Published online Mar 14, 2017. doi: 10.3748/wjg.v23.i10.1909
Peer-review started: December 12, 2016
First decision: January 10, 2017
Revised: January 20, 2017
Accepted: February 16, 2017
Article in press: February 17, 2017
Published online: March 14, 2017
Processing time: 92 Days and 0.5 Hours
To perform a meta-analysis of observational studies on inflammatory markers levels and occurrence of colorectal adenoma.
PubMed and EMBASE databases were searched until March 2016 for the articles reporting on the circulating levels of inflammatory markers, including: C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α) and risk of colorectal adenoma. Random-effects models were used to calculate summary odds ratios (ORs) with 95%CIs for the highest vs lowest category of exposure. Heterogeneity was assessed by using the Q test and I2 statistic. Subgroup analyses were also performed to test for potential source of heterogeneity.
A total of 14 case-control studies were included. Ten studies on CRP including a total of 3350 cases and 4168 controls showed non-significant summary (OR = 1.23, 95%CI: 0.98-1.54; I2 = 54%, Pheterogeneity = 0.01) in the general analysis, but significant increased odds when considering only advanced adenoma (OR = 1.59, 95%CI: 1.09-2.32; I2 = 44%, Pheterogeneity = 0.15). Subgroup and stratified analyses revealed a potential influence of smoking status and aspirin use on the association between CRP levels and colorectal adenoma. Five studies examined the association between circulating levels of TNF-α and colorectal adenoma risk, including a total of 1,568 cases and 2,832 controls. The summary OR for the highest vs the lowest category of exposure was 1.00 (95%CI: 0.77-1.29). The relationship between circulating IL-6 levels and colorectal adenoma risk was investigated in 7 studies including a total of 1936 cases and 3611 controls. The summary OR for the highest vs the lowest category of exposure was 1.19 (95%CI: 0.92-1.55).
Summary of current evidence suggests a positive association of CRP levels and advanced colorectal adenoma risk. The role of potential confounding factors should be further evaluated.
Core tip: The present study investigated the association between inflammatory markers and risk of colorectal adenoma. Ten studies on C-reactive protein (CRP) showed non-significant summary odds ratios in the general analysis. However, CRP was significantly correlated with increased risk of advanced adenoma. Subgroup and stratified analyses revealed a potential influence of smoking status and aspirin use on the association between CRP levels and colorectal adenoma risk. The results of the study may have practical value for clinicians screening for colorectal cancer.
- Citation: Godos J, Biondi A, Galvano F, Basile F, Sciacca S, Giovannucci EL, Grosso G. Markers of systemic inflammation and colorectal adenoma risk: Meta-analysis of observational studies. World J Gastroenterol 2017; 23(10): 1909-1919
- URL: https://www.wjgnet.com/1007-9327/full/v23/i10/1909.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i10.1909
Colorectal cancer represents one of the most common cancers diagnosed in developed countries and the fourth leading cause of cancer-related death globally. Chronic inflammation has been hypothesized to play an important role in several aspects of cancer initiation, promotion, and progression[1]. Indeed, existence of chronic inflammatory bowel diseases (such as Crohn’s colitis or ulcerative colitis) or regular use of anti-inflammatory medications has been demonstrated to affect risk of colorectal cancer[2,3] and colorectal adenoma, the precursor of most colorectal cancers[4,5]. However, despite evidence on potential involvement of inflammatory processes in colorectal carcinogenesis, epidemiological data regarding markers of systemic inflammation and colorectal neoplasia risk are conflicting.
Tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) are pro-inflammatory cytokine involved in cell growth, differentiation, and apoptosis, and are altered within adenoma tissues reflecting an inflammatory state[6]. C-reactive protein (CRP) is a non-specific marker of systemic inflammation produced and released into the circulation primarily by hepatocytes in response to elevations in IL-6 and TNF-α[6]. Elevated levels of CRP are typically associated with acute inflammatory conditions, such as infections, trauma, or acute flares of autoimmune diseases, and with a variety of chronic conditions[6]. Current evidence suggests that pre-diagnostic circulating CRP levels may be associated with increased risk of colorectal cancer[7]. In addition, serum levels of TNF-α have been associated with increased risk of this malignancy[8]. Results on IL-6 are inconclusive, suggesting that it is not associated with colorectal cancer risk, but the evidence is weakened by a limited number of studies[7,8]. Overall, existing summary of evidence rely on colorectal cancer risk, but findings on colorectal adenoma have not been meta-analyzed yet. Thus, the aim of this study was to systematically review and perform a meta-analysis of studies examining the association between circulating levels of CRP, TNF-α, and IL-6 and risk of colorectal adenoma.
The design, analysis, and reporting of this study followed the meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines (Online Resource 3). The reporting of this work is compliant with PRISMA guidelines.
We performed a systematic search on PubMed (http://www.ncbi.nlm.nih.gov/pubmed/) and EMBASE (http://www.embase.com/) databases of all English language studies published up to March 2016. The search terms used are shown in Online Resource 4. Inclusion criteria for the studies were: (1) having a cross-sectional, case-control, or prospective design; (2) evaluating the association between CRP, TNF-α, or IL-6 levels and colorectal adenoma comparing the highest vs the lowest category of exposure; and (3) assessing and reporting odds ratios (ORs) and the corresponding 95%CIs. Reference lists of included studies were examined for any additional article not previously identified. If more than one study was conducted on the same cohort, only the most comprehensive or most updated was included in the meta-analysis. The selection process was independently performed by two authors (Godos J and Grosso G).
Data were abstracted from each identified study by using a standardized extraction form. The following information was collected: (1) author name; (2) year of publication; (3) country; (4) number, gender, and age of participants; (5) main characteristics related to markers; (6) OR and 95%CI for the highest vs the lowest category of exposure; and (7) covariates used in adjustments.
The Newcastle-Ottawa Quality Assessment Scale was used to assess the quality of each study[9]. The instrument consists of 3 domains indicating the study quality as follows: selection (4 points), comparability (2 points), and outcome (3 points) for a total score of 9 points (9 representing the highest quality). Studies scoring 7-9 points, 3-6 points, and 0-3 points were identified as high, moderate, and low quality, respectively.
Random-effects models were used to calculate summary ORs with 95% CIs for the highest vs lowest category of exposure. The risk estimate from the most fully adjusted models in the analysis of the pooled RR was used. Heterogeneity was assessed by using the Q test and I2 statistic. The level of significance for the Q test was defined as P < 0.10. The I2 statistic represented the amount of total variation that could be attributed to heterogeneity. I2 values ≤ 25%, ≤ 50%, ≤ 75%, and > 75% indicated no, small, moderate, and significant heterogeneity, respectively. A sensitivity analysis by exclusion of one study at a time was performed to assess the stability of results and potential sources of heterogeneity. Separate analyses were conducted on adenoma advancement based on availability of datasets. According to literature investigated, advanced adenoma was defined as having diameter > 1 cm or containing villous/tubulovillous characteristics, or severe dysplasia. Subgroup analyses were also performed to test for potential source of heterogeneity according to geographical area, gender, study design, sample fasting status, measurement methods, and adjustment for main confounding factors (BMI or obesity, smoking status, family history of colorectal neoplasia, aspirin or NSAIDs use, energy intake or physical activity, and alcohol consumption). Stratified analyses were conducted by smoking status (non/former vs current), NSAIDs use (non/former vs current), and adenoma location. Publication bias was evaluated by a visual investigation of funnel plots for potential asymmetry. All analyses were performed with Review Manager (RevMan) software version 5.2 (The Nordic Cochrane Centre, The Cochrane Collaboration). Artwork was created using RevMan software. Statistical analysis was reviewed by a biostatistician (Grosso G).
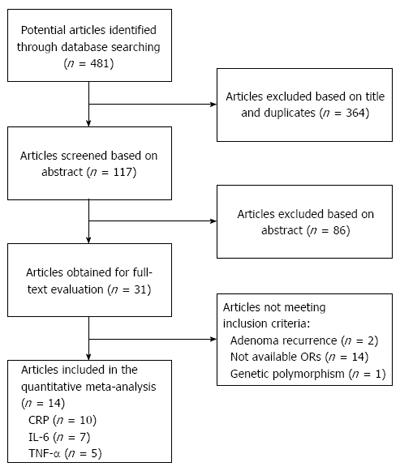
The process of identification and study selection is summarized in Figure 1. Among the initial 481 articles screened on the basis of title, 31 articles were screened for full-text examination. Seventeen studies were excluded because they assessed adenoma recurrence (n = 2), did not report risk estimates (n = 14), and reported information only on genetic polymorphisms (n = 1). A final number of 14 studies[10-23] were included for the quantitative meta-analysis (Table 1). Four studies were nested case-control studies, 9 had a case-control design, and one was cross-sectional. The studies were conducted in the United States[10,11,13,16,18,19,21-23], Japan[12,14,15,17], and the United Kingdom[20]. Four studies investigated advanced and 4 studies non-advanced adenoma. Measurements methods varied greatly between studies, including INA (Immunonephelometric Assay)[12,14,19,20], ITA (Immunoturbidimetric Assay)[13,21,23], ELISA[10,13,17,18,23], DADE-Behring method[11], Chemiluminescent Immunometric Assay[16], Cytometric Bead Array[20], Cytokine/Chemokine Magnetic Bead Panel Assay[15,18,22]. Seven studies tested markers of inflammation in plasma, 5 in serum, and 2 in serum and plasma. 10 studies collected overnight fasting samples while 4 studies did not specified fasting status. The overall quality of the studies included was high (Online Resource 5).
| Ref. | Study design | Study population | No. of controls; No of controls; gender; age | Assay method, sample type, fasting status | Exposure vs reference category | Matching factors | Adjusting factors |
| Kim et al[10] | Case-control | Patients who underwent colonoscopy and/or patients who underwent screening colonoscopy | 631; 242; M and F > 30 yr | CRP (ELISA), IL-6 (ELISA), TNF-α (ELISA), plasma, fasting | T3 (≥ 12013.1 ng/mL) vs T1 (< 2916.03 ng/mL) for CRP; T3 (≥ 0.3571 pg/mL) vs T1 (0 pg/mL) for IL-6; T3 (≥ 2.2358 pg/mL) vs T1 (< 1.3877 pg/mL) for TNF-α | NA | Age, sex, obesity |
| Tsilidis et al[11] | Nested case-control | Subjects who undergone sigmoidoscopy or colonoscopy | 269; 135, M and F 45-65 yr | CRP (Dade-Behring method), plasma, NR | Q4 (> 2.95 mg/L) vs Q1 (< 0.65 mg/L) | Age, sex, race, date of blood draw, time since last meal, type of endoscopy | Cigarette smoking status, BMI at baseline, BMI at age 21, ever use of estrogen or progesterone (women), use of aspirin or non-steroidal anti-inflammatory drugs, use of diabetes medications, family history of colorectal cancer |
| Otake et al[12] | Case-control | Subjects who underwent colonoscopy for health checkup | 635; 646; M 50-53 yr | CRP(INA), plasma/serum, fasting | Q4 (≥ 1541 ng/mL) vs Q1 (< 206 ng/mL) | NA | Age, hospital, plasma/serum status, rank in the Self Defense Forces, cigarette smoking, alcohol use, BMI, physical activity, parental colorectal cancer |
| Ognjanovic et al[13] | Case-control | Subjects who undergone screening sigmoidoscopy | 539; 271; M and F 55-69 yr | CRP (ITA), IL-6 (ELISA), serum, fasting | Q4 (> 2.00 mg/L) vs Q1 (> 0.30 mg/L) for CRP; Q4 (> 2.79 pg/mL) vs Q1 (> 1.09 pg/mL) for IL-6 | Age, sex, ethnicity, screening date, recruitment clinic | Sex, age, race, smoking status, BMI |
| Otake et al[14] | Cross-sectional | Patients who underwent colonoscopy | 26; 47; M 53-80 yr | CRP (INA), plasma, fasting | high (≥ 500 ng/mL) vs low (< 500 ng/mL) | NA | None |
| Yamaji et al[15] | Case-control | Healthy subjects who underwent screening colonoscopy | 735; 778; M 50-79 yr; F 40-79 yr | TNF-α (Cytokine/Chemokine Magnetic Bead Panel Assay), plasma, fasting | Q3 (≥ 2.98 pg/mL) vs Q1 ( ≤ 2.38 pg/mL) | Age, two screening periods | Age, sex, screening period, duration of fasting, cigarette smoking, alcohol drinking, family history of colorectal cancer, nonsteroidal anti-inflammatory drug use, BMI |
| Gunter et al[16] | Nested case-control | Subjects who undergone screening sigmoidoscopy | 396; 356; M and F 55-74 yr | CRP (Chemiluminescent Immunometric Assay), serum, NR | Q4 (≥ 4.0 mg/L) vs Q1 (< 0.8 mg/L) | Age at study entry, gender, fiscal year at study entry, race, screening center, study protocol, season of blood draw | Cigarette smoking status, BMI, use of non-steroidal anti-inflammatory drugs, diabetes, use of hormone therapy (females only), family history of colorectal cancer, educational attainment |
| Sasaki et al[17] | Case-control | Subjects who underwent colonoscopy for health checkup | 218; 118; M 52 yr cases, 51 yr controls (median) | IL-6 (ELISA), serum, fasting | Q4 (≥ 1.619 pg/mL) vs Q1 (< 0.804 pg/mL) | Age | Age, current smoking, alcohol consumption, family history of CRC, BMI, HOMA-IR, insulin |
| Vaughn et al[18] | Case-control | Patients who underwent routine colonoscopy | 1050; 401; M and F 46-66 yr | IL-6 (ELISA), TNF-α (Cytokine/ Chemokine Magnetic Bead Panel Assay), serum, fasting | T3 (> 2.71 pg/mL) vs T1 (< 1.44 pg/mL) for IL-6; T3 (> 4.46 pg/mL) vs T1 (< 3.01 pg/mL) for TNF-α | NA | Age, sex, non-steroidal anti-inflammatory use, BMI, family history of colorectal cancer, smoking status, race |
| Kong et al[19] | Case-control | Patients who underwent colonoscopy | 201; 139; M and F 46-66 yr | CRP (INA), plasma, NR | high (> 6.2 mikrog/mL) vs low (< 6.2 mikrog/mL) | NA | Age, race, sex, BMI, total energy intake, plasma cholesterol, family history of colorectal cancer in a first degree relative, hormone replacement therapy, dietary fiber, physical activity, study |
| Basavaraju et al[20] | Case-control | Patients who undergone screening colonoscopy | 319; 50; M and F 64 yr | CRP (INA), IL-6 (Cytometric Bead Array), TNF-α (Cytometric Bead Array), serum (CRP), plasma (IL-6, TNF-α), fasting | high (> 1.88) vs low ( ≤ 1.88) units NR for CRP; high (> 3.32) vs low ( ≤ 3.32) units NR for IL-6; high (> 3.53) vs low ( ≤ 3.53) units NR for TNF-α | NA | Time of recruitment, age, sex, BMI, alcohol consumption, exercise, smoking, diverticular disease, exercise, aspirin use |
| Davenport et al[21] | Case-control | Patients who underwent colonoscopy | 395; 707; M and F 49-67 yr | CRP (ITA), plasma, NR | single small tubular adenoma T3 (> 5.97 mg/L) vs T1 (< 1.59 mg/L); multiple small tubular adenoma T3 (> 6.96 mg/L) vs T1 (< 1.82 mg/L); advanced adenoma T3 (> 7.16 mg/L) vs T1 (< 1.69 mg/L) | Age, sex, race, and matched at least one of the following factors: study site, collection date of plasma, NSAID use for a minimum of three times per week for at least one year | Age, educational attainment, study site |
| Henry et al[22] | Nested case-control | Subjects who undergone colonoscopy | 97; 97; M and F ≥ 50 yr | IL-6 (Cytokine/Chemokine Magnetic Bead Panel Assay), TNF-α (Cytokine/Chemokine Magnetic Bead Panel Assay), serum, fasting | T3 vs T1 for IL-6; T3 vs T1 for TNF-α | NA | Age, sex, previous screening |
| Song et al[23] | Nested case-control | Subjects who undergone sigmoidoscopy or colonoscopy | 757; 757; F 30-55 yr | CRP (ITA), IL-6 (ELISA), plasma, fasting/non-fasting | Q5 (6.32 mg/L - median) vs Q1 (0.43 mg/L - median) for CRP; Q5 (2.64 ng/l - median) vs Q1 (0.42 ng/l - median) for IL-6 | Date of endoscopy, birth year, indication for endoscopy, time period of any prior endoscopy, month and year of blood draw, fasting status | Family history of colorectal cancer, multivitamin use, pack-years of smoking before age 30, alcohol consumption, BMI, physical activity, regular aspirin/NSAID use, postmenopausal status and hormone use, calcium intake, and Alternative Healthy Eating Index |
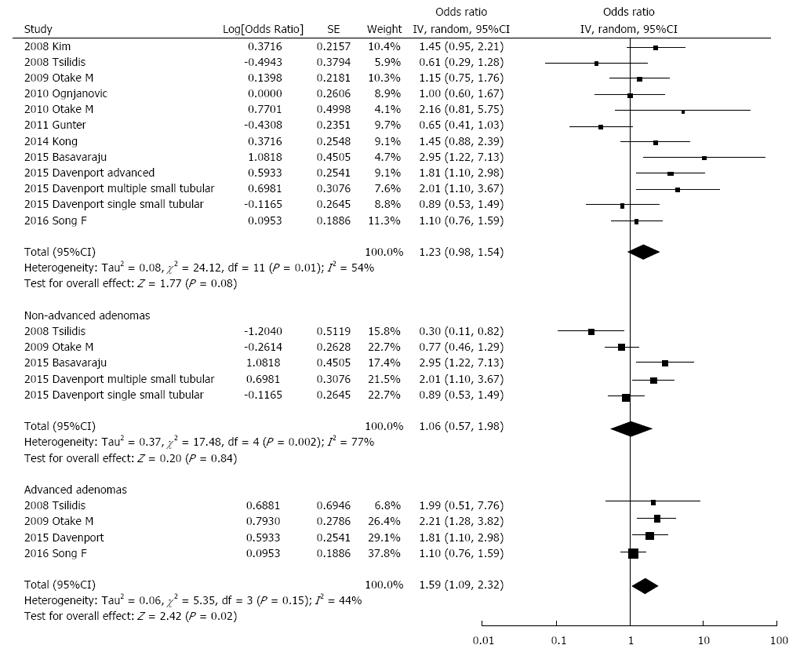
The association between CRP levels and colorectal adenoma risk was examined in 10 studies including a total of 3350 cases and 4168 controls. The summary OR for the highest vs the lowest category of exposure was 1.23 (95%CI: 0.98-1.54) with evidence of heterogeneity (I2 = 54%, Pheterogeneity = 0.01; Figure 2). Publication bias was not evident based on the symmetrical shape of funnel plot (Online Resource 1). Sensitivity analysis by excluding one study at a time showed that one study, which showed a suggestive inverse association, contributed largely to the heterogeneity[16]; after exclusion of this study, the summary OR = 1.31, 95%CI: 1.06-1.61; I2 = 40%; Pheterogeneity = 0.08). Subgroup analyses are presented in Table 2. Grouping studies by fasting status and measurement method showed statistically significant results when considering studies that collected fasting blood samples (OR = 1.38, 95%CI: 1.02-1.88; I2 = 32%, Pheterogeneity = 0.21) and INA measurement (OR = 1.54, 95%CI: 1.06-2.23; I2 = 29%, Pheterogeneity = 0.24). Moreover, including one study[23] in which blood analyses were not uniformly conducted on fasting samples but risk estimates were adjusted for fasting/non-fasting, did not change markedly results (OR = 1.29, 95%CI: 1.01-1.64; I2 = 25%, Pheterogeneity = 0.25). No significant confounders were detected when grouping by gender, sample size, geographical location, and quality score. In contrast, subgroup analysis showed significant results only for the studies not adjusting for potential confounding factors of BMI, smoking status, aspirin/NSAIDs use, and family history of colorectal neoplasia showed significant results (Table 2).
| Subgroup | CRP | IL-6 | TNF-α | |||||||||
| No. of datasets | RR (95%CI) | I2 | Pheterogeneity | No. of datasets | RR (95%CI) | I2 | Pheterogeneity | No. of datasets | RR (95%CI) | I2 | Pheterogeneity | |
| Total | 12 | 1.23 (0.98, 1.54) | 54% | 0.01 | 7 | 1.19 (0.92, 1.55) | 46% | 0.09 | 6 | 1.00 (0.77, 1.29) | 49% | 0.08 |
| Study design | ||||||||||||
| Nested case-control | 3 | 0.81 (0.54, 1.21) | 49% | 0.14 | 2 | 1.02 (0.72, 1.45) | 0% | 0.47 | 1 | 1.14 (0.55, 2.36) | NA | NA |
| Case-control | 8 | 1.39 (1.11, 1.74) | 34% | 0.16 | 5 | 1.25 (0.89, 1.78) | 59% | 0.04 | 5 | 0.98 (0.73, 1.31) | 59% | 0.05 |
| Cross-sectional | 1 | 2.16 (0.81, 5.75) | NA | NA | 0 | NA | NA | NA | 0 | NA | NA | NA |
| Gender | ||||||||||||
| Men | 2 | 1.34 (0.79, 2.28) | 25% | 0.25 | 1 | 2.06 (1.02, 4.16) | NA | NA | 1 | 0.94 (0.68, 1.30) | NA | NA |
| Women | 1 | 1.10 (0.76, 1.59) | NA | NA | 1 | 0.96 (0.65, 1.42) | NA | NA | 1 | 0.65 (0.41, 1.03) | NA | NA |
| Geographical location | ||||||||||||
| United States | 9 | 1.14 (0.89, 1.47) | 57% | 0.02 | 5 | 1.14 (0.86, 1.52) | 52% | 0.08 | 3 | 1.23 (0.87, 1.72) | 44% | 0.17 |
| United Kingdom | 1 | 2.95 (1.22, 7.13) | NA | NA | 1 | 1.00 (0.44, 2.27) | NA | NA | 1 | 0.73 (0.32, 1.67) | NA | NA |
| Japan | 2 | 1.34 (0.79, 2.28) | 25% | 0.25 | 1 | 2.06 (1.02, 4.16) | NA | NA | 2 | 0.81 (0.57, 1.16) | 39% | 0.20 |
| By adjustment for confounders | ||||||||||||
| BMI/obesity | ||||||||||||
| Yes | 8 | 1.12 (0.86, 1.45) | 54% | 0.03 | 6 | 1.19 (0.89, 1.58) | 55% | 0.05 | 5 | 0.98 (0.73, 1.31) | 59% | 0.05 |
| No | 4 | 1.54 (1.01, 2.37) | 49% | 0.12 | 1 | 1.35 (0.58, 3.14) | NA | NA | 1 | 1.14 (0.55, 2.36) | NA | NA |
| Smoking | ||||||||||||
| Yes | 6 | 1.01 (0.73, 1.40) | 56% | 0.04 | 5 | 1.03 (0.83, 1.29) | 9% | 0.35 | 4 | 0.90 (0.74, 1.09) | 0% | 0.42 |
| No | 6 | 1.48 (1.17, 1.88) | 15% | 0.32 | 2 | 1.74 (1.22, 2.49) | 0% | 0.52 | 2 | 1.51 (1.05, 2.16) | 0% | 0.39 |
| Aspirin/NSAIDs use | ||||||||||||
| Yes | 4 | 1.00 (0.58, 1.72) | 72% | 0.01 | 3 | 0.99 (0.78, 1.26) | 0% | 0.98 | 4 | 0.90 (0.74, 1.09) | 0% | 0.42 |
| No | 8 | 1.35 (1.10, 1.65) | 17% | 0.30 | 4 | 1.43 (0.93, 2.21) | 58% | 0.07 | 2 | 1.51 (1.05, 2.16) | 0% | 0.39 |
| Alcohol consumption | ||||||||||||
| Yes | 3 | 1.32 (0.87, 2.03) | 53% | 0.12 | 3 | 1.20 (0.75, 1.92) | 44% | 0.17 | 3 | 0.82 (0.64, 1.06) | 0% | 0.42 |
| No | 9 | 1.19 (0.89, 1.59) | 60% | 0.01 | 4 | 1.20 (0.83, 1.73) | 60% | 0.06 | 3 | 1.23 (0.87, 1.72) | 44% | 0.17 |
| Energy/physical activity | ||||||||||||
| Yes | 4 | 1.32 (0.97, 1.79) | 34% | 0.21 | 2 | 0.97 (0.68, 1.38) | 0% | 0.93 | 1 | 0.73 (0.32, 1.67) | NA | NA |
| No | 8 | 1.16 (0.84, 1.61) | 63% | 0.008 | 5 | 1.30 (0.92, 1.84) | 58% | 0.05 | 5 | 1.02 (0.78, 1.35) | 56% | 0.06 |
| Family history of colorectal neoplasia | ||||||||||||
| Yes | 5 | 0.98 (0.73, 1.32) | 49% | 0.10 | 3 | 1.13 (0.80, 1.61) | 47% | 0.15 | 3 | 0.90 (0.71, 1.13) | 22% | 0.28 |
| No | 7 | 1.49 (1.11, 1.99) | 43% | 0.11 | 4 | 1.24 (0.81, 1.90) | 53% | 0.10 | 3 | 1.24 (0.78, 1.97) | 38% | 0.20 |
| Sample fasting status | ||||||||||||
| Yes | 6 | 1.29 (1.01, 1.64) | 25% | 0.25 | 7 | 1.19 (0.92, 1.55) | 46% | 0.09 | 6 | 1.00 (0.77, 1.29) | 49% | 0.08 |
| No | 6 | 1.12 (0.75, 1.68) | 70% | 0.005 | 0 | NA | NA | NA | 0 | NA | NA | NA |
| Measurement method | ||||||||||||
| ELISA | 1 | 1.45 (0.95, 2.21) | NA | NA | 5 | 1.21 (0.88, 1.67) | 63% | 0.03 | 1 | 1.65 (1.09, 2.50) | NA | NA |
| INA | 4 | 1.54 (1.06, 2.23) | 29% | 0.24 | 0 | NA | NA | NA | 0 | NA | NA | NA |
| ITA | 5 | 1.26 (0.94, 1.69) | 45% | 0.12 | 0 | NA | NA | NA | 0 | NA | NA | NA |
| Cytokine/Chemokine Magnetic Bead Panel Assay | 0 | NA | NA | NA | 1 | 1.35 (0.58, 3.14) | NA | NA | 4 | 0.92 (0.76, 1.11) | 0% | 0.41 |
| Cytometric Bead Assay | 0 | NA | NA | NA | 1 | 1.00 (0.44, 2.27) | NA | NA | 1 | 0.73 (0.32, 1.67) | NA | NA |
| Dade-Behring method | 1 | 0.61 (0.29, 1.28) | NA | NA | 0 | NA | NA | NA | 0 | NA | NA | NA |
| Chemiluminescent Immunometric Assay | 1 | 0.65 (0.41, 1.03) | NA | NA | 0 | NA | NA | NA | 0 | NA | NA | NA |
Stratified analyses for such potential confounding factors were limited[12,21] but showed significant interaction with smoking status and use of aspirin/NSAIDs, whereby associations were only observed in heavy smokers and non-regular aspirin users (Online Resource 2). A stratified analysis was also conducted according to adenoma localization. No significant results for were observed for adenoma of proximal or distal colon (Online Resource 1) and for non-advanced adenoma (Figure 2). In contrast, separate analysis of the highest vs lowest CRP levels showed a significant association with increased advanced adenoma risk (OR = 1.59, 95%CI: 1.09-2.32; I2 = 44%, Pheterogeneity = 0.15; Figure 2).
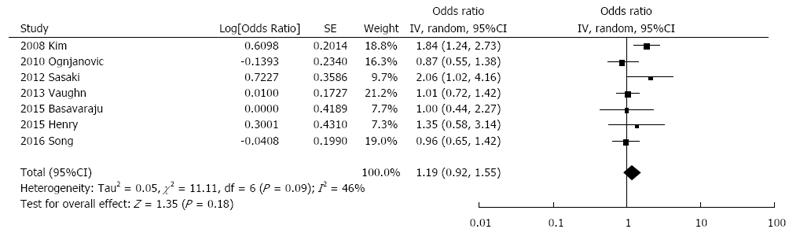
The relationship between circulating IL-6 levels and colorectal adenoma risk was investigated in 7 studies including a total of 1936 cases and 3611 controls. The summary OR for the highest vs the lowest category of exposure was 1.19 (95%CI: 0.92-1.55) with no evidence of publication bias (Online Resource 1) and little evidence of heterogeneity (I2 = 46%, Pheterogeneity = 0.09, Figure 3) and no evidence of asymmetry at funnel plot (Online Resource 1). Subgroup analyses are presented in Table 2. Grouping studies by adjustment for smoking status showed significant results for the studies that did not adjust for smoking and not significant for those that did adjust (Table 2). However, subgroup analysis for the adjustment for confounding factors, such as BMI, aspirin use, family history of colorectal cancer, and physical activity showed not-significant results. Similar results were observed for subgroup analysis controlling for IL-6 measurement.
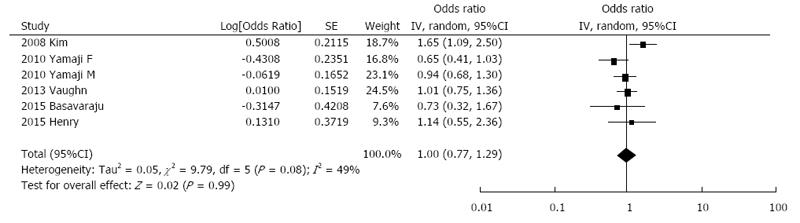
Five studies examined the association between circulating levels of TNF-α and colorectal adenoma risk, including a total of 1568 cases and 2832 controls. The summary OR for the highest vs the lowest category of exposure was 1.00 (95%CI: 0.77-1.29) with no evidence of publication bias (Online Resource 1) and little evidence of heterogeneity (I2 = 49%, Pheterogeneity = 0.08; Figure 4). Subgroup analyses showed significant results for the studies that did not adjust for smoking status while those studies adjusting did not show significant results (Table 2). Similar results were observed when pooling studies that did not adjust for aspirin/NSAIDs use (Table 2). The summary OR for the highest vs the lowest category of exposure, when grouping studies by other confounding factors (BMI, family history of colorectal cancer, physical activity, alcohol consumption) showed no significant evidence.
In this meta-analysis, circulating levels of CRP, TNF-α, and IL-6 were not appreciable associated with the risk of colorectal adenoma. Significant positive associations were observed, especially for CRP, in studies that did not adjust for smoking, aspirin use, BMI and family history. Stratified analyses also showed significant associations for CRP in the strata of heavy smokers and non-users of aspirin. In addition, CRP was significantly associated with occurrence of advanced adenoma, irrespectively of the group. These findings suggest a potential role of subclinical inflammation driven by the aforementioned factors in increasing risk of colorectal adenoma, which may be accentuated in more advanced stages of adenoma.
Experimental studies on animals have shown that colonic epithelial dysplasia provokes a cytokine-driven inflammatory response that up-regulates circulatory inflammatory markers such as TNF-α, IL-6, and, consequently, CRP production[24,25]. Thus, it is biologically plausible that an inflammatory state would occur in patients with adenoma, possibly reflected in an increase in systemic inflammatory markers. However, results observed in the studies reviewed were inconsistent. The results appeared to differ depending on whether the studies adjusted for potentially confounding factors, such as smoking, BMI, aspirin use and family history. Smoking[26], high BMI[27], and non-use of aspirin would be expected to increase levels of inflammatory markers, and these are associated with increased risk of colorectal adenoma (and cancer)[28]. Studies that did not adjust for these factors tended to show associations with inflammatory markers, whereas those that did adjust showed not significant results. This pattern suggests that uncontrolled confounding by these factors could have caused the positive associations, when observed. On the other hand, inflammation could possibly be a mediator of the effects of some of these factors. Further, when considering studies that reported fasting sample, results were significant in favor of an increased risk of colorectal adenoma for higher levels of CRP. Markers of subclinical systemic inflammatory state, especially CRP, have been hypothesized to be influenced by fasting[29] and that diet may also impact level and direction of modification[30]. Nevertheless, number of studies for such stratified analyses were limited, thus the specific role that known risk factors associated with systemic subclinical inflammation will require further investigation.
There are at least three ways that systemic inflammation may relate to colorectal adenoma. First, inflammation in the pre-neoplastic colorectal tissue (i.e., as in ulcerative colitis) could directly predispose to risk of colorectal neoplasia. Second, a neoplastic lesion (adenoma, cancer) could induce inflammation and increase inflammatory markers. Thirdly, systemic inflammation could reflect various processes, some of which may be directly or indirectly (i.e., by different mechanisms) be associated with colorectal adenoma risk (i.e., smoking habits or obesity). The first possibility is difficult to demonstrate because the studies cannot distinguish the source of the inflammation, and in most cases the adenomas were present at the time of blood draw, so a temporal relationship could not be established. The second explanation is supported by the stratified analysis that we conducted including only advanced adenoma that resulted in increased risk of disease for higher CRP levels. Most of studies combined all adenomas into an individual condition, which likely included a significant proportion of single small tubular adenoma that may not provoke an inflammatory response sufficient to raise inflammatory markers levels[10,13-19,22]. Studies exploring inflammatory markers levels over different phases of tumor progression showed increasing concentrations of several cytokines (including TNF-α) in normal mucosa, adenoma, hyperplastic polyp, and serrated adenoma[31]. Interestingly, habitual use of aspirin and NSAIDs interact with COX-2 activation that has been suggested to occur as a carcinogenic mechanism within the colonic epithelium[32]. Also, CRP and IL-6 levels have been demonstrated to vary not only between colorectal adenoma and cancer, but also by cancer stage[33]. However, evidence for markers of inflammation other than CRP was limited and future studies exploring this issue are needed.
The results of the present meta-analysis should be considered in light of some limitations. First, our meta-analysis was conducted on blood/serum markers of inflammation, but the biological effects of circulating vs local inflammatory makers may differ, and how circulating inflammation is reflecting etiologically relevant tissue inflammation will vary by factors in the study population, and by stratification of relevant factors. Second, although we included in our analyses the most adjusted models for various potential confounding factors, residual or unknown confounding may still exist. For instance, differences in the definition of advanced adenomas among study may have led to misclassification. Moreover, due to study design, results may be affected by reverse causation depending on timing of measurements. Third, number of studies and information retrieved were limited for some potential important characteristics that should be taken into account, such as adenoma location (presented in 2 studies[11,12]), multiplicity and size (not investigated in the present meta-analysis).
In conclusion, inflammation may play an important role in colorectal cancer development. Discrepancy between biological plausibility and lack of strong epidemiological difference may depend on inadequate methods of assessing inflammatory markers, with special regard to existing confounders. Although findings from this meta-analysis suggest an involvement of CRP in the early stage of colorectal cancer (i.e., advanced adenoma risk), final conclusions cannot be drawn given the nature of the studies (observational non prospective), heterogeneous determinants of systemic inflammatory markers, and the limited number of investigations involved. Future studies estimating pre-diagnostic serum/plasma markers of inflammation and sequent adenoma incidence are needed to better assess the role of chronic inflammation in malignancy development. The potentiality of using CRP as a marker to distinguish between advanced and non-advanced adenoma could be also studies to improve surveillance strategies.
Colorectal cancer represents one of the most common cancers diagnosed in developed countries and the fourth leading cause of cancer-related death globally. Chronic inflammation has been hypothesised to play an important role in the initiation and progression of colorectal cancer. Patients with ulcerative colitis and Crohn’s colitis demonstrate chronic inflammation in the gastrointestinal mucosa and are at higher risk of developing cancer. On the contrary, regular use of anti-inflammatory drugs such as aspirin has been associated with lower risk of colorectal cancer as well as colorectal adenomas,
precursors of most colorectal cancer.
Regardless the fact, that the majority of evidence implicates chronic inflammation in colorectal carcinogenesis, epidemiological data remain inconclusive about the association between systemic inflammatory markers and colorectal neoplasia risk.
The present study investigate the associations between inflammatory markers and risk of colorectal adenoma. The authors found that C-reactive protein (CRP) was significantly correlated with increased risk of advanced adenoma.
The results of the study may contribute to the improvement of the detection of advanced premalignant lesions during colorectal cancer screening.
Colorectal adenoma is a benign glandular tumour of the colon and the rectum. It is a precursor lesion of the colorectal cancer.
This systematic review and meta-analysis represents an interesting collation of evidence to date on three inflammatory markers (CRP, IL-6 and TNF-α) in relation to colorectal adenoma risk. The review is also largely well conducted and written.
| 1. | Terzić J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101-2114.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1333] [Cited by in RCA: 1539] [Article Influence: 96.2] [Reference Citation Analysis (0)] |
| 2. | Algra AM, Rothwell PM. Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol. 2012;13:518-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 658] [Cited by in RCA: 633] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 3. | Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G7-G17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 870] [Cited by in RCA: 926] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 4. | Cole BF, Logan RF, Halabi S, Benamouzig R, Sandler RS, Grainge MJ, Chaussade S, Baron JA. Aspirin for the chemoprevention of colorectal adenomas: meta-analysis of the randomized trials. J Natl Cancer Inst. 2009;101:256-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 361] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 5. | Wanders LK, Dekker E, Pullens B, Bassett P, Travis SP, East JE. Cancer risk after resection of polypoid dysplasia in patients with longstanding ulcerative colitis: a meta-analysis. Clin Gastroenterol Hepatol. 2014;12:756-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 136] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 6. | Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805-1812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 1252] [Article Influence: 54.4] [Reference Citation Analysis (2)] |
| 7. | Sinha RA, Farah BL, Singh BK, Siddique MM, Li Y, Wu Y, Ilkayeva OR, Gooding J, Ching J, Zhou J. Caffeine stimulates hepatic lipid metabolism by the autophagy-lysosomal pathway in mice. Hepatology. 2014;59:1366-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 274] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 8. | Joshi RK, Kim WJ, Lee SA. Association between obesity-related adipokines and colorectal cancer: a case-control study and meta-analysis. World J Gastroenterol. 2014;20:7941-7949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Wells GA, Shea BJ, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Proceedings of the Ottawa (Canada): Ottawa Health Research Institute; 1999; Available from: https://www.mendeley.com/catalog/newcastleottawa-scale-nos-assessing-quality-nonrandomized-studies-metaanalyses/. |
| 10. | Kim S, Keku TO, Martin C, Galanko J, Woosley JT, Schroeder JC, Satia JA, Halabi S, Sandler RS. Circulating levels of inflammatory cytokines and risk of colorectal adenomas. Cancer Res. 2008;68:323-328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 238] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 11. | Tsilidis KK, Erlinger TP, Rifai N, Hoffman S, Hoffman-Bolton J, Helzlsouer KJ, Platz EA. C-reactive protein and colorectal adenoma in the CLUE II cohort. Cancer Causes Control. 2008;19:559-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Otake T, Uezono K, Takahashi R, Fukumoto J, Tabata S, Abe H, Tajima O, Mizoue T, Ohnaka K, Kono S. C-reactive protein and colorectal adenomas: Self Defense Forces Health Study. Cancer Sci. 2009;100:709-714. [PubMed] |
| 13. | Ognjanovic S, Yamamoto J, Saltzman B, Franke A, Ognjanovic M, Yokochi L, Vogt T, Decker R, Le Marchand L. Serum CRP and IL-6, genetic variants and risk of colorectal adenoma in a multiethnic population. Cancer Causes Control. 2010;21:1131-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Otake S, Takeda H, Fujishima S, Fukui T, Orii T, Sato T, Sasaki Y, Nishise S, Kawata S. Decreased levels of plasma adiponectin associated with increased risk of colorectal cancer. World J Gastroenterol. 2010;16:1252-1257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 77] [Cited by in RCA: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 15. | Yamaji T, Iwasaki M, Sasazuki S, Tsugane S. Interaction between adiponectin and leptin influences the risk of colorectal adenoma. Cancer Res. 2010;70:5430-5437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 16. | Gunter MJ, Cross AJ, Huang WY, Stanczyk FZ, Purdue M, Xue X, Schoen R, Limburg PJ, Schatzkin A, Sinha R. A prospective evaluation of C-reactive protein levels and colorectal adenoma development. Cancer Epidemiol Biomarkers Prev. 2011;20:537-544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Sasaki Y, Takeda H, Sato T, Orii T, Nishise S, Nagino K, Iwano D, Yaoita T, Yoshizawa K, Saito H. Serum Interleukin-6, insulin, and HOMA-IR in male individuals with colorectal adenoma. Clin Cancer Res. 2012;18:392-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Vaughn CB, Ochs-Balcom HM, Nie J, Chen Z, Thompson CL, Tracy R, Li L. No Association between Circulating Levels and Genetic Variants of IL-6 and TNF-α and Colon Adenoma. Gastroenterology Res. 2013;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Kong SY, Bostick RM, Flanders WD, McClellan WM, Thyagarajan B, Gross MD, Judd S, Goodman M. Oxidative balance score, colorectal adenoma, and markers of oxidative stress and inflammation. Cancer Epidemiol Biomarkers Prev. 2014;23:545-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 20. | Basavaraju U, Shebl FM, Palmer AJ, Berry S, Hold GL, El-Omar EM, Rabkin CS. Cytokine gene polymorphisms, cytokine levels and the risk of colorectal neoplasia in a screened population of Northeast Scotland. Eur J Cancer Prev. 2015;24:296-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Davenport JR, Cai Q, Ness RM, Milne G, Zhao Z, Smalley WE, Zheng W, Shrubsole MJ. Evaluation of pro-inflammatory markers plasma C-reactive protein and urinary prostaglandin-E2 metabolite in colorectal adenoma risk. Mol Carcinog. 2016;55:1251-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Henry CJ, Sedjo RL, Rozhok A, Salstrom J, Ahnen D, Levin TR, D’Agostino R, Haffner S, DeGregori J, Byers T. Lack of significant association between serum inflammatory cytokine profiles and the presence of colorectal adenoma. BMC Cancer. 2015;15:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Song M, Mehta RS, Wu K, Fuchs CS, Ogino S, Giovannucci EL, Chan AT. Plasma Inflammatory Markers and Risk of Advanced Colorectal Adenoma in Women. Cancer Prev Res (Phila). 2016;9:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1747] [Cited by in RCA: 1808] [Article Influence: 106.4] [Reference Citation Analysis (0)] |
| 25. | Popivanova BK, Kitamura K, Wu Y, Kondo T, Kagaya T, Kaneko S, Oshima M, Fujii C, Mukaida N. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest. 2008;118:560-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 467] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 26. | Tonstad S, Cowan JL. C-reactive protein as a predictor of disease in smokers and former smokers: a review. Int J Clin Pract. 2009;63:1634-1641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 27. | Choi J, Joseph L, Pilote L. Obesity and C-reactive protein in various populations: a systematic review and meta-analysis. Obes Rev. 2013;14:232-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 521] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 28. | Keum N, Lee DH, Kim R, Greenwood DC, Giovannucci EL. Visceral adiposity and colorectal adenomas: dose-response meta-analysis of observational studies. Ann Oncol. 2015;26:1101-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 29. | Myers GL, Rifai N, Tracy RP, Roberts WL, Alexander RW, Biasucci LM, Catravas JD, Cole TG, Cooper GR, Khan BV. CDC/AHA Workshop on Markers of Inflammation and Cardiovascular Disease: Application to Clinical and Public Health Practice: report from the laboratory science discussion group. Circulation. 2004;110:e545-e549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 198] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 30. | Smidowicz A, Regula J. Effect of nutritional status and dietary patterns on human serum C-reactive protein and interleukin-6 concentrations. Adv Nutr. 2015;6:738-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 31. | Szylberg Ł, Janiczek M, Popiel A, Marszałek A. Expression of COX-2, IL-1β, TNF-α and IL-4 in epithelium of serrated adenoma, adenoma and hyperplastic polyp. Arch Med Sci. 2016;12:172-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Greenhough A, Smartt HJ, Moore AE, Roberts HR, Williams AC, Paraskeva C, Kaidi A. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis. 2009;30:377-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 848] [Cited by in RCA: 948] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 33. | Groblewska M, Mroczko B, Wereszczyńska-Siemiatkowska U, Kedra B, Lukaszewicz M, Baniukiewicz A, Szmitkowski M. Serum interleukin 6 (IL-6) and C-reactive protein (CRP) levels in colorectal adenoma and cancer patients. Clin Chem Lab Med. 2008;46:1423-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Coleman HGG S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH