Published online Apr 14, 2018. doi: 10.3748/wjg.v24.i14.1562
Peer-review started: February 6, 2018
First decision: February 26, 2018
Revised: March 3, 2018
Accepted: March 7, 2018
Article in press: March 6, 2018
Published online: April 14, 2018
Processing time: 64 Days and 22.9 Hours
To provide an updated assessment of the safety and efficacy of enhanced recovery after surgery (ERAS) protocols in elective gastric cancer (GC) surgery.
PubMed, Medline, EMBASE, World Health Organization International Trial Register, and Cochrane Library were searched up to June 2017 for all available randomized controlled trials (RCTs) comparing ERAS protocols and standard care (SC) in GC surgery. Thirteen RCTs, with a total of 1092 participants, were analyzed in this study, of whom 545 underwent ERAS protocols and 547 received SC treatment.
No significant difference was observed between ERAS and control groups regarding total complications (P = 0.88), mortality (P = 0.50) and reoperation (P = 0.49). The incidence of pulmonary infection was significantly reduced (P = 0.03) following gastrectomy. However, the readmission rate after GC surgery nearly tripled under ERAS (P = 0.009). ERAS protocols significantly decreased the length of postoperative hospital stay (P < 0.00001) and medical costs (P < 0.00001), and accelerated bowel function recovery, as measured by earlier time to the first flatus (P = 0.0004) and the first defecation (P < 0.0001). Moreover, ERAS protocols were associated with a lower level of serum inflammatory response, higher serum albumin, and superior short-term quality of life (QOL).
Collectively, ERAS results in accelerated convalescence, reduction of surgical stress and medical costs, improved nutritional status, and better QOL for GC patients. However, high-quality multicenter RCTs with large samples and long-term follow-up are needed to more precisely evaluate ERAS in radical gastrectomy.
Core tip: Enhanced recovery after surgery (ERAS) has emerged as an optimal perioperative strategy for improving clinical outcomes in gastric cancer surgery. However, numerous controversies exist with regard to ERAS practice after gastrectomy. To our knowledge, this study is the largest meta-analysis of randomized controlled trials to date, incorporating 1092 participants, of whom 545 received ERAS protocols and 547 received standard care, to assess the role of ERAS for radical gastrectomy. Our review clarified that ERAS results in accelerated convalescence, reduction of surgical stress and medical costs, improved nutritional status, and better quality of life for gastric cancer patients.
- Citation: Wang LH, Zhu RF, Gao C, Wang SL, Shen LZ. Application of enhanced recovery after gastric cancer surgery: An updated meta-analysis. World J Gastroenterol 2018; 24(14): 1562-1578
- URL: https://www.wjgnet.com/1007-9327/full/v24/i14/1562.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i14.1562
Enhanced recovery after surgery (ERAS), or fast-track surgery program, which was pioneered by Kehlet and Wilmore in the late 1990s, intends to attenuate surgical stress and accelerate postoperative functional recovery[1,2]. ERAS protocols involve a series of perioperative evidence-based interventions, the core elements of which include preoperative short fasting and carbohydrate-loaded fluids, intraoperative epidural anesthesia, minimally invasive procedures and fluid restriction, postoperative pain management, nutritional care and early ambulation[3-5]. Multimodal optimizing perioperative procedures were explored initially in the setting of elective colorectal resections, resulting in a significant reduction in overall hospital stay from 8-12 d to 2-5 d under the standard discharge criteria for conventional care[6,7]. Since then, ERAS concepts have become widely recognized and applied gradually to clinical practice. Currently, accumulating evidence highlights that the implementation of ERAS protocols in multiple surgical disciplines significantly reduces morbidity and mortality, while improving clinical outcomes without compromising patient safety[8-10].
Gastric cancer (GC) remains a major health problem in China and worldwide, and radical gastrectomy remains the most likely approach to cure GC. However, conventional perioperative care is associated with a high risk of morbidity after radical surgery, ranging from 12.5% to 39%[11-13]. Moreover, due to malnutrition of patients with gastric neoplasms and chronic comorbidities, perioperative mortality can reach up to 8.8%[14]. Postoperative complications result in prolonged inflammatory response, which is considered to have a negative influence not only on the overall survival (OS) but also on the disease-specific mortality of patients undergoing gastrectomy, even if the carcinoma is radically resected[15].
Given the strong evidence and recommendations for colorectal cancer, the application of ERAS protocols for gastrectomy procedures has been investigated in several studies[16-19]. ERAS principles combined with laparoscopic treatment for GC lead to satisfactory clinical outcomes[20-22], even in elderly patients[23,24]. Several meta-analyses have revealed that ERAS pathways in GC patients reduce the duration of hospital stay and medical costs without significantly increasing complications and hospital readmission[25-28], and the ERAS Society issued consensus guidelines for perioperative care after elective gastrectomy for GC in 2014[29].
However, there still remain numerous controversies, limitations and difficulties in ERAS practice after gastrectomy. Following the recent publication of two related high-level randomized controlled trials (RCTs)[22,30], we conducted an updated systematic review and meta-analysis to thoroughly assess the safety and efficacy of ERAS application in GC patients.
A comprehensive literature search in PubMed, Medline, EMBASE, World Health Organization International Trial Registry platform, and Cochrane Library was performed, until June 2017, independently to identify all available publications comparing the ERAS program with standard perioperative care (SC) for GC patients undergoing gastrectomy. The medical subject heading (MeSH) terms and free text terms searched for, individually and in combination, were as follows: “fast track surgery” OR “accelerated rehabilitation” OR “enhanced recovery” OR “ERAS” OR “multimodal perioperative care” AND “gastric cancer” OR “stomach carcinoma” OR “gastrectomy” OR “gastric resection.” This search strategy was able to identify all potential publications involving humans, without language restriction. Reference lists of all eligible articles were also scrutinized to identify any other related studies. Furthermore, bibliographies of systematic reviews or meta-analyses on this issue were hand-searched for additional articles that the electronic retrieval failed to capture.
The inclusion criteria for this study were: (1) evaluation of ERAS in comparison with traditional SC; (2) RCTs; (3) detailed patient data and outcomes available; (4) ERAS protocols composed of at least eight elements from consensus guidelines[29]; and (5) follow-up for at least 14 d after discharge. When more than one study reporting the same patient cohort was included in several publications, only the most recent or complete study was included.
The exclusion criteria were as follows: (1) non-comparative studies; (2) case-controlled trials, cohort studies, or retrospective studies; (3) application of less than eight items of ERAS; (4) no follow-up after discharge; and (5) other documentations that did not meet the inclusion criteria.
Following identification of citations from all potentially eligible studies, two investigators independently retrieved the full-text articles according to the inclusion criteria. Any discrepancies or divergences concerning inclusion were settled through discussion with a third reviewer until consensus was reached.
Data were extracted using a double-extraction method from each eligible study by the two investigators. Outcomes included morbidity, mortality, rates of readmission and reoperation, length of postoperative hospital stay (POHS), duration of flatus and defecation, medical costs, and postoperative inflammatory response and nutritional status, such as determined by serum C-reactive protein (CRP), interleukin-6 (IL-6) and serum albumin (ALB) concentrations.
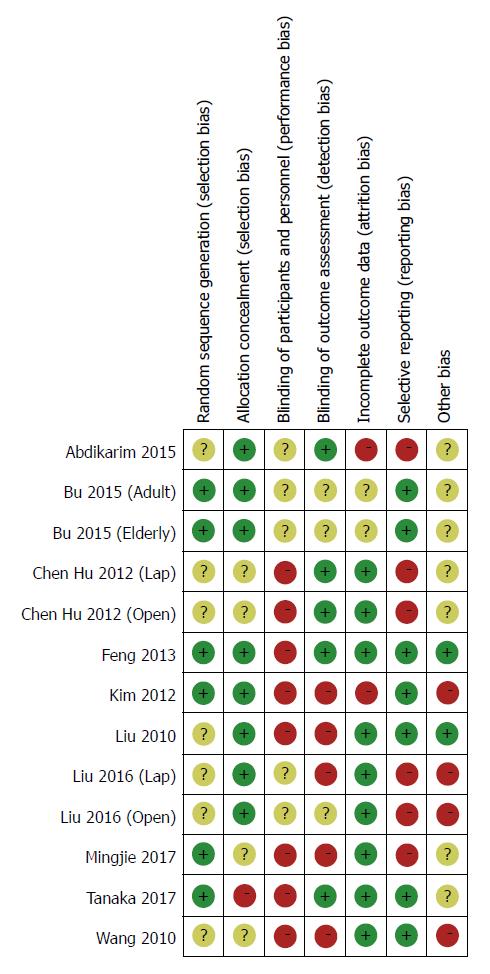
Another two investigators separately assessed the quality of identified RCTs using the criteria addressed in the Cochrane Collaboration[31]. The evaluation indices contained several aspects across randomization, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other bias. Risk of bias in each domain listed was graded as “high risk,” “low risk,” or “unclear.”
Statistical analysis was performed using the software package Review Manager Version 5.3.3 (Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark) and STATA version 12 (Stata Corp LP, College Station, TX, United States). Pooled risk ratio (RR) with 95% confidence interval (CI) was utilized to analyze dichotomous data, while continuous data were analyzed as mean differences (MDs) with 95%CIs. Heterogeneity was evaluated using the chi-square test, for which P < 0.1 was considered statistically significant. The I² value was used to quantify the impact of heterogeneity on each analysis. If the test of heterogeneity was statistically significant, the random-effects model was used; otherwise, a fixed-effects model was used. When the study did not report specific values for mean and standard deviation (SD), these were estimated using median and range based on the methods previously described[32]. In short, the median was used as a substitute for the mean. When the sample size was greater than 70, SD was estimated as range/6, and when the sample size was 15-69, SD was calculated as range/4. In the case where the interquartile range (IQR) was available, the range was estimated to be the median ± IQR.
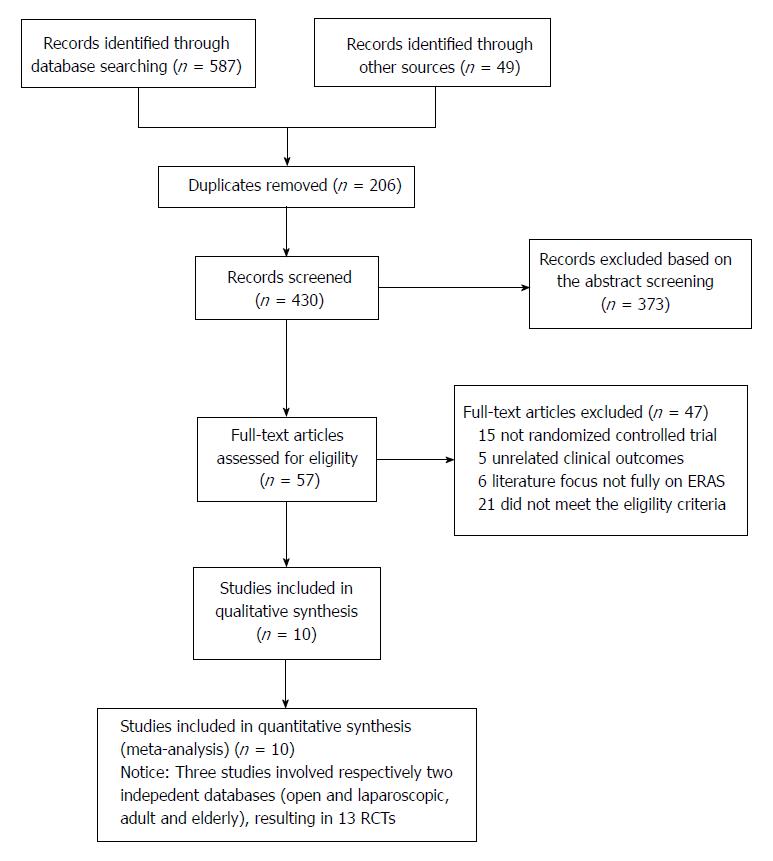
The flow chart for the selection of literature according to the predefined retrieval strategies is shown in Figure 1. Ten studies[21-24,30,33-37] published between 2010 and 2017 met the inclusion criteria. Two studies[24,34] consisted of four groups comparing ERAS protocols and SC in laparoscopic or open radical gastrectomy, respectively, for stomach cancer, while another[23] comprised four groups comparing ERAS protocols and SC in adults (aged 45-74 years) or elderly individuals (aged 75-89 years) undergoing open gastrectomy for GC. These three studies were considered to be six independent studies with reference to previous reports[26,28]. Consequently, 13 RCTs from these 10 studies were included in the current systematic review and meta-analysis.
The main characteristics of the included studies are detailed in Table 1. All studies were from a single center involving a total of 1092 participants, of whom 545 underwent the ERAS protocol and 547 received SC treatment. The sample size ranged from 41 to 256, and four studies contained more than 100 patients[22,23,30,33]. Table 2 lists the relevant elements involved in these studies regarding the implementation of ERAS pathways based on the consensus conducted in RCTs. Surgical procedures for GC with curative intent involved proximal gastrectomy, distal gastrectomy, and total gastrectomy. These included studies were implemented predominantly in Asia (China, South Korea, and Japan). Assessment of the risk of bias across all included studies is presented in Figure 2, most of which were of moderate quality. Blinding was the main risk of bias among these RCTs, as it was not easy to comply with double blinding in such procedural trials.
| Study | Year | Sample size | Age in yr | Sex, male/female | Approach | Neoadjuvant chemotherapy | Follow-up(d) | |||
| ERAS | SC | ERAS | SC | ERAS | SC | |||||
| Abdikarim et al[21] | 2015 | 30 | 31 | 63 ± 12 | 62 ± 11 | 21/9 | 20/11 | Lap | No | 30 |
| Bu et al[23]-Adult | 2015 | 64 | 64 | 62.4 ± 7.8 | 63.0 ± 7.4 | 31/33 | 35/29 | Open | No | 30 |
| Bu et al[23]-Elderly | 2015 | 64 | 64 | 80.1 ± 4.0 | 79.6 ± 3.5 | 37/27 | 40/24 | Open | No | 30 |
| Chen Hu et al[34]-Lap | 2012 | 19 | 22 | 59 (49-71) | 62.5 (45-72) | 10/9 | 10/12 | Lap | No | 28 |
| Chen Hu et al[34]-Open | 2012 | 21 | 20 | 62.5 (45-72) | 64.5 (49-75) | 9/12 | 12/8 | Open | No | 28 |
| Feng et al[33] | 2013 | 59 | 60 | 55.0 ± 11.4 | 55.8 ± 10.1 | 41/18 | 44/16 | Open | No | 28 |
| Kim et al[35] | 2012 | 22 | 22 | 52.6 ± 11.6 | 57.5 ± 14.5 | 13/9 | 15/7 | Lap | - | 14 |
| Liu et al[36] | 2010 | 33 | 30 | 60.7 ± 9.7 | 61.9 ± 8.3 | 18/15 | 16/14 | Open | No | 30 |
| Liu et al[24]-Lap | 2016 | 21 | 21 | 69.2 ± 5.1 | 70.3 ± 5.8 | 10/11 | 12/9 | Lap | No | 30 |
| Liu et al[24]-Open | 2016 | 21 | 21 | 67.8 ± 3.9 | 68.6 ± 4.9 | 9/12 | 11/10 | Open | No | 30 |
| Mingjie et al[22] | 2017 | 73 | 76 | 61 (40-75) | 63 (35-75) | 48/25 | 50/26 | Lap | No | 30 |
| Tanaka et al[30] | 2017 | 73 | 69 | 68 (29-85) | 67 (44-85) | 49/24 | 49/20 | Lap/Open | No | 30 |
| Wang et al[37] | 2010 | 45 | 47 | 58.8 ± 9.7 | 56.9 ± 9.1 | 32/13 | 29/18 | Open | No | 28 |
| Study | Year | No bowel preparation | Carbohydrate loading | No routine use of abdominal drainage | Fluid restriction | Pain management | Early mobilization | Early feeding | Others | No. of ERAS elements |
| Abdikarim et al[21] | 2015 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 11 |
| Bu et al[23] | 2015 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 14 |
| Chen Hu et al[34] | 2012 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 13 |
| Feng et al[33] | 2013 | - | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 9 |
| Kim et al[35] | 2012 | Yes | Yes | - | - | Yes | Yes | Yes | Yes | 10 |
| Liu et al[36] | 2010 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 12 |
| Liu et al[24] | 2016 | Yes | Yes | - | Yes | Yes | Yes | Yes | Yes | 11 |
| Mingjie et al[22] | 2017 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 13 |
| Tanaka et al[30] | 2017 | Yes | Yes | Yes | - | Yes | Yes | Yes | Yes | 22 |
| Wang et al[37] | 2010 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 14 |
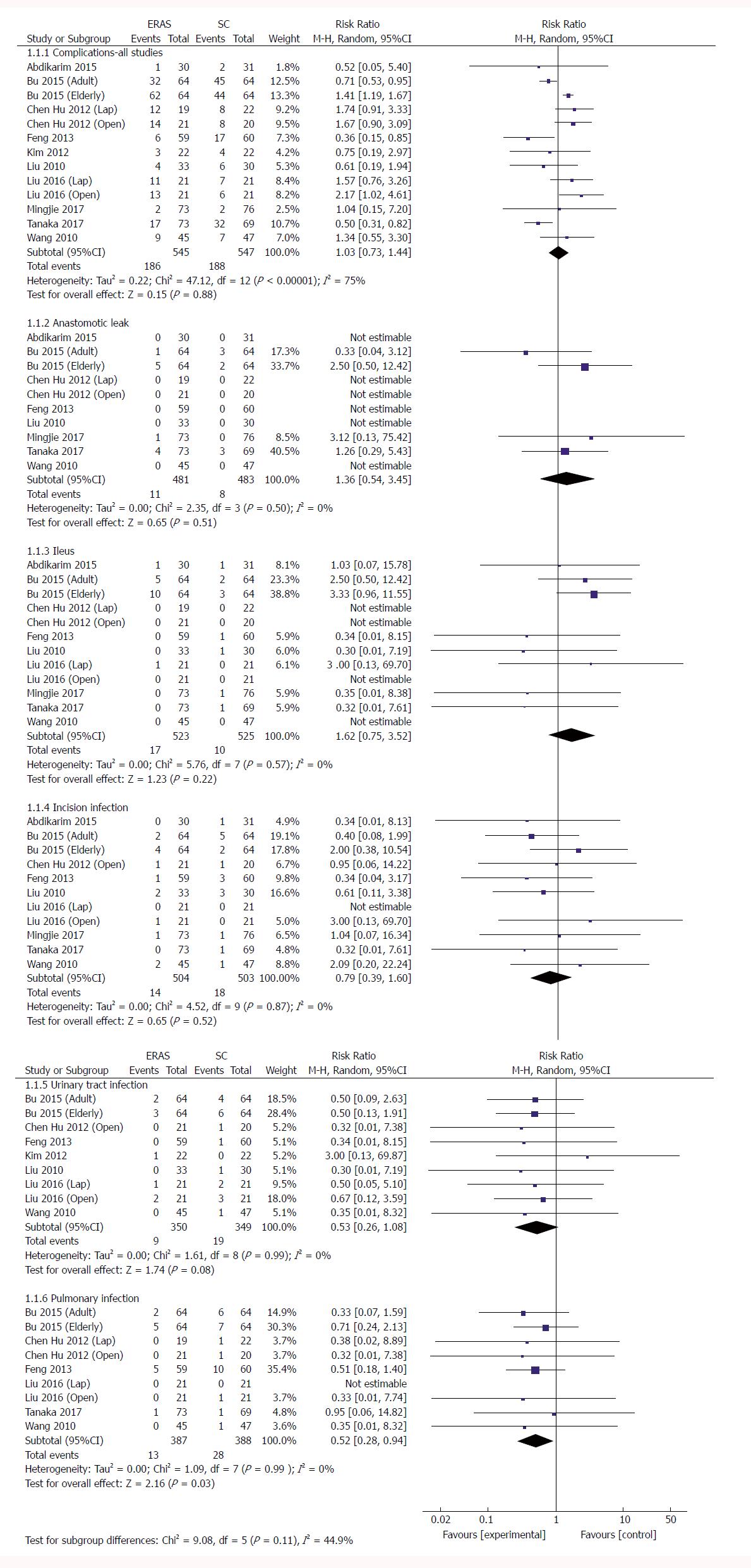
Total complications: No significant difference was demonstrated between ERAS and the control group in the 13 RCTs regarding the incidence of total complications following gastrectomy (RR: 1.03, 95%CI: 0.73-1.44, P = 0.88) (Figure 3 and Table 3), but there was significant heterogeneity among these studies (χ2 = 47.12, I2 = 75%, P < 0.00001). In five RCTs reporting a laparoscopic approach for GC[21,22,24,34,35], no significant difference in postoperative morbidity was found between the ERAS and SC groups (RR: 1.44, 95%CI: 0.93-2.23, P = 0.10), and no heterogeneity was observed (χ2 = 2.18, P = 0.70; I2 = 0). Similarly, in the open surgery RCTs[23,24,33,34,36,37], ERAS pathways did not increase the surgical complications (RR: 1.05, 95%CI: 0.68-1.63, P = 0.81), and significant heterogeneity was observed (χ2 = 31.10, P < 0.0001; I2 = 81%). However, three RCTs in the elderly[23,24] demonstrated that the incidence of complications was significantly higher in the ERAS arm than in the SC arm (RR: 1.45, 95%CI: 1.23-1.70, P < 0.00001), and no heterogeneity was found in the elderly (χ2 = 1.51, P = 0.47; I2 = 0).
| Subgroup | Studies, n | Participants, n | Statistical method | Effect estimate | Heterogeneity | |
| I2 | P value | |||||
| Total complications | 13 | 1092 | Risk ratio (M-H, random, 95%CI) | 1.03 [0.73, 1.44] | 75% | < 0.00001 |
| Anastomotic leak | 10 | 964 | Risk ratio (M-H, random, 95%CI) | 1.36 [0.54, 3.45] | 0 | 0.50 |
| Ileus | 12 | 1048 | Risk ratio (M-H, random, 95%CI) | 1.62 [0.75, 3.52] | 0 | 0.57 |
| Incision infection | 11 | 1007 | Risk ratio (M-H, random, 95%CI) | 0.79 [0.39, 1.60] | 0 | 0.87 |
| Urinary tract infection | 9 | 699 | Risk ratio (M-H, random, 95%CI) | 0.53 [0.26, 1.08] | 0 | 0.99 |
| Pulmonary infection | 9 | 775 | Risk ratio (M-H, random, 95%CI) | 0.52 [0.28, 0.94] | 0 | 0.99 |
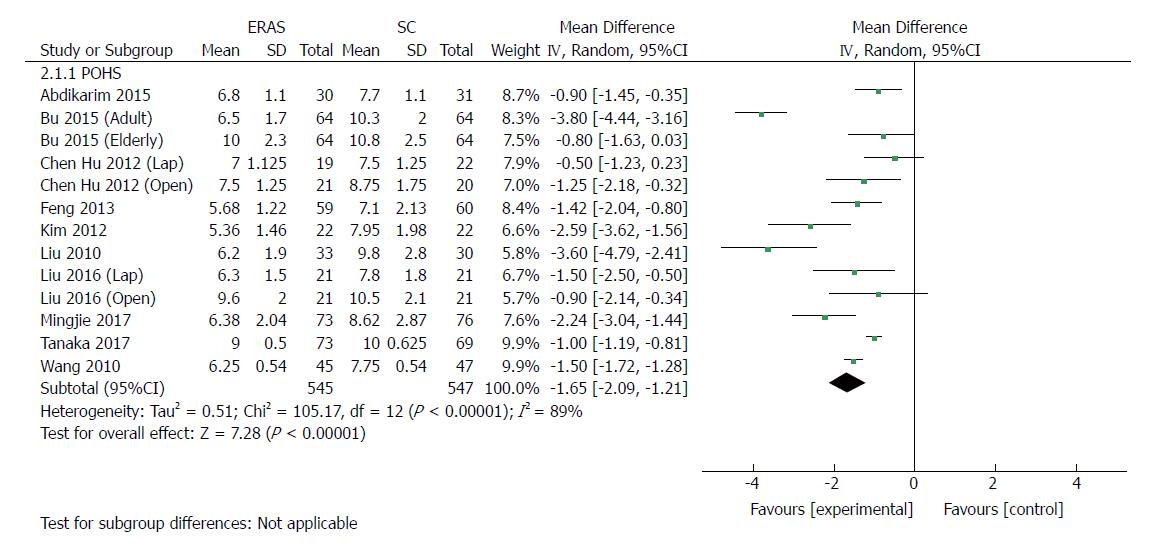
| Postoperative hospital stay | 13 | 1092 | Mean difference (IV, random, 95%CI) | -1.65 [-2.09, -1.21] | 89% | < 0.00001 |
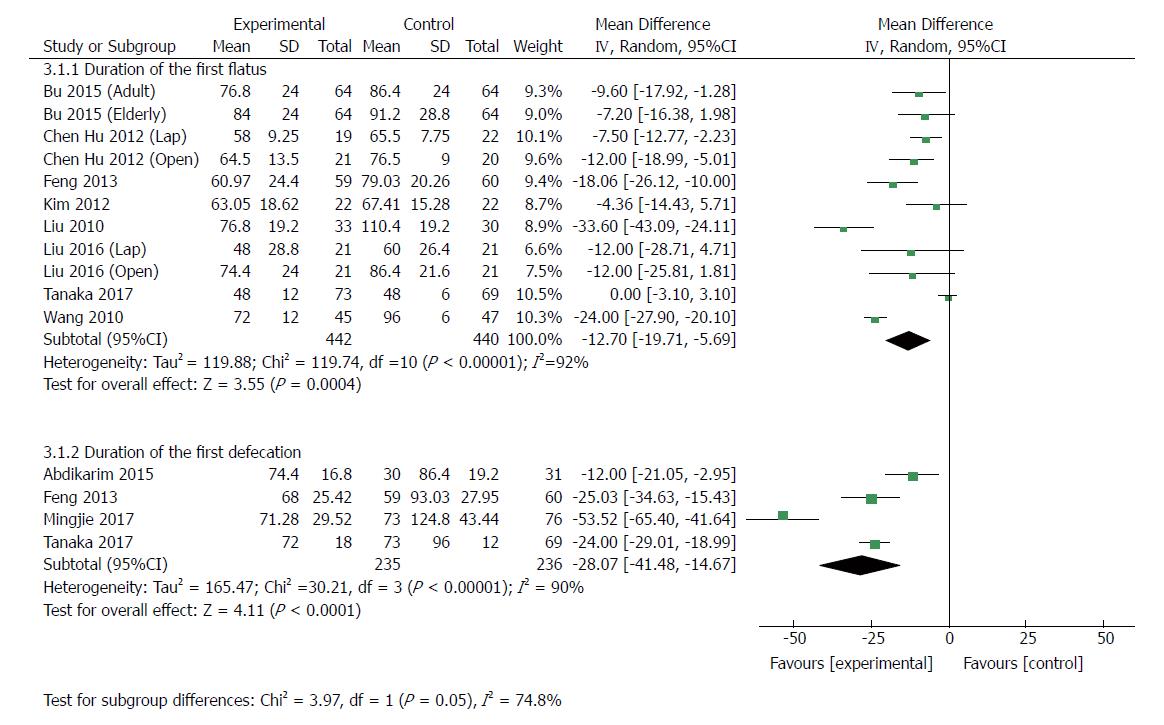
| Duration of first flatus | 11 | 882 | Mean difference (IV, random, 95%CI) | -12.70 [-19.71, -5.69] | 92% | < 0.00001 |
| Duration of first defecation | 4 | 471 | Mean difference (IV, random, 95%CI) | -28.07 [-41.48, -14.67] | 90% | < 0.00001 |
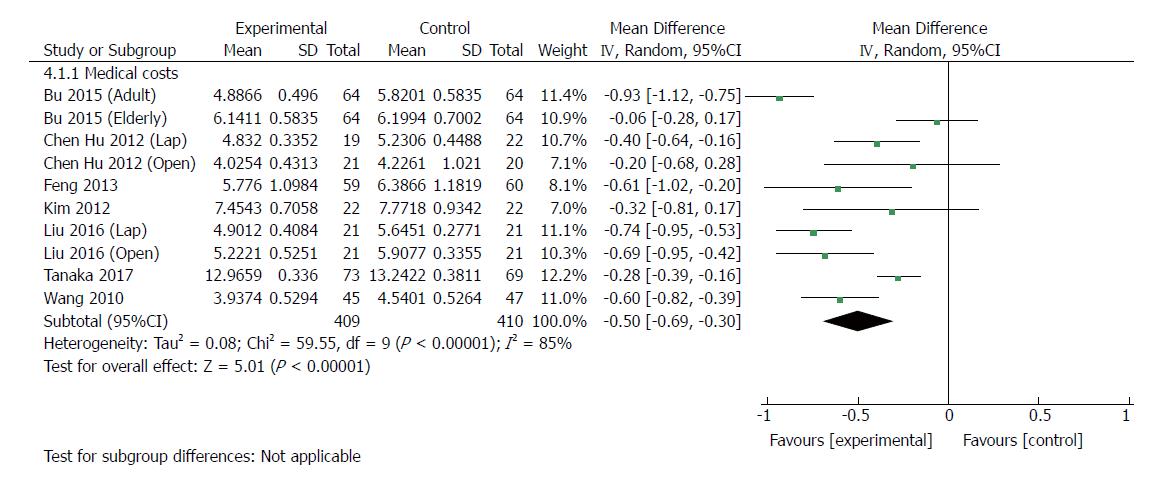
| Medical costs | 10 | 819 | Mean difference (IV, random, 95%CI) | -0.50 [-0.69, -0.30] | 85% | < 0.00001 |
| CRP | ||||||
| POD1 | 8 | 514 | Mean difference (IV, random, 95%CI) | -14.81 [-21.42, -8.21] | 72% | 0.0007 |
| POD4 | 6 | 378 | Mean difference (IV, random, 95%CI) | -19.81 [-29.64, -9.98] | 64% | 0.02 |
| POD7 | 5 | 258 | Mean difference (IV, random, 95%CI) | -21.36 [-28.81, -13.91] | 74% | 0.004 |
| IL-6 | ||||||
| POD1 | 4 | 239 | Mean difference (IV, random, 95%CI) | -61.22 [-114.58, -7.86] | 99% | < 0.00001 |
| POD4 | 3 | 147 | Mean difference (IV, random, 95%CI) | -31.50 [-55.63, -7.38] | 96% | < 0.00001 |
| POD7 | 3 | 176 | Mean difference (IV, random, 95%CI) | -26.62 [-34.23, -19.01] | 89% | 0.0001 |
| ALB | ||||||
| POD1 | 2 | 84 | Mean difference (IV, random, 95%CI) | 0.24 [-0.89, 1.36] | 0 | 0.79 |
| POD4 | 4 | 166 | Mean difference (IV, random, 95%CI) | 3.27 [2.24, 4.30] | 23% | 0.27 |
| POD7 | 4 | 166 | Mean difference (IV, random, 95%CI) | 5.68 [3.31, 8.05] | 83% | 0.0005 |
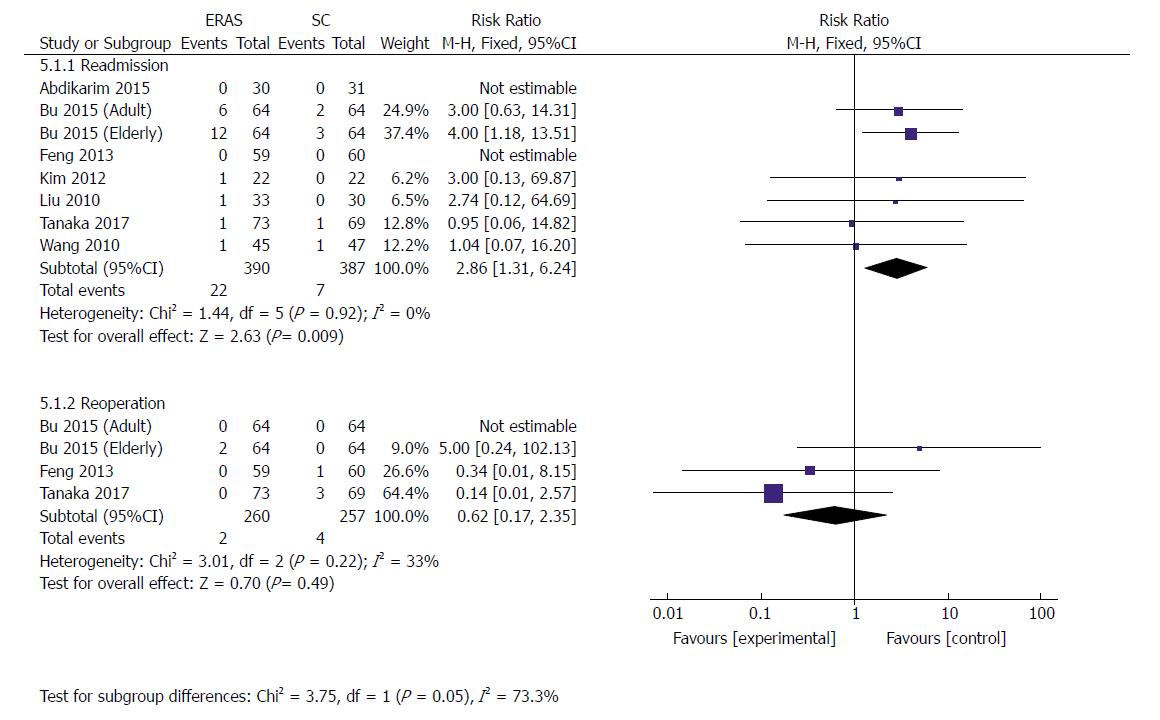
| Readmission | 8 | 777 | Risk ratio (M-H, Fixed, 95%CI) | 2.86 [1.31, 6.24] | 0 | 0.92 |
| Reoperation | 3 | 517 | Risk ratio (M-H, Fixed, 95%CI) | 0.62 [0.17, 2.35] | 33% | 0.22 |
| Quality of life | 2 | 136 | Std. mean difference (IV, Fixed, 95%CI) | -0.46 [-0.80, -0.12] | 36% | 0.21 |
Anastomotic leak: Ten RCTs[21-23,30,33,34,36,37] (964 patients) provided data on anastomotic leaks, whereby 2.3% (11/481 patients) in the ERAS group and 1.7% (8/483) in the SC group had an anastomotic leak. Pooling the results indicated that ERAS did not increase the incidence of anastomotic leaks compared with conventional care (RR: 1.36, 95%CI: 0.54-3.45, P = 0.51) (Figure 3), and heterogeneity was excluded among these trials (χ2 = 2.35, P = 0.50; I2 = 0).
Ileus: Twelve RCTs[21-24,30,33,34,36,37] (1048 patients) provided data regarding ileus: 3.3% (17/523 patients) in the ERAS group, and 1.9% (10/525) in the SC group had ileus. Pooling the results indicated that ERAS did not increase ileus compared with SC (RR: 1.62, 95%CI: 0.75-3.52, P = 0.22) (Figure 3), and no heterogeneity was observed among these trials (χ2 = 5.76, P = 0.57; I2 = 0).
Incision infection: Eleven RCTs[21-24,30,33,34,36,37] (1007 patients) reported incision infection, amounting to 2.8% (14/504 patients) in the ERAS group and 3.6% (18/503) in the SC group. Pooling the results indicated that ERAS did not increase incision infection compared with conventional care (RR: 0.79, 95%CI: 0.39-1.60, P = 0.52) (Figure 3), and there was no heterogeneity among these studies (χ2 = 4.52, P = 0.87; I2 = 0).
Urinary tract infection: Nine RCTs[23,24,33-37] (699 patients) provided data regarding urinary tract infection, which was observed in 2.6% (9/350 patients) in the ERAS group and 5.4% (19/349) in the SC group. Pooling the results indicated that ERAS did not increase urinary tract infection compared with conventional care (RR: 0.53, 95%CI: 0.26-1.08, P = 0.08) (Figure 3), and heterogeneity was excluded among these studies (χ2 = 1.61, P = 0.99; I2 = 0).
Pulmonary infection: Nine RCTs[23,24,30,33,34,37] (775 patients) reported pulmonary infection, which affected 3.4% (13/387 patients) in the ERAS group and 7.2% (28/388) in the SC group. Pooling the results indicated that ERAS decreased significantly the incidence of pulmonary infection compared with conventional care (RR: 0.52, 95%CI: 0.28-0.94, P = 0.03) (Figure 3), and there was no heterogeneity among these studies (χ2 = 1.09, P = 0.99; I2 = 0).
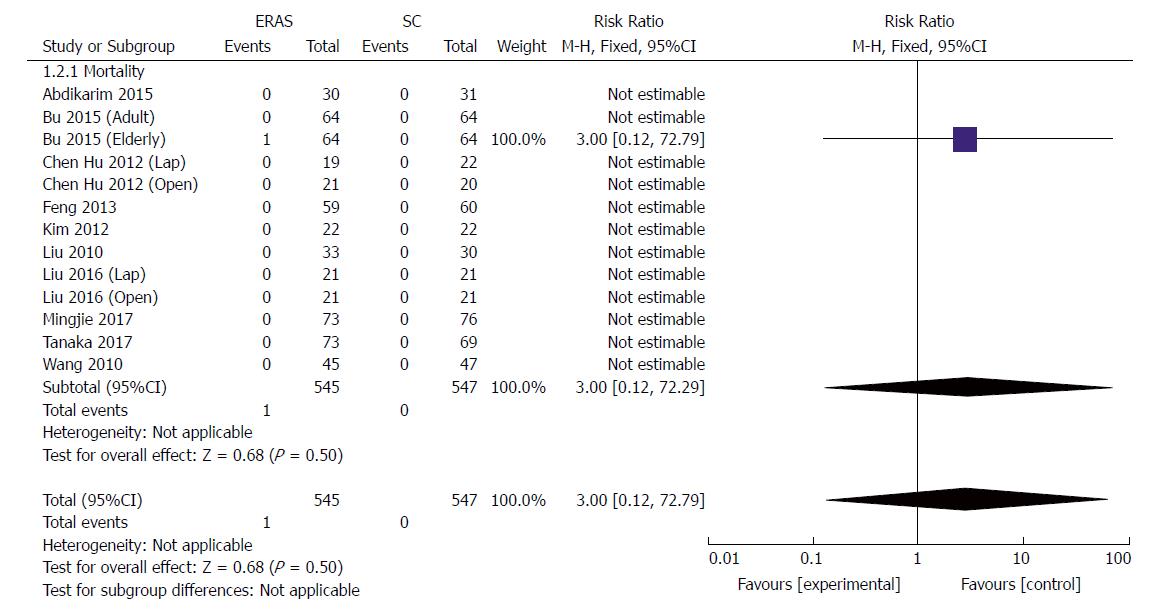
All studies reported short-term mortality after GC surgery; one patient (1/64) died of severe abdominal cavity infection in the elderly group[23]. No cases of death associated with surgery occurred in other studies during short-term follow-up. Pooling the results suggested that ERAS did not increase mortality compared with conventional care (RR: 3.0, 95%CI: 0.12-72.29, P = 0.50) (Figure 4).
All included RCTs (1092 patients) reported POHS. Ten of these studies reported a significant reduction of POHS in the ERAS group, and three reported no significant difference. The elderly group in Bu’s report[23], the laparoscopic group in Chen Hu’s study[34], and the open group of Liu’s report[24] demonstrated that patients receiving rapid rehabilitation care had POHS similar to that of the traditional care protocol. Meta-analysis revealed a significant reduction in POHS by 1.65 d with the application of the ERAS schemes compared with traditional perioperative care in pooled analysis (MD: -1.65, 95%CI: -2.09 to -1.21, P < 0.00001) (Figure 5), and the heterogeneity was significant among these studies (χ2 = 105.17, P < 0.00001; I2 = 89%). Laparoscopic surgery combined with ERAS[21,22,24,34,35] markedly reduced POHS compared with laparoscopic surgery alone (MD: -1.49, 95%CI: -2.25 to -0.74, P < 0.0001), and the heterogeneity was significant (χ2 = 18.21, P = 0.001; I2 = 78%). Similarly, there was a significant reduction in POHS observed in open surgery with ERAS[23,24,33,34,36,37] compared with open surgery alone (MD: -1.89, 95%CI: -2.69 to -1.09, P < 0.00001), and the heterogeneity was also significant (χ2 = 61.54, P < 0.00001; I2 = 90%).
Eleven RCTs[23,24,30,33-37] (882 patients) analyzed the duration of first flatus. Recovery of gut function was earlier in ERAS groups, as shown by shorter duration of the first flatus and first defecation. The MD for duration of first flatus was -12.70 (95%CI: -19.71 to -5.69, P = 0.0004), but the heterogeneity was significant among these studies (χ2 = 119.74, I2 = 92%, P < 0.0001) (Figure 6). In the patients undergoing laparoscopic gastrectomy[24,34,35], the duration of the first flatus of patients in the ERAS group was 7.20 h less than that in the control group (MD: -7.20, 95%CI: -11.70 to -2.70, P = 0.002), and there was no heterogeneity among these studies (χ2 = 0.64, P = 0.73; I2 = 0). Similarly, the first flatus was significantly earlier in the ERAS group than in the SC group (MD: -14.47, 95%CI: -23.61 to -5.33, P = 0.002) among patients undergoing open surgery[23,24,33,34,36,37], but the heterogeneity was significant (χ2 = 116.69, P < 0.00001; I2 = 94%). Four RCTs[21,22,30,33] (471 patients) reported the duration of first defecation. The MD was -28.07 (95%CI: -41.48 to -14.67, P < 0.0001) (Figure 6), and there was significant heterogeneity among the studies (χ2 = 30.21, P < 0.00001; I2 = 90%).
Ten RCTs[23,24,30,33-35,37] (819 patients) provided data regarding medical costs. The costs of hospitalization were reported in US dollars (USD) in one trial[37], Japanese yen in one trial[30], and Chinese renminbi (RMB) in six trials. All of the medical care expenses were converted to USD (http://www.xe.com) by use of the exchange rates of the aforementioned currencies on June 28, 2017. The medical costs were significantly lower with ERAS than with traditional care (MD: -5000 USD, 95%CI: -6900 to -3000, P < 0.00001) (Figure 7), and there was significant heterogeneity among trials by using the random-effects model (χ2 = 59.55, P < 0.00001; I2 = 85%). In laparoscopic groups[24,34,35], ERAS significantly decreased the medical costs compared with traditional care (MD: -5200 USD, 95%CI: -8000 to -2500, P = 0.0002), and the heterogeneity was significant (χ2 = 5.58, P = 0.06; I2 = 64%). Similarly, there was a significant reduction in medical costs in open surgery with ERAS[23,24,33,34,37] compared with open surgery alone (MD: -5300, 95%CI: -8300 to -2300, P = 0.0005), and significant heterogeneity was observed (χ2 = 37.63, P < 0.00001; I2 = 87%).
Eight RCTs[21,23,30,34-37] (777 patients) reported data concerning the readmission rate after discharge, whereby 5.6% (22/390) from ERAS groups and 1.8% (7/387) from SC groups had to be readmitted. A higher readmission rate was perceived in the ERAS group than in the control group (RR: 2.86, 95%CI: 1.31-6.24, P = 0.009) (Figure 8). There was no significant heterogeneity observed among these studies (χ2 = 1.44, P = 0.92; I2 = 0). However, sensitivity analysis showed no significant difference in readmission (RR: 2.17, 95%CI: 0.77-6.14, P = 0.14) when excluding the elderly group in Bu’s study[23], and no heterogeneity was observed (χ2 = 0.85, P = 0.93; I2 = 0).
Three RCTs[23,30,36] (517 patients) reported reoperation rates after discharge. Two patients (0.8%) in ERAS groups and four patients (1.6%) in the conventional protocol groups had to undergo reoperation because of serious complications including abdominal infection, intraabdominal bleeding, and pancreatic fistula. There was no statistical difference in the rate of reoperation between the two groups (RR: 0.62, 95%CI: 0.17-2.35, P = 0.49) (Figure 8). Heterogeneity among these studies remained moderate (χ2 = 3.01, P = 0.22; I2 = 33%).
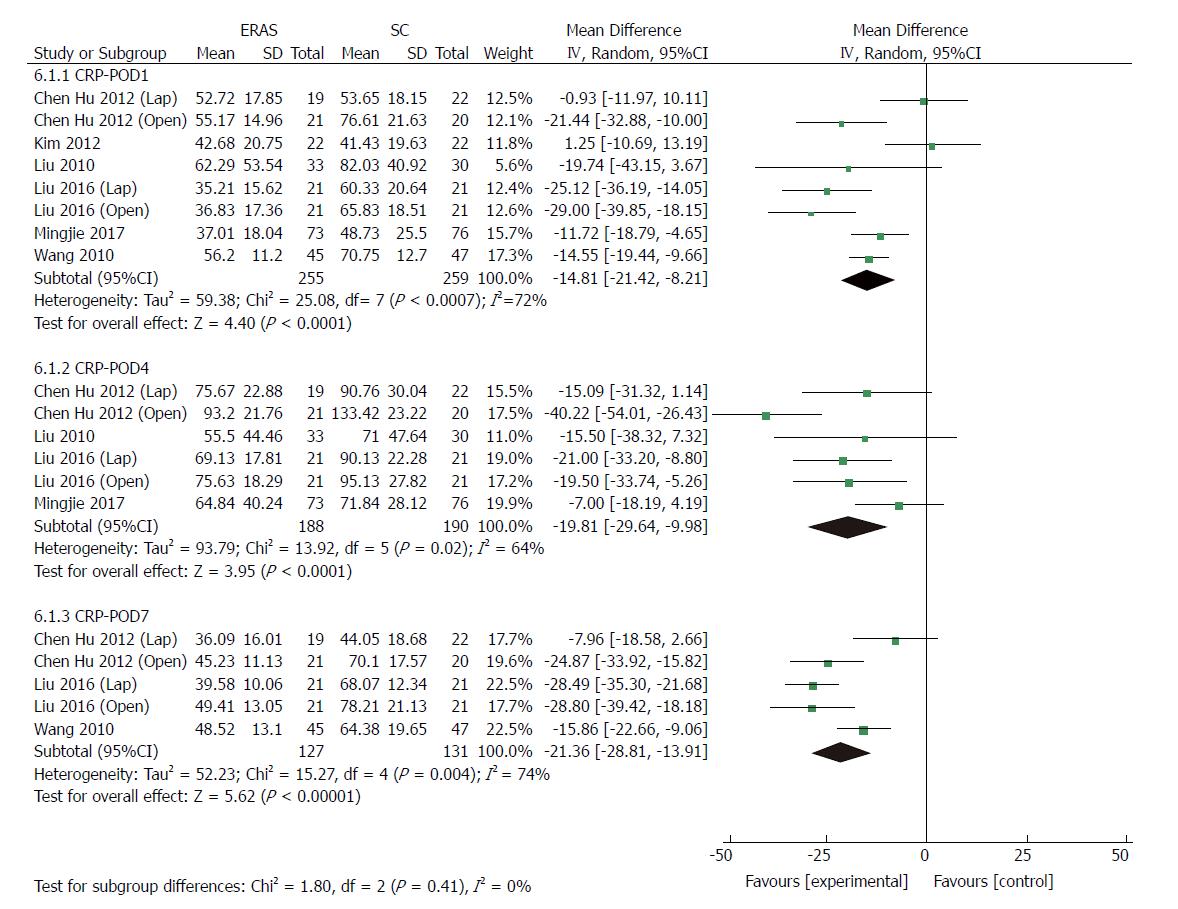
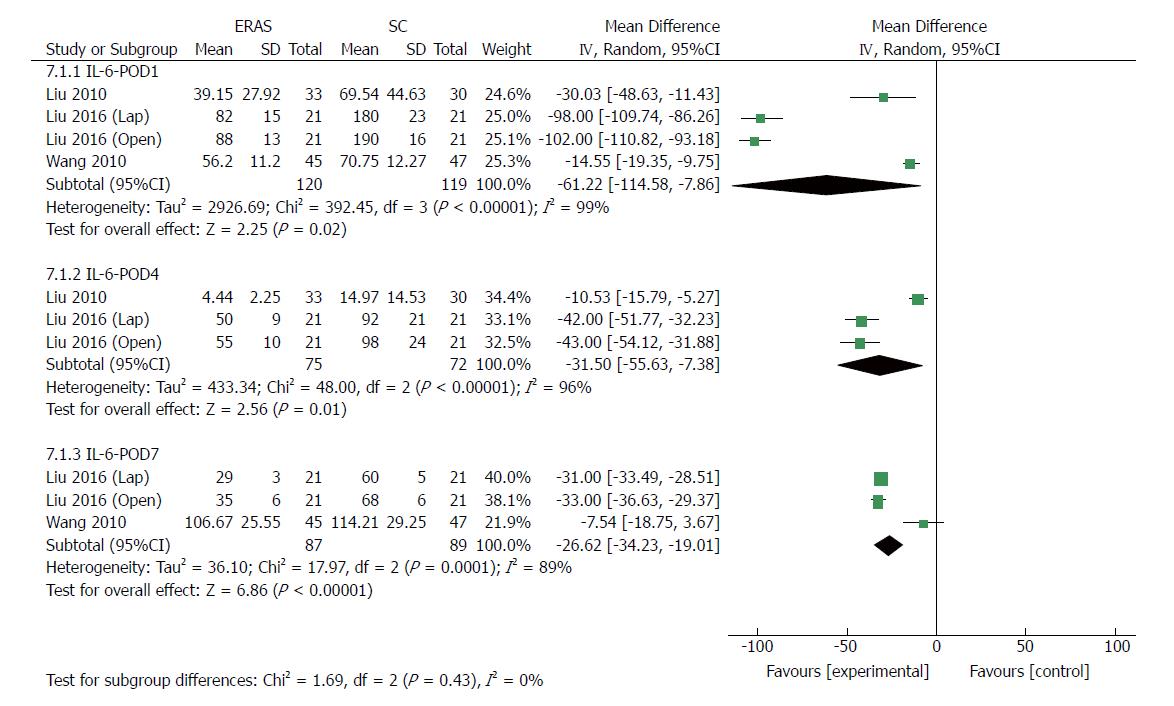
Eight RCTs[22,24,34-37] (514 patients) and four RCTs[24,36,37] (239 patients) reported CRP and IL-6 levels after gastrectomy, respectively. As markers of surgical stress-associated response, levels of CRP and IL-6 were significantly elevated after surgery. Compared with patients in the conventional care group, a milder acute-phase response was detected in the ERAS group after gastrectomy. The pooled MD using a random-effects model for serum CRP was -14.81 (95%CI: -21.42 to -8.21, P < 0.0001), -19.81 (95%CI: -29.64 to -9.98, P < 0.0001), and -21.36 (95%CI: -28.81 to -13.91, P < 0.00001) on days 1, 4 and 7 after surgery, respectively (Figure 9), and significant heterogeneity was observed among these studies (I2 = 72%, 64%, and 74% on day 1, 4 and 7 after surgery, respectively). The level of pooled MD for IL-6 was -61.22 (95%CI: -114.58 to -7.86, P = 0.02), -31.50 (95%CI: -55.63 to -7.38, P = 0.01) and -26.62 (95%CI: -34.23 to -19.01, P < 0.0001) on days 1, 4 and 7 after surgery, respectively (Figure 10), and there was a high degree of heterogeneity among these studies (I2 = 99%, 96% and 89% on day 1, 4 and 7 after surgery, respectively).
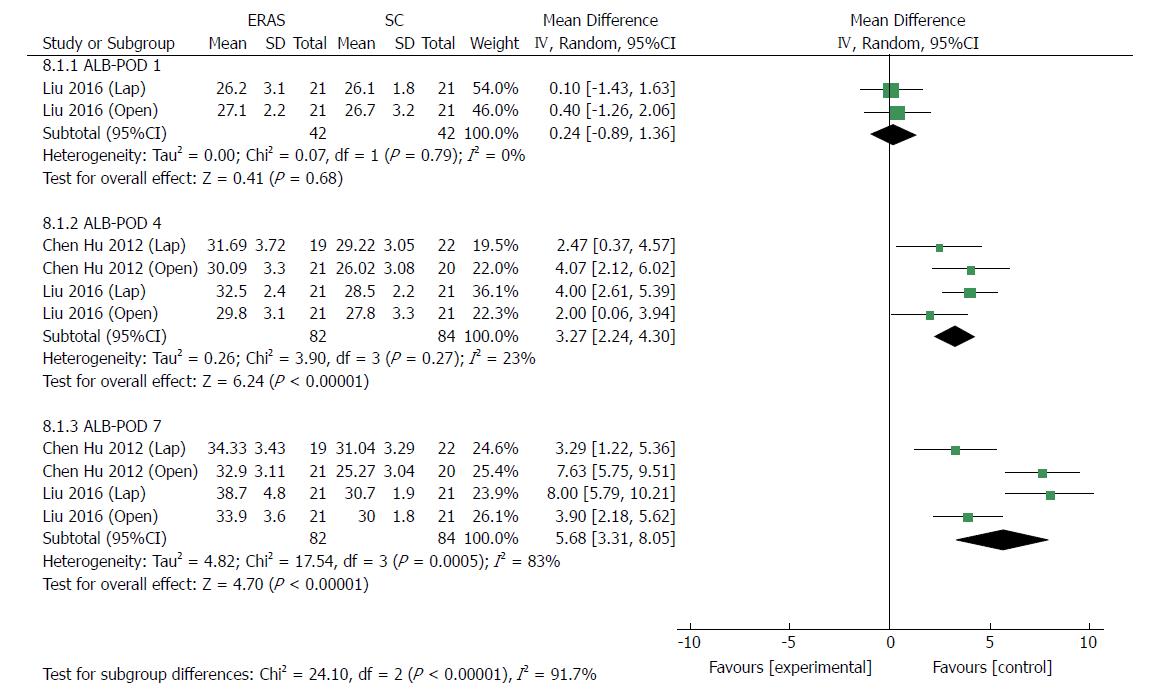
Four RCTs[24,32] reported serum ALB. In general, ALB concentration dropped significantly compared with preoperative parameters. On postoperative day (POD) 1, there was no significant difference regarding the level of ALB between the ERAS and conventional care groups (MD 0.24, 95%CI: -0.89 to 1.36, P = 0.68) (Figure 11). On PODs 4 and 7, the level of ALB was higher in the ERAS group than in the control group (MD: 3.27, 95%CI: 2.24-4.30, P < 0.00001; MD: 5.68, 95%CI: 3.31-8.05, P < 0.00001, respectively). Mild heterogeneity was detected on POD 4 (χ2 = 3.90, P = 0.27; I2 = 23%). However, there was significant heterogeneity in the outcomes on POD 7 (χ2 = 17.54, P = 0.0005; I2 = 83%) (Figure 11).
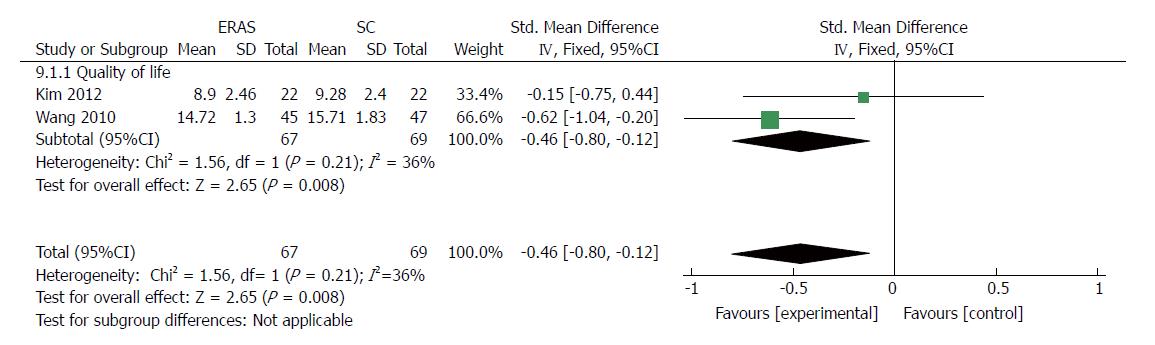
Health-related QOL was reported in two trials[35,37]. One trial checked health-related QOL with the European Organization for Research and Treatment of Cancer quality-of-life questionnaire C-30 and STO-22 at 14 d after discharge[35], while the other measured the QOL score using questionnaires at the time of discharge[37]. A significant superiority was found in the fast-track surgery protocol group compared with the conventional care program group in terms of short-term QOL using the fixed-effects model. The pooled standardized MD was -0.46 (95%CI: -0.80 to -0.12, P = 0.008) (Figure 12), and there was a mild degree of heterogeneity in the outcomes (χ2 = 1.56, P = 0.21; I2 = 36%).
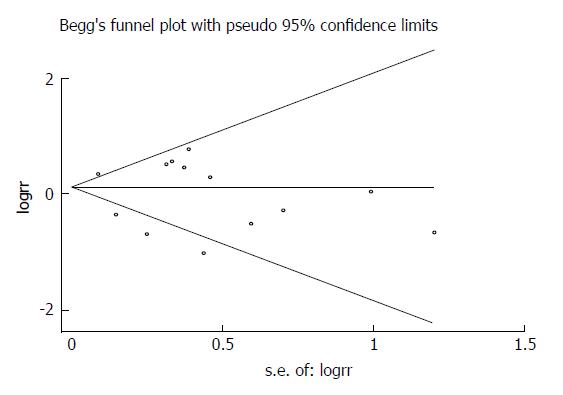
Potential publication bias was appraised graphically by using funnel plots, Begg’s test and Egger’s test. No obvious asymmetry was revealed by visual indication of the Begg’s funnel plot for postoperative total complications including all studies (Figure 13), and Begg’s test and Egger’s test indicated no significant bias was associated with publication for this meta-analysis (P = 0.55 and P = 0.435, respectively).
ERAS protocols have been gradually accepted as being able to optimize clinical outcomes, value and experience for patients with GC[22-29]. The present study is the largest meta-analysis to date, incorporating 13 RCTs enrolling 1092 participants, of whom 545 received ERAS protocols and 547 received SC for GC. Our results demonstrated that the optimized multimodal strategies significantly expedite bowel function recovery, shorten the length of POHS and reduce medical costs, and that ERAS pathways maintain comparable total complications, reoperation rates and mortality rates. The present analysis indicates that the implementation of ERAS approaches accelerates recovery, and is feasible and safe for patients with GC undergoing radical gastrectomy.
The core mechanism of ERAS is that multimodal interventions may lead to a major reduction in the undesirable sequelae of surgical injury, and stress-free surgery is the key goal of ERAS[1]. Robust evidence suggested that ERAS played an important role in attenuating the surgical stress response and accelerating the return to baseline in colorectal cancer surgery[38,39], which was afforded eloquent proof in GC surgery. The inflammatory factors, such as CRP, IL-6 and tumor necrosis factor α, are related to the extent of tissue injury caused by surgery[40,41]. In the present study, the ERAS approaches significantly reduced the concentration of CRP and IL-6 in comparison with SC on days 1, 4 and 7 after gastrectomy for GC, which was consistent with accelerated recovery. More importantly, our study suggests that the level of serum ALB after surgery in ERAS patients was significantly higher and steadier than that in SC patients, which fully demonstrates that the ERAS program could serve to improve the nutritional status of patients with GC. Good nutritional status and rapid rehabilitation after surgery allow patients to receive early postoperative multimodality therapy, including chemotherapy, thereby potentially improving their oncological outcome.
The main characteristic of ERAS is faster postoperative recovery and early discharge. However, it is noteworthy that this accelerated recovery does not come at the cost of increased medical expense. In our study, 10 RCTs reported data on medical costs and identified a mean reduction of 5000 USD in the ERAS group. If the trials with mean and imputed SD were excluded, medical expenses would be reduced by 5300 USD. Therefore, implementation of ERAS appears to have an advantage when combining clinical efficacy and cost effectiveness, which is consistent with previous reports[42,43].
More importantly, our study shows that ERAS pathways increased the readmission rate for GC patients after gastrectomy, a radically different result from previous meta-analyses[25-27]. However, sensitivity analysis, excluding the elderly patients in Bu’s study[23], indicated that there was no significant difference in readmission rates between ERAS and SC groups. To date, the evidence on the application of ERAS procedures in elderly patients with GC, especially if older than 75 years, is sparse. Only two RCTs have reported ERAS care in elderly patients with GC to date, and the age criterion for inclusion was inconsistent. Liu et al[24] confirmed that the use of ERAS in elderly patients (60-80 years) was safe and feasible, effectively reducing the stress response, speeding up the recovery of intestinal function, and improving postoperative nutritional status without increasing the complications. However, Bu et al[23] showed that implementation of the multimodal procedure in older patients (75-89 years) undergoing distal or total gastrectomy increased significantly the incidence of nausea and vomiting, gastric retention and ileus, as well as the readmission rate, in comparison with the SC group. These inconsistent results may be due to inclusion of age criterion, surgical type, and element selection.
Gerontal patients often experience underlying comorbidities and low physiological reserve, usually resulting in a high incidence of complications and delayed convalescence. Therefore, tailored perioperative care should be conducted in such a specific patient population. It was reported that a high degree of ERAS compliance was associated with fewer complications and shorter hospital stay[44,45]. Feroci et al[46] reported that male sex, advanced age (> 75 years), and American Society of Anesthesiologists’ score of grade 3 and above were correlated with lower compliance to enhanced recovery with specific reference to early removal of the urethral catheter, early oral feeding, and early ambulation in patients undergoing colorectal surgery. In our study, protocol compliance was only mentioned in studies by Feng et al[33] and Liu et al[24]. Whether the compliance of elderly GC patients with ERAS regimens affects the outcomes remains to be further investigated, although several studies have indicated that ERAS in colorectal surgery was safe and feasible, with postoperative outcomes similar to those of the younger group[47-49].
In our meta-analysis, two RCTs provided QOL data at the time of discharge[37] or 14 d after discharge[35], whereby ERAS approaches showed significant superiority in QOL over SC groups. However, many investigators prefer postoperative recovery to assess the efficacy of ERAS, which begins at the time of surgery and is complete only when the patient returns (recovers) to their baseline function or to population norms[50]. Therefore, functional status and QOL attracts more interest.
The introduction of laparoscopic surgery has dramatically lessened the impact of surgical traumas on patients and accelerated their recovery. In the past 2 decades, minimally invasive surgery and the implementation of ERAS have been considered two major revolutions in elective major abdominal surgery, both intending to minimize the surgical stress and improve patient outcomes[51]. Meta-analyses of RCTs in laparoscopic colorectal surgery have demonstrated that application of the ERAS approaches is associated with fewer complications, faster recovery of bowel function and shorter hospitalization, without increased readmissions[52,53]. Laparoscopic surgery has been recommended in the guidelines for enhanced recovery after gastrectomy[29]. In this study, we observed that laparoscopic surgery combined with ERAS markedly reduced POHS and medical costs, and speeded up the return of intestinal function in patients with GC; however, laparoscopic surgery with ERAS did not increase total complications compared with laparoscopic surgery alone.
There are undoubtedly several limitations in the present study. First, several included RCTs were smaller in size, although the total sample size of the study was greater than 1000, and a multicenter trial was lacking. Second, among the included studies there was considerable heterogeneity. No remarkable heterogeneity was found with regard to the incidence of complications (including anastomotic leaks, ileus, incision infection, urinary tract infection, and pulmonary infection), rates of readmission and reoperation, and postoperative serum ALB level (POD 1 and POD 4) and QOL. However, there was significant heterogeneity for overall complications, POHS, intestinal function recovery, medical costs, and inflammatory response indicators (I2 = 64%-99%). This substantial heterogeneity may be attributable to the clinical heterogeneity, including technical status of each institution, inclusion criteria, surgical approach, inconsistent evaluation of the outcomes, and ERAS elements used. Third, most studies excluded patients receiving neoadjuvant chemotherapy, which may increase the potential bias to a certain extent.
In conclusion, this updated meta-analysis and systematic review provides a comprehensive assessment of ERAS following gastrectomy, and demonstrates that ERAS protocols lead to accelerated recovery, reduction of surgical stress and medical costs, improved nutritional status, and better health-related QOL for GC patients. However, it appears to be associated with increased readmission rates. Further high-quality, large-sample, multicenter RCTs with long-term follow-up are needed to more precisely evaluate ERAS pathways in GC surgery.
Enhanced recovery after surgery (ERAS) has emerged as an optimal perioperative strategy for improving clinical outcomes in elective gastric cancer (GC) surgery. However, numerous controversies exist with regard to ERAS practice after radical gastrectomy.
Accumulating studies highlight that implementation of ERAS protocols reduces overall hospital stay, morbidity and mortality significantly, without compromising patient safety in multiple surgical disciplines. However, the safety and feasibility of applying ERAS in its current form in radical gastrectomy still remains to be proven by performing an updated meta-analysis.
This meta-analysis aims to provide an updated assessment of the safety and efficacy of ERAS protocols in GC surgery.
A comprehensive literature search in PubMed, Medline, EMBASE, World Health Organization International Trial Registry platform, and Cochrane Library until June 2017 was performed independently to identify all available randomized controlled trials (RCTs) comparing the ERAS program with standard perioperative care (SC) in GC surgery. Non-comparative studies, case-controlled trials, cohort studies, retrospective studies, items of ERAS applied being less than four, and no follow-up after discharge were excluded.
Thirteen RCTs, with a total of 1092 participants, were analyzed in this study, of whom 545 underwent ERAS protocols and 547 received SC treatment. ERAS protocols significantly decreased the length of postoperative hospital stay and medical costs, and accelerated bowel function recovery. Moreover, ERAS protocols were associated with a lower level of serum inflammatory response, higher serum albumin, and superior short-term quality of life. There were no significant differences regarding the incidence of total complications, mortality and reoperation following gastrectomy. However, the readmission rate after GC surgery nearly tripled under ERAS.
ERAS results in accelerated convalescence, reduction of surgical stress and medical costs, improved nutritional status, and better quality of life for GC patients, but increased the readmission rate. Furthermore, the significant heterogeneity of some results is a major limitation of this study. ERAS investigators need to proceed with caution as far as ERAS is concerned beyond colorectal cancer surgery.
This study provides an updated assessment of ERAS in GC surgery and is expected to provide guidance and reference for clinical practice, and also to provide high-level evidence for evidence-based medicine. High-quality multicenter RCTs with large samples and long-term follow-up are needed to more precisely evaluate ERAS in radical gastrectomy.
We would like to thank Dr. Mu-long Du, a member of the Biostatistic Service from the School of Public Health, Nanjing Medical University, for reviewing the statistical methods in this study.
| 1. | Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth. 1997;78:606-617. [PubMed] |
| 2. | Wilmore DW, Kehlet H. Management of patients in fast track surgery. BMJ. 2001;322:473-476. [PubMed] |
| 3. | Lassen K, Soop M, Nygren J, Cox PB, Hendry PO, Spies C, von Meyenfeldt MF, Fearon KC, Revhaug A, Norderval S, Ljungqvist O, Lobo DN, Dejong CH; Enhanced Recovery After Surgery (ERAS) Group. Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg. 2009;144:961-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 907] [Cited by in RCA: 788] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 4. | Ramírez JM, Blasco JA, Roig JV, Maeso-Martínez S, Casal JE, Esteban F, Lic DC; Spanish working group on fast track surgery. Enhanced recovery in colorectal surgery: a multicentre study. BMC Surg. 2011;11:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 5. | Kehlet H. Fast-track colonic surgery: status and perspectives. Recent Results Cancer Res. 2005;165:8-13. [PubMed] |
| 6. | Kehlet H. Fast-track colorectal surgery. Lancet. 2008;371:791-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 434] [Article Influence: 24.1] [Reference Citation Analysis (3)] |
| 7. | Kehlet H, Dahl JB. Anaesthesia, surgery, and challenges in postoperative recovery. Lancet. 2003;362:1921-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 947] [Cited by in RCA: 945] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 8. | Faucheron JL, Trilling B. Laparoscopy in combination with fast-track management is the best surgical strategy in patients undergoing colorectal resection for cancer. Tech Coloproctol. 2015;19:379-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Muallem MZ, Dimitrova D, Pietzner K, Richter R, Feldheiser A, Scharfe I, Schmeil I, Hösl TM, Mustea A, Wimberger P. Implementation of Enhanced Recovery After Surgery (ERAS) Pathways in Gynecologic Oncology. A NOGGO-AGO* survey of 144 Gynecological Departments in Germany. Anticancer Res. 2016;36:4227-4232. [PubMed] |
| 10. | Auyong DB, Allen CJ, Pahang JA, Clabeaux JJ, MacDonald KM, Hanson NA. Reduced Length of Hospitalization in Primary Total Knee Arthroplasty Patients Using an Updated Enhanced Recovery After Orthopedic Surgery (ERAS) Pathway. J Arthroplasty. 2015;30:1705-1709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 155] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 11. | Bösing NM, Goretzki PE, Röher HD. Gastric cancer: which patients benefit from systematic lymphadenectomy? Eur J Surg Oncol. 2000;26:498-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Sano T, Sasako M, Yamamoto S, Nashimoto A, Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T, Arai K, Yamamura Y. Gastric cancer surgery: morbidity and mortality results from a prospective randomized controlled trial comparing D2 and extended para-aortic lymphadenectomy--Japan Clinical Oncology Group study 9501. J Clin Oncol. 2004;22:2767-2773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 498] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 13. | Kim HH, Han SU, Kim MC, Hyung WJ, Kim W, Lee HJ, Ryu SW, Cho GS, Song KY, Ryu SY. Long-term results of laparoscopic gastrectomy for gastric cancer: a large-scale case-control and case-matched Korean multicenter study. J Clin Oncol. 2014;32:627-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 275] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 14. | Damhuis RA, Wijnhoven BP, Plaisier PW, Kirkels WJ, Kranse R, van Lanschot JJ. Comparison of 30-day, 90-day and in-hospital postoperative mortality for eight different cancer types. Br J Surg. 2012;99:1149-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 133] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 15. | Kubota T, Hiki N, Sano T, Nomura S, Nunobe S, Kumagai K, Aikou S, Watanabe R, Kosuga T, Yamaguchi T. Prognostic significance of complications after curative surgery for gastric cancer. Ann Surg Oncol. 2014;21:891-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 183] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 16. | Grantcharov TP, Kehlet H. Laparoscopic gastric surgery in an enhanced recovery programme. Br J Surg. 2010;97:1547-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | Yamada T, Hayashi T, Cho H, Yoshikawa T, Taniguchi H, Fukushima R, Tsuburaya A. Usefulness of enhanced recovery after surgery protocol as compared with conventional perioperative care in gastric surgery. Gastric Cancer. 2012;15:34-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 18. | Yamada T, Hayashi T, Aoyama T, Shirai J, Fujikawa H, Cho H, Yoshikawa T, Rino Y, Masuda M, Taniguchi H. Feasibility of enhanced recovery after surgery in gastric surgery: a retrospective study. BMC Surg. 2014;14:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Liu XX, Pan HF, Jiang ZW, Zhang S, Wang ZM, Chen P, Zhao Y, Wang G, Zhao K, Li JS. “Fast-track” and “Minimally Invasive” Surgery for Gastric Cancer. Chin Med J (Engl). 2016;129:2294-2300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Fang F, Gao J, Bi X, Han F, Wang HJ. Effect and clinical significance of fast-track surgery combined with laparoscopic radical gastrectomy on the plasma level of vascular endothelial growth factor in gastric antrum cancer. Springerplus. 2016;5:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Abdikarim I, Cao XY, Li SZ, Zhao YQ, Taupyk Y, Wang Q. Enhanced recovery after surgery with laparoscopic radical gastrectomy for stomach carcinomas. World J Gastroenterol. 2015;21:13339-13344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 22. | Mingjie X, Luyao Z, Ze T, YinQuan Z, Quan W. Laparoscopic Radical Gastrectomy for Resectable Advanced Gastric Cancer Within Enhanced Recovery Programs: A Prospective Randomized Controlled Trial. J Laparoendosc Adv Surg Tech A. 2017;27:959-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Bu J, Li N, Huang X, He S, Wen J, Wu X. Feasibility of Fast-Track Surgery in Elderly Patients with Gastric Cancer. J Gastrointest Surg. 2015;19:1391-1398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Liu G, Jian F, Wang X, Chen L. Fast-track surgery protocol in elderly patients undergoing laparoscopic radical gastrectomy for gastric cancer: a randomized controlled trial. Onco Targets Ther. 2016;9:3345-3351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Chen ZX, Liu AH, Cen Y. Fast-track program vs traditional care in surgery for gastric cancer. World J Gastroenterol. 2014;20:578-583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Beamish AJ, Chan DS, Blake PA, Karran A, Lewis WG. Systematic review and meta-analysis of enhanced recovery programmes in gastric cancer surgery. Int J Surg. 2015;19:46-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 27. | Chen S, Zou Z, Chen F, Huang Z, Li G. A meta-analysis of fast track surgery for patients with gastric cancer undergoing gastrectomy. Ann R Coll Surg Engl. 2015;97:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Ding J, Sun B, Song P, Liu S, Chen H, Feng M, Guan W. The application of enhanced recovery after surgery (ERAS)/fast-track surgery in gastrectomy for gastric cancer: a systematic review and meta-analysis. Oncotarget. 2017;8:75699-75711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 29. | Mortensen K, Nilsson M, Slim K, Schäfer M, Mariette C, Braga M, Carli F, Demartines N, Griffin SM, Lassen K; Enhanced Recovery After Surgery (ERAS®) Group. Consensus guidelines for enhanced recovery after gastrectomy: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Br J Surg. 2014;101:1209-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 548] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 30. | Tanaka R, Lee SW, Kawai M, Tashiro K, Kawashima S, Kagota S, Honda K, Uchiyama K. Protocol for enhanced recovery after surgery improves short-term outcomes for patients with gastric cancer: a randomized clinical trial. Gastric Cancer. 2017;20:861-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 31. | Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18487] [Cited by in RCA: 26340] [Article Influence: 1756.0] [Reference Citation Analysis (4)] |
| 32. | Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4895] [Cited by in RCA: 7290] [Article Influence: 347.1] [Reference Citation Analysis (1)] |
| 33. | Feng F, Ji G, Li JP, Li XH, Shi H, Zhao ZW, Wu GS, Liu XN, Zhao QC. Fast-track surgery could improve postoperative recovery in radical total gastrectomy patients. World J Gastroenterol. 2013;19:3642-3648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 66] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 34. | Chen Hu J, Xin Jiang L, Cai L, Tao Zheng H, Yuan Hu S, Bing Chen H, Chang Wu G, Fei Zhang Y, Chuan Lv Z. Preliminary experience of fast-track surgery combined with laparoscopy-assisted radical distal gastrectomy for gastric cancer. J Gastrointest Surg. 2012;16:1830-1839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 35. | Kim JW, Kim WS, Cheong JH, Hyung WJ, Choi SH, Noh SH. Safety and efficacy of fast-track surgery in laparoscopic distal gastrectomy for gastric cancer: a randomized clinical trial. World J Surg. 2012;36:2879-2887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 36. | Liu XX, Jiang ZW, Wang ZM, Li JS. Multimodal optimization of surgical care shows beneficial outcome in gastrectomy surgery. JPEN J Parenter Enteral Nutr. 2010;34:313-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 37. | Wang D, Kong Y, Zhong B, Zhou X, Zhou Y. Fast-track surgery improves postoperative recovery in patients with gastric cancer: a randomized comparison with conventional postoperative care. J Gastrointest Surg. 2010;14:620-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 142] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 38. | Ren L, Zhu D, Wei Y, Pan X, Liang L, Xu J, Zhong Y, Xue Z, Jin L, Zhan S. Enhanced Recovery After Surgery (ERAS) program attenuates stress and accelerates recovery in patients after radical resection for colorectal cancer: a prospective randomized controlled trial. World J Surg. 2012;36:407-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 170] [Article Influence: 12.1] [Reference Citation Analysis (1)] |
| 39. | Carli F. Physiologic considerations of Enhanced Recovery After Surgery (ERAS) programs: implications of the stress response. Can J Anaesth. 2015;62:110-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 40. | Feng J, Li K, Li L, Wang X, Huang M, Yang J, Hu Y. The effects of fast-track surgery on inflammation and immunity in patients undergoing colorectal surgery. Int J Colorectal Dis. 2016;31:1675-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Mari G, Crippa J, Costanzi A, Mazzola M, Rossi M, Maggioni D. ERAS Protocol Reduces IL-6 Secretion in Colorectal Laparoscopic Surgery: Results From a Randomized Clinical Trial. Surg Laparosc Endosc Percutan Tech. 2016;26:444-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 42. | Stowers MD, Lemanu DP, Hill AG. Health economics in Enhanced Recovery After Surgery programs. Can J Anaesth. 2015;62:219-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 43. | Nelson G, Kiyang LN, Chuck A, Thanh NX, Gramlich LM. Cost impact analysis of Enhanced Recovery After Surgery program implementation in Alberta colon cancer patients. Curr Oncol. 2016;23:e221-e227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 44. | ERAS Compliance Group. The Impact of Enhanced Recovery Protocol Compliance on Elective Colorectal Cancer Resection: Results From an International Registry. Ann Surg. 2015;261:1153-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 488] [Article Influence: 48.8] [Reference Citation Analysis (1)] |
| 45. | Alcántara-Moral M, Serra-Aracil X, Gil-Egea MJ, Frasson M, Flor-Lorente B, Garcia-Granero E; E. B.S.Q.-C on behalf of the collaborative Group of Coloproctology Section of The Spanish Association of Surgeons. Observational cross-sectional study of compliance with the fast track protocol in elective surgery for colon cancer in Spain. Int J Colorectal Dis. 2014;29:477-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 46. | Feroci F, Lenzi E, Baraghini M, Garzi A, Vannucchi A, Cantafio S, Scatizzi M. Fast-track surgery in real life: how patient factors influence outcomes and compliance with an enhanced recovery clinical pathway after colorectal surgery. Surg Laparosc Endosc Percutan Tech. 2013;23:259-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 47. | Bardram L, Funch-Jensen P, Kehlet H. Rapid rehabilitation in elderly patients after laparoscopic colonic resection. Br J Surg. 2000;87:1540-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 117] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 48. | Pawa N, Cathcart PL, Arulampalam TH, Tutton MG, Motson RW. Enhanced recovery program following colorectal resection in the elderly patient. World J Surg. 2012;36:415-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 49. | Baek SJ, Kim SH, Kim SY, Shin JW, Kwak JM, Kim J. The safety of a “fast-track” program after laparoscopic colorectal surgery is comparable in older patients as in younger patients. Surg Endosc. 2013;27:1225-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 50. | Neville A, Lee L, Antonescu I, Mayo NE, Vassiliou MC, Fried GM, Feldman LS. Systematic review of outcomes used to evaluate enhanced recovery after surgery. Br J Surg. 2014;101:159-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 180] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 51. | Feldman LS, Delaney CP. Laparoscopy plus enhanced recovery: optimizing the benefits of MIS through SAGES ‘SMART’ program. Surg Endosc. 2014;28:1403-1406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 52. | Lei QC, Wang XY, Zheng HZ, Xia XF, Bi JC, Gao XJ, Li N. Laparoscopic Versus Open Colorectal Resection Within Fast Track Programs: An Update Meta-Analysis Based on Randomized Controlled Trials. J Clin Med Res. 2015;7:594-601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 53. | Zhao JH, Sun JX, Huang XZ, Gao P, Chen XW, Song YX, Liu J, Cai CZ, Xu HM, Wang ZN. Meta-analysis of the laparoscopic versus open colorectal surgery within fast track surgery. Int J Colorectal Dis. 2016;31:613-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Aoyagi K, Bona S, Dai ZJ, Muhammad JS S- Editor: Wang XJ L- Editor: Filipodia E- Editor: Huang Y