Published online Nov 28, 2017. doi: 10.3748/wjg.v23.i44.7849
Peer-review started: October 11, 2017
First decision: October 18, 2017
Revised: November 3, 2017
Accepted: November 14, 2017
Article in press: November 14, 2017
Published online: November 28, 2017
Processing time: 47 Days and 16.3 Hours
To evaluate selected intestinal parameters of oxidative stress, and antioxidant capacity in adult celiac disease patients with extraintestinal manifestations.
The study involved 85 adult patients divided into the following subgroups: (1) patients with newly diagnosed celiac disease (CD) (n = 7); (2) celiac patients not adhering to a gluten-free diet (GFD) (n = 22); (3) patients with CD on the GFD (n = 31); and (4) patients with functional disorders of the gastrointestinal tract, serving as controls (n = 25). Celiac patients presented with non-classic symptoms or extraintestinal manifestations. Standard blood tests including serum antioxidant levels (uric acid, bilirubin, and vitamin D), celiac antibody levels, and histopathological status of duodenal biopsy specimens have been determined. The expression of mRNA for tumor necrosis factor α (TNF-α), interleukin 1β (IL-1β), interleukin 10 (IL-10), superoxide dismutase (SOD), heat-shock protein 70 (HSP-70), hypoxia-inducible factor 1 (HIF-1α), and BAX in the duodenal mucosa of patients was analyzed by reverse transcriptase-polymerase chain reaction.
The mean plasma uric acid level in patients with active CD (newly diagnosed and nonadherent patients) and treated celiac patients was significantly higher than in controls (260.17 ± 53.65 vs 190.8 ± 22.98, P < 0.001, and 261.7 ± 51.79 vs 190.8 ± 22.98, P < 0.001, respectively). The mean bilirubin concentration in active and treated celiac patients was significantly lower than in controls (8.23 ± 5.04 vs 10.48 ± 4.08, P < 0.05 and 8.06 ± 3.31 vs 10.48 ± 4.08, P < 0.05, respectively). The mean plasma vitamin D level was significantly lower in active celiac patients than in treated celiac patients and controls (19.37 ± 9.03 vs 25.15 ± 11.2, P < 0.05 and 19.37 ± 9.03 vs 29.67 ± 5.12, P < 0.001, respectively). The expression of TNF-α, IL-10, and HSP-70 mRNAs was significantly elevated in the celiac groups regardless of the diet when compared with controls. Patients on the GFD presented a significantly lower mRNA expression of TNF-α and IL-10 than in newly diagnosed and nonadherent patients (P < 0.05). The expression of SOD mRNA was significantly elevated in celiac patients compared with controls (P < 0.05), with a significant difference between treated and untreated patients (P < 0.05). The expression of HIF-1α mRNA and BAX mRNA was significantly higher in patients with active CD compared with controls and patients on GFD, while no difference was observed between the latter two groups.
Increased intestinal expression of HSP-70 despite GFD indicates that GFD only partially reduced oxidative stress. CD patients exhibited an oxidative imbalance and inflammatory response despite GFD. Uric acid may act as an important antioxidant in CD.
Core tip: Oxidative stress has been implicated in gliadin toxicity. Additional measures aimed at reducing oxidative imbalance may prove to be effective supplementary therapy. We demonstrated increased duodenal expression of hypoxia-inducible factor 1 (HIF-1α), heat-shock protein 70 (HSP-70), and superoxide dismutase in adult celiac patients with extraintestinal manifestations as a defensive reaction to oxidative stress. Hence, HSP-70 and HIF-1α might be potential novel biomarkers of celiac disease (CD). Increased HSP-70 expression, both in treated and untreated celiac patients, suggests that oxidative stress as well as histopathological alterations in duodenal mucosa persist despite gluten-free diet. Our data confirm the increased serum levels of uric acid in patients with CD compared with controls as a result of oxidative stress.
- Citation: Piatek-Guziewicz A, Ptak-Belowska A, Przybylska-Felus M, Pasko P, Zagrodzki P, Brzozowski T, Mach T, Zwolinska-Wcislo M. Intestinal parameters of oxidative imbalance in celiac adults with extraintestinal manifestations. World J Gastroenterol 2017; 23(44): 7849-7862
- URL: https://www.wjgnet.com/1007-9327/full/v23/i44/7849.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i44.7849
Celiac disease (CD) is an inflammatory disorder of the small intestine, which is caused by the gluten fraction of wheat or the homologous proteins from barley and rye in genetically predisposed individuals[1]. Histologically, these lesions include intraepithelial lymphocytosis, crypt hypertrophy, and villous atrophy, resulting in an inadequate absorption of micronutrients and macronutrients from the intestinal tract. The clinical presentation of CD is heterogeneous and varies with the age of patients, duration and intensity of the disease, and possible presence of extraintestinal disorders[1]. In adults, a variety of clinical manifestations have been described, including the non-classic or asymptomatic form of CD.
The pathogenesis of CD is complex and not fully understood. Besides genetic predisposition, the immunologic mechanism has been proposed because both the innate and adaptive immune responses contribute to the mucosal inflammation in patients with CD[2]. The disruption of the intestinal epithelial barrier makes it more permeable to gluten peptides, thus exacerbating the inflammatory process if gluten peptides are present in the intestinal lumen.
Recent studies have indicated a direct cytotoxic effect of gluten on enterocytes[3]. Moreover, it has been proposed that oxidative stress is one of the mechanisms responsible for gliadin toxicity[4]. Recent data have also suggested the importance of hypoxia-inducible factor 1 (HIF-1) in maintaining the functions of the intestinal epithelial barrier[5]. Although activation of HIF-1 is mainly regulated by hypoxia[6], it is now established that HIF-1 signaling can also be triggered under inflammatory conditions[7-10]. Vannay et al[11] have shown the increased mucosal expression of HIF-1α in children with untreated CD, suggesting the involvement of this signaling factor in the pathomechanism of the disease. The regulation of HIF-1 is a complex process. Among these regulatory mechanisms, a direct effect of reactive oxygen species (ROS) on the HIF-1α subunit has received a great deal of attention, but there are contradictory literature data with respect to association between HIF-1α and ROS[12]. Some studies have indicated that heat shock proteins and the family of chaperones could play important roles in the pathology of CD[13-15]. The expression of HSPs can be markedly upregulated in epithelial cells under extreme conditions by the mechanism involving the expression and release of proinflammatory mediators, such as tumor necrosis factor alpha (TNF-α) or an activation of oxidative stress[16]. All these factors can trigger apoptosis[17,18], but the decision for a cell to undergo apoptosis depends on the balance between proapoptotic and antiapoptotic signals. For instance, HSPs may exert antiapoptotic effects and contribute to preservation of intestinal epithelial barrier integrity[19]. This process can be executed either by the extrinsic or intrinsic apoptotic pathways. While the role of the extrinsic apoptotic pathway activation in the mucosa of patients with CD has been proposed in the literature, studies on the intrinsic and common apoptotic pathways in patients with CD are sparse[18].
It is likely that the development of CD depends on the balance between proinflammatory and anti-inflammatory factors, proapoptotic and antiapoptotic signals, as well as prooxidant processes and antioxidant capacity of the cell. This imbalance is reflected in an impairment of the epithelial barrier and increased permeability, leading to activation of the immune response (native and adaptive) that contributes to cell damage and villous atrophy in patients with CD.
The aim of our study was to determine the involvement of oxidative imbalance in the mechanism of mucosal injury of the small intestine and to assess the effect of oxidative stress on the course of CD in adult patients with non-classic symptoms and extraintestinal manifestations. Apart from routine blood parameters, the serum concentrations of total vitamin D, uric acid, and bilirubin were measured. Moreover, in biopsy specimens collected during endoscopy of the proximal small intestine from these groups of patients, the expression of mRNA for proinflammatory cytokines TNF-α and interleukin 1β (IL-1β) as well as an anti-inflammatory cytokine interleukin 10 (IL-10) was determined by reverse transcription–polymerase chain reaction (RT-PCR) with specific primers. Oxidative stress may influence the expression of HSP-70, another marker examined in our study. Since ROS were shown to affect the stabilization of HIF-1α RNA and activate the intrinsic apoptotic pathway associated with overexpression of proapoptotic BAX, we also examined the gene expression of HIF-1α, antioxidant enzyme SOD, and proapoptotic factor BAX in the duodenal tissues of the enrolled patients.
All individuals gave informed consent to participate in the study. The protocol of the study was approved by the Ethical Committee at Jagiellonian University Medical College in Cracow, Poland (No KBET/174/B/2013) and was run in accordance with the Declaration of Helsinki.
The study included 85 patients of the Outpatient Clinic and the Department of Gastroenterology and Hepatology of the University Hospital in Cracow (Table 1). Patients were divided into the following subgroups: (1) 7 patients with newly diagnosed CD (age range, 19-62 years; mean age, 34.7 ± 14.9 years); (2) 22 patients with CD who did not adhere to GFD (nontreated CD group; age range, 22-68 years; mean age, 38.2 ± 10.7 years); (3) 31 patients with CD who were on GFD for at least two years and who tested negative for celiac antibodies (treated CD group; age range, 28-65 years; mean age, 45.7 ± 16.1 years; mean duration, 10 ± 7.7 years); and (4) 25 patients with functional disorders of the gastrointestinal tract without abnormalities on upper gastrointestinal endoscopy and on serological and histological examinations (control group; age range 19-66 years; mean age, 38.5 ± 13.2 years). Groups 1 and 2 represented patients with active CD.
| Groups of patients | Age (yr, mean ± SD) | n (%) |
| Total | 70.74 ± 14.22 | 85 |
| Female | 71 (83.5) | |
| Male | 14 (16.5) | |
| Active CD | ||
| Newly diagnosed CD | 34.7 ± 14.9 | 7 |
| Female | 5 (71.4) | |
| Male | 2 (28.6) | |
| Nontreated CD | 38.2 ± 10.7 | 22 |
| Female | 18 (81.8) | |
| Male | 4 (18.2) | |
| Treated CD | 45.7 ± 16.1 | 31 |
| Female | 28 (90.3) | |
| Male | 3 (9.7) | |
| Control | 38.5 ± 13.2 | 25 |
| Female | 20 (80) | |
| Male | 5 (20) |
CD was diagnosed on the basis of clinical symptoms, positive test results for celiac antibodies [antitissue transglutaminase antibodies (TGAs) or antiendomysial antibodies (EmAs) or both], and the characteristic histological features of duodenal biopsies. Celiac patients presented with non-classic symptoms or extraintestinal manifestations such as iron deficiency, anemia, chronic abdominal pain without typical malabsorption syndrome, osteoporosis, osteopenia, as well as asymptomatic disease (Table 2). We excluded patients with diabetes, inflammatory bowel disease, current infectious disease, history of cancer, chronic hepatobiliary disease, chronic renal impairment, and alcohol abuse, or those who received therapy with nonsteroidal anti-inflammatory drugs, antioxidant supplements, oral contraceptives, immunosuppressants, and immunostimulants. All patients were nonsmokers.
| Groups of patients | Iron deficiency/anemia | Chronic abdominal pain | Osteopenia/osteoporosis | Menstrual disorders | Abnormal liver tests | Others |
| Active CD (n = 29) | 11 (37.9) | 7 (24.1) | 4 (13.8) | 1 (3.4) | 5 (17.2) | 1 (3.4) |
| Treated CD (n = 31) | 15 (48.4) | 4 (12.9) | 5 (16.1) | - | 5 (16.1) | 2 (6.5) |
All patients underwent upper gastrointestinal endoscopy, and at least four well-oriented duodenal specimens were taken for histological examination and determination of the IL-1β, TNF-α, IL-10, HSP-70, HIF-1α, SOD, and BAX mRNA expression in the duodenum. The degree of intestinal mucosal damage was evaluated according to the Marsh classification[20]. The histological assessment was performed by an experienced pathologist in the Department of Pathology at Jagiellonian University Medical College.
We determined the serum levels of TGAs and EmAs, blood cell count, serum activity of alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, γ-glutamyltransferase, and total protein, as well as serum levels of antioxidants: uric acid, bilirubin, and vitamin D. The TGA concentration was assessed using a commercial ELISA kit (Aesku Diagnostics GmbH, Germany), and the results were expressed as unit (U)/mL of serum. A value higher than 15 U/mL was considered positive. EmAs were assessed with immunofluorescence. A value higher than 1:10 was considered positive. The other biochemical tests were performed in the Department of Diagnostics of the University Hospital in Cracow.
Expression of IL-1β, TNF-α, IL-10, HSP-70, HIF-1α, SOD, and BAX transcripts in the human intestinal samples determined by RT-PCR
The expression of IL-1β, TNF-α, IL-10, HSP-70, HIF-1α, SOD, and BAX transcripts in human samples was determined by RT-PCR. Each specimen was immediately frozen in liquid nitrogen and stored at -80 °C until analysis. Total RNA was then isolated according to the method by Chomczynski and Sacchi[21], using Trizol Reagent (Invitrogen, Carlsbad, United States) following the manufacturer’s protocol. First-strand cDNA was synthesized from total cellular RNA (2 μg) using Reverse Transcription System (Promega, Madison, United States). The RT-PCR was carried out in an automatic DNA thermal cycler, using 1-µg cDNA and Promega PCR reagents. For amplification of IL-1β, TNF-α, IL-10, HSP-70, HIF-1α, SOD, and BAX cDNA, gene-specific primers were used (SIGMA-Aldrich St. Louis, United States) (Table 3). Amplification of control human β-actin was performed on the same samples to verify the RNA integrity. PCR products were separated by electrophoresis in 2% agarose gel containing 0.5 µg/mL of ethidium bromide and then visualized under ultraviolet light. Location of the predicted PCR product was confirmed by using the O’Gene Ruler 50 bp DNA ladder (Fermentas, Life Sciences, San Francisco, United States) as standard marker.
| Gene | Primer sequence | t | PCR product |
| IL-1β | Forward 5’-ACA TCA GCA CCT CTC AAG -3’, | 60 °C | 141 bp |
| Reverse 5’-AGT CCA CAT TCA GCA CAG -3’ | |||
| TNF-α | Forward 5’-GCC CAG GCA GTC AGA TCA TCT TC -3’, | 58 °C | 181 bp |
| Reverse 5-TGA GGT ACA GGC CCT CTG ATG G-3’ | |||
| IL-10 | Forward 5’-AGC TAT CCC AGA GCC CCA GAT CCG ATT TTG G-3’, | 60 °C | 328 bp |
| Reverse 5’-AAG CTG AGA ACC AAG ACC CAG ACA TCA AGG CG-3’ | |||
| HSP-70 | Forward: 5’-GCC CCA ACA GAT TGT TGT CTT-3’, | 59.5 °C | 111 bp |
| Reverse: 5’-CCA CCA AGC AGA CGC AGA T-3’ | |||
| HIF-1α | Forward 5’-GGT TCT CAC AGA TGA TGG TG-3’, | 60 °C | 239 bp |
| Reverse 5’-TTC TTC CTC GGC TAG TTA GG-3’ | |||
| SOD | Forward: 5’-GAA GGT GGG AAG CAT TA-3’, | 57 °C | 300 bp |
| Reverse: 5’-ACC TTT GCC CAA GTC ATC TG-3’ | |||
| BAX | Forward 5’-CGT CCA ACC CAC CCT GGT CT-3’, | 55 °C | 195 bp |
| Reverse 5’-TGG CAG CTG ACA TGT TTT CTG AC-3’ | |||
| β-actin | Forward 5’-GGG TAC ATG GTG GTG CCG-3’, | 54 °C | 307 bp |
| Reverse 5’-AGC GGG AAA TCG TGC GTG-3’ |
A statistical analysis was performed by a biomedical statistician using a nonparametric Mann-Whitney test. For the comparison of normally distributed variables between the groups, the Student’s t-test was used. The results were reported as mean ± SE or mean ± SD, and a significance level was defined as a P value of less than 0.05. The analysis was performed using Statistica 10 software (StatSoft® Inc., United States).
The results of biochemical tests are presented in Table 4. The mean leukocyte and platelet counts were similar between the celiac groups and controls. The mean red blood cell count was lower in the active CD and treated CD groups as compared with controls (4.49 ± 0.39 vs 4.7 ± 0.37, P < 0.05; 4.45 ± 0.42 vs 4.7 ± 0.37, P < 0.05, respectively). The mean hemoglobin and hematocrit levels were lower in patients with active CD than in controls (12.6 ± 1.8 vs 13.4 ± 1.4, P < 0.05; 37.6 ± 4.4 vs 41.6 ± 9.6, P < 0.05, respectively) and treated celiac patients (12.6 ± 1.8 vs 13.3 ± 1.1, P < 0.05; 37.6 ± 4.4 vs 39.5 ± 3.2, P < 0.05, respectively). Only 2 patients (3.3%) with CD were anemic (hemoglobin < 11 g/dL), but reduced mean corpuscular volume was observed in 11 patients (37.9%) with active CD (range, 59.9-81.4 fL), in 3 patients (3.2%) with treated CD (range, 80.9-81.1 fL), and in two controls (8%; range, 80.1-80.4 fL).
| Controls(n = 25) | Active CD(n = 29) | Treated CD(n = 31) | |
| WBC (103/μL) | 5.69 ± 1.55 | 5.81 ± 1.58 | 5.36 ± 1.36 |
| RBC (106 cells/μL) | 4.7 ± 0.37 | 4.49 ± 0.39a | 4.45 ± 0.42a |
| Hemoglobin (g/dL) | 13.4 ± 1.4 | 12.6 ± 1.8ac | 13.3 ± 1.1 |
| Hematocrit (%) | 41.6 ± 9.6 | 37.6 ± 4.4ac | 39.5 ± 3.2 |
| PLT (103/μL) | 248.52 ± 47.63 | 267.18 ± 96.8 | 252.52 ± 64.38 |
| Vitamin D (ng/mL) | 29.7 ± 5.1 | 19.4 ± 9.0bc | 25.2 ± 11.2a |
| Total protein (g/L) | 73.2 ± 6.1 | 71.2 ± 7.9 | 71.6 ± 3.3 |
| AST (U/L) | 18.0 ± 8.5 | 28.0 ± 19.3a | 23.2 ± 6.5a |
| ALT (U/L) | 22.0 ± 4.3 | 26.7 ± 20.1 | 24.2 ± 12.5 |
| AP (U/L) | 59.6 ± 19.7 | 62.2 ± 29.1 | 59.2 ± 27.3 |
| GGTP (U/L) | 24.4 ± 14.6 | 21.3 ± 13.6 | 21.3 ± 22.62 |
| bilirubin (μmol/L) | 10.5 ± 4.1 | 8.2 ± 5.0a | 8.1 ± 3.3a |
| Uric acid (μmol/L) | 190.8 ± 23.0 | 260.2 ± 53.7b | 261.7 ± 51.8b |
The mean serum levels of total protein, alanine aminotransferase, alkaline phosphatase, and γ-glutamyltransferase were similar to those observed in controls. The mean serum levels of aspartate aminotransferase were significantly higher in the active CD and treated CD groups compared with controls (28.0 ± 19.3 vs 18.0 ± 8.5, P < 0.05; 23.2 ± 6.5 vs 18.0 ± 8.5, P < 0.05, respectively), without significant differences between the two CD groups. Hypertransaminasemia was reported in 7 patients (24.1%) with active CD, in 5 patients (16.1%) with treated CD, and in none of the control patients.
Serum uric acid concentrations were elevated only in celiac patients, namely, in 3 patients (10.3%) with active CD and in 2 patients (6.5%) on GFD. Uric acid levels were significantly higher in the celiac groups than in controls (P < 0.001), while bilirubin levels were significantly lower in patients with CD than in controls (P < 0.05).
Reduced vitamin D levels were reported in 26 patients (89.6%) with active CD, in 21 patients (67.7%) with treated CD, and in 14 controls (56%). Moderate vitamin D deficiency (10-19 ng/mL) was reported in 11 patients (37.9%) with active CD and 12 patients (38.7%) with treated CD; severe deficiency (< 10 ng/mL) was reported in 3 patients (10.3%) with active CD and only in 1 patient (3.2%) with treated CD. Moderate to severe vitamin D deficiency was not observed in the control group. The mean vitamin D level was significantly lower in patients with active CD than in controls or treated celiac patients (P < 0.001 and P < 0.05, respectively), and was lower in treated celiac patients than in controls (P < 0.05).
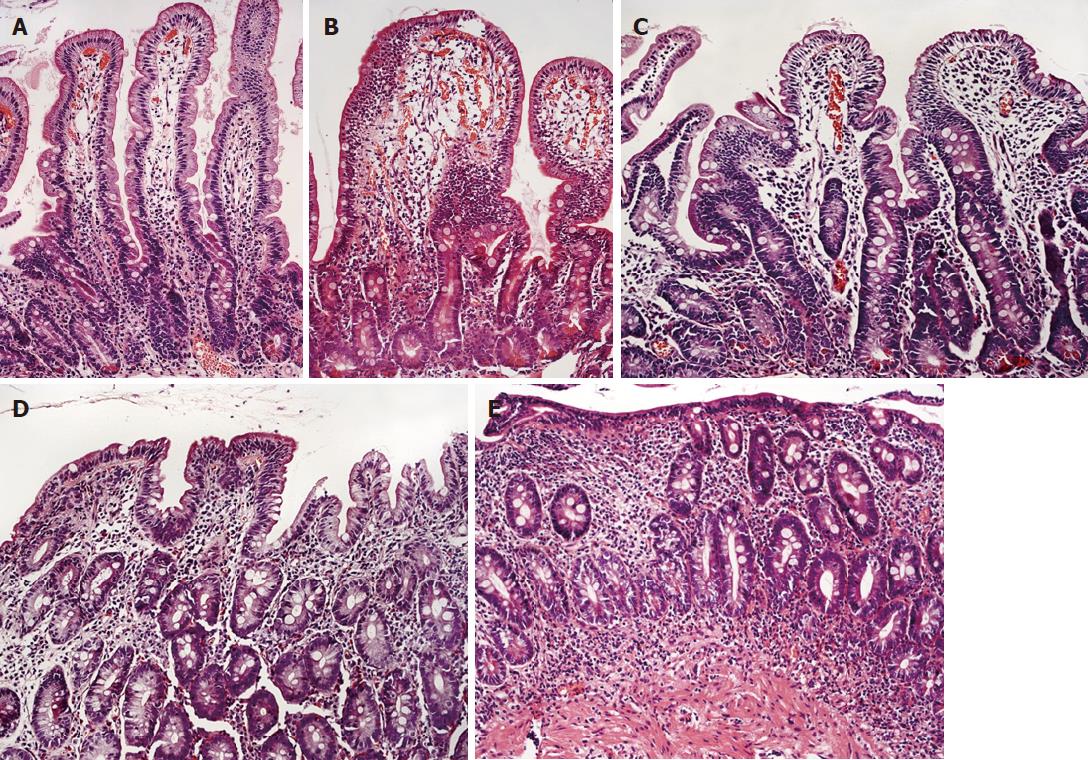
The serum levels of celiac antibodies were negative in the control group (Table 5). As expected, the significantly higher levels of celiac antibodies were observed in patients with active CD. In treated CD patients, the levels of antibodies were significantly lower compared with untreated CD patients (P < 0.001). The degree of intestinal mucosal damage evaluated according to the Marsh classification as shown in Figure 1 and Table 5 was the most severe in newly diagnosed CD patients, followed by nontreated CD patients, treated CD patients, and controls. The differences between the groups were significant. Data are presented in Table 5.
| Control (n = 25) | Treated CD (n = 31) | Nontreated CD (n = 22) | Newly diagnosed CD (n = 7) | |
| Antibody titer | ||||
| 0 | 25 | 30 (96.7) | 0 | 0 |
| 1 | 0 | 1 (3.3) | 9 (40.9) | 1 (14.3) |
| 2 | 0 | 0 | 3 (13.6) | 2 (28.6) |
| 3 | 0 | 0 | 10 (45.5) | 4 (57.1) |
| Antibody titer | 0 | 0.03 ± 0.2 | 2.0 ± 0.9bd | 2.4 ± 0.8bd |
| Degree of intestinal mucosal damage1 | ||||
| Normal mucosa 0 | 24 (96) | 13 (41.3) | 2 (9) | 1 (14.3) |
| Marsh 1 1 | 1 (4) | 5 (16.1) | 7 (31.8) | 0 |
| Marsh 2 2 | 0 | 0 | 0 | 0 |
| Marsh 3a 3 | 0 | 5 (16.1) | 6 (27.3) | 1 (14.3) |
| Marsh 3b 4 | 0 | 7 (22.6) | 5 (22.7) | 3 (42.9) |
| Marsh 3c 5 | 0 | 1 (3.2) | 2 (9) | 2 (28.6) |
| Intestinal mucosal damage1 | 0.04 ± 0.2 | 1.7 ± 1.8b | 2.5 ± 1.6bc | 3.7 ± 1.4bc |
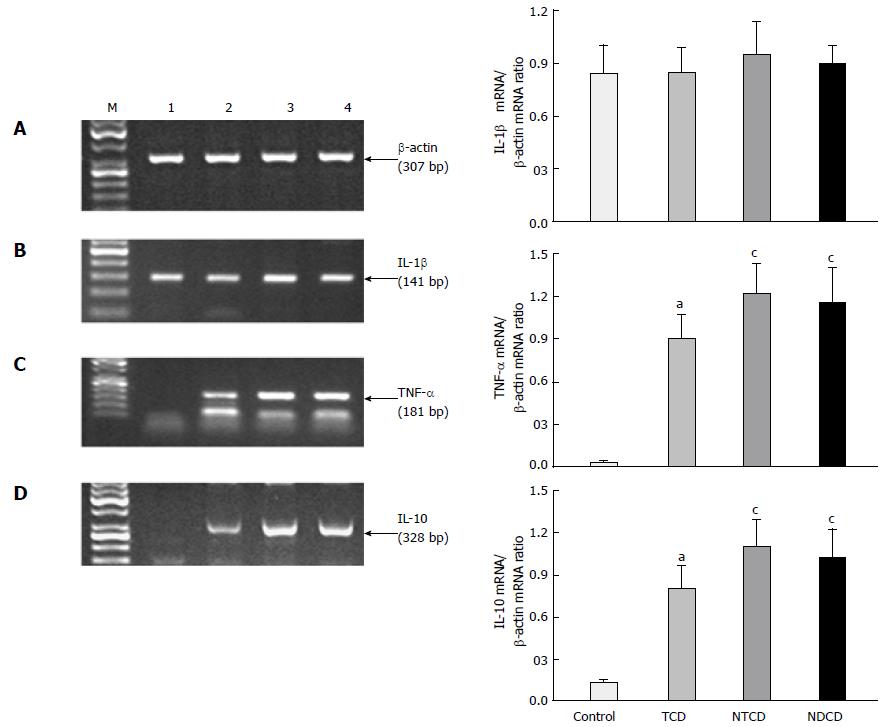
Expression of IL-1β, TNF-α, and IL-10: Figure 2 shows the mRNA expression of proinflammatory cytokines IL-1β and TNF-α and the alterations in RT-PCR mRNA expression of anti-inflammatory cytokine IL-10 in the biopsies of duodenal mucosa. The IL-1β mRNA expression was not significantly different between the study groups, although it was slightly higher in active CD as compared with controls and patients on GFD. The expression of mRNA for TNF-α was significantly increased in all celiac groups when compared with controls (P < 0.05). The TNF-α RNA expression was similar in both groups of active CD (high degree of mucosal damage), but was significantly higher than in treated patients (low grade of mucosal damage).
The expression of IL-10 mRNA in the study groups was similar to the trend observed for the expression of TNF-α mRNA. In intact intestinal mucosa, the signal for IL-10 mRNA expression was faint. However, we observed a significant increase in the IL-10 mRNA expression in the celiac groups when compared with controls (P < 0.05). Celiac patients on GFD had a lower expression of IL-10 mRNA in the mucosa than patients with active disease, and this difference based on the semi-quantitative assessment of the ratio of IL-10 mRNA expression to β-actin mRNA expression was significant (Figure 2).
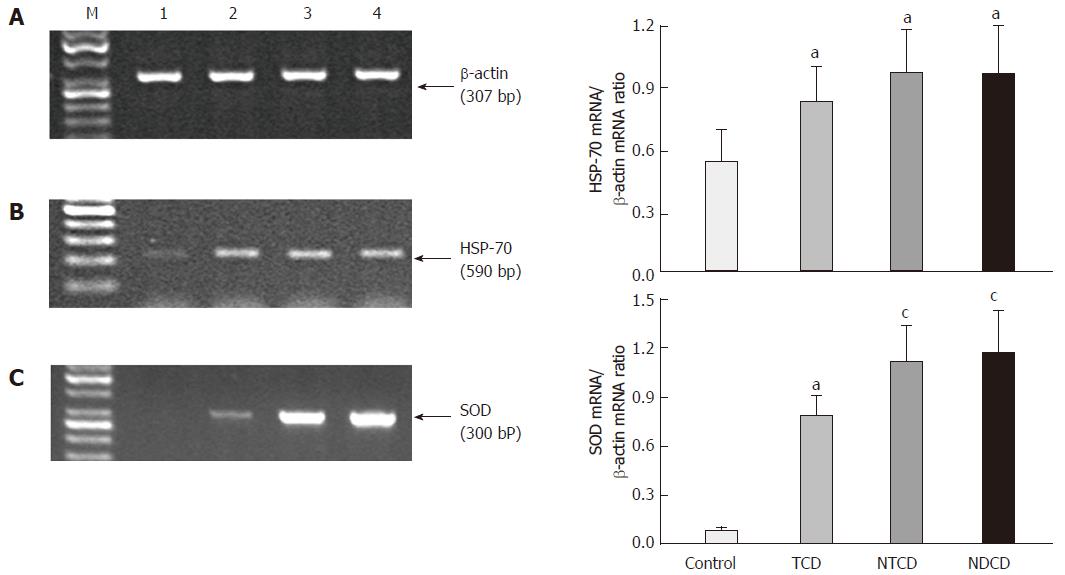
Expression of HSP-70 and SOD: As shown in Figure 3, the signal for the expression of HSP-70 mRNA was markedly increased in the celiac groups compared with controls, regardless of compliance with the diet (P < 0.05). The differences between the celiac groups were not significant.
The ratio of SOD mRNA expression to β-actin mRNA expression confirmed that the expression of this antioxidant enzyme was significantly elevated in celiac patients compared with controls (P < 0.05). The signal for SOD mRNA expression in treated CD patients was significantly lower than in untreated and newly diagnosed ones (Figure 3).
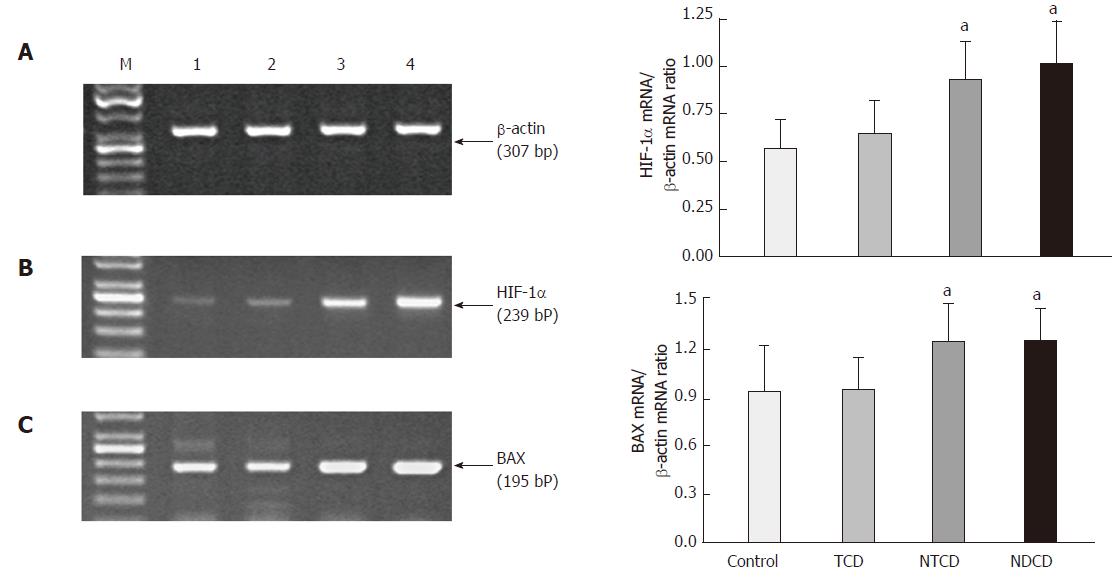
Expression of HIF-1α and BAX: The ratio of HIF-1α mRNA expression to β-actin mRNA expression confirmed that the expression of HIF-1α was significantly elevated in the mucosa of patients with active CD compared with controls and patients with treated CD (P < 0.05). HIF-1α mRNA expression was slightly increased in the duodenal mucosa of patients with treated CD compared with controls, but the difference was not significant (Figure 4). A significant increase in the expression of BAX mRNA as determined by the ratio of BAX mRNA expression to β-actin mRNA expression in the mucosa of patients with active CD was observed compared with controls and patients with treated CD (P < 0.05). We failed to observe any significant difference in the expression of BAX mRNA in the duodenal mucosa of patients with treated CD compared with controls (Figure 4).
Most studies concerning the pathomechanism of CD and intestinal changes focused on children with classic clinical symptoms of malabsorption syndrome[4,22-24]. However, malabsorption alone does not explain the pathophysiology and clinical course of numerous extraintestinal manifestations as well as non-classic symptoms that predominate in adult patients with CD. Other mechanisms have been proposed including gluten toxicity with oxidative imbalance and autoimmunity[23,24].
In this study, we have examined less extensively studied, factors implicated in CD, such as HSP-70, HIF-1α, and the proapoptotic factor BAX. We found these 3 factors to be overexpressed in active CD, with varying degrees of activity in patients on GFD. This overexpression could be triggered by oxidative imbalance linked with an increase in ROS generation. Each of these factors was shown to influence the intestinal barrier integrity. For instance, HSP-70 and HIF-1α can contribute to preservation of intestinal barrier integrity[5,19], while apoptosis manifested by the rise in the BAX expression may lead to disruption of the intestinal barrier[25]. The impaired barrier function may be involved in several immune-mediated diseases, including CD and its extraintestinal manifestations or coexisting disorders[26,27].
HSP, a known chaperone, has potential epithelial barrier protecting, antiapoptotic, and immunologic properties[19], but its role in the pathogenesis of CD remains unexplored. Our results presented in this work revealed that HSP-70, which is expressed under normal conditions, can also play a particularly important role in extreme conditions such as gluten cytotoxicity. It is noteworthy that the expression of HSP-70 was significantly increased in each celiac group in our study regardless of the degree of compliance with the diet. A few previous studies evaluated the role of HSP in intestinal pathology of patients with CD[19,28-30]. Iltanen et al[31] reported enhanced expression of epithelial cell mitochondrial HSP-65 in 80% of study children with CD and in only 7% of control subjects. Sziksz et al[19] reported an increased HSP-72 mRNA expression in the duodenal mucosa of children with untreated CD as well as children with treated CD compared with that in controls. These observations are consistent with the results of our study on HSP-70 expression in adult patients. Our results indicate that HSP-70 in adult CD patients, similarly as HSP-72 in children, was overexpressed due to oxidative stress. The increased HSP-70 expression may constitute a protective mechanism against gliadin-induced cytotoxicity associated with antiapoptotic effects, thus contributing to preservation of intestinal epithelial barrier integrity.
It should be noted, however, that significant percentage of patients with CD on GFD, in our study, showed the persistence of duodenal damage despite clinical improvement and evident decline in celiac antibodies. The main criteria for inclusion in this group involved a specialist assessment by gastroenterologist and dietitian of patients proper dietary adherence, clinical recovery and above all, the negativity of serologic markers. Interestingly, a gap has emerged between the clinical and mucosal recovery, mainly in the adult population, since when re-biopsing treated CD patients only half of them had healed mucosa, despite the negativity of celiac antibodies[32,33]. Following the GFD, the clinical symptoms and mucosal architecture usually improve very quickly in children[34], while in a mixed population including adults, the recovery of duodenal mucosa assessed by histology requires longer time to heal[35]. These previous observations seem consistent with the results of our present study because the morphological alterations persisted in some of our CD patients despite the clear disappearance of specific antibodies. The increased expression of HSP-70 in treated and untreated celiac patients indicates that oxidative stress in patients with CD may still persist despite GFD and serological and clinical remission, and may be responsible for histopathological alterations observed in our study. Finally, the enhanced expression of HSP-70 suggest incomplete elimination of all sources of gluten in modern diet. Perhaps the expression of HSP-70 could be considered as a more sensitive marker than celiac antibodies in the detection of the trace amounts of gluten in diet. Thus, HSP-70 could be considered a potential novel biomarker of this disease.
It is known that ROS and HIF-1 signaling are involved in numerous diseases including cancer, inflammatory diseases, and ischemic disorders[12]. Furthermore, the increase of ROS levels is one of the main factors stabilizing HIF-1α. It has been shown that exogenous ROS, in the form of H2O2, can enhance the synthesis of inflammatory mediators such as TNF-α and IL-1β[36], which in turn can influence the protein transcription and activity of HIF-1α under normoxia[37]. We provided evidence for the increased mucosal HIF-1α expression in untreated adult patients with active CD compared with controls and treated CD patients, while we did not observe any significant difference between treated celiac patients and controls. This observation is consistent with that of Vannay et al[11], who suggested the role of HIF-1α in the pathomechanism of CD. In addition, involvement of HIF-1 in inflammatory bowel disease has been reported[10]. The increased expression of HIF-1α in our study can be explained by the initial intestinal damage or the direct effect of gluten in diet. These data suggest that increased HIF-1α expression may be a consequence rather than a primary cause of CD. Moreover, the decreased mucosal expression of HIF-1α in treated CD may confirm the efficacy of GFD.
In general, data on the status of apoptosis in patients with CD are conflicting, but increased apoptotic cell death of intestinal epithelial cells was reported in untreated CD, as detected by DNA fragmentation assay using terminal uridine deoxynucleotidyl nick end labelling in small intestinal biopsies[38]. In that study, apoptosis was well correlated with proliferation and returned to normal in patients treated with GFD[38]. It is likely that increased apoptosis may be responsible for villous atrophy in CD. Therefore, our study included RT-PCR analysis of the proapoptotic member of the Bcl-2 family, that is, BAX, which in normal mucosa showed constitutive expression. This remains in keeping with the observation that the mucosa of healthy individuals undergoes a high rate of constitutive epithelial proliferation[17]. In our study, the expression of BAX showed a similar trend to that observed for the expression of HIF-1α. We revealed a significantly elevated BAX mRNA expression in the duodenal mucosa of patients with active CD compared with controls and patients with treated CD. Interestingly, the expression of BAX mRNA in the duodenal mucosa was not significantly different between treated CD patients and controls. Our results suggest that the increased expression of BAX results from the severity of intestinal inflammation and gluten-induced oxidative stress and leads to duodenal villous atrophy. Moreover, the decreased mucosal expression of BAX in treated CD patients may indicate the relief of inflammation and thus the efficacy of treatment. In contrast to our study, van der Woude et al[38] failed to demonstrate any changes in the expression of BAX, Bcl-2, and Bcl-xl between their study groups, which were similar to those in our study. However, Cherñavsky et al[39] found that only Bak mRNA was significantly overexpressed in the mucosa of CD patients, whereas BAX and Bcl-2 transcription levels were unchanged with respect to control mucosa.
Oxidative imbalance seems to be involved in the molecular mechanisms of CD. In normal conditions, the harmful effects of ROS are opposed by the antioxidant defense system consisting of antioxidant enzymes (glutathione peroxidase, glutathione reductase, SOD, and catalase), non-enzymatic antioxidants (such as glutathione, albumin, bilirubin, ceruloplasmin, and uric acid) as well as nutritional antioxidants (carotenoids and vitamins A, C, and E)[40]. The reduced antioxidant defense may make the inflamed mucosa more sensitive to oxidative tissue damage and may disrupt its recovery and integrity.
Using thiobarbituric acid reactive substances as a marker of oxidative stress, Odetti et al[41] showed that redox equilibrium is impaired in patients with CD. They also observed decreased serum α-tocopherol levels in patients with silent CD in comparison with controls. Earlier studies also showed that the activity of SOD is markedly increased in pediatric patients with CD, while the activity of glutathione peroxidase is significantly decreased[24].
SOD, which reduces the most abundant free radical •O2, is considered as the major intracellular antioxidant enzyme[40]. In agreement with previous data, our results demonstrated overexpression of SOD mRNA in the mucosa of celiac patients compared with controls. We observed an increased expression of SOD mRNA in active disease, and this increase was attenuated in the treated celiac group. These results suggest that the increased expression of SOD, reflecting the severity of oxidative stress in duodenal mucosa, could be a consequence of either intestinal impairment or of oxidative imbalance. Our observations may indicate that some markers of oxidative stress persist even in treated CD patients, but GFD partially counteracts the impairment of intestinal mucosa observed in active CD patients.
There is increasing experimental and clinical evidence showing that uric acid acts as an important antioxidant in vivo[42]. Interestingly, an increase in serum uric acid concentrations occurs as a physiological response to enhanced oxidative stress[43]. Despite being a major antioxidant in the human plasma, uric acid correlates with and may predict the development of conditions associated with oxidative stress such as obesity, hypertension, and cardiovascular disease[44]. Our results indicate that higher serum levels of uric acid in patients with CD compared with controls may be a consequence of oxidative stress and that uric acid may function as an antioxidant. Additional well-designed clinical studies are needed to clarify the potential use of uric acid (or uric acid precursors) in CD and to examine its role as a marker of oxidative stress and a potential therapeutic antioxidant.
In contrast to transaminases, the levels of bilirubin in patients with CD were significantly lower than in the control group. Bilirubin is an antioxidant that blocks vascular cell adhesion molecule 1 signals through ROS in vitro[45]. An Australian study[46] reported that bilirubin levels were significantly lower in severe asthma, suggesting altered regulation of inflammation in asthmatics by antioxidant vitamins and bilirubin. This observation is consistent with our results, indicating the relationship between the altered concentration of bilirubin and oxidative imbalance. However, the role of bilirubin in oxidative imbalance in CD requires further research.
A significant number of CD patients with intestinal malabsorption syndrome present vitamin D deficiency or insufficiency. In our study, vitamin D deficiency was noted in celiac patients despite the absence of clinical syndrome of malabsorption, possibly because inflammation may also lead to vitamin D deficiency. It is likely that inflammatory cytokines, such as TNF-α, cause CYP27B1-mediated conversion of 25(OH)D to 1,25(OH)2D in the intestines, thereby reducing serum 25(OH) D levels[47]. In turn, the active form of 1,25(OH)2D inhibits the proliferation and secretion of inflammatory cytokines by type 1 helper T cells, thereby reducing inflammation[48]. This inverse relationship between the activity of CD and serum vitamin D levels was observed in our study. A similar observation concerns the degree of TNF-α expression and the degree of vitamin D deficiency, which is consistent with the results obtained in previous studies in healthy individuals[49,50]. The antioxidant property of vitamin D is rather less well recognized. Cholecalciferol (vitamin D3) is likely to act as a membrane antioxidant by stabilizing the membrane against lipid peroxidation[51]. The antioxidant activity of vitamin D may involve an interaction with SOD[52]. We showed that a decrease in serum vitamin D levels in patients with CD was accompanied by an increase in the intestinal mucosal expression of TNF-α, suggesting that overexpressed TNF-α may lead to a reduction in the serum level of vitamin D. In turn, an increase in SOD expression may result from enhancement of TNF-α expression and a prominent fall in serum vitamin D levels which activate the antioxidative defense. This indicates that early diagnosis of vitamin D deficiency is particularly important in patients with CD, especially in those who do not comply with GFD. Therefore, the supplementation of vitamin D is recommended not only for bone metabolism but also for effective treatment of intestinal damage in patients with CD by reducing the oxidative stress.
A drawback of this study is a relatively small number of patients in each celiac subgroups, and definitely a further research with higher number of enrolled subjects is required to support our observations. It is noteworthy that the morphology of duodenal mucosa failed to show a full recovery despite the proper adherence to GFD, clinical improvement and the status of seroconversion, i.e. the decline in the value of antibodies in this group of patients to a negative result. Hence, further research with only subjects presenting full mucosal healing would add more to our understanding of pathomechanism of CD and intestinal recovery associated with GFD.
In conclusion, by its association with intestinal damage, the course of the disease, and perhaps extraintestinal disorders, oxidative imbalance appears to be one of the major factors implicated in the pathogenesis of CD. Our results support the hypothesis that HSP-70 may be a potential novel biomarker in CD. The increased intestinal expression of HSP-70 in patients with active CD and in treated celiac patients indicates that oxidative stress persists despite the exclusion of gluten from diet which deepens our knowledge on multifaceted mechanisms of this disease. This persistent oxidative imbalance may be responsible for sustained intestinal damage in CD despite GFD. In fact, the significant overexpression of HSP-70 despite dietary compliance may suggest refractory nature of CD. Furthermore, particularly noteworthy are non-enzymatic antioxidants, such as uric acid and bilirubin, whose concentration may be easily assessed in patients with CD.
Considering that several nutrients exert antioxidant effects and influence gene expression, they represent a useful approach for nutritional intervention in CD subjects, as confirmed by recent studies in vitro. These studies have revealed phytonutrients and docosahexaenoic acid efficacy in protection against the cytotoxic effect of gliadin[53-55].
To become aware of the usefulness of nutritional genomics as a tool for targeted medical nutrition therapy, further basic research, epidemiological studies and controlled intervention trials are needed to investigate whether some nutrients such as antioxidant vitamins modulate in vivo predisposition of chronic inflammatory conditions and thus, have a role in the therapy of celiac disease, in addition to the rigorous GFD.
Celiac disease (CD) is a common condition. The only effective treatment available is a strict life-long gluten-free diet (GFD). Untreated CD can have serious complications, such as osteoporosis or malignancy. Some patients do not report symptomatic improvement after starting treatment, and some will still have persisting symptoms after 6 to 12 mo. The literature suggests that complete normalization of duodenal lesions is exceptionally rare in adult celiac patients despite adherence to GFD.
There is an increasing body of evidence suggesting a relationship between oxidative stress and CD. It has been proposed that oxidative stress is one of the mechanisms responsible for gliadin toxicity and persistent oxidative imbalance may be responsible for sustained intestinal damage in CD despite GFD.
The assessment of the severity of oxidative stress, including the evaluation of antioxidant capacity, in patients with CD may have therapeutic implications. The indication of a proper new biomarkers useful in assessing the individual susceptibility to oxidative stress, which may help elucidate the pathogenesis of the disease and implement an appropriate treatment.
To determine the involvement of oxidative stress in the mechanism of mucosal injury of the small intestine and to assess the effect of oxidative stress on the course of CD in adult patients with non-classic symptoms and extraintestinal manifestations, we determined the expression of IL-1β, TNF-α, IL-10, HSP-70, HIF-1α, SOD and BAX transcripts in human duodenal samples by reverse transcriptase–polymerase chain reaction.
The authors found HSP-70, HIF-1α, and BAX to be overexpressed in active CD, with varying degrees of activity in patients on GFD. This overexpression could be triggered by oxidative imbalance linked with an increase in ROS generation. We observed an increased expression of SOD mRNA in active disease, and this increase was attenuated in the treated celiac group. These results suggest that the increased expression of SOD, reflecting the severity of oxidative stress in duodenal mucosa, could be a consequence of either intestinal impairment or of oxidative imbalance.
Our results indicate that oxidative stress persists even in CD patients treated with GFD. Moreover, the results suggest that HSP-70 and HIF-1α may be potential novel biomarkers of this disease. The overexpression of HSP-70 despite dietary compliance may suggest refractory nature of CD. The increased levels of uric acid in patients with CD compared with controls resulting from oxidative stress indicates that uric acid may function as an antioxidant compound.
Further research with a greater number of participants is needed to confirm our results. Further clinical studies are needed to clarify the potential therapeutic role of uric acid as an antioxidant in CD.
This study deepens the current knowledge on the role of oxidation products on the CD. By its association with intestinal damage, the course of the disease, and perhaps extraintestinal disorders, oxidative imbalance appears to be one of the major factors implicated in the pathogenesis of CD. Our observations may indicate that some markers of oxidative stress persist even in treated CD patients, but GFD partially counteracts the impairment of intestinal mucosa observed in active CD patients. Persistent oxidative imbalance may be responsible for sustained intestinal damage in adult celiac patients despite GFD. Perhaps the expression of HSP-70 could be considered as a more sensitive marker than celiac antibodies in the detection of the trace amounts of gluten in diet. Thus, HSP-70 could be considered a potential novel biomarker of this disease. Additional well-designed clinical studies are needed to clarify the potential use of uric acid (or uric acid precursors) in the diagnosis and prognosis of CD and to examine its role as a marker of oxidative stress and a potential therapeutic utility as an antioxidant. Considering that oxidative stress is involved in the molecular mechanisms of CD, additional measures aimed at reducing oxidative imbalance, such as administration of antioxidants, deserve attention as potential supplementary therapy in the treatment of CD, in addition to the rigorous GFD.
Studies comparing the different assays for antioxidant capacity measurement in patients with CD are needed to select the method of choice that would best reflect susceptibility to oxidative stress in these patients. These assays might be particularly useful in clinical practice as a tool for therapy monitoring in patients with CD. It should be hypothesized that oral antioxidant supplementation may reduce the toxic effects of peptides contained in gluten on enterocytes and help alleviate histological lesions, thus exerting beneficial effects on the course of the disease. To become aware of the usefulness of nutritional genomics as a tool for targeted medical nutrition therapy, further basic research, epidemiological studies and controlled intervention trials are needed to investigate whether some nutrients such as antioxidant vitamins modulate in vivo predisposition of chronic inflammatory conditions and thus have a role in the therapy of celiac disease, in addition to the rigorous GFD.
| 1. | Esteve M, Rosinach M, Fernández-Bañares F, Farré C, Salas A, Alsina M, Vilar P, Abad-Lacruz A, Forné M, Mariné M. Spectrum of gluten-sensitive enteropathy in first-degree relatives of patients with coeliac disease: clinical relevance of lymphocytic enteritis. Gut. 2006;55:1739-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 91] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Stepniak D, Koning F. Celiac disease--sandwiched between innate and adaptive immunity. Hum Immunol. 2006;67:460-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Conner EM, Grisham MB. Inflammation, free radicals, and antioxidants. Nutrition. 1996;12:274-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 414] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 4. | Ferretti G, Bacchetti T, Masciangelo S, Saturni L. Celiac disease, inflammation and oxidative damage: a nutrigenetic approach. Nutrients. 2012;4:243-257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 5. | Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, Haase VH. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest. 2004;114:1098-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 481] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 6. | Weidemann A, Johnson RS. Biology of HIF-1alpha. Cell Death Differ. 2008;15:621-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 749] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 7. | Hirota SA, Beck PL, MacDonald JA. Targeting hypoxia-inducible factor-1 (HIF-1) signaling in therapeutics: implications for the treatment of inflammatory bowel disease. Recent Pat Inflamm Allergy Drug Discov. 2009;3:1-16. [PubMed] |
| 8. | Yee Koh M, Spivak-Kroizman TR, Powis G. HIF-1 regulation: not so easy come, easy go. Trends Biochem Sci. 2008;33:526-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 273] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 9. | Dehne N, Brüne B. HIF-1 in the inflammatory microenvironment. Exp Cell Res. 2009;315:1791-1797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 167] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 10. | Kapoor A, Patwari AK, Kumar P, Jain A, Narayan S. Serum soluble interleukin-2 receptor, interleukin-6 and tumor necrosis factor alpha as markers of celiac disease activity. Indian J Pediatr. 2013;80:108-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Vannay A, Sziksz E, Prókai A, Veres G, Molnár K, Szakál DN, Onódy A, Korponay-Szabó IR, Szabó A, Tulassay T. Increased expression of hypoxia-inducible factor 1alpha in coeliac disease. Pediatr Res. 2010;68:118-122. [PubMed] [DOI] [Full Text] |
| 12. | Movafagh S, Crook S, Vo K. Regulation of hypoxia-inducible factor-1a by reactive oxygen species: new developments in an old debate. J Cell Biochem. 2015;116:696-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 377] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 13. | Zamanian Azodi M, Peyvandi H, Rostami-Nejad M, Safaei A, Rostami K, Vafaee R, Heidari M, Hosseini M, Zali MR. Protein-protein interaction network of celiac disease. Gastroenterol Hepatol Bed Bench. 2016;9:268-277. [PubMed] |
| 14. | Tukaj S, Görög A, Kleszczyński K, Zillikens D, Kárpáti S, Kasperkiewicz M. Autoimmunity to heat shock proteins and vitamin D status in patients with celiac disease without associated dermatitis herpetiformis. J Steroid Biochem Mol Biol. 2017;173:23-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Setty M, Discepolo V, Abadie V, Kamhawi S, Mayassi T, Kent A, Ciszewski C, Maglio M, Kistner E, Bhagat G. Distinct and Synergistic Contributions of Epithelial Stress and Adaptive Immunity to Functions of Intraepithelial Killer Cells and Active Celiac Disease. Gastroenterology. 2015;149:681-91.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 16. | van Eden W, van der Zee R, Prakken B. Heat-shock proteins induce T-cell regulation of chronic inflammation. Nat Rev Immunol. 2005;5:318-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 407] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 17. | Hall PA, Coates PJ, Ansari B, Hopwood D. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J Cell Sci. 1994;107:3569-3577. [PubMed] |
| 18. | Shalimar DM, Das P, Sreenivas V, Gupta SD, Panda SK, Makharia GK. Mechanism of villous atrophy in celiac disease: role of apoptosis and epithelial regeneration. Arch Pathol Lab Med. 2013;137:1262-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Sziksz E, Veres G, Vannay A, Prókai A, Gál K, Onody A, Korponay-Szabó IR, Reusz G, Szabó A, Tulassay T. Increased heat shock protein 72 expression in celiac disease. J Pediatr Gastroenterol Nutr. 2010;51:573-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Oberhuber G, Granditsch G, Vogelsang H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol. 1999;11:1185-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1142] [Cited by in RCA: 1224] [Article Influence: 45.3] [Reference Citation Analysis (1)] |
| 21. | Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40518] [Cited by in RCA: 39210] [Article Influence: 1005.4] [Reference Citation Analysis (0)] |
| 22. | Stojiljković V, Todorović A, Radlović N, Pejić S, Mladenović M, Kasapović J, Pajović SB. Antioxidant enzymes, glutathione and lipid peroxidation in peripheral blood of children affected by coeliac disease. Ann Clin Biochem. 2007;44:537-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Stojiljković V, Todorović A, Pejić S, Kasapović J, Saicić ZS, Radlović N, Pajović SB. Antioxidant status and lipid peroxidation in small intestinal mucosa of children with celiac disease. Clin Biochem. 2009;42:1431-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Stojiljković V, Pejić S, Kasapović J, Gavrilović L, Stojiljković S, Nikolić D, Pajović SB. Glutathione redox cycle in small intestinal mucosa and peripheral blood of pediatric celiac disease patients. An Acad Bras Cienc. 2012;84:175-184. [PubMed] |
| 25. | Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3154] [Cited by in RCA: 3575] [Article Influence: 198.6] [Reference Citation Analysis (0)] |
| 26. | Liu Z, Li N, Neu J. Tight junctions, leaky intestines, and pediatric diseases. Acta Paediatr. 2005;94:386-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 114] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 27. | Monsuur AJ, Wijmenga C. Understanding the molecular basis of celiac disease: what genetic studies reveal. Ann Med. 2006;38:578-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Partanen J, Milner C, Campbell RD, Mäki M, Lipsanen V, Koskimies S. HLA-linked heat-shock protein 70 (HSP70-2) gene polymorphism and celiac disease. Tissue Antigens. 1993;41:15-19. [PubMed] |
| 29. | Ramos-Arroyo MA, Feijoó E, Sánchez-Valverde F, Aranburu E, Irisarri N, Olivera JE, Valiente A. Heat-shock protein 70-1 and HLA class II gene polymorphisms associated with celiac disease susceptibility in Navarra (Spain). Hum Immunol. 2001;62:821-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Zanoni G, Navone R, Lunardi C, Tridente G, Bason C, Sivori S, Beri R, Dolcino M, Valletta E, Corrocher R. In celiac disease, a subset of autoantibodies against transglutaminase binds toll-like receptor 4 and induces activation of monocytes. PLoS Med. 2006;3:e358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 148] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 31. | Iltanen S, Rantala I, Laippala P, Holm K, Partanen J, Maki M. Expression of HSP-65 in jejunal epithelial cells in patients clinically suspected of coeliac disease. Autoimmunity. 1999;31:125-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Lanzini A, Lanzarotto F, Villanacci V, Mora A, Bertolazzi S, Turini D, Carella G, Malagoli A, Ferrante G, Cesana BM. Complete recovery of intestinal mucosa occurs very rarely in adult coeliac patients despite adherence to gluten-free diet. Aliment Pharmacol Ther. 2009;29:1299-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 183] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 33. | Lebwohl B, Granath F, Ekbom A, Smedby KE, Murray JA, Neugut AI, Green PH, Ludvigsson JF. Mucosal healing and risk for lymphoproliferative malignancy in celiac disease: a population-based cohort study. Ann Intern Med. 2013;159:169-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 185] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 34. | McNicholl B, Egan-Mitchell B, Stevens F, Keane R, Baker S, McCarthy CF, Fottrell PF. Mucosal recovery in treated childhood celiac disease (gluten-sensitive enteropathy). J Pediatr. 1976;89:418-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Wahab PJ, Meijer JW, Mulder CJ. Histologic follow-up of people with celiac disease on a gluten-free diet: slow and incomplete recovery. Am J Clin Pathol. 2002;118:459-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 250] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 36. | Haddad JJ, Olver RE, Land SC. Antioxidant/pro-oxidant equilibrium regulates HIF-1alpha and NF-kappa B redox sensitivity. Evidence for inhibition by glutathione oxidation in alveolar epithelial cells. J Biol Chem. 2000;275:21130-21139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 193] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 37. | Westra J, Brouwer E, Bos R, Posthumus MD, Doornbos-van der Meer B, Kallenberg CG, Limburg PC. Regulation of cytokine-induced HIF-1alpha expression in rheumatoid synovial fibroblasts. Ann N Y Acad Sci. 2007;1108:340-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 38. | Moss SF, Attia L, Scholes JV, Walters JR, Holt PR. Increased small intestinal apoptosis in coeliac disease. Gut. 1996;39:811-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 117] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 39. | Cherñavsky AC, Rubio AE, Vanzulli S, Rubinstein N, de Rosa S, Fainboim L. Evidences of the involvement of Bak, a member of the Bcl-2 family of proteins, in active coeliac disease. Autoimmunity. 2002;35:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 40. | Krinsky NI. Mechanism of action of biological antioxidants. Proc Soc Exp Biol Med. 1992;200:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 184] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 41. | Odetti P, Valentini S, Aragno I, Garibaldi S, Pronzato MA, Rolandi E, Barreca T. Oxidative stress in subjects affected by celiac disease. Free Radic Res. 1998;29:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 42. | Glantzounis GK, Tsimoyiannis EC, Kappas AM, Galaris DA. Uric acid and oxidative stress. Curr Pharm Des. 2005;11:4145-4151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 562] [Cited by in RCA: 718] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 43. | Waring WS, Webb DJ, Maxwell SR. Systemic uric acid administration increases serum antioxidant capacity in healthy volunteers. J Cardiovasc Pharmacol. 2001;38:365-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 210] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 44. | Sautin YY, Johnson RJ. Uric acid: the oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids. 2008;27:608-619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 681] [Cited by in RCA: 675] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 45. | Cook-Mills JM, McCary CA. Isoforms of vitamin E differentially regulate inflammation. Endocr Metab Immune Disord Drug Targets. 2010;10:348-366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 46. | Misso NL, Brooks-Wildhaber J, Ray S, Vally H, Thompson PJ. Plasma concentrations of dietary and nondietary antioxidants are low in severe asthma. Eur Respir J. 2005;26:257-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 47. | Schoen MS, Lindenbaum J, Roginsky MS, Holt PR. Significance of serum level of 25-hydroxycholecalciferol in gastrointestinal disease. Am J Dig Dis. 1978;23:137-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 27] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 48. | Ooi JH, McDaniel KL, Weaver V, Cantorna MT. Murine CD8+ T cells but not macrophages express the vitamin D 1α-hydroxylase. J Nutr Biochem. 2014;25:58-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 49. | Peterson CA, Heffernan ME. Serum tumor necrosis factor-alpha concentrations are negatively correlated with serum 25(OH)D concentrations in healthy women. J Inflamm (Lond). 2008;5:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 172] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 50. | Willis KS, Smith DT, Broughton KS, Larson-Meyer DE. Vitamin D status and biomarkers of inflammation in runners. Open Access J Sports Med. 2012;3:35-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 51. | Wiseman H. Vitamin D is a membrane antioxidant. Ability to inhibit iron-dependent lipid peroxidation in liposomes compared to cholesterol, ergosterol and tamoxifen and relevance to anticancer action. FEBS Lett. 1993;326:285-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 202] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 52. | Margulies SL, Kurian D, Elliott MS, Han Z. Vitamin D deficiency in patients with intestinal malabsorption syndromes--think in and outside the gut. J Dig Dis. 2015;16:617-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 53. | Bernardo D, Martínez-Abad B, Vallejo-Diez S, Montalvillo E, Benito V, Anta B, Fernández-Salazar L, Blanco-Quirós A, Garrote JA, Arranz E. Ascorbate-dependent decrease of the mucosal immune inflammatory response to gliadin in coeliac disease patients. Allergol Immunopathol (Madr). 2012;40:3-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 54. | De Stefano D, Maiuri MC, Simeon V, Grassia G, Soscia A, Cinelli MP, Carnuccio R. Lycopene, quercetin and tyrosol prevent macrophage activation induced by gliadin and IFN-gamma. Eur J Pharmacol. 2007;566:192-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 55. | Vincentini O, Quaranta MG, Viora M, Agostoni C, Silano M. Docosahexaenoic acid modulates in vitro the inflammation of celiac disease in intestinal epithelial cells via the inhibition of cPLA2. Clin Nutr. 2011;30:541-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Poland
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Jadallah KA, Ribaldone DG, Rostami-Nejad M S- Editor: Ma YJ L- Editor: A E- Editor: Ma YJ