Published online Dec 28, 2017. doi: 10.3748/wjg.v23.i48.8489
Peer-review started: October 31, 2017
First decision: November 14, 2017
Revised: November 21, 2017
Accepted: November 28, 2017
Article in press: November 28, 2017
Published online: December 28, 2017
Processing time: 57 Days and 22.2 Hours
To explore the pathogenesis of primary biliary cholangitis (PBC) by identifying candidate autoantibodies in serum samples by proteomics and bioinformatics.
Nine antimitochondrial antibody (AMA)-positive PBC patients and nine age- and sex-matched AMA-negative PBC patients were recruited. Antigen enrichment technology was applied to capture autoantigens of human intrahepatic biliary epithelial cells (HiBECs) that are recognized by autoantibodies from the sera of PBC patients. Candidate autoantigens were identified by label-free mass spectrometry. Bioinformatics analysis with MaxQuant software (version 1.5.2.8), DAVID platform, and Cytoscape v.3.0 allowed illustration of pathways potentially involved in the pathogenesis of PBC.
In total, 1081 candidate autoantigen proteins were identified from the PBC patient pool. Among them, 371 were determined to be significantly differentially expressed between AMA-positive and -negative PBC patients (P < 0.05). Fisher’s exact test was performed for enrichment analysis of Gene Ontology protein annotations (biological processes, cellular components, and molecular functions) and the Kyoto Encyclopedia of Genes and Genomes pathways. Significantly different protein categories were revealed between AMA-positive and -negative PBC patients. As expected, autoantigens related to mitochondria were highly enriched in AMA-positive PBC patients. However, lower levels of AMA were also detected in AMA-negative PBC patients. In addition, autoantigens of AMA-negative PBC patients were mainly involved in B-cell activation, recognition of phagocytosis, and complement activation.
AMA-negative PBC individuals may not exist, but rather, those patients exhibit pathogenesis pathways different from those of AMA-positive PBC. Comprehensive research is needed to confirm these observations.
Core tip: The pathogenesis of primary biliary cholangitis (PBC) is still unclear. Related studies have focused on genes, immune cells, and pathology. However, little research has been conducted to establish pathogenesis-related autoantibodies. In this study, we unraveled the pathogenesis of PBC by detecting novel autoantibodies, using proteomics. Our results suggest that the dysfunction of three pathways in human intrahepatic biliary epithelial cells might be causative in the pathogenesis of antimitochondrial antibody (AMA)-negative PBC. More interestingly, we identified AMA-negative pathology as a potential misnomer, as we detected low levels of AMA in sera of AMA-negative patients. Comprehensive research is needed to confirm these observations.
- Citation: Deng CW, Wang L, Fei YY, Hu CJ, Yang YJ, Peng LY, Zeng XF, Zhang FC, Li YZ. Exploring pathogenesis of primary biliary cholangitis by proteomics: A pilot study. World J Gastroenterol 2017; 23(48): 8489-8499
- URL: https://www.wjgnet.com/1007-9327/full/v23/i48/8489.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i48.8489
Primary biliary cholangitis (PBC) is a chronic and progressive intrahepatic cholestatic disease that can ultimately progress to severe hepatic illnesses, including hepatic cirrhosis, hepatic failure, and even hepatocellular carcinoma[1]. Currently, the pathogenesis of PBC is still unclear, but interactions between genetic predisposition and environmental triggers are considered to play an important role[2]. Several studies have found that PBC risk alleles tend to occur in loci that are related to immune function[3]. Pathological examination confirmed that the immune system is dysregulated in PBC patients. Most intriguingly, portal triads are heavily infiltrated by T cells that are responsive to mitochondrial and nuclear antigens, resulting in autoantibodies such as anti-mitochondrial antibody (AMA) found in humoral immune responses[4].
Elevated levels of serum autoantibodies are characteristic of autoimmune disease. The appearance and fluctuation of the autoantibodies not only represent the disease status but also further our understanding of the pathogenesis of the disease[5]. For example, the specific diagnostic biomarker of PBC, AMA, was confirmed to participate in injuring intrahepatic biliary epithelial cells (iBECs) and perpetuating the destructive process[6]. Interestingly, AMA cannot be detected in 10% of PBC cases, which prompted us to determine whether other pathogenesis-associated and AMA-like autoantibodies exist in AMA-negative PBC patients.
In recent years, proteomics and bioinformatics approaches have been applied to several fields, including the exploration of diagnostic markers, predictive factors, and disease pathogenesis[7-9]. Previously, we applied proteomic technologies, including high-throughput human proteome microarrays and two-dimensional difference gel electrophoresis, to explore biomarkers for PBC[7-11] in order to understand the pathogenesis of PBC. Our results were confirmed by another research team[12], which verified the scientific value of proteomics.
Dysfunction and loss of iBECs are one of the most important pathological features of PBC. A few studies have focused on the mechanism of damage to iBECs. Molecular cholangiocyte defects stymie defense against toxic bile acids, and defects in biliary bicarbonate secretion were a particular focus of investigation toward unraveling the pathogenesis of PBC[13].
In this context, we sought to perform an exploratory study by combining enrichment of antigens from iBECs, label-free mass spectrometry, and bioinformatics to explore autoantibody candidates for AMA-negative PBC. This may aid in decrypting the pathogenesis of PBC. In this pilot study, we aimed to ascertain the feasibility of the experimental approach and to obtain preliminary results that will serve as a guide for the future study.
From 2014 to 2015, 18 PBC patients were recruited from the Peking Union Medical College Hospital. They included nine AMA-positive and nine AMA-negative PBC cases, with pairs matched for age and sex. AMA and the M2 subtype of AMA were detected by an indirect immunofluorescence assay and an enzyme-linked immunosorbent assay, respectively. All enrolled patients were treatment-naïve and diagnosed according to the criteria proposed by Heathcote et al[14]. The Ethics Committee of the Peking Union Medical College Hospital approved this study. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki. Written informed consent was obtained from all participants.
Blood samples were collected under fasting conditions in the morning. Samples were drawn from the cubital vein and dispensed into 5-mL pro-coagulation tubes with gel (Becton, Dickinson and Company, United Kingdom). Blood samples were centrifuged at 1000 g for 5 min within 6 h of collection. Sera were frozen at -80 °C until use.
Human iBECS (HiBECs) were purchased from PriCell (Wuhan, China), where they were isolated from human livers obtained via organ donation from donor tissues that were not suitable for organ transplantation. HiBECs were subcultured when they reached 90%-95% confluency, and the culture medium was removed and discarded. The cell layer was rinsed briefly with 5 mL of phosphate-buffered saline solution to remove all traces of serum. Next, 1 mL of 0.25% (w/v) trypsin and 0.53 mmol/L EDTA solution was added to the flask, which was incubated in a 37 °C incubator for 5 min. Cells were observed under an inverted microscope until the cell layer dispersed. Cells were aspirated by gentle pipetting and washed twice with complete growth medium (PriCell, Wuhan, China). Cells were fed every other day with complete growth medium at 37 °C in a humidified atmosphere containing 5% CO2 and 95% air until ready for assay. HiBECs were verified by an immunofluorescence microscopic method with an antibody against cytokeratin 19.
Target antigens of potential autoantibodies were enriched using the Pierce Crosslink Immunoprecipitation Kit (Pierce Biotechnology, Rockford, United States). Briefly, equal volumes of three serum samples from AMA-positive PBC patients were pooled, resulting in three AMA-positive PBC sample pools. AMA-negative PBC samples were handled identically. All pooled samples were diluted and incubated with protein A/G agarose to capture antibodies. Then, the antibodies were cross-linked to prevent co-elution with antigens.
HiBECs were lysed using the immunoprecipitation lysis and wash buffers provided in the immunoprecipitation kit. Control agarose resin was used to pre-clear the lysate, adsorbing non-specific binding entities. To ensure the formation of target complexes, cleared lysates with excess antigens were incubated with the antibody-agarose complexes. The target antigens were eluted from these complexes, using low-pH elution buffer provided with the kit. Then, 1 M Tris pH 9.5 was added to the eluate to neutralize the pH. The final collections were stored at -80 °C until proteomic analysis.
Total target antigens (50 μg) from each sample were resuspended in 100 μL of 4% SDS and 0.1 mol/L DTT in 0.1 mol/L Tris HCl, pH 7.6 at 25 °C following a filter-aided sample preparation protocol described elsewhere[15]. Liquid chromatography (LC) was performed on an Easy-nLC System (Thermo Fisher Scientific, MA, United States). Peptides were separated on a 15-cm fused silica emitter packed in-house with the reverse phase material ReproSil-Pur C18AQ, 3-μm resin (Dr. Maisch, Germany) with a 100-min gradient from 5% to 35% of 99.9% (v/v) CH3CN, 0.1% (v/v) acetic acid. A quadrupole Orbitrap mass spectrometer (Q Exactive; Thermo Fisher Scientific, Germany) was operated in the positive-ion mode using a data-dependent “top 12” method. Survey scans and tandem mass spectrometry (MS/MS) scans were acquired at a resolution of 70000 and 17500, respectively, at 400 m/z. The top 12 most abundant isotope patterns with charges ≥ 2 from the survey scan were selected with an isolation window of 2 Thomson and fragmented by higher-energy collisional dissociation with a normalized collision energy of 25%. The maximum ion injection times for the survey scans and the MS/MS scans were 50 ms and 100 ms, respectively, and the ion target values were set to 1E6 and 1E5, respectively. Selected sequenced ions were dynamically excluded for 30 s.
Raw MS data were analyzed using MaxQuant software (version 1.5.2.8)[16]. A false discovery rate (FDR) of 0.01 and a minimum peptide length of seven amino acids for proteins and peptides were required. A time-dependent mass recalibration algorithm was used to improve the mass accuracy of precursor ions[17]. MS/MS spectra were queried using the Andromeda search engine, which is incorporated in the MaxQuant software suite, against the UniProt human database (201307, containing 88405 entries). For the search, trypsin was chosen for enzyme specificity[15], allowing for cleavage at the N-terminus of proline (trypsin/P). Cysteine carbamidomethylation was selected as a fixed modification, while protein N-terminal acetylation and methionine oxidation were selected as variable modifications. Maximally two missed cleavages were allowed. For MS and MS/MS, the tolerances of the main search for peptides were set at 7 ppm and 20 ppm, respectively. Quantification was performed in MaxQuant using the built-in label-free quantification algorithm MaxLFQ[18], enabling the “match between runs” option (time window, 2 min). Peptides that were shared between two proteins were combined and reported as one protein group. Proteins matching to the reverse database were filtered out.
For bioinformatics analysis, protein abundance information was collected to have at least two valid expression values in each group. Student’s t-test was performed for comparison of differences between AMA-positive and -negative proteins, with P < 0.05 set as the significance cut-off. Enrichment analyses were conducted for the Gene ontology (GO) biological processes (GOBP), cellular components (GOCC), and molecular functions (GOMF) categories. Kyoto Encyclopedia of genes and genomes (KEGG) pathways were analyzed through the DAVID platform[19], using the complete UniProt human protein database as a background and FDR < 0.05 as a cut-off. Heatmap analyses and visualization of significantly different proteins were conducted using complete linkage hierarchical clustering. Significantly different proteins were used as the input for STRING[20] analysis and a network was built based on high-confidence (0.8) evidence of experimental protein-protein interactions provided by the STRING database. The network was visualized using Cytoscape v.3.0[21]. Jie Dai, from Shanghai Bioprofile Technology Company Ltd., reviewed the statistical methods of this study.
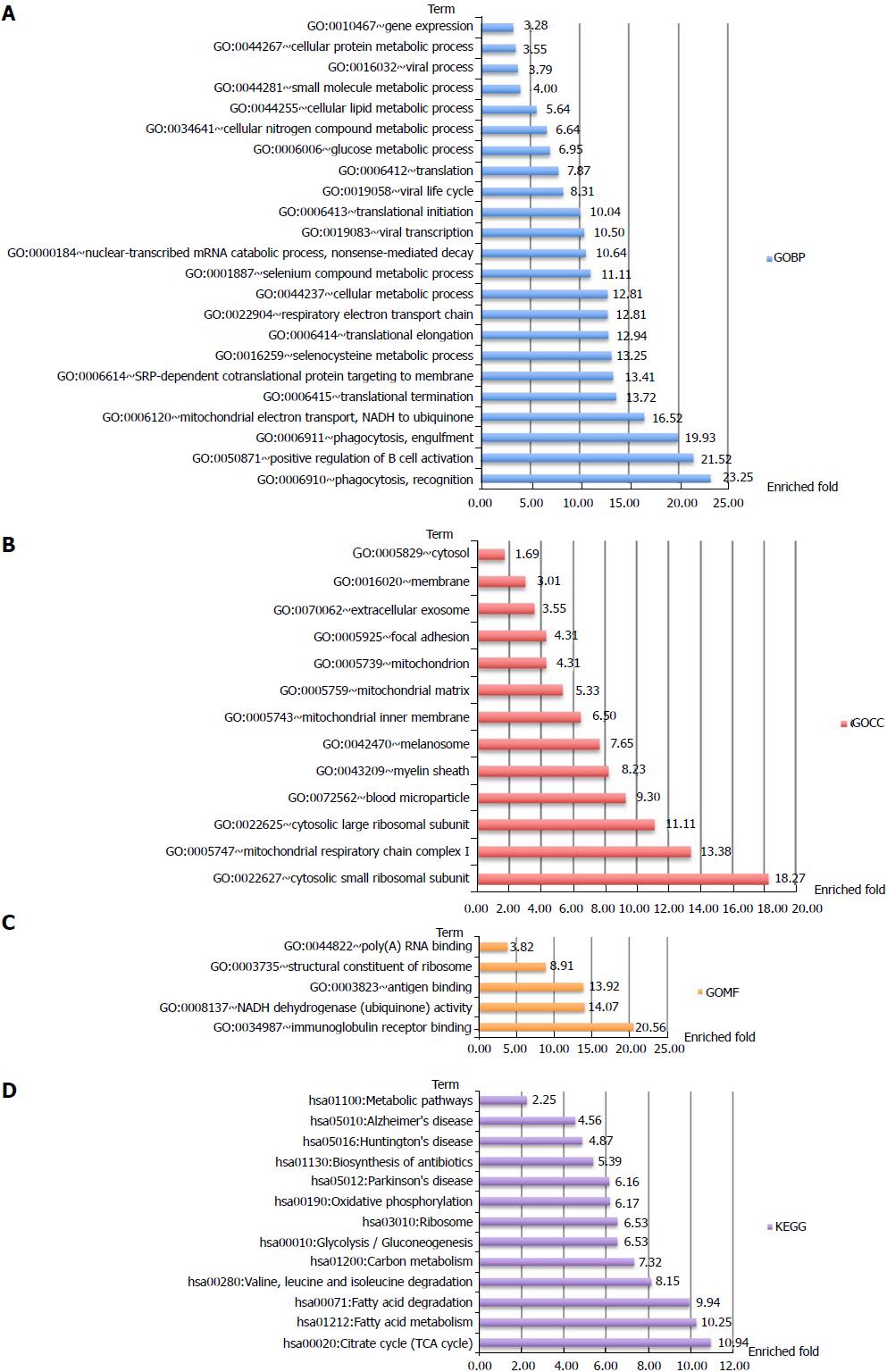
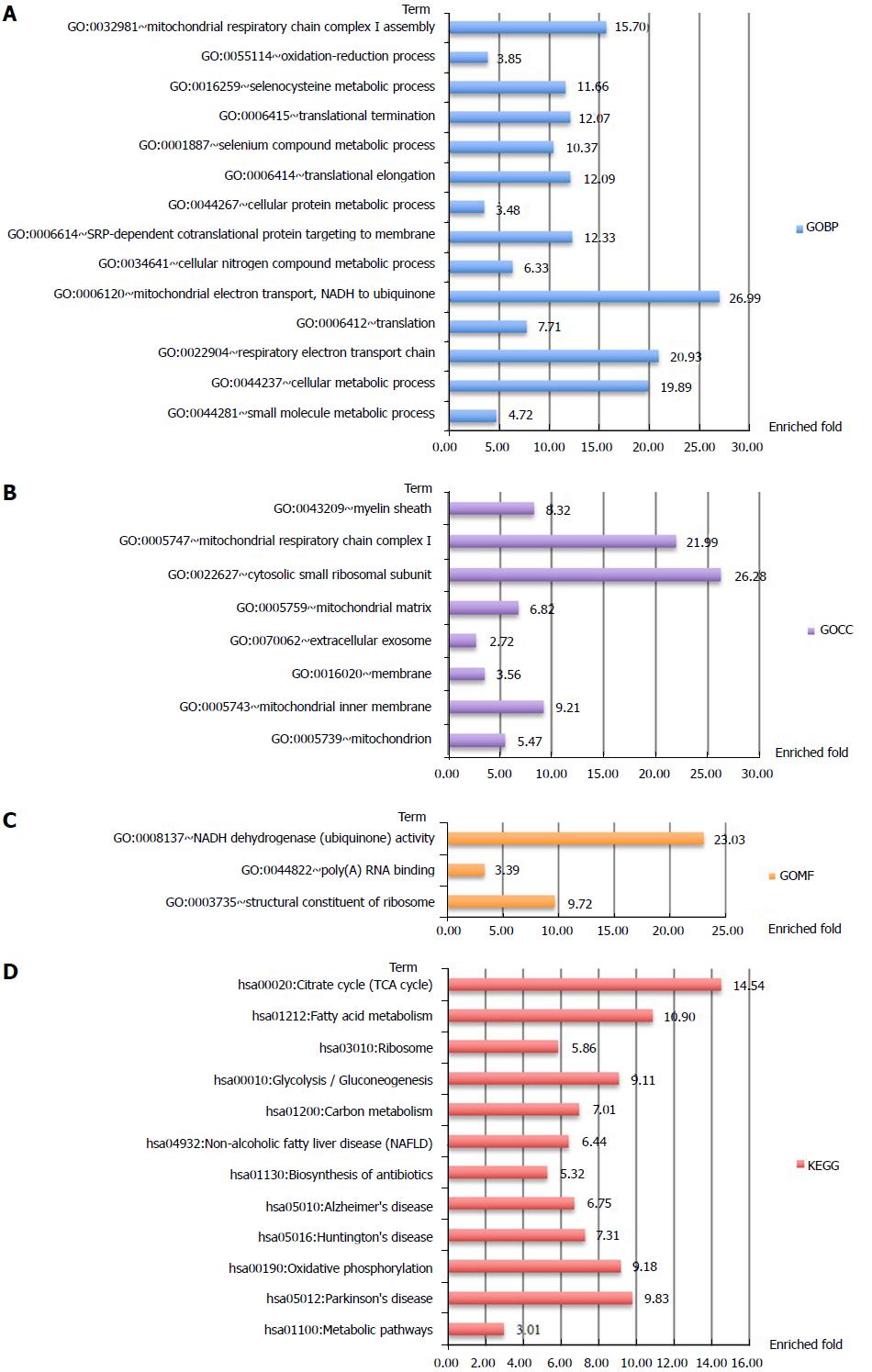
In total, 1081 autoantigen candidates were identified from all PBC patients. Among these proteins, 371 were found to be significantly differentially expressed between AMA-positive and -negative groups. Fisher’s exact test for the enrichment of GO protein annotations in the set of significantly different proteins revealed a range of protein categories (FDR < 0.05, Figure 1). As expected, the mitochondria-related biological process term was highly enriched. In addition, proteins that participated in the positive regulation of B-cell activation and phagocytic recognition and engulfment were also highly enriched.
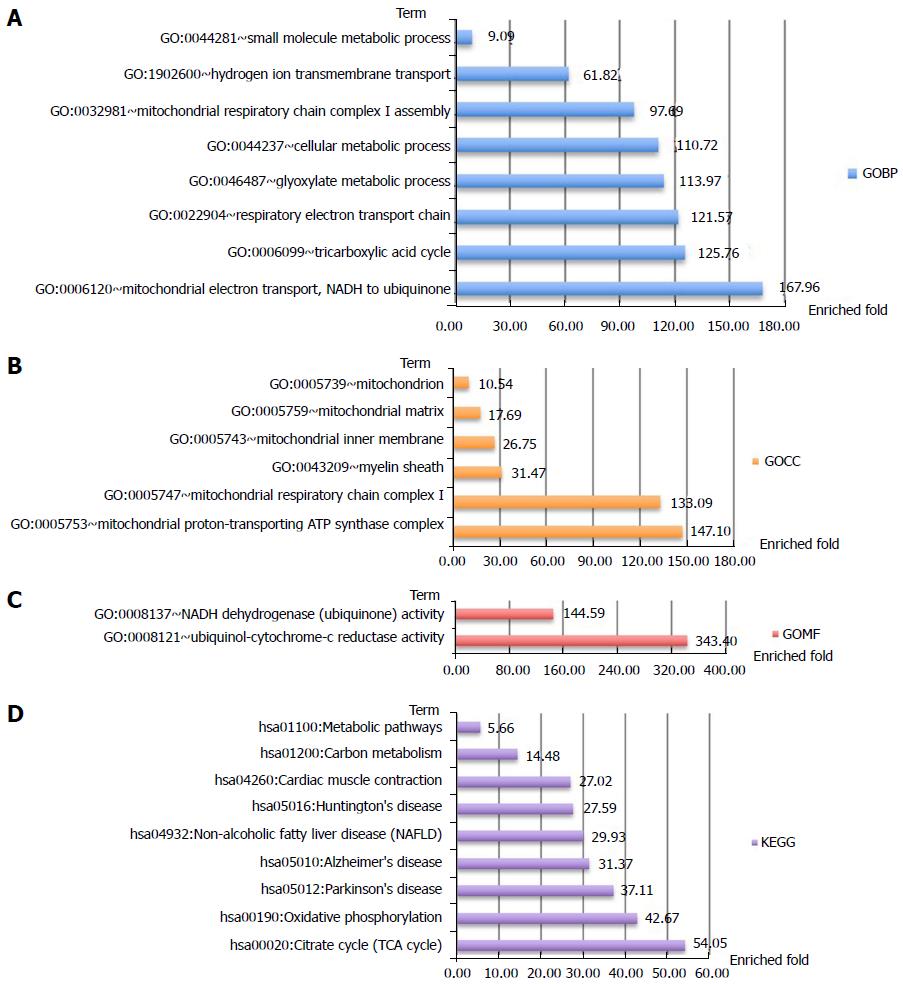
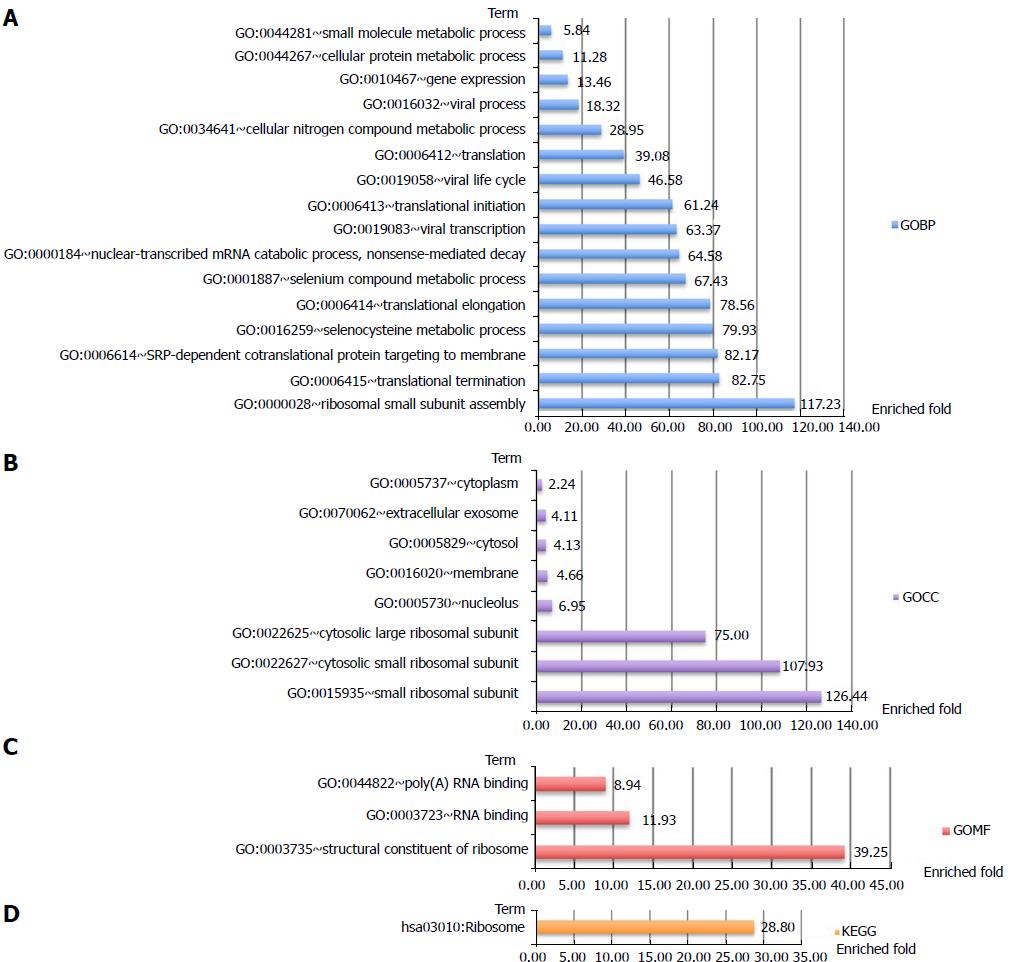
Analysis of protein-protein interactions revealed that the differential autoantigen candidates mainly interact via two hubs (Supplementary Figure 1). Enrichment analyses of GO terms and KEGG pathways highlighted differences between the two hubs (Figures 2 and 3). The candidate autoantigens of one hub mainly exist in the mitochondria, while those in the other hub reside in the cytosol. GOBP terms were also largely different between these two hubs.
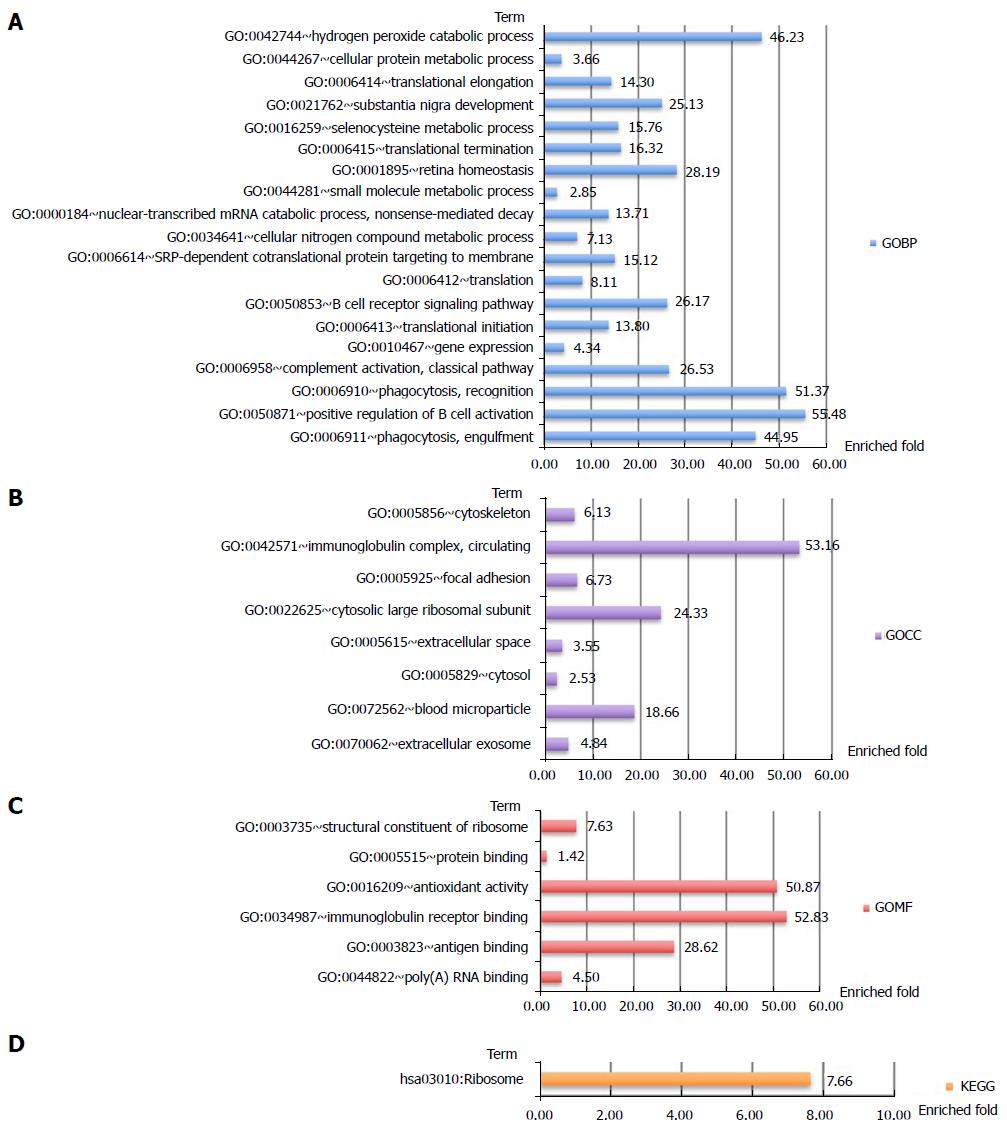
Clustering analysis was applied to examine the rationality and accuracy of the selected significant proteins (Supplementary Figure 2). Cluster 1 represents significantly upregulated proteins in AMA-negative PBC patients. They mainly participate in positive regulation of B-cell activation, recognition of phagocytosis, and complement activation (Figure 4). Cluster 2 represents significantly downregulated proteins in AMA-negative PBC patients that are upregulated in AMA-positive PBC patients. These downregulated proteins mainly participate in electron transport and cellular metabolic processes, which are mostly restricted to mitochondria (Figure 5).
In this study, 1081 autoantigen candidates were identified from AMA-positive and -negative PBC patients. Among them, 371 were determined to be significantly differentially expressed between AMA-positive and -negative groups. Further analysis of these proteins revealed autoantibody responses to autoantigens in AMA-negative PBC patients. Of note, these autoantigens participate in the biological processes of HiBECs, including positive regulation of B-cell activation, phagocytic recognition and engulfment, and complement activation.
Enrichment analysis of GO protein annotations revealed that the autoantigens of mitochondria-related biological processes were highly enriched in AMA-positive PBC patients, which matches the expectation. More importantly, lower levels of these autoantigens were also detected in AMA-negative PBC patients.
Since the establishment of AMA as the diagnostic biomarker of PBC, there has been controversy surrounding AMA-negative PBC[22]. Some studies have demonstrated that AMA cannot be detected in serum because the method applied in clinical practice is not sensitive enough[23]. Our results have confirmed this conclusion. Compared to traditional AMA detection methods such as indirect immunofluorescence and enzyme-linked immunosorbent assay, mass spectrometry was more sensitive, allowing the detection of lower levels of mitochondria-related autoantigens in AMA-negative PBC.
It seems that AMA-negative PBC technically does not exist, because AMA-related autoantibodies could be detected in these patients as well. However, some other studies did find differences between the two groups of PBC patients. It was reported that AMA-negative PBC patients tend to have lower serum levels of alkaline phosphatase and gamma-glutamyl transpeptidase, but higher levels of aspartate amino transferase[24,25]. In Japan, pruritus was observed less frequently among patients with AMA-negative PBC, but significantly higher number of patients were found to experience complications such as Sjögren’s syndrome[24]. Upon scrutinizing these results, we suggest that AMA-negative PBC might exist as a unique clinical subset of PBC. Functional analysis of the antigens found in the current study may help explain this phenomenon.
Clustering analysis revealed that most of the autoantigens in AMA-negative PBC patients participate in the biological processes of HiBECs, including recognition of phagocytosis, positive regulation of B-cell activation, and complement activation. Epithelial cells are capable of phagocytosis and antigen presentation in the immune response system[26]. The destruction of phagocytosis recognition by the immune response might lead to exposure of more antigens from HiBECs. Second, a pathology study reported that AMA-negative PBC patients exhibit a significant decrease in the number of B-cell clusters infiltrating the bile ductal regions in the early stages of bile duct damage[27]. Interestingly, there were autoantibody responses to proteins that participated in the positive regulation of B-cell activation among AMA-negative PBC patients and that might be the cause of decreased B-cell infiltration. Last but not least, epithelial cells can secrete interleukins (e.g., IL-8) to suppress inflammation[27]. If these cytokines are not activated (disturbed by an autoimmune response per the results of our study, for example), the inflammation in PBC patients will persist. In some way, the immune responses caused by the autoantigens we found may lead to dysfunction of the related pathways, which might result in the development of AMA-negative PBC, the unique clinical subtype.
This study had some limitations that should be addressed in future studies to confirm the interesting phenomena observed in this pilot study. First, a larger sample size should be analyzed. Second, simultaneous analysis of controls, a healthy cohort, and a cholestatic cohort would allow a better interpretation of the results. Last but not least, the significantly differentially expressed candidate autoantibodies identified in this study should be verified, for example, by enzyme-linked immunosorbent assay or Western blot analysis. Despite these limitations, this study provides important information for the ongoing discussion on the existence and pathogenesis of AMA-negative PBC.
In summary, the results of the current study are consistent with those in the current literature, which confirmed the feasibility and reliability of the design and technology we applied. This pilot study exploring PBC-related autoantigens demonstrated that the dysfunction of three pathways, recognition of phagocytosis, positive regulation of B-cell activation, and complement activation, in HiBECs might be causative in the pathogenesis of AMA-negative PBC. More interestingly, the existence of AMA-negative PBC was illustrated based on bioinformatics analysis. These data prompt us to launch an in-depth study using a larger sample size, including a control cohort, to verify the candidate autoantigens.
Currently, the pathogenesis of primary biliary cholangitis (PBC) is still unclear. The appearance and fluctuation of autoantibodies not only represent the disease status but also further our understanding of the pathogenesis of the disease. The specific diagnostic biomarker of PBC, anti-mitochondrial antibody (AMA), was confirmed to participate in injuring intrahepatic biliary epithelial cells (iBECs) and perpetuating the destructive process. Exploring novel autoantibodies for PBC will help unravel the underlying mechanism of pathogenesis.
AMA cannot be detected in 10% of PBC cases, which prompted us to determine whether other pathogenesis-associated and AMA-like autoantibodies exist in AMA-negative PBC patients. This may aid in decrypting the pathogenesis of PBC.
We sought to carry out an exploratory study using a novel proteomics approach that combines the enrichment of antigens from human BECs (HiBECs), label-free mass spectrometry, and bioinformatics to identify candidate autoantibodies for AMA-negative PBC. In this pilot study, we aimed to ascertain the feasibility of this experimental approach and obtain preliminary results that will guide future larger-scale and in-depth studies. In addition, the novel experimental roadmap established here can be applied in related studies in future.
Eighteen PBC patients were recruited from the Peking Union Medical College Hospital. They included nine AMA-positive and nine AMA-negative PBC cases, with pairs matched for age and sex. Sera were collected from patients and frozen at -80°C until use. Human iBECs (HiBECs) were subcultured and lysed to provide sufficient target antigens for candidate autoantibodies enriched from the sera of PBC patients. Cell lysates and enriched autoantibodies were mixed and incubated under various conditions. Candidate autoantigens were eluted and subsequently identified by label-free mass spectrometry. Student’s t-test was performed for comparison of differences between AMA-positive and -negative proteins, with P < 0.05 set as the significance cut-off. Enrichment analyses were conducted for the Gene Ontology (GO) biological processes (GOBP), cellular components (GOCC), and molecular functions (GOMF) categories. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways were analyzed through the DAVID platform, using the complete UniProt human protein database as a background and an FDR < 0.05 as a cut-off. Heatmap analyses and visualization of significantly different proteins were conducted by complete linkage hierarchical clustering. Significantly differentially expressed proteins were used as the input for STRING analysis and a network was built based on high-confidence (0.8) evidence of experimental protein-protein interactions provided by the STRING database. The network was visualized using Cytoscape v.3.0. This is the first time that enrichment of antigens from HiBECs, label-free mass spectrometry, and bioinformatics were combined to explore candidate pathogenesis-associated autoantibodies for AMA-negative PBC.
In total, 1081 autoantigen candidates were identified from all PBC patients. Among these, 371 were significantly differentially expressed between AMA-positive and -negative groups. Fisher’s exact test for the enrichment of GO protein annotations in the set of significantly different proteins revealed a range of protein categories (FDR < 0.05). As expected, the mitochondria-related biological process term was highly enriched. In addition, proteins that participate in the positive regulation of B-cell activation and phagocytic recognition and engulfment were highly enriched. Clustering analysis was applied to examine the rationality and accuracy of the selected proteins. Cluster 1 represents significantly upregulated proteins in AMA-negative PBC patients. They mainly participate in positive regulation of B-cell activation, recognition of phagocytosis, and complement activation. Cluster 2 represents significantly downregulated proteins in AMA-negative PBC patients that are upregulated in AMA-positive PBC patients. These downregulated proteins mainly participate in electron transport and cellular metabolic processes, which are mostly restricted to mitochondria.
This pilot study exploring PBC-related autoantigens demonstrated that the dysfunction of three pathways in HiBECs might be causative in the pathogenesis of AMA-negative PBC, including phagocytosis recognition, positive regulation of B-cell activation, and complement activation. More interestingly, the controversy of the existence of AMA-negative PBC was illustrated based on bioinformatics analysis. AMA cannot be detected in sera of PBC patients because the method applied in clinical practice is not sensitive enough. However, AMA-negative PBC might exist as a unique clinical subset of PBC. This is the first study to combine enrichment of antigens from HiBECs, label-free mass spectrometry, and bioinformatics to explore candidate autoantibodies for AMA-negative PBC. Of note, the results of the current pilot study are consistent with those in research publications, confirming the feasibility and reliability of the design and technology we applied.
The existence of serum autoantibody-negative autoimmune disease can be verified using proteomic technology, including enrichment of antigens from the HiBECs, label-free mass spectrometry, and bioinformatics. For future studies, the design needs to be optimized, including a larger sample size containing healthy and cholestatic cohorts, and the candidate autoantigens should be verified by Western blot analysis or enzyme-linked immunosorbent assay.
| 1. | Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med. 2005;353:1261-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 965] [Cited by in RCA: 940] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 2. | Carey EJ, Ali AH, Lindor KD. Primary biliary cirrhosis. Lancet. 2015;386:1565-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 407] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 3. | Joshita S, Umemura T, Tanaka E, Ota M. Genetic Contribution to the Pathogenesis of Primary Biliary Cholangitis. J Immunol Res. 2017;2017:3073504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Hirschfield GM, Gershwin ME. The immunobiology and pathophysiology of primary biliary cirrhosis. Annu Rev Pathol. 2013;8:303-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 235] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 5. | Ma WT, Chang C, Gershwin ME, Lian ZX. Development of autoantibodies precedes clinical manifestations of autoimmune diseases: A comprehensive review. J Autoimmun. 2017;83:95-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 101] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 6. | Patel A, Seetharam A. Primary Biliary Cholangitis: Disease Pathogenesis and Implications for Established and Novel Therapeutics. J Clin Exp Hepatol. 2016;6:311-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Deng C, Hu C, Wang L, Zhang S, Li P, Wu Z, Chen S, Zhang F, Li Y. Serological comparative proteomics analysis of mitochondrial autoantibody-negative and -positive primary biliary cirrhosis. Electrophoresis. 2015;36:1588-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Deng C, Lin M, Hu C, Li Y, Gao Y, Cheng X, Zhang F, Dong M, Li Y. Establishing a serologic decision tree model of extrapulmonary tuberculosis by MALDI-TOF MS analysis. Diagn Microbiol Infect Dis. 2011;71:144-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Deng C, Lin M, Hu C, Li Y, Gao Y, Cheng X, Zhang F, Dong M, Li Y. Exploring serological classification tree model of active pulmonary tuberculosis by magnetic beads pretreatment and MALDI-TOF MS analysis. Scand J Immunol. 2011;74:397-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Hu CJ, Song G, Huang W, Liu GZ, Deng CW, Zeng HP, Wang L, Zhang FC, Zhang X, Jeong JS. Identification of new autoantigens for primary biliary cirrhosis using human proteome microarrays. Mol Cell Proteomics. 2012;11:669-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 11. | Song G, Hu C, Zhu H, Wang L, Zhang F, Li Y, Wu L. New centromere autoantigens identified in systemic sclerosis using centromere protein microarrays. J Rheumatol. 2013;40:461-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Norman GL, Yang CY, Ostendorff HP, Shums Z, Lim MJ, Wang J, Awad A, Hirschfield GM, Milkiewicz P, Bloch DB. Anti-kelch-like 12 and anti-hexokinase 1: novel autoantibodies in primary biliary cirrhosis. Liver Int. 2015;35:642-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Pinzani M, Luong TV. Pathogenesis of biliary fibrosis. Biochim Biophys Acta. 2017; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 14. | Selmi C, Lleo A, Pasini S, Zuin M, Gershwin ME. Innate immunity and primary biliary cirrhosis. Curr Mol Med. 2009;9:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Wiśniewski JR, Zougman A, Nagaraj N, Mann M. Universal sample preparation method for proteome analysis. Nat Methods. 2009;6:359-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5054] [Cited by in RCA: 6444] [Article Influence: 379.1] [Reference Citation Analysis (0)] |
| 16. | Cox J, Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol. 2008;26:1367-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9849] [Cited by in RCA: 11931] [Article Influence: 662.8] [Reference Citation Analysis (0)] |
| 17. | Cox J, Mann M. Computational principles of determining and improving mass precision and accuracy for proteome measurements in an Orbitrap. J Am Soc Mass Spectrom. 2009;20:1477-1485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Cox J, Hein MY, Luber CA, Paron I, Nagaraj N, Mann M. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol Cell Proteomics. 2014;13:2513-2526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2967] [Cited by in RCA: 3985] [Article Influence: 332.1] [Reference Citation Analysis (0)] |
| 19. | Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24735] [Cited by in RCA: 28598] [Article Influence: 1682.2] [Reference Citation Analysis (0)] |
| 20. | von Mering C, Jensen LJ, Snel B, Hooper SD, Krupp M, Foglierini M, Jouffre N, Huynen MA, Bork P. STRING: known and predicted protein-protein associations, integrated and transferred across organisms. Nucleic Acids Res. 2005;33:D433-D437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 945] [Cited by in RCA: 1151] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 21. | Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498-2504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24663] [Cited by in RCA: 35800] [Article Influence: 1627.3] [Reference Citation Analysis (7)] |
| 22. | Juliusson G, Imam M, Björnsson ES, Talwalkar JA, Lindor KD. Long-term outcomes in antimitochondrial antibody negative primary biliary cirrhosis. Scand J Gastroenterol. 2016;51:745-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Mendes F, Lindor KD. Antimitochondrial antibody-negative primary biliary cirrhosis. Gastroenterol Clin North Am. 2008;37:479-484, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Sakauchi F, Mori M, Zeniya M, Toda G. Antimitochondrial antibody negative primary biliary cirrhosis in Japan: utilization of clinical data when patients applied to receive public financial aid. J Epidemiol. 2006;16:30-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Michieletti P, Wanless IR, Katz A, Scheuer PJ, Yeaman SJ, Bassendine MF, Palmer JM, Heathcote EJ. Antimitochondrial antibody negative primary biliary cirrhosis: a distinct syndrome of autoimmune cholangitis. Gut. 1994;35:260-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 141] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Rong GH, Yang GX, Ando Y, Zhang W, He XS, Leung PS, Coppel RL, Ansari AA, Zhong R, Gershwin ME. Human intrahepatic biliary epithelial cells engulf blebs from their apoptotic peers. Clin Exp Immunol. 2013;172:95-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Jin Q, Moritoki Y, Lleo A, Tsuneyama K, Invernizzi P, Moritoki H, Kikuchi K, Lian ZX, Hirschfield GM, Ansari AA. Comparative analysis of portal cell infiltrates in antimitochondrial autoantibody-positive versus antimitochondrial autoantibody-negative primary biliary cirrhosis. Hepatology. 2012;55:1495-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Bao ZJ, Gazouli M, Kreisel W, Tanabe S S- Editor: Gong ZM L- Editor: Wang TQ E- Editor: Ma YJ