the preventive use of somatostatin, which can inhibit pancreatic juice secretion and reduce hemorrhage risk by decreasing the portal vein pressure[33,34].
In conclusion, according to our study, 3D-printed coplanar template-assisted iodine-125 seed implantation therapy appears to be safe and effective and may improve the implantation accuracy and similarity between the postoperative dosimetric parameters and treatment planning values. The key limitations of our study include the small sample size and short follow-up period. However, the endpoints of the study included dosimeter and safety results. The long-term curative effect and complications associated with this approach will be evaluated in a future study.
Patients with advanced pancreatic cancer frequently suffer from intractable pain secondary to tumor perineural invasion. This pain can be relieved by traditional external radiotherapy but with significant systemic side effects. To reduce these side effects, in previous studies, radioactive iodine-125 seeds acting as the source of radiation were implanted in pancreatic lesions under computed tomography (CT) guidance, and this approach demonstrated efficacy for local tumor control and pain relief. However, the special location of the pancreas and fragile adjacent organ increase implantation difficulty and lead to inconsistent dosimetric parameters before and after implantation.
3D-printed template is a new iodine-125 seed implantation guidance tool, which can improve implantation accuracy and radiotherapy dosimeters in superficial organs. However, few studies have sufficient information on its application in profound organs, especially the pancreas. As the pancreatic dosimetric parameters are unsatisfactory by using the traditional free hand way, 3D-printed template might be an applicable guidance tool.
The main objective of our study was to evaluate the efficacy and safety of this new guidance tool in the treatment of advanced pancreatic cancer. The preliminary results demonstrated that the procedure is safe and the brachytherapy dosimeters are better in the 3D-printed template group.
In this retrospective study, 25 patients with advanced unresectable pancreatic carcinoma were treated with iodine-125 seed implantation. Of these, 12 implantations were assisted by a 3D-printed coplanar template (group A), and the remaining 13 implantations were performed without template guidance and selected as a control group (group B). For group A, the template was made of polymethyl methacrylate (PMMA). Its shape and size were designed and printed based on the entry point and puncture angle. All procedures were performed under CT guidance. The postoperative CT images were sent to TPS to calculate the D90, V90, V100, and V150. For both groups, the dosimetric data were collected and compared. Standard chemotherapy was performed in all patients 1 week after the implantation.
Iodine-125 seeds were successfully inserted into all pancreatic tumors in groups A and B. For group A, the median pre- and postoperative D90 values were 155.32 ± 8.05 Gy and 154.82 ± 16.43 Gy, respectively; the difference between the values was minimal and not statistically significant (P = 0.91). The postoperative V90, V100, and V150 values were 94.64% ± 2.35%, 91.05% ± 4.06%, and 64.54% ± 13.40%, respectively. Among these, V90 and V100 were slightly lower than planned, and the reasons for this might include the following: (1) altered location and morphology of the pancreatic lesion due to the presence of implantation needles; and (2) the presence of an intratumor necrosis area into which the iodine-125 seeds might migrate due to gravity. Nevertheless, the position of the iodine-125 seeds changed only slightly, and the average postoperative V100 was still greater than 90% and likely did not influence prognosis. The dose of the 3D coplanar template guide group exceeded that of the group B, and the difference was statistically significant (P = 0.00). No major procedure-related complications were observed. For group A, mild hemorrhage was observed in 1 patient with a peritoneal local hematoma duo to mesenteric vein injury.
According to our study, 3D-printed coplanar template-assisted iodine-125 seed implantation therapy appears to be safe and effective and may improve the implantation accuracy and similarity between the postoperative dosimetric parameters and treatment planning values. 3D-printed coplanar template may be an ideal assistive tool for the iodine-125 seed implantation not only in superficial organs but also in profound organs, e.g., the pancreas.
The key limitations of our study include the small sample size and short follow-up period. However, the endpoints of the study included dosimeter and safety results. The long-term curative effect and complications associated with this approach will be evaluated in a future case-control prospective study.
| 1. | Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol. 2016;22:9694-9705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1003] [Cited by in RCA: 994] [Article Influence: 99.4] [Reference Citation Analysis (33)] |
| 2. | Tucker ON, Rela M. Controversies in the management of borderline resectable proximal pancreatic adenocarcinoma with vascular involvement. HPB Surg. 2008;2008:839503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, Niedergethmann M, Zülke C, Fahlke J, Arning MB. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1180] [Cited by in RCA: 1422] [Article Influence: 109.4] [Reference Citation Analysis (0)] |
| 4. | Cunningham D, Chau I, Stocken DD, Valle JW, Smith D, Steward W, Harper PG, Dunn J, Tudur-Smith C, West J. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2009;27:5513-5518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 585] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 5. | Colucci G, Labianca R, Di Costanzo F, Gebbia V, Cartenì G, Massidda B, Dapretto E, Manzione L, Piazza E, Sannicolò M, Ciaparrone M, Cavanna L, Giuliani F, Maiello E, Testa A, Pederzoli P, Falconi M, Gallo C, Di Maio M, Perrone F; Gruppo Oncologico Italia Meridionale (GOIM); Gruppo Italiano per lo Studio dei Carcinomi dell’Apparato Digerente (GISCAD); Gruppo Oncologico Italiano di Ricerca Clinica (GOIRC). Randomized phase III trial of gemcitabine plus cisplatin compared with single-agent gemcitabine as first-line treatment of patients with advanced pancreatic cancer: the GIP-1 study. J Clin Oncol. 2010;28:1645-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 236] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 6. | Ebrahimi G, Rasch CRN, van Tienhoven G. Pain relief after a short course of palliative radiotherapy in pancreatic cancer, the Academic Medical Center (AMC) experience. Acta Oncol. 2018;57:697-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Lahoud MJ, Kourie HR, Antoun J, El Osta L, Ghosn M. Road map for pain management in pancreatic cancer: A review. World J Gastrointest Oncol. 2016;8:599-606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Minsky BD, Hilaris B, Fuks Z. The role of radiation therapy in the control of pain from pancreatic carcinoma. J Pain Symptom Manage. 1988;3:199-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Zhongmin W, Yu L, Fenju L, Kemin C, Gang H. Clinical efficacy of CT-guided iodine-125 seed implantation therapy in patients with advanced pancreatic cancer. Eur Radiol. 2010;20:1786-1791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 10. | Yu YP, Yu Q, Guo JM, Jiang HT, Di XY, Zhu Y. Effectiveness and security of CT-guided percutaneous implantation of (125)I seeds in pancreatic carcinoma. Br J Radiol. 2014;87:20130642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Han Q, Deng M, Lv Y, Dai G. Survival of patients with advanced pancreatic cancer after iodine125 seeds implantation brachytherapy: A meta-analysis. Medicine (Baltimore). 2017;96:e5719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Huang MW, Liu SM, Zheng L, Shi Y, Zhang J, Li YS, Yu GY, Zhang JG. A digital model individual template and CT-guided 125I seed implants for malignant tumors of the head and neck. J Radiat Res. 2012;53:973-977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Omary RA, Bettmann MA, Cardella JF, Bakal CW, Schwartzberg MS, Sacks D, Rholl KS, Meranze SG, Lewis CA. Quality improvement guidelines for the reporting and archiving of interventional radiology procedures. J Vasc Interv Radiol. 2002;13:879-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Niu H, Zhang X, Wang B, Zhou Z, Wang J, Xu Z. The clinical utility of image-guided iodine-125 seed in patients with unresectable pancreatic cancer. Tumour Biol. 2016;37:2219-2223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Wang H, Wang J, Jiang Y, Li J, Tian S, Ran W, Xiu D, Gao Y. The investigation of 125I seed implantation as a salvage modality for unresectable pancreatic carcinoma. J Exp Clin Cancer Res. 2013;32:106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Ma JX, Jin ZD, Si PR, Liu Y, Lu Z, Wu HY, Pan X, Wang LW, Gong YF, Gao J. Continuous and low-energy 125I seed irradiation changes DNA methyltransferases expression patterns and inhibits pancreatic cancer tumor growth. J Exp Clin Cancer Res. 2011;30:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Wang ZM, Lu J, Zhang LY, Lin XZ, Chen KM, Chen ZJ, Liu FJ, Yan FH, Teng GJ, Mao AW. Biological effects of low-dose-rate irradiation of pancreatic carcinoma cells in vitro using 125I seeds. World J Gastroenterol. 2015;21:2336-2342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Hinnen KA, van Vulpen M. Predictors in the outcome of 125I brachytherapy as monotherapy for prostate cancer. Expert Rev Anticancer Ther. 2011;11:115-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Liu K, Zhang Q, Li X, Zhao C, Quan X, Zhao R, Chen Z, Li Y. Preliminary application of a multi-level 3D printing drill guide template for pedicle screw placement in severe and rigid scoliosis. Eur Spine J. 2017;26:1684-1689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 20. | Huang J, Li G, Wang W, Wu K, Le T. 3D printing guiding stent graft fenestration: A novel technique for fenestration in endovascular aneurysm repair. Vascular. 2017;25:442-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Malik HH, Darwood AR, Shaunak S, Kulatilake P, El-Hilly AA, Mulki O, Baskaradas A. Three-dimensional printing in surgery: a review of current surgical applications. J Surg Res. 2015;199:512-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 207] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 22. | Zhang L, Li M, Li Z, Kedeer X, Wang L, Fan Z, Chen C. Three-dimensional printing of navigational template in localization of pulmonary nodule: A pilot study. J Thorac Cardiovasc Surg. 2017;154:2113-2119.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Lindegaard JC, Madsen ML, Traberg A, Meisner B, Nielsen SK, Tanderup K, Spejlborg H, Fokdal LU, Nørrevang O. Individualised 3D printed vaginal template for MRI guided brachytherapy in locally advanced cervical cancer. Radiother Oncol. 2016;118:173-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 24. | Dwivedi DK, Chatzinoff Y, Zhang Y, Yuan Q, Fulkerson M, Chopra R, Brugarolas J, Cadeddu JA, Kapur P, Pedrosa I. Development of a Patient-specific Tumor Mold Using Magnetic Resonance Imaging and 3-Dimensional Printing Technology for Targeted Tissue Procurement and Radiomics Analysis of Renal Masses. Urology. 2018;112:209-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 25. | Marro A, Bandukwala T, Mak W. Three-Dimensional Printing and Medical Imaging: A Review of the Methods and Applications. Curr Probl Diagn Radiol. 2016;45:2-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 195] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 26. | Park SH, Kang BK, Lee JE, Chun SW, Jang K, Kim YH, Jeong MA, Kim Y, Kang K, Lee NK. Design and Fabrication of a Thin-Walled Free-Form Scaffold on the Basis of Medical Image Data and a 3D Printed Template: Its Potential Use in Bile Duct Regeneration. ACS Appl Mater Interfaces. 2017;9:12290-12298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Hongtao Z, Xuemin D, Huimin Y, Zeyang W, Lijuan Z, Jinxin Z, Zezhou L, Aixia S, Juan W. Dosimetry study of three-dimensional print template-guided precision 125I seed implantation. J Cancer Res Ther. 2016;12:C159-C165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Han T, Yang X, Xu Y, Zheng Z, Yan Y, Wang N. Therapeutic value of 3-D printing template-assisted 125I-seed implantation in the treatment of malignant liver tumors. Onco Targets Ther. 2017;10:3277-3283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Hsu MY, Pan KT, Chen CM, Lui KW, Chu SY, Hung CF, Huang YT, Tseng JH. Trans-organ versus trans-mesenteric computed tomography-guided percutaneous fine-needle aspiration biopsy of pancreatic masses: feasibility and safety. Clin Radiol. 2014;69:1050-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Hsu MY, Pan KT, Chen CM, Lui KW, Chu SY, Lin YY, Hung CF, Huang YT, Tseng JH. CT-guided percutaneous core-needle biopsy of pancreatic masses: comparison of the standard mesenteric/retroperitoneal versus the trans-organ approaches. Clin Radiol. 2016;71:507-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Strobl FF, Schwarz JB, Haeussler SM, Paprottka PM, Rist C, Thierfelder KM, Boeck S, Heinemann V, Reiser MF, Trumm CG. Percutaneous CT fluoroscopy-guided core biopsy of pancreatic lesions: technical and clinical outcome of 104 procedures during a 10-year period. Acta Radiol. 2017;58:906-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Tyng CJ, Almeida MF, Barbosa PN, Bitencourt AG, Berg JA, Maciel MS, Coimbra FJ, Schiavon LH, Begnami MD, Guimarães MD. Computed tomography-guided percutaneous core needle biopsy in pancreatic tumor diagnosis. World J Gastroenterol. 2015;21:3579-3586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 33. | Connor S, Alexakis N, Garden OJ, Leandros E, Bramis J, Wigmore SJ. Meta-analysis of the value of somatostatin and its analogues in reducing complications associated with pancreatic surgery. Br J Surg. 2005;92:1059-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 113] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 34. | D’Amico G, Politi F, Morabito A, D’Antoni A, Guerrera D, Giannuoli G, Traina M, Vizzini G, Pasta L, Pagliaro L. Octreotide compared with placebo in a treatment strategy for early rebleeding in cirrhosis. A double blind, randomized pragmatic trial. Hepatology. 1998;28:1206-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 40] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Gobejishvili L, Morling JR, Okada S S- Editor: Ma RY L- Editor: Wang TQ E- Editor: Yin SY