Published online Dec 14, 2018. doi: 10.3748/wjg.v24.i46.5271
Peer-review started: September 12, 2018
First decision: October 14, 2018
Revised: October 20, 2018
Accepted: November 9, 2018
Article in press: November 9, 2018
Published online: December 14, 2018
Processing time: 92 Days and 15.6 Hours
To assess the correlation between the efficacy of lusutrombopag and clinical characteristics in patients with chronic liver disease.
In this retrospective, multicenter study, which conducted at four locations in Japan, 50 thrombocytopenic patients with chronic liver disease were enrolled. All patients received oral lusutrombopag (3.0 mg/d for 7 d) for chronic liver disease. We assessed the increase in platelet count after the trial drug administration. A treatment response was defined as a platelet count ≥ 5 × 104/μL and an increased platelet count ≥ 2 × 104/μL from baseline after drug administration. We evaluated the response to lusutrombopag compared to baseline clinical characteristics in patients with chronic liver disease.
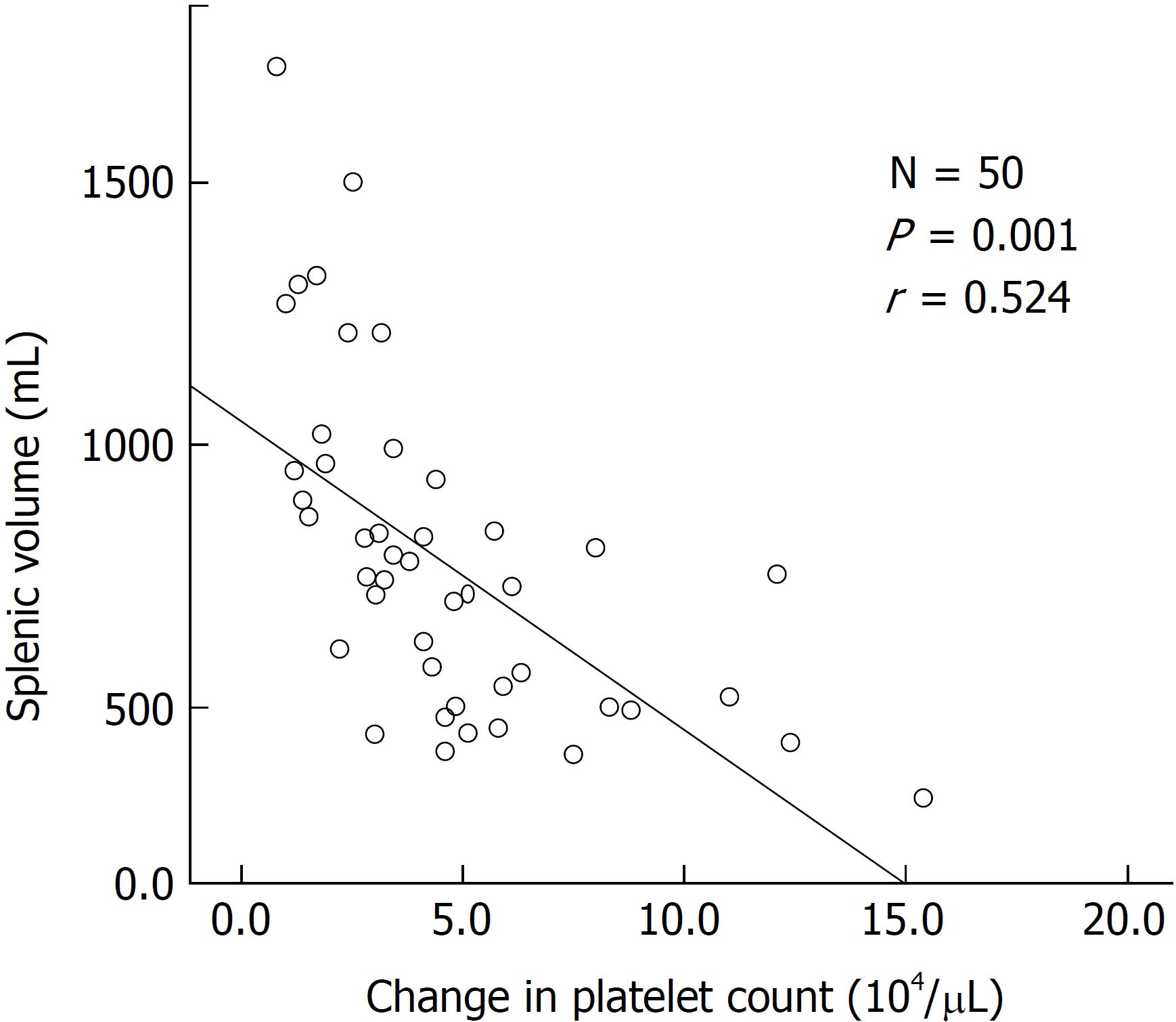
The numbers of responders and non-responders were 40 (80.0%) and 10 (20.0%), respectively. The patients were divided into a responder and non-responder group, and we added factors that may correspond to successful treatment with lusutrombopag. Splenic volume and body weight were lower in the responder group than in the non-responder group. White blood cell count and hemoglobin level were higher in responders compared with non-responders. Using a logistic regression model to assess the relationship between response to lusutrombopag and clinical characteristics, multivariate analysis confirmed that splenic volume was an independent factor that predicted the response of platelet counts (P = 0.025; odds ratio = 11.2; 95% confidence interval: 1.354-103.0). Splenic volume negatively correlated to changes in platelet count (r = -0.524, P = 0.001).
Splenic volume influences the change in platelet counts after administration of lusutrombopag in patients with chronic liver disease.
Core tip: Lusutrombopag is an oral, small-molecule thrombopoietin receptor agonist used for the treatment of thrombocytopenic patients with chronic liver diseases. However, the response to this drug is unpredictable. The study aimed to assess the correlation between the clinical characteristics of patients with chronic liver disease and the efficacy of lusutrombopag treatment. Splenic volume influences the change in platelet counts after administration of lusutrombopag in patients with chronic liver disease. Splenic volume increase is negatively related to changes in the platelet count.
- Citation: Uojima H, Arase Y, Itokawa N, Atsukawa M, Satoh T, Miyazaki K, Hidaka H, Sung JH, Kako M, Tsuruya K, Kagawa T, Iwakiri K, Horie R, Koizumi W. Relationship between response to lusutrombopag and splenic volume. World J Gastroenterol 2018; 24(46): 5271-5279
- URL: https://www.wjgnet.com/1007-9327/full/v24/i46/5271.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i46.5271
Thrombocytopenia is a common complication of end-stage liver disease that leads to portal hypertension[1,2]. Severe thrombocytopenia can significantly increase a patient’s risk of bleeding, especially during surgery or other invasive procedures[3]. Some cirrhotic patients with severe thrombocytopenia require blood transfusions prior to an invasive surgery[4]. However, platelet transfusion poses some risks, such as bacterial contamination, severe allergic reaction, and alloimmunization to platelets[5]. Therefore, to minimize the administration of transfusion, additional methods for reducing the incidence of bleeding events in thrombocytopenic patients are required.
Lusutrombopag is an oral, small-molecule thrombopoietin (TPO) receptor agonist (TPO-RA) used for the treatment of thrombocytopenic patients with chronic liver disease. This new drug promotes thrombopoiesis via the human TPO receptor on the surface of bone marrow cells[6]. TPO production is dependent on functional liver cells, and TPO in the peripheral blood in advanced-stage liver disease severely is reduced. Therefore, patients with inadequate TPO production due to chronic liver disease are the principal targets of treatment with lusutrombopag[7].
However, the response to this drug is unpredictable, assuming that the drug may exert more dramatic effects in patients with thrombocytopenia associated with severe liver disease prior to invasive surgery. Therefore, the study aimed to assess the correlation between the clinical characteristics of patients with chronic liver disease and the efficacy of lusutrombopag treatment.
This study was approved by the Institutional Review Board Ethics Committee at all four institutes. This study is registered in the UMIN Clinical Trials Registry as UMIN 000031354.
This multicenter retrospective study was conducted at four locations in Japan. Enrollment commenced in February 2015 and ended at the end of March 2018. This study enrolled thrombocytopenic patients, who received oral lusutrombopag (3.0 mg/d for 7 d), and from whom blood samples to analyze changes in platelet counts were collected on days 1, 5, 12 (or the maximum count), and 28, according to the manufacturer’s prescription guidelines. The enrolled thrombocytopenic patients had chronic liver disease, which was composed of either cirrhosis or non-cirrhosis. Cirrhosis in these patients were caused by hepatitis C, hepatitis B, alcoholic or non-alcoholic steatohepatitis, primary biliary cholangitis, and autoimmune hepatitis. Non-cirrhotic patients were composed of patients with idiopathic portal hypertension, Budd-Chiari syndrome, or an extrahepatic portal venous obstruction. The diagnosis of cirrhotic and non-cirrhotic diseases was based on laboratory results and imaging tests that revealed a hepatic cirrhotic appearance, gastroesophageal varices, ascites, splenomegaly, portal vein obstruction, and/or portal collateral circulation. Liver biopsy was not performed for any of the patients with liver cirrhosis because of the high risk of life-threatening complications. Any patients with hematologic disease, past history of thromboembolism, who underwent blood or platelet transfusions in the previous 2 wk, or those who had changes in their doses of conventional drugs were excluded.
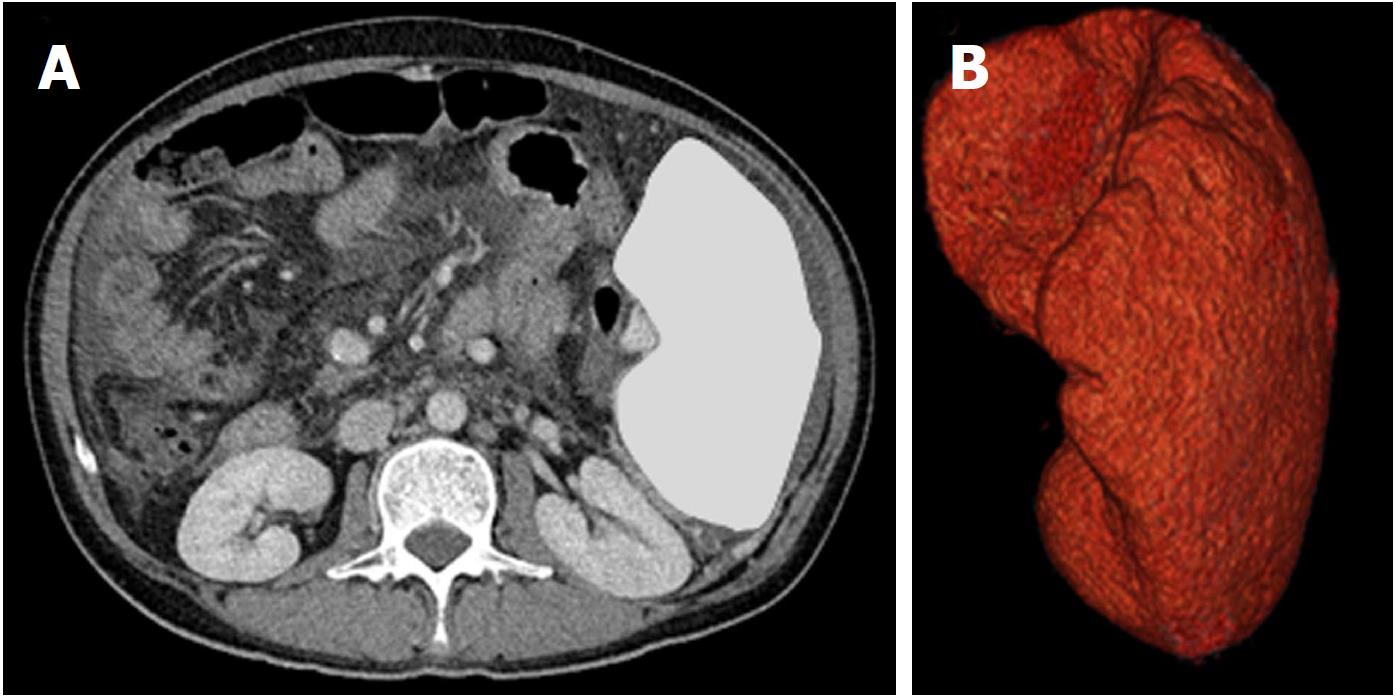
General characteristics, demographic information, procedures, and all laboratory data, except for serological data, were obtained during the administration of the trial drug. Laboratory tests included: Platelet count, hemoglobin, white blood cell count, percent prothrombin time, aspartate aminotransferase (AST), alanine aminotransferase (ALT), total protein level, albumin, blood urea nitrogen, creatinine, total bilirubin, HbA1c, α-fetoprotein (AFP), and protein induced by vitamin K absence-II (PIVKA-II). Two non-invasive fibrosis markers and an algorithm-based score model were calculated as follows: (1) AST to platelet ratio index (APRI): APRI = [AST (/35 IU/L)/platelets (103/μL)] × 100; (2) Fibrosis 4 (FIB-4) index: FIB-4 = [AST (IU/L) × Age (years)]/[ALT (IU/L) × platelets (103/μL)]; (3) Model for end-stage liver disease (MELD): MELD = 3.78 × loge [serum bilirubin (mg/dL)] + 11.2 × loge [PT-INR] + 9.57 × loge [serum creatinine (mg/dL)] + 6.43. Calculation of splenic volume, portal vein diameter, and splenic vein diameter in patients with chronic liver disease was conducted via abdominal computed tomography (CT) on a multidetector scanner during a 6-mo period. After image acquisition, the data were transferred to an image processing workstation. Five-millimeter slices from enhanced CT images of the entire spleen were obtained and outlined using a semi-automatic segmentation technique by an experienced radiological technologist. Volume software on the workstation computer were used to calculate the total splenic volume by adding the slice thickness to determine the volume for each section of the spleen[8] (Figure 1).
