Published online Nov 28, 2016. doi: 10.3748/wjg.v22.i44.9734
Peer-review started: June 29, 2016
First decision: July 29, 2016
Revised: August 12, 2016
Accepted: September 6, 2016
Article in press: September 6, 2016
Published online: November 28, 2016
Processing time: 151 Days and 7.1 Hours
To evaluate how mucosal bacteria impact on the spontaneous and muramyl dipeptide (MDP)-induced inflammation in Crohn’s disease (CD) and ulcerative colitis (UC).
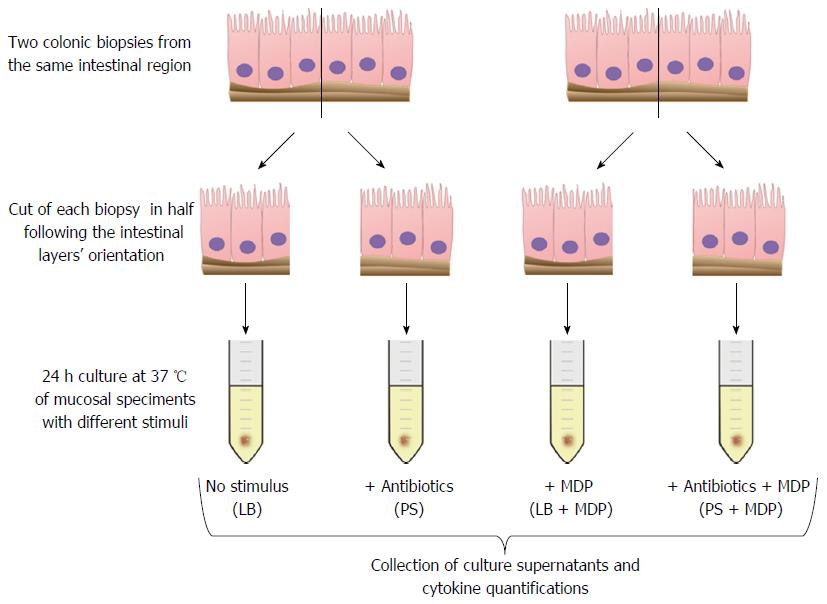
Colonic mucosal biopsies were collected from children with active or remissive CD, UC and controls. Two tissue samples were taken from inflamed mucosal segments (in patients with active disease) or from non-inflamed mucosa [in patients in remission or in healthy controls (HC)]. Experiments were performed in the presence or absence of antibiotics, to assess whether the disease-associated microbiota can modulate the cytokine response ex vivo. For this purpose, each specimen was half-cut to compare spontaneous and MDP-induced inflammation in the presence of live bacteria (LB) or antibiotics. After 24 h of culture, an array of 17 cytokines was assessed in supernatants. Statistical analyses were performed to find significant differences in single cytokines or in patterns of cytokine response in the different groups.
We demonstrated that subjects with CD display a spontaneous production of inflammatory cytokines including granulocyte-colony stimulating factor (G-CSF), interleukin (IL) 6, IL8, IL10 and IL12, that was not significantly influenced by the addition of antibiotics. UC specimens also displayed a trend of increased spontaneous secretion of several cytokines, which however was not significant due to broader variability among patients. After the addition of antibiotics, spontaneous IL8 secretion was significantly higher in UC than in controls. In HC, a trend towards the weakening of spontaneous IL8 production was observed in the presence of live mucosal bacteria with respect to the presence of antibiotics. In contrast, in the presence of LB UC showed an increasing trend of spontaneous IL8 production, while MDP stimulation resulted in lower IL8 production in the presence of antibiotics. We also showed that subjects with CD seem to have a lowered production of IL8 in response to MDP in the presence of LB. Only with the addition of antibiotics, likely reducing the contribution of LB, multivariate statistical analysis could identify the combination of measures of G-CSF, tumor necrosis factor alpha, IL4 and IL17 as a good discriminator between CD and UC.
We showed that the presence of LB or antibiotics can significantly influence the inflammatory response ex vivo in inflammatory bowel diseases.
Core tip: Even though previous studies have already considered cytokine secretions as marker of an inflammatory condition and mucosal imbalance in Crohn’s disease (CD) subjects, they did not take into account the autochthonous colonization of the intestinal mucosa by the disease-associated microbiota. In this work we investigated whether the microbiota can modulate the ex vivo cytokine response, in the presence or absence of antibiotics, or if a selection of cytokines could discriminate different inflammatory bowel diseases types. Through a multivariate logistic model we identified, only in specimens treated with antibiotics, a specific cytokine profile able to discriminate CD from ulcerative colitis.
- Citation: Loganes C, Valencic E, Pin A, Marini E, Martelossi S, Naviglio S, De Leo L, Not T, Monasta L, Tommasini A, Marcuzzi A. Ex vivo response to mucosal bacteria and muramyl dipeptide in inflammatory bowel disease. World J Gastroenterol 2016; 22(44): 9734-9743
- URL: https://www.wjgnet.com/1007-9327/full/v22/i44/9734.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i44.9734
Inflammatory bowel diseases (IBD) are intestinal disorders characterized by a complex inflammatory process that may involve any part of the digestive tract. IBD include mainly Crohn’s disease (CD) and ulcerative colitis (UC), which are characterized by a different spectrum of clinical and pathological features[1,2].
In CD, the inflammation is extended through the whole intestinal wall of the gut with a segmental distribution, while in UC the inflammatory process is continuous and predominantly confined to the colonic mucosa[2].
IBD are characterized by an aberrant activation of the mucosal immune system with reaction to luminal content in genetically susceptible subjects[3], resulting in a chronic inflammatory state with dysregulation of several cytokines[4-6].
Cytokines are important mediators in the gut-associated immune system, both in physiological conditions and in disease[7]. In normal conditions, the mucosal immune activation reflects an active balance between tolerance of luminal content and response to commensal and pathogenic bacteria. Indeed, this basal activation is associated with a predominance of cytokines such as interleukin (IL) 10 and transforming growth factor beta and the production of antimicrobial peptides that can contribute to the shaping of the “healthy” microbiota. In pathological conditions, other cytokines and mediators are produced, which can, at the same time, lead to tissue injury and altered regulation of the microbiota and dysbiosis[4,8-10].
Chronic tissue damage results in clinical intestinal symptoms in IBD patients (abdominal cramping and pain, diarrhea, constipation and bowel obstruction, nausea and vomiting), even though extra-intestinal manifestations (weight loss, fever, asthenia and arthralgias) and immune disorders are also associated with this pathologies[11].
Despite the large number of studies and clinical analyses, several issues remain to be solved regarding the immune pathogenesis of IBD. In particular, the therapy of IBD is still based mainly on anti-inflammatory and immunosuppressive therapies, while mounting evidence supports the idea that defective immunity may underlie IBD pathogenesis, especially in cases with an early onset in childhood[12]. In fact, excessive inflammation in IBD could either result from a hyper-response of the immune system or it may just represent a compensatory response to luminal contest in the background of defective immunity[13].
Indeed, CD has been alternatively classified as an autoinflammatory disorder or a disease with primary immune deficiency[14].
On the one hand, some studies suggested that CD is an autoinflammatory disease, characterized by an increase in the inflammatory response due to nucleotide-binding oligomerization domain-containing protein 2 (NOD2) variants, even though data supporting this hypothesis are controversial. NOD2 is an intracellular receptor involved in gastrointestinal immunity; it detects bacterial components and has been identified as the first susceptibility gene for CD[15-17]. The binding of muramyl dipeptide (MDP) to NOD2 leads to the downstream activation of mitogen-activated protein kinases and nuclear factor-kappa beta (NF-κB) signaling, which in turn stimulates the transcription of several immune response genes. However, early studies have shown the association of the most common mutation 3020insC with a reduced activation of NF-κB[18]. In contrast, later investigations proposed that the pro-inflammatory action is caused by a dysregulated autophagy[19].
On the other hand, several studies have found evidence that CD patients present immune response defects. In fact, a decreased bacterial clearance by the phagocytic system has been observed in peripheral blood cells from patients with CD; furthermore, NOD2-/- mice exhibit a reduced production of antimicrobial peptides by Paneth cells that leads to an imbalance in the microbiota in the presence of certain microbial pathogens, and intestinal inflammation can be prevented in these models by the forced expression of defensins in the intestinal cells[20].
For these reasons, we wanted to describe what happens in the disease itself, not by using simple animal models, but by studying the relationships between the mucosal immune system, influenced by its multigenic background, and the intestinal microbiota colonizing the diseased epithelium.
In these conditions, we tried to understand how the inflammatory response to stimuli such as MDP is influenced ex vivo by the presence of live or antibiotic-inactivated bacteria.
We performed an ex vivo culture of colonic explants to investigate the mucosal response both in spontaneous conditions and after the stimulation with MDP.
Forty-eight children with IBD and eight control subjects were enrolled. Participation in the study was proposed to all patients with IBD or their guardians when the performance of a colonoscopy was needed for clinical assessment, required for the diagnosis or the follow-up of their intestinal disease.
Patients with IBD were divided into four groups based on the type of intestinal disorder and on endoscopic mucosal disease activity: active Crohn's disease (CD, n = 23), remissive Crohn's disease (rCD, n = 3), active ulcerative colitis (UC, n = 15) and remissive ulcerative colitis (rUC, n = 7). Disease activity scores, namely the Pediatric Crohn’s Disease Activity Index and the Pediatric Ulcerative Colitis Activity Index were also calculated. Any other recent acute disease or chronic inflammatory conditions are reported in these patients.
Control subjects were selected among children who underwent colonoscopy as part of their clinical investigations for various reasons not related to inflammatory enteropathies, such as routine evaluation of juvenile intestinal polyps, autoimmune gastritis, unexplained abdominal symptoms and weight loss. They had evidence of normal, non-inflamed mucosa and were thus considered healthy controls (HC).
Colonic mucosal biopsies were taken during colonoscopy. For each subject, two specimens were collected from the same mucosal area, in addition to the biopsies obtained for clinical purposes. In patients with active disease, two inflamed biopsies were collected, while in patients with inactive disease or HC two biopsies were taken from non-inflamed areas.
All subjects were recruited from the Gastroenterology and Clinical Nutrition Unit, Institute for Maternal and Child Health - IRCCS “Burlo Garofolo”, Trieste, Italy and written informed consent was obtained from patients and donors (or their guardians) according to a protocol approved by the Independent Ethical Board at the Institute (n.185/08, 19/08/2008).
Immediately after collection, biopsies were cut in half following the intestinal layers’ orientation and split in two tubes containing a physiological saline solution. Once they reached the laboratory, the specimens were transferred to sterile tubes with culture medium (X-VIVO, Lonza, Verviers, Belgium) supplemented with 10% human AB serum (Sigma-Aldrich, Milano, Italy) and 2 mmol/L L-glutamine (EuroClone, Milano, Italy). The intestinal tissue was exposed to 250 ng/mL MDP (N-Acetylmuramyl-L-alanyl-D-isoglutamine hydrate, Sigma-Aldrich) or medium alone, in the presence or absence of antibiotics: 100 U/mL penicillin (EuroClone) and 0.1 mg/mL streptomycin (EuroClone).
After 24 h culture at 37 °C in a humidified atmosphere (95% air and 5% CO2), supernatants were collected and stored at -20 °C for cytokine analysis.
The cytokines and the chemokines released in the culture medium were measured using the Bio-Plex Pro® Human Cytokine 17-plex Assay (BioRad, Hemel Hempstead, United Kingdom), according to the manufacturer’s instructions.
Analytes examined by this method were IL1β, IL2, IL4, IL5, IL6, IL7, IL8, IL10, IL12 (p70), IL13, IL17, granulocyte-colony stimulating factor (G-CSF), GM-CSF, IFNγ, MCP1 (MCAF), MIP1β, and TNFα.
Samples were analyzed with the Bio-Plex® 200 reader and data were managed using Bio-Plex Manager® software, which returned data as Median Fluorescence Intensity and concentration (pg/mL).
All statistical analyses were carried out using Stata/IC 14.1 (StataCorp LP, College Station, United States) and GraphPad Prism software version 5 (GraphPad, San Diego, United States). Values assumed by cytokines were described as medians and interquartile ranges and represented with box plots using Tukey’s whiskers. Statistical significance was set as P < 0.05 (a), P < 0.01 (b) and P < 0.001 (c).
Associations between cytokines and specific conditions (HC vs IBD, and CD vs UC) were analyzed firstly by univariate logistic regression and then by multivariate logistic regression. For the multiple comparisons carried out by univariate logistic regression, considering the relatively small sample size, which implied having relatively large P values, we decided not to apply any correction to the P value for statistical significance: we realize that we carried out independent univariate logistic regressions on different outcomes and separately for 17 cytokines, chemokines and growth factors. However, this just is considered as a preliminary step for the selection of cytokines to be included in the multivariate analysis: significant variables (P < 0.05 in the univariate analysis) were entered into multivariate analysis. Multivariate logistic regression analysis was employed to determine cytokine profiles associated with the different forms of IBD. Receiver operating characteristic (ROC) curves were constructed to evaluate the performance of the multivariate models. The area under the curve (AUC), sensitivity and specificity were also calculated. For each model, we identified the optimal cut-off by maximizing the sum of sensitivity and specificity.
Clinical characteristics of all patients included in the study, obtained from the medical sheets and from the colonoscopy records, are reported in Supplementary Table 1 and Supplementary Table 2 and summarized in Table 1.
| Patients (n = 56) | Age (yr) (mean ± SD) | Male/female | Active/remission | |
| IBD (n = 48) | CD (n = 26) | 11 ± 3.08 | 16/10 | 23/3 |
| UC (n = 22) | 15 ± 3.88 | 12/10 | 15/7 | |
| CONTROLS (n = 8) | HC (n = 8) | 10 ± 4.60 | 4/4 | - |
Ex vivo culture of biopsies is thought to reproduce both the immunological and the microbiologic background of the disease. However, to isolate the contribution of live bacteria (LB) to inflammation, biopsies were stimulated in absence or in presence of antibiotics. Thus, for each sample we examined four conditions: basal with LB or with antibiotics (PS), and MDP-stimulated with (PS + MDP) or without antibiotics (LB + MDP).
