Published online Apr 7, 2017. doi: 10.3748/wjg.v23.i13.2355
Peer-review started: December 30, 2016
First decision: January 19, 2017
Revised: February 20, 2017
Accepted: March 15, 2017
Article in press: March 15, 2017
Published online: April 7, 2017
Processing time: 98 Days and 23.8 Hours
To investigate the influence of interferon-free antivirus therapy on lipid profiles in chronic hepatitis C virus genotype 1b (HCV1b) infection.
Interferon-free antiviral agents were used to treat 276 patients with chronic HCV1b infection, and changes in serum lipids of those who achieved sustained virologic response (SVR) were examined. The treatment regimen included 24 wk of daclatasvir plus asunaprevir (DCV + ASV) or 12 wk of sofosbuvir plus ledipasvir (SOF + LDV). SVR was achieved in 121 (85.8%) of 141 patients treated with DCV + ASV and 132 (97.8%) of 135 patients treated with SOF + LDV. In the two patient groups (DCV + ASV-SVR and SOF + LDV-SVR), serum total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglycerides were measured at baseline during treatment and at 4 and 12 wk after treatment. Then, longitudinal changes in lipid profiles were analyzed.
Serum levels of TC, LDL-C, and HDL-C were significantly increased throughout the observation period in both the DCV + ASV-SVR and SOF + LDV-SVR groups. During antivirus treatment, the increases in TC and LDL-C were significantly greater in the SOF + LDV-SVR group than in the DCV + ASV-SVR group (P < 0.001). At 4 and 12 wk after the therapy, serum levels of TC and LDL-C were similar between the two groups and were significantly greater than those at baseline. Approximately 75%-80% of the increase in TC was derived from an increased LDL-C. In multiple regression analysis, the difference in therapy protocol (DCA + ASV or SOF + LDV) was an independent predictor that was significantly associated with the increase in TC and LDL-C at 4 wk of therapy.
Serum cholesterol significantly increased during SOF + LDV treatment. After treatment, HCV elimination was associated with a similar increase in cholesterol regardless of the therapy protocol.
Core tip: Hepatitis C virus genotype 1b (HCV1b) infection is associated with a decrease in serum cholesterol. However, little is known about the changes in lipid metabolism caused by anti-HCV therapy. Changes in serum lipid profiles were examined during and after two types of interferon-free antiviral therapy for chronic HCV1b infection. Total cholesterol, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol levels increased post therapy regardless of the regimen. However, the change in cholesterol during the treatment markedly varied according to the therapy protocol. These findings indicated that each anti-HCV regimen had a unique influence on lipid profiles during treatment.
- Citation: Endo D, Satoh K, Shimada N, Hokari A, Aizawa Y. Impact of interferon-free antivirus therapy on lipid profiles in patients with chronic hepatitis C genotype 1b. World J Gastroenterol 2017; 23(13): 2355-2364
- URL: https://www.wjgnet.com/1007-9327/full/v23/i13/2355.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i13.2355
Hepatitis C virus (HCV) is one of the most harmful viruses to human health[1,2]. Chronic HCV infection causes sustained inflammation of the liver, which can progress to chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma[3,4]. Until just a few years ago, anti-HCV therapy was limited to interferon (IFN)-based regimens, which can cause severe side effects and result in an unsatisfactory cure rate[5,6]. However, several oral anti-HCV drugs (direct acting antivirals; DAAs) have been developed over the last several years[7-10]. The combination of DAAs brought a revolutionary change to anti-HCV therapy. Now, HCV can be eliminated from the infected host within 12 wk of DAA combination therapy without noticeable side effects with tremendously high probability. The sustained viral response (SVR) rate in chronic HCV genotype 1b (HCV1b) infection is now reported to be 95%-100%[10].
Several critical steps of the HCV life cycle are closely associated with lipid metabolism[11-13]. HCV particles in the peripheral blood are coated with lipids. About 108 HCV particles are assumed to be secreted from the liver into peripheral blood every 24 h[14]. This finding suggested that lipid metabolism is somewhat shifted towards the production of HCV particles. Therefore, HCV may interfere with host lipid metabolism in order to enhance the replication process.
Many studies of serum lipid profiles in chronic HCV infection reported significant decreases in serum total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C), while some reported a decrease in high-density lipoprotein cholesterol (HDL-C)[15-19]. Meanwhile, a decrease in serum triglycerides (TG) in chronic HCV infection remains an issue of debate[15]. Serum cholesterol levels in patients who achieved SVR with IFN-based therapy were reported to increase after therapy[16-18]. However, the change in cholesterol metabolism during IFN-based therapy is not easily defined because the nutritional state is worsened as a side effect of IFN-based therapy that may severely influence serum lipid levels[6]. On the contrary, IFN-free DAA combination therapy had no influence on the nutritional state of the host. Therefore, the change in serum lipid profiles during and after therapy are more clearly defined in DAA combination therapy.
A few recent reports suggested that serum cholesterol level is increased soon after initiation of sofosbuvir plus ledipasvir (SOF + LDV) therapy and is decreased from the baseline level after treatment failure[19]. However, these changes were not reported in other DAA combination regimens. Sofosbuvir is a nucleotide analog that inhibits HCV NS5B polymerase. Other DAAs inhibit the activities of NS3-4 HCV protease, the NS5A HCV replication complex, or NS5B polymerase. Changes in serum cholesterol in response to DAA combination therapy may indicate cancellation of the suppressive effect of HCV proliferation. Moreover, DAA by itself may impact lipid metabolism.
