Published online Jan 14, 2017. doi: 10.3748/wjg.v23.i2.336
Peer-review started: August 3, 2016
First decision: September 12, 2016
Revised: October 3, 2016
Accepted: October 19, 2016
Article in press: October 19, 2016
Published online: January 14, 2017
Processing time: 162 Days and 20 Hours
To confirm previous conclusions on Saccharomyces cerevisiae (S. cerevisiae) CNCM I-3856 for irritable bowel syndrome (IBS) management.
An individual patient data meta-analysis was performed on two randomized clinical trials studying the effect of S. cerevisiae CNCM I-3856 supplementation on gastrointestinal (GI) symptoms in IBS subjects. A total of 579 IBS subjects were included. Outcomes were the daily Likert scale scores of abdominal pain/discomfort and bloating [area under the curve (AUC) and weekly means], responder status, and bowel movements (stool frequency and consistency). Statistical analyses were conducted in Intent to Treat (ITT) population, IBS-C subjects and IBS-C subjects with an abdominal pain/discomfort score higher than or equal to 2 at baseline (“IBS-C ≥ 2 subpopulation”).
S. cerevisiae CNCM I-3856 significantly improved abdominal pain/discomfort and bloating during the second month of supplementation [AUC (W5-W8)] with improvement up to the minimal clinically relevant threshold of 10%: a 12.3% reduction of abdominal pain/discomfort in the ITT population compared to the Placebo group (P = 0.0134) has been observed. In the IBS-C ≥ 2 subpopulation, there were a 13.1% reduction of abdominal pain/discomfort and a 14.9% reduction of bloating compared to the Placebo group (P = 0.0194 and P = 0.0145, respectively). GI symptoms significantly decreased during supplementation but no statistical differences were reported between groups at the end of the supplementation period. Responder status was defined as a subject who experienced a decrease of 1 arbitrary unit (a.u.) or 50% of the abdominal discomfort score from baseline for at least 2 wk out of the last 4 wk of the study. A significant difference between groups was reported in the ITT population, when considering the first definition: subjects in the Active group had 1.510 higher odds to be a responder (reduction of 1 a.u. of abdominal pain/discomfort) compared with subjects in the Placebo group (P = 0.0240). At the end of supplementation period, stool consistency in the Active group of the ITT population was significantly improved and classified as “normal” compared to Placebo (respectively 3.13 ± 1.197 a.u. vs 2.58 ± 1.020 a.u., P = 0.0003). Similar results were seen in the IBS-C ≥ 2 subpopulation (Active group: 3.14 ± 1.219 a.u. vs Placebo group: 2.59 ± 1.017 a.u., P = 0.0009).
This meta-analysis supports previous data linking S. cerevisiae I-3856 and improvement of GI symptoms, in IBS overall population and in the IBS-C and IBS-C ≥ 2 subpopulations.
Core tip: Since the gut microbiota is considered to play a role in the pathophysiology of Irritable Bowel Syndrome, the use of probiotics has gained interest for the management of gastrointestinal symptoms of irritable bowel syndrome (IBS). However, the properties of probiotics are strain-dependent. We performed an individual patient data meta-analysis to assess the effectiveness of Saccharomyces cerevisiae CNCM I-3856 on the management of IBS symptoms.
- Citation: Cayzeele-Decherf A, Pélerin F, Leuillet S, Douillard B, Housez B, Cazaubiel M, Jacobson GK, Jüsten P, Desreumaux P. Saccharomyces cerevisiae CNCM I-3856 in irritable bowel syndrome: An individual subject meta-analysis. World J Gastroenterol 2017; 23(2): 336-344
- URL: https://www.wjgnet.com/1007-9327/full/v23/i2/336.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i2.336
Irritable bowel syndrome (IBS) is a common functional gastrointestinal (GI) disorder with high prevalence, affecting up to 20% of the population in some geographical areas[1]. Diverse GI symptoms occur in IBS, mainly abdominal pain, bloating and altered bowel habits. Subjects also report some extra-GI symptoms and an impaired quality of life[2]. According to Rome III criteria, IBS diagnosis is based on the presence of abdominal pain and/or discomfort, associated with at least two of the following: improvement with defecation, onset associated with a change in frequency of stool, onset associated with a change in form of stool[3]. The diagnosis is confirmed by the absence of any organic disorder. Some IBS subtypes are distinguished depending on the predominant stool pattern: constipation-predominant IBS (IBS-C), diarrhea-predominant IBS (IBS-D), mixed IBS (IBS-M) alternating constipation and diarrhea, and an un-subtyped IBS. To date, the pathogenesis of IBS is still not clear. Different mechanisms may be implicated, such as altered GI motility, visceral hypersensitivity, intestinal barrier disorder, dysfunction of the brain-gut axis, intestinal inflammation and disruption of the intestinal flora[2,4]. When investigating IBS, the gut microbiota is of great interest. First, some IBS cases begin after an acute episode of a GI infection, which can alter the gut microbiota’s diversity[5,6]. Secondly, the intestinal microbiota is thought to be involved in most of the mechanisms of IBS[7]. Due to a lack of satisfactory pharmacological treatments suitable for long-term administration[8], both patients and caregivers turn to alternative methods for the management of IBS. In such an approach, probiotics prove to be an interesting and promising option. Their supplementation may help to counterbalance mechanisms involved in IBS[5,9] and to alleviate associated symptoms. Today the challenge is to identify which probiotic strains are relevant for IBS management and the conditions for their use (preparations, doses, duration).
Saccharomyces cerevisiae (S. cerevisiae) CNCM I-3856, a proprietary yeast strain from Lesaffre, has been studied for its beneficial properties in alleviating GI symptoms in IBS subjects. Two randomized clinical trials (RCTs) have been conducted and published[10,11] involving a total of 579 IBS subjects. We decided to perform an individual patient data (IPD) meta-analysis to confirm the previous findings and strengthen conclusions on this particular yeast strain.
Methods for statistical analyses were pre-specified in a Statistical Analysis Plan. We evaluated manuscripts that studied the effect of S. cerevisiae CNCM I-3856 supplementation on GI symptoms in IBS subjects. Only the two aforementioned clinical trials were identified and included in this meta-analysis.
The I-3856 strain of Sacharomyces cerevisiae is a proprietary, well-characterized strain of Lesaffre, registered in the French National Collection of Cultures of Microorganisms (CNCM). The S. cerevisiae species has been characterized by using phenotypic (API® ID32C, Biomerieux SAS) and genotypic referenced methods (genetic amplification and sequencing of 26S DNA)[12,13]. Moreover, the strain I-3856 is identified thanks to the Polymerase Chain Reaction (PCR) Interdelta typing technique[14], and other genetic methods (e.g., complete genome sequencing).
All data were provided by the sponsor of the trials. Uniform collection methods were employed in the two trials.
Outcomes were defined as the daily Likert scale scores of abdominal pain/discomfort and bloating (calculated on a weekly basis) over the common study period of both studies (8 wk) and also expressed as total Area Under the Curve (AUC) [total AUC (W0-W8) and total AUC (W5-W8)]. According to the abdominal score at baseline, responder status was considered. A subject was defined as responder if he/she experienced a decrease of 1 arbitrary unit (a.u.) or 50% of the abdominal discomfort score from baseline for at least 2 wk out of the last 4 wk of the study. Bowel movements, i.e., stool frequency and consistency, were also studied.
Statistical analyses were conducted, in first instance, on the Intent to Treat (ITT) population. Two subpopulations of interest where then derived from this population: IBS-C subjects (IBS subjects with hard or lumpy stools ≥ 25% and loose (mushy) or watery stools < 25% of bowel movements[3]) and IBS-C subjects with an abdominal pain/discomfort score higher than or equal to 2 at baseline (referred as “IBS-C ≥ 2 subpopulation” in this paper). In both RCTs of this meta-analysis, subjects were included if they reported a score of abdominal pain/discomfort ranging between 1 and 6 (on a 7-point Likert scale) at baseline. Nevertheless, only subjects presenting a score ≥ 2 and < 6 at baseline have been considered in the IBS-C ≥ 2 subpopulation. IBS typology can vary a lot from one individual to another, inducing great variability in symptoms scores intensity. The supplementation with S. cerevisiae CNCM I-3856 aims to be administered to healthy subjects with a medium level of abdominal pain/discomfort. IBS patients who need a follow-up at hospital because of high-level of pain (≥ 6) are out of the scope of the present supplementation. Inversely, subjects with low level of symptoms intensity (strictly below 2) can’t be considered as being improvable. In the ITT population, outcomes throughout the study period were analyzed using a fixed-effect repeated measure model including the factors Product, Week, IBS Type, Study and interactions Product*Week, Product*Study and Product*Week*Study[15,16]. An Analysis of Covariance (ANCOVA), including factors Product, IBS Type, Study, Product*Study and the baseline value as a covariate, was conducted to analyze total AUCs. Finally, a Generalized Linear Model (GLM, binomial distribution) including factors Product, Study, IBS Type and interaction Product*Study was conducted to assess the responder status. In the subpopulations, the same statistical models, without the IBS Type effect, were run. Statistical analyses were performed using SAS® software version 9.3 (SAS Institute Inc., Cary, NC, United States).
The two clinical trials were published in 2015 and 2016 in two different peer-reviewed journals. Characteristics of the trials are shown in Table 1.
| Trial | Type of IBS | Criteria | Sample size | Probiotic | Probiotic dosage1 | Control | Duration of treatment | Follow-up |
| Pineton de Chambrun et al[10] 2015 | All types | Rome III | 200 | Saccharomyces cerevisiae CNCM I-3856 | 1 capsule (500 mg) of S. cerevisiae/d | Placebo | 8 wk | 3 wk |
| 8 × 109 CFU/g | ||||||||
| Spiller et al[11] 2015 | All types | Rome III | 379 | Saccharomyces cerevisiae CNCM I-3856 | 2 capsules of 500 mg of S. cerevisiae/d | Placebo | 12 wk | - |
| 8 × 109 CFU/g |
Data were obtained from 579 subjects, diagnosed with IBS according to Rome III criteria[3]. All IBS subtypes were represented in the two trials. Patient characteristics for the 2 trials are listed in Table 2. There are no significant differences at baseline between the Placebo and Active groups on the total population, nor on the IBS-C subpopulation and the IBS-C ≥ 2 subpopulation.
| Placebo group | Active group | |
| Subjects, n | 287 | 292 |
| Age (yr), mean ± SD | 45.3 ± 14.03 | 44.4 ± 14.79 |
| Subject weight (kg), mean ± SD | 64.6 ± 12.37 | 65.8 ± 13.75 |
| Subject height (cm), mean ± SD | 164.3 ± 7.75 | 164.9 ± 8.19 |
| Subject BMI (kg/m²), mean ± SD | 23.9 ± 3.93 | 24.1 ± 4.53 |
| Heart rate (bpm), mean ± SD | 70.2 ± 9.10 | 70.6 ± 9.08 |
| Female | 244 (85.0) | 245 (83.9) |
| Smoker | 65 (22.6) | 75 (25.7) |
| IBS-C | 134 (46.7) | 130 (44.5) |
| IBS-C with an abdominal pain/discomfort score ≥ 2 at baseline | 111 (38.7) | 111 (38.0) |
| IBS-D | 55 (19.2) | 56 (19.2) |
| IBS-M | 96 (33.4) | 103 (35.3) |
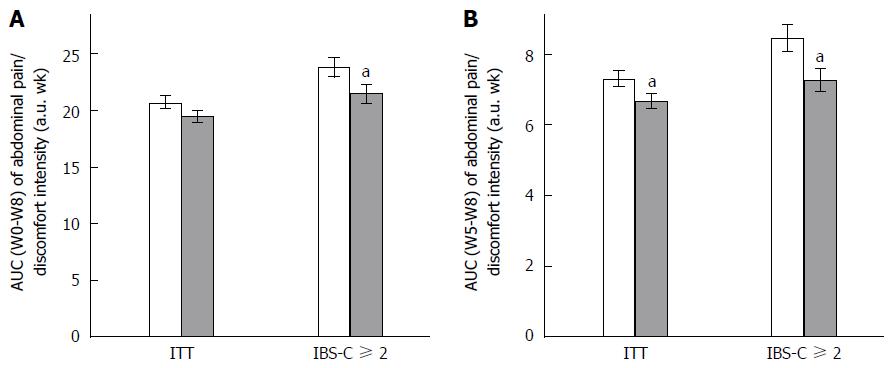
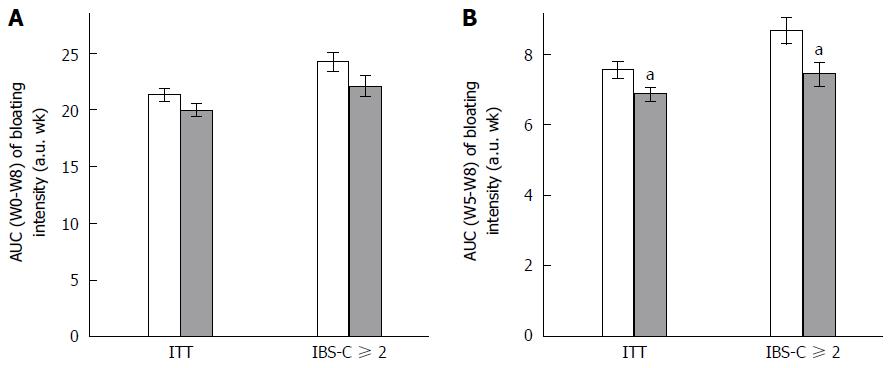
Any change in GI symptoms throughout the supplementation period was analyzed using AUC. Data are presented in Figure 1 (abdominal pain/discomfort) and Figure 2 (bloating).
Over the 2 mo of supplementation, no differences were determined by AUC (W0-W8) analysis of abdominal pain/discomfort and bloating between Active and Placebo groups of the ITT population and the IBS-C subpopulation.
A significant difference between the Active and Placebo groups was observed in the IBS-C ≥ 2 subpopulation on abdominal pain/discomfort (P = 0.0417, diff[95%CI] = -2.096 [-4.112;-0.080]) for AUC (W0-W8); and a trend was reported on bloating (P = 0.0540) in favor of the Active group.
Considering the second month of supplementation [AUC (W5-W8)], S. cerevisiae CNCM I-3856 improved GI symptoms in all analyzed populations. Abdominal pain/discomfort was significantly reduced in the Active group compared to the Placebo group in the ITT population (-12.3%, P = 0.0134, diff[95%CI] = -0.728 [-1.305;-0.152]) and in the IBS-C ≥ 2 subpopulation (-13.1%, P = 0.0194, diff[95%CI] = -1.144 [-2.100;-0.187]). A trend was reported in the IBS-C subpopulation (-10.4%, P = 0.0544). Bloating was significantly decreased during the second month of supplementation in the ITT population (-9.2%, P = 0.0105, diff[95%CI] = -0.743 [-1.311;-0.175]); in the IBS-C population (-12.3%, P = 0.0463, diff[95%CI] = -0.864 [-1.714;-0.014]); and in the IBS-C ≥ 2 subpopulation (-14.9%, P = 0.0145, diff[95%CI] = -1.192 [-2.143;-0.240]).
In a complementary approach, daily Likert scale scores of abdominal pain/discomfort and bloating were analyzed on a weekly basis. At baseline, in the ITT population, the two groups were not different regarding the abdominal pain/discomfort scores (3.15 ± 1.163 a.u. in the Active group vs 3.16 ± 1.110 a.u. in the Placebo group, P = 0.9714). Abdominal pain/discomfort was significantly reduced throughout the study in the two study groups (between W0 and W8, P < 0.0001 in both groups). At W8, lower abdominal pain/discomfort scores were reported in the Active group compared to Placebo but the difference did not achieve statistical significance (Active group: 2.14 ± 1.292 a.u., Placebo group: 2.31 ± 1.355 a.u., P = 0.1052). In the IBS-C subpopulation, abdominal pain/discomfort significantly decreased between W0 and W8 in both groups (Active group: 3.25 ± 1.177 a.u. vs 2.23 ± 1.231 a.u.; Placebo group: 3.22 ± 1.159 a.u. vs 2.40 ± 1.420 a.u., P < 0.0001 in both groups). Similar evolution was observed for bloating over the supplementation period (Active group: 3.31 ± 1.272 a.u. at W0 vs 2.25 ± 1.257 a.u. at W8; Placebo group: 3.36 ± 1.179 a.u. at W0 vs 2.54 ± 1.390 a.u. at W8, P < 0.0001 in both groups).
In the IBS-C ≥ 2 subpopulation, slightly higher abdominal pain/discomfort was expressed at baseline (3.54 ± 1.005 a.u. in the Active group; 3.54 ± 0.918 a.u. in the Placebo group) as compared to the IBS-C subpopulation. Abdominal pain/discomfort scores significantly decreased from W0 to W8 in both groups (Active group: -1.21 ± 1.249 a.u.; Placebo group: -0.91 ± 1.340 a.u., P < 0.0001 in both groups). Bloating significantly improved throughout the supplementation period in both study groups (between W0 and W8, P < 0.0001 in both groups).
Two definitions of responder status were applied in this meta-analysis. In the ITT population, subjects in the Active group had 1.510 higher odds to be a responder compared to subjects in the Placebo group (P = 0.0240, OR[95%CI] = 1.510 [1.056;2.161]) when responders were defined by a reduction of 1 a.u. of abdominal pain/discomfort score from W0 for at least 2 wk between W5 and W8. This difference was not significant anymore when responders were defined by a reduction of 50% of abdominal pain/discomfort score.
No significant differences were reported between the Active and Placebo groups in both IBS-C and IBS-C ≥ 2 subpopulations.
Bowel movements are not reported in the ITT population because of the variety of stool patterns in regard to the different IBS subtypes present in the study population.
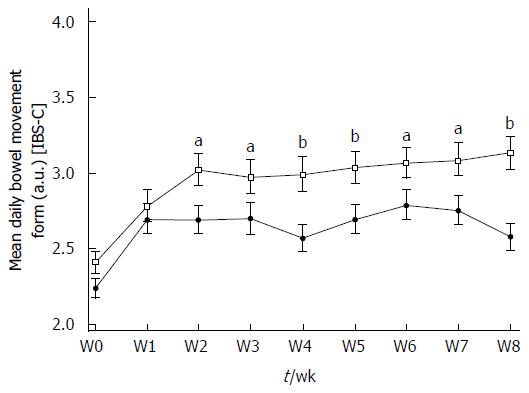
Stool consistency statistically improved in the Active and Placebo groups of the IBS-C subpopulation during the supplementation period. There was a statistically significant increase in the Bristol Stool Scale (BSS) score between W0 and W8 in the Active group (2.41 ± 0.817 a.u. vs 3.13 ± 1.197 a.u., P < 0.0001, diff[95%CI] = 0.752 [0.391;1.114]) and in the Placebo group (2.24 ± 0.714 a.u. vs 2.58 ± 1.020 a.u., P = 0.0266, diff[95%CI] = 0.402 [0.032;0.771]) (Figure 3). Over the 8 wk of supplementation, a significant product effect was reported in the IBS-C subpopulation in favor of S. cerevisiae CNCM I-3856 (P < 0.0001) and stool consistency was significantly different from Placebo from W2 to W8. At the end of the supplementation period, stool consistency in the Active group was significantly higher and classified as “normal” compared to the Placebo group (respectively 3.13 ± 1.197 a.u. vs 2.58 ± 1.020 a.u., P = 0.0003, diff[95%CI]: 0.510 [0.231-0.788]).
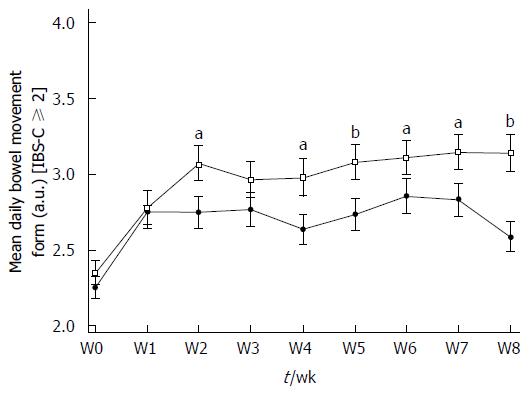
In the IBS-C ≥ 2 subpopulation, stool consistency improved in the same way. BSS scores increased in Active and Placebo groups between W0 and W8. A statistically significant difference was reported in the Active group (W0: 2.35 ± 0.832 a.u. vs W8: 3.14 ± 1.219 a.u., P < 0.0001, diff[95%CI]: 0.808 [0.411-1.204]) but not in the Placebo group (2.25 ± 0.728 a.u. vs 2.59 ± 1.017 a.u., P = 0.0865) (Figure 4). A significant product effect in favor of S. cerevisiae CNCM I-3856 was apparent in the IBS-C ≥ 2 subpopulation over the supplementation period (P = 0.0017) and stool consistency was significantly different from Placebo during the second part of the supplementation (P < 0.05 from W4 to W8).
Statistically significant product effects were irregularly observed on stool frequency in the IBS-C subpopulation and the IBS-C ≥ 2 subpopulation in favor of the Active group. However the non-persistence of the effect and the absence of clinical relevance between Active vs Placebo did not demonstrate any benefits from S. cerevisiae CNCM I-3856 supplementation on this outcome.
Based on the data of two RCTs investigating the benefits of S. cerevisiae CNCM I-3856 on IBS symptoms management, this IPD meta-analysis confirmed some promising qualities of this yeast strain to alleviate GI symptoms.
Analysis of GI symptoms (abdominal pain/discomfort and bloating) on a week-by-week basis demonstrated a great variability in patient complaints over the supplementation period which has already been discussed by different authors[17,18]. No significant benefits of S. cerevisiae CNCM I-3856 supplementation were observed during the first month of supplementation compared to placebo. This point has been previously reported by Pineton de Chambrun et al[10] who mentioned a potential delay of action of S. cerevisiae. The yeast may positively impact abdominal pain/discomfort due to an analgesic effect, with a delay before significant effectiveness as reported in animal studies[19]. In rats, some significant product effects were noted over the second month of study but the analgesic effect was transitory and limited to the time of product administration.
From a clinical point of view, AUC data provide relevant information in regard to overall subjective feelings. First, the AUC presents an overview of GI symptom intensity during the study period. This provides an indication of symptom alleviation over the supplementation period. The present analysis hardly succeeded in demonstrating a beneficial effect of S. cerevisiae CNCM I-3856 on the global AUC [AUC (W0-W8)] for abdominal pain/discomfort and bloating. Statistically positive effects were reported in the IBS-C ≥ 2 subpopulation, but not in the ITT population and IBS-C subpopulation. An AUC was also calculated over the second month of supplementation. This period was chosen in order to account for the aforementioned delay for significant action of the probiotic. Analysis on this specific period [AUC (W5-W8)] confirmed the product effect associated with yeast supplementation, in comparison to placebo, in all analyzed populations. Additionally, every statistically significant difference or trend was related to an improvement in the Active group from 9.2% and up to 14.9% compared to Placebo. This observation is of great interest from a clinical point of view: the 10% difference is considered as a minimal clinically relevant threshold[20]. This means that S. cerevisiae CNCM I-3856 improves IBS symptoms in a significant and relevant manner in IBS patients, in a global IBS population and in the IBS-C subtype during the second month of consumption. Another approach in validating the clinical benefits of therapy consists of analyzing the percentage of patients who positively respond to the treatment. Various definitions of responder status are suggested by the literature[21]. In addition, food and drug authorities have since established some complete definitions for responder status[22,23]. Two different definitions of responder status were applied in the two RCTs[10,11]. They were re-used in the present meta-analysis for consistency. Conclusions on responder status were limited to the ITT population who experienced an improvement of 1 a.u. of abdominal pain/discomfort for at least 50% of the second month of supplementation.
One point limiting the positive conclusions on S. cerevisiae CNCM I-3856 relates to the placebo response commonly reported in GI disorders. A meta-analysis of placebo-controlled RCTs conducted in IBS patients reported a mean placebo response rate of 37.5%[24]. This is consistent with previous meta-analysis from Patel et al[25] who observed that 40.2% of patients respond to placebo. The placebo effect is one factor influencing the placebo response rate, but it does not affect it alone. The authors of this meta-analysis tried to determine the confounding factors which may influence the placebo response rate (duration of therapy, run-in phase, single or multi-centre study, etc.); but, inconsistent data were reported[24,25].
Today, probiotics are an alternative approach being extensively researched for the management of symptoms in IBS patients. While the effects of probiotics on IBS physiopathology are still under scrutiny[9], many clinical trials investigating the benefits of one or more bacterial strains in IBS have been published. Increased resources are now available to examine probiotic benefits in IBS management. It remains difficult to clearly conclude efficacy and set up recommendations for probiotics supplementation because of the poor repeatability of the data and the difficulty in comparing studies. Indeed, great heterogeneity is observed between trials in terms of variety of strains used, probiotic dosage, treatment duration, outcome definitions, and statistical analyses. This point is highlighted in literature reviews and meta-analyses[26-28]. In the absence of comparable studies, meta-analyses are performed to integrate qualitative clinical trials irrespective of the probiotic strains studied[26-31]. All authors globally agree as to the benefits of probiotics supplementation in IBS patients. Neither individual probiotic nor combination of strains was, however, identified as effective by itself and conclusions were drawn for probiotics in general. Some authors directed their meta-analyses specifically to Lactobacillus strains[32] or to Lactobacillus rhamnosus GG in children[33]. Only a few trials were included in these meta-analyses (six trials investigating Lactobacillus strains and three trials investigating Lactobacillus rhamnosus GG in children), but their conclusions can be considered relevant in light of the studies selected. Both authors concluded on beneficial effect of Lactobacillus spp on IBS symptoms, namely on frequency and intensity of abdominal pain. However, no recommendations for dose and treatment duration were proposed.
In regards to these meta-analyses, the present IPD meta-analysis demonstrates two advantages. First, the two RCTs were designed to evaluate the same probiotic strain, i.e., S. cerevisiae CNCM I-3856, and the methodologies were very similar. Study designs were double-blind, randomized, placebo-controlled to achieve high quality studies, and recommendations for treatment trial design were applied[21]. These points strengthen the integration of data and their analyses as a whole. Second, individual data on all subjects were compiled to perform new statistical analyses, which provide a more robust result than meta-analyses based on aggregate data. The pooling of the two original individual trials[10,11] for meta-analysis increased the sample size and thus the power of the statistical analyses, in particular in the context of the analysis in the IBS-C subpopulation. In addition, this meta-analysis allowed answering and confirming questions not initially raised by both studies and consolidated previous results reported on S. cerevisiae CNCM I-3856.
In conclusion, this IPD meta-analysis confirms previous data linking S. cerevisiae CNCM I-3856 supplementation and improvement of GI symptoms in IBS overall population and in the IBS-C and IBS-C ≥ 2 subpopulations. Despite great similarities in study designs, the two RCTs considered in the meta-analysis differ in terms of endpoints. The present observations would gain in being validated by a new RCT designed according to these meta-analysis conclusions.
Irritable bowel syndrome (IBS) is a common functional gastrointestinal (GI) disorder with high prevalence. Its diagnosis is based on the presence of abdominal pain and/or discomfort associated with at least two of the following: improvement with defecation; onset associated with a change in frequency of stool; onset associated with a change in form of stool. With the lack of satisfactory pharmacological treatments suitable for long-term administration, an alternative approach such as probiotic use may be of interest for IBS management.
The intestinal microbiota appears to play a key role in IBS, even if its implication is not clearly identified. Probiotics are live microorganisms that confer a health benefit to the host. The present meta-analysis focuses on yeast strain Saccharomyces cerevisiae (S. cerevisiae) CNCM I-3856.
Two randomized clinical trials have been included in this individual patient data meta-analysis. Data on 579 subjects were compiled and analyzed to increase the power of the initial statistical analyses. Results confirm the efficacy of S. cerevisiae CNCM I-3856 supplementation in subjects suffering from IBS for the improvement of GI symptoms. Interesting results were reported in subjects characterized by IBS-C or by IBS-C with score of abdominal pain/discomfort ≥ 2 at baseline.
The results suggest that S. cerevisiae CNCM I-3856 may be an alternative approach to deal with GI symptoms associated with IBS.
IBS is defined as recurrent abdominal pain or discomfort at least 3 d per month in the last 3 mo associated with 2 or more of the following: 1. Improvement with defecation, 2. Onset associated with a change in frequency of stool, 3. Onset associated with a change in form (appearance) of stool.
The study contents are interesting, being focused on the efficacy of S. cerevisiae CNCM I-3856 supplementation in subjects suffering from IBS for the improvement of GI symptoms. The title and abstract accurately convey the study contents. The objectives of the study are clear. The methods are appropriate and well described, the data are sound and controlled. The discussion and conclusions are well balanced and adequately supported by the data.
| 1. | Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol. 2014;6:71-80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 423] [Article Influence: 35.3] [Reference Citation Analysis (1)] |
| 2. | Soares RL. Irritable bowel syndrome: a clinical review. World J Gastroenterol. 2014;20:12144-12160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 128] [Cited by in RCA: 136] [Article Influence: 11.3] [Reference Citation Analysis (10)] |
| 3. | Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3413] [Cited by in RCA: 3409] [Article Influence: 170.5] [Reference Citation Analysis (4)] |
| 4. | Saha L. Irritable bowel syndrome: pathogenesis, diagnosis, treatment, and evidence-based medicine. World J Gastroenterol. 2014;20:6759-6773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 318] [Cited by in RCA: 315] [Article Influence: 26.3] [Reference Citation Analysis (16)] |
| 5. | Simrén M, Barbara G, Flint HJ, Spiegel BM, Spiller RC, Vanner S, Verdu EF, Whorwell PJ, Zoetendal EG. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. 2013;62:159-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 655] [Cited by in RCA: 660] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 6. | Bennet SM, Ohman L, Simren M. Gut microbiota as potential orchestrators of irritable bowel syndrome. Gut Liver. 2015;9:318-331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 7. | Lee KN, Lee OY. Intestinal microbiota in pathophysiology and management of irritable bowel syndrome. World J Gastroenterol. 2014;20:8886-8897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 47] [Reference Citation Analysis (0)] |
| 8. | Jadallah KA, Kullab SM, Sanders DS. Constipation-predominant irritable bowel syndrome: a review of current and emerging drug therapies. World J Gastroenterol. 2014;20:8898-8909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 9. | Santos AR, Whorwell PJ. Irritable bowel syndrome: the problem and the problem of treating it - is there a role for probiotics? Proc Nutr Soc. 2014;73:470-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Pineton de Chambrun G, Neut C, Chau A, Cazaubiel M, Pelerin F, Justen P, Desreumaux P. A randomized clinical trial of Saccharomyces cerevisiae versus placebo in the irritable bowel syndrome. Dig Liver Dis. 2015;47:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 11. | Spiller R, Pélerin F, Cayzeele Decherf A, Maudet C, Housez B, Cazaubiel M, Jüsten P. Randomized double blind placebo-controlled trial of Saccharomyces cerevisiae CNCM I-3856 in irritable bowel syndrome: improvement in abdominal pain and bloating in those with predominant constipation. United European Gastroenterol J. 2016;4:353-362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 12. | Kurtzman CP, Robnett CJ. Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie Van Leeuwenhoek. 1998;73:331-371. [PubMed] |
| 13. | Kurtzman CP, Robnett CJ. Identification of clinically important ascomycetous yeasts based on nucleotide divergence in the 5’ end of the large-subunit (26S) ribosomal DNA gene. J Clin Microbiol. 1997;35:1216-1223. [PubMed] |
| 14. | Standardization ECf. Animal feeding stuffs: PCR typing of probiotic strains of Saccharomyces cerevisiae (yeast). . |
| 15. | Borenstein M, Hedges L, Rothstein H. Meta-analysis: Fixed effect vs random effects. Available from: http://www.Meta-Analysis.com. |
| 16. | Whitehead A. Meta-Analysis of Controlled Clinical Trials: John Wiley and Sons, Ltd, 2003. . |
| 17. | Shah E, Pimentel M. Placebo effect in clinical trial design for irritable bowel syndrome. J Neurogastroenterol Motil. 2014;20:163-170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Musial F, Klosterhalfen S, Enck P. Placebo responses in patients with gastrointestinal disorders. World J Gastroenterol. 2007;13:3425-3429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Rousseaux C, Bouguen G, Dubuquoy C, Dubuquoy L, Vandekerckove P, Desreumaux P. 274 Sacharomyces Cerevisiae Cncm I-3856 Decreases Intestinal Pain Through PPAR Alpha Activation in the Gut. Gastroenterology. 2010;138:S-51. [DOI] [Full Text] |
| 20. | Chang L, Lembo A, Sultan S. American Gastroenterological Association Institute Technical Review on the pharmacological management of irritable bowel syndrome. Gastroenterology. 2014;147:1149-1172.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (1)] |
| 21. | Irvine EJ, Whitehead WE, Chey WD, Matsueda K, Shaw M, Talley NJ, Veldhuyzen van Zanten SJ. Design of treatment trials for functional gastrointestinal disorders. Gastroenterology. 2006;130:1538-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 246] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 22. | Food and Drug Administration. Guidance for Industry: Irritable Bowel Syndrome - Clinical Evaluation of Drugs for Treatment. US Department of Health and Human services, Food and Drug Administration, Center for Drug Evalution and research (CDER), 2012: 17. . |
| 23. | European Medicines Agency. Guideline on the evaluation of medicinal products for the treatment of irritable bowel syndrome. European Medicines Agency, 2014: 18. . |
| 24. | Ford AC, Moayyedi P. Meta-analysis: factors affecting placebo response rate in the irritable bowel syndrome. Aliment Pharmacol Ther. 2010;32:144-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 177] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 25. | Patel SM, Stason WB, Legedza A, Ock SM, Kaptchuk TJ, Conboy L, Canenguez K, Park JK, Kelly E, Jacobson E. The placebo effect in irritable bowel syndrome trials: a meta-analysis. Neurogastroenterol Motil. 2005;17:332-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 219] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 26. | Hoveyda N, Heneghan C, Mahtani KR, Perera R, Roberts N, Glasziou P. A systematic review and meta-analysis: probiotics in the treatment of irritable bowel syndrome. BMC Gastroenterol. 2009;9:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 186] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 27. | McFarland LV, Dublin S. Meta-analysis of probiotics for the treatment of irritable bowel syndrome. World J Gastroenterol. 2008;14:2650-2661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 241] [Cited by in RCA: 199] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 28. | Moayyedi P, Ford AC, Talley NJ, Cremonini F, Foxx-Orenstein AE, Brandt LJ, Quigley EM. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59:325-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 456] [Article Influence: 28.5] [Reference Citation Analysis (1)] |
| 29. | Didari T, Mozaffari S, Nikfar S, Abdollahi M. Effectiveness of probiotics in irritable bowel syndrome: Updated systematic review with meta-analysis. World J Gastroenterol. 2015;21:3072-3084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 266] [Cited by in RCA: 232] [Article Influence: 21.1] [Reference Citation Analysis (5)] |
| 30. | Ford AC, Quigley EM, Lacy BE, Lembo AJ, Saito YA, Schiller LR, Soffer EE, Spiegel BM, Moayyedi P. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1547-1561; quiz 1546, 1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 496] [Article Influence: 41.3] [Reference Citation Analysis (1)] |
| 31. | Ortiz-Lucas M, Tobías A, Saz P, Sebastián JJ. Effect of probiotic species on irritable bowel syndrome symptoms: A bring up to date meta-analysis. Rev Esp Enferm Dig. 2013;105:19-36. [PubMed] |
| 32. | Tiequn B, Guanqun C, Shuo Z. Therapeutic effects of Lactobacillus in treating irritable bowel syndrome: a meta-analysis. Intern Med. 2015;54:243-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 33. | Horvath A, Dziechciarz P, Szajewska H. Meta-analysis: Lactobacillus rhamnosus GG for abdominal pain-related functional gastrointestinal disorders in childhood. Aliment Pharmacol Ther. 2011;33:1302-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: France
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Lakatos PL, Pani SP, Specchia ML S- Editor: Qi Y L- Editor: A E- Editor: Liu WX