Published online Apr 7, 2017. doi: 10.3748/wjg.v23.i13.2337
Peer-review started: October 12, 2016
First decision: December 2, 2016
Revised: January 9, 2017
Accepted: February 8, 2017
Article in press: February 8, 2017
Published online: April 7, 2017
Processing time: 178 Days and 7.3 Hours
To determine the potential roles of CD4 and microRNA (miR)-145 in gastric cancer.
The levels of CD44 and miR-145 were determined in gastric cancer cells. Quantitative real-time polymerase chain reaction was used to measure to the level of CD44 mRNA. A luciferase reporter assay and western blotting were performed to examine the effect of miR-145 on CD44 expression. Tumor sphere and MTT assays were carried out to evaluate the self-renewal and chemo-resistance properties of gastric cancer cells.
The expression of CD44 was greatly increased and miR-145 was decreased in gastric cancer cells that were highly enriched in cancer stem cells (CSCs). The results demonstrated that miR-145 regulated CD44 by targeting directly the CD44 3’-untranslated region (3’-UTR). In gastric cancer cells, overexpression of miR-145 repressed the activity of the CD44 3’-UTR, and disruption of miR-145/CD44 3’-UTR interactions abrogated the silencing effects. In addition, miR-145 inhibition stimulated CD44 3’-UTR activity and disruption of miR-145/CD44 3’-UTR interactions abrogated this stimulatory effect. Enforced CD44 expression greatly increased tumor sphere formation and chemo-resistance in gastric cancer cells. Furthermore, the inhibition of CSCs and the chemo-sensitivity of gastric cancer cells treated with miR-145 were significantly abrogated by overexpression of CD44.
miR-145 targeting of CD44 plays critical roles in the regulation of tumor growth and chemo-resistance in gastric cancer.
Core tip: The levels of CD44 and miR-145 are related strongly to stemness properties in gastric cancer. The aim of this investigation was to determine the underlying molecular mechanism involved in this relationship. The findings demonstrated that miR-145 regulates CD44 expression by directly targeting its 3’-untranslated region, which might play a critical role in the regulation of tumor growth and chemo-resistance in gastric cancer.
- Citation: Zeng JF, Ma XQ, Wang LP, Wang W. MicroRNA-145 exerts tumor-suppressive and chemo-resistance lowering effects by targeting CD44 in gastric cancer. World J Gastroenterol 2017; 23(13): 2337-2345
- URL: https://www.wjgnet.com/1007-9327/full/v23/i13/2337.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i13.2337
Despite advances in medical technology to improve gastric cancer outcome, gastric cancer remains the fourth most common cancer worldwide[1]. The 5-year survival rate in gastric cancer patients is still less than 35%, and it remains the third leading cause of cancer-related death[1,2]. Seventy percent of gastric cancer-related deaths occur in developing countries, with approximately 40% occurring in China[3]. In China, this low survival rate is mainly the result of the disappointing early detection rate, tumor recurrence, and high chemotherapy resistance[4]. Accumulating evidence indicates that a subset of cancer cells with high self-renewal and stemness properties, known as cancer stem cells (CSCs), are the key contributors to chemo-resistance, and are responsible for tumor progression and recurrence after conventional therapy[5].
CD44, an integral cell membrane glycoprotein, was identified initially as a lymphocyte homing receptor on circulating lymphocytes, and exhibits homing, adhesion, and migration functions[6,7]. CD44 participates in a wide variety of cellular functions, including lymphocyte activation, recirculation and homing, hematopoiesis, and tumor metastasis[8]. The protein is not only involved in cell-cell adhesion, cell-matrix interactions, and tumor survival, but also has been accepted as a CSC marker for gastric cancer in many studies[9]. CD44 expression is upregulated in advanced gastric lesions[10]. Depletion of CD44 inhibited the stem cell-like properties, which was accompanied by the downregulation of Oct4[10]. Conversely, CD44+ gastric cancer cells showed the stem cell properties of self-renewal and the ability to form differentiated progeny[11,12]. CD44 is highly polymorphic, possesses a number of alternative splice variants, and undergoes extensive post-translational modifications[13].
