Published online Jan 21, 2018. doi: 10.3748/wjg.v24.i3.408
Peer-review started: November 11, 2017
First decision: December 13, 2017
Revised: December 20, 2017
Accepted: December 26, 2017
Article in press: December 26, 2017
Published online: January 21, 2018
Processing time: 70 Days and 21 Hours
To evaluate the use of fully covered self-expandable metal stents (FCSEMSs) for pancreatic duct strictures in children with chronic pancreatitis.
Eight patients with refractory benign dominant stricture of the main pancreatic duct (MPD) were enrolled through chart reviews between December 2014 and June 2017 in a single center. Endoscopic retrograde cholangiopancreatography (ERCP) with placement of a 6-mm FCSEMS with dual flaps was performed. Endoscopic removal of FCSEMSs was performed with a snare or rat-tooth forceps. All procedures were performed by a pediatric gastroenterologist. For the assessment of outcomes, technical and clinical success, adverse events, and stent patency were evaluated retrospectively.
The placement and removal of the FCSEMSs were successful in all 8 patients. Five patients were boys and 3 were girls. The median age at initial FCSEMS placement was 12 years (range, 5-18 years). The diameters of all the inserted stents were 6 mm, and the lengths were 4-7 cm. The median indwelling time was 6 mo (range, 3-10 mo). No pancreatic sepsis, pancreatitis, cholestasis, or mortality occurred. There was no proximal and distal migration. All subjects showed a patent stent. On follow-up ERCP, the mean diameter of the stricture improved from 1.1 mm to 2.8 mm (P < 0.05), whereas that of upstream dilation improved from 8.4 mm to 6.3 mm (P < 0.05).
This initial experience showed that temporary FCSEMS placement is feasible and safe for the management of refractory benign MPD stricture in children.
Core tip: This study reports the initial experience with the use of fully covered self-expandable metal stents (FCSEMSs) for recurrent benign pancreatic duct strictures in children. We indwelled 6-mm FCSEMSs with dual flaps in the pancreatic duct of pediatric patients for 6 mo. The placement and removal of the FCSEMSs were technically and clinically successful. There were no adverse events or stent obstruction. The findings support the applicability of FCSEMSs to the pediatric population.
- Citation: Jeong IS, Lee SH, Oh SH, Park DH, Kim KM. Metal stents placement for refractory pancreatic duct stricture in children. World J Gastroenterol 2018; 24(3): 408-414
- URL: https://www.wjgnet.com/1007-9327/full/v24/i3/408.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i3.408
Plastic stents (PSs) or metal stents have been widely used for the management of biliary and pancreatic duct strictures in endoscopic interventions[1]. The fully covered self-expandable metal stent (FCSEMS) was designed to compensate for the defects of PSs and uncovered self-expandable metal stents (SEMSs). The SEMS has the advantages of longer patency and resolution of main pancreatic duct (MPD) strictures because its lumen diameter is larger than that of the PS[2,3]. Meanwhile, the FCSEMS has been shown to improve tissue embedding by wrapping around the frame of the uncovered SEMSs[4].
As SEMSs were initially used for malignant biliary obstruction[5], FCSEMSs have been widely used for the management of biliary and pancreatic duct strictures in adults[6]. FCSEMSs have proven to be a particularly useful intervention for malignant biliary obstruction[7,8], and their use in the management of benign biliary strictures has been expanding steadily[9]. In recent studies, FCSEMSs were increasingly used for the management of MPD strictures refractory to PS placement in adults[10].
In general, studies on the application of stents in pediatric patients are lacking. However, most pediatric studies of endoscopic retrograde cholangiopancreatography (ERCP), including our previous study, investigated the use of PSs; therefore, stenting is an important indication in pediatric ERCP[11-13]. One pediatric study showed that PS placement was effective for reducing the recurrence of pancreatitis[14].
For these reasons, FCSEMSs may be useful even in pediatric populations. In this study, we evaluated the feasibility, safety, and therapeutic effect of FCSEMSs for the management of benign MPD strictures in pediatric patients with chronic pancreatitis (CP).
