Published online Dec 28, 2017. doi: 10.3748/wjg.v23.i48.8512
Peer-review started: July 25, 2017
First decision: September 14, 2017
Revised: September 29, 2017
Accepted: November 22, 2017
Article in press: November 22, 2017
Published online: December 28, 2017
Processing time: 154 Days and 23.7 Hours
To investigate the inhibitory effect of astragaloside IV on the pathological functions of cancer-associated fibroblasts, and to explore the underlying mechanism.
Paired gastric normal fibroblast (GNF) and gastric cancer-associated fibroblast (GCAF) cultures were established from resected tissues. GCAFs were treated with vehicle control or different concentrations of astragaloside IV. Conditioned media were prepared from GNFs, GCAFs, control-treated GCAFs, and astragaloside IV-treated GCAFs, and used to culture BGC-823 human gastric cancer cells. Proliferation, migration and invasion capacities of BGC-823 cells were determined by MTT, wound healing, and Transwell invasion assays, respectively. The action mechanism of astragaloside IV was investigated by detecting the expression of microRNAs and the expression and secretion of the oncogenic factor, macrophage colony-stimulating factor (M-CSF), and the tumor suppressive factor, tissue inhibitor of metalloproteinase 2 (TIMP2), in different groups of GCAFs. The expression of the oncogenic pluripotency factors SOX2 and NANOG in BGC-823 cells cultured with different conditioned media was also examined.
GCAFs displayed higher capacities to induce BGC-823 cell proliferation, migration, and invasion than GNFs (P < 0.01). Astragaloside IV treatment strongly inhibited the proliferation-, migration- and invasion-promoting capacities of GCAFs (P < 0.05 for 10 μmol/L, P < 0.01 for 20 μmol/L and 40 μmol/L). Compared with GNFs, GCAFs expressed a lower level of microRNA-214 (P < 0.01) and a higher level of microRNA-301a (P < 0.01). Astragaloside IV treatment significantly up-regulated microRNA-214 expression (P < 0.01) and down-regulated microRNA-301a expression (P < 0.01) in GCAFs. Reestablishing the microRNA expression balance subsequently suppressed M-CSF production (P < 0.01) and secretion (P < 0.05), and elevated TIMP2 production (P < 0.01) and secretion (P < 0.05). Consequently, the ability of GCAFs to increase SOX2 and NANOG expression in BGC-823 cells was abolished by astragaloside IV.
Astragaloside IV can inhibit the pathological functions of GCAFs by correcting their dysregulation of microRNA expression, and it is promisingly a potent therapeutic agent regulating tumor microenvironment.
Core tip: Most of chemotherapeutic agents directly act on cancer cells. However, direct drug attacks on cancer cells have the drawbacks of accelerating cancer evolution, chemoresistance, recurrence, and metastasis. Cancer-associated fibroblasts considerably contribute to cancer initiation and progression by providing nourishment and support for cancer cells. Blocking the pathological functions of cancer-associated fibroblasts can eliminate the conditions suitable for cancer cell survival and expansion, representing a novel effective anti-cancer strategy. Unfortunately, few effective drugs have been found against cancer-associated fibroblasts. Here, astragaloside IV significantly inhibited the pathological functions of gastric cancer-associated fibroblasts, suggesting its valuable therapeutic application in gastric carcinoma.
- Citation: Wang ZF, Ma DG, Zhu Z, Mu YP, Yang YY, Feng L, Yang H, Liang JQ, Liu YY, Liu L, Lu HW. Astragaloside IV inhibits pathological functions of gastric cancer-associated fibroblasts. World J Gastroenterol 2017; 23(48): 8512-8525
- URL: https://www.wjgnet.com/1007-9327/full/v23/i48/8512.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i48.8512
Cancer is a major burden of disease worldwide. Every year, approximately 14 million new cases are reported and 8.2 million cancer-related deaths occur[1]. Chemotherapy forms the mainstay of cancer treatment, particularly for patients who do not respond to local excision or radiation treatment[2]. Currently, most chemotherapeutic agents directly act on cancer cells. However, this approach has two serious drawbacks. First, because cancer cells have unstable genomes, repeated drug attacks aggravate their genomic instability and cause new mutations in their genomes, leading to acceleration of cancer evolution[3,4]. Second, some commonly used chemotherapeutic drugs induce the conversion of differentiated cancer cells to cancer stem cells, thus provoking treatment resistance and speeding up cancer metastasis[5,6]. Therefore, new strategies and drugs are urgently required to effectively treat cancer.
Cancer malignancy is caused not only by genetic and epigenetic abnormalities in cancer cells, but more profoundly, by the stimulation and support from the tumor microenvironment[7,8]. Within the tumor microenvironment, cancer-associated fibroblasts, which can accompany metastatic cancer cells to the invasive front and the tumor-interstitium interface, constitute the dominant cell population[8]. They secrete soluble factors, such as oncogenic cytokines, chemokines, and matrix metalloproteinases, into the microenvironment, thus creating suitable conditions for the activation and maintenance of cancer cell survival, proliferation, migration, invasion, and treatment resistance[7,9]. Due to their stable genomes with few mutations, cancer-associated fibroblasts are proper drug targets. Inhibition of the nourishment and support provided by cancer-associated fibroblasts with proper drugs can destroy the “fertile soil” for cancer cell survival and expansion, representing a robust strategy to control cancer. However, few effective drugs have been found against cancer-associated fibroblasts.
Traditional Chinese medicine and pharmacology is a valuable source of knowledge warranting in-depth exploration. It has accumulated rich anti-cancer experience over thousands of years of clinical practice. Based on the unique concept of holism, traditional Chinese medicine investigates the mechanisms of cancer initiation and progression by considering the interactions among the tumor, human body, and surrounding environment instead of merely focusing on cancer cells[10]. Used under the guidance of traditional Chinese medicine theories, Chinese herbs can not only kill cancer cells, but more excellently, can regulate and improve patients’ internal environment to restore various tissues and cells to their normal states and enhance their functions[11]. Consequently, the body’s internal environment becomes unfavorable for tumor initiation and progression; the tumor microenvironment is unable to nourish cancer cells and support their growth; the cancer cells encounter conditions unsuitable for their survival. Thus, traditional Chinese herbs are a promising source of cancer-associated fibroblast inhibitors.
Radix astragali is a crucial Chinese herb prescribed for strengthening the constitutions of patients and eliminating toxins from their bodies. It is frequently present in anti-cancer formulas[12]. Astragaloside IV is a cycloartane-type triterpene glycoside isolated from Radix astragali. It has been reported to suppress matrix metalloproteinase-1 expression in dermal fibroblasts[13] and reduce collagen I/III secretion by skin fibroblasts[14], exhibiting an ability to affect fibroblast functions. Considering this property of astragaloside IV, we speculated that it can inhibit the pathological functions of cancer-associated fibroblasts. In the present study, for the first time, we treated gastric cancer-associated fibroblasts (GCAFs) with astragaloside IV, observed its effect on the malignancy-promoting functions of GCAFs, and explored the mechanism underlying the effect of astragaloside IV on GCAFs.
Human gastric carcinoma BGC-823 cell line was purchased from the Cell Bank of Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai, China). Astragaloside IV was provided by Standard Technology Co., Ltd. (Shanghai, China) and dissolved in DMSO to a concentration of 20 mM as a stock solution. The stock solution was diluted with RPMI-1640 medium when used and the final DMSO concentration did not exceed 0.2% (v/v). A mouse monoclonal antibody against pan-cytokeratin (pan-CK) and rabbit polyclonal antibodies against E-cadherin, vimentin, PDGFR-β, and alpha smooth muscle actin (α-SMA) were obtained from Abcam (Cambridge, United Kingdom). Rabbit polyclonal antibodies against macrophage colony-stimulating factor (M-CSF), tissue inhibitor of metalloproteinase 2 (TIMP2), SRY-box 2 (SOX2), NANOG, GAPDH, and β-actin, as well as CY3-conjugated goat anti-mouse IgG, CY3-conjugated goat anti-rabbit IgG, and HRP-conjugated goat anti-rabbit IgG were obtained from Elabscience Biotechnology (Wuhan, Hubei, China). The 2’-O-methyl chemically modified oligonucleotides for microRNA-214 inhibitors (anti-miR-214), anti-miR-214 negative control (214-NC), microRNA-301a mimic (miR-301a mimic), and miR-301a mimic negative control (301a-NC) were produced by GenePharma (Suzhou, Jiangsu, China).
Gastric tumor tissue and adjacent normal tissue (at least 2 cm from the outer tumor margin) were obtained from a patient with moderately differentiated gastric adenocarcinoma during gastric surgery and immediately transported to the laboratory. The fresh tissue samples were minced into 0.5-1 mm3 fragments, seeded in 60-mm culture dishes in the presence of RPMI-1640 supplemented with 20% fetal bovine serum (FBS), and cultured at 37 °C in a humid atmosphere containing 5% CO2. The culture medium was changed twice a week for 2-3 wk. Under these conditions, fibroblasts grew out from tissue fragments while other cells were mostly retained in the fragments. After reaching confluence, monolayers were trypsinized and passaged at a split ratio of 1:2 (passage 1). The fibroblasts were then sub-cultured for another 3-4 passages until the cultures were free of epithelial cell contamination and subsequently maintained in RPMI-1640 supplemented with 10% FBS, 2% penicillin, and 2% streptomycin (complete RPMI-1640 medium). GNFs and GCAFs were both used between passage 5 and 8.
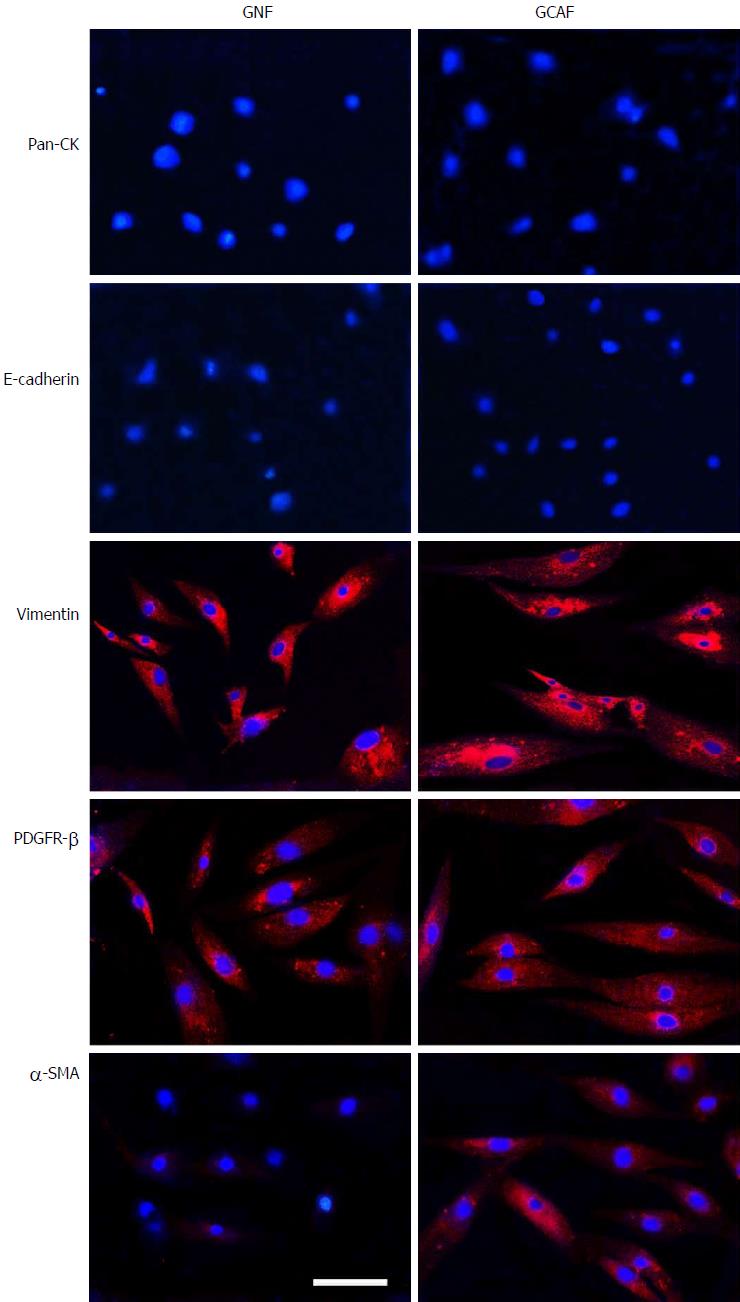
Gastric normal fibroblasts (GNFs) and GCAFs grown on glass coverslips were washed with cold phosphate-buffered saline (PBS), fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100, and then incubated with primary antibodies to pan-CK (1:100), E-cadherin (1:100), vimentin (1:150), PDGFR-β (1:100), and α-SMA (1:100) overnight at 4 °C. The cells were subsequently incubated with fluorescence-conjugated secondary antibodies for 1 h at 37 °C. After three PBS washes, cell nuclei were counterstained with DAPI for 5 min; the cells were examined with a Nikon Eclipse Ti-S Epifluorescence microscope equipped with a DS-L2 digital camera (Nikon).
To prepare conditioned media from GNFs and GCAFs, GNFs and GCAFs were plated into 60-mm dishes (5 × 105 cells) and cultured with complete RPMI-1640 medium. Twelve hours later, the medium was replaced with 4 mL fresh RPMI-1640 medium for an additional 48 h culture. The supernatants were collected, centrifuged at 1000 rpm, filtered with 0.1 μm membranes, and supplemented with 3% FBS.
To prepare conditioned media from astragaloside IV-treated GCAFs, GCAFs were treated with 10, 20, or 40 μmol/L astragaloside IV or 0.2% DMSO (vehicle control) for 72 h. Then, the cells were collected and cultured in complete RPMI-1640 medium in 60-mm dishes (5 × 105 cells) for 12 h. Thereafter, the medium was replaced with 4 mL fresh RPMI-1640 medium for an additional 48 h culture. The supernatants were collected, centrifuged at 1000 rpm, filtered with 0.1 μm membranes, and supplemented with 3% FBS.
BGC-823 cells were maintained in complete RPMI-1640 medium. At 80% confluence, the cells were collected, allocated into different groups, and cultured with appropriate conditioned media for 48 h.
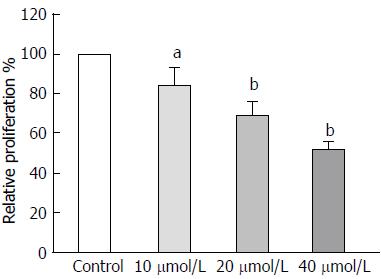
Cells were seeded in each well of 96-well plates in 200 μL complete RPMI-1640 medium and cultured at 37 °C. After 48 h of culture, MTT solution (5 mg/mL) was added and incubated at 37 °C for 4 h. Then, the medium was absorbed off and 100 μL of dimethyl sulfoxide was added to each well. Following vibrating on a shaker for 10 min, the plates were measured for absorbance at 490 nm wavelength.