MicroRNAs (miRNA) are noncoding small RNAs that function as a crucial post-transcriptional regulatory mechanism for various cellular functions. Emerging data indicate that miRNAs play pivotal roles in regulating most biological processes in both normal development and in various diseases, including cancer[14]. They act as cancer signatures, oncogenes, or tumor suppressors by targeting their downstream targets. MiRNAs are also involved in many aspects of gastric cancer progression[15]. Multiple miRNAs have been implicated in the pathogenesis of gastric cancer. For example, Petrocca et al[16] demonstrated that the miR-106b-25 cluster is involved in E2F1 post-transcription in the development of TGFβ resistance gastric cancer positively. In addition, Li et al[17] reported that miR-25 regulates gastric cancer cell migration, invasion, and proliferation positively by targeting transducer of epidermal growth factor receptor 2, 1 (EGFR2, 1) directly. Furthermore, miR-20a and miR-17 were shown to be upregulated in gastric cancer tissues[18]. miR-21-5p was also identified a useful predictor of recurrence in early gastric cancer[19].
miR-145, a tumor-suppressive miRNA, is associated with tumor growth and metastasis in several types of cancer. Recently, Chen et al[20] showed that miR-145 regulates cell migration and invasion in gastric cancer primarily by targeting fascin actin-bundling protein 1 (FSCN1) directly. Furthermore, miR-145 regulates embryonic stem cell differentiation and tunes the expressions of multiple stemness genes simultaneously, including KLF4, Oct4, and Sox2[21]. However, the potential mechanism of miR-145 in gastric CSC properties and chemo-resistance is unclear. In the current study, we found that miR-145 is decreased, while the expression of CD44 is markedly increased, in gastric cancer cells with stemness properties. As a target, CD44 is regulated directly by miR-145. Overexpression of miR-145 in gastric cancer greatly inhibited gastric cancer cell stemness properties and chemo-resistance. We also found that the tumor suppressive and chemo-resistance lowering effects of miR-145 in gastric cancer cells were significantly reversed by overexpression of CD44. These findings demonstrated, for the first time, that miR-145 inhibits the stem-like properties of gastric cancer mainly by targeting CD44 directly.
The human CD44 3’-untranslated region (UTR) was amplified from MGC-803 cDNA by polymerase chain reaction (PCR) amplification using the following primer pairs: 5’-TACGAGCTCCACCTACACCATTATCTTGGAAAGA-3’ (Forward); 5’-TCAACGCGTCCAATAAGTGCTTTCAACTCAGCA-3’ (Reverse). The CD44 3’UTR was cloned downstream of the luciferase coding sequence in the pMIR-REPORT (Ambion) vector at the Sac I/Mlu I restriction sites to construct the human CD44-3’UTR-luciferase reporter. Mutations were introduced into the miRNA-binding sites using a QuikChange Mutagenesis Kit (TransGen, Beijing, China). The mutation primers were as follows: 5’-ACTTGAAAGAAAGTCGACATTAGGCCACTAT-3’ (Forward); 5’-GACTTTCTTTCAAGTTGAAAAGAAAATAAAAAG-3’ (Reverse) (mutation sites underlined). For the CD44 expression plasmids, sequences were amplified by PCR using the following primers: 5’-TACACGCGTATGGACAAGTTTTGGTGGCA-3’ (Forward); 5’-TCAGCTAGCCACCCCAATCTTCATGTCCAC-3’ (Reverse). The amplified fragment was cloned into the Mlu I /Nhe I sites in the pLV-CS 2.0.
The human gastric cancer cell line, MGC-803, was purchased from the Institute of Cell Biology (Shanghai, China, http://www.cellbank.org.cn). Cells were maintained in Roswell Park Memorial Institute-1640 (RPMI-1640) medium. All cell culture media were supplemented with 10% fetal bovine serum, and 1% penicillin-streptomycin (all from Invitrogen, Carlsbad, CA, United States).
Tumor sphere cultures were grown in ultralow attachment six-well plates (Corning, Lowell, MA, United States) using a cell suspension (500 cells/mL) in serum-free DMEM/F12 media (Invitrogen), supplemented with 20 ng/mL epidermal growth factor (EGF, Sigma-Aldrich), 4 μg/mL insulin (Sigma-Aldrich), B27 supplement (1 ×, Invitrogen), and 1% penicillin-streptomycin in a humidified incubator at 37 °C in 5% CO2.
Cells were transfected with pWT-CD44-3’UTR-luc or pMT-CD44-3’UTR-luc (WT, wild type; MT, mutant type), β-galactosidase, and miR-145 mimics, or an miR-145 inhibitor (RiboBio, Guangzhou, China) using Lipofectamine 2000 transfection reagent (Invitrogen). Luciferase activity was measured 36 h after transfection, and the transfection efficiency was normalized to internal βgalactosidase activity.
Total RNA was extracted using the TRIZOL Reagent (Invitrogen) and reverse transcribed with R-PCR Quick Master Mix (Toyoba) to produce cDNA. QPCR was performed using SYBR Green-based detection in a LightCycler®480 (Roche) according to the manufacturer’s instructions using the following primer pairs: CD44 (NM_000610.3) (Forward: 5’-CTCATGGATCTGAATCAGATGGA-3’, Reverse: 5’-ACTGCAATGCAAACTGCAAGA-3’); GAPDH (glyceraldehyde-3 phosphate dehydrogenase, NM_001289745.1) (Forward: 5’-TCTCCTCTGACTTCAACAGCGA-3’, Reverse: 5’-GTCCACCACCCTGTTGCTGT-3’). GAPDH levels were used as normalization controls.
The MTT assay (Cell titer 96® Aqueous One Solution Cell Proliferation Assay, Promega) was used to assess the rates of resistance to drugs. Briefly, MGC-803 cells were transfected with or without miR-145 or/and CD44, and after 12 h of transfection the gastric cancer cells (2 × 103/well) were seeded in 96-well plates. The cells were then treated with the indicated concentration of chemotherapeutic drugs [5-FU (5-Fluorouracil, Sigma-Aldrich) and cisplatin (Sigma-Aldrich)]. The MTT assay was performed 72 h later using the iMarkmicroplate Absorbance Reader (Bio-RAD, Richmond, CA, United States), according to the manufacturer’s instructions.
Western blots were performed according to previously described protocols[22]. The Immobilon Western Chemiluminescent HRP Substrate Kit (Millipore) was used to evaluate the results. The primary antibodies were CD44 (Abcam, Cambridge, 1:3000), and β-actin (Sigma-Aldrich, 1:5000).
Results are expressed as the mean ± SEM. Statistical significance was determined by Student’s t-test or a one-way or two-way analysis of variance followed by Tukey’s test, as appropriate, using Graphpad Prism statistical software (Graphpad Software). P < 0.05 was considered statistically significant.
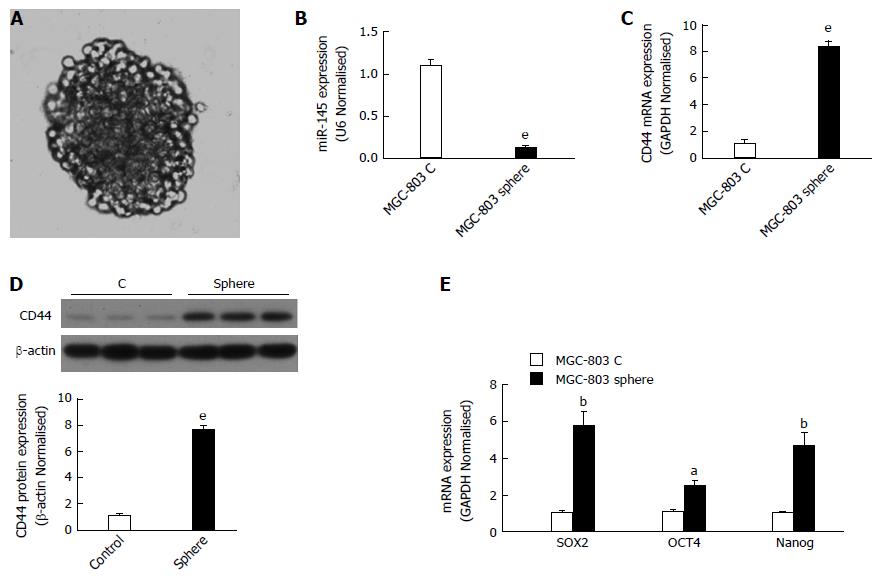
The tumor sphere assay has been used widely to identity stem cells in vitro. Tumor spheres of MGC-803 cells were cultured as described in the Materials and Methods section. Tumor spheres with a tight appearance were observed in serum-free medium (Figure 1). To investigate the function of miR-145 and CD44 in gastric cancer, we first determined the expressions of miR-145 and CD44 in monolayer MGC-803 cells and MGC-803 spheres using qPCR. The results showed that miR-145 expression was significantly inhibited in MGC-803 spheres compared with monolayer MGC-803 cells (by 87.9%, Figure 1B, P < 0.001). In addition, the spheres expressed much higher levels of CD44 mRNA and protein than the monolayer cells. When calculated as fold changes relative to the monolayer MGC-803 cells, CD44 mRNA and protein expression levels increased by approximately 8-fold and 7-fold, respectively (Figure 1C and D, P < 0.001). Moreover, the results showed that the expression of other CSC markers, such as Sox2, Nanog, and Oct4, increased significantly in sphere cells (Figure 1E, P < 0.05, P < 0.01).
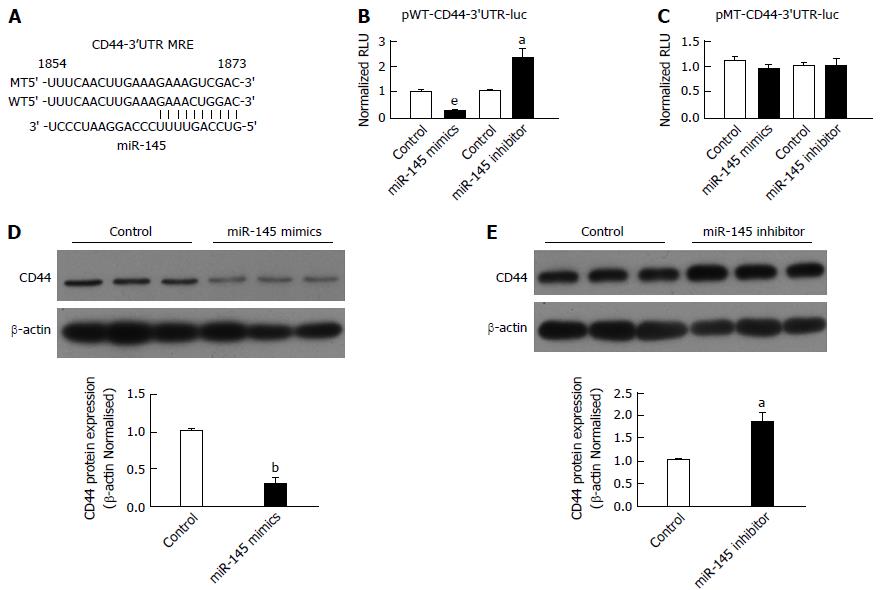
The above results indicated an inverse relationship between the expression of miR-145 and CD44. The prediction of miRNA-recognition element (MRE) sites for miR-145 on the CD44-3’ UTR was performed using the TargetScan (http://www.targetscan.org) algorithms (Figure 2A). To determine whether miR-145 regulated CD44 through an interaction with the CD44-3’ UTR, we first co-transfected chemically synthesized miR-145 mimics or a miR-145 inhibitor with luciferase reporter pWT-CD44-3’ UTR in MGC-803 cells. Transfection of the miR-145 mimics reduced the CD44-3’ UTR activity significantly (Figure 2B, P < 0.001). Conversely, inhibition of miR-145 resulted in a significant increase in CD44-3’ UTR activity (Figure 2B, P < 0.05). To verify the specificity of the interactions, we mutated the MRE site for miR-145 on the CD44-3’ UTR. Using the mutant, we demonstrated that mutation of the MRE for miR-145 abrogated the regulatory effects of the miR-145 mimics or miR-145 inhibitor (Figure 2C). To further evaluate the regulation of miR-145 on CD44 expression, we transfected miR-145 mimics in MGC-803 cells. Consistent with the results from the luciferase reporter assay, CD44 expression was downregulated by 71.74% in MGC-803 cells transfected with miR-145 mimics (Figure 2D, P < 0.01). In contrast, miR-145 inhibition resulted in significant increases in CD44 expression (Figure 2D, P < 0.05). Take together, these results showed that miR-145 regulated CD44 expression by targeting the CD44 3’ UTR (Figure 2).
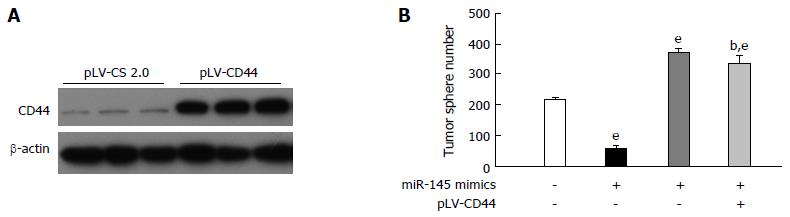
miR-145 is known to exert suppressive effects on many cancer types, including gastric cancer[23]. The tumor sphere assay was used to investigate whether repression of CD44 is necessary for miR-145 to inhibit gastric cancer cells. The plasmid pLV-CD44 was constructed and the overexpression of CD44 was confirmed (Figure 3A). The tumor sphere assay has been used widely to identify the self-renewal properties of stem cells in vitro. As expected, miR-145 mimics significantly decreased tumor sphere formation in MGC-803 cells with an efficiency of 74.74% (Figure 3B, P < 0.001). Conversely, overexpression of CD44 resulted in a significant increase in tumor sphere formation (Figure 3B, P < 0.001). Furthermore, simultaneous re-expression of CD44 compromised miR-145-suppressed tumor sphere formation in MGC-803 cells (Figure 3B, P < 0.001), to a higher level than that in the control (Figure 3B, P < 0.01). Collectively, the above results demonstrated that repression of CD44 is necessary for miR-145 to inhibit the self-renewal properties of gastric cancer cells (Figure 3).
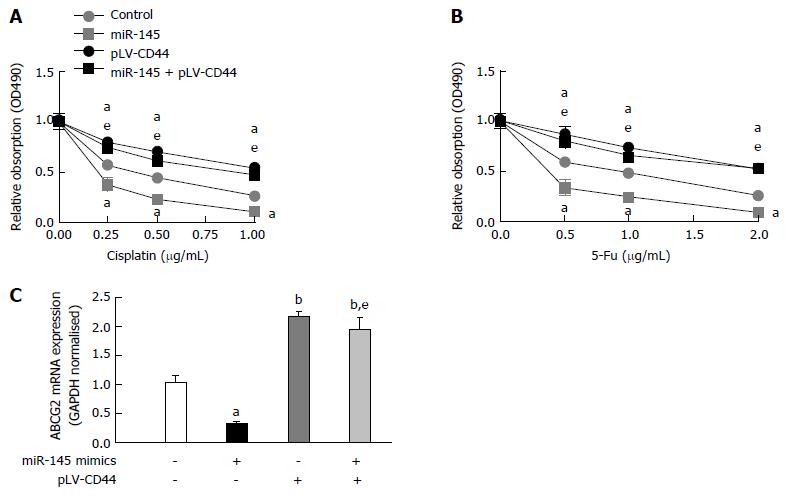
A large proportion of patients with gastric cancer fail chemotherapeutic approaches because of intrinsic or acquired drug resistance, particularly multidrug resistance. Chemo-resistance is another important characteristic of CSCs. We next investigated whether miR-145 or miR-145-regulated CD44 were involved in the chemo-resistance of gastric cancer cells. For this purpose, MGC-803 cells were transfected with or without miR-145 mimics and/or pLV-CD44 for 24 h and then various concentrations of two chemotherapeutic drugs, 5-FU and cisplatin, were used to treat the cells. As shown in Figure 4A and B, miR-145 enhanced the cells’ chemo-sensitivity to these drugs. Conversely, overexpression of CD44 resulted in a significant increase in chemo-resistance (Figure 4A and B, P < 0.05). Furthermore, simultaneous re-expression of CD44 compromised the chemo-sensitivity mediated by miR-145 in MGC-803 cells (Figure 4A and B, P < 0.001), and the cells were more sensitive than the controls (Figure 4A and B, P < 0.05). Collectively, the above results demonstrated that repression of CD44 is necessary for the chemo-resistance lowering effect of miR-145 in gastric cancer cells.
Drug resistance is closely related to increased drug efflux mediated by an energy-dependent mechanism involving the ABC (ATP binding cassette) transporters, mainly ABCB1 (ATP binding cassette subfamily B member 1), ABCC1 (ATP binding cassette subfamily C member 1), and ABCG2 (ATP binding cassette subfamily G member)[24]. Moreover, it has been reported that ABCG2 plays an important role in regulating chemo-resistance in gastric cancer[22]. To evaluate the role of ABCG2 in miR-145 regulated gastric cancer cell chemo-resistance, the expression of ABCG2 was determined in MGC-803 cells. As shown in Figure 3C, ABCG2 expression was repressed in MGC-803 cells following treatment with miR-145 mimics (P < 0.05). Interestingly, overexpression of CD44 resulted in a significant upregulation of ABCG2 expression (Figure 4C, P < 0.01). Furthermore, simultaneous re-expression of CD44 compromised the down-regulation mediated by miR-145 in MGC-803 cells (Figure 4C, P < 0.001). Collectively, the above results demonstrated that the involvement of ABCG2 is associated with the chemo-resistance lowering effect of miR-145 in gastric cancer cells.
Although many factors might contribute to the relapse and chemo-resistance of gastric cancer, it is reasonable to speculate that gastric CSCs play a critical role in these processes. Our results further suggested that the tumor suppressor miR-145, oncogene CD44, and their relationship are involved critically in regulating gastric cancer development.
SOX2, OCT4, and Nanog comprise the core transcriptional network responsible for the regulation of stem cell self-renewal and pluripotency[25,26]. Several groups demonstrated that Sox2, OCT-4, and Nanog are enriched in gastric CSCs. Gastric CSCs identified using the CD44 surface marker in MKN-45 gastric carcinoma cells had elevated levels of Nanog, Sox2, and Oct4[27]. In our experimental system, the tumor spheres expressed much higher levels of Sox2, OCT-4, and Nanog (Figure 1E). This demonstrated that the spheres enrich the cancer gastric CSCs population. At the same time, miR-145 expression was repressed in the spheres (Figure 1B). We speculated that miR-145 plays an inhibitory role in the stemness properties of gastric cancer cells.
There is a large body of evidence showing that deregulation of miRNAs is associated with various human cancers, including gastric cancer. However, the underlying mechanisms by which miRNAs modulate carcinogenesis remain obscure. Previous studies reported that miR-145 is downregulated in various human malignancies, including breast cancer, lung cancer, and gastric cancer[28-30]. Lu et al[31] found that miR-145 functions as a tumor suppressor and targets two oncogenes, ANGPT2 and NEDD9, in renal cell carcinoma. Other studies have shown that miR-145 suppresses cell migration and invasion by inhibiting N-cadherin and FSCN1 in gastric cancer cells[20,23]. The current data showed that miR-145 also regulates the expression of CD44 by targeting the CD44 3’UTR. The latter was confirmed by the following observations; (1) there is an inverse correlation between miR-145 and CD44 expression in gastric tumor spheres; (2) miR-145 regulates CD44 protein expression in MGC-803; and (3) the CD44 3’UTR is regulated by miR-145.
CD44 is a useful marker for identifying and isolating gastric CSCs from a panel of human gastric cancer cell lines, and CD44-positive gastric cancer cells exhibited the stem cell properties of self-renewal and chemo-resistance[10-12]. The expression of CD44 was correlated positively with a more aggressive tumor phenotype and poorer overall prognosis[32]. It is suggested that CD44 is not only a cell surface marker, but also might be a driving factor in the development of CSCs[10]. Recently, one report highlighted the value of changing the perspective of CD44 expression from that of a simple marker to a signaling molecule[33]. In the present study, CD44 overexpression increased the self-renewal activity and enhanced the chemo-resistance of gastric cancer cells. CD44 regulated the expression of Oct4 and phospho-ERK positively, both of which are vital for regulating the pluripotency of CSCs[9]. ERK activity plays an important role in regulating ABCG2 expression[22]. Our results demonstrated that overexpression of CD44 stimulates ABCG2 mRNA expression (Figure 4C). We speculated that the upregulation of ABCG2 by CD44 is mediated by ERK. It was reported that ABCG2 not only plays a major role in multidrug resistance but also could be characterized as a CSCs marker[34]. The more precise mechanisms by which CD44 stimulates ABCG2 expression warrant further investigation. In addition to the well-known effects of ABCG2 on cytotoxic and targeted agents, ABCG2 is also increasingly linked with failure of photodynamic therapy and is a CSCs marker[34,35]. Interestingly, ABCG2 is reported to regulate self-renewal and stem cell marker expression, but not tumorigenicity or radiation resistance, in glioma cells; however, the role of ABCG2 in resistance to radiation therapy remains to be further investigated[36]. Furthermore, overexpression of CD44 abolished the inhibitory and chemo-sensitive effects of miR-145 in gastric cancer cells. Collectively, these findings demonstrated that miR-145 suppresses cell self-renewal properties and improves chemo-sensitivity in gastric cancer primarily by targeting CD44 directly. Thus, miR-145’s targeting of CD44 could make it a potential target for preventing recurrence and chemo-resistance in patients with gastric cancer; however, this needs to be further verified using more gastric cell lines and in vivo assays.
Current research on gastric cancer uses tumor sphere culture, luciferase reporter assays, and chemo-resistance assays to discover the potential mechanism of gastric cancer pathogenesis. Cancer stem cells (CSCs), or cancer cells with stem cell-like properties, have been reported in many human tumors, including gastric cancer, and are considered to be responsible for tumor initiation, progression, chemo-resistance, metastasis, and relapse. CD44, either individually or in combination with other molecules, is used to identify or isolate CSCs from solid and hematological tumors. MicroRNAs (miRNAs) have emerged as critical factors in the regulation of CSCs. From a therapeutic point of view, the elucidation of miRNA networks could help to develop drugs that reverse, delay, or prevent gastric carcinogenesis.
miR-145 regulates embryonic stem cell differentiation and simultaneously targets stemness genes. miR-145 is associated with tumor growth and metastasis in gastric cancer. CD44 expression is upregulated in advanced gastric lesions. Depletion of CD44 inhibited stem cell-like properties, which was accompanied by downregulation of stemness gene expression. However, the underlying mechanism is unclear.
Numerous reports on miRNAs have provided a new avenue to understand the regulatory mechanism in CSCs. Understanding how CSCs are regulated is important for the development of novel mechanism-based therapeutics that specifically target CSCs. The present investigation found that miR-145, which targets CD44, plays a critical role in the inhibition of gastric cancer cells with stem cell properties.
CD44 and gastric cancer have a close relationship. If miR-145 can inhibit CD44 expression and thus abrogate the stem cell-like properties, this should improve chemo-resistance and limit the recurrence of gastric cancer.
This is an interesting study describing a novel mechanism by which miR-145 modulates gastric cancer cell growth and chemo-resistance through direct inhibition of CD44 expression. The aim is clearly stated, the findings are well described, and the data are convincing.
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21467] [Article Influence: 1951.5] [Reference Citation Analysis (6)] |
| 2. | Wilke H, Stahl M. [Therapy in gastric cancer. From an oncological perspective]. Chirurg. 2009;80:1023-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Wadhwa R, Song S, Lee JS, Yao Y, Wei Q, Ajani JA. Gastric cancer-molecular and clinical dimensions. Nat Rev Clin Oncol. 2013;10:643-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 343] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 4. | Robb WB, Mariette C. Predicting the response to chemotherapy in gastric adenocarcinoma: who benefits from neoadjuvant chemotherapy? Recent Results Cancer Res. 2012;196:241-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Saikawa Y, Fukuda K, Takahashi T, Nakamura R, Takeuchi H, Kitagawa Y. Gastric carcinogenesis and the cancer stem cell hypothesis. Gastric Cancer. 2010;13:11-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Haynes BF, Liao HX, Patton KL. The transmembrane hyaluronate receptor (CD44): multiple functions, multiple forms. Cancer Cells. 1991;3:347-350. [PubMed] |
| 7. | Chanmee T, Ontong P, Kimata K, Itano N. Key Roles of Hyaluronan and Its CD44 Receptor in the Stemness and Survival of Cancer Stem Cells. Front Oncol. 2015;5:180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 152] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 8. | Xu H, Tian Y, Yuan X, Wu H, Liu Q, Pestell RG, Wu K. The role of CD44 in epithelial-mesenchymal transition and cancer development. Onco Targets Ther. 2015;8:3783-3792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 153] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 9. | Brungs D, Aghmesheh M, Vine KL, Becker TM, Carolan MG, Ranson M. Gastric cancer stem cells: evidence, potential markers, and clinical implications. J Gastroenterol. 2016;51:313-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 10. | Zhang X, Hua R, Wang X, Huang M, Gan L, Wu Z, Zhang J, Wang H, Cheng Y, Li J. Identification of stem-like cells and clinical significance of candidate stem cell markers in gastric cancer. Oncotarget. 2016;7:9815-9831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 11. | Chen W, Zhang X, Chu C, Cheung WL, Ng L, Lam S, Chow A, Lau T, Chen M, Li Y. Identification of CD44+ cancer stem cells in human gastric cancer. Hepatogastroenterology. 2013;60:949-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 12. | Takaishi S, Okumura T, Tu S, Wang SS, Shibata W, Vigneshwaran R, Gordon SA, Shimada Y, Wang TC. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells. 2009;27:1006-1020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 820] [Cited by in RCA: 825] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 13. | Cichy J, Puré E. The liberation of CD44. J Cell Biol. 2003;161:839-843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 210] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 14. | Zheng N, Yang P, Wang Z, Zhou Q. OncomicroRNAs-Mediated Tumorigenesis: Implication in Cancer Diagnosis and Targeted Therapy. Curr Cancer Drug Targets. 2017;17:40-47. [PubMed] |
| 15. | Jiang C, Chen X, Alattar M, Wei J, Liu H. MicroRNAs in tumorigenesis, metastasis, diagnosis and prognosis of gastric cancer. Cancer Gene Ther. 2015;22:291-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 16. | Petrocca F, Visone R, Onelli MR, Shah MH, Nicoloso MS, de Martino I, Iliopoulos D, Pilozzi E, Liu CG, Negrini M. E2F1-regulated microRNAs impair TGFbeta-dependent cell-cycle arrest and apoptosis in gastric cancer. Cancer Cell. 2008;13:272-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 678] [Cited by in RCA: 700] [Article Influence: 38.9] [Reference Citation Analysis (1)] |
| 17. | Li BS, Zuo QF, Zhao YL, Xiao B, Zhuang Y, Mao XH, Wu C, Yang SM, Zeng H, Zou QM. MicroRNA-25 promotes gastric cancer migration, invasion and proliferation by directly targeting transducer of ERBB2, 1 and correlates with poor survival. Oncogene. 2015;34:2556-2565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 18. | Zhang X, Kong Y, Xu X, Xing H, Zhang Y, Han F, Li W, Yang Q, Zeng J, Jia J. F-box protein FBXO31 is down-regulated in gastric cancer and negatively regulated by miR-17 and miR-20a. Oncotarget. 2014;5:6178-6190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Park SK, Park YS, Ahn JY, Do EJ, Kim D, Kim JE, Jung K, Byeon JS, Ye BD, Yang DH. MiR 21-5p as a predictor of recurrence in young gastric cancer patients. J Gastroenterol Hepatol. 2016;31:1429-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Chen JJ, Cai WY, Liu XW, Luo QC, Chen G, Huang WF, Li N, Cai JC. Reverse Correlation between MicroRNA-145 and FSCN1 Affecting Gastric Cancer Migration and Invasion. PLoS One. 2015;10:e0126890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Xu N, Papagiannakopoulos T, Pan G, Thomson JA, Kosik KS. MicroRNA-145 regulates OCT4, SOX2, and KLF4 and represses pluripotency in human embryonic stem cells. Cell. 2009;137:647-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 884] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 22. | Huang W, Wan C, Luo Q, Huang Z, Luo Q. Genistein-inhibited cancer stem cell-like properties and reduced chemoresistance of gastric cancer. Int J Mol Sci. 2014;15:3432-3443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 23. | Gao P, Xing AY, Zhou GY, Zhang TG, Zhang JP, Gao C, Li H, Shi DB. The molecular mechanism of microRNA-145 to suppress invasion-metastasis cascade in gastric cancer. Oncogene. 2013;32:491-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 24. | Peña-Solórzano D, Stark SA, König B, Sierra CA, Ochoa-Puentes C. ABCG2/BCRP: Specific and Nonspecific Modulators. Med Res Rev. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 25. | Kanehisa M, Goto S, Sato Y, Kawashima M, Furumichi M, Tanabe M. Data, information, knowledge and principle: back to metabolism in KEGG. Nucleic Acids Res. 2014;42:D199-D205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2292] [Cited by in RCA: 2416] [Article Influence: 185.8] [Reference Citation Analysis (0)] |
| 26. | Kaufhold S, Garbán H, Bonavida B. Yin Yang 1 is associated with cancer stem cell transcription factors (SOX2, OCT4, BMI1) and clinical implication. J Exp Clin Cancer Res. 2016;35:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 123] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 27. | Golestaneh AF, Atashi A, Langroudi L, Shafiee A, Ghaemi N, Soleimani M. miRNAs expressed differently in cancer stem cells and cancer cells of human gastric cancer cell line MKN-45. Cell Biochem Funct. 2012;30:411-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Chen Z, Zeng H, Guo Y, Liu P, Pan H, Deng A, Hu J. miRNA-145 inhibits non-small cell lung cancer cell proliferation by targeting c-Myc. J Exp Clin Cancer Res. 2010;29:151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 202] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 29. | Götte M, Mohr C, Koo CY, Stock C, Vaske AK, Viola M, Ibrahim SA, Peddibhotla S, Teng YH, Low JY. miR-145-dependent targeting of junctional adhesion molecule A and modulation of fascin expression are associated with reduced breast cancer cell motility and invasiveness. Oncogene. 2010;29:6569-6580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 172] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 30. | Takagi T, Iio A, Nakagawa Y, Naoe T, Tanigawa N, Akao Y. Decreased expression of microRNA-143 and -145 in human gastric cancers. Oncology. 2009;77:12-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 243] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 31. | Lu R, Ji Z, Li X, Zhai Q, Zhao C, Jiang Z, Zhang S, Nie L, Yu Z. miR-145 functions as tumor suppressor and targets two oncogenes, ANGPT2 and NEDD9, in renal cell carcinoma. J Cancer Res Clin Oncol. 2014;140:387-397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 32. | Wang W, Dong LP, Zhang N, Zhao CH. Role of cancer stem cell marker CD44 in gastric cancer: a meta-analysis. Int J Clin Exp Med. 2014;7:5059-5066. [PubMed] |
| 33. | Jordan AR, Racine RR, Hennig MJ, Lokeshwar VB. The Role of CD44 in Disease Pathophysiology and Targeted Treatment. Front Immunol. 2015;6:182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 207] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 34. | Jiang Y, He Y, Li H, Li HN, Zhang L, Hu W, Sun YM, Chen FL, Jin XM. Expressions of putative cancer stem cell markers ABCB1, ABCG2, and CD133 are correlated with the degree of differentiation of gastric cancer. Gastric Cancer. 2012;15:440-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 35. | Westover D, Li F. New trends for overcoming ABCG2/BCRP-mediated resistance to cancer therapies. J Exp Clin Cancer Res. 2015;34:159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 36. | Wee B, Pietras A, Ozawa T, Bazzoli E, Podlaha O, Antczak C, Westermark B, Nelander S, Uhrbom L, Forsberg-Nilsson K. ABCG2 regulates self-renewal and stem cell marker expression but not tumorigenicity or radiation resistance of glioma cells. Sci Rep. 2016;6:25956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Kaabachi W, McCoy MJ S- Editor: Yu J L- Editor: Stewart G E- Editor: Zhang FF