This was a retrospective study of data collected through medical chart reviews between December 2014 and June 2017 at Asan Medical Center Children’s Hospital in Seoul, South Korea. Eight patients with CP and benign dominant MPD stricture refractory to PS placement were enrolled (Table 1). Patients with MPD dilatation due to a malignant cause or trauma were excluded. Five patients were boys and 3 were girls. Their median age was 12 years (range, 5-18 years). The patients in this study had pancreatic divisum and/or gene mutations. The genetic mutations were PRSS1 p.Gly208Ala, PRSS1 p.Arg122His, SPINK1 p.Asn34Ser, SPINK1 p.ThrT53Ilefs*41, SPINK1 IVS3(+2)T>C, and CFTR p.Gln1352His. The strictures were located at the pancreatic head in 5 patients, at the pancreatic neck in 2 patients, and at the pancreatic body in 1 patient. The subjects had a history of previous PS trials after endoscopic sphincterotomy. The median number of days and total duration of PS placement were 2 d (range, 1-7 d) and 57 d (range, 1-431 d), respectively. The stricture was verified using imaging methods [ultrasonography, computed tomography, endoscopic retrograde pancreatography (ERP), or magnetic resonance cholangiography]. A dominant stricture was defined when the contrast medium on the ERP film was not washed out and the upstream MPD dilation was ≥ 6 mm in diameter[15].
| Case no. | Sex | Anatomical abnormality | Genetic mutation | No. of previous plastic1 stent placements | Placement time for each plastic stent (d), median (range) | Age at first insertion of a FCSEMS (yr) | Location of the stricture | Diameter and length of the stent (mm/cm) |
| 1 | F | Pancreas divisum | PRSS1 | 2 | 25.5 (19-32) | 17 | Head | 6/4 |
| 2 | M | - | SPINK1 | 7 | 42.0 (4-93) | 12 | Head | 6/5 |
| 3 | M | Incomplete pancreas divisum | - | 4 | 126.5 (63-431) | 10 | Head | 6/6 |
| 4 | M | Pancreas divisum | CFTR | 3 | 57.0 (1-109) | 12 | Body | 6/7 |
| 5 | F | - | PRSS1 | 1 | 46.0 | 18 | Neck | 6/7 |
| 6 | M | - | PRSS1 | 2 | 58 (24-92) | 5 | Head | 6/4 |
| 7 | F | - | SPINK1 | 1 | 87 | 9 | Head | 6/4 |
| 8 | M | - | SPINK1 | 1 | 29 | 16 | Neck | 6/7 |
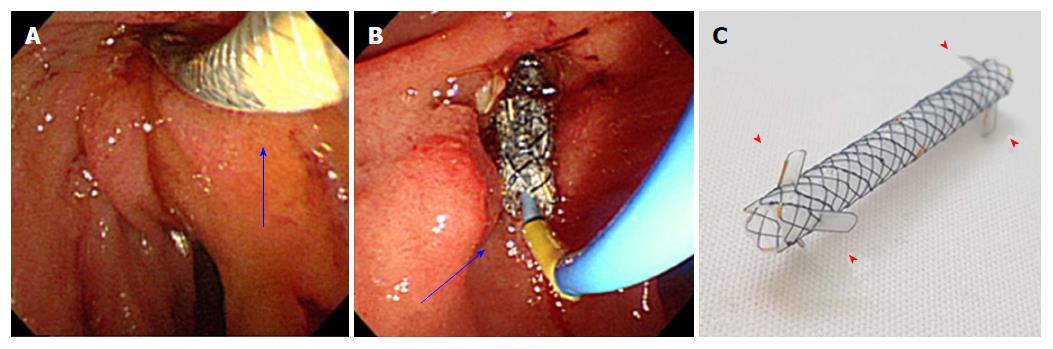
The first session of placement and removal of FCSEMS was performed by an adult and pediatric gastroenterologist. Thereafter, all procedures were performed by a pediatric gastroenterologist. ERP was performed using either an adult duodenoscope (JF; Olympus Optical, Tokyo, Japan) or a therapeutic duodenoscope (TJF, Olympus Optical, Tokyo, Japan). Commercially available 6-mm FCSEMSs (Hanarostent Biliary dual flap; M.I. Tech, Seoul, South Korea) that consisted of nitinol material with a silicone covering membrane were used (Figure 1). After the endoscopic sphincterotomy, which had been described elsewhere, dilatation of the stricture was performed with a Soehendra stent retriever (Wilson Cook Medical, Winston-Salem, NC, United States) and/or a Hurricane dilator balloon (Boston Scientific, Natick, MA, United States) prior to stent placement. The insertion length of the stent was determined such that at least 1 cm of ampulla and normal area at the end of the stenosis were each covered. Repositioning was carried out until the stent was fully inflated and placed in the targeted location. On the basis of previous studies in adults[16], stent removal was planned with rat-tooth forceps at 6 mo after placement, unless adverse events or episodes of pancreatitis had occurred. Informed consent was obtained from the parents of the patients, after the risks and benefits of the procedure and alternative treatments were explained. The study protocol was approved by the institutional review board of Asan Medical Center (2017-0210).
Feasibility was evaluated in terms of technical success, migration, and patency. Technical success was determined in accordance with the success of stent placement and removal. Successful stenting was attained when the stent passed through the stricture and the mesh spread easily, followed by a flow of pancreatic juice or washing fluid into the lumen of the stent. Successful removal was attained when the entire stent was removed without ductal leakage during contrast medium injection. Stent patency was assessed via visual inspection when the FCSEMS was removed. Efficacy was evaluated according to the improvements of the stricture and reduction of pancreatic pain and pancreatitis. Stricture improvement was evaluated by measuring the diameter of the stricture and the upstream dilation of the MPD as shown on pancreatography after the removal of the first metal stent. Safety was evaluated based on the occurrence of adverse events such as pancreatitis, cholestasis, bowel perforation, sepsis, or mortality. Blood pressure, heart rate, body temperature, laboratory tests (complete blood count and C-reactive protein, total bilirubin, direct bilirubin, amylase, and lipase levels), and abdominal radiographs were conducted at 3 d and 14-21 d after FCSEMS placement to evaluate for short-term adverse events.
The Wilcoxon signed-rank test was used to compare changes in the diameter of the pancreatic duct between pre- and post-FCSEMS measurements. The IBM SPSS Statistics ver. 23.0 (IBM Co., Armonk, NY, United States) software was used for statistical analysis, and the stenting duration and patency are expressed as median values and ranges. P values < 0.05 were considered statistically significant.
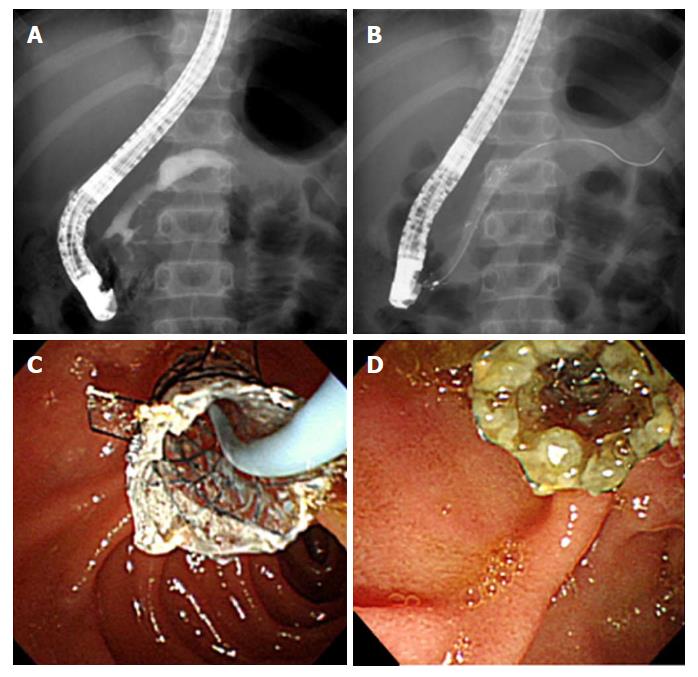
In all 8 patients, the placement of the FCSEMS with dual flaps was technically successful. The diameters of all inserted stents were 6 mm, and the lengths were 4-7 cm. The stents were successfully removed in all patients. The median indwelling time of stenting was 6 mo (range, 3-10 mo; Table 2). One patient had the stent removed 4 mo later than the planned time of removal because of personal reasons. However, stent embedding and occlusion did not develop (Figure 2). The patency of the stent lumen in the 8 patients with an FCSEMS had been maintained upon assessment at the time of stent removal. No stent migrations were observed.
Recurrent pancreatitis and pancreatic pain did not develop in 4 patients during FCSEMS placement. In 1 patient, pain developed 3 mo after stenting, resulting in an early removal that revealed remnant stones blocking the distal MPD, although the lumen of the FCSEMS was patent. Following the removal of the stone and reinsertion of the stent, the pain disappeared and statistically significant improvements in stricture and upstream dilation were observed. The mean stricture diameter was 1.1 ± 0.3 cm before stenting and 2.8 ± 0.9 cm after stenting (P < 0.05). The diameter of the upstream dilatation showed improvement from 8.4 ± 2.5 cm to 6.3 ± 1.6 cm after stenting (P < 0.05).
No adverse events such as pancreatitis, cholestasis, sepsis, bowel perforation, or mortality occurred during FCSEMS placement (Table 3).
| Case no. | Stent migration | Stent patency | Pancreatitis | Cholestasis | Bowel perforation | Sepsis | Mortality |
| 1 | N | Y | N | N | N | N | N |
| 2 | N | Y | N | N | N | N | N |
| 3 | N | Y | N | N | N | N | N |
| 4 | N | Y | N | N | N | N | N |
| 5 | N | Y | N | N | N | N | N |
| 6 | N | Y | N | N | N | N | N |
| 7 | N | Y | N | N | N | N | N |
| 8 | N | Y | N | N | N | N | N |
We, herein, report the first case series of the use of FCSEMSs in pediatric patients with CP and MPD strictures refractory to PS placement. Despite the small sample size and lack of long-term follow-up, this preliminary study presents evidence of the feasibility of the use of FCSEMS even in children, with technical and functional success. The disadvantages of PSs include migration and occlusion by sludge, resulting in necessary replacement at around 3 mo[17]. In a previous study, FCSEMSs in the management of MPD stricture showed efficacy and safety at 6-mo intervals in adults[16]. We suggest that our short-term outcomes are similar to the results for adults in terms of the improvement of strictures and the safety of the procedure[17]. Uncovered SEMSs are associated with tissue ingrowth through the stent mesh, which makes their removal difficult[18]. Tissue hyperplasia was reported in 23% of patients with partially covered stents[19]. When the stent was pulled using rat-tooth forceps at the scheduled removal time, the stent moved easily out of the pancreatic duct without resistance.
Initially, the introduction of FCSEMSs solved the tissue ingrowth issue but led to greater stent migration, with an incidence as high as 41%[20]. To overcome this challenge, fins and flaps have been incorporated into these devices[8,21]. During our procedure with a dual-flapped FCSEMS, we did not encounter any bleeding, ulcers, or migration problems. In terms of bioavailability, the nitinol materials and silicone membranes of the FCSEMSs applied in the present study series have been used safely in a previous study on the drainage of walled-off necrosis in children[22].
The major cause of CP in pediatric patients is hereditary pancreatitis rather than gallstones and alcoholism[23,24]. Furthermore, because the pancreas reserve of pediatric patients may worsen over time, considering total pancreatectomy with autologous islet transplantation (TP-IAT) may be much more important in children[25]. Surgery can result in a reduction of chronic pain through TP-IAT, which was shown to have a more protective effect against negative developments such as postoperative diabetes in preadolescence[26]. However, insulin dependency was still present in approximately 40% of postoperative patients[27]. Surgical burdens including morbidity, gastric motility dysfunction, and endocrine and exocrine replacement may also occur[28,29]. Given that the cause of CP differs between adults and children, adult guidelines for treating this condition as well as surgical recommendations cannot be automatically applied to pediatric cases. Therefore, until these problems are definitively resolved, endoscopic rather than surgical treatment should be considered in children.
In conclusion, this study showed that the use of FCSEMSs is feasible and safe and may be effective for the management of children with refractory benign MPD strictures. A future long-term investigation with a greater number of patients is warranted.
Plastic stents (PSs) or metal stents have been widely used for the management of biliary and pancreatic duct strictures in endoscopic interventions. The fully covered self-expandable metal stent (FCSEMS) was designed to compensate for the defects of PSs and uncovered self-expandable metal stents (SEMSs). FCSEMSs have been widely used for the management of biliary and pancreatic duct strictures in adults. In general, studies on the application of stents in pediatric patients are lacking. This study reported our experience with using FCSEMS as part of the treatment for benign main pancreatic duct (MPD) strictures in pediatric patients with chronic pancreatitis (CP).
The major cause of CP in pediatric patients is hereditary pancreatitis rather than gallstones and alcoholism, unlike in adults. Pediatric patients experience mental and physical repetitive pain from complications of MPD stricture during their long life. Stenting is an important indication in pediatric ERCP. In a previous study, the use of FCSEMSs in the management of MPD stricture showed efficacy and safety at 6-mo intervals in adults. We report that our short-term outcomes in children are similar to the results obtained for adults, in terms of the improvement of strictures and the safety of the procedure.
This study evaluated the feasibility, safety, and therapeutic effect of FCSEMSs for the management of benign MPD strictures in pediatric patients with CP.
This was a retrospective study of data collected through medical chart reviews between December 2014 and June 2017 at Asan Medical Center Children’s Hospital in Seoul, Korea. Eight patients with CP and benign dominant MPD stricture refractory to PS placement were enrolled. A dominant stricture was defined when the contrast medium on the endoscopic retrograde pancreatography film was not washed out and the upstream MPD dilation was ≥ 6 mm in diameter. Feasibility was evaluated based on technical success, migration, and patency. Technical success was determined in accordance with the success of stent placement and removal. Stricture improvement was evaluated by measuring the diameter of the stricture and the upstream dilation of the MPD. Safety was evaluated based on the occurrence of adverse events at 3 d and 14-21 d after FCSEMS placement. The Wilcoxon signed-rank test was used to compare changes in the diameter of the pancreatic duct between pre- and post-FCSEMS measurements. The IBM SPSS Statistics ver. 23.0 software was used for statistical analysis, and the stenting duration and patency are expressed as median values and ranges.
In all 8 patients, the placement of the 6-mm FCSEMS with dual flaps was technically successful. The median indwelling time of stenting was 6 mo (range, 3-10 mo). Stent embedding and occlusion did not develop. The patency of the stent lumen in the 8 patients with an FCSEMS had been maintained upon assessment at the time of stent removal. No stent migrations, adverse events, and mortalities were observed. After the removal of the stone and insertion of the stent, the pain disappeared and statistically significant improvements in stricture and upstream dilation were observed. The mean stricture diameter was 1.1 ± 0.3 cm before stenting and 2.8 ± 0.9 cm after stenting (P < 0.05). The diameter of the upstream dilatation showed improvement from 8.4 ± 2.5 cm to 6.3 ± 1.6 cm after stenting (P < 0.05). A future study with a larger sample size could clarify the usefulness of FCSEMS in children with benign MPD dilatation.
This is the first study to investigate the efficacy and safety of the placement of the 6-mm FCSEMS with dual flaps in children. Despite the technical success and satisfactory clinical outcomes in these 8 children, the present study had certain limitations, including its retrospective design, relatively small sample size, and short follow-up period. In this study, we report our experience with using FCSEMS as part of the management of benign MPD strictures in pediatric patients with CP, providing valuable information about useful treatment for such patients. FCSEMS placement is an effective and safe management strategy for MPD strictures in pediatric patients with CP.
We report the first case series of the use of FCSEMSs in the pediatric population with CP and MPD strictures refractory to PS placement. Despite the small sample size and lack of long-term follow-up, this preliminary study presents evidence of the feasibility of using FCSEMS even in children, with technical and functional success. A future long-term investigation with a greater number of patients is warranted.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Fu D S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y
| 1. | Mangiavillano B, Pagano N, Baron TH, Arena M, Iabichino G, Consolo P, Opocher E, Luigiano C. Biliary and pancreatic stenting: Devices and insertion techniques in therapeutic endoscopic retrograde cholangiopancreatography and endoscopic ultrasonography. World J Gastrointest Endosc. 2016;8:143-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Almadi MA, Barkun A, Martel M. Plastic vs. Self-Expandable Metal Stents for Palliation in Malignant Biliary Obstruction: A Series of Meta-Analyses. Am J Gastroenterol. 2017;112:260-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 201] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 3. | Seicean A, Vultur S. Endoscopic therapy in chronic pancreatitis: current perspectives. Clin Exp Gastroenterol. 2014;8:1-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Shin HP, Kim MH, Jung SW, Kim JC, Choi EK, Han J, Lee SS, Seo DW, Lee SK. Endoscopic removal of biliary self-expandable metallic stents: a prospective study. Endoscopy. 2006;38:1250-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Neuhaus H, Hagenmüller F, Classen M. Self-expanding biliary stents: preliminary clinical experience. Endoscopy. 1989;21:225-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 59] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Hawes RH. Diagnostic and therapeutic uses of ERCP in pancreatic and biliary tract malignancies. Gastrointest Endosc. 2002;56:S201-S205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Eum J, Park DH, Ryu CH, Kim HJ, Lee SS, Seo DW, Lee SK, Kim MH. EUS-guided biliary drainage with a fully covered metal stent as a novel route for natural orifice transluminal endoscopic biliary interventions: a pilot study (with videos). Gastrointest Endosc. 2010;72:1279-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Tsuchiya T, Itoi T, Gotoda T, Kuraoka K, Sofuni A, Itokawa F, Kurihara T, Ishii K, Tsuji S, Ikeuchi N. A multicenter prospective study of the short-term outcome of a newly developed partially covered self-expandable metallic biliary stent (WallFlex(®)). Dig Dis Sci. 2011;56:1889-1895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Tarantino I, Mangiavillano B, Di Mitri R, Barresi L, Mocciaro F, Granata A, Masci E, Curcio G, Di Pisa M, Marino A. Fully covered self-expandable metallic stents in benign biliary strictures: a multicenter study on efficacy and safety. Endoscopy. 2012;44:923-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Park DH, Kim MH, Moon SH, Lee SS, Seo DW, Lee SK. Feasibility and safety of placement of a newly designed, fully covered self-expandable metal stent for refractory benign pancreatic ductal strictures: a pilot study (with video). Gastrointest Endosc. 2008;68:1182-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | Troendle DM, Barth BA. Pediatric Considerations in Endoscopic Retrograde Cholangiopancreatography. Gastrointest Endosc Clin N Am. 2016;26:119-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Cho JM, Jeong IS, Kim HJ, Oh SH, Kim KM. Early adverse events and long-term outcomes of endoscopic sphincterotomy in a pediatric population: a single-center experience. Endoscopy. 2017;49:438-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Agarwal J, Nageshwar Reddy D, Talukdar R, Lakhtakia S, Ramchandani M, Tandan M, Gupta R, Pratap N, Rao GV. ERCP in the management of pancreatic diseases in children. Gastrointest Endosc. 2014;79:271-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Oracz G, Pertkiewicz J, Kierkus J, Dadalski M, Socha J, Ryzko J. Efficiency of pancreatic duct stenting therapy in children with chronic pancreatitis. Gastrointest Endosc. 2014;80:1022-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Dumonceau JM, Delhaye M, Tringali A, Dominguez-Munoz JE, Poley JW, Arvanitaki M, Costamagna G, Costea F, Devière J, Eisendrath P. Endoscopic treatment of chronic pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2012;44:784-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 190] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 16. | Ogura T, Onda S, Takagi W, Kitano M, Sano T, Okuda A, Miyano A, Masuda D, Takeuchi T, Fukunishi S. Placement of a 6 mm, fully covered metal stent for main pancreatic head duct stricture due to chronic pancreatitis: a pilot study (with video). Therap Adv Gastroenterol. 2016;9:722-728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | England RE, Martin DF, Morris J, Sheridan MB, Frost R, Freeman A, Lawrie B, Deakin M, Fraser I, Smith K. A prospective randomised multicentre trial comparing 10 Fr Teflon Tannenbaum stents with 10 Fr polyethylene Cotton-Leung stents in patients with malignant common duct strictures. Gut. 2000;46:395-400. [PubMed] |
| 18. | Cantù P, Villa F, Baroni S, Brunati S. Comment to “Precut sphincterotomy, repeated cannulation and post-ERCP pancreatitis in patients with bile duct stone disease”. Dig Liver Dis. 2012;44:625-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Deviere J, Cremer M, Baize M, Love J, Sugai B, Vandermeeren A. Management of common bile duct stricture caused by chronic pancreatitis with metal mesh self expandable stents. Gut. 1994;35:122-126. [PubMed] |
| 20. | Devière J, Nageshwar Reddy D, Püspök A, Ponchon T, Bruno MJ, Bourke MJ, Neuhaus H, Roy A, González-Huix Lladó F, Barkun AN. Successful management of benign biliary strictures with fully covered self-expanding metal stents. Gastroenterology. 2014;147:385-395; quiz e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 173] [Article Influence: 14.4] [Reference Citation Analysis (1)] |
| 21. | Park DH, Lee SS, Lee TH, Ryu CH, Kim HJ, Seo DW, Park SH, Lee SK, Kim MH, Kim SJ. Anchoring flap versus flared end, fully covered self-expandable metal stents to prevent migration in patients with benign biliary strictures: a multicenter, prospective, comparative pilot study (with videos). Gastrointest Endosc. 2011;73:64-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 22. | Nabi Z, Lakhtakia S, Basha J, Chavan R, Ramchandani M, Gupta R, Kalapala R, Darisetty S, Talukdar R, Reddy DN. Endoscopic Ultrasound-guided Drainage of Walled-off Necrosis in Children With Fully Covered Self-expanding Metal Stents. J Pediatr Gastroenterol Nutr. 2017;64:592-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Schwarzenberg SJ, Bellin M, Husain SZ, Ahuja M, Barth B, Davis H, Durie PR, Fishman DS, Freedman SD, Gariepy CE. Pediatric chronic pancreatitis is associated with genetic risk factors and substantial disease burden. J Pediatr. 2015;166:890-896.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 145] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 24. | Lee YJ, Kim KM, Choi JH, Lee BH, Kim GH, Yoo HW. High incidence of PRSS1 and SPINK1 mutations in Korean children with acute recurrent and chronic pancreatitis. J Pediatr Gastroenterol Nutr. 2011;52:478-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Chinnakotla S, Beilman GJ, Dunn TB, Bellin MD, Freeman ML, Radosevich DM, Arain M, Amateau SK, Mallery JS, Schwarzenberg SJ. Factors Predicting Outcomes After a Total Pancreatectomy and Islet Autotransplantation Lessons Learned From Over 500 Cases. Ann Surg. 2015;262:610-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 26. | Bellin MD, Carlson AM, Kobayashi T, Gruessner AC, Hering BJ, Moran A, Sutherland DE. Outcome after pancreatectomy and islet autotransplantation in a pediatric population. J Pediatr Gastroenterol Nutr. 2008;47:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Kobayashi T, Manivel JC, Bellin MD, Carlson AM, Moran A, Freeman ML, Hering BJ, Sutherland DE. Correlation of pancreatic histopathologic findings and islet yield in children with chronic pancreatitis undergoing total pancreatectomy and islet autotransplantation. Pancreas. 2010;39:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Bhayani NH, Enomoto LM, Miller JL, Ortenzi G, Kaifi JT, Kimchi ET, Staveley-O’Carroll KF, Gusani NJ. Morbidity of total pancreatectomy with islet cell auto-transplantation compared to total pancreatectomy alone. HPB (Oxford). 2014;16:522-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Radomski M, Zureikat AH. Total pancreatectomy and islet cell autotransplantation: outcomes, controversies and new techniques. JOP. 2015;16:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |