Published online Nov 7, 2017. doi: 10.3748/wjg.v23.i41.7470
Peer-review started: July 25, 2017
First decision: August 30, 2017
Revised: September 10, 2017
Accepted: September 29, 2017
Article in press: September 28, 2017
Published online: November 7, 2017
Processing time: 104 Days and 20.9 Hours
To compare the outcomes of transcatheter superior mesenteric artery (SMA) urokinase infusion and transjugular intrahepatic portosystemic shunt (TIPS) for acute portal vein thrombosis (PVT) in cirrhosis.
From January 2013 to December 2014, patients with liver cirrhosis and acute symptomatic PVT who met the inclusion criteria were randomly assigned to either an SMA group or a TIPS group. The two groups accepted transcatheter selective SMA urokinase infusion therapy and TIPS, respectively. The total follow-up time was 24 mo. The primary outcome measure was the change in portal vein patency status which was evaluated by angio-computed tomography or Doppler ultrasound. Secondary outcomes were rebleeding and hepatic encephalopathy.
A total of 40 patients were enrolled, with 20 assigned to the SMA group and 20 to the TIPS group. The symptoms of all patients in the two groups improved within 48 h. PVT was improved in 17 (85%) patients in the SMA group and 14 (70%) patients in the TIPS group. The main portal vein (MPV) thrombosis was significantly reduced in both groups (P < 0.001), and there was no significant difference between them (P = 0.304). In the SMA group, superior mesenteric vein (SMV) thrombosis and splenic vein (SV) thrombosis were significantly reduced (P = 0.048 and P = 0.02), which did not occur in the TIPS group. At 6-, 12-, and 24-mo follow-up, in the SMA group and the TIPS group, the cumulative rates free of the first episode of rebleeding were 80%, 65%, and 45% vs 90%, 80%, and 60%, respectively (P = 0.320); the cumulative rates free of the first episode of hepatic encephalopathy were 85%, 80%, and 65% vs 50%, 40%, and 35%, respectively (P = 0.022).
Transcatheter selective SMA urokinase infusion and TIPS are safe and effective for acute symptomatic PVT in cirrhosis.
Core tip: Transcatheter selective superior mesenteric artery urokinase infusion therapy and transjugular intrahepatic portosystemic shunt can both significantly reduce acute portal vein thrombosis in cirrhosis, and there was no significant difference between them. Moreover, the two strategies did not result in serious adverse events such as bleeding.
- Citation: Jiang TT, Luo XP, Sun JM, Gao J. Clinical outcomes of transcatheter selective superior mesenteric artery urokinase infusion therapy vs transjugular intrahepatic portosystemic shunt in patients with cirrhosis and acute portal vein thrombosis. World J Gastroenterol 2017; 23(41): 7470-7477
- URL: https://www.wjgnet.com/1007-9327/full/v23/i41/7470.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i41.7470
Portal vein thrombosis (PVT) is defined as thrombosis occurring in the trunk of the portal vein or portal branches (mesenteric and splenic veins)[1]. The prevalence of non-neoplastic PVT in liver cirrhosis ranges from 7.2% to 17%[2-4], and is higher at the decompensated or advanced stage[3,5]. Acute thrombosis can be severe and may lead to mesenteric ischemia and variceal bleeding. Recent studies have shown that acute PVT influences the outcomes of liver cirrhosis and results in worse survival and a significant increase in the risk of gastroesophageal variceal rebleeding[6-10].
Up to now, there has been no consensus on the treatment of liver cirrhosis with acute PVT in any guideline. Anticoagulation is usually used as a first-line treatment, and has been confirmed to be effective by some studies[11,12], whereas systemic and local thrombolysis, percutaneous portal vein recanalization, and transjugular intrahepatic portosystemic shunt (TIPS) are often considered as second-line choices[13]. Recently, endovascular selective catheterization thrombolytic therapy has been increasingly successfully implemented, which can be administered directly via percutaneous transhepatic or transjugular intrahepatic route or indirectly via the superior mesenteric artery (SMA) through femoral or radial artery infusion of thrombolytic agents[14,15]. The aim of this study was to compare the clinical outcomes of transcatheter selective SMA urokinase infusion therapy and TIPS in patients with cirrhosis and acute PVT to evaluate their effectiveness and safety.
From January 2013 to December 2014, all patients with cirrhosis and acute PVT treated at the Second Hospital of Chongqing Medical University were enrolled. All patients provided informed consent before admission. The ethics committee approved our research program. The inclusion criteria were as follows: (1) patients with liver cirrhosis; (2) clear thrombosis in the portal vein system as seen through Doppler ultrasound, angio-computed tomography (CTA), and/or angio-magnetic resonance imaging analyses (MRA); (3) onset of acute thrombosis within 1 wk; (4) continued abdominal pain or abdominal distension. The exclusion criteria were as follows: (1) contraindications to anticoagulation therapy; (2) severe cardiac or lung disease; (3) previous TIPS or thrombolytic therapy; (4) PVT after liver transplantation; (5) malignant tumor; and (6) refusing interventional therapy or follow-up.
Before the study, clinical symptoms, physical examination, and laboratory examination were recorded. All patients had an endoscopy to check for varices prior to thrombolysis and band ligation would be used to prevent variceal bleeding in patients with varices.
Doppler ultrasound, CTA, and/or MRA were used to estimate thrombosis from the aspects of location and severity. The PVT location was divided into the main portal vein (MPV), superior mesenteric vein (SMV), and splenic vein (SV). The PVT severity was divided into four levels: grade 0 (thrombus deficiency), grade I (MPV thrombus < 50% or only SMV and SV thrombus existed), grade II (MPV thrombus accounted for 50%-100%), and grade III (complete blocking or cavernous transformation of the portal vein).
Using randomly generated numbers, patients who met the inclusion criteria were randomly allocated to the SMA group or the TIPS group.
In the SMA group, we used the transfemoral approach for transcatheter selective SMA urokinase infusion therapy. Transcatheter thrombolysis was completed as follows: a catheter was selected for the SMA and remained in place. Contrast agent was injected through the SMA catheter. The first impact volume was 500000 IU. After the completion of angiography, urokinase was continuously pumped via the indwelling SMA catheter, depending on the patient’s weight (250000-500000 IU, twice daily, for a total of 15000 IU/kg/d). Thrombolysis time depended on the improvement of the patient’s clinical symptoms, a decrease in D-dimer, and imaging data. Anticoagulant therapy was completed as follows: patients were given subcutaneous heparin (2000 IU, twice daily) to maintain an international normalized ratio (INR) between 2 and 3. Patients were constantly monitored during thrombolytic therapy, including D-dimer, coagulation function, and routine blood tests. In addition, at intervals of 72 h and before removal of the catheter, angiography was re-performed. We considered ending thrombolysis and removing the indwelling SMA catheter when: (1) Superior mesenteric venography showed improvement of portal vein and SMV obstruction; (2) CT-enhanced examination showed that the PVT had obviously absorbed (stale thrombus occupying < 50% after treatment); or (3) vascular recanalization was achieved. After leaving the hospital, the patients continued taking oral warfarin for at least 3 mo.
In the TIPS group, TIPS was performed by two experienced physicians in accordance with standard procedures. After balloons were used to expand the obstructive passageway, a covered stent was embedded into the passageway. Additional bare-metal stents were placed into other obstructive veins of the portal venous system. After the procedure, the patients were given hypodermic low-molecular-weight heparin to prevent acute thrombosis (6000 IU, three time daily, consecutively for 1 wk). After discharge, all patients were treated with warfarin to achieve an INR of 2 to 3 for 6 mo.
All patients were followed at 1, 3, 6, 12, 18, and 24 mo after the procedure. Physical examinations and laboratory testing (coagulation function, routine blood test) were performed in both groups at each arranged follow-up visit, and CTA or Doppler ultrasound was performed at 6, 12, and 18 mo or whenever clinically required (e.g., for ascites, black stools, or abdominal pain). During follow-up, no blind method was adopted for patients taking warfarin.
The primary outcome measure was the change in portal vein patency status, which was evaluated by CTA or Doppler ultrasound. Secondary outcomes were rebleeding and hepatic encephalopathy. Changes in portal vein patency status were defined as follows: (1) recanalization, with complete disappearance or reconstruction of cavernous transformation; (2) improved, with recanalization improvement from grade III or II to grade II or I, or disappearance of an SMV or SV thrombus; (3) stable; and (4) worsened, with worsening from grade I or II to grade II or III, progression of thrombus to cavernous transformation, or formation of an SMV or SV thrombus.
Statistical analyses were performed using SPSS software version 19.0. All tests of significance were two-sided, and a P value < 0.05 was considered significant. A Student’s t-test was used to compare the differences in continuous variables between the two groups. Bivariate associations between categorical variables were analyzed with the χ2 test and Fisher exact test. Category ordinal variables, including Child-Pugh class and MPV thrombus severity, were analyzed using the Mann-Whitney U test. Survival analysis was performed by the Kaplan-Meier method and compared by log-rank test.
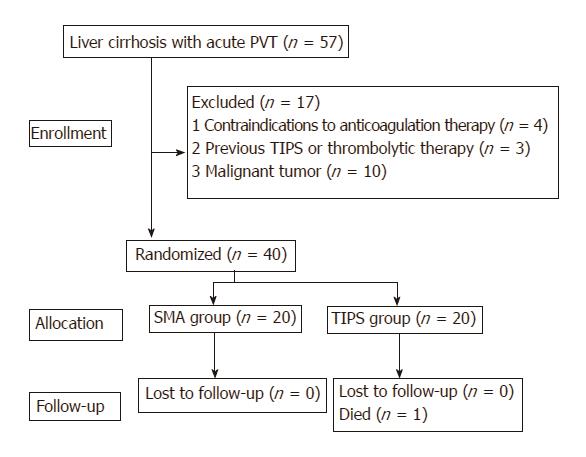
A total of 40 patients were included in our study. Figure 1 shows the patient inclusion and exclusion process. Twenty patients were assigned to the SMA group, and the other 20 patients were assigned to the TIPS group. Between the two groups, the baseline characteristics (i.e., age, sex, etiology, liver function, splenectomy, and status of PVT) had no significant difference (Table 1). There were 29 (72.5%) men and 11 (27.5%) women. The mean patient age was 49.3 ± 11.0 years (range, 27-72 yr). There were 31 (77.5%) patients with cirrhosis caused by hepatitis B virus (HBV), 1 patient (2.5%) by hepatitis C virus (HCV), 6 patients (15%) by alcohol, 1 patient (2.5%) by an autoimmune disorder, and 1 patient (2.5%) had a cryptogenic etiology. The average Child-Pugh score was 8.98 ± 1.79, and the average MELD score was 8.56 ± 5.98. There was no significant difference between the two groups in the MPV status; SMV thrombosis and SV thrombosis occurred in 16 patients and 15 patients, respectively.
| Characteristic | SMA group (n = 20) | TIPS group (n = 20) | P value |
| Age (yr)1 | 48.4 ± 13.2 | 50.1 ± 8.6 | 0.632 |
| Male sex | 14 | 15 | 1.0003 |
| Etiology | 0.1563 | ||
| HBV | 14 | 17 | |
| HCV | 0 | 1 | |
| Alcohol | 5 | 1 | |
| Autoimmune | 0 | 1 | |
| Cryptogenic | 1 | 0 | |
| Child-Pugh class | 0.0652 | ||
| A | 6 | 1 | |
| B | 8 | 9 | |
| C | 6 | 10 | |
| Child-Pugh score1 | 8.6 ± 1.7 | 9.4 ± 1.8 | 0.135 |
| MELD score1 | 8.0 ± 6.9 | 9.1 ± 5.1 | 0.582 |
| Splenectomy | 6 | 3 | 0.4513 |
| Past esophageal or gastric varices | 13 | 18 | 0.1273 |
| Past gastrointestinal bleeding | 12 | 18 | 0.0653 |
| MPV thrombus | 0.9382 | ||
| Grade I | 6 | 7 | |
| Grade II | 13 | 11 | |
| Grade III | 1 | 2 | |
| SMV thrombus | 11 | 5 | 0.1053 |
| SV thrombus | 8 | 7 | 1.0003 |
The technical success rate was 100% in both groups, and no fatal complications occurred.
In the SMA group, after treatment for 24 h, abdominal pain and abdominal distension were reduced by 80.0% (16/20), and 15.0% (3/20) of patients had no re-aggravation of their symptoms. At 48 h after the start of treatment, all of the 20 patients had a definite improvement in symptoms, and no patient had abdominal pain or distention before the end of thrombolysis (SMA catheter withdrawal). The mean time of indwelling SMA catheter placement was 8.75 ± 2.31 d, and the average dose of urokinase was 3705000.0 ± 1437000.1 IU. Before removing the catheter, contrast studies showed that in 15 cases, PVT had completely disappeared; another five cases of PVT had been absorbed (compared with before treatment, residual thrombus was < 10%).
During the follow-up period, no patients died. Oral warfarin (1.5-3.0 mg/d) for at least 3 mo was administered to patients to achieve an INR of 2 to 3. One patient stopped using warfarin and was treated with vitamin K1 after discharge for 1 mo; this patient had an INR of 9.27 and an activated partial thromboplastin time of 66.8 s during follow-up. Two patients were diagnosed with malignant tumors.
In the TIPS group, after treatment for 24 h, abdominal pain and abdominal distension were reduced by 75.0% (15/20), and 15.0% (3/20) of patients maintained the original symptoms. At 48 h after the start of treatment, all of the 20 patients had a definite improvement in symptoms.
The average value of portal pressure decreased from 46.30 ± 11.50 mmHg to 32.30 ± 7.47 mmHg. The last ultrasound scan before discharge showed that PVT had completely disappeared in 14 cases, and another 6 cases of PVT had been absorbed. During the follow-up period, one patient died of liver failure 14 mo after treatment. Ten patients had gastrointestinal rebleeding. Six patients had hepatic encephalopathy. Shunt dysfunction occurred in eight patients, six of whom underwent stent recanalization. Three patients were diagnosed with hepatocellular carcinoma, and one patient was diagnosed with suspected liver cancer.
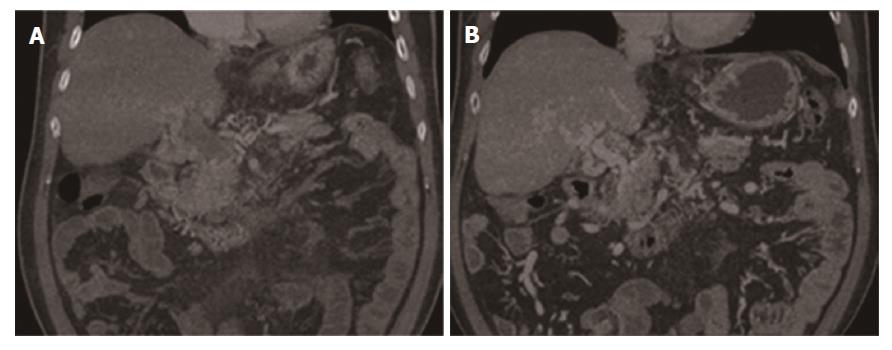
Changes in PVT: At the 6-mo follow-up, in the SMA group, 15 patients maintained sustained recanalization, four improved, and one remained stable; no patient had a worsened condition. In the TIPS group, 12 patients maintained sustained recanalization, four improved, and four remained stable; no patient had a worsened condition (P = 0.239). At the 12-mo follow-up, in the SMA group, compared with the 6-mo follow-up, the patients' status of thrombus did not change. In the TIPS group, 13 patients achieved continuous recanalization, two improved, two remained stable, and three worsened due to shunt dysfunction (P = 0.307). At the 24-mo follow-up, 13 patients maintained sustained recanalization, four improved, three remained stable, and no one worsened in the SMA group. In the TIPS group, 11 patients maintained sustained recanalization, three improved, two remained stable, and four worsened (one died of liver failure). For the thrombolytic result, there was no significant difference between the two groups (P = 0.304) (Table 2). One patient with MPV complete blocking and cavernous transformation (grade III), with severe abdominal pain and black stool, was treated by transcatheter SMA urokinase infusion therapy; 6 mo later, the MPV achieved sustained recanalization (Figure 2). In summary, the thrombus was significantly reduced in both groups (P < 0.001) (Tables 3 and 4) and patients with grade I and II PVT benefitted the most. In addition, in the SMA group, SMV thrombosis and SV thrombosis were significantly reduced (P = 0.048, P = 0.02).
| SMA group (n = 20) | TIPS group (n = 20) | ||||||
| Time (mo) | 6 | 12 | 24 | 6 | 12 | 24 | P value |
| Recanalization | 15 | 15 | 13 | 12 | 13 | 11 | 0.239 (6 mo) |
| Improved | 4 | 4 | 4 | 4 | 2 | 3 | 0.307 (12 mo) |
| Stable | 1 | 1 | 3 | 4 | 2 | 2 | 0.304 (24 mo) |
| Worse | 0 | 0 | 0 | 0 | 3 | 4 | |
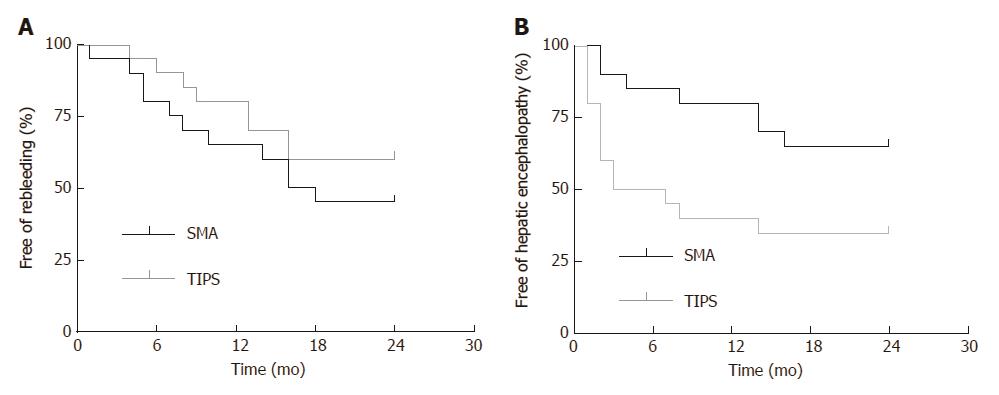
Rebleeding: A total of 11 patients in the SMA group and eight patients in the TIPS group had rebleeding. In the SMA group, recurrent variceal bleeding occurred in seven patients; no explicit causation was found in the remaining four patients. In the TIPS group, gastrointestinal bleeding after shunt dysfunction occurred in four patients; no explicit causation was found in any of the four patients. The cumulative rates free of the first episode of rebleeding at months 6, 12, and 24 in the SMA group and in the TIPS group were 80%, 65%, and 45% vs 90%, 80%, and 60%, respectively (P = 0.320) (Figure 3A).
Hepatic encephalopathy: Hepatic encephalopathy occurred in seven and 13 patients in the SMA group and the TIPS group, respectively. The cumulative rates free of the first episode of hepatic encephalopathy at months 6, 12, and 24 in the SMA group and in the TIPS group were 85%, 80%, and 65% vs 50%, 40%, and 35%, respectively (P = 0.022) (Figure 3B).
To date, the primary treatments for PVT include anticoagulation, thrombectomy, thrombolysis, and TIPS[16]. Although there is general agreement that anticoagulant therapy is needed for most symptomatic cases of PVT[17], there is no consensus on the treatment of liver cirrhosis with acute PVT. It was reported that simple anticoagulant therapy had a better curative effect on the patients with mild symptoms and limited scope of thrombus[18,19]. Compared to anticoagulation therapy, catheter-directed thrombolysis and TIPS are used infrequently, but they can: (1) decrease the risk of rebleeding in cirrhotic patients with previous variceal bleeding; (2) increase the rate of portal vein recanalization; and (3) be used in patients with cavernous transformation of the portal vein[14,20-23].
In our study, acute PVT greatly improved after catheter-directed thrombolysis and TIPS, and there was no significant difference between the two groups. Evidence about the use of thrombolytics for the treatment of acute PVT in patients with cirrhosis is relatively rare; some studies showed that PVT disappearance occurs in 100% of cases, with no recurrence during follow-up[14]. Acute PVT in 70% of patients was improved after TIPS treatment, which is consistent with previous studies that PVT disappearance occurred in 57% to 100% of patients[22,24]. We consider that this is consistent with the view that a reduced portal vein flow velocity is the key risk factor for PVT formation[7,14,25]. Patients with concomitant cirrhosis have significantly slower portal vein flow rates than the healthy population[14]. In addition, old thrombus often cannot be cleared completely, which aggravates local blood circulation disorder. Moreover, we observed that SMV thrombolysis and SV thrombolysis disappeared significantly in the SMA group, and no phenomenon occurred in the TIPS group. Previous reports showed that the treatment of acute PV-SMV thrombosis by transcatheter superior mesenteric artery catheter urokinase infusion therapy via the radial artery was effective[14]. This method is simple, easy to operate, and very safe. Because the thrombolytic agent circulates into the branch of the intestinal vein, the treatment of mesenteric venous thrombosis is better. All in all, acute PVT improved after SMA and TIPS; in addition, transcatheter selective SMA infusion therapy is better for the treatment of fresh thrombus in the mesentery.
Another problem that we are particularly concerned about is rebleeding. We observed that the rates of rebleeding were lower and the time to rebleeding was delayed in the TIPS group compared with the SMA group, although there was no significant difference between the two groups (P = 0.320). It has been confirmed that TIPS decreases the incidence of rebleeding as the second-line treatment for variceal hemorrhage[22,25,26]. In our study, infusion of relatively low-dose urokinase, no simultaneous peripheral venous infusion, and close monitoring of blood coagulation (which may cause the incidence of rebleeding) kept complications relatively low.
In the TIPS group, the patency rate in 24 mo was consistent with those reported in previous studies using covered stents[27]. TIPS is related to an increased risk of hepatic encephalopathy[22,23]. Our study showed that occurrences of hepatic encephalopathy in the TIPS group were markedly higher than those in the SMA group, and 50% of patients with hepatic encephalopathy, which was caused by the portosystemic shunting, had this occurrence within of 6 mo after TIPS treatment, whereas patients in the SMA group had hepatic encephalopathy caused by deterioration of liver function.
There are some limitations to our study. First, the lack of a large sample of participants is the major limitation, and further large-scale studies are needed. Second, our study was not a double-blind study, because patients in our study needed to be closely followed for blood clotting, and to detect complications that may be life-threatening.
In conclusion, transcatheter SMA infusion therapy and TIPS are both safe and effective treatments for patients with cirrhosis and acute PVT, particularly for grade I and II PVT, and transcatheter SMA urokinase infusion therapy is more ideal for the treatment of fresh thrombus in the mesentery.
Acute portal vein thrombosis is a common complication of cirrhosis and would lead to adverse prognosis. Endovascular selective catheterization thrombolytic therapy and transjugular intrahepatic portosystemic shunt has been increasingly successfully implemented, which brings new opportunities for the treatment of acute portal vein thrombosis (PVT).
The aim of this study was to compare the clinical outcomes of transcatheter selective superior mesenteric artery (SMA) urokinase infusion therapy and transjugular intrahepatic portosystemic shunt (TIPS) in patients with cirrhosis and acute PVT to evaluate their effectiveness and safety for acute PVT.
To evaluate the effectiveness and safety of transcatheter selective SMA urokinase infusion therapy and TIPS in patients with cirrhosis and acute PVT to provide theoretical support for the implementation of new therapies.
A randomized controlled trial was performed, and the total follow-up time was 24 mo. The outcome measures were the change in portal vein patency status, rebleeding, and hepatic encephalopathy.
Both treatments can quickly relieve symptoms within 48 h. The main portal vein thrombosis was significantly reduced in both groups and there was no significant difference between them. No fatal complications occurred.
Transcatheter SMA infusion therapy and TIPS are both safe and effective treatments for patients with cirrhosis and acute PVT, particularly for grade I and II PVT, and transcatheter SMA urokinase infusion therapy is more ideal for the treatment of fresh thrombus in the mesentery.
Further large-scale studies are needed. It is better to have a separate anticoagulant group as a control.
| 1. | Primignani M. Portal vein thrombosis, revisited. Dig Liver Dis. 2010;42:163-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 2. | Violi F, Corazza RG, Caldwell SH, Perticone F, Gatta A, Angelico M, Farcomeni A, Masotti M, Napoleone L, Vestri A. Portal vein thrombosis relevance on liver cirrhosis: Italian Venous Thrombotic Events Registry. Intern Emerg Med. 2016;11:1059-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 3. | Stine JG, Shah PM, Cornella SL, Rudnick SR, Ghabril MS, Stukenborg GJ, Northup PG. Portal vein thrombosis, mortality and hepatic decompensation in patients with cirrhosis: A meta-analysis. World J Hepatol. 2015;7:2774-2780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 4. | AmanN , Gul H, Roghani IS, Afridi Z. Frequency of portal vein thrombosis in cirrhosis on ultrasound. J Med Sci. 2015;23:11-13. |
| 5. | Turon F, Baiges A, Garcia-Criado A, Nuñez I, Gilabert R, Bru C, Hernández-Gea V. Portal Vein Thrombosis in Patients with Cirrhosis. Incidence and Factors Associated with Its Development. J Hepatol. 2016;64:S260. [DOI] [Full Text] |
| 6. | Qi X, Li H, Liu X, Yao H, Han G, Hu F, Shao L, Guo X. Novel insights into the development of portal vein thrombosis in cirrhosis patients. Expert Rev Gastroenterol Hepatol. 2015;9:1421-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Qi X, Dai J, Yang M, Ren W, Jia J, Guo X. Association between Portal Vein Thrombosis and Survival in Non-Liver-Transplant Patients with Liver Cirrhosis: A Systematic Review of the Literature. Gastroenterol Res Pract. 2015;2015:480842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Qi X, Dai J, Jia J, Ren W, Yang M, Li H, Fan D, Guo X. Association between portal vein thrombosis and survival of liver transplant recipients: a systematic review and meta-analysis of observational studies. J Gastrointestin Liver Dis. 2015;24:51-59, 4 p following 59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Qi X, Su C, Ren W, Yang M, Jia J, Dai J, Xu W, Guo X. Association between portal vein thrombosis and risk of bleeding in liver cirrhosis: A systematic review of the literature. Clin Res Hepatol Gastroenterol. 2015;39:683-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Zang L, Sun Z, Li W, Liu X. [Meta-analysis of risk factors of gastroesophageal varices rebleeding after therapeutic endoscopy]. Zhonghua Gan Zang Bing Za Zhi. 2015;23:275-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Loffredo L, Pastori D, Farcomeni A, Violi F. Effects of Anticoagulants in Patients With Cirrhosis and Portal Vein Thrombosis: A Systematic Review and Meta-analysis. Gastroenterology. 2017;153:480-487.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 311] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 12. | TononM , Piano S, Sacerdoti D, Dalla Valle F, Grbec M, Spiezia L, AngeliP . Efficacy and safety of treatment of acute nonmalignant portal vein thrombosis with subcutaneous fondaparinux in patients with cirrhosis and marked thrombocytopenia. Hepatology. 2015;62:591A. [DOI] [Full Text] |
| 13. | Tsochatzis EA, Senzolo M, Germani G, Gatt A, Burroughs AK. Systematic review: portal vein thrombosis in cirrhosis. Aliment Pharmacol Ther. 2010;31:366-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 226] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 14. | Wang MQ, Guo LP, Lin HY, Liu FY, Duan F, Wang ZJ. Transradial approach for transcatheter selective superior mesenteric artery urokinase infusion therapy in patients with acute extensive portal and superior mesenteric vein thrombosis. Cardiovasc Intervent Radiol. 2010;33:80-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Wang MQ, Liu FY, Duan F, Wang ZJ, Song P, Fan QS. Acute symptomatic mesenteric venous thrombosis: treatment by catheter-directed thrombolysis with transjugular intrahepatic route. Abdom Imaging. 2011;36:390-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Sharma AM, Zhu D, Henry Z. Portal vein thrombosis: When to treat and how? Vasc Med. 2016;21:61-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu. EASL Clinical Practice Guidelines: Vascular diseases of the liver. J Hepatol. 2016;64:179-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 564] [Article Influence: 56.4] [Reference Citation Analysis (3)] |
| 18. | Harnik IG, Brandt LJ. Mesenteric venous thrombosis. Vasc Med. 2010;15:407-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 19. | Bergqvist D, Svensson PJ. Treatment of mesenteric vein thrombosis. Semin Vasc Surg. 2010;23:65-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Liu K, Li WD, Du XL, Li CL, Li XQ. Comparison of Systemic Thrombolysis Versus Indirect Thrombolysis via the Superior Mesenteric Artery in Patients with Acute Portal Vein Thrombosis. Ann Vasc Surg. 2017;39:264-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Qi X, Han G, Fan D. The preferable treatment for cirrhotic portal vein thrombosis: anticoagulation or transjugular intrahepatic portosystemic shunt? Hepatology. 2010;51:713-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Han G, Qi X, He C, Yin Z, Wang J, Xia J, Yang Z, Bai M, Meng X, Niu J. Transjugular intrahepatic portosystemic shunt for portal vein thrombosis with symptomatic portal hypertension in liver cirrhosis. J Hepatol. 2011;54:78-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 188] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 23. | Luca A, Miraglia R, Caruso S, Milazzo M, Sapere C, Maruzzelli L, Vizzini G, Tuzzolino F, Gridelli B, Bosch J. Short- and long-term effects of the transjugular intrahepatic portosystemic shunt on portal vein thrombosis in patients with cirrhosis. Gut. 2011;60:846-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 201] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 24. | D’Avola D, Bilbao JI, Zozaya G, Pardo F, Rotellar F, Iñarrairaegui M, Quiroga J, Sangro B, Herrero JI. Efficacy of transjugular intrahepatic portosystemic shunt to prevent total portal vein thrombosis in cirrhotic patients awaiting for liver transplantation. Transplant Proc. 2012;44:2603-2605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Stine JG, Wang J, Shah PM, Argo CK, Intagliata N, Uflacker A, Caldwell SH, Northup PG. Decreased portal vein velocity is predictive of the development of portal vein thrombosis: A matched case-control study. Liver Int. 2017; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 119] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 26. | Qi X, He C, Guo W, Yin Z, Wang J, Wang Z, Niu J, Bai M, Yang Z, Fan D. Transjugular intrahepatic portosystemic shunt for portal vein thrombosis with variceal bleeding in liver cirrhosis: outcomes and predictors in a prospective cohort study. Liver Int. 2016;36:667-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 27. | Perarnau JM, Le Gouge A, Nicolas C, d’Alteroche L, Borentain P, Saliba F, Minello A, Anty R, Chagneau-Derrode C, Bernard PH. Covered vs. uncovered stents for transjugular intrahepatic portosystemic shunt: a randomized controlled trial. J Hepatol. 2014;60:962-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 159] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Memeo R, Tripathi D S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Huang Y