Published online Dec 28, 2017. doi: 10.3748/wjg.v23.i48.8526
Peer-review started: October 30, 2017
First decision: November 21, 2017
Revised: November 27, 2017
Accepted: December 13, 2017
Article in press: December 13, 2017
Published online: December 28, 2017
Processing time: 60 Days and 0.4 Hours
To determine the prevalence, characteristics and clinical course of pancreatic cystic neoplasms (PCNs) in liver transplantation (LT) recipients.
We retrospectively studied consecutive patients who underwent LT between January 1998 to April 2016. Clinical and laboratory data were obtained from patient medical records. Imaging findings on computed tomography and magnetic resonance cholangiopancreatography were reviewed by two radiologists.
During the study period, 872 patients underwent cadaveric LT. Pancreatic cysts were identified in 53/872 (6.1%) and 31/53 (58.5%) were PCNs [28 intraductal papillary mucinous neoplasm (IPMN), 2 mucinous cystic neoplasm (MCN), 1 serous cystadenoma]. Patients with PCNs exhibited less male predominance (55% vs 73%, P = 0.03) compared to patients without pancreatic cysts. Thirteen patients (42%) were diagnosed with PCN pre-LT while 18 patients (58%) developed PCN post-LT. The median size of PCNs was 13mm [interquartile range (IQR) 10-20 mm]. All IPMNs were side-branch type. Most PCNs were found in the head and body of pancreas (37% each), followed by the tail (25%). Five patients underwent further evaluation with endoscopic ultrasound. Progress imaging was performed on 81% of patients. PCNs remained stable in size and number in all but 2 patients. During a median follow up of 39 mo (IQR 26-58 mo), the 2 (6%) patients with MCN underwent pancreatectomy. No PCN patient developed pancreatic adenocarcinoma, while 5 died from illnesses unrelated to the PCN. Among patients without PCN, 1/841 (0.1%) developed pancreatic adenocarcinoma.
The prevalence of PCNs in LT recipients was similar to the general population (3.6%, 31/872). Side-branch IPMNs do not appear to have accelerated malignant potential in post-LT patients, indicating the current surveillance guidelines are applicable to this group.
Core tip: The prevalence of pancreatic cystic neoplasms (PCNs) in liver transplantation (LT) recipients is similar to that of the general population and PCNs do not appear to behave more aggressively in this setting. Our results suggest that current surveillance guidelines can be safely applied in the immunosuppressed LT population.
- Citation: Liu K, Joshi V, van Camp L, Yang QW, Baars JE, Strasser SI, McCaughan GW, Majumdar A, Saxena P, Kaffes AJ. Prevalence and outcomes of pancreatic cystic neoplasms in liver transplant recipients. World J Gastroenterol 2017; 23(48): 8526-8532
- URL: https://www.wjgnet.com/1007-9327/full/v23/i48/8526.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i48.8526
Pancreatic cystic lesions are a common incidental finding in asymptomatic adults undergoing abdominal imaging, with a quoted prevalence of 2%-13%[1,2]. While 80% of pancreatic cysts are non-neoplastic (simple cysts, pseudocysts, retention cysts), some are considered to be premalignant lesions and require surveillance[3]. These cysts with neoplastic potential are classified as pancreatic cystic neoplasms (PCNs) of which the two most common types are intraductal papillary mucinous neoplasm (IPMN) and mucinous cystic neoplasm (MCNs). Currently, the assessment of malignant progression risk in PCNs mainly focuses on cyst type and characteristics including size, presence of a solid component and main pancreatic duct dilatation[4,5].
The prevalence of PCNs has been reported to be twice as common in cirrhotic patients compared to the general population while their risk of pancreatic cancer is increased five-fold[6,7]. Liver transplantation (LT) is the treatment of choice for appropriately selected patients with end-stage liver cirrhosis. Limited data exist regarding whether LT and its ensuing immunosuppression influences the incidence and risk of malignant progression of PCNs. Furthermore, patient populations and immunosuppression protocols differ between LT centers worldwide, which may influence the prevalence and natural history of PCNs. LT recipients undergo regular cross-sectional abdominal imaging [computed tomography (CT) and/or magnetic resonance imaging (MRI)] as a part of hepatocellular carcinoma (HCC) screening and LT work up. The aim of this study was to determine the prevalence, characteristics and clinical course of PCNs in LT recipients in a large Australian LT center.
A retrospective review of medical records for all LT recipients from January 1998 to April 2016 at Royal Prince Alfred Hospital, a quaternary state-wide LT referral center in Sydney, Australia was performed. Patient demographics, etiology of liver disease, and radiology reports of CT or MRI scans were reviewed by electronic medical records. Those with pancreatic lesions reported on abdominal imaging had their scans reviewed and further data were collected including: clinical findings including history of pancreatitis, laboratory values, histology reports, and results of fluid analysis, if available. Clinical pancreatitis was defined by the presence of two of the following three criteria: (1) characteristic abdominal pain; (2) elevated (≥ 3 times the upper limit of normal) levels of serum amylase and/or lipase; or (3) characteristic findings on a CT scan[8]. Pancreatitis was attributed to the PCN if no other etiology was present e.g. alcohol abuse (alcoholic pancreatitis) or choledocholithiasis (gallstone pancreatitis). Patients with known chronic pancreatic disease were excluded. Outcomes measures included growth of the PCN, the development of pancreatic adenocarcinoma, and requirement for surgical resection and mortality. Patients were otherwise followed until the date of their last abdominal CT or MRI scan. The study protocol was approved by the Sydney Local Health District Human Research Ethics Committee (X15-0426 and LNR/15/RPAH/570).
Abdominal imaging (either CT, MRI or both), for all patients with a pancreatic cystic lesion was reviewed by two independent radiologists. CT was performed on either GE 16 or 64 slice or Siemens SOMATOM Plus 4 spiral scanners depending on year. Either single portal venous phase (70 s after injection) or multiphase images were obtained with pre-contrast, arterial (40 s after injection), portal-venous (70 s after injection) and delayed (five minutes after injection). Magnetic resonance cholangiopancreatography (MRCP) was performed on either Siemens (3T) or GE (1.5T) magnets depending on inpatient or outpatient status. The institution’s MRCP standard protocol for both magnets includes axial and coronal T2-weighted single shot fast spin echo breath-hold, respiratory triggered axial T2-weighted fast recovery fast spine echo (FRFSE), heavily-weighted axial T2 with fat suppression, axial T1-weighted pancreas in and out of phase, axial T1 pancreas fat suppressed, three-dimensional and thick slab MRCP respiratory triggered. Where indicated, gadolinium (Gadovist, Bayer Group, Germany) was administered at a rate of 1.5mL/s with pancreatic (20-40 s after injection), portal venous (65-85 s after injection) and delayed phase (three minutes and five minutes after injection) sequences obtained. Review of images was performed on a PACS-integrated workstation (Centricity RA 1000, GE Healthcare, Little Chalfont, United Kingdom).
Characterization of pancreatic cysts on imaging was performed using generally accepted imaging criteria[9,10]. A presumed imaging diagnosis of a mucinous cystadenoma was made if the cyst was unilocular with or without mild septations, located in the body or tail and with the signal intensity of simple fluid on MRI. If a unilocular or multilocular cyst demonstrated communication with a non-dilated main pancreatic duct, then a side-branch IPMN was diagnosed. If the cyst was comprised of numerous small cysts, with or without central calcification and no ductal communication on imaging, then a favored diagnosis of serous cystadenoma was given. A lesion was classified as a pseudocyst if a unilocular cyst was seen with layering debris and/or features of acute or chronic pancreatitis. In the absence of the above imaging features, a diagnosis of an undifferentiated pancreatic cyst was made. Final cyst diagnoses were also governed by results of cyst fluid analysis, cytology or histology in patients who underwent endoscopic ultrasound (EUS) with cyst aspiration or resection.
PCNs were evaluated for cyst size, multiplicity, location in the pancreas (head, body and tail). For patients with multiple PCNs, data from the largest cyst were recorded. The presence of worrisome features was also assessed including: size > 30 mm, thickened or enhancing cyst walls, dilated pancreatic duct, or solid component to the cyst[5]. The evolution of PCNs was assessed for changes in cyst size and characteristics in patients who underwent subsequent imaging.
Categorical data was analyzed by the Pearson χ2 test or Fisher’s Exact test where appropriate. The Mann Whitney U test was used to analyze continuous data with non-normal distribution. Statistical analyses were performed using SPSS v.22.0 software (IBM, Armonk, NY, United States). A result was considered statistically significant if P < 0.05.
During the study period, 872 patients underwent cadaveric LT. Median age at time of LT was 60 years [interquartile range (IQR) 55-66 years]. The majority of patients were male (630/872, 72%). The most common cause of liver disease was hepatitis C (35%), followed by alcohol (15%), hepatitis B (13%), primary sclerosing cholangitis (9%), and non-alcoholic fatty liver disease (4%). HCC complicated the liver disease in 14% (126/872) of patients pre-LT.
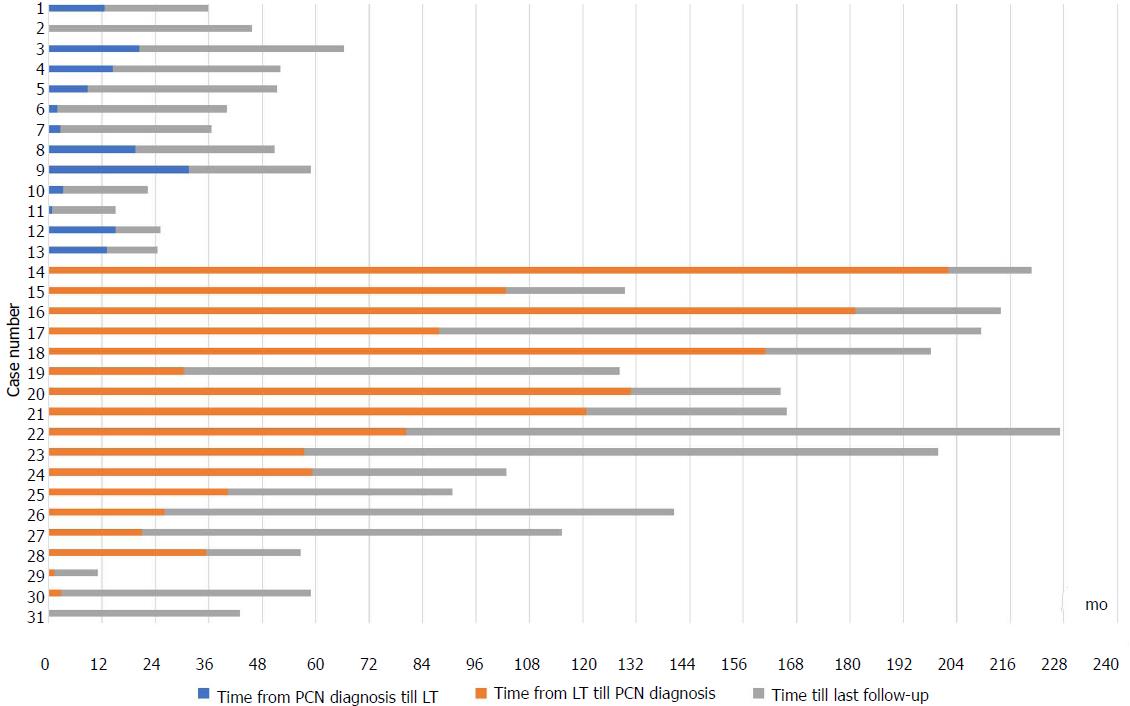
Pancreatic cysts were identified in 53/872 (6.1%) of which 31 (3.6%) were PCNs (28 IPMNs, two MCNs, one serous cystadenoma). The other cystic lesions included: undifferentiated cyst (13/53), simple cysts (6/53) and pseudocysts (2/53). All PCNs were discovered incidentally (71% on CT, 29% on MRCP), with no prior history of pancreatitis. All undifferentiated cysts were < 30 mm in size, well-defined, round and exhibited a homogenous fluid signal. Most PCNs (22/31, 71%) were diagnosed in the last five years of the study period while 27/31 (87%) were diagnosed in the last ten years. Thirteen patients (42%) were diagnosed with PCN pre-LT while 18 patients (58%) developed PCN post-LT (Figure 1). The median age at cyst discovery was 59 years (IQR 56-62 years). Patients with PCNs exhibited less male predominance (55% vs 73%, P = 0.03) and were comparable in terms of age at time of LT (median 62 years vs 60 years, P = 0.18) compared to those without PCNs. There were no significant differences in etiology of liver disease in patients with and without PCN (P = 0.65). Presence of HCC also did not differ between the two groups (10% PCN group vs 15% no PCN, P = 0.44). The median white cell count in patients at time of PCN discovery was 5.0 × 109/L (IQR 4.1-7.1 × 109/L, normal laboratory range 4.0-10.0 × 109/L). Patient characteristics comparing patients with and without PCNs are shown in Table 1.
| Characteristic | All Patients, n = 872 | Patients with PCN, n = 31 | Patients without PCN, n = 841 | P value |
| Age at LT (yr) | 60 (55-66) | 62 (59-66) | 60 (54-66) | 0.18 |
| Age at cyst PCN discovery (yr) | N/A | 59 (56-62) | N/A | N/A |
| Males | 630 (72) | 17 (56) | 613 (73) | 0.03a |
| Cause of liver disease | 0.65 | |||
| Hepatitis C | 304 (35) | 8 (26) | 296 (35) | |
| Alcoholic liver disease | 134 (15) | 2 (7) | 132 (16) | |
| Hepatitis B | 109 (13) | 3 (10) | 106 (13) | |
| Primary sclerosing cholangitis | 79 (9) | 4 (13) | 75 (9) | |
| Non-alcoholic fatty liver disease | 39 (4) | 3 (10) | 36 (4) | |
| Primary biliary cholangitis | 38 (4) | 2 (7) | 36 (4) | |
| Autoimmune hepatitis | 31 (4) | 2 (7) | 29 (3) | |
| Cryptogenic | 19 (2) | 1 (3) | 18 (2) | |
| Other | 118 (14) | 6 (19) | 112 (13) | |
| Concomitant HCC | 126 (14) | 3 (10) | 123 (14) | 0.44 |
The majority of patients with PCNs had multiple cystic lesions (20/31, 65%). Most PCNs occurred in the head of pancreas (37%) and body (37%) followed by the tail (25%). The median size of PCNs at initial diagnosis was 13 mm (IQR 10-20 mm). All 28 IPMNs were side-branch type. No PCNs exhibited worrisome features in the form of having a solid component, thickened or enhancing wall or pancreatic duct dilatation. However, three PCNs were > 30 mm in size: an IPMN at 35 mm, a MCN at 40 mm and a serous cystadenoma at 35 mm. Five patients (16%) underwent further evaluation with EUS evaluation which confirmed the suspected lesion seen on imaging (three side-branch IPMNs, two MCNs). Only one patient underwent cyst aspiration with cytology and fluid analysis. At time of cyst discovery, the median serum Ca 19.9 was 48 U/mL (IQR 35.5-112.5 U/mL, reference range < 37 U/mL) and the median serum lipase was 48 U/L (IQR 44-75 U/L, reference range 13-60 U/L).
Patients with PCN were followed up for a median duration of 40 mo from date of cyst discovery (IQR 27-59 mo). Surveillance with CT, MRCP and/or EUS was performed on 25/31 (81%) patients. Two of the remaining six patients who did not have surveillance had only recently been diagnosed with PCNs and were not yet due for repeat imaging according to guidelines[5]. Of the patients with subsequent imaging, PCNs remained stable in size in 20/25 (80%) patients, reduced in size in 3/25 (12%) patients and increased in size in 2/25 (8%) patients. No patients developed clinical pancreatitis during follow-up.
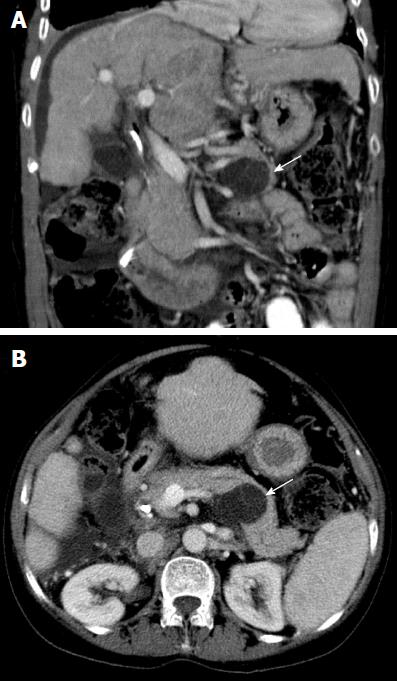
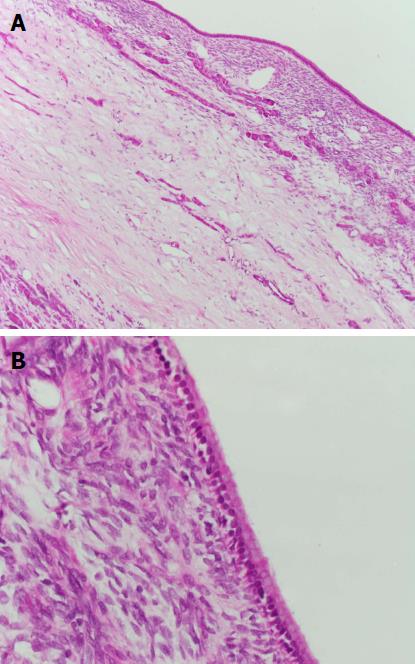
No patient with a PCN developed pancreatic adenocarcinoma. Two patients (6%) underwent surgical resection of their PCN. Both patients had a MCN in the tail of the pancreas and underwent distal pancreatectomy and splenectomy without operative complications. The first patient was a 38-year-old woman whose MCN was discovered on routine LT assessment (for primary biliary cholangitis) initially measuring 9 mm in diameter. This subsequently grew to 17 mm and 26 mm at two and five years post-LT, respectively, which prompted the decision to resect. Histopathology revealed a MCN with intermediate dysplasia. The second patient, a 61-year-old woman, had her MCN discovered on LT assessment (for primary sclerosing cholangitis) initially measuring 40mm in diameter. The lesion grew further to 47 mm and was subsequently resected post-LT with histopathology showing a MCN with low grade dysplasia (Figures 2 and 3). Surgical resection was not considered for the other two aforementioned patients with PCNs > 30 mm in size (one 35 mm IPMN, one 35 mm serous cystadenoma) due to significant medical co-morbidities.
During the follow-up period, five patients died due to acute pathologies or post-LT complications unrelated to their PCNs. Incidentally, among the patients without PCN, 1/841 (0.1%) developed metastatic pancreatic adenocarcinoma eight years after LT for hepatitis C and HCC.
In this large cohort study of 872 LT patients, the prevalence of PCNs was found to be 3.6%. This is similar to the rate of incidental PCNs in the general population found on abdominal imaging for other indications[1,2]. Although PCNs were commonly encountered, cyst size > 30 mm (10%) and cyst enlargement on surveillance imaging (6%) were uncommon, while the presence of other worrisome features was not seen. Accordingly, the rate of pancreatic adenocarcinoma in our study was low with no cases seen among patients with PCNs.
Patients with cirrhosis have been shown to be immune suppressed[11,12]. Furthermore, patients who undergo LT remain on life-long immunosuppressive drugs. Based on our results, the immunosuppressive effect of cirrhosis pre-LT and anti-rejection medications post-LT does not seem to alter the natural course of PCNs nor increase the risk of malignant transformation. Hence current guidelines for surveillance of PCNs can likely be safely applied in the LT population.
Our findings are consistent with other studies of PCNs in the LT setting. Vidhyarkorn et al[13] reported a prevalence of 3.9% (70/1778 patients) for pancreatic cystic lesions demonstrated on CT or MRI among LT recipients. Despite showing an increase in the average size and number of PCNs by 4.5mm and 1.4, respectively during the follow-up period, only one patient developed a mixed acinar-neuroendocrine carcinoma 9 years post-LT. In a French study of LT waitlist patients, IPMN was identified in 6.6% (14/212 patients) using MRI. No worrisome features, pancreatic resections or pancreatic adenocarcinomas were observed[14]. In contrast, Lennon et al[15] followed 297 patients with side-branch IPMN and found high-risk features in up to 17%, potentially related to a higher proportion of patients undergoing EUS. There was no significant difference in risk of progression when comparing 23 patients who had undergone LT versus 274 patients without a history of immunosuppression. All the above authors concluded that LT does not appear to increase the risk of malignancy in patients with PCNs despite immunosuppression.
The strength of our study is the large cohort of LT patients (872 patients over 18 years) with a relatively large number of PCNs (28 IPMNs versus 14-23 in other studies). While previous studies in the LT population have focused on IPMNs[13-15], our study is the first to describe MCNs in this setting. The two patients with MCNs were middle-aged females with single lesions located in the tail of the pancreas - hallmark characteristics of MCNs in the general population[3,4]. Surgical resection of MCNs is recommended for all surgically fit patients[5]. Both patients with MCN in our study underwent pancreatectomy post-LT with significant dysplasia seen on histopathology, however it remains unknown whether the behavior of MCNs is altered by immunosuppression. Clearly, further studies with a focus on MCNs in immunosuppressed patients are needed.
A limitation of the present study is its retrospective nature which impacts on the accuracy and completeness of the data, as well as the potential influence of length time bias. However, retrospective data collection provided the advantage of capturing patients with PCNs over an 18-year period of LT. Furthermore, our study outcomes of PCN growth, pancreatic adenocarcinoma, surgical resection and mortality are hard endpoints which minimizes other potential biases. Although the imaging of patients without PCNs described on their initial radiological reports was not re-reviewed, all scans at our institution are reported formally by at least one experienced radiologist. Our data also suggest that awareness and identification of PCNs is increasing with most (72%) of our PCNs diagnosed in the last five years of the study period. However, the recency of these diagnoses has meant that our follow-up period in this study was relatively short (median 40 mo) which limited our ability to capture events such as cyst growth and malignancy transformation.
In conclusion, the prevalence of PCNs was 3.6% in our cohort of Australian LT patients. This is similar to that of the general population. All PCNs were discovered incidentally and over half of PCNs developed post-LT. All patients with IPMN exhibited a benign course in this study while 2/2 MCN (6% of PCNs) patients underwent surgical resection. No cases of pancreatic adenocarcinoma occurred in patients with pre-existing PCNs. These results suggest that current surveillance guidelines can likely be safely applied in the LT population.
Pancreatic cystic neoplasms (PCNs) are a common incidental finding and their behaviour in liver transplantation (LT) recipients has not been well studied.
By studying PCNs in the LT setting, we can observe whether the immunosuppressive effects of cirrhosis (pre-LT) and anti-rejection medications (post-LT) have any impact on PCN development and progression. This would provide important information on whether current screening and surveillance for the general population can be safely applied to these patients.
We aimed to determine the prevalence, characteristics and clinical course of PCNs in LT recipients.
Consecutive patients who underwent LT between January 1998 to April 2016 were retrospectively studied. Clinical and laboratory data and imaging findings on computed tomography and/or magnetic resonance cholangiopancreatography were assessed.
The prevalence of PCNs was 3.6% in our cohort of Australian LT patients which is similar to that of the general population. All PCNs were discovered incidentally and over half of PCNs developed post-LT. Only 3/31 (10%) PCNs exhibited worrisome features. All patients with intraductal papillary mucinous neoplasm (IPMN) exhibited a benign course in this study while 2/2 mucinous cystic neoplasm (MCN) (6% of PCNs) patients underwent surgical resection. No cases of pancreatic adenocarcinoma occurred in patients with pre-existing PCNs.
This is the first cohort study of PCNs in Australian LT recipients. It is also the first study to describe MCNs in the LT setting. Our results suggest that current surveillance guidelines can likely be safely applied in the LT population. Our data also suggest that awareness and identification of PCNs is increasing with most being identified in the last five years of our study period.
It would be important to pool experience from multiple centers to improve our knowledge of the behavior of non-IPMN PCNs in the context of immunosuppression.
We would to thank Dr. Catriona McKenzie for her assistance with obtaining histopathology pictures for our manuscript.
| 1. | Lee KS, Sekhar A, Rofsky NM, Pedrosa I. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol. 2010;105:2079-2084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 446] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 2. | de Jong K, Nio CY, Hermans JJ, Dijkgraaf MG, Gouma DJ, van Eijck CH, van Heel E, Klass G, Fockens P, Bruno MJ. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin Gastroenterol Hepatol. 2010;8:806-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 386] [Article Influence: 24.1] [Reference Citation Analysis (1)] |
| 3. | Brugge WR. Diagnosis and management of cystic lesions of the pancreas. J Gastrointest Oncol. 2015;6:375-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 43] [Reference Citation Analysis (0)] |
| 4. | Scheiman JM, Hwang JH, Moayyedi P. American gastroenterological association technical review on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148:824-848.e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 314] [Article Influence: 28.5] [Reference Citation Analysis (1)] |
| 5. | Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1714] [Cited by in RCA: 1645] [Article Influence: 117.5] [Reference Citation Analysis (0)] |
| 6. | Zerboni G, Capurso G, Di Pietropaolo M, Carbonetti F, Iannicelli E, Marignani M, Delle Fave G. The prevalence of pancreatic cystic lesions in patients with liver cirrhosis is double that in controls. United European Gastroenterol J. 2017;5:1007-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Kalaitzakis E, Gunnarsdottir SA, Josefsson A, Björnsson E. Increased risk for malignant neoplasms among patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9:168-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Muddana V, Whitcomb DC, Papachristou GI. Current management and novel insights in acute pancreatitis. Expert Rev Gastroenterol Hepatol. 2009;3:435-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Kalb B, Sarmiento JM, Kooby DA, Adsay NV, Martin DR. MR imaging of cystic lesions of the pancreas. Radiographics. 2009;29:1749-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 10. | Sahani DV, Kambadakone A, Macari M, Takahashi N, Chari S, Fernandez-del Castillo C. Diagnosis and management of cystic pancreatic lesions. AJR Am J Roentgenol. 2013;200:343-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 11. | Piconese S, Timperi E, Barnaba V. 'Hardcore' OX40+ immunosuppressive regulatory T cells in hepatic cirrhosis and cancer. Oncoimmunology. 2014;3:e29257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | O’Brien AJ, Fullerton JN, Massey KA, Auld G, Sewell G, James S, Newson J, Karra E, Winstanley A, Alazawi W. Immunosuppression in acutely decompensated cirrhosis is mediated by prostaglandin E2. Nat Med. 2014;20:518-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 243] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 13. | Vidhyarkorn S, Siripongsakun S, Yu J, Sayre J, Agopian VG, Durazo F, Lu DS. Longterm follow-up of small pancreatic cystic lesions in liver transplant recipients. Liver Transpl. 2017;23:324-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Laurent L, Vullierme MP, Rebours V, Maire F, Hentic O, Francoz C, Durand F, Ruszniewski P, Lévy P. Estimation of the prevalence of intraductal papillary mucinous neoplasm of the pancreas in the French population through patients waiting for liver transplantation. United European Gastroenterol J. 2017;5:499-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Lennon AM, Victor D, Zaheer A, Ostovaneh MR, Jeh J, Law JK, Rezaee N, Molin MD, Ahn YJ, Wu W. Liver transplant patients have a risk of progression similar to that of sporadic patients with branch duct intraductal papillary mucinous neoplasms. Liver Transpl. 2014;20:1462-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Gong JS, Gupta R, Matsuda Y S- Editor: Chen K L- Editor: A E- Editor: Ma YJ