We investigated an increase in platelet count after the trial drug administration. We evaluated the response to lusutrombopag compared to baseline clinical characteristics in patients with chronic liver disease. According to a phase 3 trial, a treatment response was defined as a platelet count ≥ 5 × 104/μL with an increased platelet count ≥ 2 × 104/μL from baseline after drug administration[6-9].
We also evaluated adverse events at each visit. The data on all adverse events was collected from the start of the administration to up to 90 d after the first dosing. The severity of any adverse events was graded using CTCAE (Common Terminology Criteria for Adverse Events), version 4.0.
Our estimated response rate in thrombocytopenic patients was 83% based on previous studies[9]. With an alpha of 0.05 and power of 80%, 50 patients were required for this trial. Data were analyzed using the SPSS v.24.0 software package (IBM Corp., Armonk, NY, United States). All data are expressed as mean and standard deviation (SD). Continuous variables in the responder and non-responder groups were compared using the unpaired t-test. The paired t-test was used to compare paired data. Correlations between the changes in platelet count and clinical characteristics were determined using Pearson’s r coefficient. Receiver operating characteristic (ROC) curves were constructed to establish sensitivity-specificity relationships. Univariate and multivariate analyses with logistic regression models were used to calculate the odds ratios (OR) and 95% confidence intervals (CI) to assess the correlation between response and clinical characteristics. We categorized patients into two groups by each variable using a median value. Variables that achieved statistical significance (P < 0.05) or marginal significance (P < 0.15) on univariate analysis were entered into a multivariate Cox proportion hazard model to identify significant independent factors. All differences with a P-value < 0.05 were considered statistically significant. The statistical methods of this study had been reviewed by the Institute of Biomedical Research, Sapporo Higashi Tokushukai Hospital, Hokkaido, Japan.
A total of 59 patients were treated with lusutrombopag from February 2015 through March 2018 in the four study centers. Of these, 6 patients did not meet the above inclusion criteria (3 patients received an insufficient dose of the trial drug during the period of the study, 2 patients had a change in the doses of their conventional drugs, and 1 patient received a platelet transfusion before day 12). When we assessed the remaining 53 patients for eligibility, 3 patients were excluded because of missing data regarding their splenic volume and/or their blood samples. The data of the remaining 50 patients are summarized in Table 1. The mean age was 65.9 ± 10.2 years (range, 48-83 years), 34 (68.0%) patients were male, and 26 (52.0%) patients had liver cancer. The mean body weight was 64.7 ± 12.1 kg (range, 46.5-93.0 kg), and the mean height was 1.63 ± 0.10 m (range, 1.20-1.81 m). Forty-eight (96.0%) patients had liver cirrhosis caused by hepatic viruses (n = 21) and non-hepatic viruses (n = 27). The mean platelet count was 4.48 ± 1.07 × 104/μL (range, 3-7 × 104/μL), albumin was 3.46 ± 0.54 g/dL (range, 2.2-4.5 g/dL), serum creatinine was 0.82 ± 0.21 mg/dL (range, 0.49-1.61 dL), AST was 47.4 ± 25.6 IU/L (range, 15-133 IU/L), total bilirubin was 1.41 ± 0.83 g/dL (range, 0.6-4.9 g/dL), and AFP was 33.4 ± 82.7 ng/mL (range, 2-479 ng/mL). According to non-invasive fibrosis markers and an algorithm-based score model, the mean MELD score was 7.26 ± 3.05 (range, 2-16), APRI was 31.3 ± 17.3 (range, 11-80), and the FIB-4 index was 12.4 ± 5.49 (range, 4.51-25.7). The numbers and proportions of invasive procedures in radiofrequency ablation, interventional radiology, and endoscopic therapy were 14 (28%), 10 (20%), and 22 (44%), respectively.
| All | Responder | Non-responder | P value | |
| n | 50 | 40 | 10 | |
| Age (yr) | 65.9 ± 10.2 | 66.2 ± 10.2 | 64.7 ± 10.4 | 0.638 |
| Gender: Male, n (%) | 34 (68.0) | 25 (63.9) | 9 (90.0) | 0.138 |
| Height (m) | 1.63 ± 0.10 | 1.62 ± 0.11 | 1.64 ± 0.08 | 0.695 |
| Weight (kg) | 64.7 ± 12.1 | 63.0 ± 11.3 | 71.6 ± 13.4 | 0.044 |
| Body mass index (kg/m2) | 24.5 ± 3.92 | 24.3 ± 4.10 | 25.2 ± 3.25 | 0.548 |
| Etiology (n) | 0.717 | |||
| Cirrhotic/Non-cirrhotic | 48/2 | 39/1 | 9/1 | |
| HCV/HBV/Alcohol/Non-viral | 15/6/15/12 | 13/4/11/11 | 2/2/4/1 | 0.717 |
| IPH/EHO | 1/1 | 1/0 | 0/1 | |
| Child-Pugh | 6.94 ± 1.57 | 6.98 ± 1.62 | 6.80 ± 1.40 | 0.756 |
| MELD | 7.26 ± 3.05 | 7.02 ± 2.98 | 8.20 ± 3.33 | 0.281 |
| Liver cancer, n (%) | 26 (52.0) | 22 (55.2) | 4 (40.0) | 0.490 |
| Splenic volume (mL) | 741 ± 325 | 653 ± 267 | 1092 ± 314 | <0.001 |
| Spleen vein diameter (mm) | 10.8 ± 3.60 | 10.3 ± 3.47 | 12.8 ± 3.69 | 0.059 |
| Portal diameter (mm) | 12.6 ± 3.45 | 12.3 ± 3.46 | 14.1 ± 3.12 | 0.143 |
| Procedure: RFA/IVR/Endoscopy/Others (n) | 14/10/22/4 | 12/8/18/2 | 2/2/4/2 | 0.273 |
| APRI | 31.3 ± 17.3 | 31.7 ± 15.6 | 29.0 ± 23.8 | 0.661 |
| FIB-4 index | 12.4 ± 5.49 | 12.8 ± 5.65 | 10.9 ± 4.71 | 0.315 |
| White blood cells (/μL) | 3149 ± 1039 | 3330 ± 1051 | 2480 ± 681 | 0.020 |
| Hemoglobin (g/dL) | 12.0 ± 2.14 | 12.4 ± 1.84 | 10.7 ± 2.82 | 0.026 |
| Platelets (× 104/μL) | 4.48 ± 1.07 | 4.50 ± 1.12 | 4.35 ± 0.82 | 0.778 |
| Prothrombin time (%) | 66.7 ± 12.5 | 67.1 ± 11.6 | 64.6 ± 16.3 | 0.578 |
| Total protein (g/dL) | 7.00 ± 0.70 | 7.02 ± 0.69 | 6.93 ± 0.75 | 0.726 |
| Serum albumin (g/dL) | 3.46 ± 0.54 | 3.39 ± 0.53 | 3.72 ± 0.56 | 0.089 |
| BUN (mg/dL) | 15.8 ± 5.76 | 15.5 ± 5.53 | 17.3 ± 6.69 | 0.277 |
| Serum creatinine (mg/dL) | 0.82 ± 0.21 | 0.80 ± 0.20 | 0.86 ± 0.26 | 0.443 |
| AST (IU/L) | 47.4 ± 25.6 | 48.4 ± 23.2 | 43.6 ± 35.2 | 0.602 |
| ALT (IU/L) | 36.7 ± 22.9 | 37.5 ± 21.3 | 34.2 ± 30.2 | 0.700 |
| Total bilirubin (g/dL) | 1.41 ± 0.83 | 1.40 ± 0.85 | 1.45 ± 0.76 | 0.873 |
| HbA1c (%) | 5.98 ± 1.15 | 5.97 ± 1.15 | 6.03 ± 1.23 | 0.901 |
| Ammonia (μg/dL) | 66.7 ± 42.0 | 64.3 ± 37.3 | 75.9 ± 58.5 | 0.442 |
| AFP (ng/mL) | 33.4 ± 82.7 | 29.0 ± 80.8 | 54.2 ± 95.2 | 0.441 |
| PIVKA- II (mAU/mL) | 48.1 ± 68.4 | 51.4 ± 75.7 | 32.8 ± 26.0 | 0.493 |
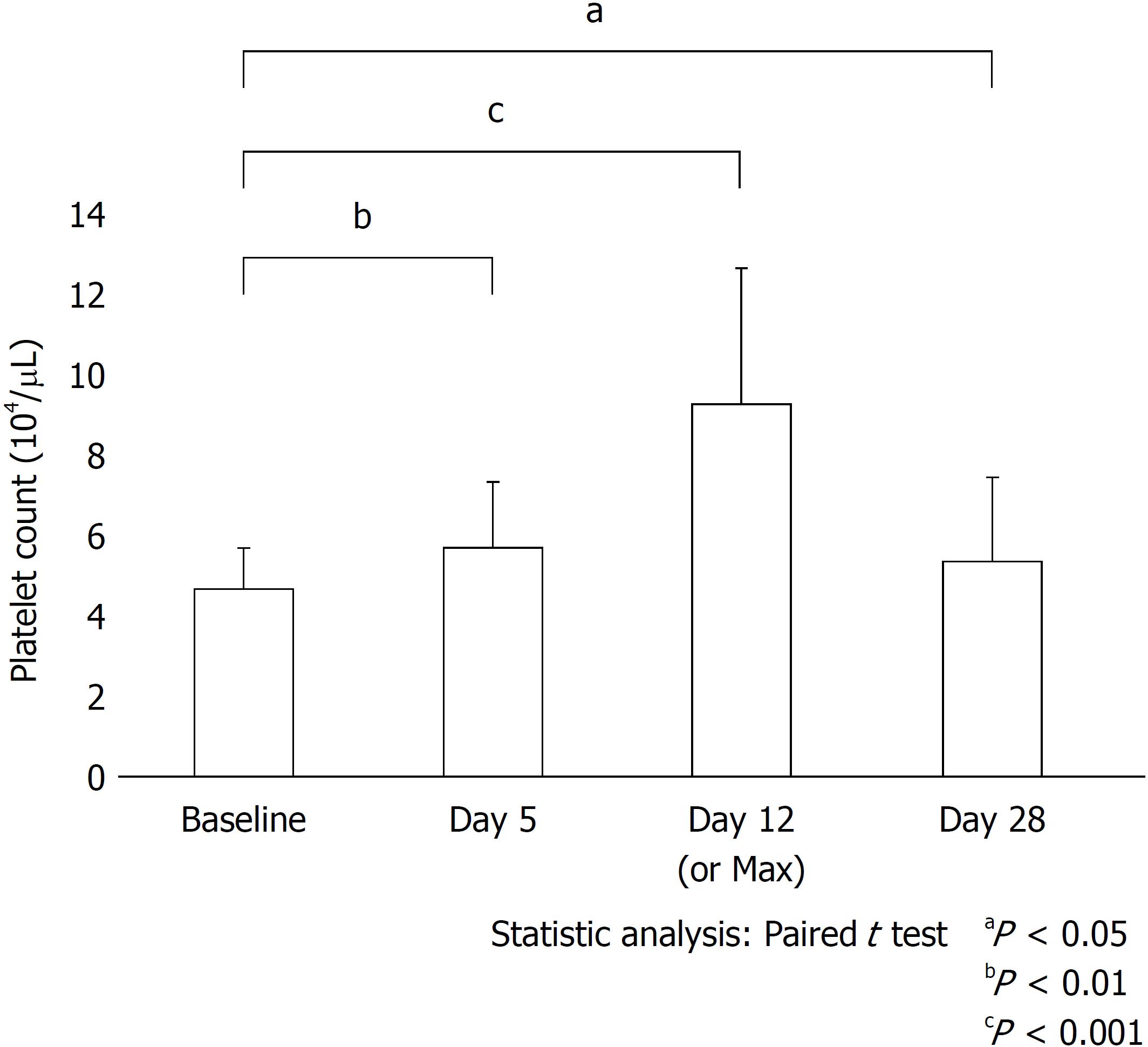
The numbers and proportions of patients in the responder and non-responder groups were 40 (80%) and 10 (20%), respectively. After treatment with oral lusutrombopag for all enrolled patients, a significant increase was observed in the mean platelet count from 4.49 ± 1.03 × 104/μL pre-treatment to 9.13 ± 3.50 × 104/μL post-treatment. Changes in platelet counts on days 5, 12, and 28 were 1.16, 4.64, and 1.02 (P = 0.001, P < 0.001, and P = 0.003, respectively) (Figure 2). All patients had sufficient platelet counts (platelet count ≥ 5 × 104/μL) except 2 patients (platelet count < 5 × 104/μL) who received blood transfusions prior to an elective invasive procedure. After the invasive procedures, there were no instances of spontaneous bleeding.
In the non-responder group (n = 10), 2 patients received platelet transfusion prior to an elective invasive procedure. One patient was a woman with HCV-related liver cirrhosis, and the splenic volume was 890 mL. Her pre-treatment, post-treatment, and post-platelet transfusion platelet counts were 4.1, 5.5, and 6.5 × 104/μL, respectively. Another patient was a man with HBV-related liver cirrhosis, and the splenic volume was 1720 mL. His platelet counts pre-treatment, post-treatment, and post-platelet transfusions were 4.0, 4.8, and 5.2 × 104/μL, respectively.
Splenic volume was significantly lower in responders compared with non-responders (653.0 ± 267 mL vs 1,092 ± 314 mL, P < 0.0001) (Table 1). Body weight was lower in the responder group compared to the non-responder group (63.0 ± 11.3 kg vs 71.6 ± 13.4 kg, P = 0.044). White blood cell and hemoglobin counts were higher in the responder group than in the non-responder group (3330 ± 1051/μL vs 2480 ± 681/μL, P = 0.020, and 12.4 ± 1.84 g/dL vs 10.7 ± 2.82 g/dL, P = 0.026, respectively). Furthermore, the responder group had slightly more males than females (P = 0.138). No significant difference was found between the two groups with respect to age, body mass index, etiology, the presence of liver cancer, the Child-Pugh score, the MELD score, and the APRI and FIB-4 indices. No significant differences between the groups were found with respect to platelets, serum albumin, serum creatinine, AST, ALT, and AFP.
We evaluated the relationship between response to lusutrombopag and baseline clinical characteristics using a logistic regression model (Table 2). Splenic volume was identified as significant predictor in the univariate analysis. Multivariate analysis confirmed that splenic volume was an independent factor that predicted platelet response (P = 0.025, OR = 11.2; 95%CI: 1.354-103.0).
| Univariate analysis | Multiple analysis | ||||
| Variable | OR (95%CI) | P value | OR (95%CI) | P value | |
| Splenic size (mL) | 1: < 723 | 13.5 (1.556-117.1) | 0.018 | 11.2 (1.354-103.0) | 0.025 |
| 2: ≥ 723 | |||||
| Gender | 1: Male | ||||
| 2: Female | 5.41 (0.621-40.95) | 0.368 | |||
| Age (yr) | 1: <69 | 1.11 (0.270-4.550) | 0.168 | ||
| 2: ≥ 69 | |||||
| Platelets (×104/μL) | 1: < 4.5 | ||||
| 2: ≥ 4.5 | 2.02 (0.442-9.412) | 0.212 | |||
| Hemoglobin (g/dL) | 1: < 12.4 | ||||
| 2: ≥ 12.4 | 2.581 (0.582-11.42) | 0.212 | |||
| BUN (mg/dL) | 1: < 15.2 | 1.66 (0.405-9.412) | 0.190 | ||
| 2: ≥ 15.2 | |||||
| Child-Pugh | 1: < 7 | 1.56 (0.582-11.42) | 0.212 | ||
| 2: ≥ 7 | |||||
| White blood cells (/μl) | 1: < 3000 | ||||
| 2: ≥ 3000 | 4.70 (0.878-25.22) | 0.072 | |||
| Weight (kg) | 1: < 62 | 2.85 (0.643-12.64) | 0.168 | ||
| 2: ≥ 62 | |||||
| Ammonia (μg/dL) | 1: < 56.5 | 1.43 (0.347-5.851) | 0.623 | ||
| 2: ≥ 56.5 | |||||
Splenic volume was associated with a change in platelet count (r = -0.524, P = 0.001) (Figure 3). Splenic volume increase was negatively related to changes in the platelet count.
