Published online Dec 21, 2017. doi: 10.3748/wjg.v23.i47.8308
Peer-review started: September 7, 2017
First decision: October 10, 2017
Revised: November 3, 2017
Accepted: November 14, 2017
Article in press: November 14, 2017
Published online: December 21, 2017
Processing time: 105 Days and 0.5 Hours
To investigate the effects of Panax notoginseng (PN) on microvascular injury in colitis, its mechanisms, initial administration time and dosage.
Dextran sodium sulfate (DSS)- or iodoacetamide (IA)-induced rat colitis models were used to evaluate and investigate the effects of ethanol extract of PN on microvascular injuries and their related mechanisms. PN administration was initiated at 3 and 7 d after the model was established at doses of 0.5, 1.0 and 2.0 g/kg for 7 d. The severity of colitis was evaluated by disease activity index (DAI). The pathological lesions were observed under a microscope. Microvessel density (MVD) was evaluated by immunohistochemistry. Vascular permeability was evaluated using the Evans blue method. The serum concentrations of cytokines, including vascular endothelial growth factor (VEGF)A121, VEGFA165, interleukin (IL)-4, IL-6, IL-10 and tumor necrosis factor (TNF)-α, were detected by enzyme-linked immunosorbent assay. Myeloperoxidase (MPO) and superoxide dismutase (SOD) were measured to evaluate the level of oxidative stress. Expression of hypoxia-inducible factor (HIF)-1α protein was detected by western blotting.
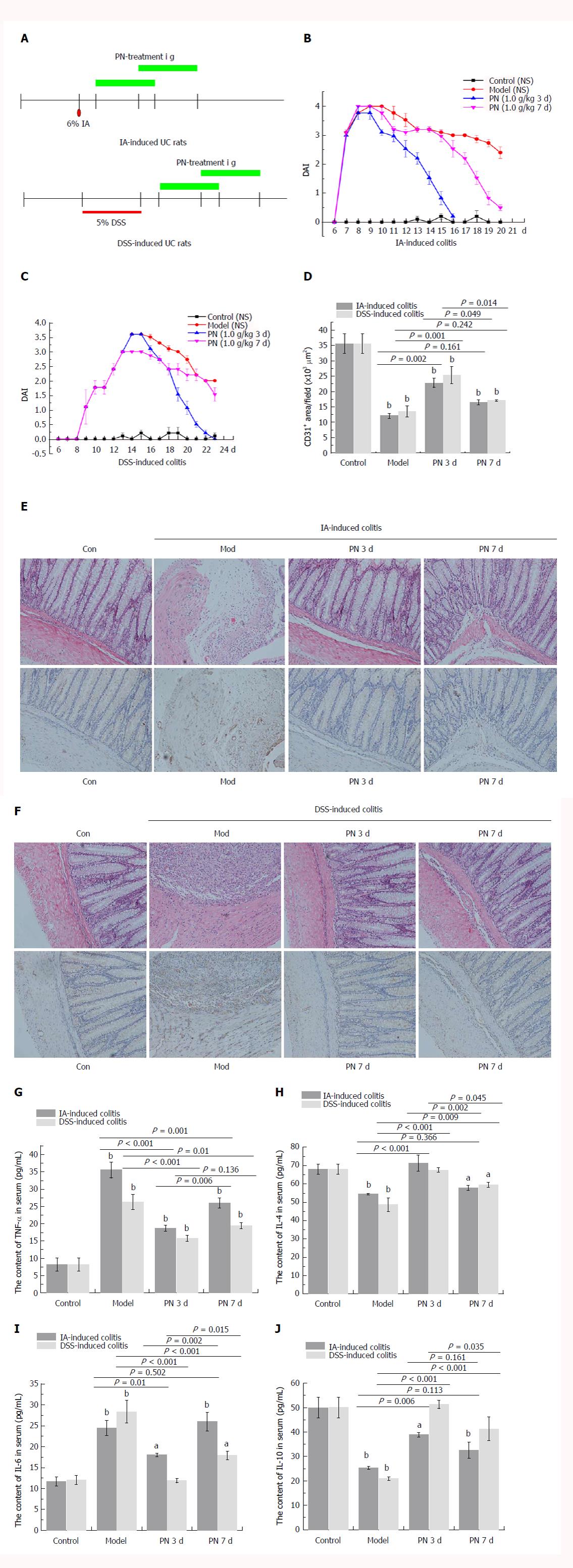
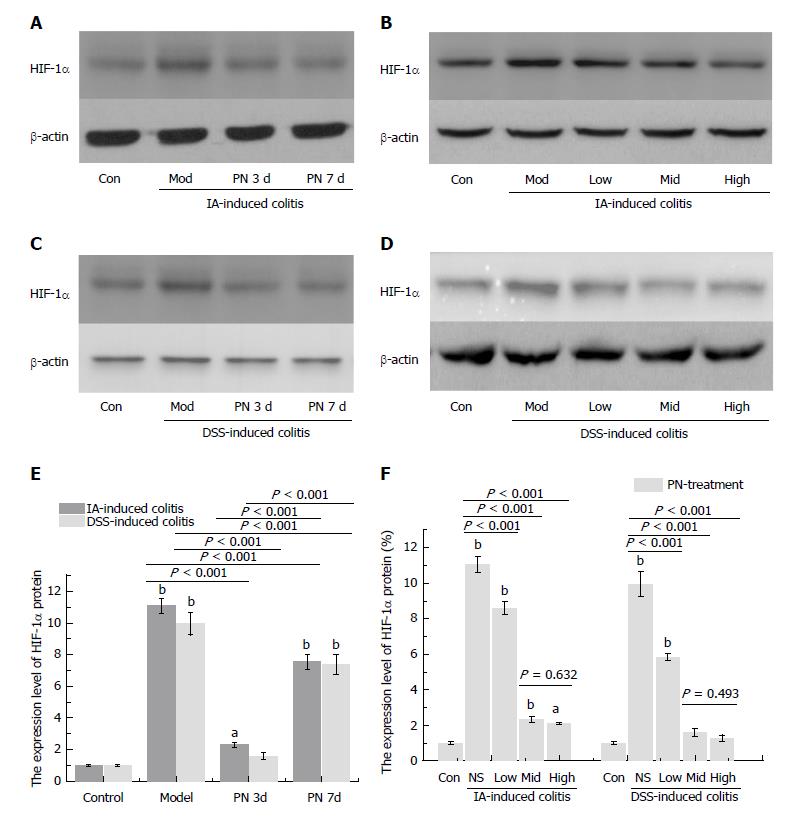
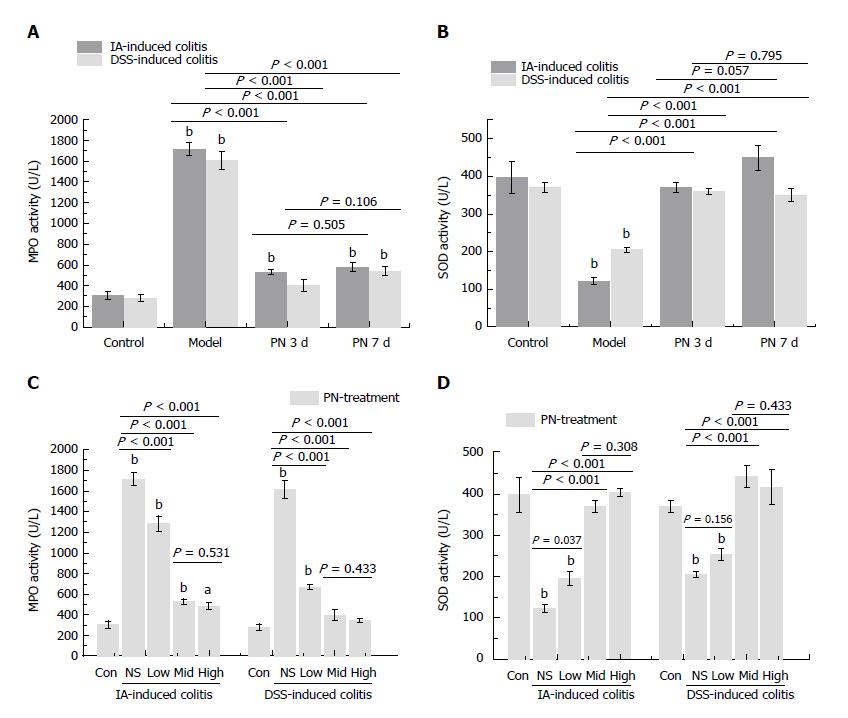
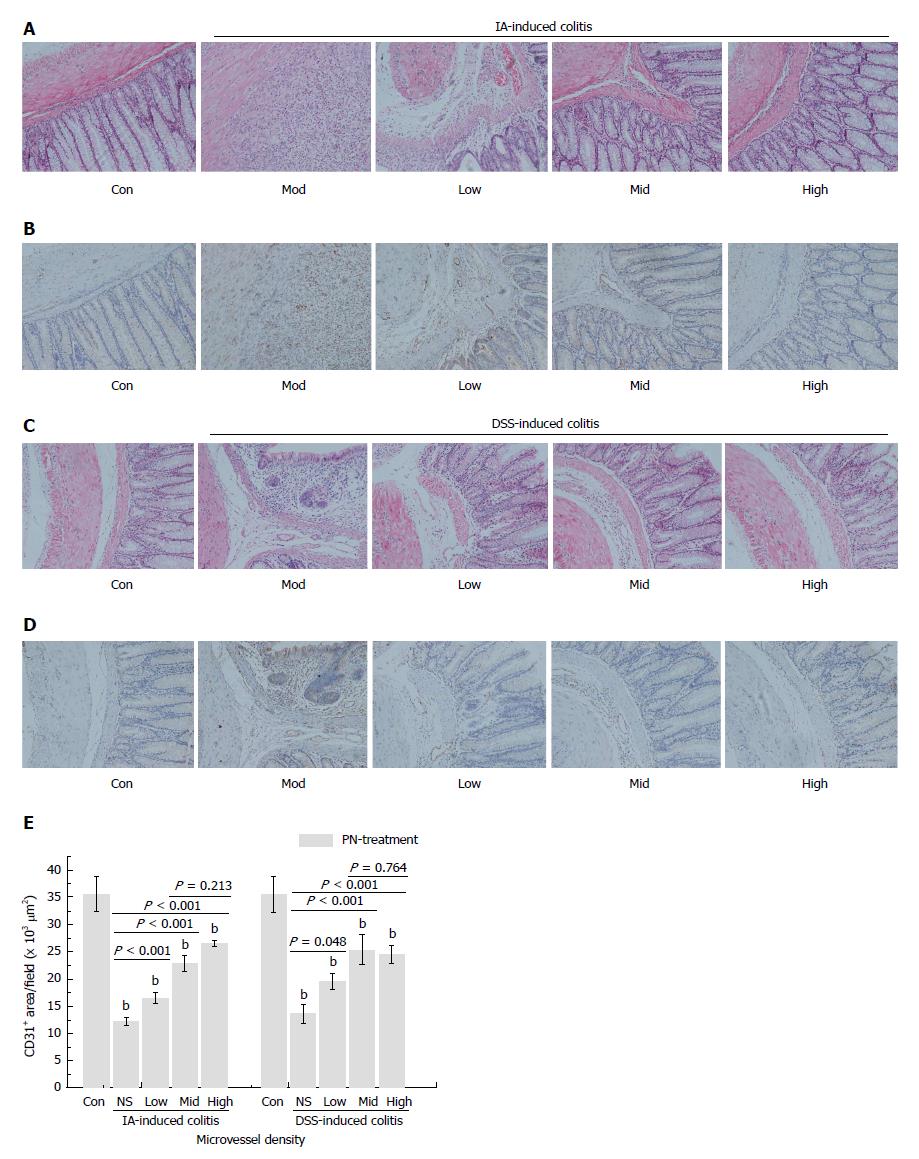
Obvious colonic inflammation and injuries of mucosa and microvessels were observed in DSS- and IA-induced colitis groups. DAI scores, serum concentrations of VEGFA121, VEGFA165, VEGFA165/VEGFA121, IL-6 and TNF-α, and concentrations of MPO and HIF-1α in the colon were significantly higher while serum concentrations of IL-4 and IL-10 and MVD in colon were significantly lower in the colitis model groups than in the normal control group. PN promoted repair of injuries of colonic mucosa and microvessels, attenuated inflammation, and decreased DAI scores in rats with colitis. PN also decreased the serum concentrations of VEGFA121, VEGFA165, VEGFA165/VEGFA121, IL-6 and TNF-α, and concentrations of MPO and HIF-1α in the colon, and increased the serum concentrations of IL-4 and IL-10 as well as the concentration of SOD in the colon. The efficacy of PN was dosage dependent. In addition, DAI scores in the group administered PN on day 3 were significantly lower than in the group administered PN on day 7.
PN repairs vascular injury in experimental colitis via attenuating inflammation and oxidative stress in the colonic mucosa. Efficacy is related to initial administration time and dose.
Core tip:Panax notoginseng (PN) is a traditional Chinese medicine used to treat ulcerative colitis, but its mechanisms are unclear. In our study, we found that PN promoted repair of injuries of colonic mucosa and microvessels in rat colitis. PN decreased concentrations of vascular endothelial growth factor, interleukin (IL)-6 and tumor necrosis factor-α while it increased the concentrations of IL-4 and IL-10 in serum. It also decreased concentrations of myeloperoxidase and hypoxia-inducible factor-1α while it increased the concentration of superoxide dismutase in colon. So it is concluded that PN repairs mucosal and vascular injuries in rat colitis via attenuating inflammation and oxidative stress in the colonic mucosa.
- Citation: Wang SY, Tao P, Hu HY, Yuan JY, Zhao L, Sun BY, Zhang WJ, Lin J. Effects of initiating time and dosage of Panax notoginseng on mucosal microvascular injury in experimental colitis. World J Gastroenterol 2017; 23(47): 8308-8320
- URL: https://www.wjgnet.com/1007-9327/full/v23/i47/8308.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i47.8308
Microvessels are a major component of the colonic mucosa that nourish the colonic tissue and clear metabolic waste via controlling the intestinal blood flow[1]. They also play an important role in maintaining normal intestinal permeability[2]. Recent studies have found that injury of the colonic microvessels precedes injury of the colonic mucosa in the development of experimental colitis[3]. The increased vascular permeability aggravates the early endothelial injury[4], which induces hypoxia of the colonic mucosa and further oxidative stress[5,6].
Previous studies have demonstrated that colonic mucosal hypoxia induced by mucosal vascular injury plays an important role in the pathogenesis of ulcerative colitis (UC)[7]. Our previous study found that colonic mucosal injury in rats with experimental colitis improved along with repair of the mucosal microvascular injury[8]. Therefore, microvascular injury is essential to the development of UC and could be a new therapeutic target. However, there are no drugs that can promote effective microvascular repair.
Panax notoginseng (PN) is a common Chinese herbal medicine that has long been used to treat vascular lesions[9]. Some studies have found that it promotes repair of vascular damage via proangiogenic and anti-apoptotic effects[10,11]. Animal experiments have shown that PN attenuates colonic mucosal injury and promotes mucosal repair in mouse models of colitis[12], but its mechanism of action is unclear. It is hypothesized that PN repair of mucosal injury could be related to its promotion of repair of microvascular injury. In this study, we investigated the mechanism of PN repair of colonic mucosal injury in rats with colitis from the aspect of promotion of vascular repair. We also investigated the relationship between dose and initial time of administration of PN and its efficacy.
Sprague-Dawley rats (120-140 g) were from Shanghai SLAC Laboratory Animal Co. Ltd. (Shanghai, China). The Institutional Animal Care and Use Committee of Shanghai University of Traditional Chinese Medicine approved all procedures. Rats were housed in a pathogen-free environment and allowed to acclimate to the environment for 7 d before inclusion in an experiment.
Iodoacetamide (IA; purity > 99%), dextran sodium sulfate (DSS), formamide (purity > 99%) and Evans blue (dye content > 75%) were purchased from Sigma-Aldrich (St. Louis, MO, United States). Enzyme-linked immunosorbent assay (ELISA) kits for interleukin (IL)-4, IL-6, IL-10 and tumor necrosis factor (TNF)-α were purchased form R&D Systems (Minneapolis, MN, United States). ELISA kits for vascular endothelial growth factor (VEGF)A165 and VEGFA121 were purchased from Cloud-Clone Corp. (Katy, TX, United States). CD31 antibody (ab23680) and goat anti-mouse antibody were purchased from Abcam (Cambridge, United Kingdom). Myeloperoxidase (MPO) and superoxide dismutase (SOD) assay kits were purchased from Cell Signaling Technology (Danvers, MA, United States).
PN was purchased from Shanghai Huayu Traditional Chinese Medicine Co. Ltd. (Shanghai, China) and authenticated by Professor Yang Dong (Shanghai University of Traditional Chinese Medicine). PN (0.9 kg) was dissolved in tap water (9 L), boiled at 100 °C for 3 h, filtered through a sieve (150 μm), extracted with absolute ethanol, and dried in a freeze dryer. The brown extract powder of PN was stored at -20 °C.
Two rat models of colitis were used in this study. One was induced by 0.1 mL 6% IA dissolved in 1% methylcellulose given to rats once by enema (7 cm above the anus). The other was induced by DSS, in which rats were allowed free access to purified water containing 5% DSS (w/v) for 7 d. DSS solution was prepared daily.
Rats were divided into control, low-dose, medium-dose and high-dose groups (n = 6 each). For the intervention groups, PN was intragastrically administered to rats with IA-induced or DSS-induced colitis once daily for seven consecutive days at doses of 0.5, 1.0 or 2.0 g/kg. For the control groups, normal saline was given to the corresponding rats. The initial administration times of PN were 3 and 7 d after establishing the colitis models. Three independent experiments were performed in triplicate.
Before, during and after treatment, the severity of colitis was evaluated with disease activity index (DAI), as described previously[13]. The parameters of DAI included weight loss (0, none; 1, 0%-5%; 2, 5%-10%; 3, 10%-20%; 4, > 20%), stool consistency (0, none; 2, loose stool; 4, watery), and bleeding (0, none; 1, trace; 2, mild occult blood; 3, obvious occult blood; 4, gross bleeding).
Segments of colon were fixed in formalin buffer and embedded in paraffin. Sections of 5 μm thick were deparaffinized and stained with hematoxylin and eosin (HE). The histological changes were assessed by a pathologist.
Five-micrometer-thick paraffin-embedded sections of colon were deparaffinized, subjected to heat-mediated antigen retrieval, and blocked with goat serum. The tissues were incubated with the primary anti-CD31 antibody (1:200, v/v) overnight at 4 °C. After three 5-min washes, the horseradish peroxidase (HRP)-labeled secondary antibody (1: 300) was added and the samples were incubated at 37 °C for 1 h. The sections were counterstained with hematoxylin for 1 min at room temperature to visualize the endothelial cell nuclei. Three fields with CD31-positive cells in each section were chosen to assess microvessel density (MVD). Areas of highest vascularization were selected by scanning the sections at low magnification. Stained microvessels were counted in a single 200 × field within the selected field by three observers without previous knowledge of control groups. The following cellular structures were considered as countable microvessels: (1) stained lumen; (2) stained endothelial cell; and (3) stained endothelial cell cluster (1 and 2 were clearly separated from adjacent strained lumens, colonic mucosal cells and other connective tissue elements). The MVD value was calculated as the average vessel counts in three selected areas within a microscopic field.
Vascular permeability (VP) of vessels in colonic mucosa was evaluated by the Evans blue method, as described previously[14]. Rats were anesthetized with intraperitoneal injection of sodium pentobarbital. Evans blue (1 mg/100 g) was injected intravenously 15 min before autopsy. Evans blue was extracted from the 1-cm segment of colonic tissue using formamide and measured by spectrophotometry at 610 nm. Results were expressed as OD value per milligram of colon.
Blood samples were collected from the abdominal aorta. The serum concentrations of VEGFA165 (Cloud-Clone Corp.), VEGFA121 (Cloud-Clone Corp.), IL-4 (R&D Systems), IL-6 (R&D Systems), IL-10 (R&D Systems) and TNF-α (R&D Systems) were detected using the appropriate ELISA kits.
SOD activity was measured using the SOD assay kit (Cell Signaling Technology). Tissue homogenate was prepared by vortex homogenizer, heated at 95 °C for 40 min and centrifuged at 178 × g at 4 °C for 10 min. Ethanol-chloroform mixture (5:3, v/v) was used to extract SOD in the homogenate for total SOD activity assay. MPO activity was measured using the MPO assay kit (Cell Signaling Technology). Tissue homogenate was prepared by vortex homogenizer, heated at 95 °C for 40 min and centrifuged at 714 × g at 4 °C for 10 min. The samples were added to phosphate buffer containing 30 mM H2O2 (pH 7.0) and incubated for 10 min. The enzymatic activity of SOD and MPO was expressed by the decrease of OD240.
Hypoxia-inducible factor (HIF)-1α was measured by western blotting, as described previously[15]. Colonic tissue was cut into pieces and homogenized in 5-fold volumes of ice-cold homogenizing buffer (0.1 mmol/L NaCl, 0.1 mol/L Tris-HCl, and 0.001 mol/L EDTA) containing 1 mmol/L phenylmethylsulfonyl fluoride, 1 mg/mL aprotinin and 0.1 mmol/L leupeptin at 3000 × g and 4 °C for 1 h. Bovine serum albumin was used to estimate the protein content in supernatants. The protein samples (60 μg in each sample) were subjected to SDS-PAGE and transferred to polyvinylidene fluoride membranes using a transfer apparatus (Bio-Rad, Hercules, CA, United States). The membranes were blocked for 2 h, then the primary antibody anti-HIF-1α was added and incubated at 4 °C overnight, and the corresponding HRP-conjugated secondary antibody (Cell Signaling Technology) was added and incubated for 1 h. Protein-antibody complexes were detected by Clarity Western ECL Substrate (Bio-Rad), and results were authenticated with the ImageJ software (Gene Co. Ltd., China).
Data were presented as the mean ± SD. One-way analysis of variance or general linear model with repeated measures was used to analyze the data sets with three or more groups, and least significant difference post hoc test for multiple comparisons. Student’s t-test was used to analyze data sets with two groups. P < 0.05 was considered significant.
After the colitis model was established, PN administration (1.0 g/kg) was initiated at days 3 and 7 for seven consecutive days (Figure 1A). Compared with the day 7 group, DAI scores, injuries of colonic mucosa and microvessels, serum concentrations of pro-inflammatory cytokines (IL-6 and TNF-α), and expression of HIF-1α and MPO were significantly lower (Figure 1B, C, E-G and I; Figure 2A, C and E; Figure 3A), and serum concentrations of anti-inflammatory cytokines (IL-4 and IL-10), MVD and SOD were significantly higher (Figure 1D, H and J; Figure 3C) in the day 3 group. The earlier PN was administered, the more effective it was in treating acute colitis.
The rats in the IA- and DSS-induced experimental colitis groups had obvious injuries of the colonic mucosa and microvessels, as well as lower MVD. After being treated with PN (0.5, 1.0 and 2.0 g/kg) for seven consecutive days, colonic mucosal injuries and microvessels significantly improved and MVD increased compared with the model groups. The efficacy of PN in attenuating mucosal and microvascular injuries was dose dependent (Figure 4A-E). The effects of medium- and high-dose PN were superior to those of low-dose PN, but there were no significant differences between the medium- and high-dose groups (Figure 4A-E).
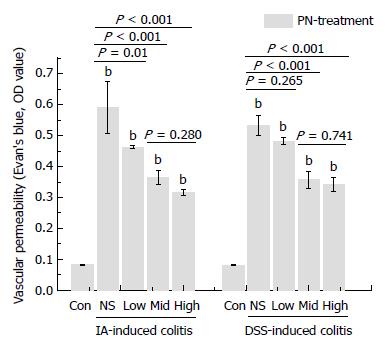
VP increased significantly in the groups with experimental colitis induced by DSS and IA compared with the normal control group. PN improved impaired VP in a dose-dependent manner. The effects of medium and high doses of PN were superior to those of low dose PN, but there were no significant differences between the medium- and high-dose groups (Figure 5).
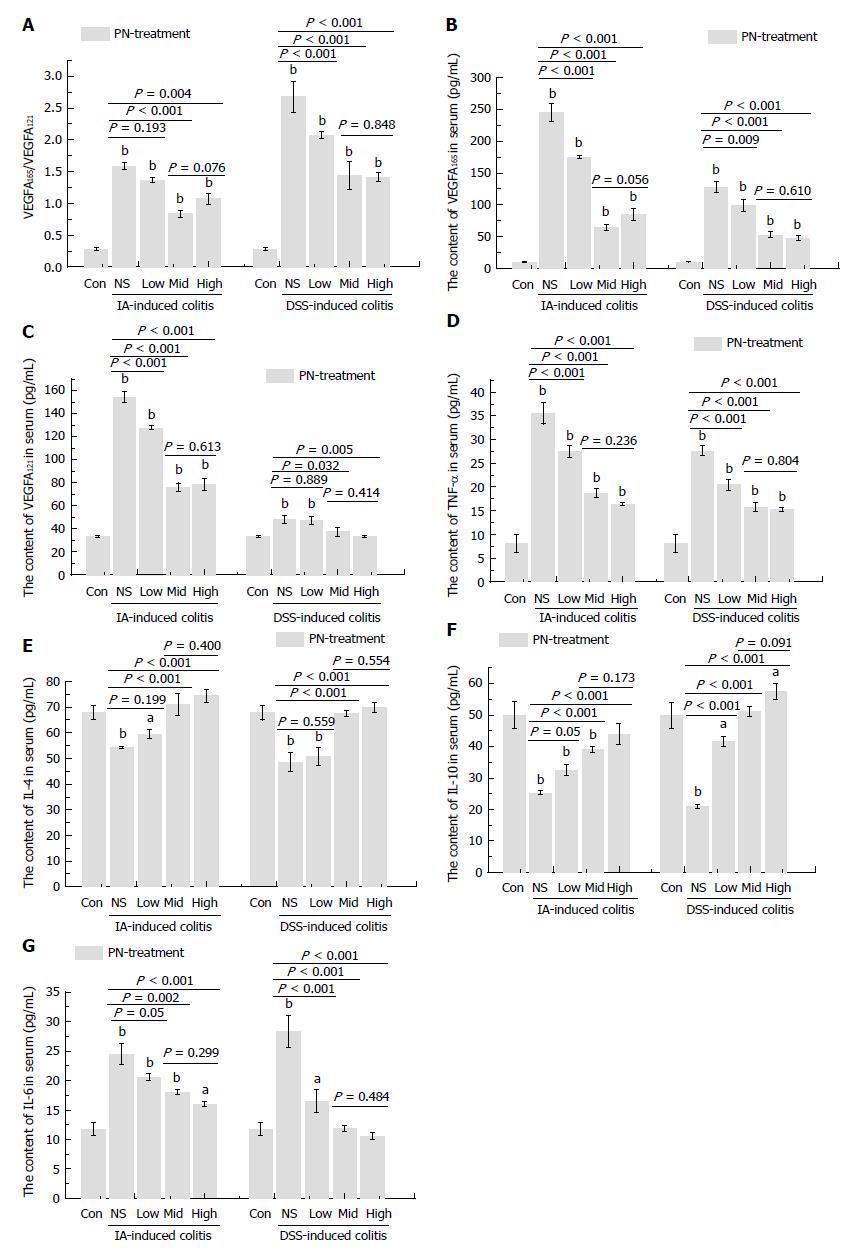
The serum concentrations of VEGFA165 and VEGFA121 and the ratio of VEGFA165/VEGFA121 increased significantly in the groups with experimental colitis induced by IA and DSS compared with the normal control group. PN significantly decreased elevated VEGFA165, VEGFA121 and VEGFA165/VEGFA121 ratio in a dose-dependent manner in rats with experimental colitis. The effects in the medium- and high-dose groups were superior to those of the low-dose group, but there were no significant differences between the medium- and high-dose groups (Figure 6A-C).
The pro-inflammatory cytokines (IL-6 and TNF-α) increased significantly and the anti-inflammatory cytokines (IL-4 and IL-10) decreased significantly in the experimental colitis groups compared with the normal control group. PN down-regulated elevated IL-6 and TNF-α and up-regulated reduced IL-4 and IL-10 in a dose-dependent manner compared with the model control group (Figure 6D-G). The effects of medium and high dose PN were superior to those of low dose PN, but there were no significant differences between the medium- and high-dose groups.
Expression of HIF-1α in colonic mucosa was significantly up-regulated in the experimental colitis groups compared with the normal control group. PN down-regulated increased expression of HIF-1α in a dose-dependent manner compared with the model control group (Figure 2B, D and F). The effects of medium and high dose PN were superior to those of low dose PN, but there were no significant differences between the medium- and high-dose groups (Figure 2B, D and F).
The activity of MPO and SOD in colonic tissue was used to evaluate the anti-oxidative effect of PN. The activity of MPO increased and the activity of SOD decreased in the colon in the experimental colitis groups compared with the normal control group. PN down-regulated elevated MPO and up-regulated decreased SOD in a dose-dependent manner compared with the control group. The effects of medium and high dose PN were superior to those of low dose PN, but there were no significant differences between the medium- and high-dose groups (Figure 3B and D).
PN, also known as Sanqi or Tianqi, is a common Chinese herbal medicine with various activities and is used to treat cardiovascular diseases, pain, inflammation and hemorrhagic injury[16]. In recent years, PN has been used to treat UC, with the effects of promoting repair of mucosal injury and attenuating inflammatory responses[17]. However, its mechanism of action is unclear.
It is well known that the efficacy of a drug is closely related to the initial administration time and its dose[18]. In previous studies, the initial time of PN administration was usually based on personal experience rather than experimental evidence, which affected the standardization and efficacy of PN treatment. We found that initiating PN at day 3 after establishing the colitis models was more effective than initiating at day 7, demonstrating improved mucosal injury, microvascular impairment, inflammatory response and hypoxia. In our other study that has not been published, we found mild mucosal injury and increased vessel permeability and concentrations of TNF-α, IL-6 and MPO on day 3 in a colitis model. This suggested that the changes of vessel permeability and inflammatory cytokines occurred in the early stage of colitis and preceded mucosal injury. That may be why early initiation of PN treatment (day 3) improved colitis more than initiating treatment on day 7. In addition, the efficacy of PN was dose dependent. The efficacy of medium and high doses was superior to that of the low dose, but there were no significant differences between the medium and high doses. This provided empirical evidence for using PN early and choosing the optimal dose in UC treatment.
Maintaining oxygen supply and metabolic clearance are the two crucial roles of colonic vessels. The severity of active UC is associated with mucosal hypoxia, which may result from increased oxygen consumption of inflammatory cells and decreased oxygen supply caused by vascular dysfunction[19,20]. The imbalance between oxygen consumption and supply, as well as excessive serum cytokines, leads to increased epithelial cell apoptosis and consequent impairment of mucosal barrier function[2,19]. Therefore, the levels of serum cytokines and expression of hypoxia- and oxidative stress-related proteins in colonic mucosa could reflect the status of hypoxia.
The serum concentrations of cytokines, including anti-inflammatory (IL-4 and IL-10) and pro-inflammatory (L-6 and TNF-α) cytokines are indicators of inflammatory status[21,22]. In addition, HIF-1α is a crucial marker protein expressed under hypoxic conditions[23,24]. Its expression increases when hypoxia occurs in tissues and is usually used to assess the severity of hypoxia[25,26]. MPO and SOD, two kinds of oxidative enzymes, are crucial markers used to assess oxidative stress[27,28]. They could reflect the severity of oxidative stress and hypoxia in colonic mucosa[19,29]. VEGF, especially VEGFA, is an important cytokine implicated in angiogenesis[8]. VEGFA121 and VEGFA165, two isoforms of VEGF, are closely correlated to the angiogenesis of colonic microvessels[8,30].
Our previous study demonstrated that increased ratio of VEGFA165/VEGFA121 was in proportion to impairment of microvessels. In the present study, 7-d PN treatment reduced serum concentrations of VEGFA165 and VEGFA121 and the ratio of VEGFA165/VEGFA121, and attenuated impairment of microvessels compared with the colitis groups. PN down-regulated the expression of MPO and HIF-1α while up-regulating the expression of SOD in colonic mucosa. These findings demonstrate that PN attenuates hypoxia and oxidative stress in colonic mucosa. The changes in VEGFA isoforms, HIF-1α, MPO and SOD were dose dependent. The effects of medium and high doses were superior to those of low dose, but there were no significant differences between the medium and high doses. This suggested that the effects of PN reached a plateau when the dose was increased to a certain value. The optimal dose of PN is 1.0 g/kg for treating experimental colitis in rats.
In summary, PN is promising in UC treatment. It could improve hypoxia and relieve oxidative stress in the colon, attenuate vessel impairment and/or promote angiogenesis, and finally promote repair of colonic mucosa. Early use of PN at an optimal dose might yield better efficacy. However, there are still some unresolved problems in the present study that need further study. For example, what is the real effective component in PN for UC treatment? What are the mechanisms of PN attenuating oxidative stress and regulating angiogenesis? These are important questions for using PN for treatment of UC and warrant exploration in future studies.
Panax notoginseng (PN) is a Chinese herbal medicine commonly used to treat ulcerative colitis (UC) and vascular diseases. Microvascular injury plays an important role in the pathogenesis of UC, but PN’s effects on microvascular injury in UC are unclear. To clarify the effects of PN on microvascular injury is important for treating UC.
The effects of PN on microvascular injury in colitis, its initial administration time, its dosage and its related mechanisms were investigated. These are important questions for using PN for treatment of UC.
To clarify the effects of PN on microvascular injury and related affecting factors, as well as its mechanisms.
Dextran sodium sulfate (DSS)- or iodoacetamide (IA)-induced rat colitis models were used. PN administration was initiated at 3 d and 7 d after the model was established at doses of 0.5, 1.0 and 2.0 g/kg for seven consecutive days. The severity of colitis was evaluated by disease activity index (DAI). The pathological lesions were observed under microscope. Microvessel density (MVD) was evaluated by immunohistochemistry. Vascular permeability was evaluated using the Evans blue method. The serum concentrations of vascular endothelial growth factor (VEGF)A121, VEGFA165, interleukin (IL)-4, IL-6, IL-10 and tumor necrosis factor (TNF)-α were detected by enzyme-linked immunosorbent assay. Myeloperoxidase (MPO) and superoxide dismutase (SOD) were measured to evaluate the level of oxidative stress. Expression of hypoxia-inducible factor (HIF)-1α protein was detected by western blotting. One-way ANOVA or general linear model with repeated measures was used to analyze the data sets with three or more groups, and least significant difference post hoc test for multiple comparisons. Student’s t-test was used to analyze data sets with two groups. P < 0.05 was considered significant.
Obvious colonic inflammation and injuries of colonic mucosa and microvessels were observed in DSS- and IA-induced colitis in rats. DAI scores, the serum concentrations of VEGFA121, VEGFA165, VEGFA165/VEGFA121, IL-6 and TNF-α, and the concentrations of MPO and HIF-1α in the colon were significantly higher while the serum concentrations of IL-4 and IL-10 and MVD in colon were significantly lower in the colitis model groups than in the normal control group. PN promoted repair of the injuries of colonic mucosa and microvessels, attenuated inflammation and decreased DAI scores in rats with colitis. PN decreased the serum concentrations of VEGFA121, VEGFA165, VEGFA165/VEGFA121, IL-6 and TNF-α and the concentrations of MPO and HIF-1α in the colon. It also increased the serum concentrations of IL-4 and IL-10 as well as the concentration of SOD in the colon. The efficacy of PN was dosage dependent. In addition, DAI scores in the group initiating PN administration on day 3 were significantly lower than in the group initiating PN administration on day 7.
PN repaired microvessel injury in experimental colitis via attenuating inflammation and oxidative stress in the colonic mucosa. The efficacy of PN was related to the initial administration time and the dose.
Finding the real effective component in PN and clarifying the mechanisms of PN attenuating oxidative stress and regulating angiogenesis will be conducted in the future studies.
The authors thank all technical staff who provided help in the study and Dr. Ying Dong who provided help in biostatistics.
| 1. | Kaelin WG Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2073] [Cited by in RCA: 2530] [Article Influence: 140.6] [Reference Citation Analysis (0)] |
| 2. | Saijo H, Tatsumi N, Arihiro S, Kato T, Okabe M, Tajiri H, Hashimoto H. Microangiopathy triggers, and inducible nitric oxide synthase exacerbates dextran sulfate sodium-induced colitis. Lab Invest. 2015;95:728-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Gutierrez LS, Ling J, Nye D, Papathomas K, Dickinson C. Thrombospondin peptide ABT-898 inhibits inflammation and angiogenesis in a colitis model. World J Gastroenterol. 2015;21:6157-6166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Tolstanova G, Deng X, French SW, Lungo W, Paunovic B, Khomenko T, Ahluwalia A, Kaplan T, Dacosta-Iyer M, Tarnawski A. Early endothelial damage and increased colonic vascular permeability in the development of experimental ulcerative colitis in rats and mice. Lab Invest. 2012;92:9-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Pinzón-Daza ML, Cuellar-Saenz Y, Nualart F, Ondo-Mendez A, Del Riesgo L, Castillo-Rivera F, Garzón R. Oxidative Stress Promotes Doxorubicin-Induced Pgp and BCRP Expression in Colon Cancer Cells Under Hypoxic Conditions. J Cell Biochem. 2017;118:1868-1878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 6. | Slattery ML, Lundgreen A, Welbourn B, Wolff RK, Corcoran C. Oxidative balance and colon and rectal cancer: interaction of lifestyle factors and genes. Mutat Res. 2012;734:30-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Deng X, Szabo S, Khomenko T, Tolstanova G, Paunovic B, French SW, Sandor Z. Novel pharmacologic approaches to the prevention and treatment of ulcerative colitis. Curr Pharm Des. 2013;19:17-28. [PubMed] |
| 8. | Shiying W, Boyun S, Jianye Y, Wanjun Z, Ping T, Jiang L, Hongyi H. The Different Effects of VEGFA121 and VEGFA165 on Regulating Angiogenesis Depend on Phosphorylation Sites of VEGFR2. Inflamm Bowel Dis. 2017;23:603-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Ng TB. Pharmacological activity of sanchi ginseng (Panax notoginseng). J Pharm Pharmacol. 2006;58:1007-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 296] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 10. | Yang BR, Cheung KK, Zhou X, Xie RF, Cheng PP, Wu S, Zhou ZY, Tang JY, Hoi PM, Wang YH. Amelioration of acute myocardial infarction by saponins from flower buds of Panax notoginseng via pro-angiogenesis and anti-apoptosis. J Ethnopharmacol. 2016;181:50-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 11. | Lei Y, Tao LL, Wang GL. [Effect of extracts from Panax ginseng, Panax notoginseng, and Ligusticum chuanxiong on vascular smooth muscle cells of aging and hypertension rats]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2012;32:1374-1379. [PubMed] |
| 12. | Wen XD, Wang CZ, Yu C, Zhao L, Zhang Z, Matin A, Wang Y, Li P, Xiao SY, Du W. Panax notoginseng attenuates experimental colitis in the azoxymethane/dextran sulfate sodium mouse model. Phytother Res. 2014;28:892-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Dai YC, Zheng L, Zhang YL, Chen X, Chen DL, Wang LJ, Tang ZP. Jianpi Qingchang decoction regulates intestinal motility of dextran sulfate sodium-induced colitis through reducing autophagy of interstitial cells of Cajal. World J Gastroenterol. 2017;23:4724-4734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Yamamoto A, Itoh T, Nasu R, Nishida R. Sodium alginate ameliorates indomethacin-induced gastrointestinal mucosal injury via inhibiting translocation in rats. World J Gastroenterol. 2014;20:2641-2652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Glover LE, Bowers BE, Saeedi B, Ehrentraut SF, Campbell EL, Bayless AJ, Dobrinskikh E, Kendrick AA, Kelly CJ, Burgess A. Control of creatine metabolism by HIF is an endogenous mechanism of barrier regulation in colitis. Proc Natl Acad Sci USA. 2013;110:19820-19825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 16. | Wang T, Guo R, Zhou G, Zhou X, Kou Z, Sui F, Li C, Tang L, Wang Z. Traditional uses, botany, phytochemistry, pharmacology and toxicology of Panax notoginseng (Burk.) F.H. Chen: A review. J Ethnopharmacol. 2016;188:234-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 313] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 17. | Triantafillidis JK, Triantafyllidi A, Vagianos C, Papalois A. Favorable results from the use of herbal and plant products in inflammatory bowel disease: evidence from experimental animal studies. Ann Gastroenterol. 2016;29:268-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 18. | Wang M, Lei Y. Time-effect relationship of extracts from ginseng, notoginseng and chuanxiong on vascular endothelial cells senescence. Chin J Integr Med. 2014;20:758-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Stupin A, Cosic A, Novak S, Vesel M, Jukic I, Popovic B, Karalic K, Loncaric Z, Drenjancevic I. Reduced Dietary Selenium Impairs Vascular Function by Increasing Oxidative Stress in Sprague-Dawley Rat Aortas. Int J Environ Res Public Health. 2017;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Cummins EP, Crean D. Hypoxia and inflammatory bowel disease. Microbes Infect. 2017;19:210-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 21. | Liu MY, Yang ZY, Dai WK, Huang JQ, Li YH, Zhang J, Qiu CZ, Wei C, Zhou Q, Sun X. Protective effect of Bifidobacterium infantis CGMCC313-2 on ovalbumin-induced airway asthma and β-lactoglobulin-induced intestinal food allergy mouse models. World J Gastroenterol. 2017;23:2149-2158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 22. | Almeida Junior LD, Quaglio AEV, de Almeida Costa CAR, Di Stasi LC. Intestinal anti-inflammatory activity of Ground Cherry (Physalis angulata L.) standardized CO2 phytopharmaceutical preparation. World J Gastroenterol. 2017;23:4369-4380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Sun X, Liu YD, Gao W, Shen SH, Li M. HIF-1α -1790G>A polymorphism significantly increases the risk of digestive tract cancer: a meta-analysis. World J Gastroenterol. 2015;21:1641-1649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Kim JH, Hwang YJ, Han SH, Lee YE, Kim S, Kim YJ, Cho JH, Kwon KA, Kim JH, Kim SH. Dexamethasone inhibits hypoxia-induced epithelial-mesenchymal transition in colon cancer. World J Gastroenterol. 2015;21:9887-9899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Okamoto R, Watanabe M. Role of epithelial cells in the pathogenesis and treatment of inflammatory bowel disease. J Gastroenterol. 2016;51:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 155] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 26. | Flannigan KL, Agbor TA, Motta JP, Ferraz JG, Wang R, Buret AG, Wallace JL. Proresolution effects of hydrogen sulfide during colitis are mediated through hypoxia-inducible factor-1α. FASEB J. 2015;29:1591-1602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Zheng XY, Lv YF, Li S, Li Q, Zhang QN, Zhang XT, Hao ZM. Recombinant adeno-associated virus carrying thymosin β4 suppresses experimental colitis in mice. World J Gastroenterol. 2017;23:242-255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Wang T, Leng YF, Zhang Y, Xue X, Kang YQ, Zhang Y. Oxidative stress and hypoxia-induced factor 1α expression in gastric ischemia. World J Gastroenterol. 2011;17:1915-1922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Chen Z, Wang J, Yang W, Chen J, Meng Y, Geng B, Cui Q, Yang J. FAM3A mediates PPARγ’s protection in liver ischemia-reperfusion injury by activating Akt survival pathway and repressing inflammation and oxidative stress. Oncotarget. 2017;8:49882-49896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Xin H, Zhong C, Nudleman E, Ferrara N. Evidence for Pro-angiogenic Functions of VEGF-Ax. Cell. 2016;167:275-284.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Doherty GA, Ziogas GE S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Huang Y