In this retrospective study, longitudinal changes in serum lipid profiles were examined in chronic HCV1b patients who were treated with a combination of daclatasvir and asunaprevir (DCA + ASV) or SOF + LDV and achieved SVR. Then, the differences in serum lipid profiles between the two regimens were clarified.
The study cohort included 276 patients with chronic infection with hepatitis C genotype 1b (HCV1b) who were treated with combination IFN-free DAAs at the Jikei University Katsushika Medical Center and Otakanomori Hospital from October 2014 to July 2016. Of 276 subjects, 141 were treated with DCV + ASV, while 135 were treated with SOF + LDV. The protocol of DCV + ASV therapy was oral administration of 60 mg of DCV (Daklinza®, Bristol-Myers-Squibb, New York, United States) once daily and 100 mg of ASV (Sunvepra®, Bristol-Myers-Squibb) twice daily for 24 wk. That of SOF + LDV was oral administration of one tablet of combination drug (Harvoni®, Gilead sciences, California, United States) containing 400 mg of SOF and 90 mg of LDV once a day for 12 wk.
The virological efficacy in the patients who completed the anti-HCV therapy regimen was determined by negativity of serum HCV RNA at 12 wk after treatment termination (SVR12). SVR12 was achieved in 121 (85.8%) of 141 patients treated with DCV + ASV and 132 (97.8%) of 135 patients treated with SOF + LDV. The patients who achieved SVR12 were categorized as DCV + ASV-SVR or SOF + LDV-SVR group and were selected as the subjects of the following study. In addition, 11 patients who completed the protocol of DCV + ASV therapy but did not achieve SVR (DCV + ASV-nSVR group) comprised the subjects of a supplemental study.
In total, for 264 patients, lipid profiles were examined in this study; coinfection of human immunodeficiency virus as well as complication of hepatocellular carcinoma or decompensated cirrhosis was not included. A cholesterol-lowering drug was given to two patients treated with DCV-ASV. Twenty five patients were given oral anti-diabetic drugs for mild/moderate type 2 diabetes mellitus (13 patients treated with DCV + ASV and 12 patients treated with SOF + LDV). History of coronary disease was found in two patients (one patient treated with DCV + ASV and another treated with SOF + LDV). At the beginning of anti-HCV therapy, hypercholesterolemia (LDL-C > 160 mg/dL) was found in three patients (one patient in DCV + ASV and two patients in SOF + LDV). In all 276 patients, addition or withdrawal of drugs that potentially influence the lipid metabolism was not performed during the study period.
The study protocol was approved by the Institutional Review Board of the Jikei University Katsushika Medical Center and conducted in compliance with the tenets of the Declaration of Helsinki 2004. A written informed consent for participation in this study was obtained from all the patients.
In this retrospective study, serum lipid profiles of the patients who achieved SVR were examined. Fasting blood samples were collected and serum lipid profiles were examined at baseline and at 4 wk (4W), 8 wk, (8W), and 12 wk (12W) during therapy. For patients in the SOF + LDV-SVR group, 12W was the end point of therapy. Of the patients in the DCV + ASV-SVR group, serum lipid levels were measured at 24 wk (24W) of treatment (the end of DCV + ASV therapy). Thereafter, serum lipid levels of both groups were measured at 4 and 12 wk after completion of treatment (post 4 wk; P4W, post 12 wk; P12W, respectively). P12W was set as the endpoint of this study. Longitudinal changes in serum lipid profiles throughout the examination period were investigated.
In order to compare changes in serum lipids between the DCV + ASV-SVR and SOF + LDV-SVR groups, the value of baseline serum TC or LDL-C was subtracted from the value of TC or LDL-C at 4W, 8W, 12W, 24W (DCV + ASV-SVR group only), P4W, and P12W. Then, the differences between the subtracted data expressed as ΔTC or ΔLDL-C for the DCV + ASV-SVR and SOF + LDV-SVR groups were compared. Finally, the significance of the therapy regimen on the value of ΔTC, ΔLDL-C at 4W was determined by multiple linear regression analysis.
As a supplemental study, longitudinal changes in the cholesterol level of the DCV + ASV-nSVR group patients were examined in the same manner.
Demographic data, including sex, age, and basic laboratory data before therapy, were collected. The basic laboratory data included aspartate 2-oxoglutarate aminotransferase (AST), alanine 2-oxoglutarate aminotransferase (ALT), albumin (Alb), total bilirubin (TB), triglycerides (TG), prothrombin time (PT), hemoglobin (Hb), and platelet count (PLT). The fibrosis-4 index was calculated according to the following equation: [age (years) × AST (U/L)]/[PLT (104/μL) × 10 × ALT (U/L)1/2].
The quantity of HCV RNA was determined using the COBAS® Ampliprep/COBAS® TaqMan® HCV Test, v2.0 (Roche Molecular Diagnostics, Pleasanton, CA, United States). HCV RNA was examined every 4 wk until the endpoint of this study. The serum levels of TC and HDL-C were directly quantified using commercial kits (Kyowa Medex Co., Ltd., Tokyo, Japan), while LDL-C was calculated according to the Friedewald equation.
Continuous data are expressed as means ± SD. The Student’s t-test or χ2 test was used to compare data between the two groups (DCV + ASV-SVR group vs SOF + LDV-SVR group). Longitudinal changes in the same group were compared using the paired Student’s t-test. Furthermore, in order to clarify the importance of the therapy regimen on ΔTC and ΔLDL-C at 4W, significant predictors of these values at 4W were elucidated by multiple linear regression analysis. In the multiple regression analysis, ΔTC and ΔLDL-C at 4W were set as dependent variables, and baseline laboratory data, age, sex, and therapy protocol were designated as independent variables, with male assigned a value of 0, female a value of 1, DCV + ASV therapy a value of 0, and SOF + LDV therapy a value of 1. Among the baseline laboratory data, ALT and AST were strongly correlated (R2 = 0.77). Therefore, AST was omitted as a candidate independent variable. Then, the most suitable model was constructed by step-wise selection of the independent variables.
All statistical analyses were performed using STATISTICA software, version 6 (StatSoft Japan Inc. Tokyo, Japan). A two-tailed probability (P) value of < 0.1 was considered as a tendency, while P values < 0.05 were considered significant. P values less than 0.05 to 0.01 were expressed as P < 0.05, less than 0.01 to 0.001 as P < 0.01, and less than 0.001 as P < 0.001.
Patient characteristics are shown in Table 1. The mean patient age was 68.4 ± 11.8 years for the DCV + ASV-SVR group and 66.7 ± 13.1 years for the SOF + LDV-SVR group. There were 59 males (48.7%) and 62 females (51.3%) in the DCV + ASV-SVR group, and 49 males (37.1%) and 83 females (62.9%) in the SOF + LDV-SVR group. The female/male ratio tended to be higher in the SOF + LDV-SVR group, but the difference did not reach significance. In the DCV + ASV-SVR and SOF + LDV-SVR groups, the baseline levels of TC were 163.7 ± 33.3 and 171.1 ± 34.9 mg/dL, LDL-C levels were 82.8 ± 27.2 and 89.2 ± 28.5 mg/dL, HDL-C levels were 55.5 ± 18.4 and 58.7 ± 17.6 mg/dL, and TG levels were 113.4 ± 83.0 and 112 ± 58.9 mg/dL, respectively.
| Therapy regimen | DCV + ASV | SOF + LDV | P value |
| Number | 121 | 132 | |
| SVR 12 | 85.8% | 97.7% | |
| Gender (M:F) | 59:62 | 49:83 | 0.075 |
| mean ± SD | mean ± SD | ||
| Age (yr) | 68.4 ± 11.8 | 66.7 ± 13.1 | 0.280 |
| AST (IU/L) | 56.4 ± 39.6 | 51.7 ± 33.3 | 0.300 |
| ALT (IU/L) | 51.9 ± 40.5 | 46.5 ± 38.3 | 0.270 |
| Hb (g/dL) | 13.3 ± 1.7 | 13.3 ± 1.7 | 0.830 |
| Plt (104/μL) | 13.9 ± 5.9 | 15.5 ± 7.0 | < 0.05 |
| Alb (g/dL) | 3.9 ± 0.53 | 4.0 ± 0.45 | < 0.05 |
| T-Bil (mg/dL) | 0.87 ± 0.57 | 0.76 ± 0.39 | 0.086 |
| PT (%) | 88.6 ± 15.6 | 90.4 ± 13.7 | 0.330 |
| TC (mg/dL) | 163.7 ± 33.3 | 171.1 ± 34.9 | 0.087 |
| TG (mg/dL) | 113.4 ± 83.0 | 112 ± 58.9 | 0.870 |
| LDL-C (mg/dL) | 82.8 ± 27.2 | 89.2 ± 28.5 | 0.068 |
| HDL-C (mg/dL) | 55.5 ± 18.4 | 58.7 ± 17.6 | 0.160 |
| HCV-RN A (log•IU/mL) | 5.9 ± 0.78 | 5.9 ± 0.87 | 0.960 |
| Fib4 index | 4.9 ± 3.3 | 4.7 ± 4.0 | 0.640 |
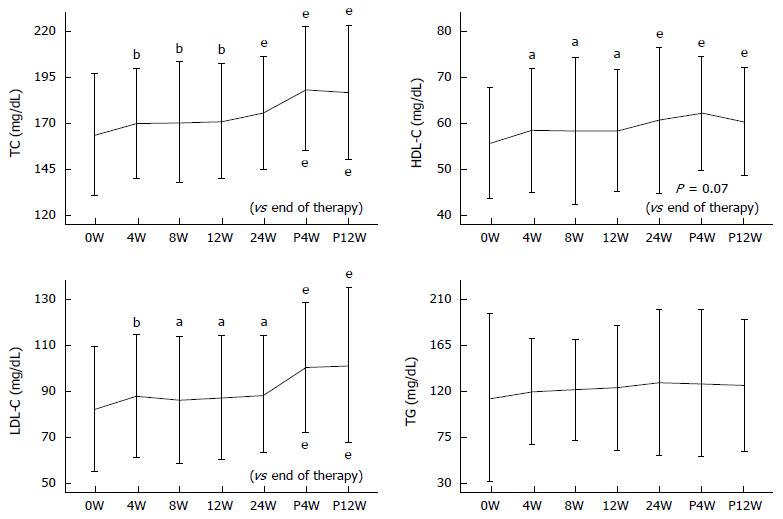
Longitudinal changes in serum TC, LDL-C, HDL-C, and TG levels in the DCV + ASV-SVR group are shown in Figure 1. Serum TC levels at 4W, 8W, 12W, 24W, P4W, and P12W were 169.67 ± 30.0, 170.55 ± 32.76, 170.93 ± 31.52, 175.38 ± 30.68, 188.77 ± 33.65, and 186.83 ± 36.7 mg/dL, respectively. TC was significantly increased throughout the observation period. A further significant increase in TC at P4W and P12W, as compared to that at the end of therapy, was a feature of DCV + ASV-SVR group.
Longitudinal changes in LDL-C were similar to those of TC. Serum LDL-C levels at 4W, 8W, 12W, 24W, P4W, and P12W were 88.26 ± 26.7, 86.67 ± 27.56, 87.8 ± 27.08, 89.06 ± 25.47, 100.95 ± 28.33, and 101.71 ± 33.87 mg/dL, respectively. Serum LDL-C markedly increased after termination of therapy.
HDL-C levels were significantly increased throughout the treatment period. However, no further increase after the end of therapy was observed. The serum levels of HDL-C at 4W, 8W, 12W, 24W, P4W, and P12W were 58.42 ± 18.24, 58.18 ± 18.12, 58.34 ± 17.41, 60.56 ± 17.82, 62.11 ± 18.37, and 60.46 ± 16.97 mg/dL, respectively. Meanwhile, there was no significant change in serum TG levels.
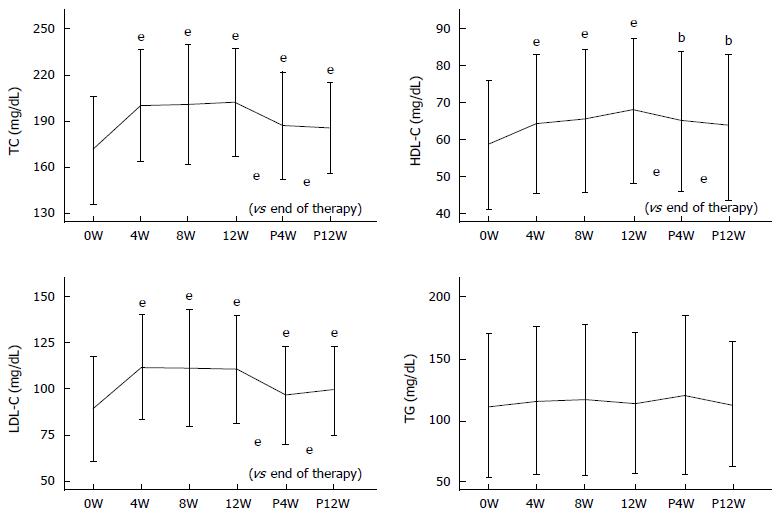
Longitudinal changes in serum TC, LDL-C, HDL-C, and TG levels in the SOF + LDV-SVR group are shown in Figure 2. Serum TC levels at 4W, 8W, 12W, P4W, and P12W were 200.07 ± 36.18, 200.71 ± 39.08, 202.2 ± 35.28, 187.11 ± 35.02, and 185.57 ± 29.54 mg/dL, respectively. A significant increase in TC was observed throughout the examination period (P < 0.001). TC was markedly increased from the early stage of therapy, which lasted until the end of therapy. Then, the TC level was sharply decreased after termination of therapy (P < 0.001).
Changes in LDL-C were quite similar to those of TC. Serum LDL-C levels at 4W, 8W, 12W, P4W, and P12W were 112.12 ± 28.6, 111.54 ± 31.77, 111.0 ± 29.38, 96.51 ± 26.55, and 99.58 ± 24.18 mg/dL, respectively. Throughout the examination period, the increase in LDL-C, as compared to baseline levels, was significant (P < 0.001). Then, levels significantly decreased after therapy, as compared to the end of therapy (P < 0.001).
Similarly, serum HDL-C was significantly increased throughout the examination period. Serum HDL-C levels at 4W, 8W, 12W, P4W, and P12W were 64.35 ± 18.66, 65.47 ± 19.31, 67.91 ± 19.54, 65.19 ± 19.18, and 63.69 ± 19.59 mg/dL, respectively. After the end of therapy, HDL-C levels were decreased, as compared to during therapy (P < 0.001). On the contrary, there were no significant changes in serum TG levels.
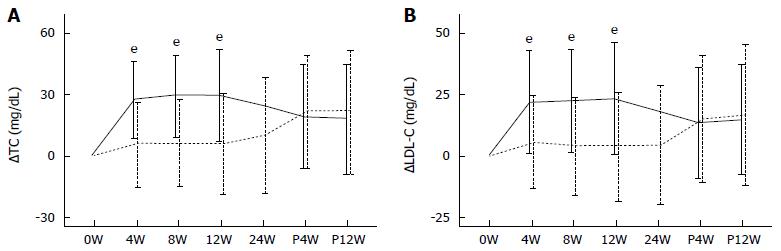
In the DCV + ASV-SVR group, ΔTC values at 4W, 8W, 12W, 24W, P4W, and P12W were 6.03 ± 20.59, 6.12 ± 20.55, 6.07 ± 23.79, 10.11 ± 27.79, 21.73 ± 27.47, and 22.01 ± 29.76 mg/dL, respectively. In the SOF + LDV-SVR group, ΔTC values at 4W, 8W, 12W, P4W, and P12W were 27.37 ± 18.59, 29.36 ± 19.9, 29.5 ± 22.35, 19.43 ± 25.17, and 18.52 ± 25.9 mg/dL, respectively. ΔTC was constantly higher in the SOF + LDV-SVR group than in the DCV + ASV-SVR group during treatment. At the time of therapy termination, ΔTC was still significantly higher in SOF + LDV-SVR group (P < 0.001), whereas post-treatment ΔTC values were similar between the groups (Figure 3A).
The ΔLDL-C values in the DCV + ASV-SVR group at 4W, 8W, 12W, 24W, P4W, and P12W were 5.5 ± 18.73, 4.05 ± 19.49, 4.08 ± 21.85, 4.44 ± 23.66, 14.82 ± 25.39, and 16.79 ± 28.51 mg/dL, respectively. In the SOF + LDV-SVR group, the ΔLDL-C values at 4W, 8W, 12W, P4W, and P12W were 21.76 ± 20.37, 22.03 ± 20.25, 23.09 ± 22.1, 13.41 ± 22.25, and 15.1 ± 21.43 mg/dL, respectively. The increase in LDL-C during treatment was significantly greater in the SOF + LDV-SVR group than in the DCV + ASV-SVR group. At the time of therapy termination, ΔLDL-C in SOF + LDV-SVR group was still significantly higher than that in DCV + ASV-SVR group (P < 0.001). However, the increase in LDL-C after the end of treatment was similar between the groups (Figure 3B).
The independent factors significantly affecting ΔTC at 4W were therapy protocol, baseline ALT, baseline quantity of HCV RNA, and baseline albumin. The corrected R2 in this model was 29.0% (Table 2). The most suitable regression equation was as follows: ΔTC at 4W (mg/mL) = 19.79 + 22.44 (therapy protocol: DCV + ASV, 0; SOF + LDV, 1) - 0.065 ALT (IU/L) + 4.54 HCV RNA (log•IU/mL) - 9.63 albumin (g/dL).
| B | SE | P value | |
| ΔTC at 4W | |||
| Constant | 19.79 | 12.10 | 0.103 |
| Therapy protocol (DCV + ASV: 0, SOF + LDV:1) | 22.44 | 2.45 | < 0.001 |
| ALT | -0.065 | 0.03 | 0.033 |
| HCV-RNA | 4.540 | 1.50 | 0.003 |
| Alb | -9.630 | 2.53 | < 0.001 |
| ΔLDL-C at 4W | |||
| Constant | 49.38 | 11.32 | < 0.001 |
| Therapy protocol (DCV + ASV: 0, SOF + LDV:1) | 17.5 | 2.40 | < 0.001 |
| Alb | -10.67 | 2.68 | < 0.001 |
| T-Bil | -12.17 | 2.82 | < 0.001 |
| TG | 0.063 | 0.016 | < 0.001 |
The significant variables of LDL-C were therapy protocol, baseline albumin, baseline TB, and baseline TG. The corrected R2 in this model was 26.7% (Table 2). The most suitable regression equation was as follows: ΔLDL-C at 4W (mg/mL) = 49.38 + 17.5 (therapy protocol: DCV + ASV, 0; SOF + LDV, 1) - 10.67 albumin (g/dL) - 12.17 TB (mg/mL) + 0.063 TG (mg/mL).
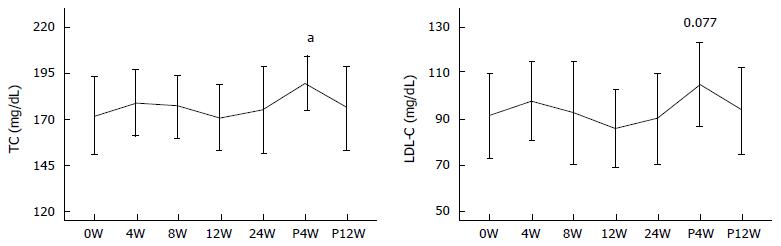
There were four males and seven females in this group. The age, HCV RNA, TC, and LDL-C in this group were 71.7 ± 7.0 years, 6.6 ± 0.27 log•IU/mL, 172.2 ± 21.1 mg/dL and 91.6 ± 18.5 mg/dL, respectively.
In all the patients, serum HCV RNA disappeared till 8W of therapy, and negativity of serum HCV RNA persisted till the end of therapy except in two patients in whom HCV RNA reappeared at 20W of therapy. The serum TC levels at 4W, 8W, 12W, 24W, P4W, and P12W were 179.1 ± 17.73, 177.27 ± 16.76, 170.91 ± 17.6, 175.27 ± 23.56, 189.82 ± 14.63 and 176.18 ± 22.4 mg/dL, respectively. Significant changes in TC levels were not observed except for a transient increase at P4W (P = 0.021). Serum LDL-C levels at 4W, 8W, 12W, 24W, P4W, and P12W were 97.55 ± 16.97, 92.6 ± 22.49, 85.75 ± 17.02, 89.84 ± 19.58, 104.82 ± 18.01 and 93.65 ± 18.89 mg/dL, respectively. Although significant changes in LDL-C levels were not observed, LDL-C tended to increase transiently at P4W (Figure 4).
In this study, we observed the impact of IFN-free antiviral regimens on lipid metabolism as indicated by changes in serum cholesterol in patients with chronic HCV1b infection. Comparisons of serum lipid profiles after treatment showed similar increases in TC and LDL-C levels between the DCV + ASV-SVR and SOF + LDV-SVR groups. The increase in TC at P12W was 22.01 mg/dL in the DCV + ASV-SVR group and 18.52 mg/dL in the SOF + LDV-SVR group. Similar increases in TC levels of patients who achieved SVR with IFN-based therapy were reported[16-18]. Thus, an increase in TC of about 20 mg/dL was considered to be derived by clearance of HCV, regardless of the therapy regimen[16-18]. It is well known that lipid metabolism is disrupted by chronic HCV infection[20,21]. Therefore, cancellation of the suppressive effect of chronic HCV infection on lipid metabolism may have brought about the increase in TC of 20 mg/dL[16-18]. Additionally, these data indicated LDL-C was the major contributor (75%-80%) to the increase in TC. This finding suggested that the increase in cholesterol in this situation is considered to be primarily caused by an increase in LDL-C.
Meanwhile, the increases in cholesterol levels during treatment were dependent on the treatment regimen[22]. Although the increases in TC and LDL-C levels were features of both DCV + ASV and SOF + LDV treatment, the increase in cholesterol during treatment was much greater in the SOF + LDV-SVR group than in the DCV + ASV-SVR group[22]. The differences in the increase in cholesterol between DCV + ASV-SVR and SOF + LDV-SVR groups may not be simply explained by cancellation of disturbed lipid metabolism caused by HCV. In our patients, rapid virological response (no serum HCV RNA detected) rate at 4W was 89.3% in the DCV + ASV-SVR group and 87.9% in the SOF + LDV-SVR group. There was no difference in the rapid virological response rate between groups. Serum HCV RNA at 8W was not detected in any of the patients. Moreover, at the time of therapy termination, when HCV was completely abolished from hepatocytes, serum cholesterol remained significantly higher in SOF + LDV-SVR group. Therefore, the difference in anti-viral efficacy between the two regimens was not likely to be involved in the extent of the increase in serum cholesterol.
Interestingly, the behavior of serum cholesterol differed between the SOF + LDV-SVR and DCV + ASV-SVR groups. The increases in TC and LDL-C levels were slight during the therapy in the DCV + ASV-SVR group. Then, further increases in TC and LDL-C levels were observed after therapy termination at P4W and P12W. This finding suggested that DCV + ASV therapy somewhat inhibited the increase in serum cholesterol. In contrast, TC and LDL-C levels were greatly increased from the early stage of SOF + LDV therapy till the end of treatment. Thereafter, TC and LDL-C levels rapidly decreased. Therefore, SOF-LDV is believed to accelerate the increase in serum cholesterol. After therapy termination, similar degree of increase in cholesterol was observed regardless of the DAA regimen.
These findings suggested that elimination of HCV was not the sole factor contributing to the changes in serum cholesterol during DAA therapy. Instead, DAAs by themselves may have pharmacological actions on lipid metabolism[22]. This hypothesis that DAAs have pharmacological effects on lipid metabolism was strengthened by the changes in the serum cholesterol in DCV + ASV-nSVR group. In this group, serum cholesterol did not increase during the therapy, but it transiently increased at P4W. The behavior of serum cholesterol in this group differed from that in DCV + ASV-SVR group. This finding further suggested that DCV + ASV therapy suppressed the increase in serum cholesterol during therapy because transient increase in cholesterol was evoked despite replication of activated HCV after removal of DCV + ASV. However, relatively small number of patients in the DCV + ASV-nSVR group may limit the value of this supplemental study.
Using multiple linear regression analysis of factors contributing to the degree of the increase in TC and LDL-C levels at 4W of therapy, the choice of therapy regimen was elucidated as a strong contributing factor to the increase in TC and LDL-C levels. Thus, selection of the therapy regimen had a deep impact on the change in serum cholesterol during anti-HCV treatment. As mentioned above, the therapy regimen (DCV + ASV vs SOF + LDV) was not associated with the difference in final virological efficacy in these patients because we selected only the SVR patients in this retrospective study. From this finding, we concluded that SOF + LDV therapy strongly induced an increase in serum cholesterol, especially LDL-C, while the pharmacological effect of DCV + ASV may result in a suppression of increase in serum cholesterol. In this study, no further change in cholesterol was observed in the mid or late stage of SOF + LDV or DCV + ASV therapy. This finding may indicate that there was a limitation of SOF + LDV or DCV + ASV action on serum cholesterol.
Although the mechanisms underlying the opposite effects of SOF + LDV and DCV + ASV on serum cholesterol levels remain unclear, there is a difference in the inhibitory manner on HCV proliferation. Both DCV and LDV are inhibitors of the NS5A protein[23,24]; ASV is an inhibitor of the NS3/4A protease[25], while SOF is a nucleotide analog that inhibits the activity of NS5B polymerase[26,27], which might affect the viral kinetics during the very early phase of treatment in different way. In addition, the potency of each drug exerts different inhibitory effects. SOF has been used as a backbone in many regimens and has shown to be stronger in viral inhibition. Therefore, SOF-LDV had shorter treatment time with better efficacy.
The viral kinetic of DCV was biphasic, with decline phase of HCV starting from the first 12 h of the treatment. In the second phase, decline of HCV slowed down[28]. However, more prominent suppression of HCV could be expected at 2 d of therapy in SOF-combination. Thus, the potency of SOF could rapidly eradicate HCV. This may be related to prominent increase in the cholesterol in SOF + LDV therapy because with earlier viral reduction, a better reduction of hepatic inflammation could be seen in SOF based combination therapy[29,30]. As cholesterol is an indicator of liver function, cholesterol levels were decreased in HCV-related chronic hepatitis. Therefore, the more prominent increase in serum cholesterol in the SOF + LDV-SVR group might represent faster improvement in liver pathology or inflammation in the SOF + LDV-SVR group than in the DCV + ASV-SVR group. This hypothesis is particularly well applied for the differences in the cholesterol between the early stage of SOF + LDV and DCV + ASV therapy.
However, increase in cholesterol was still significantly higher at the time of therapy termination in the SOF + LDV-SVR group than in the DCV + ASV-SVR group. In addition, using this mechanism, it might be difficult to explain the transient elevation in cholesterol shortly after the therapy termination in the DCV + ASV-nSVR group. These findings suggested that there might be novel pharmacological effects of DAAs on the cholesterol metabolism. This issue remains to be solved in the future.
Hashimoto et al[22] recently examined factors affecting the increase in LDL-C in the early stage of DCV + ASV or SOF + LDV treatment and reported that factors promoting an increase in LDL-C of 25 mg/dL or more at 4 wk of therapy were therapy regimen (SOF + LDV) and change in the quantity of the HCV core antigen. The greater the decrease in the amount of HCV core antigen, the greater the increase in LDL-C.
In the present study, factors promoting an increase in TC at 4W were therapy regimen (SOF + LDV) and baseline quantity of HCV RNA. As HCV RNA was not detected in the majority of patients, the decrease in HCV RNA at 4W was approximately the same as the baseline quantity of HCV RNA. Therefore, our data was quite similar to those reported by Hashimoto et al[22]. However, factors positively affecting the increase in LDL-C were therapy regimen (SOF + LDV) and baseline TG, but not baseline quantity of HCV RNA. The difference between the precedent study and our study may be due to differences in patient classification, methods to quantify HCV activity, or the statistical methods used to evaluate the factors affecting the increase in LDL-C.
In summary, the increase in serum cholesterol was different between the SOF + LDV and DCV + ASV therapies. SOF + LDV therapy strongly increased cholesterol, while DCV + ASV may have suppressive effect on increase in cholesterol. The potency of eradication of HCV with SOF + LDV may be associated with more prominent increase in cholesterol during treatment. In addition, SOF + LDV or DCV + ASV therapy might have its own pharmacological effects on serum cholesterol during the process of HCV eradication. In order to clarify the mechanisms of DAAs in serum cholesterol, further investigations are required.
In conclusion, SOF + LDV and DCV + ASV therapies had different influences on serum cholesterol levels.
We thank the faculty and staff of Jikei University Katsushika Medical Center for excellent assistance.
Hepatitis C virus (HCV) infection is considered to alter host lipid metabolism. With chronic HCV infection, serum cholesterol is decreased, while clearance of HCV with anti-viral therapy results in a considerable increase in serum cholesterol. However, the change in serum cholesterol during anti-viral treatment was not fully examined.
We investigated the longitudinal changes in serum lipid profiles during and after daclatasvir plus asunaprevir (DCV + ASV) and sofosbuvir plus ledipasvir (SOF + LDV) treatment in patients with chronic infection with hepatitis C genotype 1b. The change in serum lipids with these therapies has not been extensively studied.
The authors confirm the differential action of SOF + LDV from DCV + ASV therapy on serum levels of total cholesterol, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol in patients with chronic HCV infection who were treated with SOF + LDV or DCV + ASV.
This approach can be applied to evaluate the action of anti-HCV regimens other than DCV + ASV or SOF + LDV in chronic HCV infection caused by HCV genotypes other than 1b.
DAAs (direct-acting antivirals) are oral anti-HCV drugs that specifically inhibit critical steps in the HCV reproductive process.
The findings of this study should be useful to further elucidate the significance of the anti-HCV therapy regimen on the alteration of serum lipid profiles in the process of HCV elimination.
| 1. | Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36:S35-S46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 442] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 2. | Hoofnagle JH. Course and outcome of hepatitis C. Hepatology. 2002;36:S21-S29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 372] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 3. | Casiraghi MA, De Paschale M, Romanò L, Biffi R, Assi A, Binelli G, Zanetti AR. Long-term outcome (35 years) of hepatitis C after acquisition of infection through mini transfusions of blood given at birth. Hepatology. 2004;39:90-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 107] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3249] [Cited by in RCA: 3630] [Article Influence: 259.3] [Reference Citation Analysis (12)] |
| 5. | Raison CL, Demetrashvili M, Capuron L, Miller AH. Neuropsychiatric adverse effects of interferon-alpha: recognition and management. CNS Drugs. 2005;19:105-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 343] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 6. | Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, Miller AH. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 567] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 7. | Omata M, Nishiguchi S, Ueno Y, Mochizuki H, Izumi N, Ikeda F, Toyoda H, Yokosuka O, Nirei K, Genda T. Sofosbuvir plus ribavirin in Japanese patients with chronic genotype 2 HCV infection: an open-label, phase 3 trial. J Viral Hepat. 2014;21:762-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 165] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 8. | Kumada H, Chayama K, Rodrigues L, Suzuki F, Ikeda K, Toyoda H, Sato K, Karino Y, Matsuzaki Y, Kioka K. Randomized phase 3 trial of ombitasvir/paritaprevir/ritonavir for hepatitis C virus genotype 1b-infected Japanese patients with or without cirrhosis. Hepatology. 2015;62:1037-1046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 9. | Kumada H, Suzuki Y, Ikeda K, Toyota J, Karino Y, Chayama K, Kawakami Y, Ido A, Yamamoto K, Takaguchi K. Daclatasvir plus asunaprevir for chronic HCV genotype 1b infection. Hepatology. 2014;59:2083-2091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 468] [Cited by in RCA: 453] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 10. | Mizokami M, Yokosuka O, Takehara T, Sakamoto N, Korenaga M, Mochizuki H, Nakane K, Enomoto H, Ikeda F, Yanase M. Ledipasvir and sofosbuvir fixed-dose combination with and without ribavirin for 12 weeks in treatment-naive and previously treated Japanese patients with genotype 1 hepatitis C: an open-label, randomised, phase 3 trial. Lancet Infect Dis. 2015;15:645-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 303] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 11. | Syed GH, Amako Y, Siddiqui A. Hepatitis C virus hijacks host lipid metabolism. Trends Endocrinol Metab. 2010;21:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 293] [Article Influence: 18.3] [Reference Citation Analysis (4)] |
| 12. | Nakamuta M, Yada R, Fujino T, Yada M, Higuchi N, Tanaka M, Miyazaki M, Kohjima M, Kato M, Yoshimoto T. Changes in the expression of cholesterol metabolism-associated genes in HCV-infected liver: a novel target for therapy? Int J Mol Med. 2009;24:825-828. [PubMed] |
| 13. | Miyanari Y, Atsuzawa K, Usuda N, Watashi K, Hishiki T, Zayas M, Bartenschlager R, Wakita T, Hijikata M, Shimotohno K. The lipid droplet is an important organelle for hepatitis C virus production. Nat Cell Biol. 2007;9:1089-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 937] [Cited by in RCA: 994] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 14. | Aizawa Y, Seki N, Nagano T, Abe H. Chronic hepatitis C virus infection and lipoprotein metabolism. World J Gastroenterol. 2015;21:10299-10313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Dai CY, Chuang WL, Ho CK, Hsieh MY, Huang JF, Lee LP, Hou NJ, Lin ZY, Chen SC, Hsieh MY. Associations between hepatitis C viremia and low serum triglyceride and cholesterol levels: a community-based study. J Hepatol. 2008;49:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 114] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Jang ES, Won JE, Jung JI, Lee SH, Kim JW, Jeong SH. The effect of antiviral therapy on serum cholesterol levels in chronic hepatitis C. Gut Liver. 2011;5:356-362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Kuo YH, Chuang TW, Hung CH, Chen CH, Wang JH, Hu TH, Lu SN, Lee CM. Reversal of hypolipidemia in chronic hepatitis C patients after successful antiviral therapy. J Formos Med Assoc. 2011;110:363-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Corey KE, Kane E, Munroe C, Barlow LL, Zheng H, Chung RT. Hepatitis C virus infection and its clearance alter circulating lipids: implications for long-term follow-up. Hepatology. 2009;50:1030-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 155] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 19. | Meissner EG, Lee YJ, Osinusi A, Sims Z, Qin J, Sturdevant D, McHutchison J, Subramanian M, Sampson M, Naggie S. Effect of sofosbuvir and ribavirin treatment on peripheral and hepatic lipid metabolism in chronic hepatitis C virus, genotype 1-infected patients. Hepatology. 2015;61:790-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 121] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 20. | Negro F, Sanyal AJ. Hepatitis C virus, steatosis and lipid abnormalities: clinical and pathogenic data. Liver Int. 2009;29 Suppl 2:26-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 134] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 21. | Lerat H, Higgs M, Pawlotsky JM. Animal models in the study of hepatitis C virus-associated liver pathologies. Expert Rev Gastroenterol Hepatol. 2011;5:341-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Hashimoto S, Yatsuhashi H, Abiru S, Yamasaki K, Komori A, Nagaoka S, Saeki A, Uchida S, Bekki S, Kugiyama Y. Rapid Increase in Serum Low-Density Lipoprotein Cholesterol Concentration during Hepatitis C Interferon-Free Treatment. PLoS One. 2016;11:e0163644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 23. | Gao M, Nettles RE, Belema M, Snyder LB, Nguyen VN, Fridell RA, Serrano-Wu MH, Langley DR, Sun JH, O’Boyle DR. Chemical genetics strategy identifies an HCV NS5A inhibitor with a potent clinical effect. Nature. 2010;465:96-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 773] [Cited by in RCA: 767] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 24. | Ivanenkov YA, Aladinskiy VA, Bushkov NA, Ayginin AA, Majouga AG, Ivachtchenko AV. Small-molecule inhibitors of hepatitis C virus (HCV) non-structural protein 5A (NS5A): a patent review (2010-2015). Expert Opin Ther Pat. 2017;27:401-414. [PubMed] |
| 25. | McPhee F, Sheaffer AK, Friborg J, Hernandez D, Falk P, Zhai G, Levine S, Chaniewski S, Yu F, Barry D. Preclinical Profile and Characterization of the Hepatitis C Virus NS3 Protease Inhibitor Asunaprevir (BMS-650032). Antimicrob Agents Chemother. 2012;56:5387-5396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 164] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 26. | Saigo K, Ozaki S, Ueda K, Ryo R, Tatsumi E, Yamaguchi N. [An aged patient with acute myelogenous leukemia complicated with liver cirrhosis: successful treatment with low-dose cytosine arabinoside]. Gan To Kagaku Ryoho. 1989;16:2441-2444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Tatum H, Thuluvath PJ, Lawitz E, Martorell C, DeMicco M, Cohen S, Rustgi V, Ravendhran N, Ghalib R, Hanson J. A randomized, placebo-controlled study of the NS5B inhibitor beclabuvir with peginterferon/ribavirin for HCV genotype 1. J Viral Hepat. 2015;22:658-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Chatterjee A, Smith PF, Perelson AS. Hepatitis C viral kinetics: the past, present, and future. Clin Liver Dis. 2013;17:13-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Dahari H, Canini L, Graw F, Uprichard SL, Araújo ES, Penaranda G, Coquet E, Chiche L, Riso A, Renou C. HCV kinetic and modeling analyses indicate similar time to cure among sofosbuvir combination regimens with daclatasvir, simeprevir or ledipasvir. J Hepatol. 2016;64:1232-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (3)] |
| 30. | Lau KG, Benhamou Y. Complete cure after three weeks of all-oral triple-direct acting antiviral (DAA) regimens in non-dnhotic chronic hepatitis C genceype 1 b ChlnaH subjects (SODAPI STUDY). Hepatology. 2015;6:1394A. |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Bunchorntavakul C, Komolmit P, Tanaka Y S- Editor: Qi Y L- Editor: A E- Editor: Wang CH