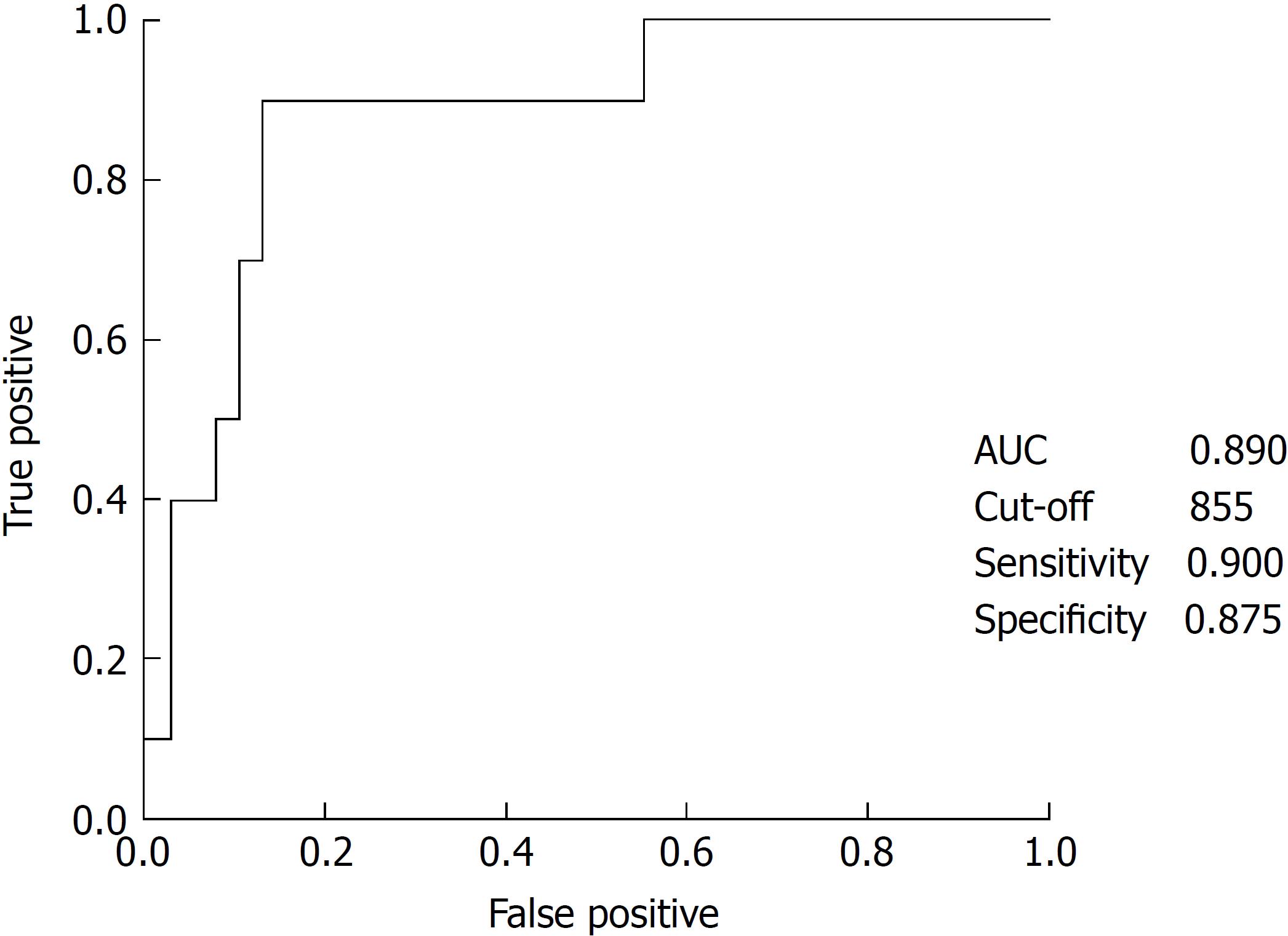
The respective cut-off point for splenic volume was estimated using ROC curves for responders after administration of the trial drug (Figure 4). A splenic volume cut-off of 855 mL predicted responders with a sensitivity of 90% and specificity of 87.5%.
The numbers and proportions of patients with a splenic volume < 855 mL and splenic volume ≥ 855 mL were 36 (72.0%) and 14 (28.0%), respectively.
In the splenic volume < 855 mL group, the mean increase in platelet count from baseline administration of lusutrombopag was 5.68 ± 3.04 × 103/μL (P < 0.0001), with 97.2% (35/36) of patients as responders. In the splenic volume ≥ 855 mL group, the mean increase in platelet count from baseline was 2.03 ± 1.03 × 103/μL (P = 0.008), with 35.7% (5/14) of patients as responders. Unpaired t-test confirmed that the change in platelet count was significantly different between the groups (P < 0.001).
The incidence of adverse events and adverse drug reactions was summarized in Table 3. Fever, AST increase, gastrointestinal disorders, chest pain, hypertension, and thromboembolic events were reported in 9 (25.7%) patients during treatment.
| Fever | Any grade | 2 (4.0) |
| ≥ Grade 3 | 0 | |
| Aspartate aminotransferase increased | Any grade | 2 (4.0) |
| ≥ Grade 3 | 0 | |
| Gastrointestinal disorders | Any grade | 1 (2.0) |
| ≥ Grade 3 | 0 | |
| Chest pain | Any grade | 1 (2.0) |
| ≥ Grade 3 | 0 | |
| Hypertension | Any grade | 1 (2.0) |
| ≥ Grade 3 | 0 | |
| Portal thrombosis | Any grade | 0 |
| ≥ Grade 3 | 2 (4.0) |
Although there were no treatment-related deaths, some serious adverse events (≥ grade 3) were reported. There were two thromboembolic events that affected portal hypertension and liver function. Portal embolic events required admission for treatment of thromboembolism.
According to any grade AE, no significant differences were found between patients with and without adverse events with respect to clinical characteristics. According to ≥ grade 3 AE, 2 patients with portal vein thrombosis were diagnosed as having Child-Pugh class B and had a history of previous variceal bleeding with subsequent endoscopic treatment.
The results of this study revealed that the response to lusutrombopag was related to splenic volume in chronic liver disease. Lusutrombopag, an oral, small molecule TPO-RA, binds to the TPO receptor and acts on the transmembrane domain of human TPO receptors expressed in megakaryocytes to increase platelet production[6]. One of the reasons for thrombocytopenia is decreased levels or activity of the platelet growth factor thrombopoietin, which can lead to insufficient activation of the TPO receptor in severe chronic liver disease[2]. Therefore, TPO-RAs are particularly useful in patients with advanced chronic hepatitis or liver cirrhosis.
On the other hand, in patients with splenomegaly, the main causes of thrombocytopenia are increased splenic sequestration and destruction of platelets, whereas reduced platelet count seems to be greatly related to increased splenic size[10-14]. The present study revealed that increased splenic volume is related to negative changes in platelet count. In particular, splenic volume > 855 mL was associated with an insufficient increase in platelet count after the administration of appropriate doses of lusutrombopag for 7 d. We hypothesized that severe splenomegaly could reduce the efficacy seen in platelet counts due to increased splenic sequestration and destruction in spite of the TPO-RA causing sufficient platelet production. Based on the results of this phase 2b study, a dose-related increase in the maximum platelet count and duration of the maintenance of the increase in platelet count was reported[15].
When the dose of this treatment drug can be increased in case of severe splenomegaly, a treatment response may be achieved.
We recommend combination therapy with partial splenic artery embolization (PSE) and TPO-RA to increase platelet production in patients with a splenic volume > 855 mL, who remain at risk of bleeding following invasive surgery. PSE is a useful method used for patients with splenomegaly and with accompanying portal hypertension. However, a large infarcted splenic volume is a risk factor for complications, including intra-abdominal abscess, portal vein thrombosis, septic shock, and post-embolization syndrome[16,17]. Previous studies reported infarcted splenic volumes between 388 and 540 g[18-20], and the therapeutic effect of PSE on severe splenomegaly is sometimes insufficient. Although it is enough for patients with mild splenomegaly to undergo PSE alone, thrombocytopenic patients with severe splenomegaly should be administered with lusutrombopag after an appropriate infarcted splenic volume is achieved.
We should consider that splenic volume is influenced by sex, height, and weight. In this cohort, 90% of the non-responders were composed of men, and body weight was significantly higher in the non-responder group compared to the responder group (P = 0.044). Previous reports have shown that splenic length and volume have independent correlations with sex, height, and weight[19]. Therefore, the influential parameters for splenic volume need to be considered in patient cohorts. Furthermore, patients enrolled in this study had a relatively large spleen size. If they had presented with mild or moderate spleen size, the results would likely have been different.
TPO-RAs are generally well tolerated, with common adverse events including AST increase, gastrointestinal disorders, and hypertension[21]. However, thromboembolic events have been reported in patients treated with TPO-RAs, the majority of whom had pre-existing cardiovascular or thromboembolic risk factors[21,22]. In particular, the administration of TPO-RAs in patients with advanced cirrhosis requires close monitoring. Severe chronic liver disease is considered as a hypercoagulable state, with increased bleeding tendency due to severe homeostatic disruption[23]. The phase-3 randomized controlled trials ENABLE-1 and ENABLE-2 studied the efficacy of TPO-RAs in patients with chronic hepatitis C and thrombocytopenia and revealed that thromboembolic complications were more frequently observed in the group treated with TPO-RAs compared to the control group[24,25]. In the present study, thromboembolic events were reported in two (4.0%) patients during the observation period, requiring hospital admission. Two patients with portal vein thrombosis were diagnosed as having Child-Pugh class B and had a history of previous variceal bleeding with subsequent endoscopic treatment. This suggests that patients should be carefully monitored during treatment so that potentially life-threatening thromboembolic events can be prevented or treated.
TPO-RAs can increase platelet count and decrease bleeding events. Our final objective was to minimize the administration of transfusion for reducing the incidence of bleeding events in thrombocytopenic patients. However, strategies to reduce or avoid platelet transfusions were not achieved by the present study where only the relationship between the response to lusutrombopag and splenic volume was showed. For increasing platelet counts, we can select the optimal therapy from blood transfusion, PSE, splenectomy, and TPO-RA. The most suitable method for patients is selected by careful discussion and consideration of the benefits and disadvantages of each method. Comparative clinical trial data are needed in making correct treatment choices while maximizing efficacy and safety.
This study had some limitations. First, the study employed a retrospective design. Second, there are multiple factors which can cause thrombocytopenia in patients with chronic liver disease. These factors include anti-platelet antibodies, levels or activity of thrombopoietin, and bone marrow suppression of thrombopoiesis due to myelodysplastic syndromes and/or direct myelosuppressive effects of HCV infection. In this study, the evaluation of influences of these factors was lacking. Therefore, further studies are warranted. Third, the number of patients was relatively small comparing the responder and non-responder groups. This could have led to a selection bias.
This study demonstrated that splenic volume influences the response to lusutrombopag in chronic liver disease. Larger spleen size appears to reduce the effect of lusutrombopag in terms of platelet count. This is the first report to assess the factors that affect the response to lusutrombopag in patients with chronic liver disease. Splenic volume should be taken into consideration when administering lusutrombopag to ensure that patients receive optimal treatment.
Lusutrombopag is an oral, small-molecule thrombopoietin (TPO) receptor agonist (TPO-RA) used for the treatment of thrombocytopenic patients with chronic liver diseases. TPO in the peripheral blood in advanced-stage liver disease was been reduced severely. Therefore, patients with inadequate TPO production due to chronic liver disease are the principal targets of lusutrombopag.
The response to lusutrombopag is unpredictable, assuming that the drug exerts more dramatic effects in thrombocytopenia associated with severe liver disease prior to invasive surgery.
The study aimed to assess the correlation between the clinical characteristics of patients with chronic liver disease and the efficacy of lusutrombopag treatment.
This multicenter retrospective study was conducted at four locations in Japan. This study enrolled thrombocytopenic patients who received oral lusutrombopag. We evaluated the response to lusutrombopag compared to baseline clinical characteristics in patients with chronic liver disease.
Splenic volume and body weight were lower in the responder group than in the non-responder group. Using a logistic regression model to assess the relationship between response to lusutrombopag and clinical characteristics, multivariate analysis confirmed that splenic volume was an independent factor that predicted the response of platelet counts (P = 0.025, odds ratio: 11.2; 95%CI: 1.354-103.0). Splenic volume was negatively correlated to changes in platelet count (r = -0.524, P = 0.001).
Splenic volume influences change in platelet counts after administration of lusutrombopag in patients with chronic liver disease. Larger spleen size appears to reduce the effect of lusutrombopag in terms of platelet count. Splenic volume should be taken into consideration when administering lusutrombopag to ensure that patients receive the optimal treatment. This is the first report to assess the factors that affect the response to lusutrombopag in patients with chronic liver disease. There are multiple factors which can cause thrombocytopenia in patients with chronic liver disease. These factors include anti-platelet antibodies, levels or activity of thrombopoietin, and bone marrow suppression of thrombopoiesis due to myelodysplastic syndromes and/or direct myelosuppressive effects of HCV infection. In this study, the evaluation of influences of these factors was lacking. Therefore, further studies are warranted.
Splenic volume should be taken into consideration when administering lusutrombopag to ensure that patients receive optimal treatment. It is not clear whether or not the combination therapy improves the long-term prognosis of these patients. Therefore, future long-term observational studies are warranted. Further studies are desired to assess multiple factors include anti-platelet antibodies, levels or activity of thrombopoietin, and bone marrow suppression of thrombopoiesis due to myelodysplastic syndromes and/or direct myelosuppressive effects of HCV infection.
We thank Ayumu Sugitani at the Institute of Biomedical Research, Sapporo Higashi Tokushukai Hospital, Hokkaido, Japan for assistance with the statistical analyses, and Robert E. Brandt, Founder, CEO, and CME, of MedEd Japan, for editing and formatting the manuscript.
| 1. | Afdhal N, McHutchison J, Brown R, Jacobson I, Manns M, Poordad F, Weksler B, Esteban R. Thrombocytopenia associated with chronic liver disease. J Hepatol. 2008;48:1000-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 408] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 2. | Poordad F. Review article: thrombocytopenia in chronic liver disease. Aliment Pharmacol Ther. 2007;26 Suppl 1:5-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Stiegler G, Stohlawetz P, Peck-Radosavljevic M, Jilma B, Pidlich J, Wichlas M, Höcker P, Panzer S. Direct evidence for an increase in thrombopoiesis after liver transplantation. Eur J Clin Invest. 1998;28:755-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Gangireddy VG, Kanneganti PC, Sridhar S, Talla S, Coleman T. Management of thrombocytopenia in advanced liver disease. Can J Gastroenterol Hepatol. 2014;28:558-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Peck-Radosavljevic M. Thrombocytopenia in liver disease. Can J Gastroenterol. 2000;14 Suppl D:60D-66D. [PubMed] |
| 6. | Kim ES. Lusutrombopag: First Global Approval. Drugs. 2016;76:155-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 7. | Katsube T, Ishibashi T, Kano T, Wajima T. Population Pharmacokinetic and Pharmacodynamic Modeling of Lusutrombopag, a Newly Developed Oral Thrombopoietin Receptor Agonist, in Healthy Subjects. Clin Pharmacokinet. 2016;55:1423-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Hidaka H, Nakazawa T, Wang G, Kokubu S, Minamino T, Takada J, Tanaka Y, Okuwaki Y, Watanabe M, Shibuya A. Reliability and validity of splenic volume measurement by 3-D ultrasound. Hepatol Res. 2010;40:979-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Hidaka H. Lusutrombopag reduces the need for platelet transfusion in thrombocytopenic patients undergoing invasive procedures. Clin Gastroenterol Hepatol. 2018;In press. [RCA] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 10. | Eichner ER. Splenic function: normal, too much and too little. Am J Med. 1979;66:311-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 111] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Dahal S, Upadhyay S, Banjade R, Dhakal P, Khanal N, Bhatt VR. Thrombocytopenia in Patients with Chronic Hepatitis C Virus Infection. Mediterr J Hematol Infect Dis. 2017;9:e2017019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Mitchell O, Feldman DM, Diakow M, Sigal SH. The pathophysiology of thrombocytopenia in chronic liver disease. Hepat Med. 2016;8:39-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (1)] |
| 13. | Djordjević J, Svorcan P, Vrinić D, Dapcević B. [Splenomegaly and thrombocytopenia in patients with liver cirrhosis]. Vojnosanit Pregl. 2010;67:166-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Patel AG, Parker JE, Wallwork B, Kau KB, Donaldson N, Rhodes MR, O’Rourke N, Nathanson L, Fielding G. Massive splenomegaly is associated with significant morbidity after laparoscopic splenectomy. Ann Surg. 2003;238:235-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 99] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Tateishi R, Seike M, Kudo M, Tamai H, Kawazoe S, Katsube T, Ochiai T, Fukuhara T, Kano T, Tanaka K. A randomized controlled trial of lusutrombopag in Japanese patients with chronic liver disease undergoing radiofrequency ablation. J Gastroenterol. 2018; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 16. | Hayashi H, Beppu T, Shirabe K, Maehara Y, Baba H. Management of thrombocytopenia due to liver cirrhosis: a review. World J Gastroenterol. 2014;20:2595-2605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 82] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
| 17. | Witters P, Freson K, Verslype C, Peerlinck K, Hoylaerts M, Nevens F, Van Geet C, Cassiman D. Review article: blood platelet number and function in chronic liver disease and cirrhosis. Aliment Pharmacol Ther. 2008;27:1017-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 125] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 18. | Hayashi H, Beppu T, Okabe K, Masuda T, Okabe H, Baba H. Risk factors for complications after partial splenic embolization for liver cirrhosis. Br J Surg. 2008;95:744-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Chow KU, Luxembourg B, Seifried E, Bonig H. Spleen Size Is Significantly Influenced by Body Height and Sex: Establishment of Normal Values for Spleen Size at US with a Cohort of 1200 Healthy Individuals. Radiology. 2016;279:306-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 132] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 20. | Hayashi H, Beppu T, Masuda T, Mizumoto T, Takahashi M, Ishiko T, Takamori H, Kanemitsu K, Hirota M, Baba H. Predictive factors for platelet increase after partial splenic embolization in liver cirrhosis patients. J Gastroenterol Hepatol. 2007;22:1638-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Afdhal NH, Dusheiko GM, Giannini EG, Chen PJ, Han KH, Mohsin A, Rodriguez-Torres M, Rugina S, Bakulin I, Lawitz E. Eltrombopag increases platelet numbers in thrombocytopenic patients with HCV infection and cirrhosis, allowing for effective antiviral therapy. Gastroenterology. 2014;146:442-452.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 22. | Grotzinger KM, Younossi ZM, Giannini EG, Chen PJ, Rendas-Baum R, Theodore D. Health-related quality of life in thrombocytopenic patients with chronic hepatitis C with or without cirrhosis in the ENABLE-1 and ENABLE-2 studies. Health Qual Life Outcomes. 2016;14:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Mihăilă RG, Cipăian RC. Eltrombopag in chronic hepatitis C. World J Gastroenterol. 2014;20:12517-12521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Wong RSM, Saleh MN, Khelif A, Salama A, Portella MSO, Burgess P, Bussel JB. Safety and efficacy of long-term treatment of chronic/persistent ITP with eltrombopag: final results of the EXTEND study. Blood. 2017;130:2527-2536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 242] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 25. | Elgebaly AS, Ashal GE, Elfil M, Menshawy A. Tolerability and Efficacy of Eltrombopag in Chronic Immune Thrombocytopenia: Meta-Analysis of Randomized Controlled Trials. Clin Appl Thromb Hemost. 2017;23:928-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Manenti A, Sterpetti AV, Zapater P S- Editor: Wang XJ L- Editor: Wang TQ E- Editor: Yin SY