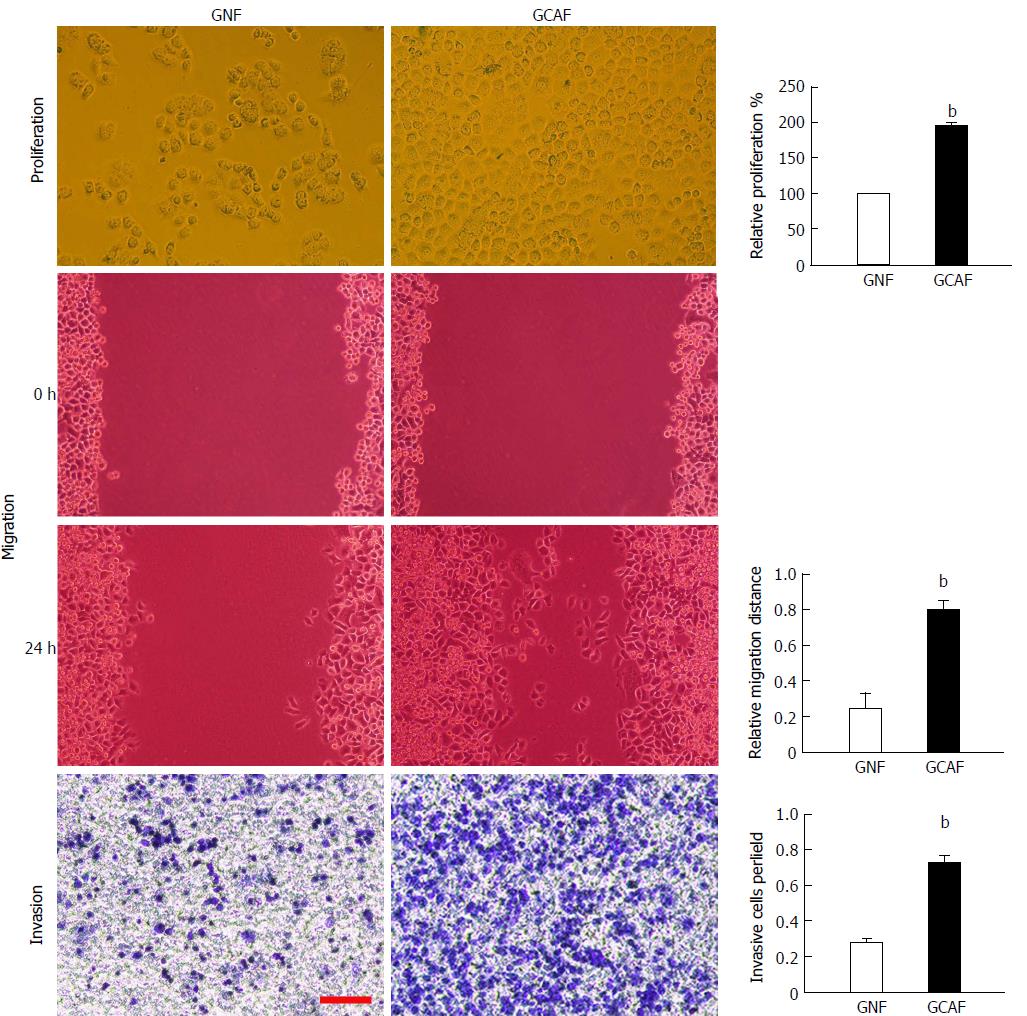
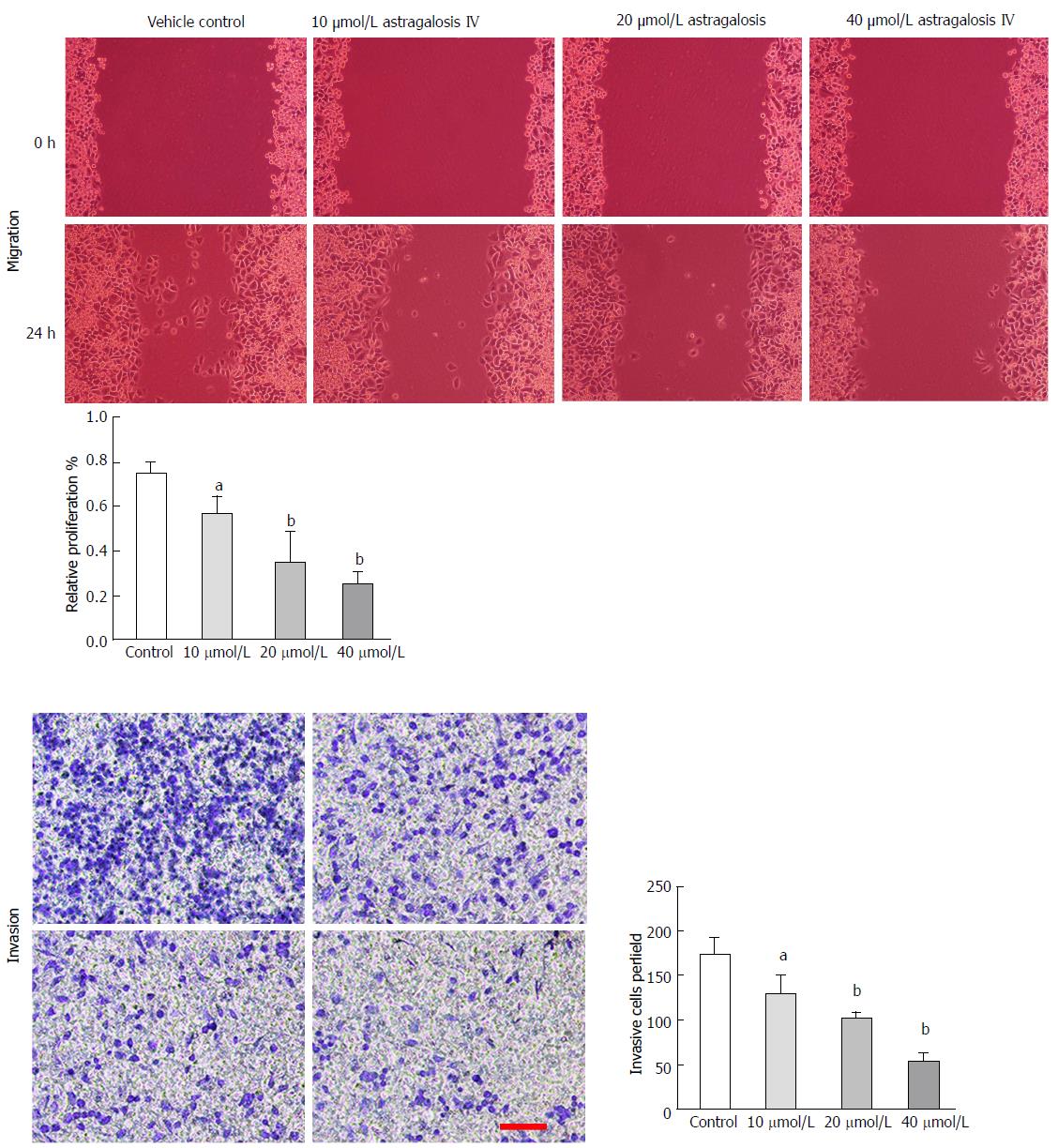
Cell migration capacity was determined by wound healing assay. BGC-823 cells were seeded in 6-well plates at 5 × 105 cells per well to grow into a monolayer. The monolayers were wounded by scratching lines with a plastic tip. The wells were then washed twice with PBS to remove any debris, and photographed under a microscope. Thereafter, the plates were incubated at 37 °C under 5% CO2 for 24 h with RPMI-1640, after which the cells were observed and photographed. The distance that the cells had migrated was measured on the photographs. The relative migration distance was calculated as follows: 1-(mean remained breadth/mean wounded breadth)[15].
Invasion assay was performed using 24-well Transwell chambers (polycarbonate membrane, 8 μm pore size; Costar, Cambridge, MA, United States). The upper surfaces of the Transwell membranes were pre-coated with Matrigel (BD Biosciences, San Jose, CA, United States) which was allowed to solidify at 37 °C for 4 h. Thereafter, 1.5 × 105 BGC-823 cells suspended in 150 μL RPMI-1640 were seeded into each upper chamber, and 600 μL of RPMI-1640 supplemented with 20% FBS were added into each lower chamber. The plates were incubated for 24 h at 37 °C, and then the media were removed from the Transwell chambers and the cells on the upper surfaces of the Transwell membranes were wiped off. Cells that had migrated to the lower surfaces of the Transwell membranes were fixed and stained with crystal violet, and the number of cells in five randomly selected fields at × 200 magnification was counted.
Total RNA was isolated using RNAiso Reagent (TaKaRa, Dalian, Liaoning, China) according to the manufacturer’s protocol. RNA concentrations were measured using a NanoDrop 2000 (Thermo Scientific, Wilmington, DE, United States). To evaluate the expression of microRNAs, reverse transcription and qPCR were performed with the TransScript Green miRNA Two-Step qRT-PCR SuperMix (Transgene, Beijing, China) using U6 as an endogenous control. The qPCR cycling condition was as follows: 94 °C for 30 s, and 40 cycles of 94 °C for 5 s and 60 °C for 34 s. To evaluate the expression of SOX2 and NANOG mRNAs, reverse transcription was performed with a PrimeScript RT reagent kit with gDNA Eraser (TaKaRa) using 1 μg of total RNA from each sample, followed by qPCR with the SYBR Premix Ex Taq (TaKaRa) using GAPDH as an endogenous control. The qPCR cycling condition was as follows: 95 °C for 30 s, and 40 cycles of 95 °C for 5 s and 60 °C for 31 s. Relative expression levels of each gene were calculated using the ΔΔCt method. Primers used are listed in Table 1.
| Gene name | Forward primer (F) |
| Reverse primer (R) | |
| U6 | F: 5’-TTCCTCCGCAAGGATGACACGC-3’ |
| R: Universal miRNA qPCR primer | |
| miR-214 | F: 5’-CAGGCACAGACAGGCAGT-3’ |
| R: Universal miRNA qPCR primer | |
| miR-301a | F:5’-GGCAGTGCAATAGTATTGT-3’ |
| R: Universal miRNA qPCR primer | |
| miR-34b | F: 5’-TAGGCAGTGTCATTAGCTGATTG-3’ |
| R: Universal miRNA qPCR primer | |
| miR-31 | F: GCCGCAGGCAAGATGCTGGC |
| R: Universal miRNA qPCR primer | |
| miR-155 | F: TTAATGCTAATCGTGATAGGGGT |
| R: Universal miRNA qPCR primer | |
| miR-200b | F: GCGGCTAATACTGCCTGGT |
| R: Universal miRNA qPCR primer | |
| miR-16 | F: GTAGCAGCACGTAAATATTGGCG |
| R: Universal miRNA qPCR primer | |
| miR-148a | F: AGCAGTTCAGTGCACTACAG |
| R: Universal miRNA qPCR primer | |
| GAPDH | F: 5’-TGTCCCCACTGCCAACGTGTCA-3’ |
| R: 5’-GCGTCAAAGGTGGAGGAGTGGGT-3’ | |
| SOX2 | F: 5’-TACAGCATGTCCTACTCGCAG-3’ |
| R: 5’-GAGGAAGAGGTAACCACAGGG-3’ | |
| NANOG | F: 5’-CCAACATCCTGAACCTCAGCTAC-3’ |
| R: 5’-GCCTTCTGCGTCACACATT-3’ |
Oligonucleotides for anti-miR-214, 214-NC, miR-301a mimic, and 301a-NC were transfected with siRNA-Mate at a final concentration of 50 nM. After 48 h of transfection, cells were harvested for further experiment.
Western blot procedures were conducted as previously reported[16]. Antibodies against M-CSF (1:3000), TIMP2 (1:500), SOX2 (1:2000), NANOG (1:2000), GAPDH (1:2000), and β-actin (1:2000) were used as primary antibodies. HRP-conjugated goat anti-rabbit IgG diluted in 0.5% non-fat milk was used as secondary antibody.
The M-CSF and TIMP2 concentrations in the conditioned media were measured using ELISA kits (Baizhi, Beijing, China) according to the manufacturer’s instructions.
Results are summarized as mean ± SD. Student’s t-test and one-way analysis of variance were used to analyze the data and the significance level was set at P < 0.05. The statistical analyses were performed with SPSS 17.0 software (SPSS, Chicago, IL, United States).
First, we assessed the cultured GNFs and GCAFs through immunofluorescence staining for the epithelial cell markers pan-CK and E-cadherin as well as the mesenchymal cell markers vimentin and PDGFR-β. The results revealed that GNFs and GCAFs were negative for pan-CK and E-cadherin and positive for vimentin and PDGFR-β (Figure 1). Furthermore, to identify GCAFs, we evaluated the cells for expression of α-SMA, a marker of activated fibroblasts typically expressed strongly in cancer-associated fibroblasts but weakly in quiescent fibroblasts[17]. Expression of α-SMA was considerably higher in GCAFs than in paracancerous GNFs (Figure 1). These results indicate that paired GNF and GCAF cultures were successfully established.
To determine whether GCAFs influence the proliferation, migration, and invasion abilities of gastric cancer cells, BGC-823 cells were cultured with GNF- or GCAF-conditioned medium and then subjected to MTT, wound healing, and Transwell invasion assays. The results showed that BGC-823 cells cultured in the GCAF-conditioned medium exhibited significantly higher proliferation, migration, and invasion abilities than cells cultured in the GNF-conditioned medium (P < 0.01) (Figure 2), indicating that GCAFs promote the malignant behaviors of gastric cancer cells.
To determine whether astragaloside IV affects the malignancy-promoting capacities of GCAFs, GCAFs were treated with vehicle control or increasing concentrations of astragaloside IV (10, 20, or 40 μmol/L). Then, conditioned media were prepared to culture BGC-823 cells. BGC-823 cells cultured in the conditioned media from astragaloside IV-treated GCAFs showed lower proliferation, migration, and invasion abilities than BGC-823 cells cultured in the conditioned medium from control-treated GCAFs (P < 0.05 for 10 μmol/L group, P < 0.01 for 20 μmol/L and 40 μmol/L groups) (Figures 3 and 4), indicating that astragaloside IV inhibits the malignancy-promoting capacities of GCAFs.
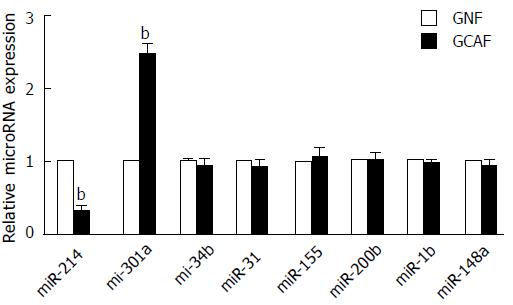
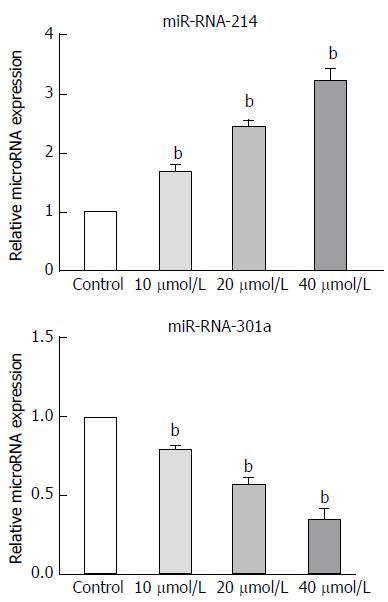
Recent studies have shown that the dysregulation of microRNA expression substantially contributes to the malignancy-promoting capacities of cancer-associated fibroblasts[18]. In our study, we selected eight microRNAs that are related to the functions of cancer-associated fibroblasts, and evaluated their expression in GNFs and GCAFs using RT-qPCR. GCAFs exhibited lower expression of miR-214 and higher expression of miR-301a than GNFs (P < 0.01) (Figure 5). Treatment of GCAFs with astragaloside IV effectively up-regulated miR-214 and down-regulated miR-301a expressions (P < 0.01) (Figure 6).
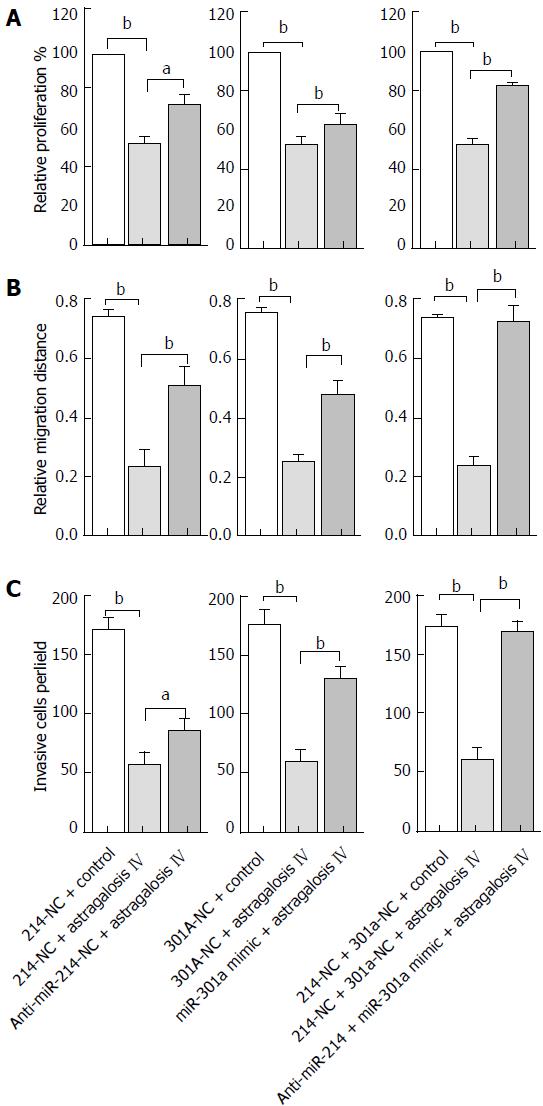
To determine whether miR-214 and miR-301a are involved in astragaloside IV-induced changes in GCAF functions, GCAFs were transfected with an anti-miR-214 or miR-301a mimic, or co-transfected with anti-miR-214 and miR-301a mimic. Then, the cells were treated with 40 μM astragaloside IV and conditioned media were prepared to culture BGC-823 cells.
MTT assay revealed that anti-miR-214 or miR-301a mimic transfection partially reversed astragaloside IV-induced inhibition of proliferation-promoting capacity of GCAFs (P < 0.05) (Figure 7A). Moreover, anti-miR-214 and miR-301a mimic co-transfection significantly reduced astragaloside IV-induced inhibition of proliferation-promoting capacity of GCAFs (P < 0.01) (Figure 7A). The results signify that miR-214 and miR-301a participate in astragaloside IV-induced inhibition of proliferation-promoting capacity of GCAFs.
Wound healing assay similarly showed that anti-miR-214 or miR-301a mimic transfection partially reversed astragaloside IV-induced inhibition of migration-promoting capacity of GCAFs (P < 0.01) (Figure 7B). Additionally, anti-miR-214 and miR-301a mimic co-transfection completely reversed astragaloside IV-induced inhibition of migration-promoting capacity of GCAFs (P < 0.01) (Figure 7B). These results reveal that miR-214 and miR-301a mediate astragaloside IV-induced inhibition of migration-promoting capacity of GCAFs.
Transwell invasion assay also revealed that anti-miR-214 or miR-301a mimic transfection partially reversed astragaloside IV-induced inhibition of invasion-promoting capacity of GCAFs (P < 0.05) (Figure 7C). Moreover, anti-miR-214 and miR-301a mimic co-transfection completely reversed astragaloside IV-induced inhibition of invasion-promoting capacity of GCAFs (P < 0.01) (Figure 7C). These results indicate that miR-214 and miR-301a mediate astragaloside IV-induced inhibition of invasion-promoting capacity of GCAFs.
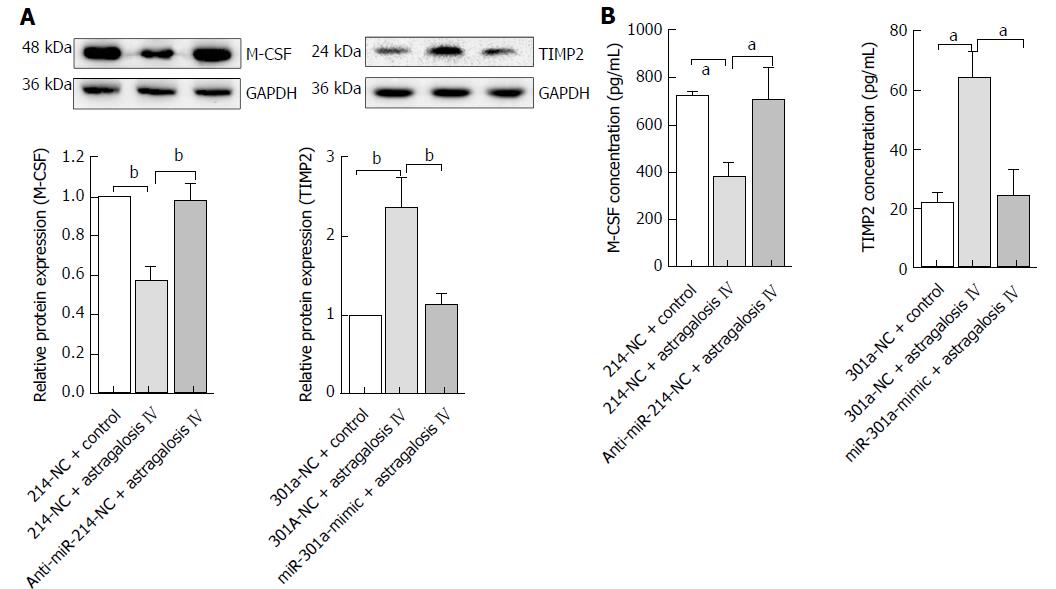
Astragaloside IV reduces M-CSF expression and increases TIMP2 expression in GCAFs
M-CSF and TIMP2 are validated targets of miR-214 and miR-301a, respectively. We investigated whether astragaloside IV-induced changes in microRNA expression affected M-CSF and TIMP2 expression in GCAFs. Western blot analysis showed that astragaloside IV treatment reduced M-CSF and increased TIMP2 protein expression in GCAFs (P < 0.01), and that pre-transfection of GCAFs with anti-miR-214 and miR-301a mimic prevented astragaloside IV from altering M-CSF and TIMP2 expression, respectively (Figure 8A). ELISA also confirmed that astragaloside IV treatment suppressed M-CSF and elevated TIMP2 protein secretion by GCAFs (P < 0.05) (Figure 8B).
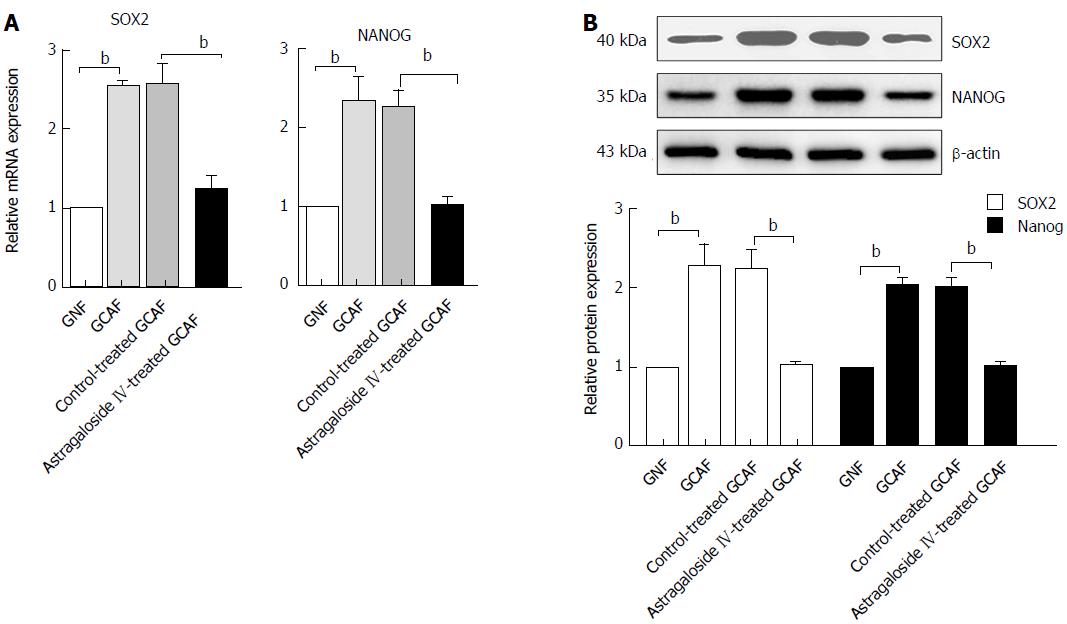
Astragaloside IV prevents GCAFs from up-regulating SOX2 and NANOG in BGC-823 cells
M-CSF and TIMP2 both participate in regulating cancer stem cell phenotype in the tumor microenvironment. We next explored whether treatment of GCAFs with astragaloside IV influenced the expression of the oncogenic pluripotency factors SOX2 and NANOG in gastric cancer cells. RT-qPCR and Western blot analysis revealed that BGC-823 cells cultured in the GCAF-conditioned medium expressed higher levels of SOX2 and NANOG than those cultured in the GNF-conditioned medium (P < 0.01), and that BGC-823 cells cultured in the conditioned medium from astragaloside IV-treated GCAFs expressed similar levels of SOX2 and NANOG to those cultured in the GNF-conditioned medium (Figure 9). These results indicate that astragaloside IV prevents GCAFs from up-regulating the oncogenic pluripotency factors SOX2 and NANOG in gastric cancer cells.
Cancer-associated fibroblasts, the most abundant cells in the tumor stroma, differ from normal fibroblasts in expression profiles and functions. They produce and secrete high levels of factors that promote cancer cell proliferation, migration, and invasion, simultaneously synthesizing and releasing low levels of factors that maintain tissue homeostasis. Thus, they create an environment favorable for tumor survival and progression[7,9]. Blocking the pathological functions of cancer-associated fibroblasts can diminish the nourishment and support they provide for cancer cells. Some researchers have attempted to inhibit the pathological functions of cancer-associated fibroblasts by targeting fibroblast activation proteins on the membranes of cancer-associated fibroblasts[19,20]. However, the efficacy of this strategy is unsatisfactorily low. For example, neither sibrotuzumab (a monoclonal antibody) nor talabostat (a small-molecule inhibitor of fibroblast activation protein) generated tumor responses in patients with metastatic colorectal cancer during phase II clinical trials[19,20]. One likely reason for the unsatisfactory outcome is that cancer-associated fibroblasts have complex pathological mechanisms, and thus it is difficult to completely inhibit their pathological functions by targeting only one molecule. Notably, in the present study, astragaloside IV strongly inhibited GCAFs from supporting gastric cancer cells by correcting the dysregulated expression of multiple oncogenic and tumor suppressive molecules, exhibiting potential as an effective new drug against cancer-associated fibroblassts.
Astragaloside IV is a main bioactive component of Radix astragali, a commonly used nontoxic Chinese herb. Apart from its anti-inflammatory and antiviral activities, astragaloside IV also has chemoprotective effects on human somatic cells. For example, at 100 μmol/L, it can protect dopaminergic neurons against 6-hydroxydopamine-induced degeneration[21]; at 250 μmol/L, it can prevent glucose-induced podocyte apoptosis[22]; at 100 μmol/L, it can protect ventricular myocytes from isoproterenol-induced hypertrophy without causing cytotoxicity[23]. In the current study, astragaloside IV strongly inhibited the proliferation-, migration- and invasion-promoting capacities of GCAFs at a concentration of only 40 μmol/L, suggesting that astragaloside IV could be considered a safe therapeutic agent for gastric malignancy. This property may provide astragaloside IV with an advantage over cytotoxic chemotherapy drugs, which form the traditional mainstay of cancer drug treatment despite their substantial injuries to multiple tissues and organs of patients[24].
MicroRNAs, a class of small non-coding RNAs that typically suppress the translation and reduce the stability of mRNAs, are involved in cancer initiation and progression[25]. Down-regulation of miR-214 has been reported in cervical[26], hepatocellular[27], and gastric[28] carcinomas. In particular, decreased miR-214 expression in gastric carcinoma tissues is associated with lymph node metastasis and tumor size[28]. Overexpression of miR-301a has also been observed in gastric cancer tissues, and it is positively associated with tumor size, invasion depth, and lymph node metastasis[29]. However, the expression and functions of the two microRNAs in GCAFs have rarely been reported. In the current study, we found that miR-214 expression was decreased and miR-301a expression increased in GCAFs as compared with GNFs, and that astragaloside IV inhibited the migration- and invasion-promoting capacities of GCAFs by enhancing miR-214 and reducing miR-301a expression. Thus, the dysregulated expression of the two microRNAs is a critical cause of the pathological functions of GCAFs; correcting microRNA expression abnormalities in GCAFs is an important anti-cancer mechanism of astragaloside IV.
M-CSF has been experimentally proved to be a direct target of miR-214 by a recent study[28]. In addition to regulating macrophage cell proliferation and differentiation, M-CSF also plays a vital role in promoting tumor progression. In the colon tumor microenvironment, M-CSF released by macrophages activates the tumorigenicity of non-cancer stem cell subpopulations by triggering their cancer stem cell properties[30]. In Lewis lung carcinoma, M-CSF secreted by lung cancer cells induces the pro-tumoral function of stromal myeloid cells to facilitate tumor invasion[31]. In mice bearing breast cancer xenografts, administration of M-CSF neutralizing antibody reduced primary tumor growth[32]. In the present study, we found that GCAFs produced and secreted M-CSF protein, suggesting that M-CSF is also an oncogenic factor in the gastric cancer microenvironment. Treatment of GCAFs with astragaloside IV effectively suppressed M-CSF production and secretion. This effect can reduce the oncogenic signals that gastric cancer cells receive from the surrounding microenvironment, thereby inhibiting the malignant progression of gastric cancer.
Pre-transfection of GCAFs with anti-miR-214 prevented astragaloside IV from suppressing M-CSF production and secretion, indicating that astragaloside IV suppresses M-CSF protein expression in GCAFs by increasing miR-214 expression. This result is consistent with the study of Wang et al[28] who observed a decrease in M-CSF protein expression after transfection of miR-214 precursor into gastric cancer cells, and it provides another evidence for the role of miR-214 in targeting M-CSF to regulate cell behaviors.
Matrix metallaproteinases in the tumor microenvironment are crucial oncogenic factors. They can degrade the protein components of extracellular matrices to destroy the physical barriers to metastasis, thus promoting cancer cell invasion and entry into blood or lymphatic vessels[33]. TIMP2 is a critical tumor suppressive factor that can inhibit the activity of matrix metallopeptidases by binding with a 1:1 stoichiometry to their active sites[34]. We found that astragaloside IV significantly elevated the production and secretion of TIMP2 by GCAFs. TIMP2 released from GCAFs by astragaloside IV stimulation could effectively inhibit the activity of matrix metallaproteinases and disrupt gastric cancer cell migration and invasion. Furthermore, purified TIMP2 protein showed an anti-proliferation activity against human cancer cells in a latest study[35]. Thus, astragaloside IV may be able to repress gastric cancer cell proliferation by increasing the concentration of TIMP2 in the tumor microenvironment.
We found that astragaloside IV failed to elevate TIMP2 protein expression and secretion levels when GCAFs were pre-transfected with miR-301a mimic. A study by Liang et al[36] has shown that TIMP2 is a direct target of miR-301a, which can bind to the 3’-untranslated region of TIMP2 mRNA to inhibit its translation. Therefore, astragaloside IV did not directly increase TIMP2 production and secretion by GCAFs; instead, it acted by down-regulating miR-301a expression to relieve miR-301a-induced inhibition of TIMP2 translation. This result suggests a potential use of astragaloside IV as an epigenetic therapeutic agent for gastric cancer.
SOX2 and NANOG are two critical stemness markers. Their overexpression contributes greatly to the maintenance of cancer stem cell phenotypes in multiple types of tumors[37,38]. In this study, GCAFs significantly up-regulated SOX2 and NANOG expression in gastric cancer cells, suggesting that promoting the generation of gastric cancer stem cells and supporting their malignant phenotypes is an important oncogenic mechanism of GCAFs. Treatment with astragaloside IV prevented GCAFs from inducing SOX2 and NANOG up-regulation in gastric cancer cells, suggesting that astragaloside IV could impair the niche of gastric cancer stem cells by acting on GCAFs, and thus may radically inhibit gastric cancer progression.
The activity of astragaloside IV to prevent GCAFs from up-regulating SOX2 and NANOG in gastric cancer cells is largely due to its ability to change M-CSF and TIMP2 secretion levels by GCAFs. M-CSF present in the tumor microenvironment acts as a dedifferentiating factor. It can enhance the stemness of cancer cells by increasing their expression of the pluripotent factors SOX2 and NANOG[39,40]. The tumor suppressor TIMP2 acts as a differentiating factor in multiple cancers. It can inhibit the malignant behaviors of cancer stem cells through two pathways: direct down-regulation of the genes associated with cancer stem cell characteristics[41], and indirectly through inhibiting matrix metalloproteinases from activating pluripotent factor expression[42]. Astragaloside IV treatment significantly reduced M-CSF secretion and increased TIMP2 secretion by GCAFs, making gastric cancer cells receive less dedifferentiating signals and more differentiating signals. Thus, the expression of SOX2 and NANOG in gastric cancer cells was decreased.
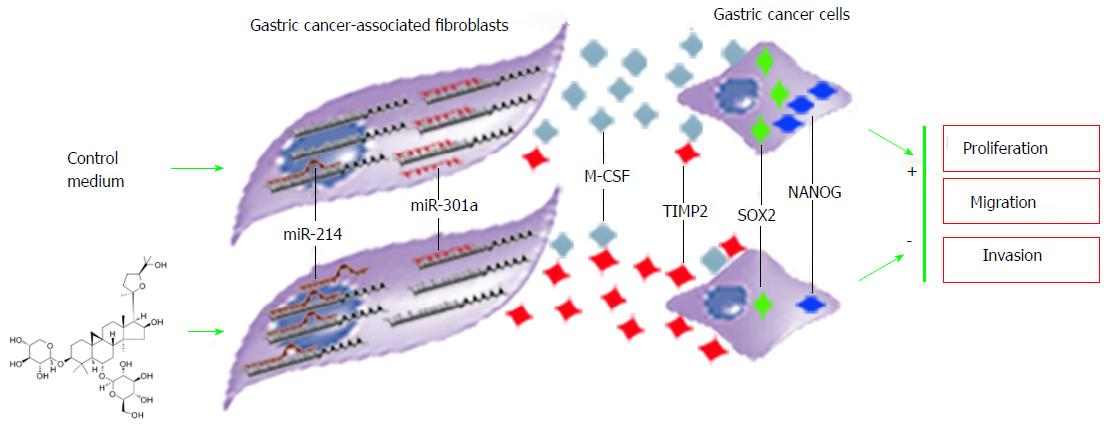
Astragaloside IV regulated the microRNA expression of GCAFs in a bidirectional pattern. It increased the expression of abnormally down-regulated miR-214 and simultaneously reduced the expression of abnormally up-regulated miR-301a. Reestablishment of the microRNA expression balance subsequently suppressed the production and secretion of the oncogenic factor M-CSF as well as elevated the production and secretion of the tumor suppressive factor TIMP2. Consequently, the nourishment and support for gastric cancer cells from GCAFs diminished considerably, and gastric cancer cells lost the environment suitable for proliferation, migration, and invasion (Figure 10). These effects are consistent with the properties of the traditional Chinese herb Radix astragali of “eliminating toxins” and “strengthening constitutions”.
Our results confirm the scientific validity of traditional Chinese medicine and highlight the feasibility, effectiveness, and uniqueness of the methods guided by the traditional Chinese medicine concept of holism. Traditional Chinese medicine and pharmacology is truly a valuable source of knowledge, warranting in-depth exploration for the improvement of cancer treatment strategies.
Currently, most chemotherapeutic agents perform by directly acting on cancer cells. They have limited efficacy and generate adverse reactions. Cancer-associated fibroblasts, the dominant cell population within cancer stroma, create favorable conditions for cancer initiation and progression. Preventing cancer-associated fibroblasts from nourishing and supporting cancer cells is a novel and effective anti-cancer pathway.
So far, few effective drugs have been found against cancer-associated fibroblasts. Developing effective inhibitors of cancer-associated fibroblasts is an urgent issue for cancer therapy.
Chinese herbs can regulate and improve patients’ internal environment to restore various tissues and cells to their normal states and enhance their functions. It is promising to find effective inhibitors of cancer-associated fibroblasts from Chinese herbs. Astragaloside IV is a cycloartane-type triterpene glycoside isolated from Radix astragali. Here, for the first time, we studied whether astragaloside IV can inhibit the pathological functions of cancer-associated fibroblasts, and explored the mechanism underlying the inhibitory effect. The research will provide valuable information on the feasibility of developing cancer-associated fibroblast inhibitors from Chinese herbs.
Paired gastric normal fibroblast (GNF) and gastric cancer-associated fibroblast (GCAF) cultures were established from resected tissues. GCAFs were treated with vehicle control or different concentrations of astragaloside IV. Conditioned media were prepared from GNFs, GCAFs, control-treated GCAFs, and astragaloside IV-treated GCAFs, and used to culture BGC-823 human gastric cancer cells. Proliferation, migration, and invasion capacities of BGC-823 cells were determined with MTT, wound healing, and Transwell invasion assays, respectively. The expression of microRNAs in the GCAFs was detected by RT-qPCR. The expression and secretion of the oncogenic factor M-CSF and the tumor suppressive factor TIMP2 in different groups of GCAFs were determined by Western blot and ELISA analysis, respectively. The expression of the oncogenic pluripotency factors SOX2 and NANOG in BGC-823 cells cultured with different conditioned media was also examined by RT-qPCR and Western blot analysis.
GCAFs displayed higher capacities to induce BGC-823 cell proliferation, migration, and invasion than GNFs. Astragaloside IV treatment strongly inhibited the proliferation-, migration- and invasion-promoting capacities of GCAFs. Compared with GNFs, GCAFs expressed a lower level of mir-RNA-214 and a higher level of microRNA-301a. Astragaloside IV treatment significantly up-regulated microRNA-214 expression and down-regulated microRNA-301a expression in GCAFs. Reestablishing the microRNA expression balance subsequently suppressed M-CSF production and secretion, and elevated TIMP2 production and secretion. Consequently, the ability of GCAFs to increase SOX2 and NANOG expression in BGC-823 cells was abolished by astragaloside IV. These results demonstrate that astragaloside IV is promisingly a potent therapeutic agent regulating tumor microenvironment.
This study shows for the first time that astragaloside IV can effectively inhibit the pathological functions of cancer-associated fibroblasts, and that astragaloside IV is useful for cancer therapy by regulating tumor microenvironment.
Previous studies focused on investigating the abnormal mir-RNA expression in cancer cells. Our results showed that the dysregulation in mir-RNA expression is an important cause of pathological functions of cancer-associated fibroblasts. Correcting the mir-RNA expression dysregulation in cancer associated fibroblasts is a new anti-cancer mechanism of Chinese herbs. Chinese herbs are a valuable source for developing new drugs regulating tumor microenvironment.
The method used in this study was to culture gastric cancer cells with the conditioned medium from GCAFs. In the culture system, GCAFs promoted the malignant behaviors of gastric cancer cells. Astragaloside IV inhibited the malignancy-promoting capacity of GCAFs through down-regulating the expression of oncogenic factors and up-regulating the expression of tumor suppressive factors. Also, we found that promoting the generation of gastric cancer stem cells and supporting their malignant phenotypes are an important oncogenic mechanism of GCAFs. Importantly, some Chinese herbs can destroy the niche of gastric cancer stem cells by acting on GCAFs.
The concept of holism of traditional Chinese medicine is valuable for guidance of developing new anti-cancer drugs. Non-toxic Chinese herbs may have great values in cancer therapy by regulating the tumor microenvironment. Chinese herbs that can regulate the tumor microenvironment are a promising resource for the development of novel anti-cancer drugs.
| 1. | Søreide K, Sandvik OM, Søreide JA, Giljaca V, Jureckova A, Bulusu VR. Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol. 2016;40:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 579] [Cited by in RCA: 559] [Article Influence: 55.9] [Reference Citation Analysis (1)] |
| 2. | Kreso A, O’Brien CA, van Galen P, Gan OI, Notta F, Brown AM, Ng K, Ma J, Wienholds E, Dunant C. Variable clonal repopulation dynamics influence chemotherapy response in colorectal cancer. Science. 2013;339:543-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 584] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 3. | Rosenberg SM, Shee C, Frisch RL, Hastings PJ. Stress-induced mutation via DNA breaks in Escherichia coli: a molecular mechanism with implications for evolution and medicine. Bioessays. 2012;34:885-892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 4. | Burrell RA, Swanton C. The evolution of the unstable cancer genome. Curr Opin Genet Dev. 2014;24:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | Safa AR, Saadatzadeh MR, Cohen-Gadol AA, Pollok KE, Bijangi-Vishehsaraei K. Glioblastoma stem cells (GSCs) epigenetic plasticity and interconversion between differentiated non-GSCs and GSCs. Genes Dis. 2015;2:152-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 171] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 6. | Martins-Neves SR, Paiva-Oliveira DI, Wijers-Koster PM, Abrunhosa AJ, Fontes-Ribeiro C, Bovée JV, Cleton-Jansen AM, Gomes CM. Chemotherapy induces stemness in osteosarcoma cells through activation of Wnt/β-catenin signaling. Cancer Lett. 2016;370:286-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 7. | Han Y, Zhang Y, Jia T, Sun Y. Molecular mechanism underlying the tumor-promoting functions of carcinoma-associated fibroblasts. Tumour Biol. 2015;36:1385-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 8. | Hu C, Wang Z, Zhai L, Yang M, Shan L, Chai C, Liu M, Wang L. Effects of cancer-associated fibroblasts on the migration and invasion abilities of SGC-7901 gastric cancer cells. Oncol Lett. 2013;5:609-612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Shimoda M, Mellody KT, Orimo A. Carcinoma-associated fibroblasts are a rate-limiting determinant for tumour progression. Semin Cell Dev Biol. 2010;21:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 279] [Article Influence: 16.4] [Reference Citation Analysis (1)] |
| 10. | Qi F, Zhao L, Zhou A, Zhang B, Li A, Wang Z, Han J. The advantages of using traditional Chinese medicine as an adjunctive therapy in the whole course of cancer treatment instead of only terminal stage of cancer. Biosci Trends. 2015;9:16-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 340] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 11. | Muluye RA, Bian Y, Alemu PN. Anti-inflammatory and Antimicrobial Effects of Heat-Clearing Chinese Herbs: A Current Review. J Tradit Complement Med. 2014;4:93-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 12. | Jung Y, Jerng U, Lee S. A systematic review of anticancer effects of Radix Astragali. Chin J Integr Med. 2016;22:225-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Yang B, Ji C, Chen X, Cui L, Bi Z, Wan Y, Xu J. Protective effect of astragaloside IV against matrix metalloproteinase-1 expression in ultraviolet-irradiated human dermal fibroblasts. Arch Pharm Res. 2011;34:1553-1560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Chen X, Peng LH, Li N, Li QM, Li P, Fung KP, Leung PC, Gao JQ. The healing and anti-scar effects of astragaloside IV on the wound repair in vitro and in vivo. J Ethnopharmacol. 2012;139:721-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | Bettenworth D, Lenz P, Krausewitz P, Brückner M, Ketelhut S, Domagk D, Kemper B. Quantitative stain-free and continuous multimodal monitoring of wound healing in vitro with digital holographic microscopy. PLoS One. 2014;9:e107317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Wang Z, Dao R, Bao L, Dong Y, Wang H, Han P, Yue Y, Yu H. Epigenetic reprogramming of human lung cancer cells with the extract of bovine parthenogenetic oocytes. J Cell Mol Med. 2014;18:1807-1815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Herrera M, Herrera A, Domínguez G, Silva J, García V, García JM, Gómez I, Soldevilla B, Muñoz C, Provencio M. Cancer-associated fibroblast and M2 macrophage markers together predict outcome in colorectal cancer patients. Cancer Sci. 2013;104:437-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 247] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 18. | Nouraee N, Mowla SJ, Calin GA. Tracking miRNAs’ footprints in tumor-microenvironment interactions: Insights and implications for targeted cancer therapy. Genes Chromosomes Cancer. 2015;54:335-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Hofheinz RD, al-Batran SE, Hartmann F, Hartung G, Jäger D, Renner C, Tanswell P, Kunz U, Amelsberg A, Kuthan H. Stromal antigen targeting by a humanised monoclonal antibody: an early phase II trial of sibrotuzumab in patients with metastatic colorectal cancer. Onkologie. 2003;26:44-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 245] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 20. | Narra K, Mullins SR, Lee HO, Strzemkowski-Brun B, Magalong K, Christiansen VJ, McKee PA, Egleston B, Cohen SJ, Weiner LM. Phase II trial of single agent Val-boroPro (Talabostat) inhibiting Fibroblast Activation Protein in patients with metastatic colorectal cancer. Cancer Biol Ther. 2007;6:1691-1699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 219] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 21. | Chan WS, Durairajan SS, Lu JH, Wang Y, Xie LX, Kum WF, Koo I, Yung KK, Li M. Neuroprotective effects of Astragaloside IV in 6-hydroxydopamine-treated primary nigral cell culture. Neurochem Int. 2009;55:414-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 22. | Gui D, Guo Y, Wang F, Liu W, Chen J, Chen Y, Huang J, Wang N. Astragaloside IV, a novel antioxidant, prevents glucose-induced podocyte apoptosis in vitro and in vivo. PLoS One. 2012;7:e39824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 23. | Zhang S, Tang F, Yang Y, Lu M, Luan A, Zhang J, Yang J, Wang H. Astragaloside IV protects against isoproterenol-induced cardiac hypertrophy by regulating NF-κB/PGC-1α signaling mediated energy biosynthesis. PLoS One. 2015;10:e0118759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Harrold K, Gould D, Drey N. The management of cytotoxic chemotherapy extravasation: a systematic review of the literature to evaluate the evidence underpinning contemporary practice. Eur J Cancer Care (Engl). 2015;24:771-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Calin GA, Croce CM. MicroRNA-cancer connection: the beginning of a new tale. Cancer Res. 2006;66:7390-7394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 787] [Cited by in RCA: 816] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 26. | Peng RQ, Wan HY, Li HF, Liu M, Li X, Tang H. MicroRNA-214 suppresses growth and invasiveness of cervical cancer cells by targeting UDP-N-acetyl-α-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase 7. J Biol Chem. 2012;287:14301-14309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 27. | Wang J, Li J, Wang X, Zheng C, Ma W. Downregulation of microRNA-214 and overexpression of FGFR-1 contribute to hepatocellular carcinoma metastasis. Biochem Biophys Res Commun. 2013;439:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 28. | Wang YW, Shi DB, Chen X, Gao C, Gao P. Clinicopathological significance of microRNA-214 in gastric cancer and its effect on cell biological behaviour. PLoS One. 2014;9:e91307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 29. | Xu XD, He XJ, Tao HQ, Zhang W, Wang YY, Ye ZY, Zhao ZS. Abnormal expression of miR-301a in gastric cancer associated with progression and poor prognosis. J Surg Oncol. 2013;108:197-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Yamashina T, Baghdadi M, Yoneda A, Kinoshita I, Suzu S, Dosaka-Akita H, Jinushi M. Cancer stem-like cells derived from chemoresistant tumors have a unique capacity to prime tumorigenic myeloid cells. Cancer Res. 2014;74:2698-2709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 31. | Park J, Lee SE, Hur J, Hong EB, Choi JI, Yang JM, Kim JY, Kim YC, Cho HJ, Peters JM. M-CSF from Cancer Cells Induces Fatty Acid Synthase and PPARβ/δ Activation in Tumor Myeloid Cells, Leading to Tumor Progression. Cell Rep. 2015;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 32. | Paulus P, Stanley ER, Schäfer R, Abraham D, Aharinejad S. Colony-stimulating factor-1 antibody reverses chemoresistance in human MCF-7 breast cancer xenografts. Cancer Res. 2006;66:4349-4356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 175] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 33. | Brown GT, Murray GI. Current mechanistic insights into the roles of matrix metalloproteinases in tumour invasion and metastasis. J Pathol. 2015;237:273-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 192] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 34. | Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387-2392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2074] [Cited by in RCA: 2098] [Article Influence: 87.4] [Reference Citation Analysis (0)] |
| 35. | Radisky ES, Raeeszadeh-Sarmazdeh M, Radisky DC. Therapeutic Potential of Matrix Metalloproteinase Inhibition in Breast Cancer. J Cell Biochem. 2017;118:3531-3548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 114] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 36. | Liang B, Yin JJ, Zhan XR. MiR-301a promotes cell proliferation by directly targeting TIMP2 in multiple myeloma. Int J Clin Exp Pathol. 2015;8:9168-9174. [PubMed] |
| 37. | Hütz K, Mejías-Luque R, Farsakova K, Ogris M, Krebs S, Anton M, Vieth M, Schüller U, Schneider MR, Blum H. The stem cell factor SOX2 regulates the tumorigenic potential in human gastric cancer cells. Carcinogenesis. 2014;35:942-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 38. | Lin T, Ding YQ, Li JM. Overexpression of Nanog protein is associated with poor prognosis in gastric adenocarcinoma. Med Oncol. 2012;29:878-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 39. | Liao CP, Chen LY, Luethy A, Kim Y, Kani K, MacLeod AR, Gross ME. Androgen receptor in cancer-associated fibroblasts influences stemness in cancer cells. Endocr Relat Cancer. 2017;24:157-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 40. | Raggi C, Mousa HS, Correnti M, Sica A, Invernizzi P. Cancer stem cells and tumor-associated macrophages: a roadmap for multitargeting strategies. Oncogene. 2016;35:671-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 41. | Han H, Bourboulia D, Jensen-Taubman S, Isaac B, Wei B, Stetler-Stevenson WG. An endogenous inhibitor of angiogenesis inversely correlates with side population phenotype and function in human lung cancer cells. Oncogene. 2014;33:1198-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 42. | Kessenbrock K, Wang CY, Werb Z. Matrix metalloproteinases in stem cell regulation and cancer. Matrix Biol. 2015;44-46:184-190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 166] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Morris DL, Yoon S S- Editor: Gong ZM L- Editor: Wang TQ E- Editor: Ma YJ