Published online Jan 21, 2018. doi: 10.3748/wjg.v24.i3.415
Peer-review started: October 25, 2017
First decision: November 21, 2017
Revised: December 18, 2017
Accepted: December 26, 2017
Article in press: December 26, 2017
Published online: January 21, 2018
Processing time: 86 Days and 19.2 Hours
To optimize the hepatobiliary phase delay time (HBP-DT) of Gd-EOB-DTPA-enhanced magnetic resonance imaging (GED-MRI) for more efficient identification of hepatocellular carcinoma (HCC) occurring in different degrees of cirrhosis assessed by Child-Pugh (CP) score.
The liver parenchyma signal intensity (LPSI), the liver parenchyma (LP)/HCC signal ratios, and the visibility of HCC at HBP-DT of 5, 10, 15, 20, and 25 min (i.e., DT-5, DT-10, DT-15, DT-20, and DT-25 ) after injection of Gd-EOB-DTPA were collected and analyzed in 73 patients with cirrhosis of different degrees of severity (including 42 patients suffering from HCC) and 18 healthy adult controls.
The LPSI increased with HBP-DT more significantly in the healthy group than in the cirrhosis group (F = 17.361, P < 0.001). The LP/HCC signal ratios had a significant difference (F = 12.453, P < 0.001) among various HBP-DT points, as well as between CP-A and CP-B/C subgroups (F = 9.761, P < 0.001). The constituent ratios of HCC foci identified as obvious hypointensity (+++), moderate hypointensity (++), and mild hypointensity or isointensity (+/-) kept stable from DT-10 to DT-25: 90.6%, 9.4%, and 0.0% in the CP-A subgroup; 50.0%, 50.0%, and 0.0% in the CP-B subgroup; and 0.0%, 0.0%, and 100.0% in the CP-C subgroup, respectively.
The severity of liver cirrhosis has significant negative influence on the HCC visualization by GED-MRI. DT-10 is more efficient and practical than other HBP-DT points to identify most of HCC foci emerging in CP-A cirrhosis, as well as in CP-B cirrhosis; but an HBP-DT of 15 min or longer seems more appropriate than DT-10 for visualization of HCC in patients with CP-C cirrhosis.
Core tip: In order to optimize the hepatobiliary phase delay time (HBP-DT) of Gd-EOB-DTPA-enhanced magnetic resonance imaging for more efficient identification of hepatocellular carcinoma (HCC) in cirrhosis of different degrees of severity, we analyzed the signal intensity and ratios between liver parenchyma and HCC, and the percentages of HCC visibility at a series of HBP-DT points in those patients. The severity of cirrhosis was shown to negatively influence HCC visibility, but DT-10 is already enough and more efficient than longer DT to identify HCC in Child-Pugh (CP)-A and CP-B cirrhosis. DT-15 or longer DT seems more appropriate for HCC visibility in patients with CP-C cirrhosis.
- Citation: Wu JW, Yu YC, Qu XL, Zhang Y, Gao H. Optimization of hepatobiliary phase delay time of Gd-EOB-DTPA-enhanced magnetic resonance imaging for identification of hepatocellular carcinoma in patients with cirrhosis of different degrees of severity. World J Gastroenterol 2018; 24(3): 415-423
- URL: https://www.wjgnet.com/1007-9327/full/v24/i3/415.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i3.415
Gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid (Gd-EOB-DTPA, Primovist; Bayer Schering Pharma, Berlin, Germany) is a modern contrast medium which can be taken mainly by hepatocytes with relative specificity. It has been used more and more frequently in magnetic resonance imaging (MRI) to provide better diagnosis, differential diagnosis, assessment of hepatic function, and imaging of bile ducts in patients with liver-occupying lesions[1-3]. Gd-EOB-DTPA-enhanced MRI (GED-MRI) is proven to have marked advantages over contrast-enhanced computed tomography (CT) in finding smaller hepatocellular carcinoma (HCC), especially those with a diameter less than 20 mm, as well as the recurrent HCC after various antitumor treatments[4,5]. However, a disadvantage of GED-MRI is that it needs remarkably long time for scanning, in which the hepatobiliary phase delay time (HBP-DT) is usually set at 15 to 20 min or longer[6,7]. For the purpose of applying GED-MRI more efficiently and rationally in complex clinical background, it is very necessary to optimize the HBP-DT in different groups of patients, especially in those with liver-occupying lesions under various stages of cirrhosis[8,9]. A study reported that an HBP-DT of 10 min after Gd-EOB-DTPA injection was sufficient for hepatic lesion characterization in patients with normal liver function and without cirrhosis[10]. However, whether an HBP-DT of 10 min is suitable for patients with liver dysfunction that correlates with cirrhosis is unknown. Another study concluded that an HBP-DT of 15 min was sufficient for patients with mild liver dysfunction classified as grade A of Child-Pugh score (CP-A); on the other hand, an HBP-DT longer than 5 min was meaningless for lesion characterization in patients with moderate or severe liver dysfunction classified as CP-B or CP-C[11]. Clinically, these conclusions are worthy of further investigation and discussion. In the current study, we attempted to identify more efficient and practical HBP-DT of GED-MRI for detection of HCC in the context of different grades of cirrhosis.
Totally, 73 patients (49 males and 24 females) with a median age of 47 years (range, 23-65 years) who suffered from cirrhosis caused by chronic hepatitis B (CHB) were included in this study. The numbers of patients classified as CP-A, B, and C were 43, 25, and 5, respectively. Eighteen healthy adults were selected as controls, with a male-to-female ratio of 1:1 and a median age was 43 years (range, 23-62 years).
Forty-two of the 73 patients with CHB-related cirrhosis were identified as having HCC, of whom 36 were diagnosed by histopathologic examination using samples from hepatectomy (n = 26), liver transplantation (n = 4), or liver biopsy (n = 6). The rest six patients with HCC were diagnosed clinically by the long history of CHB, typical imaging, and a high level of serum alpha-fetoprotein. In the 36 patients with histopathologic examination, moderately to lowly differentiated HCC was confirmed in 34 patients, moderately differentiated HCC was found in one patient, and the degree of differentiation could not be distinguished in the last patient.
Totally, 47 HCC foci were found in the 42 patients with HCC, and there were 32, 12, and 3 HCC foci in patients with CP-A (n = 27), CP-B (n = 12), and CP-C (n = 3) disease, respectively. The median diameter of these HCC foci was 2.1 cm (range, 0.5-4.4 cm).
According to the features and mechanism of GED-MRI, patients who had the following status were excluded from this study: (1) the diameter of one HCC focus, or the sum of diameters of all HCC foci was more than 5 cm; (2) there was cancer embolus or thrombus in the portal vein or its main branch; (3) existence of obstructive jaundice; (4) allergy to Gd-EOB-DTPA; (5) acute/subacute-on-chronic liver failure and severe impairment of renal function; and (6) those who could not cooperate well with the MRI operators during GED-MRI.
After fasting for at least 6 hours and learning how to breathe and breath-hold correctly in accordance with the order of MRI operator, all subjects underwent MRI of the upper abdomen using a 3.0-T scanner (HDxt; GE Medical Systems) with an 8-channel phased array coil. Parallel imaging using the Array Spatial Sensitivity Encoding Technique (ASSET) were done at first, followed by the axial breath-holding three-dimensional fast spoiled gradient-echo (3D FSPGR) T1 weighted imaging, FSE-XL fat-suppression respiratory-triggered T2 weighted imaging, SE/EPI diffuse weighted imaging, and magnetic resonance cholangiopancreatography.
For contrast-enhanced MRI, Gd-EOB-DTPA was injected as a bolus at 0.025 mmol/kg at a rate of 1.0 mL/s, followed by the same volume of physiological saline flush at a rate of 2.0 mL/s. The dynamic and delayed imaging in the HBP at 5, 10, 15, 20, and 25 min (i.e., at DT-5, DT-10, DT-15, DT-20 and DT-25) was sequentially executed after injection of Gd-EOB-DTPA using an axial fat-suppressed liver acceleration volume acquisition (LAVA) sequence (repetition time = 2.8 ms; echo time = 1.2-1.3 ms; flip angle = 11°; frequency bandwidth of 83.33; field of view = 400-480 mm; acquisition matrix = 224 × 224; ASSET3.00PH; slice thickness = 2.6 mm; phase field of view = 1.0; acquisition time = 12-16 s).
The liver parenchyma signal intensity (LPSI) of the regions of interest from the centers of the left external lobe, left internal lobe, right anterior lobe, and right rear lobe at the level of the porta hepatis, with each area being about 100 mm2, was measured on LAVA images at DT-5, DT-10, DT-15, DT-20, and DT-25 after Gd-EOB-DTPA injection. The regions of interest were away from the vessels, bile ducts, and lesions to avoid the disturbance on signal intensity. The mean value with a standard deviation (mean ± SD) of LPSI was calculated and compared.
The signal intensity of each HCC focus and corresponding adjacent LP at any HBP-DT point was measured, and the signal ratios of LP to each HCC focus at all HBP-DT points were calculated as follows: signal ratio = (mean signal intensity of LP)/(signal intensity of HCC focus).
The strength of HCC visualization at each HBP-DT point was assessed by naked eyes, and compared among patients with different grades of CP score. The HCC visualization was subjectively divided into three degrees: obvious hypointensity (+++), moderate hypointensity (++), and hypointensity or isointensity (+/-). All the interpretation of HCC visualization and data processing were done by two senior doctors separately. If there was any disagreement between the two doctors, the data would be presented to a third senior doctor and discussed by the three doctors together to reach a final diagnosis.
The statistical software SPSS19.0 was used to process the data in this study. Repeated measures two-way analysis of variance (TW-ANOVA) was used to compare the mean LPSI between healthy controls and the cirrhotic group, and the mean LP/HCC signal ratios between subgroups with CP-A cirrhosis and CP-B/C cirrhosis. Repeated measures one-way ANOVA (OW-ANOVA) was used to compare the mean LP/HCC signal ratios at each HBP-DT point in patients suffering from cirrhosis overlapped with HCC, and Huynh-Feldt correction was adopted when the data did not satisfy spherical symmetry. P < 0.05 was regarded as having statistical significance.
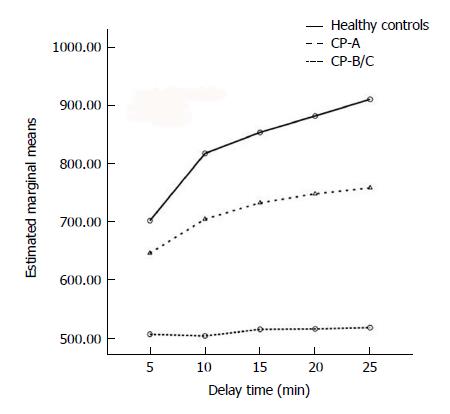
Repeated measures TW-ANOVA and Huynh-Feldt correction showed that the LPSI increased time-dependently in each group included in this study (F = 77.109, P < 0.001; Figure 1). There was a significant difference for the ascending trends of LPSI among different groups (F = 17.361, P < 0.001). The strongest LPSI with a markedly ascending trend was seen in healthy controls. Moderate LPSI with a slowly ascending trend was seen in the CP-A subgroup. Mild LPSI without a significantly ascending trend was seen in the CP-B/C subgroup (F = 47.685, P < 0.001).
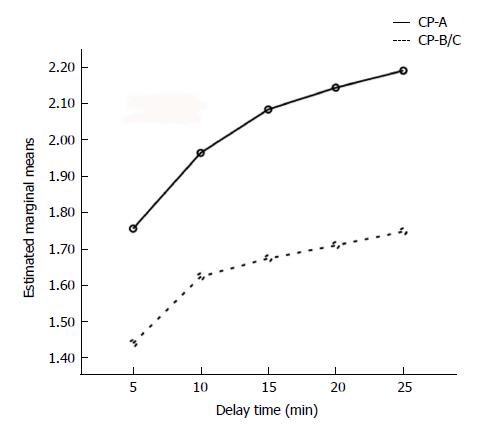
In the group of HCC coexistent with cirrhosis, there were 32 and 15 HCC foci in the CP-A subgroup and CP-B/C subgroup, respectively. Repeated measures TW-ANOVA and Huynh-Feldt correction showed that the LP/HCC signal ratios increased with HBP-DT in both of the two subgroups (F = 8.201, P < 0.006; Figure 2), with higher signal ratios in the CP-A subgroup (F = 9.761, P < 0.001). After DT-10, the curve of signal ratios escalated more slowly in the CP-B/C subgroup than in the CP-A subgroup.
The MRI images obtained at DT-5 were not assessed for visualization, because much Gd-EOB-DTPA had detained in hepatic vessels and extracellular space at this moment. Accordingly, some of the HCC foci at DT-5 usually appeared as mild hypointensity or isointensity, and thus were difficult for naked eyes to identify.
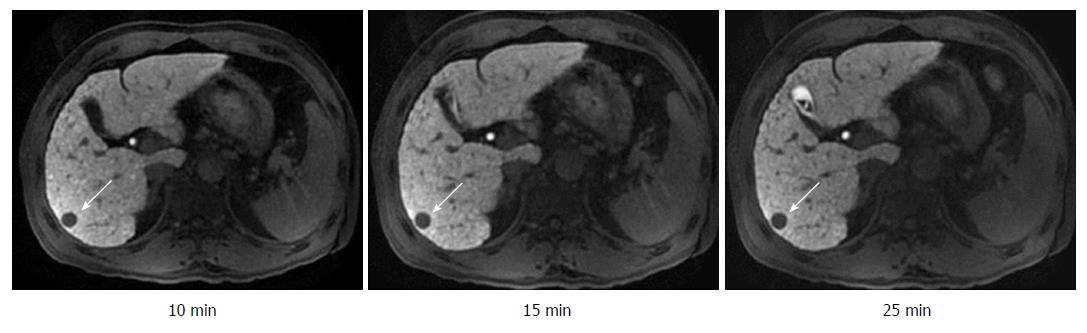
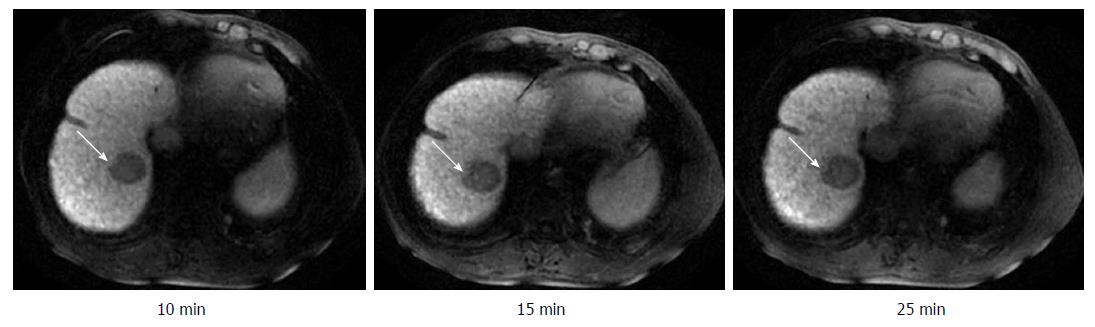
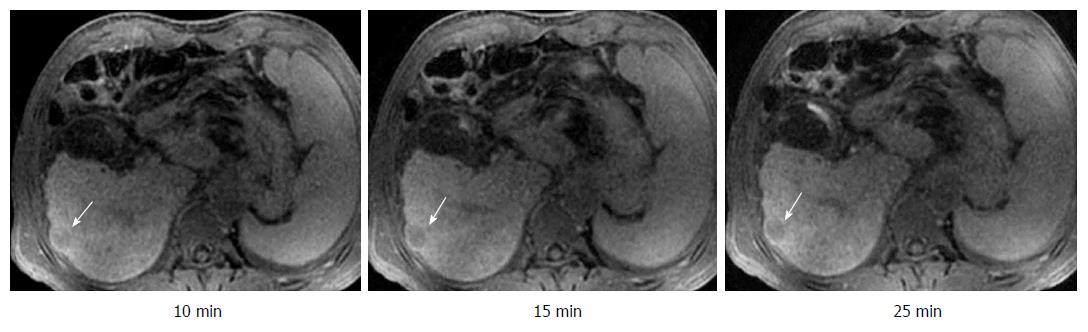
The visualization rates of HCC foci at DT-10, DT-15, DT-20, and DT-25 kept invariable in the same subgroup (Table 1). On the other hand, there was a significant difference between the CP-A subgroup and CP-B/C subgroup for the constituent ratios of HCC visualization at any corresponding HBP-DT point (Table 1). The constituent ratios of obvious hypointensity (+++) and moderate hypointensity (++) were 90.6% and 9.4% in the CP-A subgroup (Figure 3), 50.0% and 50.0% in the CP-B subgroup (Figure 4), respectively, and there were no HCC foci with mild hypointensity or isointensity (+/-) in both of the two subgroups. It was worth noting that two HCC foci from two patients with CP-C cirrhosis showed only a little increase of visualization with the extension of HBP-DT (Figure 5), and another HCC focus from another patient with CP-C cirrhosis kept the status of isointensity at any HBP-DT point.
| Grade of CP score | Sum of HCC foci | Signal intensity | HBP-DT | ||||
| 5 min | 10 min | 15 min | 20 min | 25 min | |||
| A | 32 | +++ | 0 | 29 (90.6) | 29 (90.6) | 29 (90.6) | 29 (90.6) |
| ++ | 24 (75) | 3 (9.4) | 3(9.4) | 3 (9.4) | 3 (9.4) | ||
| +/- | 8 (25) | 0 | 0 | 0 | 0 | ||
| B | 12 | +++ | 0 | 6 (50.0) | 6 (50.0) | 6 (50.0) | 6 (50.0) |
| ++ | 7 (58.3) | 6 (50.0) | 6 (50.0) | 6 (50.0) | 6 (50.0) | ||
| +/- | 5 (41.7) | 0 | 0 | 0 | 0 | ||
| C | 3 | +++ | 0 | 0 | 0 | 0 | 0 |
| ++ | 0 | 0 | 0 | 0 | 0 | ||
| +/- | 3 (100.0) | 3 (100.0) | 3 (100.0) | 3 (100.0) | 3 (100.0) | ||
Gd-EOB-DTPA is a kind of hepatocyte-specific contrast medium for enhanced MRI, which combines the advantages of both routine MRI contrast media and hepatocyte-specific contrast media. Compared to routine contrast-enhanced CT or MRI scan, GED-MRI could provide not only more accurate diagnosis and differential diagnosis for space-occupying lesions[12-16], early HCC, and hyperplastic nodules of the liver[17,18], but also more accurate assessment of liver function in clinic[19,20].
The key mechanism for Gd-EOB-DTPA to identify HCC is to create a detectable signal contrast between LP and HCC foci in the HBP. After injection, nearly 50% of Gd-EOB-DTPA are taken by hepatocytes through organic anion transporting polypeptide (OATP) 1B1 and 1B3, which are expressed on the hepatocellular membrane, thus contributing to the increase of LPSI. HCC loci usually appear to be hypointense because such lesions could not take or only absorbed a little Gd-EOB-DTP, and thus could be identified by naked eyes[21-23].
Gd-EOB-DTPA started to be taken by hepatocytes from one and a half minutes after injection, and mounted the peak at about 20 min after injection[6]. Some researchers reported that the signal contrast between the liver and spleen reached the peak at a delay time of 60 min, and concluded that it would decrease the contrast of signals between the liver and spleen if the GED-MRI pictures were collected before DT-20 of the HBP[7]. However, an important problem is that many patients will feel discomfort if the delay time lasts too long, which makes them difficult to cooperate with operators, and then interferes with the procedure of GED-MRI and decreases its accuracy of diagnosis. Accordingly, it is necessary to optimize, especially shorten the scanning time of GED-MRI to a rational extent.
The severity of cirrhosis and liver function impairment could significantly influence the uptake of Gd-EOB-DTPA by LP. Tamada et al[24] reported that the LPSI increased significantly during 10 to 20 min of HBP-DT in healthy controls and patients with CP-A or CP-B cirrhosis, but had no significant increase after the portal venous phase. Our investigation showed that the LPSI increased with the time from DT-5 to DT-25 after injection of Gd-EOB-DTPA in healthy controls, but increased relatively slowly in patients with cirrhosis, especially in patients with CP-B/C cirrhosis. This finding suggested that the ability to uptake Gd-EOB-DTPA decreased in liver with cirrhosis, which would lead to a decrease of signal contrast between LP and HCC foci and thus impair the visualization by naked eyes and detection rate of HCC foci.
Most experts believe that 20-30 min of HBP-DT was the best choice for the identification of liver-occupying lesions, but some experts reported that 15 min of HBP-DT was enough to find HCC foci, in spite of less enhancement of LP in CP-C patients than in CP-B or CP-A patients[17]. In contrast, our research showed that DT-10 was indeed enough to guarantee the identification of all HCC foci in 27 patients with CP-A cirrhosis. Although the LP/HCC signal ratios were relatively low in 12 patients with CP-B cirrhosis, all HCC foci were also clearly displayed at DT-10, DT-15, DT-20, and DT-25. These findings showed that DT-10 in GED-MRI could not only sufficiently ensure the identification of HCC foci in patients with CP-A and CP-B cirrhosis, but also markedly shorten the duration of MRI scanning. Although the contrast of signal intensity between LP and HCC foci increased after DT-15, it really had no significant influence on the detection rate of HCC foci. Accordingly, 15 min or longer HBP-DT was not only unnecessary in clinic in patients with CP-A or CP-B cirrhosis, but also decreased the compliance of patients and efficiency of diagnosis during GED-MRI.
The signal contrast was not satisfactory between LP and HCC foci at DT-5, because there was much Gd-EOB-DTPA detained in hepatic vessels, extracellular space, and HCC foci at this time point. Accordingly, although more than half of HCC foci could be visualized at DT-5, we did not recommend images obtained at this time point as the evidence to exclude the diagnosis of HCC.
On the other hand, the signal contrast between LP and HCC foci improved very slowly with time in two patients with CP-C cirrhosis (See Figure 5), and the signal intensity of HCC focus was nearly equal to that of LP in another patient with CP-C cirrhosis, which made the HCC focus very difficult to be visualized. The poor enhancement of LP at any HBP-DT point in the three patients contributed to the poor signal contrast between LP and HCC foci. These results showed that GED-MRI had no significant advantages over other strategies to identify HCC in patients with CP-C cirrhosis. This conclusion is in accordance with the published findings[24-26]. How to optimize the MRI procedure and improve the visualization of HCC foci in patients with CP-C cirrhosis is an important issue awaiting further investigation.
It should be pointed out that many researchers reported that some HCC cells had the ability to take Gd-EOB-DTPA[27-31], but this ability was very poor compared with normal hepatocytes. The ability of HCC foci to take Gd-EOB-DTPA was associated with several factors, including the differentiation degree of HCC and genetic polymorphisms of OATP1B1[31], OATP1B3 (OATP8)[27-30], other OATPs, and hepatocyte nuclear factor 4A (HNF-4A)[27]. A few well-differentiated HCCs could take a bit of Gd-EOB-DTPA, and a very few poorly differentiated HCCs could express OATP1B3 which endowed HCC cells with the ability to take some Gd-EOB-DTPA[29,30]. Yamashita et al[27] reported that nearly 15% of HCC foci could take Gd-EOB-DTPA in HBP, which was the result of increased expression of OATP1B3 and HNF-4A. Narita et al[28] believed that the expression of OATP1B3 was the determinant factor for the uptake of Gd-EOB-DTPA by some HCC foci. Other investigators also reported that the signal intensity and visualization of some HCC foci might be interfered by the ability of HCC cells to take Gd-EOB-DTPA[29,30] and the genetic polymorphism of OATP1B1[31]. In a recent retrospective study, Miura et al[32] reported that 14 patients had high HCC with the LP/HCC signal ratios ≤ 1.0. Clinicopathological analysis revealed low-grade malignancy in high HCC compared with low HCC, and the expression of OATP1B3 was a key mechanism for the hyperintensity in the HBP of GED-MRI. But in our study, we did not find the high HCC lesions except one isointense HCC lesion in a patient with CP-C cirrhosis. It should be noted that borderline lesions of HCC might also show hypo-, iso-, or hyperintensity in the HBP of GED-MRI, but the hypointense borderline lesions had the highest risk to progress into typical HCC[33].
In summary, our research demonstrated that LPSI in the HBP of GED-MRI was lower in patients with cirrhosis than in healthy controls, but had no significant influence on the visualization rate of HCC foci in patients with CP-A or CP-B cirrhosis. In most of the patients with CP-A or CP-B cirrhosis, DT-10 already satisfied the detection of HCC, and it was not very necessary to prolong the HBP-DT up to 15-25 min. In patients with CP-C cirrhosis, GED-MRI usually had no obvious advantage in detecting HCC; but in some of those cases, a longer HBP-DT of 15-25 min might improve the visualization to a certain extent. Based on the above-mentioned findings and in order to balance the accuracy of diagnosis, efficiency of MRI process, and compliance of the patients, it is advisable to individualize HBP-DT according to the status of liver function for the detection of HCC in patients with cirrhosis. In view of the evidence that HCC foci could take a certain proportion of Gd-EOB-DTPA in some patients, it is very necessary in the future to design larger studies including more cases to analyze the correlation between uptake of Gd-EOB-DTPA and genetic polymorphism or/and expression status of OATP, in order to further clarify the appropriate patients who should receive the examination of GED-MRI and optimize the HBP-DT individually.
Gd-EOB-DTPA-enhanced magnetic resonance imaging (GED-MRI) has significant advantages in finding smaller HCC lesions. However, it is believed that GED-MRI may need remarkably long time for scanning, in which the hepatobiliary phase delay time (HBP-DT) is usually set at 15 to 20 min or longer, and many patients could not cooperate well with the operators during the long process of scanning. Recently, a study reported that DT-10 was sufficient for hepatic lesion characterization in patients with normal liver function and without cirrhosis, and another study concluded that DT-15 was sufficient for patients with mild liver dysfunction classified as cirrhosis of Child-Pugh A (CP-A). However, which HBP-DT is both more efficient and more practical for patients with liver dysfunction that correlates with different degrees of severity of cirrhosis is unknown. Accordingly, we attempted to gather new clinical evidence to optimize the HBP-DT of GED-MRI for detection of HCC in the context of different grades of cirrhosis.
The main topics and key problems of the study include: (1) whether and how the severity of liver cirrhosis will influence the signal intensity of liver parenchyma (LPSI) in the process of GED-MRI examination? (2) whether and how the features of LPSI of liver cirrhosis will interfere with the visibility of HCC? and (3) which HBP-DT will provide the more efficient examination without the discount of diagnostic accuracy in the different context of liver cirrhosis? The findings in the current study will provide first hand information for the answers of above-mentioned questions, and thus contribute to the improvement of rational, efficient, and individual application of GED-MRI.
This study aimed to optimize the HBP-DT of GED-MRI for more efficient identification of HCC occurring in different degrees of cirrhosis assessed by CP score without any discount of diagnostic accuracy. Through the systematic assessment of correlations among liver LPSI, liver parenchyma (LP)/HCC signal ratios, and percentage of visibility of HCC lesions at a series of HBP-DT points in the background of liver cirrhosis with different CP scores, it was demonstrated that the HBP-DT of GED-MRI surely could be optimized in the context of liver cirrhosis with different CP scores. Based on those findings and the existence of heterogeneity in the liver function tests, severity of fibrosis, polymorphism of organic anion transporting polypeptide (OATP), and other potential factors that may interfere with the visibility of HCC, a big-sample multicenter prospective study is needed in the future.
This study is a retrospective analysis about how to improve the application of GED-MRI to diagnose HCC occurring in the background of different degrees of cirrhosis. Forty-two patients with HCC from 73 patients with CHB-related cirrhosis were included in this study according to the criteria of inclusion and exclusion. The history and CP scores of CHB-related cirrhosis, the features of LPSI, LP/HCC signal ratios, visibility, and its percentages of HCC lesions at a series of HBP-DT points were systematically collected and compared. The two-way analysis of variance (TW-ANOVA), repeated measures one-way ANOVA (OW-ANOVA), and Huynh-Feldt correction were used to do the statistical analyses of the data. These research methods were the routine ways adopted widely in the clinical investigation.
The main findings in this study are as follows. First, the LPSI was found to increase time-dependently both in healthy controls and in patients with HCC overlapped on cirrhosis. Second, the LP/HCC signal ratios had a significant difference among various HBP-DT points, as well as between the CP-A and CP-B/C subgroups. Third, the constituent ratios of HCC foci identified as obvious hypointensity (+++), moderate hypointensity (++), and mild hypointensity/isointensity (+/-) kept stable from DT-10 to DT-25 in each subgroups, but had a difference among subgroups with cirrhosis of CP-A, CP-B, or CP-C. To our knowledge, this is the first time to report that HCC visibility at DT-10 is equal to that at DT-15 or longer DT; that is, compared to longer DT, DT-10 had the same diagnostic accuracy, but showed more efficient diagnosis of HCC existing in the background of both CP-A cirrhosis and CP-B cirrhosis. The problems that remain to be solved in the future include: (1) whether DT-10 will still keep the same high efficiency and accuracy for identification of HCC overlapped on CP-A and CP-B cirrhosis when this concept is applied to more patients; (2) whether and what kind of OATP polymorphism will interfere with the diagnostic efficacy of GED-MRI when used for identification of HCC in Chinese patients; and (3) how to improve the detection of HCC lesions presenting as mild hypointensity or even isointensity in patients with CP-C cirrhosis.
The conclusions from this study are summarized as follows. First, the severity of liver cirrhosis has significant negative influence on the HCC visualization by GED-MRI. Second, DT-10 is more efficient and practical than other HBP-DT points to identify most of HCC foci emerging in CP-A cirrhosis, as well as in CP-B cirrhosis. This is the most important new finding of this study. Third, an HBP-DT of 15 min or longer seems more appropriate than DT-10 for visualization of HCC in patients with CP-C cirrhosis. Based on the new findings of this study, we proposed that DT-10 should be chosen as the most appropriate HBP-DT point of GED-MRI for most of the patients with HCC overlapped on CP-A and CP-B cirrhosis, but a longer DT should be used for patients with CP-C patients.
This study told clinicians that the status of liver cirrhosis should be assessed carefully before GED-MRI examination in order to choose the most efficient HBP-DT point without any discount of accuracy which is based on the visibility of HCC lesions. It was shown in this study that DT-10 was the optimal HBP-DT which could satisfy the requirement of HCC diagnosis in most of the patients suffering from both CP-A/B liver cirrhosis and HCC, without the necessity of longer HBP-DT. On the other hand, a longer HBP-DT ≥ 15 min might improve the visibility of some HCCs overlapped on CP-C cirrhosis. These interesting findings need to be further confirmed in more patients in the future, and the best method to attain this objective is well-designed multicenter big-sample prospective study.
| 1. | Ippolito D, Colombo M, Trattenero C, Bonaffini PA, Talei Franzesi C, Fior D, Sironi S. Diagnostic Value of Semiquantitative Analysis of Dynamic Susceptibility Contrast Magnetic Resonance Imaging with GD-EOB-DTPA in Focal Liver Lesions Characterization: A Feasibility Study. Gastroenterol Res Pract. 2015;2015:630273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Erra P, Puglia M, Ragozzino A, Maurea S, Liuzzi R, Sabino G, Barbuto L, Cuocolo A, Imbriaco M. Appearance of hepatocellular carcinoma on gadoxetic acid-enhanced hepato-biliary phase MR imaging: a systematic review. Radiol Med. 2015;120:1002-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Xiao YD, Paudel R, Liu H, Zhang B, Ma C, Zhou SK. Gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging: A potential utility for the evaluation of regional liver function impairment following transcatheter arterial chemoembolization. Oncol Lett. 2015;9:1191-1196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Saba L, di Martino M, de Cecco CN, Catalano C, Piga M. Diagnostic confidence of computed tomography and magnetic resonance in focal liver pathology. Eur J Gastroenterol Hepatol. 2015;27:97-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Imbriaco M, De Luca S, Coppola M, Fusari M, Klain M, Puglia M, Mainenti P, Liuzzi R, Maurea S. Diagnostic Accuracy of Gd-EOB-DTPA for Detection Hepatocellular Carcinoma (HCC): A Comparative Study with Dynamic Contrast Enhanced Magnetic Resonance Imaging (MRI) and Dynamic Contrast Enhanced Computed Tomography (CT). Pol J Radiol. 2017;82:50-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Vogl TJ, Kümmel S, Hammerstingl R, Schellenbeck M, Schumacher G, Balzer T, Schwarz W, Müller PK, Bechstein WO, Mack MG. Liver tumors: comparison of MR imaging with Gd-EOB-DTPA and Gd-DTPA. Radiology. 1996;200:59-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 366] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 7. | Akimoto S, Mori H, Fujii T, Furuya K. [Optimal scan timing for Gd-EOB-DTPA enhanced liver dynamic MR imaging]. Nihon Hoshasen Gijutsu Gakkai Zasshi. 2009;65:626-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Kim MY, Kim YK, Park HJ, Park MJ, Lee WJ, Choi D. Diagnosis of focal liver lesions with gadoxetic acid-enhanced MRI: is a shortened delay time possible by adding diffusion-weighted imaging? J Magn Reson Imaging. 2014;39:31-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Esterson YB, Flusberg M, Oh S, Mazzariol F, Rozenblit AM, Chernyak V. Improved parenchymal liver enhancement with extended delay on Gd-EOB-DTPA-enhanced MRI in patients with parenchymal liver disease: associated clinical and imaging factors. Clin Radiol. 2015;70:723-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | van Kessel CS, Veldhuis WB, van den Bosch MA, van Leeuwen MS. MR liver imaging with Gd-EOB-DTPA: a delay time of 10 minutes is sufficient for lesion characterisation. Eur Radiol. 2012;22:2153-2160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Liang M, Zhao J, Xie B, Li C, Yin X, Cheng L, Wang J, Zhang L. MR liver imaging with Gd-EOB-DTPA: The need for different delay times of the hepatobiliary phase in patients with different liver function. Eur J Radiol. 2016;85:546-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Chou CT, Chen YL, Wu HK, Chen RC. Characterization of hyperintense nodules on precontrast T1-weighted MRI: utility of gadoxetic acid-enhanced hepatocyte-phase imaging. J Magn Reson Imaging. 2011;33:625-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Bartolozzi C, Battaglia V, Bargellini I, Bozzi E, Campani D, Pollina LE, Filipponi F. Contrast-enhanced magnetic resonance imaging of 102 nodules in cirrhosis: correlation with histological findings on explanted livers. Abdom Imaging. 2013;38:290-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 14. | Zhang T, Lu J, Zhang X, Liang H, Miao X, Jiang J, Ding D, Yang X. [Diagnostic value of hepatobiliary phase imaging with GD-EOB-DTPA for hepatocellular carcinomas in cirrhosis]. Zhonghua Yi Xue Za Zhi. 2014;94:517-520. [PubMed] |
| 15. | Yoneda N, Matsui O, Kitao A, Kozaka K, Gabata T, Sasaki M, Nakanuma Y, Murata K, Tani T. Beta-catenin-activated hepatocellular adenoma showing hyperintensity on hepatobiliary-phase gadoxetic-enhanced magnetic resonance imaging and overexpression of OATP8. Jpn J Radiol. 2012;30:777-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Fujiwara H, Sekine S, Onaya H, Shimada K, Mikata R, Arai Y. Ring-like enhancement of focal nodular hyperplasia with hepatobiliary-phase Gd-EOB-DTPA-enhanced magnetic resonance imaging: radiological-pathological correlation. Jpn J Radiol. 2011;29:739-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Nakamura S, Awai K, Utsunomiya D, Namimoto T, Nakaura T, Morita K, Yamashita Y. Chronological evaluation of liver enhancement in patients with chronic liver disease at Gd-EOB-DTPA-enhanced 3-T MR imaging: does liver function correlate with enhancement? Jpn J Radiol. 2012;30:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Lee NK, Kim S, Lee JW, Lee SH, Kang DH, Kim GH, Seo HI. Biliary MR imaging with Gd-EOB-DTPA and its clinical applications. Radiographics. 2009;29:1707-1724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 161] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 19. | Motosugi U, Ichikawa T, Sou H, Sano K, Tominaga L, Kitamura T, Araki T. Liver parenchymal enhancement of hepatocyte-phase images in Gd-EOB-DTPA-enhanced MR imaging: which biological markers of the liver function affect the enhancement? J Magn Reson Imaging. 2009;30:1042-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 201] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 20. | Yamada A, Hara T, Li F, Fujinaga Y, Ueda K, Kadoya M, Doi K. Quantitative evaluation of liver function with use of gadoxetate disodium-enhanced MR imaging. Radiology. 2011;260:727-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 173] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 21. | Sahani DV, Agarwal S, Chung RT. The double-edged sword of functional liver imaging. Radiology. 2012;264:621-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Kudo M. Will Gd-EOB-MRI change the diagnostic algorithm in hepatocellular carcinoma? Oncology. 2010;78 Suppl 1:87-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Chou CT, Chen YL, Su WW, Wu HK, Chen RC. Characterization of cirrhotic nodules with gadoxetic acid-enhanced magnetic resonance imaging: the efficacy of hepatocyte-phase imaging. J Magn Reson Imaging. 2010;32:895-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Tamada T, Ito K, Higaki A, Yoshida K, Kanki A, Sato T, Higashi H, Sone T. Gd-EOB-DTPA-enhanced MR imaging: evaluation of hepatic enhancement effects in normal and cirrhotic livers. Eur J Radiol. 2011;80:e311-e316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 25. | Okada M, Ishii K, Numata K, Hyodo T, Kumano S, Kitano M, Kudo M, Murakami T. Can the biliary enhancement of Gd-EOB-DTPA predict the degree of liver function? Hepatobiliary Pancreat Dis Int. 2012;11:307-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Kim JY, Lee SS, Byun JH, Kim SY, Park SH, Shin YM, Lee MG. Biologic factors affecting HCC conspicuity in hepatobiliary phase imaging with liver-specific contrast agents. AJR Am J Roentgenol. 2013;201:322-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Yamashita T, Kitao A, Matsui O, Hayashi T, Nio K, Kondo M, Ohno N, Miyati T, Okada H, Yamashita T. Gd-EOB-DTPA-enhanced magnetic resonance imaging and alpha-fetoprotein predict prognosis of early-stage hepatocellular carcinoma. Hepatology. 2014;60:1674-1685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 28. | Narita M, Hatano E, Arizono S, Miyagawa-Hayashino A, Isoda H, Kitamura K, Taura K, Yasuchika K, Nitta T, Ikai I. Expression of OATP1B3 determines uptake of Gd-EOB-DTPA in hepatocellular carcinoma. J Gastroenterol. 2009;44:793-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 282] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 29. | Kitao A, Matsui O, Yoneda N, Kozaka K, Shinmura R, Koda W, Kobayashi S, Gabata T, Zen Y, Yamashita T. The uptake transporter OATP8 expression decreases during multistep hepatocarcinogenesis: correlation with gadoxetic acid enhanced MR imaging. Eur Radiol. 2011;21:2056-2066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 209] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 30. | Kitao A, Zen Y, Matsui O, Gabata T, Kobayashi S, Koda W, Kozaka K, Yoneda N, Yamashita T, Kaneko S. Hepatocellular carcinoma: signal intensity at gadoxetic acid-enhanced MR Imaging--correlation with molecular transporters and histopathologic features. Radiology. 2010;256:817-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 285] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 31. | Nassif A, Jia J, Keiser M, Oswald S, Modess C, Nagel S, Weitschies W, Hosten N, Siegmund W, Kühn JP. Visualization of hepatic uptake transporter function in healthy subjects by using gadoxetic acid-enhanced MR imaging. Radiology. 2012;264:741-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 32. | Miura T, Ban D, Tanaka S, Mogushi K, Kudo A, Matsumura S, Mitsunori Y, Ochiai T, Tanaka H, Tanabe M. Distinct clinicopathological phenotype of hepatocellular carcinoma with ethoxybenzyl-magnetic resonance imaging hyperintensity: association with gene expression signature. Am J Surg. 2015;210:561-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 33. | Kobayashi S, Matsui O, Gabata T, Koda W, Minami T, Ryu Y, Kozaka K, Kitao A. Relationship between signal intensity on hepatobiliary phase of gadolinium ethoxybenzyl diethylenetriaminepentaacetic acid (Gd-EOB-DTPA)-enhanced MR imaging and prognosis of borderline lesions of hepatocellular carcinoma. Eur J Radiol. 2012;81:3002-3009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Sakaguchi T S- Editor: Chen K L- Editor: Wang TQ E- Editor: Huang Y