Published online Mar 14, 2017. doi: 10.3748/wjg.v23.i10.1828
Peer-review started: October 27, 2016
First decision: December 28, 2016
Revised: January 6, 2017
Accepted: January 18, 2017
Article in press: January 18, 2017
Published online: March 14, 2017
Processing time: 138 Days and 6.3 Hours
To establish and evaluate an experimental porcine model of fistula-in-ano.
Twelve healthy pigs were randomly divided into two groups. Under general anesthesia, the experimental group underwent rubber band ligation surgery, and the control group underwent an artificial damage technique. Clinical magnetic resonance imaging (MRI) and histopathological evaluation were performed on the 38th d and 48th d after surgery in both groups, respectively.
There were no significant differences between the experimental group and the control group in general characteristics such as body weight, gender, and the number of fistula (P > 0.05). In the experimental group, 15 fistulas were confirmed clinically, 13 complex fistulas were confirmed by MRI, and 11 complex fistulas were confirmed by histopathology. The success rate in the porcine complex fistula model establishment was 83.33%. Among the 18 fistulas in the control group, 5 fistulas were confirmed clinically, 4 complex fistulas were confirmed by MRI, and 3 fistulas were confirmed by histopathology. The success rate in the porcine fistula model establishment was 27.78%. Thus, the success rate of the rubber band ligation group was significantly higher than the control group (P < 0.05).
Rubber band ligation is a stable and reliable method to establish complex fistula-in-ano models. Large animal models of complex anal fistulas can be used for the diagnosis and treatment of anal fistulas.
Core tip: Different new surgical methods for fistula-in-ano such as fibrin sealant or AFP plug should be used to establish animal models before a clinical trial begins. We established an experimental porcine anal fistula model using a rubber band ligation method which may provide a possible platform for anorectal fistula research. This surgical method using rubber band ligation is more stable and reliable than an artificial damage technique. Our porcine model of fistula-in-ano was confirmed by histopathology and anatomically similar to humans. This porcine model of fistula-in-ano can be used in the diagnosis and treatment of anal fistulas.
- Citation: A Ba-Bai-Ke-Re MMTJ, Chen H, Liu X, Wang YH. Experimental porcine model of complex fistula-in-ano. World J Gastroenterol 2017; 23(10): 1828-1835
- URL: https://www.wjgnet.com/1007-9327/full/v23/i10/1828.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i10.1828
Fistula-in-ano is a common disorder in general surgery. A simple, open fistulectomy or fistulotomy is effective for simple or low fistulas. However, complex or multiple-tract fistulas are difficult to treat due to a high rate of recurrence and a significant risk of incontinence. Surgery remains the only method of healing anal fistulas. To date, various treatment methods have been used for fistula-in-ano due to the lack of a standard treatment. Numerous surgical procedures including fistulotomy, the Seton cutting procedure, fibrin glue, endoanal advancement flaps, and ligation of the intersphincteric fistula track have been performed. The main aim of anal fistula treatment is to eradicate internal and external openings while preserving anorectal continence. Many treatments can result in sphincter damage and fecal incontinence. For example, use of the Seton cutting procedure is associated with healing rates of approximately 80%-100%, but fecal incontinence rates of up to 60%[1-3]. Fistulotomy results in healing in approximately 75%-100% of patients, but impairs continence in up to 60%, especially in those with a complex fistula[4-6]. Fistulotomy, although extremely effective in treatment of anal fistulas, is not a feasible option when the fistula tract incorporates a significant amount of internal and external anal sphincter, as is the case in many high trans-sphincteric fistulas[7,8]. The mucosal advancement flap, a technically precise operation, is the best established alternative to fistulotomy, with reported success rates ranging from 37% to 89%[9,10] and with incontinence rates ranging from 9% to 21%[9,11]. Fibrin glue injection through the fistula tract is a minimally invasive, sphincter-sparing alternative, but long-term recurrence reaches 69%-100%[12]. To date, there is no standard management method for anal fistulas as none of these procedures has a high chance of success and no risk, and depends on the patients’ anatomy, fistula anatomy and the patient’s wishes. A variety of new surgical procedures for anorectal fistula or anal gland infection[13] should be performed in animals in order to minimize the possible risk to humans. Small animal models of anal fistula have not been well described in terms of the pathogenesis and healing of anorectal fistulas. Buchanan et al[14] successfully established large animal models of anal fistulas and described possible surgical techniques. Although their research provided an important experimental platform for an anal fistula model using large animals, the exact pathogenesis of fistula formation was not described[15]. Different and novel surgical methods, such as fibrin sealant or AFP plug, should be undertaken using animal models before clinical trials are performed. It is necessary to establish large animal models in this research field. Ideal animals used in fistula models are monkeys or dogs[16,17]. However, monkey or dog experiments are not practical. The main purpose of the present prospective study was to establish an experimental porcine anal fistula model using a rubber band ligation method and to provide a possible platform for anorectal fistula research.
This research was conducted in accordance with animal ethics regulation laws published in 1986 and an animal protection law of the People’s Republic of China. Care and handling of the animals were in compliance with the “Guide for the Care and Use of Laboratory Animals”. Written approval was obtained from the animal experiment ethics committee of The First Affiliated Hospital of Xinjiang Medical University. All aspects of this study, including animal husbandry, were undertaken at the First Affiliated Hospital of Xinjiang Medical University. Considering the similarities of the internal and external sphincter to human anorectal structure, pigs were selected for this research. Twelve healthy pigs (with an average weight of 39-45 kg), chosen from the experimental animal center of the First Affiliated Hospital of Xinjiang Medical University, were used to establish an anal fistula model using rubber band ligation surgery. All pigs were randomly divided into two groups in accordance with a computerized random number table. Under general anesthesia, the experimental group underwent rubber band ligation surgery, and the control group underwent an artificial damage technique. An intramuscular injection of the anesthetic Zoteil 50 at 1 mL/kg was administered. Two mL of lidocaine was administered to the local area for efficient general anesthesia.
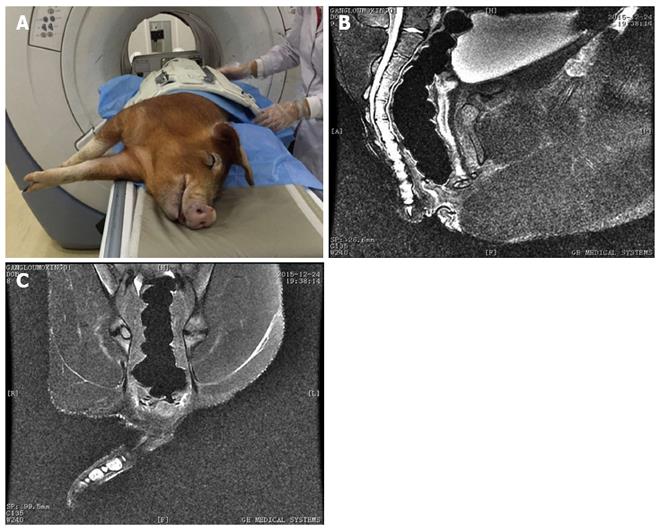
Under general anesthesia, six experimental animals underwent rubber band ligation surgery, and 18 models of fistula-in-ano were created. The control group underwent an artificial damage technique. There were no significant differences between the experimental group and the control group regarding general characteristics such as body weight, gender, and number of fistula (P > 0.05) (Table 1). All animals received a visual examination, palpation, and anoscopy in the right lateral position after general anesthesia and disinfection. On the 28th d after surgery, the rubber bands were removed in the experimental group. Clinical magnetic resonance imaging (MRI) and histopathological evaluation were performed on the 38th d and 48th d after surgery in both groups, respectively. Clinical and histopathological evaluations were also carried out in both groups.
| Number of animals | Number of fistulas | Male/female ratio | Average weight | |
| Experimental group | 6 | 18 | 3/3 | 39.3 ± 1.1 |
| Control group | 6 | 18 | 4/2 | 38.9 ± 1.2 |
| χ2/t value | 0.3429 | 0.6019 | ||
| P value | 0.5582 | 0.5606 |
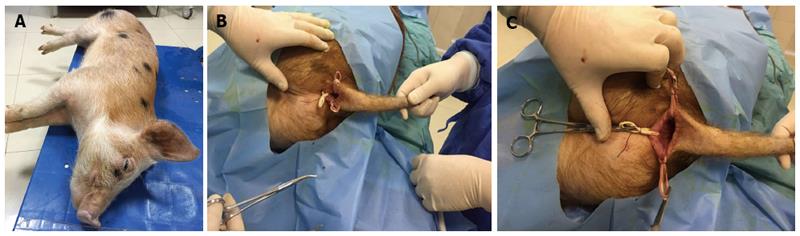
Modeling methods in the experimental group: All animals in the experimental group underwent regular disinfection, visual anus examination, and diagnostic anoscopy under general anesthesia. A 0.5 cm minimal incision in the anus was made in the lithotomy position at the 3, 9, and 12 o’clock positions. The 6 o’clock position was not used as it was extremely close to the pig’s tail and coccyx, and to avoid the risk of sepsis. A rubber band Seton was directly crossed through the anal sphincter and inserted into the sphincter using forceps. The incision was fixed using a gauze bandage. All animals were placed in the postoperative care unit until they regained consciousness. Vital signs were monitored after surgery.
Modeling methods in the control group: All perioperative care, preparation, and incisions in the control group were the same as for the experimental group. Forceps were directly crossed through the anal sphincter and inserted into the sphincter. No additional materials were inserted into this artificial canal. Following the establishment of these two models, all animals received postoperative monitoring and a standard diet.
Postoperative intervention: All wounds in the two groups were observed and dressed daily. All Setons were extracted on the 28th d under general anesthesia. As anus sonography is unstable, all animals underwent anorectal MRI after Seton extraction in order to determine anatomical structure on the 38th d after surgery according to the diagnostic guide for MRI[18]. On the 48th d after surgery, fistulas were resected again and histopathological examinations were performed. Following these procedures, all animals were given their usual diet.
The animal protocol in this research was designed to minimize animal pain and discomfort. The pigs were acclimatized to laboratory conditions (23 °C, 12h/12h light/dark, 50% humidity, ad libitum access to food and water) for eight weeks prior to experimentation. Intragastric gavage was carried out in conscious animals, using straight gavage needles appropriate for animal size (35-45 g body weight: 22 gauge, 1 inch length, 1.25 mm ball diameter). All animals were euthanized by barbiturate overdose (intravenous injection, 150 mg/kg pentobarbital sodium) for tissue collection.
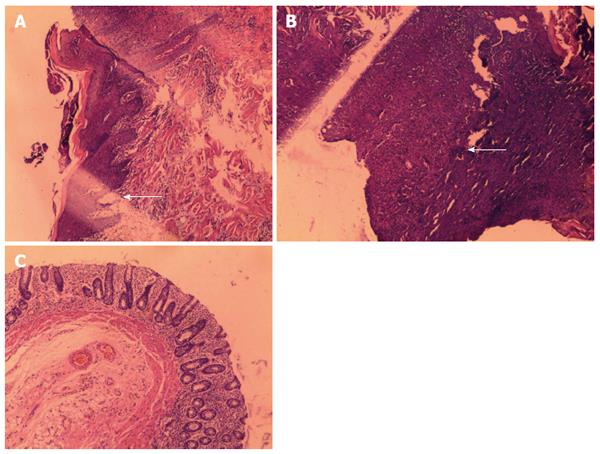
All animals were reexamined under general anesthesia to determine the clinical state of the created fistulas. Fistula creation was considered successful when a clear outer opening on clinical observation or obvious structures on MRI were observed. The basic structure of the fistula and hyperplastic tissue or fibroblast components were considered histopathological confirmation of the fistula model (Figures 1, 2 and 3). The end point of fistula healing was the disappearance of the lumen on MRI or clinical observation.
Numerical data were expressed as the median and ranges. Excel data analysis was performed using SPSS 19.0 software. The χ2 test was used to compare related data. P < 0.05 was considered statistically significant.
Following surgery, all animals were alive with no obvious adverse effects and no postoperative complications such as abscess or general infection. There were no significant differences between the experimental group and the control group regarding general characteristics such as body weight, gender, and number of fistulas (P > 0.05). In the experimental group, 15 fistulas were confirmed clinically, 13 complex fistulas (7 lower type and 6 hilar type) were confirmed by MRI, and 11 fistulas were confirmed by histopathology. All 11 fistulas assessed histologically had a lumen and surrounding granulation tissue. The granulation tissue was similar to that seen in human fistula-in-ano. The clinical success rate of the porcine fistula model was 83.33%. In the control group of 18 fistulas, 5 fistulas were confirmed clinically, 4 fistulas were confirmed by MRI, and 3 fistulas were confirmed by histopathology. The clinical success rate of the porcine fistula model was 27.78%. The success rate of the rubber band ligation group was significantly better than the control group (P < 0.05) (Table 2).
| Group | Animals | No. of fistulas | Number of successful clinical models | MRI showing typical characteristics of complex fistulas | Pathological features showing fixed fistulas |
| Experimental group | 6 | 18 | 15 | 13 | 11 |
| Control group | 6 | 18 | 5 | 4 | 3 |
| χ2 value | 9.1125 | 7.1331 | 7.4805 | ||
| P value | 0.0025 | 0.0076 | 0.0062 |
Anorectal fistula is an abnormal channel between the anal canal and perianal skin due to chronic infection, and is also called an anal fistula, or fistula-in-ano. Highly complex anal fistulas are very common and difficult to treat in clinical practice[19-21]. The recurrence rate of highly complex fistulas is high, and the healing time is long. Satisfactory defecation control after traditional open surgery causes major difficulties in the treatment of this disease. Although anal fistulas are caused by chronic infections, it is not the same as a typical superficial chronic infection. The traditional treatment of anal fistulas, such as fistulotomy, fistulectomy, or Seton ligation therapy, can be associated with longer healing time and significant pain in patients. In addition, care can be complicated by anal dysfunction or even fecal incontinence[22,23]. According to previous research, a mucosal advancement flap is an alternative method for repairing fistula-in-ano; however, this procedure requires demanding technical support and has a high postoperative recurrence rate. Although fibrin glue treatment is effective for fistulas, according to a recent meta-analysis, the success rate varies. These surgical procedures have obvious shortcomings, and a standard procedure is needed for treating anal fistulas. Reducing recurrence and avoiding postoperative incontinence are the final objectives of most new minimally invasive surgical methods[24,25]. However, animal experiments should be carried out to confirm the safety and efficacy of the method before it is put into clinical application.
With the application of new concepts in perianal anatomy, anorectal physiology, and the pathogenesis of anal fistulas, there is an increasing tendency to perform new therapeutic methods, and there has been great progress in anorectal studies. Most of the studies on anorectal diseases were based on clinical aspects such as diagnosis or clinical treatment. However, there is less information on basic research, such as animal experiments. Due to technical difficulties and high research cost, it is not easy to carry out basic research on large animals. The purpose of establishing the present animal model was to fill this gap and provide a platform for further research in this field. This study provides a feasible animal model for further study on the diagnosis and treatment of anal fistulas.
In this study, we confirmed that there were no significant differences between the experimental group and the control group regarding the general characteristics such as body weight, gender, and number of fistulas (P > 0.05). In the experimental group, 15 fistulas were confirmed clinically, 13 complex fistulas were confirmed by MRI, and 11 fistulas were confirmed by histopathology. The clinical success rate of the porcine fistula model establishment was 83.33%. This result was encouraging. In the control group of 18 fistulas, 5 fistulas were confirmed clinically, 4 fistulas were confirmed by MRI, and 3 fistulas were confirmed by histopathology. The clinical success rate of the porcine fistula model was 27.78%. The success rate in the rubber band ligation group was significantly higher than that in the control group (P < 0.05). The final results indicated that the rubber band ligation method was a better animal model of fistula-in-ano. We consider that use of the rubber band ligation technique is stable and reliable, and better than the artificial damage technique.
As it is impossible to resect the anal canal in humans, the ideal experimental animal is the monkey. These animals have typical anal gland structures similar to humans; however, considering the national animal protection laws, we selected pigs instead and obtained satisfactory models of fistula-in-ano. As shown by MRI and histopathological analysis, we established 15 anal fistula models. We found scar granulation tissue near the fistula. The morphology of the anal muscle was very similar to human internal and external anal sphincter structures. Model fistulas in the experimental group had rich granulation tissue confirmed by MRI and histopathology.
We designed the anal fistula model using pigs as a large animal experimental model of fistula-in-ano is essential when histopathologic evaluations and reproducibility are required. This study presents a successful large animal experimental model of fistula-in-ano, which satisfies the need for new management alternatives such as an anal fistula plug or stem cells.
We believe that the rubber band ligation procedure creates a better fistula model. This model had good tolerance. We observed the experimental animals for 60 d; 15 did not heal naturally, and the three remaining fistulas self-healed. Self-healing may be related to the anti-infection ability of the porcine itself, but the exact mechanism is unclear[26-28] and requires further study. We found that the anal fistulas established using the rubber band ligation method simulated the pathophysiology of a clinical anal fistula. Compared with the animal model using BLAKE silicone drains by Aikawa et al[29], the rubber band ligation model was easily created and observed. In the aforementioned research, the authors confirmed the construction of a fistula according to clinical manifestations. However, they did not describe how the animal model was established or observed using measurement methods such as MRI or histopathology. We conclude that the rubber band ligation method was reliable and resulted in less damage to the normal surrounding tissues. Marianas[30] constructed an anal fistula model using steel wire. However, the author was unable to detect possible changes in the surrounding tissues and the state of the sphincter. We believe that steel wire can easily lead to unnecessary damage to normal tissues. In the present study, we also found many epithelial cells, fibroblasts, inflammatory cells, and muscle cell proliferation around the fistula. It is possible that the created porcine fistula model may be associated with the epithelization of chronic inflamed tissue. We also conclude that simple injury does not create a good anal fistula model. It is necessary to provide enough time for the granulation process near the artificial fistula.
With regard to durability and follow-up in this study, we were unable to demonstrate durability (longer length of time, stability without Seton) due to resection of the anus and sphincter at the end of the experiment. Some fistulas healed in the control group; thus, these animals showed good healing of damage in the anorectal area. Therefore, it is difficult to determine whether a stable fistula can be obtained after the Seton is removed. Further investigations are required to obtain satisfactory fistulas. In addition, we were unable to detect the length of the puborectalis muscle which is closely related to the diagnosis of complex fistula-in-ano.
To date, complex human anal fistulas have not been effectively resolved[31-33]. Many anorectal surgeons have attempted to use minimally invasive surgical treatments for anal fistulas, but have failed. Our research team also tried to determine the efficacy of minimally invasive surgery, but the results after a long follow-up period indicated that the final success rate was unsatisfactory[34]. In order to obtain more precise proof regarding pathogenesis, further animal studies and other fundamental researches are required. The porcine anus is anatomically similar to humans, which was confirmed by MRI and histology. We conclude that use of rubber band ligation to establish a complex anorectal fistula was stable and reliable. Rubber band ligation was better than the artificial damage technique. Furthermore, the experimental porcine anal fistula model using rubber band ligation can be used in the diagnosis and treatment of anal fistulas. Large animal models of anal fistula can also be used for other anorectal diseases such as hemorrhoids, anal fissure and anorectal abscess formation.
Compared with the animal model using BLAKE silicone drains by Aikawa et al[29], the rubber band ligation model was easily created and observed. We searched for articles on animal anal fistula models using PubMed and Web of Science databases, however, we only identified 4 relevant articles. The researchers in these studies confirmed the establishment of fistula according to clinical manifestations only. They did not describe how the animal model was established or the measurement methods used such as pathophysiology, MRI and histopathology. In our research, we determined that the rubber band ligation method was reliable using MRI or histopathology. Although we were unable to determine the pathophysiologic evidence of the model, we believe that imaging and pathology assessment were more practical than pathophysiology. In addition, the pathophysiologic determination of fistula is not easy. Anal fistula models established using the rubber band ligation method could simulate the pathophysiology of clinical anal fistulas. We will carry out further studies in order to obtain pathophysiologic evidence of rubber band ligation in the near future.
The authors would like to thank the members of the animal experiment center of Xinjiang Medical University for their technical support; Dr. Turgunjan Tuerxun for his careful language assistance; and Xiang Yan in Xinjiang Medical University for his assistance in careful statistical analysis.
Fistula-in-ano is a common disorder in general surgery. A simple open fistulectomy or fistulotomy is effective for simple or low fistula. However, complex or multiple tract higher fistulas are difficult to treat due to a high rate of recurrence and a significant risk of incontinence. Different new surgical methods such as fibrin sealant or AFP plug should be carried out using animal models before clinical trials are performed. There is still a need to establish large animal models in this research field.
The main purpose of this prospective study was to establish an experimental porcine anal fistula model using a rubber band ligation method and to provide a possible platform for anorectal fistula research.
Surgery using rubber band ligation was stable and reliable. This method was better than the artificial damage technique. Fistulas in the experimental group had rich granulation tissue, which was confirmed by magnetic resonance imaging (MRI) and histopathology.
In order to obtain more precise proof regarding pathogenesis, further animal studies and other fundamental researches are needed. The porcine anus is anatomically similar to humans, which was confirmed by MRI and histology. Furthermore, the experimental porcine or large animal models of anal fistula model can be used in the diagnosis and treatment of anal fistulas.
Animal models are a central component of studies aimed at understanding the pathophysiological or anatomical bases of certain diseases, and are essential in the development of new, more effective therapies which are desperately needed.
It is a good original research about the establishment and assess of an experimental porcine model of fistula-in-ano in large animal models. They demonstrate that the surgical method using rubber ligation to establish complex fistula-in-ano was stable and reliable. This method was better than the artificial damage process.
| 1. | Rizzo JA, Naig AL, Johnson EK. Anorectal abscess and fistula-in-ano: evidence-based management. Surg Clin North Am. 2010;90:45-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | Ho KS, Tsang C, Seow-Choen F, Ho YH, Tang CL, Heah SM, Eu KW. Prospective randomised trial comparing ayurvedic cutting seton and fistulotomy for low fistula-in-ano. Tech Coloproctol. 2001;5:137-141. [PubMed] |
| 3. | Ommer A, Herold A, Berg E, Fürst A, Sailer M, Schiedeck T. Cryptoglandular anal fistulas. Dtsch Arztebl Int. 2011;108:707-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Shawki S, Wexner SD. Idiopathic fistula-in-ano. World J Gastroenterol. 2011;17:3277-3285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 5. | Whiteford MH, Kilkenny J, Hyman N, Buie WD, Cohen J, Orsay C, Dunn G, Perry WB, Ellis CN, Rakinic J. Practice parameters for the treatment of perianal abscess and fistula-in-ano (revised). Dis Colon Rectum. 2005;48:1337-1342. [PubMed] |
| 6. | Deeba S, Aziz O, Sains PS, Darzi A. Fistula-in-ano: advances in treatment. Am J Surg. 2008;196:95-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Jordán J, Roig JV, García-Armengol J, García-Granero E, Solana A, Lledó S. Risk factors for recurrence and incontinence after anal fistula surgery. Colorectal Dis. 2010;12:254-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | van Koperen PJ, Safiruddin F, Bemelman WA, Slors JF. Outcome of surgical treatment for fistula in ano in Crohn’s disease. Br J Surg. 2009;96:675-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 9. | Athanasiadis S, Köhler A, Nafe M. Treatment of high anal fistulae by primary occlusion of the internal ostium, drainage of the intersphincteric space, and mucosal advancement flap. Int J Colorectal Dis. 1994;9:153-157. [PubMed] |
| 10. | van der Hagen SJ, Baeten CG, Soeters PB, van Gemert WG. Long-term outcome following mucosal advancement flap for high perianal fistulas and fistulotomy for low perianal fistulas: recurrent perianal fistulas: failure of treatment or recurrent patient disease? Int J Colorectal Dis. 2006;21:784-790. [PubMed] |
| 11. | Mizrahi N, Wexner SD, Zmora O, Da Silva G, Efron J, Weiss EG, Vernava AM, Nogueras JJ. Endorectal advancement flap: are there predictors of failure? Dis Colon Rectum. 2002;45:1616-1621. [PubMed] |
| 12. | Loungnarath R, Dietz DW, Mutch MG, Birnbaum EH, Kodner IJ, Fleshman JW. Fibrin glue treatment of complex anal fistulas has low success rate. Dis Colon Rectum. 2004;47:432-436. [PubMed] |
| 13. | McColl I. The comparative anatomy and pathology of anal glands. Arris and Gale lecture delivered at the Royal College of Surgeons of England on 25th February 1965. Ann R Coll Surg Engl. 1967;40:36-67. [PubMed] |
| 14. | Buchanan GN, Sibbons P, Osborn M, Bartram CI, Ansari T, Halligan S, Cohen CR. Experimental model of fistula-in-ano. Dis Colon Rectum. 2005;48:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Harvey CE. Perianal fistula in the dog. Vet Rec. 1972;91:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Greiner TP. Surgical treatment of canine perianal fistulas. Vet Med Small Anim Clin. 1981;76:663-664. [PubMed] |
| 18. | Lunniss PJ, Armstrong P, Barker PG, Reznek RH, Phillips RK. Magnetic resonance imaging of anal fistulae. Lancet. 1992;340:394-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 115] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Lenisa L, Espìn-Basany E, Rusconi A, Mascheroni L, Escoll-Rufino J, Lozoya-Trujillo R, Vallribera-Valls F, Mégevand J. Anal fistula plug is a valid alternative option for the treatment of complex anal fistula in the long term. Int J Colorectal Dis. 2010;25:1487-1493. [PubMed] |
| 20. | de la Portilla F, Rada R, Jiménez-Rodríguez R, Díaz-Pavón JM, Sánchez-Gil JM. Evaluation of a new synthetic plug in the treatment of anal fistulas: results of a pilot study. Dis Colon Rectum. 2011;54:1419-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Ommer A, Herold A, Joos A, Schmidt C, Weyand G, Bussen D. Gore BioA Fistula Plug in the treatment of high anal fistulas--initial results from a German multicenter-study. Ger Med Sci. 2012;10:Doc13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 22. | Ratto C, Litta F, Parello A, Donisi L, Zaccone G, De Simone V. Gore Bio-A® Fistula Plug: a new sphincter-sparing procedure for complex anal fistula. Colorectal Dis. 2012;14:e264-e269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Chan S, McCullough J, Schizas A, Vasas P, Engledow A, Windsor A, Williams A, Cohen CR. Initial experience of treating anal fistula with the Surgisis anal fistula plug. Tech Coloproctol. 2012;16:201-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Han JG, Xu HM, Song WL, Jin ML, Gao JS, Wang ZJ, Yang XQ. Histologic analysis of acellular dermal matrix in the treatment of anal fistula in an animal model. J Am Coll Surg. 2009;208:1099-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Adedeji OA, Bailey CA, Varma JS. Porcine dermal collagen graft in abdominal-wall reconstruction. Br J Plast Surg. 2002;55:85-86. [PubMed] |
| 26. | O’Riordan JM, Datta I, Johnston C, Baxter NN. A systematic review of the anal fistula plug for patients with Crohn’s and non-Crohn’s related fistula-in-ano. Dis Colon Rectum. 2012;55:351-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 27. | van Koperen PJ, D’Hoore A, Wolthuis AM, Bemelman WA, Slors JF. Anal fistula plug for closure of difficult anorectal fistula: a prospective study. Dis Colon Rectum. 2007;50:2168-2172. [PubMed] |
| 28. | Schwandner O, Stadler F, Dietl O, Wirsching RP, Fuerst A. Initial experience on efficacy in closure of cryptoglandular and Crohn’s transsphincteric fistulas by the use of the anal fistula plug. Int J Colorectal Dis. 2008;23:319-324. [PubMed] |
| 29. | Aikawa M, Miyazawa M, Okada K, Akimoto N, Koyama I, Yamaguchi S, Ikada Y. A newly designed anal fistula plug: clinicopathological study in an experimental iatrogenic fistula model. Int Surg. 2013;98:122-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Arakakia MS, Henrique C, dos Santos M, Falc GR, Cassinob CP, Nakamurab RK, Gomesb NF, Coutinho Santosb RG. Experimental model of anal fistula in rats. J Coloproctol. 2013;33:135-138. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Ky AJ, Sylla P, Steinhagen R, Steinhagen E, Khaitov S, Ly EK. Collagen fistula plug for the treatment of anal fistulas. Dis Colon Rectum. 2008;51:838-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 32. | Lawes DA, Efron JE, Abbas M, Heppell J, Young-Fadok TM. Early experience with the bioabsorbable anal fistula plug. World J Surg. 2008;32:1157-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 33. | Christoforidis D, Etzioni DA, Goldberg SM, Madoff RD, Mellgren A. Treatment of complex anal fistulas with the collagen fistula plug. Dis Colon Rectum. 2008;51:1482-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 34. | A ba-bai-ke-re MM, Wen H, Huang HG, Chu H, Lu M, Chang ZS, Ai EH, Fan K. Randomized controlled trial of minimally invasive surgery using acellular dermal matrix for complex anorectal fistula. World J Gastroenterol. 2010;16:3279-3286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Munoz M, Ren JA S- Editor: Qi Y L- Editor: Ma JY E- Editor: Zhang FF