Published online Feb 21, 2017. doi: 10.3748/wjg.v23.i7.1215
Peer-review started: October 20, 2016
First decision: November 9, 2016
Revised: November 26, 2016
Accepted: January 11, 2017
Article in press: January 11, 2017
Published online: February 21, 2017
Processing time: 123 Days and 21.4 Hours
To investigate the surgical therapies for gastric cancer (GC) patients of age 85 or older in a multicenter survey.
Therapeutic opportunities for elderly GC patients have expanded in conjunction with extended life expectancy. However, the number of cases encountered in a single institution is usually very small and surgical therapies for elderly GC patients have not yet been standardized completely. In the present study, a total of 134 GC patients of age 85 or older who underwent surgery in 9 related facilities were retrospectively investigated. The relationships between surgical therapies and clinicopathological or prognostic features were analyzed.
Eighty-nine of the patients (66%) presented with a comorbidity, and 26 (19% overall) presented with more than two comorbidities. Radical lymphadenectomy was performed in 59 patients (44%), and no patient received pre- or post-operative chemotherapy. Forty of the patients (30%) experienced perioperative complications, but no surgical or perioperative mortality occurred. Laparoscopic surgery was performed in only 12 of the patients (9.0%). Univariate and multivariate analyses of the 113 patients who underwent R0 or R1 resection identified the factors of pT3/4 and limited lymphadenectomy as predictive of worse prognosis (HR = 4.68, P = 0.02 and HR =2.19, P = 0.05, respectively). Non-cancer-specific death was more common in cStage I patients than in cStage II or III patients. Limited lymphadenectomy correlated with worse cancer-specific survival (P = 0.01), particularly in cStage II patients (P < 0.01). There were no relationships between limited lymphadenectomy and any comorbidities, except for cerebrovascular disease (P = 0.07).
Non-cancer-specific death was not negligible, particularly in cStage I, and gastrectomy with radical lymphadenectomy appears to be an effective treatment for cStage II elderly GC patients.
Core tip: Therapeutic opportunities for elderly gastric cancer (GC) patients have expanded. This multicenter study investigated surgical therapies for GC patients of age 85 or older. Cancer-specific and overall survival rates were 100% and 56% in cStage I. The factors of pT3/4 and limited lymphadenectomy were predictive of worse prognosis. Cancer-specific survival in cStage II with radical lymphadenectomy was significantly better, but did not significantly benefit cStage III. Only cerebrovascular disease was related with limited lymphadenectomy. Non-cancer-specific death was not negligible, particularly in cStage I, and gastrectomy with radical lymphadenectomy appeared to be an effective treatment for cStage II elderly patients.
- Citation: Konishi H, Ichikawa D, Itoh H, Fukuda K, Kakihara N, Takemura M, Okugawa K, Uchiyama K, Nakata M, Nishi H, Kosuga T, Komatsu S, Okamoto K, Otsuji E. Surgery for gastric cancer patients of age 85 and older: Multicenter survey. World J Gastroenterol 2017; 23(7): 1215-1223
- URL: https://www.wjgnet.com/1007-9327/full/v23/i7/1215.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i7.1215
The elderly population is increasing worldwide, and life expectancy has also consistently increased in most countries[1]. In Japan, the average lifetime of women is 87 years while that of men is 81 years, and the life expectancies of 85-year-old women and men are 8.4 and 6.2 years, respectively[2]. Therefore, gastric cancer (GC) patients of age 85 or older may undergo radical gastrectomy with the aim of achieving 5-year survival. On the other hand, elderly patients may present with existing functional decline in major organs and comorbidities[1,3]. The rate of non-cancer-specific death in elderly patients has generally been increasing, and post-operative disorders following gastrectomy may indirectly influence the cause of death[4-6]. Therefore, the decision to perform surgery on elderly GC patients needs to be made carefully.
Standard therapeutic strategies for GC patients in Japan are selected according to the Japanese Gastric Cancer Treatment Guidelines[7]; however, these guidelines are not standardized for elderly GC patients, particularly those aged 85 or older. Although some retrospective studies of GC patients in single institutions have evaluated gastrectomy for its feasibility and safety among the elderly patient population[8-10], few have investigated patients of age 85 or older[11,12].
We previously reported the surgical outcomes of gastrectomy for elderly GC patients and concluded that the outcomes were similar to those in non-elderly patients[13]. However, it remains unclear as to whether radical gastrectomy has prognostic significance in elderly GC patients. There are some limitations in the selection of therapeutic strategies for elderly GC patients, due to the small number of cases that a single institution usually encounters. Therefore, we collected data on GC patients of age 85 or older who underwent surgery in our related hospitals, and herein report our findings on the surgical therapies, clinicopathological features and survival.
A total of 134 GC patients of age 85 or older who underwent surgery in any of our related hospitals (9 facilities) between 2000 and 2014 were retrospectively registered. Thirty-six patients were treated at hospital 1, 21 at hospital 2, 20 at hospital 3, 12 at hospital 4, 11 at hospital 5, 9 at hospital 6, 10 at hospital 7, 4 at hospital 8, and 11 at hospital 9. Clinical and pathological stages were determined based on the Japanese Classification of Gastric Carcinoma 3rd edition[14]. No patients in the present study received neo-adjuvant or adjuvant chemotherapy. Some clinicopathological and prognostic data were not usable, as they were outdated. The median length of follow-up for censored cases was 19.5 mo (range: 1-88 mo).
Written informed consent for surgery was obtained from all patients in each institution; however, it was confirmed that written informed consent for participation in the present study was not always necessary because this was a retrospective non-interventional study.
Standard operability for each case was decided according to the Japanese Gastric Cancer Treatment Guidelines[7]. The operative procedure and extent of resection or lymphadenectomy were ultimately selected by each institution based on the clinical stage and location of the cancer. The extent of lymphadenectomy was re-evaluated using data obtained from the dissected lymph nodes and pre-operative clinical staging that was based on the Japanese Classification of Gastric Carcinoma 3rd edition[14]; briefly, radical lymphadenectomy was adapted to cT1N0 patients who underwent D1 or more extended lymphadenectomy and to cN+ or cT2-4 patients who underwent D2 lymphadenectomy. In the present study, splenectomy in total gastrectomy was not related to the extent of lymphadenectomy because significance of splenectomy due to No.10 or 11d lymph node dissection was controversial.
The status of residual tumors after surgery was also described as the R status, according to the Japanese Classification of Gastric Carcinoma 3rd edition[14]. R0 denoted curative resection, R1 denoted resection with a microscopic residual tumor (positive in the resection margin, or CY+), and R2 denoted resection with a macroscopic residual tumor. In the present study, patients with bypass or un-resected surgery were included among the R2 cases.
All statistical analyses were performed using StatView 5.0 J software (SAS Institute Inc., Cary, NC, United States). Survival curves for overall survival, cancer-specific survival and non-original disease-specific death were derived using the Kaplan-Meier method and compared by the stratified Log-rank test. A multivariate survival analysis was performed using Cox’s proportional hazard regression model. A P-value less than 0.05 was considered significant.
The characteristics and perisurgical outcomes of the total 134 patients analyzed in this study are shown in Table 1. All patients were diagnosed with adenocarcinoma, and 57 patients (43%) were female. Comorbidities were present in 89 of the patients (66%), and 26 of the patients (19%) had more than one comorbidity. Hypertension was the most frequent comorbidity (52/134, 39%), followed by cardiovascular disease (36/134, 27%), respiratory disease (13/134, 10%) and diabetes mellitus (13/134, 10%). The mean post-operative hospital stay was 29 d (range: 9-305 d), and no operative or perioperative mortalities occurred, excluding gastric cancer-specific death.
| Total patients (n = 134) | |
| Age (yr) | 87.4 (85-97) |
| Sex (male/female) | 77/57 |
| Post-operative hospital stay (d) | 29 (9-305) |
| Comorbidity | 89 (66.0) |
| Hypertension | 52 (39.0) |
| Cardiovascular disease | 36 (27) |
| Respiratory disease | 13 (9.7) |
| Cerebrovascular disease | 9 (6.7) |
| Diabetes mellitus | 13 (9.7) |
| Renal dysfunction | 4 (3.0) |
| Other | 4 (3.0) |
| Number of comorbidities | |
| 0 | 45 |
| 1/2 | 63/18 |
| 3/4 | 6/2 |
| Operation | |
| Distal | 84 |
| Proximal | 5 |
| Total | 34 |
| Partial | 4 |
| Bypass | 4 |
| Unresectable | 3 |
| Procedure | |
| Open | 122 |
| Laparoscopy | 12 |
| Lymphadenectomy | |
| D0/D1 | 17/52 |
| D1+/D2 | 40/25 |
| Lymphadenectomy | |
| Radical/limited | 59/75 |
| Number of resected LN | 23 (0-67) |
| Operative time (min) | 206 (62-426) |
| Bleeding (g) | 263 (10-1855) |
| Residual tumor | |
| R0/1/2 | 103/10/21 |
Distal gastrectomy was performed on 84 patients, proximal gastrectomy on 5, total gastrectomy on 34, and partial gastrectomy on 4 (Table 1). Neither pre- nor post-operative chemotherapy was performed on any patient. The original lesions were un-resectable in 7 patients, and bypass surgery was performed on 4 of these patients. Laparoscopic surgery was performed on only 12 patients (12/134, 9.0%), including 9 distal and 3 total gastrectomies. The rates of R0+1 resection, radical lymphadenectomy and post-operative complications in laparoscopic surgery were 100% (12/12), 75% (9/12) and 17% (2/12), respectively.
Table 2 shows the clinicopathological features and post-operative complications, with the Clavien-Dindo classification of more than grade II[15,16]. Complications were present in 40 of the total 134 patients (30%). Although the frequencies of anastomotic leakage (12/134, 9.0%) and pneumonia (7/134, 5.2%) were slightly high, no lethal complications or re-operations occurred.
| Total patients, n =134 | ||
| Location | L/M/U | 59/51/24 |
| Macroscopic type | 0/1/2/3/4/5/unknown | 35/8/35/25/10/3/18 |
| cT | 1-2/3-4 | 64/70 |
| cN | -/+ | 70/64 |
| cStage | I/II/III/IV | 54/36/32/12 |
| pT | 1-2/3-4 | 62/72 |
| pN | -/+/unknown | 59/68/7 |
| pStage | I/II/III/IV | 49/29/39/17 |
| Differentiation | Well/moderate/poor/unknown | 46/17/55/16 |
| Lymphatic invasion | - +/unknown | 38/79/17 |
| Venous invasion | -/+/unknown | 55/62/17 |
| Complications | -/+ | 94/40 |
| Surgical site infection | 11 (8.2) | |
| Anastomotic leakage | 12 (9.0) | |
| Anastomotic stenosis | 4 (3.0) | |
| Pneumonia | 7 (5.2) | |
| Pancreatic fistula | 3 (2.2) | |
| Intestinal hypoperistalsis | 2 (1.5) | |
| Cardiac complication | 1 (0.7) | |
| Brain complication | 1 (0.7) | |
| Other | 2 (1.5) | |
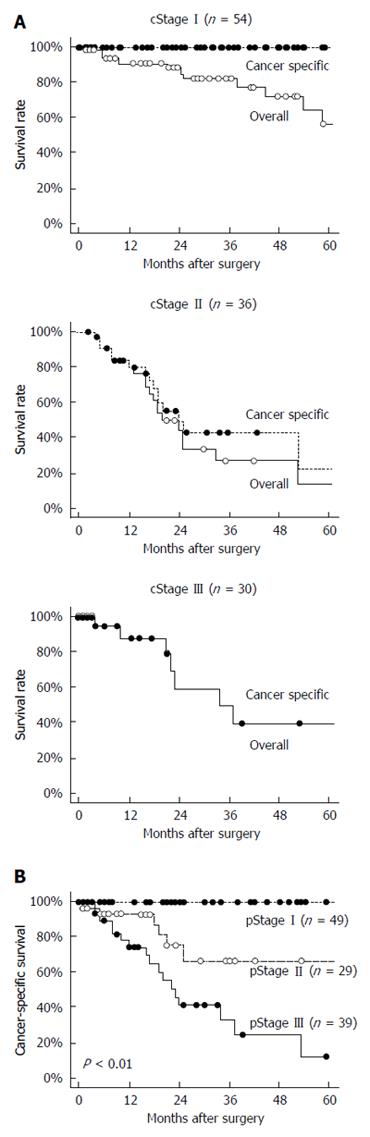
Overall survival and cancer-specific survival in cStage I/II/III patients are shown in Figure 1A. In cStage I patients, the 5-year cancer-specific survival rate was 100%, whereas the overall survival rate was remarkably worse (at 56%) because of non-cancer-specific deaths. The cStage II or III patients showed similar overall and cancer-specific survival rates, again with the rate of cancer-specific deaths higher than that of non-cancer-specific deaths. Cancer-specific survival was significantly different in each pStage (P < 0.01; Figure 1B).
Table 3 shows the relationships between survival and clinicopathological features for the 113 patients applicable for these analyses, after exclusion of those patients who underwent R2 resection. Limited lymphadenectomy (P = 0.01), cT3-4 (P < 0.01), pT3-4 (P < 0.01), pN+ (P < 0.01), pStage III-VI (P < 0.01), and positive venous invasion (P < 0.01) were identified as worse prognostic factors in the univariate analysis, whereas only an advanced pT factor and limited lymphadenectomy appeared to be prognostic factors in the multivariate analysis (HR = 4.68, 95%CI: 1.29-20.7, P = 0.02 and HR = 2.19, 95%CI: 1.00-4.97, P = 0.05, respectively).
| n = 113 | 5-yr survival | Univariate | Multivariate | |||
| P value | HR | 95%CI | P value | |||
| Sex | ||||||
| Male | 65 | 33 | 0.17 | - | ||
| Female | 48 | 48 | ||||
| Comorbidities | ||||||
| < 2 | 93 | 35 | 0.35 | - | ||
| ≥ 2 | 20 | 63 | ||||
| Operation | ||||||
| Total | 29 | 36 | 0.13 | - | ||
| Others | 84 | 41 | ||||
| Procedure | ||||||
| Open | 101 | 37 | 0.10 | - | ||
| Laparoscopy | 12 | 62 | ||||
| Lymphadenectomy | ||||||
| Radical | 59 | 43 | 0.01 | 1.00 | 1.00-4.97 | 0.05 |
| Limited | 54 | 35 | 2.19 | |||
| Operative time (min) | ||||||
| < 240 | 76 | 32 | 0.34 | - | ||
| ≥ 240 | 33 | 58 | ||||
| Unknown | 4 | |||||
| Bleeding (g) | ||||||
| < 400 | 91 | 40 | 0.12 | - | ||
| ≥ 400 | 18 | 27 | ||||
| Unknown | 4 | |||||
| cT | ||||||
| 1-2 | 63 | 49 | < 0.01 | 1.16 | 0.41-3.23 | 0.78 |
| 3-4 | 50 | 27 | 1.00 | |||
| cN | ||||||
| Absent | 67 | 45 | 0.43 | - | ||
| Present | 46 | 35 | ||||
| cStage | ||||||
| I-II | 87 | 40 | 0.59 | - | ||
| III- IV | 26 | 37 | ||||
| pT | ||||||
| 1-2 | 62 | 58 | < 0.01 | 1.00 | 1.29-20.7 | 0.02 |
| 3-4 | 51 | 16 | 4.68 | |||
| pN | ||||||
| Absent | 58 | 56 | < 0.01 | 1.00 | 0.68-5.62 | 0.24 |
| Present | 55 | 23 | 1.84 | |||
| pStage | ||||||
| I-II | 78 | 55 | < 0.01 | - | ||
| III- IV | 35 | 9 | ||||
| Residual tumor | ||||||
| R0 | 103 | 41 | 0.06 | 1.14 | 0.39-3.87 | 0.81 |
| R1 | 10 | 31 | 1.00 | |||
| Lymphatic invasion | ||||||
| Absent | 37 | 55 | 0.09 | 1.49 | 0.43-5.10 | 0.53 |
| Present | 67 | 29 | 1.00 | |||
| Unknown | 9 | |||||
| Venous invasion | ||||||
| Absent | 53 | 58 | < 0.01 | 1.00 | 0.75-5.80 | 0.17 |
| Present | 51 | 15 | 2.00 | |||
| Unknown | 9 | |||||
| Complications | ||||||
| Absent | 81 | 34 | 0.31 | - | ||
| Present | 32 | 51 | ||||
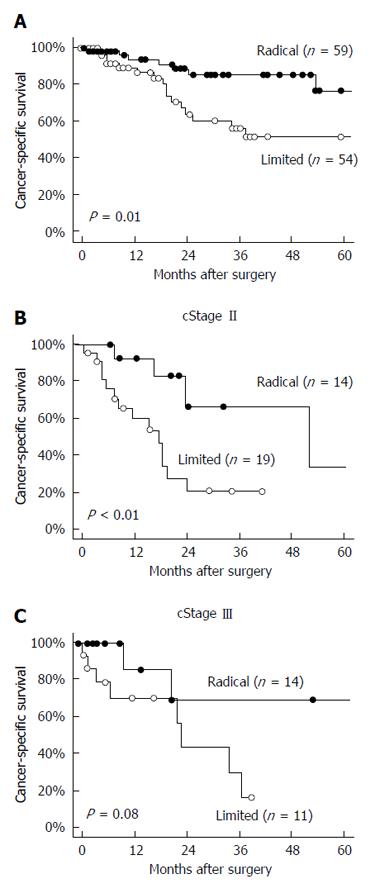
Cancer-specific survival in the patients who underwent radical lymphadenectomy was significantly better than in those who underwent limited lymphadenectomy (P = 0.01; Figure 2A). In a subgroup analysis, the cancer-specific survival in cStage II patients who underwent radical lymphadenectomy was also significantly better than in those who underwent limited lymphadenectomy (P < 0.01; Figure 2B); however, the difference was not significant in the cStage III patients (P = 0.08; Figure 2C).
Most comorbidities were not associated with limited lymphadenectomy, and only the presence of cerebrovascular disease was found to be associated (P = 0.07; Table 4).
| n = 113 | Lymphadenectomy | P value | ||
| Radical | Limited | |||
| Comorbidity | ||||
| Present | 75 | 45 | 30 | 0.02 |
| Absent | 38 | 14 | 24 | |
| Hypertension | ||||
| Present | 43 | 28 | 15 | 0.03 |
| Absent | 70 | 31 | 39 | |
| Cardiovascular disease | ||||
| Present | 31 | 14 | 17 | 0.36 |
| Absent | 82 | 45 | 37 | |
| Respiratory disease | ||||
| Present | 11 | 7 | 4 | 0.42 |
| Absent | 102 | 52 | 50 | |
| Cerebrovascular disease | ||||
| Present | 6 | 1 | 5 | 0.07 |
| Absent | 107 | 58 | 49 | |
| Diabetes mellitus | ||||
| Present | 12 | 7 | 5 | 0.65 |
| Absent | 101 | 52 | 49 | |
| Renal dysfunction | ||||
| Present | 3 | 3 | 0 | 0.09 |
| Absent | 110 | 56 | 54 | |
| Number of comorbidities | ||||
| < 2 | 93 | 47 | 46 | 0.44 |
| ≥ 2 | 20 | 12 | 8 | |
| cStage | ||||
| I-II | 87 | 45 | 42 | 0.85 |
| III- IV | 26 | 14 | 12 | |
| Operation | ||||
| Total | 29 | 14 | 15 | 0.62 |
| Other | 84 | 45 | 39 | |
Life-span has been extended for the elderly by advances in medical treatments[1,2]. In conjunction, therapeutic opportunities for elderly cancer patients, including those with GC, have also increased[1]. Therapeutic strategies for GC in Japan are standardized by the Japanese Gastric Cancer Association Guidelines[7]; however, strategies for elderly patients are not clearly stated. An important issue is that systemic conditions and previous histories generally vary among elderly patients[3-6,17], and generally the number of elderly patients is still smaller than of young patients treated in a single Japanese institution. Therefore, comprehensive data on GC patients, particularly those aged 85 or older, have not been reported in detail[11,12,18-20].
In the present study, we retrospectively collected information on GC patients of age 85 or older who underwent surgery in our related hospitals in order to investigate therapeutic strategies for elderly GC patients. We were unable to confirm information on the frequency of patients treated by non-surgical therapies or untreated due to their general condition. Therefore, although this set of data may be slightly biased due to regional characteristics or therapeutic strategies in each institution, the average frequency of GC patients aged 85 or older treated by surgery was almost similar among all the institutions (at 2.6%, range: 2.2%-3.8%).
There were some distinct characteristics noted for the treatment of elderly GC patients compared with younger GC patients. Neo-adjuvant or adjuvant chemotherapy was not performed in any of the elderly GC patients, and the frequencies of laparoscopic gastrectomy and radical lymphadenectomy were slightly low for the elderly GC patients as well. Although lymphadenectomy was limited in some patients, cancer-specific survival in cStage I was remarkably favorable and the leading cause of death was non-cancer-specific death, specifically due to pneumonia or cardiovascular event. These non-cancer-specific events occurred equally among the elderly patients. Therefore, gastrectomy with limited lymphadenectomy or less-invasive laparoscopic surgery is permissible, at least for elderly patients with cStage I[21]. On the other hand, among the elderly patients with cStage II or III, non-cancer-specific death was not of greater clinical importance than cancer-specific death. Cancer-specific survival in patients who underwent radical lymphadenectomy was significantly better than in those who underwent limited lymphadenectomy. In subgroup analysis, this tendency was more significant in cStage II patients than in cStage III patients.
There was no positive relationship found between the limited lymphadenectomy and the presence or number of comorbidities or the extent of resection; however, the patients with cerebrovascular disease were more likely to have undergone limited lymphadenectomy.
As reported by the Japanese Association of Clinical Cancer Centers, the 5-year relative survival rates of GC patients treated with any surgical therapy between 2004 and 2007 were 96% in cStage I, 66.9% in cStage II, 48.1% in cStage III and 15.7% in cStage IV[22]. When the results of the present study are generally compared with results of younger GC patients, the cancer-specific survival rate in cStage I elderly patients is acceptable, regardless of the surgical extent; however, non-cancer-specific death was frequent in the elderly patient population of the present study and appeared to be affected by surgical therapy and previous histories[3,4,6]. On the other hand, the survival rate of cStage III elderly patients was slightly low compared to that which is known for younger GC patients. We attributed this discrepancy to the lack of adjuvant chemotherapy[23,24] and the stage migration of cStage IV patients due to limited lymphadenectomy[25,26]. The survival rate in cStage II elderly patients was similar to that in younger patients[20]. Moreover, cancer-specific survival in cStage II patients who underwent radical lymphadenectomy was significantly better than in those who underwent limited lymphadenectomy. This result indicates that radical lymphadenectomy can improve survival in cStage II elderly patients[10,12].
There were some limitations to the present study, particularly related to the small number of cases, which must be considered when interpreting the findings. Stage migration should be considered because limited lymphadenectomy was performed more frequently[23,24]. The rate of cancer-specific survival in cStage I patients was very high, regardless of lymphadenectomy; however, survival among cStage II or III patients who underwent limited lymphadenectomy was expected to be improved by stage migration. Therefore, this feature may have affected the results of the present study. Furthermore, overall and cancer-specific survivals were not fully divided by clinical stages, particularly for the cStage II or III patients. The clinical staging data may have been influenced by a limitation in imaging techniques, the criteria used to reach a diagnosis in each institution, or short follow-up length. In the present study, we were unable to reconfirm details on clinical staging or prognosis in each institution.
In conclusion, although the present study was performed using limited case samples and with some biases, the results obtained showed a trend of elderly GC patients towards surgical therapy. A less-invasive gastrectomy will be permissible, at least for cStage I elderly patients, and surgical therapy with radical lymphadenectomy may be effective for cStage II elderly patients; however, further studies on non-cancer-specific death or chemotherapy are needed.
The elderly population is increasing worldwide, and therapeutic opportunities for elderly gastric cancer (GC) patients have also expanded. On the other hand, elderly patients may present with existing functional decline in major organs and comorbidities. Although retrospective studies using information of single institutions have evaluated gastrectomy among elderly patient populations, therapeutic strategies for elderly GC patients, particularly those aged 85 or older, are not standardized due to the generally small number of cases.
GC patients of age 85 or older have undergone radical gastrectomy with the aim of achieving 5-year survival. However, the decision to perform surgery on an elderly GC patient needs to be made carefully, because the rate of non-cancer-specific death will be increasing generally and post-operative disorders following gastrectomy may indirectly influence the cause of death. The research hotspot is to examine optimized surgical therapies for elderly GC patients in each clinical disease stage by using a multicenter survey approach.
There are some limitations in the selection of therapeutic strategies for elderly GC patients due to the small number of cases in a single institution. In the present study, the data for 134 GC patients of age 85 or older who underwent surgery were collected from our 9 related hospitals. The cancer-specific survival rate for cStage I elderly patients was acceptable, regardless of the surgical extent; however, the rate of non-cancer-specific deaths due to pneumonia or cardiovascular event was frequent and not negligible. On the other hand, the rate of non-cancer-specific death was not high in cStage II or III patients, and cancer-specific survival with radical lymphadenectomy was significantly better than that achieved with limited lymphadenectomy in cStage II patients.
The data in this study suggests that a less-invasive gastrectomy will be permissible for elderly patients, at least for those with cStage I GC. Furthermore, this study also provides readers important information regarding surgical therapy with radical lymphadenectomy in elderly GC patients, particularly as related to its effectiveness in cStage II elderly patients.
A limited lymphadenectomy for GC is considered for lymphadenectomy less frequently than standard resection, but it has been successfully performed in selected patients with poor general condition or of elderly age. Although the definitive indication of limited lymphadenectomy is not determined, such a limited therapy will be acceptable for some patients, such as the oldest-old patients, after careful considerations of the therapeutic quality or prognosis.
The authors do a good work, they collected data on GC patients aged 85 or older who underwent surgery in their related hospitals, and examined surgical therapies, clinicopathological features, and survival, which give us some treatment advice for elder gastric cancer patients.
| 1. | Lichtman SM, Hurria A, Jacobsen PB. Geriatric oncology: an overview. J Clin Oncol. 2014;32:2521-2522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 2. | Ministry of Health, Labour and Welfare. Abridged life tables for Japan 2014. Available from: http://www.mhlw.go.jp/toukei/saikin/hw/life/life14/index.html. |
| 3. | Extermann M, Overcash J, Lyman GH, Parr J, Balducci L. Comorbidity and functional status are independent in older cancer patients. J Clin Oncol. 1998;16:1582-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 751] [Cited by in RCA: 711] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 4. | Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118:219-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 929] [Cited by in RCA: 938] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 5. | Smetana GW, Lawrence VA, Cornell JE; American College of Physicians. Preoperative pulmonary risk stratification for noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med. 2006;144:581-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 621] [Cited by in RCA: 546] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 6. | Endo S, Yoshikawa Y, Hatanaka N, Dousei T, Yamada T, Nishijima J, Kamiike W. Prognostic factors for gastrectomy in elderly patients. Int Surg. 2014;99:166-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Japanese Gastric Cancer Association. Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2014 (version 4). 2014;. |
| 8. | Gretschel S, Estevez-Schwarz L, Hünerbein M, Schneider U, Schlag PM. Gastric cancer surgery in elderly patients. World J Surg. 2006;30:1468-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Kunisaki C, Akiyama H, Nomura M, Matsuda G, Otsuka Y, Ono HA, Shimada H. Comparison of surgical outcomes of gastric cancer in elderly and middle-aged patients. Am J Surg. 2006;191:216-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Bandoh T, Isoyama T, Toyoshima H. Total gastrectomy for gastric cancer in the elderly. Surgery. 1991;109:136-142. [PubMed] |
| 11. | Endo S, Dousei T, Yoshikawa Y, Hatanaka N, Kamiike W, Nishijima J. Prognosis of gastric carcinoma patients aged 85 years or older who underwent surgery or who received best supportive care only. Int J Clin Oncol. 2013;18:1014-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Kiyokawa T, Hiki N, Nunobe S, Honda M, Ohashi M, Sano T, Yamaguchi T. Feasibility of Gastrectomy with Standard Lymphadenectomy for Patients Over 85 Years Old with Gastric Cancer. Ann Surg Oncol. 2015;22:3962-3969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Takeshita H, Ichikawa D, Komatsu S, Kubota T, Okamoto K, Shiozaki A, Fujiwara H, Otsuji E. Surgical outcomes of gastrectomy for elderly patients with gastric cancer. World J Surg. 2013;37:2891-2898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (1)] |
| 14. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2947] [Article Influence: 196.5] [Reference Citation Analysis (1)] |
| 15. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 26103] [Article Influence: 1186.5] [Reference Citation Analysis (2)] |
| 16. | Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6210] [Cited by in RCA: 9198] [Article Influence: 541.1] [Reference Citation Analysis (1)] |
| 17. | Yamada H, Shinohara T, Takeshita M, Umesaki T, Fujimori Y, Yamagishi K. Postoperative complications in the oldest old gastric cancer patients. Int J Surg. 2013;11:467-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Isobe T, Hashimoto K, Kizaki J, Miyagi M, Aoyagi K, Koufuji K, Shirouzu K. Surgical procedures, complications, and prognosis for gastric cancer in the very elderly (& gt; 85): a retrospective study. Kurume Med J. 2012;59:61-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Ishigami S, Natsugoe S, Hokita S, Iwashige H, Saihara T, Tokushige M, Aikou T. Strategy of gastric cancer in patients 85 years old and older. Hepatogastroenterology. 1999;46:2091-2095. [PubMed] |
| 20. | Endo S, Yoshikawa Y, Hatanaka N, Tominaga H, Shimizu Y, Hiraoka K, Nishitani A, Irei T, Nakashima S, Park MH. Treatment for gastric carcinoma in the oldest old patients. Gastric Cancer. 2011;14:139-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Kwon IG, Cho I, Guner A, Kim HI, Noh SH, Hyung WJ. Minimally invasive surgery as a treatment option for gastric cancer in the elderly: comparison with open surgery for patients 80 years and older. Surg Endosc. 2015;29:2321-2330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Ministry of Health, Labour and Welfare. The 5-years relative survival rate of gastric cancer patients with any surgical therapies in 2004-2007. Available from: http://www.gunma-cc.jp/sarukihan/seizonritu/seizonritu2007c.html. |
| 23. | Sun DS, Jeon EK, Won HS, Park JC, Shim BY, Park SY, Hong YS, Kim HK, Ko YH. Outcomes in elderly patients treated with a single-agent or combination regimen as first-line chemotherapy for recurrent or metastatic gastric cancer. Gastric Cancer. 2015;18:644-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Desai AM, Lichtman SM. Systemic therapy of non-colorectal gastrointestinal malignancies in the elderly. Cancer Biol Med. 2015;12:284-291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 25. | Schwarz RE, Smith DD. Clinical impact of lymphadenectomy extent in resectable gastric cancer of advanced stage. Ann Surg Oncol. 2007;14:317-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 211] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 26. | Bunt AM, Hermans J, Smit VT, van de Velde CJ, Fleuren GJ, Bruijn JA. Surgical/pathologic-stage migration confounds comparisons of gastric cancer survival rates between Japan and Western countries. J Clin Oncol. 1995;13:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 189] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Garcia-Olmo D, Huang CM, Lee JI, Nagahara H, Yang MH S- Editor: Gong ZM L- Editor: A E- Editor: Wang CH