After a 24 h culture, the media were harvested to proceed with the quantification of 17 analytes, including cytokines, chemokines and growth factors (Figure 1).
The levels of three cytokines (IL5, IL7 and IL13) were under the lower limit of detection in all evaluated conditions and were thus excluded.
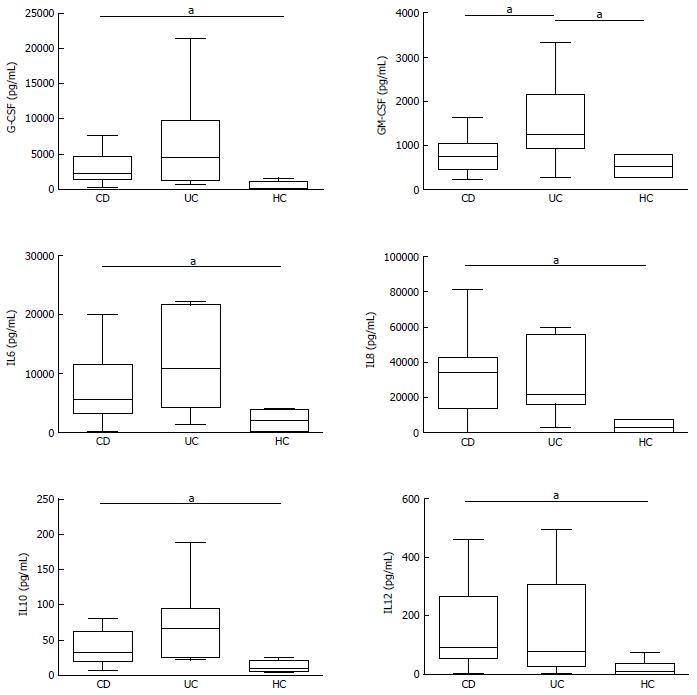
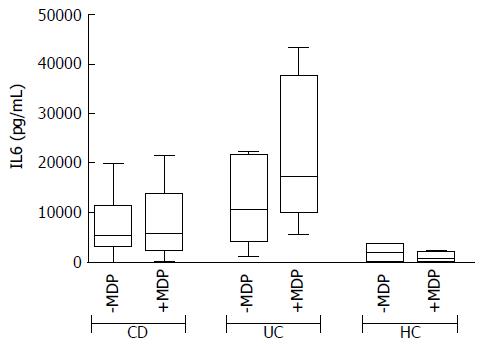
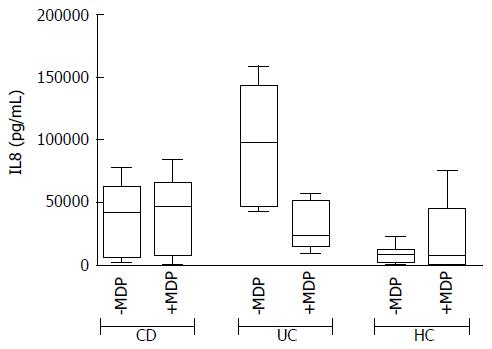
In basal conditions with live mucosal bacteria, five cytokines had levels significantly higher in CD than in controls (HC) (CD vs HC: G-CSF, P = 0.024, IL6 P = 0.037, IL8 P = 0.033, IL10 P = 0.049, IL12, P = 0.028); levels of most cytokines tended to be even higher in UC, but because of the high variability among patients, differences did not reach significance, except for GM-CSF, whose levels were higher in UC compared with both HC (P = 0.031) and CD (P = 0.034) (Figure 2).
Levels of IL1β, IL2, IL4, IL17, IFNγ, TNFα, MCP1 and MIP1β did not differ significantly between the three studied groups, even if median levels of several cytokines such as IL2, IL4 and TNFα tended to be higher in UC than in HC. In general, HC showed a narrower variability compared to patients (Supplementary Figure 1).
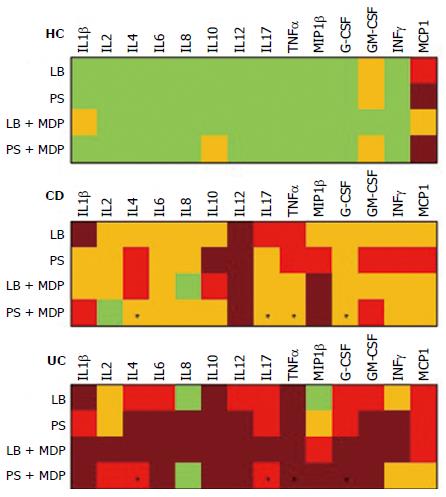
Median levels of all cytokines in the different groups and conditions are rendered in Figure 3 with a color analog scale (CAS): for each cytokine, values 0-24% of the higher value are displayed in green, 25%-49% in orange, 50%-74% in red and above 75% in dark red.
In specimens from patients with endoscopically inactive disease, almost all cytokines were detected, as expected, at lower levels than in active disease. The decrease is significant in UC for GM-CSF (P < 0.01), IL6 (P < 0.05), IL10 (P < 0.05) and TNFα (P < 0.05) (Data not shown).
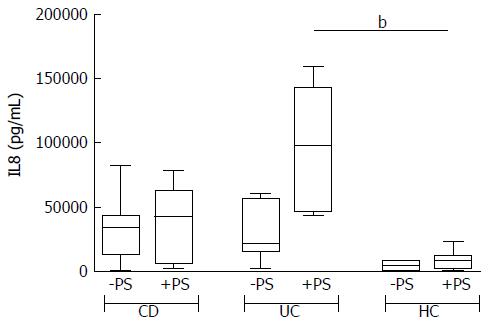
The addition of antibiotics in the bioptic culture to inhibit mucosal bacteria did not lead to relevant changes in the secretion pattern (Supplementary Figure 2), with the exception of IL8 in UC samples. The trend of IL8 increase after antibiotics in UC is so clear that the difference compared with HC becomes significant (Figure 4).
MDP was used to investigate the function of the NOD2-pathway ex vivo in CD compared with UC and HC.
In general, the response to MDP appeared to be higher in UC than in CD (see the CAS in Figure 3). After stimulation with MDP in the presence of LB, even though the differences are not significant, there is a clear trend of increased secretion of IL2, IL6, IL8, IL17 and IFNγ in UC, while no change was observed in CD. Indeed, even if in a smaller scale, HC tended to secrete reduced amounts of IL2, IL6 and IFNγ (Figure 5).
However, after the addition of antibiotics, this pattern was subverted and MDP stimulation failed to produce an evident increase in cytokine secretion in any group and it was even possible to note a reduced trend of secretion for IL8 in UC (Figure 6).
No variation was noted in the remaining cytokines, whose values are reported in supplementary Tables 3 and 4.
Multivariate logistic regression were carried out to assess if any cytokine combination was associated with IBD related outcomes.
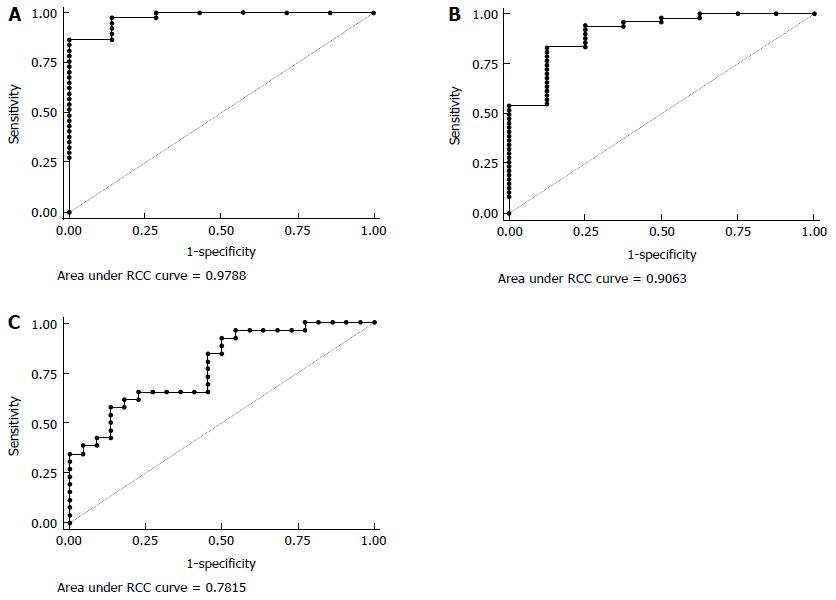
Only experiments in the presence of antibiotics provided profiles significantly associated with IBD. In particular, without MDP stimulation, IL2, IL4 and IL8 were simultaneously associated with IBD in a multivariate analysis (Table 2). ROC analysis (Figure 7A) shows that the AUC was 0.98 (95%CI: 0.93-1.00), and we could have a sensitivity of 100% with a specificity of 71%.
| Variables | OR | 95%CI | P value | Regression coefficients |
| IL2 | 1.553421 | 1.011162-2.386481 | 0.044 | 0.440460 |
| IL4 | 0.473238 | 0.228780-0.978906 | 0.044 | -0.748157 |
| IL8 | 1.000267 | 1.000030-1.000504 | 0.027 | 0.000267 |
| Constant | 0.065259 | 0.003533-1.205600 | 0.067 | -2.729393 |
After MDP stimulation another set of cytokines (MCP1, IL8 and IL10) was significantly associated with IBD (Table 3). The AUC for this model was 0.91 (95%CI: 0.79-1.00) (Figure 7B). Keeping sensitivity for the detection of IBD at 94%, we obtained a specificity of 75%, which would permit to identify 45 IBD patients out of 48, with 2 false positives out of 8 HC.
| Variables | OR | 95%CI | P value | Regression coefficients |
| MCP1 | 0.997248 | 0.995067-0.999434 | 0.014 | -0.0027555 |
| IL8 | 1.000094 | 1.000019-1.000170 | 0.014 | 0.0000944 |
| IL10 | 1.098966 | 1.009008-1.196944 | 0.030 | 0.0943694 |
| Constant | 0.529026 | 0.108222-2.586054 | 0.432 | -0.636717 |
After MDP stimulation it was also possible to discriminate between CD and UC based on the measure of G-CSF, TNFα, IL4 and IL17 (Table 4). According to the ROC analysis (Figure 7C), the AUC was 0.78 (95%CI: 0.65-0.91), and we could obtain a sensitivity on CD of 92.31% with a specificity of 50%.
| Variables | OR | 95%CI | P value | Regression coefficients |
| G-CSF | 0.999703 | 0.999484-0.999921 | 0.008 | -0.0002975 |
| TNFα | 0.990492 | 0.981420-0.999649 | 0.042 | -0.0095533 |
| IL4 | 1.155281 | 1.012918-1.317651 | 0.031 | 0.1443433 |
| IL17 | 1.009361 | 1.000308-1.018497 | 0.043 | 0.0093177 |
| Constant | 1.043247 | 0.333344-3.264992 | 0.924 | 0.042338 |
It is well known that subjects with active IBD display increased secretion of several inflammatory cytokines in the intestinal mucosa. For example, aberrant secretions of IL8 after challenge with E. coli or other intestinal bacteria were recorded by Edward and colleagues and considered as a marker of the mucosal imbalance typical of CD[21]. The authors, however, did not take into account the autochthonous colonization of the intestinal mucosa by the disease-associated microbiota. In the present work, we compared the cytokine profile of the intestinal mucosa in the presence or absence of antibiotics, to assess whether disease-associated microbiota can modulate the cytokine response ex vivo. We demonstrated that subjects with CD display a spontaneous production of inflammatory cytokines besides IL8, and including G-CSF, IL6, IL10 and IL12. The addition of antibiotics to the culture produced only a slight trend towards increased spontaneous cytokine secretion, both in CD and in UC, but no difference was significant, except for increased IL8 secretion in UC. These data may support the idea that antibiotics can influence UC, as suggested by other authors[22]. However, whilst some protocols that include metronidazole in vivo are shown to have protective effects on disease activity[22], other combinations had no effect[23]. Actually, it is well known that the risk of CD can be raised by previous use of antibiotics in childhood, but this is not believed to occur for UC[24]. Thus, the significance of increased levels of IL8 in UC after the addition of antibiotics remains without a clear explanation.
Even if the difference is not significant, there is a trend toward higher concentrations of inflammatory cytokines in UC if compared to CD. Actually, this is not surprising if we consider that we are sampling just superficial specimens where UC is expected to be more expressed than CD, which, on the contrary, may extend the inflammatory process throughout the whole intestinal wall[25].
Of note, in HC, the IL8 secretion with or without MDP is reduced in the presence of LB if compared to antibiotics. This effect is likely due to a modulatory effect of LB on mucosal immunity. In contrast, samples from UC showed a trend of increase of cytokines such as IL6 and IL8 when stimulated with MDP in the presence of LB. However, in the presence of antibiotics, stimulation with MDP was associated with decreased IL8 secretion. Thus, antibiotics alone seem to increase IL8 production in UC, either because of a loss of the protective effect of mucosal bacteria or for the release of pro-inflammatory bacterial compounds. Conversely, we observed a reduction of IL8 production in UC when MDP is used together with antibiotics. Even though there is no clear explanation for this phenomenon, we can argue that in this case antibiotics and MDP-induced peptides may synergize in controlling both protective and harmful bacteria[26].
We also showed that subjects with CD seem to have a lowered production of IL8 in response to MDP in the presence of LB. Although it is not clear whether defects in NOD2 signaling might play a role in this finding, these results support the idea that CD is not usually associated with a hyper-inflammatory response to MDP, i.e., it is not just an autoinflammatory disorder. Recent data suggest that inflammation in CD may be the result of compensatory responses to defective immunity[27].
Although it was not the primary aim of our work, we could demonstrate that culture with antibiotics is the only ex vivo condition in which it was possible to identify profiles of cytokine secretions able to discriminate between CD and UC with good sensitivity and specificity. Indeed, the combination of measures of G-CSF, TNFα, IL4 and IL17 was shown to identify CD with respect to UC with sensitivity and specificity respectively of 92% and 50%. Rather than being used to assist diagnosis, which relies only on clinical and histological features, these results highlight once more the importance of taking into account the presence of mucosal bacteria when dealing with ex vivo analyses of IBD.
Among the limitations of this study, we need to mention the small sample size. The decision not to apply corrections to the p values for significance for multiple univariate comparisons was a consequence of this constrain and it should be taken into account.
However, despite the small sample size, valid results have been obtained with this ex vivo mucosal culture, that allowed us to discern the two IBD forms.
In conclusion, we showed that CD and UC have distinct cytokine profiles in the intestinal mucosa and that the mucosal-associated microbiota can differentially impact on the inflammatory response in the two conditions.
Inflammatory bowel diseases (IBD) are associated with an unbalanced crosstalk between the mucosal immune system and the luminal content. Production of cytokines and antimicrobial-peptides in gut mucosa may contribute to the shaping of an altered intestinal microbiota and, conversely, microbes can translocate across the epithelium and induce an excessive inflammatory response and recruit adaptive immunity. Treatment of IBD with antibiotics, however, gave inconsistent results as different bacterial species may have protective or harmful effects.
Studies on the pathogenesis of IBD should involve the specific disease-associated microbiota together with the mucosal immunity, with its genetic background. Thus, ex vivo experiments that bring together these two factors are of particular importance to unravel the pathogenesis of IBD.
By reproducing four different conditions, we could dissect the effect of disease-associated live or antibiotic-inactivated bacteria on the mucosal response to muramyl dipeptide (MDP). We showed that live bacteria affect the response to MDP in different ways in Crohn’s disease (CD) and in ulcerative colitis (UC).
Ex vivo analyses of the inflammatory response in the presence of antibiotics and soluble stimuli may allow studying the action of different antibiotics in patients with CD or UC.
MDP represents a pro-inflammatory stimulus used to mimic the inflammatory activation.
Some strengths of the article include: an adequate sample to test the hypothesis, novel study looking at the autochthonous colonization of the intestinal mucosa by the disease-associated microbiota, and seemingly good methods to address the question. It also provides a novel method to identify CD and UC based off of specific cytokines markers.
| 1. | Vermeire S. Towards a novel molecular classification of IBD. Dig Dis. 2012;30:425-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Vermeire S, Van Assche G, Rutgeerts P. Classification of inflammatory bowel disease: the old and the new. Curr Opin Gastroenterol. 2012;28:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Nagalingam NA, Lynch SV. Role of the microbiota in inflammatory bowel diseases. Inflamm Bowel Dis. 2012;18:968-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 221] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 4. | Sanchez-Munoz F, Dominguez-Lopez A, Yamamoto-Furusho JK. Role of cytokines in inflammatory bowel disease. World J Gastroenterol. 2008;14:4280-4288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 440] [Cited by in RCA: 532] [Article Influence: 29.6] [Reference Citation Analysis (11)] |
| 5. | Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet. 2012;380:1590-1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1347] [Cited by in RCA: 1580] [Article Influence: 112.9] [Reference Citation Analysis (0)] |
| 6. | Geremia A, Biancheri P, Allan P, Corazza GR, Di Sabatino A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun Rev. 2014;13:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 743] [Article Influence: 57.2] [Reference Citation Analysis (2)] |
| 7. | Kleiner G, Zanin V, Monasta L, Crovella S, Caruso L, Milani D, Marcuzzi A. Pediatric patients with inflammatory bowel disease exhibit increased serum levels of proinflammatory cytokines and chemokines, but decreased circulating levels of macrophage inhibitory protein-1β, interleukin-2 and interleukin-17. Exp Ther Med. 2015;9:2047-2052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Strober W, Fuss IJ. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1756-1767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 887] [Cited by in RCA: 873] [Article Influence: 58.2] [Reference Citation Analysis (0)] |
| 9. | Manoharan I, Suryawanshi A, Hong Y, Ranganathan P, Shanmugam A, Ahmad S, Swafford D, Manicassamy B, Ramesh G, Koni PA. Homeostatic PPARα Signaling Limits Inflammatory Responses to Commensal Microbiota in the Intestine. J Immunol. 2016;196:4739-4749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 10. | Al Nabhani Z, Lepage P, Mauny P, Montcuquet N, Roy M, Le Roux K, Dussaillant M, Berrebi D, Hugot JP, Barreau F. Nod2 Deficiency Leads to a Specific and Transmissible Mucosa-associated Microbial Dysbiosis Which Is Independent of the Mucosal Barrier Defect. J Crohns Colitis. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | de Souza HS, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol. 2016;13:13-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1185] [Cited by in RCA: 1188] [Article Influence: 118.8] [Reference Citation Analysis (3)] |
| 12. | Bianco AM, Zanin V, Girardelli M, Magnolato A, Martelossi S, Tommasini A, Marcuzzi A, Crovella S. A common genetic background could explain early-onset Crohn’s disease. Med Hypotheses. 2012;78:520-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Notarangelo LD, Tommasini A. Defective and excessive immunities in pediatric diseases. Curr Pharm Des. 2012;18:5729-5734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Tontini GE, Vecchi M, Pastorelli L, Neurath MF, Neumann H. Differential diagnosis in inflammatory bowel disease colitis: state of the art and future perspectives. World J Gastroenterol. 2015;21:21-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 120] [Cited by in RCA: 155] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 15. | Hugot JP, Chamaillard M, Zouali H, Lesage S, Cézard JP, Belaiche J, Almer S, Tysk C, O’Morain CA, Gassull M. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411:599-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4223] [Cited by in RCA: 3932] [Article Influence: 157.3] [Reference Citation Analysis (0)] |
| 16. | Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001;411:603-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3555] [Cited by in RCA: 3498] [Article Influence: 139.9] [Reference Citation Analysis (1)] |
| 17. | Naser SA, Arce M, Khaja A, Fernandez M, Naser N, Elwasila S, Thanigachalam S. Role of ATG16L, NOD2 and IL23R in Crohn’s disease pathogenesis. World J Gastroenterol. 2012;18:412-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 62] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (1)] |
| 18. | Cho JH. The Nod2 gene in Crohn’s disease: implications for future research into the genetics and immunology of Crohn’s disease. Inflamm Bowel Dis. 2001;7:271-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Stappenbeck TS, Rioux JD, Mizoguchi A, Saitoh T, Huett A, Darfeuille-Michaud A, Wileman T, Mizushima N, Carding S, Akira S. Crohn disease: a current perspective on genetics, autophagy and immunity. Autophagy. 2011;7:355-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 44] [Reference Citation Analysis (0)] |
| 20. | Biswas A, Liu YJ, Hao L, Mizoguchi A, Salzman NH, Bevins CL, Kobayashi KS. Induction and rescue of Nod2-dependent Th1-driven granulomatous inflammation of the ileum. Proc Natl Acad Sci USA. 2010;107:14739-14744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 141] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 21. | Edwards LA, Lucas M, Edwards EA, Torrente F, Heuschkel RB, Klein NJ, Murch SH, Bajaj-Elliott M, Phillips AD. Aberrant response to commensal Bacteroides thetaiotaomicron in Crohn’s disease: an ex vivo human organ culture study. Inflamm Bowel Dis. 2011;17:1201-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Sato K, Chiba T, Ohkusa T. Serial changes of cytokines in active ulcerative colitis: effects of antibiotic combination therapy. Hepatogastroenterology. 2009;56:1016-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | Mantzaris GJ, Archavlis E, Christoforidis P, Kourtessas D, Amberiadis P, Florakis N, Petraki K, Spiliadi C, Triantafyllou G. A prospective randomized controlled trial of oral ciprofloxacin in acute ulcerative colitis. Am J Gastroenterol. 1997;92:454-456. [PubMed] |
| 24. | Ungaro R, Bernstein CN, Gearry R, Hviid A, Kolho KL, Kronman MP, Shaw S, Van Kruiningen H, Colombel JF, Atreja A. Antibiotics associated with increased risk of new-onset Crohn’s disease but not ulcerative colitis: a meta-analysis. Am J Gastroenterol. 2014;109:1728-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 270] [Article Influence: 22.5] [Reference Citation Analysis (1)] |
| 25. | Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066-2078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 2256] [Article Influence: 132.7] [Reference Citation Analysis (10)] |
| 26. | Reinoso Webb C, Koboziev I, Furr KL, Grisham MB. Protective and pro-inflammatory roles of intestinal bacteria. Pathophysiology. 2016;23:67-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 27. | Marks DJ, Rahman FZ, Sewell GW, Segal AW. Crohn’s disease: an immune deficiency state. Clin Rev Allergy Immunol. 2010;38:20-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Kojima N S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF