Published online Feb 21, 2017. doi: 10.3748/wjg.v23.i7.1224
Peer-review started: September 19, 2016
First decision: October 28, 2016
Revised: December 3, 2016
Accepted: January 18, 2017
Article in press: January 18, 2017
Published online: February 21, 2017
Processing time: 154 Days and 16.9 Hours
To investigate incidence and survival of post-transplant lymphoproliferative disorder (PTLD) patients after liver transplantation.
A cross-sectional survey was conducted among patients who underwent liver transplantation at Shiraz Transplant Center (Shiraz, Iran) between August 2004 and March 2015. Clinical and laboratory data of patients were collected using a data gathering form.
There were 40 cases of PTLD in the pediatric age group and 13 cases in the adult group. The incidence of PTLD was 6.25% in pediatric patients and 1.18% in adult liver transplant recipients. The post-PTLD survival of patients at 6 mo was 75.1% ± 6%, at 1 year was 68.9% ± 6.5% and at 5 years was 39.2% ± 14.2%. Higher serum tacrolimus level was associated with lower post-PTLD survival in pediatric patients (OR = 1.07, 95%CI: 1.006-1.15, P = 0.032). A serum tacrolimus level over 11.1 ng/mL was predictive of post PTLD survival (sensitivity = 90%, specificity = 52%, area under the curve = 0.738, P = 0.035).
Incidence of PTLD in our liver transplant patients is comparable to other centers. Transplant physicians may consider adjustment of tacrolimus dose to maintain its serum level below this cutoff point.
Core tip: Post-transplant lymphoproliferative disorder (PTLD) is one of the complications that may occur after liver transplantation. The present study is a survival analysis of liver transplant patients after PTLD development. The incidence of PTLD was 6.25% in pediatric patients and 1.18% in adult liver transplant recipients. The main new finding is association of serum tacrolimus level with post-PTLD survival. Higher serum tacrolimus level was associated with lower post-PTLD survival in pediatric patients.
- Citation: Eshraghian A, Imanieh MH, Dehghani SM, Nikeghbalian S, Shamsaeefar A, Barshans F, Kazemi K, Geramizadeh B, Malek-Hosseini SA. Post-transplant lymphoproliferative disorder after liver transplantation: Incidence, long-term survival and impact of serum tacrolimus level. World J Gastroenterol 2017; 23(7): 1224-1232
- URL: https://www.wjgnet.com/1007-9327/full/v23/i7/1224.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i7.1224
Liver transplantation is an established modality of treatment for end-stage liver diseases of various etiologies. Despite considerable improvement in outcomes of patients, complications frequently occur after transplantation that may have negative impact on survival[1]. Post-transplant lymphoproliferative disorder (PTLD) is one of the complications that may occur after liver transplantation and threatens both graft and patient survival. PTLD is generally believed to be a consequence of relative immunodeficiency state secondary to immunosuppressive regimens in these patients[2]. Immunosuppressive therapy results in depressed T-cell function that predisposes patients to lymphoid proliferation[3]. Epstein-Barr virus (EBV) infection is the other major risk factor for development of PTLD after liver transplantation and the majority of cases (60%-70%) are EBV-positive[4]. While EBV infection has minimal consequences in normal subjects, in liver transplant recipients it is associated with a spectrum of disorders, ranging from reactive monoclonal hyperplasia to aggressive malignant lymphoma[5]. In immunocompetent subjects, the EBV genome remains latent in resting memory B cells after immortalization[6]. However, after transplantation, long-term immunosuppressive therapy results in depressed T-cell function and lack of T-cell inhibition on B-cell proliferation[7]. This may lead to uncontrolled B-cell proliferation and subsequent hyperplasia, and even malignant transformation.
PTLD is more frequently encountered in pediatric patients, and younger age by itself is a known risk factor for PTLD despite controversies[8]. Another proposed risk factor for PTLD development after liver transplantation is hepatitis C virus infection[9]. With a mortality rate ranging from 12% to 60% in different studies, PTLD has imposed considerable negative impact on transplant patients until recently[10-12]. However, outcomes of patients and survival rates have been substantially improved by using new modalities for treatments, such as rituximab (a chimeric anti-CD20 monoclonal antibody) and sirolimus, in addition to reduced-dose immunosuppression[13-15].
This study aimed to investigate incidence, risk factors (including impact of immunosuppressive regimen) and survival of PTLD patients after liver transplantation in Iranian patients.
Shiraz Organ Transplant Center (Shiraz, Iran) is a leading transplant center in Iran, with considerable annual cases of liver transplantation for both adult and pediatric patients. A cross-sectional survey was conducted among the adult and pediatric patients (< 18 years) who underwent liver transplantation at Shiraz Transplant Center between August 2004 and March 2015. Clinical and laboratory data of patients were collected using a data gathering form containing information regarding age, sex, underlying liver disease, type of allograft (deceased donor, living related donor, split liver transplantation), time of liver transplantation and time of PTLD development, survival of patients from date of liver transplantation, survival after PTLD diagnosis, immunosuppressive regimen and dosage, rejection episodes, EBV status before and after transplantation, presenting sign and symptoms, PTLD histology, multi-organ involvement, modality of treatment, response to therapy, and serum level of calcineurin inhibitors (including tacrolimus and cyclosporine). All patients received intravenous methylprednisolone as induction of immunosuppression. Patients received tacrolimus, cyclosporine, mycophenolate mofetil and prednisolone as immunosuppressive therapy during their follow-up. Serum tacrolimus level was measured periodically during follow-up for each patient and the last measured serum tacrolimus levels before diagnosis of PTLD were recorded and analyzed for each patient. Patients were treated with rituximab or chemotherapy based on grade, type and invasiveness of PTLD. Change from tacrolimus to sirolimus was applied for all of the patients diagnosed with PTLD. Thirteen patients with positivity for cytomegalovirus (CMV)-DNA were treated with ganciclovir or valganciclovir.
Diagnosis of PTLD was confirmed by tissue biopsies reviewed by expert pathologists. World Health Organization (WHO) classification for tumors of lymphoid tissue was used for PTLD classification[16]. While diagnosis of PTLD was confirmed, patients underwent staging work-up, including CT scans (abdomen, chest and pelvis) and bone marrow aspiration and biopsy to detect possibility of multiple organ involvement. Frozen section or paraffin immunoperoxidase staining or flow cytometry were applied for immunophenotyping of B-cell- and T-cell-associated antigens, as previously described.
Whether PTLD is monoclonal or polyclonal was determined by flow cytometry on fresh cell suspensions checking immunoglobulin light chain restriction, by immunoperoxidase staining on frozen or paraffin-embedded tissues or by southern blot on frozen tissues checking immunoglobulin or T-cell receptor gene rearrangements[17].
Based on these classification, one patient had nodular sclerosis Hodgkin disease, one had plasmacytoma, and others had polymorphic and monomorphic B cell lymphoma.
The study protocol was approved by the institutional review board of Shiraz University of Medical Sciences. The study protocol was carried out in accordance with the Helsinki Declaration as revised in Seoul 2008. Written informed consent was obtained from patients.
Comparisons of continuous variables were performed with the Student’s t-test, and categorical variables were compared using the chi-square test. Non-parametric Mann-Whitney test was used when appropriate. Data were presented using mean ± standard deviation for numeric variables, and percent and counts for categorical variables. Kaplan-Meier estimates were used for analysis of time to PTLD development and survival after PTLD diagnosis. Kaplan-Meier and Cox regression analyses were used to calculate the influence of probable risk factors on PTLD development and survival. Rejection episodes were considered a time varying statistical variable, and rejections that occurred after PTLD development were excluded. Statistical analysis was performed with SPSS 16.0 (SPSS Inc., Chicago, IL, United States). A P value of < 0.05 were considered statistically significant.
Overall, 53 patients were diagnosed with PTLD. There were 40 cases of PTLD in the pediatric age group and 13 cases in the adult group. The incidence of PTLD was 6.25% in the pediatric patients and 1.18% in the adult liver transplant recipients. The baseline characteristics of PTLD patients are outlined in Table 1.
| Pediatrics | Adults | Overall | |
| Number | 40 | 13 | 53 |
| Mean age in years | 5.05 ± 4.43 | 42 ± 13.39 | 14.11 ± 17.71 |
| Sex, male/female | 23/17 | 10/3 | 33/20 |
| Allograft | |||
| Living donor | 28 | 0 | 28 |
| Deceased donor | 12 | 13 | 25 |
| Presenting sign and symptoms | |||
| LAP | 18 | 9 | 27 |
| Fever | 13 | 1 | 14 |
| Abdominal pain | 15 | 3 | 18 |
| Diarrhea | 5 | 0 | 5 |
| Weight loss | 0 | 1 | 1 |
| Cough and dyspnea | 1 | 0 | 1 |
| Bowel obstruction | 1 | 0 | 1 |
| Unilateral weakness | 1 | 0 | 1 |
| Underlying liver disease | |||
| HBV cirrhosis | 0 | 5 | 5 |
| Cryptogenic cirrhosis | 0 | 2 | 2 |
| PSC | 0 | 2 | 2 |
| HCV cirrhosis | 0 | 1 | 1 |
| AIH | 2 | 1 | 3 |
| Wilson’s disease | 1 | 0 | 1 |
| PFIC | 5 | 0 | 5 |
| Crigler-Najjar syndrome | 8 | 0 | 8 |
| Biliary atresia | 12 | 0 | 12 |
| Tyrosinemia | 10 | 0 | 10 |
| Budd-Chiari syndrome | 1 | 1 | 2 |
| Liver metastasis | 0 | 1 | 1 |
| Neonatal hepatitis | 1 | 0 | 1 |
| Immunosuppressive regimen | |||
| Prednisolone | 39 | 12 | 49 |
| Tacrolimus | 36 | 10 | 46 |
| Mycophenolate mofetil | 15 | 11 | 26 |
| Cyclosporine | 1 | 4 | 5 |
| Sirolimus | 35 | 8 | 43 |
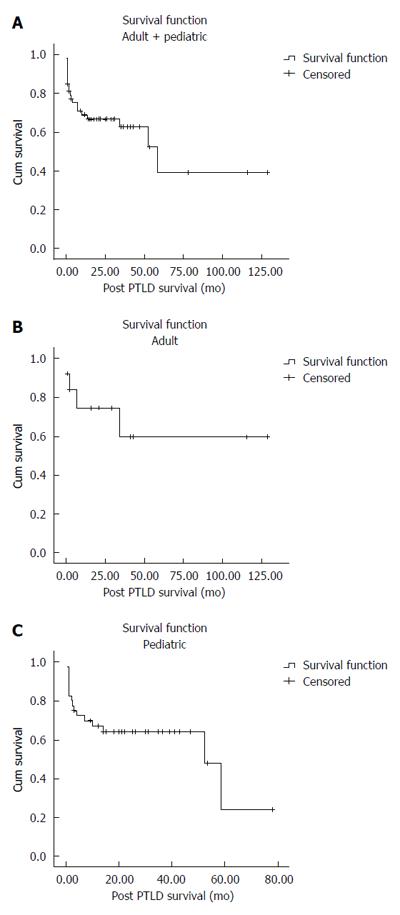
The mean overall (adult and pediatric) post-PTLD survival was 66.29 ± 11.86 mo. The post-PTLD survival of patients at 6 mo was 75.1% ± 6%, at 1 year was 68.9% ± 6.5% and at 5 years was 39.2% ± 14.2% (Figure 1A).
The mean post-PTLD survival in adult patients was 82.94 ± 18.58 mo. The post-PTLD survival of adult patients at 6 mo was 83.9% ± 10.4%, at 1 year was 74.6% ± 12.8% and at 5 years was 59.7% ± 16.8% (Figure 1B).
The mean post-PTLD survival in pediatric patients was 42.61 ± 6.1 mo. The post-PTLD survival of pediatric patients at 6 mo was 72.4% ± 7.1%, at 1 year was 67.1% ± 7.5% and at 5 years was 24.1% ± 18.6% (Figure 1C).
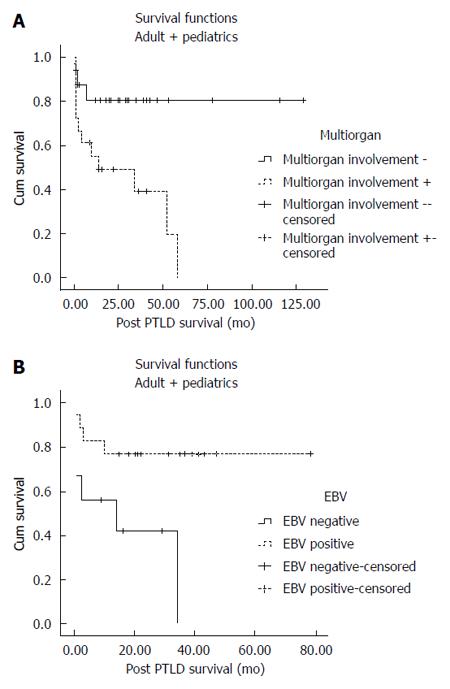
When both pediatric and adult patients were analyzed altogether, multi-organ involvement was significantly associated with lower post-PTLD survival (104.25 ± 9.08 mo vs 27.13 ± 6.30 mo, P = 0.002) (Figure 2A). EBV-positive patients with PTLD had significantly higher mean survival compared to EBV-negative PTLD patients (60.58 ± 7.62 mo vs 16.72 ± 5.66 mo, P = 0.018) (Figure 2B). Other variables including sex, CMV status, rejection episodes, time to PTLD before or after 1 year, and type of allograft had no significant effect on post-PTLD survival (Table 2).
| Mean survival in mo | P value | |
| Sex | 0.902 | |
| Male | 65.65 ± 13.18 | |
| Female | 36.06 ± 5.30 | |
| Multi-organ involvement | 0.002 | |
| (+) | 27.13 ± 6.30 | |
| (-) | 104.25 ± 9.08 | |
| CMV status | 0.370 | |
| CMV-positive | 51.98 ± 10.50 | |
| CMV-negative | 23.29 ± 5.76 | |
| EBV status | 0.002 | |
| EBV-positive | 60.58 ± 7.62 | |
| EBV-negative | 16.72 ± 5.66 | |
| Rejection episode | 0.762 | |
| (+) | 64.90 ± 13.78 | |
| (-) | 65.86 ± 15.76 | |
| Time to PTLD development in years | 0.704 | |
| ≤ 1 | 62.18 ± 14.03 | |
| ≥ 1 | 75.13 ± 12.49 | |
| Type of allograft | 0.904 | |
| Living donor | 50.56 ± 6.95 | |
| Deceased donor | 60.32 ± 14.33 |
We also analyzed the influence of different risk factors on pediatric PTLD patients separately. Multi-organ involvement and EBV negativity were significantly associated with lower mean post-PTLD survival in pediatric patients (Table 3). Higher serum tacrolimus level was associated with lower post-PTLD survival in pediatric patients (OR = 1.07, 95%CI: 1.006-1.15, P = 0.032) (Table 4).
| Mean survival in mo | P value | |
| Sex | 0.749 | |
| Male | 41.41 ± 7.38 | |
| Female | 35.85 ± 5.76 | |
| Multi-organ involvement | 0.002 | |
| (+) | 25.82 ± 6.90 | |
| (-) | 67.62 ± 5.56 | |
| CMV status | 0.139 | |
| CMV-positive | 58.82 ± 9.56 | |
| CMV-negative | 19.35 ± 6.21 | |
| EBV status | 0.002 | |
| EBV-positive | 60.58 ± 7.62 | |
| EBV-negative | 5.58 ± 2.72 | |
| Rejection episode | 0.888 | |
| (+) | 43.61 ± 8.49 | |
| (-) | 36.02 ± 5.24 | |
| Time to PTLD development in years | 0.326 | |
| ≤ 1 | 39.72 ± 6.86 | |
| ≥ 1 | 36.45 ± 5.30 | |
| Type of allograft | 0.806 | |
| Living donor | 50.56 ± 6.95 | |
| Deceased donor | 37.37 ± 8.11 |
| Mean | OR | 95%CI | P value | |
| Age in years | 5.05 | 0.94 | 0.82-1.08 | 0.434 |
| Time to PTLD in months | 15.63 | 0.96 | 0.91-1.02 | 0.242 |
| Tacrolimus level | 14.99 | 1.07 | 1.006-1.15 | 0.032 |
| Tacrolimus dose | 3.81 | 1.06 | 0.67-1.66 | 0.797 |
| Prednisolone dose | 10.12 | 0.99 | 0.86-1.13 | 0.897 |
Patients were divided into those who developed PTLD in ≤ 1 year and those who developed PTLD in ≥ 1 year. Age, post-PTLD survival, serum tacrolimus level, tacrolimus dose and prednisolone dose were not correlated with time to PTLD development in pediatric patients (P > 0.05) (Table 5). However, multi-organ involvement was more common in patients who developed PTLD within 1 year after liver transplantation (P = 0.007) (Table 6). Multi-organ involvement was also more common in pediatric patients who developed PTLD within 1 year after liver transplantation (P = 0.007) (Table 6).
| Mean rank PTLD development ≤ 1 yr | Mean rank PTLD development ≥ 1 yr | U value | Z score | P value | |
| Age | 19.85 | 21.85 | 158 | -0.50 | 0.61 |
| Post-PTLD survival | 20.19 | 21.15 | 167 | -0.24 | 0.80 |
| Tacrolimus level | 16.57 | 14.61 | 86 | -0.54 | 0.58 |
| Tacrolimus dose | 19.84 | 18.85 | 154 | -0.27 | 0.78 |
| Prednisolone dose | 20.56 | 18.88 | 154 | -0.44 | 0.65 |
| Alive patient | Deceased patient | ||||
| Age | 23.19 | 16.47 | 127.5 | -1.78 | 0.74 |
| Tacrolimus level | 13.62 | 21.00 | 55 | -2.11 | 0.03 |
| Tacrolimus dose | 18.96 | 20.43 | 175 | -0.40 | 0.68 |
| Prednisolone dose | 19.65 | 20.57 | 171 | -0.25 | 0.79 |
| Mean time to PTLD | 23.12 | 16.56 | 129 | -1.74 | 0.08 |
| Multi-organ (+) | Multi-organ (-) | ||||
| Age | 19.50 | 19.50 | 176 | 0.00 | 1.00 |
| Tacrolimus level | 16.50 | 14.74 | 97 | -0.54 | 0.58 |
| Tacrolimus dose | 20.27 | 18.14 | 146 | -0.60 | 0.54 |
| Prednisolone dose | 19.81 | 19.27 | 171 | -0.15 | 0.87 |
| Post-PTLD survival | 15.59 | 22.34 | 113 | -1.85 | 0.06 |
| Mean time to PTLD | 13.62 | 23.77 | 82 | -2.78 | 0.005 |
| PTLD development ≤ 1 yr | PTLD development ≥ 1 yr | P value | |
| All PTLD patients | |||
| Sex | 0.150 | ||
| Male | 17 | 16 | |
| Female | 14 | 6 | |
| Multi-organ involvement | 0.007 | ||
| (+) | 15 | 3 | |
| (-) | 14 | 18 | |
| CMV status | 0.186 | ||
| CMV-positive | 9 | 4 | |
| CMV-negative | 11 | 1 | |
| EBV status | 0.296 | ||
| EBV-positive | 12 | 5 | |
| EBV-negative | 8 | 1 | |
| Rejection episode | 0.399 | ||
| (+) | 15 | 9 | |
| (-) | 16 | 13 | |
| Mortality | 0.324 | ||
| (+) | 13 | 7 | |
| (-) | 18 | 15 | |
| Type of allograft | 0.118 | ||
| Living donor | 19 | 9 | |
| Deceased donor | 12 | 13 | |
| Pediatric PTLD patients | |||
| Sex | 0.496 | ||
| Male | 15 | 8 | |
| Female | 12 | 5 | |
| Multi-organ involvement | 0.018 | ||
| (+) | 14 | 2 | |
| (-) | 11 | 11 | |
| CMV status | 0.368 | ||
| CMV-positive | 9 | 3 | |
| CMV-negative | 9 | 1 | |
| EBV status | 0.184 | ||
| EBV-positive | 12 | 5 | |
| EBV-negative | 6 | 0 | |
| Rejection episode | 0.587 | ||
| (+) | 13 | 6 | |
| (-) | 14 | 7 | |
| Mortality | 0.120 | ||
| (+) | 13 | 3 | |
| (-) | 14 | 10 | |
| Type of allograft | 0.609 | ||
| Living donor | 19 | 9 | |
| Deceased donor | 8 | 4 |
Multi-organ involvement was associated with increased mortality after PTLD development (P < 0.05) (Table 7). EBV-positive patients with PTLD had lower mortality when compared to EBV-negative patients (P < 0.05) (Table 7).
| Alive patient | Deceased patient | P value | |
| All PTLD patients | |||
| Sex | 0.491 | ||
| Male | 10 | 13 | |
| Female | 13 | 7 | |
| Multi-organ involvement | 0.001 | ||
| (+) | 6 | 12 | |
| (-) | 26 | 6 | |
| CMV status | 0.284 | ||
| CMV-positive | 9 | 4 | |
| CMV-negative | 6 | 6 | |
| EBV status | 0.042 | ||
| EBV-positive | 13 | 4 | |
| EBV-negative | 3 | 6 | |
| Rejection episode | 0.600 | ||
| (+) | 15 | 9 | |
| (-) | 18 | 11 | |
| Type of allograft | 0.485 | ||
| Living donor | 18 | 10 | |
| Deceased donor | 15 | 10 | |
| Pediatric PTLD patients | |||
| Sex | 0.424 | ||
| Male | 13 | 10 | |
| Female | 11 | 6 | |
| Multi-organ involvement | 0.001 | ||
| (+) | 5 | 11 | |
| (-) | 19 | 3 | |
| CMV status | 0.110 | ||
| CMV-positive | 9 | 3 | |
| CMV-negative | 4 | 6 | |
| EBV status | 0.018 | ||
| EBV-positive | 13 | 4 | |
| EBV-negative | 1 | 5 | |
| Rejection episode | 0.525 | ||
| (+) | 11 | 8 | |
| (-) | 13 | 8 | |
| Type of allograft | 0.309 | ||
| Living donor | 18 | 10 | |
| Deceased donor | 6 | 6 |
Multi-organ involvement was not associated with age, serum tacrolimus level, tacrolimus dose, prednisolone dose in univariate analysis (Table 5). EBV-positive patients were less likely to have multi-organ involvement in comparison with EBV- negative patients (P = 0.008) (Table 8). Pediatric patients who received liver allograft from deceased donors were more likely to develop PTLD with multi-organ involvement when compared to those receiving liver allograft from living donors (P = 0.019) (Table 8).
| Multi-organ involvement (+) | Multi-organ involvement (-) | P value | |
| All PTLD patients | |||
| Sex | 0.421 | ||
| Male | 12 | 19 | |
| Female | 6 | 13 | |
| CMV status | 0.418 | ||
| CMV-positive | 6 | 7 | |
| CMV-negative | 7 | 5 | |
| EBV status | 0.008 | ||
| EBV-positive | 5 | 11 | |
| EBV-negative | 8 | 1 | |
| Rejection episode | 0.448 | ||
| (+) | 9 | 14 | |
| (-) | 9 | 18 | |
| Type of allograft | 0.235 | ||
| Living donor | 8 | 19 | |
| Deceased donor | 10 | 13 | |
| Pediatric PTL patients | |||
| Sex | 0.206 | ||
| Male | 11 | 11 | |
| Female | 5 | 11 | |
| CMV status | 0.335 | ||
| CMV-positive | 5 | 7 | |
| CMV-negative | 6 | 4 | |
| EBV status | 0.006 | ||
| EBV-positive | 5 | 11 | |
| EBV-negative | 6 | 0 | |
| Rejection episode | 0.520 | ||
| (+) | 8 | 10 | |
| (-) | 8 | 12 | |
| Type of allograft | 0.019 | ||
| Living donor | 8 | 19 | |
| Deceased donor | 8 | 3 |
To estimate a cutoff point value for tacrolimus level in relation to post-PTLD survival in pediatric patients, we used receiver operating characteristic (ROC) curve analysis. A serum tacrolimus of over 11.1 ng/mL was predictive of post-PTLD survival (sensitivity = 90%, specificity = 52%, area under the curve = 0.738, P = 0.035).
The present study is one of the largest series of patients with PTLD after liver transplantation. Our study showed that the incidence of PTLD following pediatric liver transplantation was much higher than for adult liver transplantation (6.25% in pediatrics and 1.18% in adults). While previous studies reported PTLD incidence of up to 20% after pediatric liver transplantation[18], recent reported incidence from different studies are lower and range from 10% to 5.5%[19,20]. Since PTLD is mainly considered as a result of interaction of immunosuppression and EBV infection, the decreased incidence in pediatric patients may be secondary to the better monitoring of patients, especially for immunosuppressive regimen and EBV infection. In pediatric patients, our reported incidence is comparable to other studies; however, due to unexplained reasons the incidence of PTLD after adult liver transplantation in our study was lower than previous reports from other centers[21].
Mean post-PTLD survival was higher in the adult patients than in the pediatric patients. This observation is probably due to the long-term survival (> 10 years) of 2 of our adult patients. In a recent study conducted in our center, the 1-year and 5-year overall survival of pediatric liver transplant recipients was found to be 73% and 66% respectively[22]. In this way, the 1-year post-PTLD survival in the pediatric age group is nearly equal to the overall survival of our pediatric patients. However, it should be noted that the 5-year post-PTLD survival in pediatric patients has dramatically declined to 24.1%.
Due to small numbers of adult PTLD patients, the analyses were performed on either pediatric patients or adult plus pediatric patients. We investigated the impact of different variables on post-PTLD survival. As expected, multi-organ involvement was associated with a lower post-PTLD survival and increased mortality. EBV-positive patients had higher mean post-PTLD survival in comparison with EBV-negative subjects. EBV positivity was also associated with lower mortality, especially among the pediatric age group. These findings may be jeopardized by our other finding that EBV-positive patients had lower probability of multi-organ involvement.
Although up to 30% of PTLD patients are EBV-negative, EBV has been generally considered as responsible for most cases of PTLD. However, the influence of recipient EBV status on outcomes of PTLD patients is conflicting. Some studies have shown that EBV-negative PTLD patients have more malignant appearing disease with an aggressive course and higher mortality rate[23,24]. In univariate analysis, our findings are inconsistent with these mentioned results. However, in regression analysis, EBV status was not associated with post-PTLD survival. Several other studies showed that EBV status had no significant impact on outcomes of PTLD patients, including their survival[25-28]. EBV status was not associated with time of PTLD development in our study. This finding is in contrast with previous reports showing that EBV-negative PTLD occurs later after liver transplantation when compared to EBV-positive PTLD patients[29,30].
Immunosuppressive therapy has been reported to be associated with PTLD development. Treatment of rejection episodes with steroid or OKT3 were risk factors of PTLD development, especially during 1 year after treatment[31,32]. Reducing dose of immunosuppressive medications is another treatment strategy used on PTLD patients in some studies[33,34]. In our study, rejection episode, steroid dose and tacrolimus dose were not associated with PTLD survival, while higher serum tacrolimus level was associated with lower survival. Finally, we showed that a serum tacrolimus cutoff value of over 11.1 ng/mL is associated with post-PTLD survival, having a high sensitivity but a rather low specificity in pediatric patients. Therefore, it might be suggested that transplant physicians consider adjustment of tacrolimus dose to maintain its serum level around this cutoff point.
Although a PTLD series has been published from our center previously[35], this study is the first that evaluates incidence, survival and associated factors influencing survival of PTLD patients after liver transplantation. This study is also the first that shows the association between serum tacrolimus level and post-PTLD survival, and suggests a serum tacrolimus cutoff point value to adjust tacrolimus dose.
Post-transplant lymphoproliferative disorder (PTLD) is one of the complications after liver transplantation and may threaten both graft and patient survival. This study aimed to investigate incidence and survival of PTLD patients after liver transplantation.
Few studies with considerable number of patients have reported survival of PTLD patients after liver transplantation. This study aimed to investigate incidence, risk factors (including impact of immunosuppressive regimen) and survival of PTLD patients after liver transplantation in Iranian patients.
Multi-organ involvement was associated with a lower post-PTLD survival and increased mortality. Epstein-Barr virus (EBV)-positive patients had higher mean post-PTLD survival in comparison with EBV-negative subjects. EBV status was not associated with time of PTLD development in our study. This finding is in contrast with previous reports showing that EBV-negative PTLD occurs later after liver transplantation when compared to EBV-positive PTLD patients. We showed that a serum tacrolimus cutoff value of 11.1 ng/mL is associated with post-PTLD survival.
Adjustment of tacrolimus level to lower than 11.1 ng/mL may help improve post-PTLD survival of patients.
The reviewer has read with interest the manuscript entitled, “Post-transplant lymphoproliferative disorder after liver transplantation: incidence, long-term survival and impact of serum tacrolimus level”. Eshraghian and colleagues performed a retrospective single-center study with a wide recruitment period, including 53 liver transplant patients who developed PTLD (40 pediatric and 13 adult cases). The authors evaluated the risk factors affecting post-PTLD survival of patients. They found that EBV-negative recipients and multi-organ involvement are the two main risk factors of lower post-PTLD survival. They further found within a pediatric recipient cohort that higher serum tacrolimus level was associated with poor survival after PTLD development.
| 1. | Starzl TE, Fung JJ. Themes of liver transplantation. Hepatology. 2010;51:1869-1884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 2. | Penn I. Posttransplantation de novo tumors in liver allograft recipients. Liver Transpl Surg. 1996;2:52-59. [PubMed] |
| 3. | Vaysberg M, Lambert SL, Krams SM, Martinez OM. Activation of the JAK/STAT pathway in Epstein Barr virus+-associated posttransplant lymphoproliferative disease: role of interferon-gamma. Am J Transplant. 2009;9:2292-2302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Loren AW, Porter DL, Stadtmauer EA, Tsai DE. Post-transplant lymphoproliferative disorder: a review. Bone Marrow Transplant. 2003;31:145-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 182] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 5. | Cohen JI. Epstein-Barr virus lymphoproliferative disease associated with acquired immunodeficiency. Medicine (Baltimore). 1991;70:137-160. [PubMed] |
| 6. | Thorley-Lawson DA, Gross A. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N Engl J Med. 2004;350:1328-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 735] [Cited by in RCA: 757] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 7. | Evens AM, Roy R, Sterrenberg D, Moll MZ, Chadburn A, Gordon LI. Post-transplantation lymphoproliferative disorders: diagnosis, prognosis, and current approaches to therapy. Curr Oncol Rep. 2010;12:383-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | Duvoux C, Pageaux GP, Vanlemmens C, Roudot-Thoraval F, Vincens-Rolland AL, Hézode C, Gaulard P, Miguet JP, Larrey D, Dhumeaux D. Risk factors for lymphoproliferative disorders after liver transplantation in adults: an analysis of 480 patients. Transplantation. 2002;74:1103-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | McLaughlin K, Wajstaub S, Marotta P, Adams P, Grant DR, Wall WJ, Jevnikar AM, Rizkalla KS. Increased risk for posttransplant lymphoproliferative disease in recipients of liver transplants with hepatitis C. Liver Transpl. 2000;6:570-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 57] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Guthery SL, Heubi JE, Bucuvalas JC, Gross TG, Ryckman FC, Alonso MH, Balistreri WF, Hornung RW. Determination of risk factors for Epstein-Barr virus-associated posttransplant lymphoproliferative disorder in pediatric liver transplant recipients using objective case ascertainment. Transplantation. 2003;75:987-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Newell KA, Alonso EM, Whitington PF, Bruce DS, Millis JM, Piper JB, Woodle ES, Kelly SM, Koeppen H, Hart J. Posttransplant lymphoproliferative disease in pediatric liver transplantation. Interplay between primary Epstein-Barr virus infection and immunosuppression. Transplantation. 1996;62:370-375. [PubMed] |
| 12. | Cacciarelli TV, Reyes J, Jaffe R, Mazariegos GV, Jain A, Fung JJ, Green M. Primary tacrolimus (FK506) therapy and the long-term risk of post-transplant lymphoproliferative disease in pediatric liver transplant recipients. Pediatr Transplant. 2001;5:359-364. [PubMed] |
| 13. | González-Barca E, Domingo-Domenech E, Capote FJ, Gómez-Codina J, Salar A, Bailen A, Ribera JM, López A, Briones J, Muñoz A. Prospective phase II trial of extended treatment with rituximab in patients with B-cell post-transplant lymphoproliferative disease. Haematologica. 2007;92:1489-1494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 100] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Choquet S, Leblond V, Herbrecht R, Socié G, Stoppa AM, Vandenberghe P, Fischer A, Morschhauser F, Salles G, Feremans W. Efficacy and safety of rituximab in B-cell post-transplantation lymphoproliferative disorders: results of a prospective multicenter phase 2 study. Blood. 2006;107:3053-3057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 302] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 15. | Majewski M, Korecka M, Kossev P, Li S, Goldman J, Moore J, Silberstein LE, Nowell PC, Schuler W, Shaw LM. The immunosuppressive macrolide RAD inhibits growth of human Epstein-Barr virus-transformed B lymphocytes in vitro and in vivo: A potential approach to prevention and treatment of posttransplant lymphoproliferative disorders. Proc Natl Acad Sci USA. 2000;97:4285-4290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 164] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Harris N, Swerdlow S, Frizzera G, Knowles D. Post-transplant lymphoproliferative disorders. World Health Organization classification of tumours. Pathology and genetics of tumours of hematopoietic and lymphoid tissues. Lyon: IARC Press 2001; 264-269. |
| 17. | Lust JA. Molecular genetics and lymphoproliferative disorders. J Clin Lab Anal. 1996;10:359-367. [PubMed] |
| 18. | Cox KL, Lawrence-Miyasaki LS, Garcia-Kennedy R, Lennette ET, Martinez OM, Krams SM, Berquist WE, So SK, Esquivel CO. An increased incidence of Epstein-Barr virus infection and lymphoproliferative disorder in young children on FK506 after liver transplantation. Transplantation. 1995;59:524-529. [PubMed] |
| 19. | Koch DG, Christiansen L, Lazarchick J, Stuart R, Willner IR, Reuben A. Posttransplantation lymphoproliferative disorder--the great mimic in liver transplantation: appraisal of the clinicopathologic spectrum and the role of Epstein-Barr virus. Liver Transpl. 2007;13:904-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Fernández MC, Bes D, De Dávila M, López S, Cambaceres C, Dip M, Imventarza O. Post-transplant lymphoproliferative disorder after pediatric liver transplantation: characteristics and outcome. Pediatr Transplant. 2009;13:307-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Leblond V, Choquet S. Lymphoproliferative disorders after liver transplantation. J Hepatol. 2004;40:728-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Haseli N, Hassanzadeh J, Dehghani SM, Bahador A, Malek Hosseini SA. Long-term survival and its related factors in pediatric liver transplant recipients of shiraz transplant center, shiraz, iran in 2012. Hepat Mon. 2013;13:e10257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Ghobrial IM, Habermann TM, Macon WR, Ristow KM, Larson TS, Walker RC, Ansell SM, Gores GJ, Stegall MD, McGregor CG. Differences between early and late posttransplant lymphoproliferative disorders in solid organ transplant patients: are they two different diseases? Transplantation. 2005;79:244-247. [PubMed] |
| 24. | Leblond V, Davi F, Charlotte F, Dorent R, Bitker MO, Sutton L, Gandjbakhch I, Binet JL, Raphael M. Posttransplant lymphoproliferative disorders not associated with Epstein-Barr virus: a distinct entity? J Clin Oncol. 1998;16:2052-2059. [PubMed] |
| 25. | Evens AM, David KA, Helenowski I, Nelson B, Kaufman D, Kircher SM, Gimelfarb A, Hattersley E, Mauro LA, Jovanovic B. Multicenter analysis of 80 solid organ transplantation recipients with post-transplantation lymphoproliferative disease: outcomes and prognostic factors in the modern era. J Clin Oncol. 2010;28:1038-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 254] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 26. | Kremers WK, Devarbhavi HC, Wiesner RH, Krom RA, Macon WR, Habermann TM. Post-transplant lymphoproliferative disorders following liver transplantation: incidence, risk factors and survival. Am J Transplant. 2006;6:1017-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Maecker B, Jack T, Zimmermann M, Abdul-Khaliq H, Burdelski M, Fuchs A, Hoyer P, Koepf S, Kraemer U, Laube GF. CNS or bone marrow involvement as risk factors for poor survival in post-transplantation lymphoproliferative disorders in children after solid organ transplantation. J Clin Oncol. 2007;25:4902-4908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 96] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 28. | Elstrom RL, Andreadis C, Aqui NA, Ahya VN, Bloom RD, Brozena SC, Olthoff KM, Schuster SJ, Nasta SD, Stadtmauer EA. Treatment of PTLD with rituximab or chemotherapy. Am J Transplant. 2006;6:569-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 29. | Dotti G, Fiocchi R, Motta T, Mammana C, Gotti E, Riva S, Cornelli P, Gridelli B, Viero P, Oldani E. Lymphomas occurring late after solid-organ transplantation: influence of treatment on the clinical outcome. Transplantation. 2002;74:1095-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 30. | Nelson BP, Nalesnik MA, Bahler DW, Locker J, Fung JJ, Swerdlow SH. Epstein-Barr virus-negative post-transplant lymphoproliferative disorders: a distinct entity? Am J Surg Pathol. 2000;24:375-385. [PubMed] |
| 31. | Schubert S, Renner C, Hammer M, Abdul-Khaliq H, Lehmkuhl HB, Berger F, Hetzer R, Reinke P. Relationship of immunosuppression to Epstein-Barr viral load and lymphoproliferative disease in pediatric heart transplant patients. J Heart Lung Transplant. 2008;27:100-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 32. | Swinnen LJ, Costanzo-Nordin MR, Fisher SG, O’Sullivan EJ, Johnson MR, Heroux AL, Dizikes GJ, Pifarre R, Fisher RI. Increased incidence of lymphoproliferative disorder after immunosuppression with the monoclonal antibody OKT3 in cardiac-transplant recipients. N Engl J Med. 1990;323:1723-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 715] [Cited by in RCA: 615] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 33. | Ganschow R, Schulz T, Meyer T, Broering DC, Burdelski M. Low-dose immunosuppression reduces the incidence of post-transplant lymphoproliferative disease in pediatric liver graft recipients. J Pediatr Gastroenterol Nutr. 2004;38:198-203. [PubMed] |
| 34. | Molmenti EP, Nagata DE, Roden JS, Squires RH, Molmenti H, Fasola CG, Winick N, Tomlinson G, Lopez MJ, D’Amico L. Post-transplant lymphoproliferative syndrome in the pediatric liver transplant population. Am J Transplant. 2001;1:356-359. [PubMed] |
| 35. | Geramizadeh B, Malek-Hosseini SA, Bahador A, Salahi H, Nikeghbalian S, Sharifian M, Lankarani KB, Imanieh MH, Dehghani M. Post-transplantation lymphoproliferative disorder after liver transplantation: report of 5 cases among more than 550 liver transplants in Iran. Arch Iran Med. 2010;13:417-419. [PubMed] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Iran
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): C, C, C
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Kin T, Salvadori M, Suzuki M S- Editor: Qi Y L- Editor: Filipodia E- Editor: Wang CH