Published online Nov 28, 2016. doi: 10.3748/wjg.v22.i44.9744
Peer-review started: May 30, 2016
First decision: August 8, 2016
Revised: September 9, 2016
Accepted: October 10, 2016
Article in press: October 10, 2016
Published online: November 28, 2016
Processing time: 181 Days and 21.4 Hours
To evaluate the efficacy of peripheral blood concentrations of angiopoietins (Ang) as cirrhosis biomarkers of chronic hepatitis C (CHC).
Ang1 and Ang2 serum levels were measured by enzyme-linked immunosorbent assays (ELISA) in samples from 179 cirrhotic and non-cirrhotic CHC patients, classified according to the METAVIR system. Groups were compared by non-parametric Mann-Whitney U test. Subsequently, the association of peripheral concentrations of angiopoietins with the stage of fibrosis was analyzed using Spearman correlation test. Finally, the accuracy, sensitivity and specificity of circulating angiopoietins for cirrhosis diagnosis were determined by the study of the respective area under the curve of receiver operator characteristics (AUC-ROC).
Peripheral blood concentrations of Ang1 and Ang2 in CHC patients were significantly related to fibrosis. While Ang1 was decreased in cirrhotic subjects compared to non-cirrhotic (P < 0.0001), Ang2 was significantly increased as CHC progressed to the end stage of liver disease (P < 0.0001). Consequently, Ang2/Ang1 ratio was notably amplified and significantly correlated with fibrosis (P < 0.0001). Interestingly, the individual performance of each angiopoietin for the diagnosis of cirrhosis reached notable AUC-ROC values (above 0.7, both), but the Ang2/Ang1 ratio was much better (AUC-ROC = 0.810) and displayed outstanding values of sensitivity (71%), specificity (84%) and accuracy (82.1%) at the optimal cut-off (10.33). Furthermore, Ang2/Ang1 ratio improved the performance of many other previously described biomarkers or scores of liver cirrhosis in CHC.
Ang2/Ang1 ratio might constitute a useful tool for monitoring the progression of chronic liver disease towards cirrhosis and play an important role as therapeutic target.
Core tip: Chronic hepatitis C (CHC) is the leading cause of cirrhosis and hepatocellular carcinoma and monitoring of liver fibrosis is essential for the prognosis and treatment of these patients. Liver biopsy, the gold standard for fibrosis determination, is invasive and costly. Therefore, novel reliable non-invasive biomarkers are crucial for CHC management. Angiogenesis is closely related to the pathogenesis of the disease and angiopoietins play a relevant role in this process. Interestingly, this study confirms the valuable association of circulating angiopoitein-1 (Ang1) and angiopoitein-2 (Ang2) levels with CHC progression and reveals the outstanding role of Ang2/Ang1 ratio as potential non-invasive biomarker of cirrhosis.
- Citation: Hernández-Bartolomé A, López-Rodríguez R, Borque MJ, González-Moreno L, Real-Martínez Y, García-Buey L, Moreno-Otero R, Sanz-Cameno P. Angiopoietin-2/angiopoietin-1 as non-invasive biomarker of cirrhosis in chronic hepatitis C. World J Gastroenterol 2016; 22(44): 9744-9751
- URL: https://www.wjgnet.com/1007-9327/full/v22/i44/9744.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i44.9744
Chronic liver disease (CLD) caused by hepatitis C virus (HCV) is an important public health problem worldwide. Nowadays, the number of patients with HCV-related cirrhosis is increasing and at this stage of the disease serious complications, such as bleeding esophageal varices or hepatocellular carcinoma (HCC) development, can take place[1-4]. Although the new medication based on direct-acting antivirals (DAAs) is very efficient for chronic hepatitis C (CHC) treatment, the access of numerous patients to these novel therapies is difficult because of their elevated cost. In addition, the silent course of disease often leads to many undiagnosed subjects[4-6].
An important feature of CHC progression is the persistence of HCV in the liver, which perpetuates the inflammatory response and deregulates other repairing processes, leading to angiogenesis, fibrosis, cirrhosis and HCC. Liver fibrosis is characterized by the replacement of hepatocytes by extracellular matrix (ECM), particularly collagen and several extracellular matrix proteins whose organization in non-soluble complex polymers generates the architectural and functional disorganization of the liver[7-10]. Simultaneously, chronic liver injury leads to the development of abnormal intrahepatic vasculature in a fundamental attempt to reestablish the metabolic interchange between blood and the injured tissue[11-14]. Indeed, pathological angiogenesis has been reported in diverse CLD and in the context of different inflammatory, fibrotic, and ischemic conditions as well as in HCC[13,15-18].
Among the mechanisms that closely modulate the angiogenic process, the Angiopoietins/Tie2 system is considered to play a pivotal role during the late phase of angiogenesis and is responsible for the maturation of newly formed vascular structures[19-21]. The correct regulation of the tyrosine kinase Tie2 is essential for normal vascular development[22,23]. Angiopoitein-1 (Ang1) and angiopoitein-2 (Ang2) have similar affinity toward Tie2 but their effects are quite different and context dependent[24-26]. Interestingly, the balance between both angiopoietins is altered in several CLD diseases, with its highest manifestation in HCC[13,27].
The knowledge of the fibrosis stage and progression rate is crucial for prognosis and treatment of CHC patients[28], but it is quite difficult to achieve since liver biopsy, the unique clinically accepted tool to evaluate the advance of the disease, has many drawbacks such as its invasiveness and elevated cost[29-31]. Therefore, alternative strategies are being actively investigated in order to reduce or avoid the need of liver biopsies for the assessment of liver disease[32,33].
In this regard, the close relationship between liver fibrosis and pathological angiogenesis, together with the observed imbalance of angiopoietins levels in different CLD, pointed us to evaluate the usefulness of these angiogenic factors as non-invasive biomarkers of CHC progression[34,35]. Therefore, this study was designed to assess the levels of Ang-1 and Ang-2 in the serum of CHC patients with or without cirrhosis and to estimate their potential diagnostic value.
The study included 179 serum samples from CHC patients without human immunodeficiency virus (HIV), hepatitis B or other liver diseases who had undergone liver biopsy for clinical purposes and gave written informed consent for their experimental use.
The study protocol was approved by the Clinical Research Ethics Committee of Hospital Universitario de La Princesa and adhered to the rules of the Declaration of Helsinki. The diagnosis of CHC was confirmed by the presence of serum HCV-RNA assayed with the reverse transcription polymerase chain reaction (RT-PCR) method (Amplicor Roche Molecular System, Branchburg, NJ). The genotype of HCV was determined by reverse-hybridization line probe assay (INNO-LiPAHCV; Innogenetics, Zwijndreht, Belgium). Also, immediately prior to liver biopsy, a blood sample was taken from each patient to analyse routine biochemical and clinical parameters using standard methods.
Liver biopsy tissue was obtained from all patients by percutaneous needle extraction (HepafixH, B. Braun Melsungen AG, Melsungen, Germany) under ecographic control. All liver biopsy specimens were fixed in 5% buffered formalin and embedded in paraffin for routine anatomo-pathological examination. Liver fibrosis was staged as F0 to F4 according to the METAVIR classification system[36]. In order to simplify, 3 patients with F0 were included in the F1 group.
Concentrations of Ang1 and Ang2 were measured in serum samples from all patients with CHC obtained on the same day that they had undergone percutaneous liver biopsy. According to the manufacturer’s directions, levels of Ang1 and Ang2 in serum were evaluated using human enzyme-linked immunosorbent assay (ELISA) Kits (Quantikine: R&D Systems, Minneapolis, MN). Once the reaction was stopped, the absorbance of each well was determined using a microplate reader (BioRad). Concentrations of Ang1 and Ang2 were obtained from the standard curve. All assays were done by duplicate and the mean concentration was calculated.
All data were analyzed with SPSS version 16.0 software (SPSS Inc., Chicago, IL, United States) and expressed as median values or in percentages, except for age (median and range). Comparisons of Ang1 and Ang2 serum levels between groups of cirrhotic and non-cirrhotic patients were performed by non-parametric Mann-Whitney U test. The association of angiopoietins with liver fibrosis was analyzed by the Spearman correlation test. Two-tailed P values below 0.05 were considered statistically significant. Receiver operating characteristics (ROC) curves were applied to evaluate the diagnostic precision of angiopoietins and their ratio (Ang2/Ang1) to identify CHC patients with cirrhosis. In addition, different parameters of clinical relevance, such as sensitivity (Se), specificity (Sp), positive predictive value (PPV), negative predictive value (NPV), positive and negative likelihood ratio (LR), and accuracy (ACC), at different cut-off values (Youden Index - which corresponds to the maximum sum of sensitivity and specificity, 90% sensitivity or 90% specificity) were also determined. Statistic differences among different cirrhosis indices were calculated by De Long test (version 12.3.0.0, MedCalc, MariaKerke, Belgium).
Demographic and clinical characteristics of CHC patients are shown in Table 1. Compared with non-cirrhotic, patients with cirrhosis had lower levels of platelets and albumin but higher levels of bilirubin and transaminases [except alkaline phosphatase (ALP)]. No significant differences were found with regard to cholesterol, age, viral load or gender.
| Patients | Cirrhotic (n = 31) | Non-cirrhotic (n = 148) | P value |
| Sex (M/F) | 23/8 | 92/56 | 0.20 |
| Age (yr) | 48 (24-63) | 44 (22-67) | 0.23 |
| Viral load (× 105 IU/mL) | 5.0 (3.5-11.0) | 7.1 (1.8-13.0) | 0.68 |
| HCV genotype, n (%) | - | ||
| 1 | 27 (87.1) | 117 (79.1) | |
| Non-1 | 4 (12.9) | 31 (20.9) | |
| Stage of liver fibrosis, n (%) | - | ||
| F1 | - | 42 (28.4) | |
| F2 | - | 66 (44.6) | |
| F3 | - | 40 (27.0) | |
| F4 | 31 (100.0) | - | |
| AST (UI/L) | 99.0 (60.0-123.0) | 48.0 (33.0-65.0) | < 0.001 |
| ALT (UI/L) | 116.0 (87.0-161.0) | 74.0 (55.3-111.5) | < 0.001 |
| ALP (UI/L) | 116.0 (75.0-220.0) | 126.5 (77.5-164.8) | 0.39 |
| GGT (UI/L) | 91.0 (68.0-172.0) | 36.0 (23.0-65.8) | < 0.001 |
| INR | 1.1 (1.1-1.2) | 1.0 (0.9-1.1) | < 0.001 |
| Bilirubin (mg/dL) | 0.9 (0.7-1.1) | 0.6 (0.5-0.8) | < 0.001 |
| Platelet count (× 109/L) | 141.0 (120.0-167.0) | 204.0 (167.0-241.0) | < 0.001 |
| Cholesterol total (mg/dL) | 163.0 (141.0-180.0) | 171.0 (154.3-191.5) | 0.06 |
| Albumin (g/dL) | 4.2 (4.0-4.4) | 4.3 (4.2-4.6) | 0.03 |
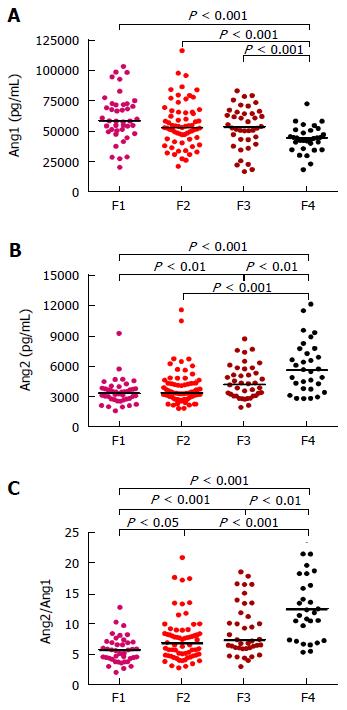
In order to measure serum concentrations of Ang1 and Ang2 in the peripheral blood of CHC patients ELISA assays were performed. Interestingly, the concentration of Ang1 decreased progressively in relation to the stage of liver fibrosis whereas Ang2 levels showed the opposite tendency (Figure 1). Furthermore, the concentration of Ang1 in the serum of cirrhotic patients was significantly lower when compared to the non-cirrhotic groups (P < 0.001); On the contrary, Ang2 serum levels were considerably higher in patients with cirrhosis (P < 0.01, Figure 1). Hence, differences among fibrosis stages were more evident for Ang2/Ang1 ratio, which was further able to significantly discriminate F > 1.
Consequently, Spearman correlation revealed an important association of circulating levels of angiopoietins with fibrosis stage, in accordance to the results shown above: while Ang1 levels were inversely related, Ang2 and Ang2/Ang1 ratio were directly associated (P < 0.0001, all) as Table 2 shows.
| n | Rho spearman coefficient | P value | |
| Ang1 | 179 | -0.297 | 5.25 × 10-5 |
| Ang2 | 179 | 0.402 | 2.37 × 10-9 |
| Ang2/Ang1 | 179 | 0.474 | 2.14 × 10-11 |
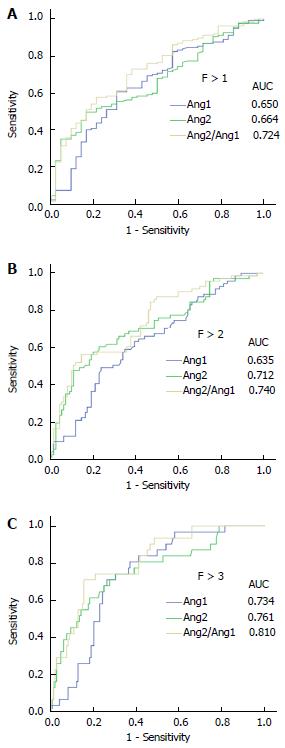
Subsequently, receiver operating curve analyses were performed to demonstrate the diagnostic validity of each individual angiopoietin or combined as a ratio to classify liver fibrosis in CHC. As shown in Figure 2, both angiopoietins had a high power to differentiate patients with F > 1, F > 2 or cirrhosis. Indeed, the area under the curve (AUC) of Ang1 was 0.734, with a sensitivity 70.97% and a specificity 73.65% at its optimal cut-off for cirrhosis staging; likewise, Ang2 had an AUC of 0.761 for diagnosing CHC patients with cirrhosis, with a sensitivity 74.19% and a specificity 69.59% at the value corresponding to Youden index. Importantly, Ang2/Ang1 ratio displayed the highest precision in discriminating cirrhotic patients (Figure 2 and Table 3) with an AUC of 0.810, a sensitivity of 70.97% and a specificity of 84.46% at the optimal cut-off (10.33). Furthermore, the simultaneous analysis of both angiopoietins as a ratio greatly improves other clinically relevant parameters, such as positive likelihood ratio and accuracy (4.57 and 82.1, respectively). Although other cut-offs were inspected in order to improve sensitivity or specificity to 90% (data not shown), optimal criterion (Youden index) displayed better clinical results (Table 3).
| AUC-ROC (95%CI) | Criterion | Se | 95%CI | Sp | 95%CI | +LR | 95% CI | -LR | 95%CI | +PV | 95%CI | -PV | 95%CI | Cost | ACC | |
| F > 1 | ||||||||||||||||
| Ang1 | 0.650 (0.575 -0.720) | ≤ 54337.63 | 60.58% | 51.9-68.8 | 69.05% | 52.9-82.4 | 1.96 | 1.2-3.1 | 0.57 | 0.4-0.8 | 64.40 | 52.9-74.7 | 65.5 | 55.2-74.8 | 0.35 | 65.00% |
| Ang2 | 0.664 (0.589-0.732) | > 4041.67 | 49.64% | 41.0-58.3 | 83.33% | 68.6-93.0 | 2.98 | 1.5-6.0 | 0.60 | 0.5-0.7 | 73.30 | 60.1-84.1 | 64.2 | 55.0-72.7 | 0.33 | 67.20% |
| Ang2/ Ang1 | 0.724 (0.652- 0.788) | > 7.162 | 57.66% | 48.9-66.1 | 78.57% | 63.2-89.7 | 2.69 | 1.5-4.9 | 0.54 | 0.4-0.7 | 71.30 | 59.2-81.5 | 66.8 | 57.1-75.5 | 0.32 | 68.50% |
| F > 2 | ||||||||||||||||
| Ang1 | 0.635 (0.560-0.705) | ≤ 47573.25 | 49.3% | 37.2-61.4 | 75.93% | 66.7-83.6 | 2.05 | 1.4-3.1 | 0.67 | 0.5-0.9 | 41.80 | 28.7-55.9 | 81.0 | 73.0-87.5 | 0.31 | 69.00% |
| Ang2 | 0.712 (0.639-0.777) | > 4256 | 60.56% | 48.3-72.0 | 76.85% | 67.8-84.4 | 2.62 | 1.8-3.9 | 0.51 | 0.4-0.7 | 47.90 | 34.7-61.3 | 84.7 | 77.0-90.6 | 0.27 | 72.60% |
| Ang2/ Ang1 | 0.740 (0.669-0.802) | > 9.262 | 56.34% | 44.0-68.1 | 85.19% | 77.1-91.3 | 3.80 | 2.3-6.2 | 0.51 | 0.4-0.7 | 57.20 | 41.7-71.7 | 84.7 | 77.5-90.4 | 0.22 | 77.70% |
| F > 3 | ||||||||||||||||
| Ang1 | 0.734 (0.663-0.797) | ≤ 47573.25 | 70.97% | 52.0-85.8 | 73.65% | 65.8-80.5 | 2.69 | 1.9-3.8 | 0.39 | 0.2-0.7 | 37.90 | 25.9-51.2 | 91.8 | 85.3-96.1 | 0.27 | 73.20% |
| Ang2 | 0.761 (0.691-0.821) | > 4256 | 74.19% | 55.4-88.1 | 69.59% | 61.5-76.9 | 2.44 | 1.8-3.4 | 0.37 | 0.2-0.7 | 35.60 | 24.5-48.1 | 92.2 | 85.6-96.5 | 0.30 | 70.40% |
| Ang2/ Ang1 | 0.810 (0.744- 0.864) | > 10.33 | 70.97% | 52.0-85.8 | 84.46% | 77.6-89.9 | 4.57 | 2.9-7.1 | 0.34 | 0.2-0.6 | 50.90 | 35.8-65.9 | 92.8 | 87.0-96.5 | 0.18 | 82.00% |
Finally, the efficacy of Ang2/Ang1 for cirrhosis staging was compared with other previously described non-invasive serum markers [aspartate aminotransferase to alanine aminotransferase ratio (AAR), aspartate aminotransferase-to-platelet ratio index (APRI), fibrosis index based on the four factors (FIB4), fibrosis index (FI) and fibrosis-cirrhosis index (FCI)]. As Table 4 shows, angiopoietins ratio performs better than AAR (P = 0.01) and similar to the other indices (P > 0.05).
| Indices | AUC-ROC (95%CI) | Standard error | P value |
| Cirrhosis | |||
| Ang2/Ang1 | 0.810 (0.744-0.864) | 0.040 | - |
| AAR | 0.643 (0.568-0.713) | 0.050 | 0.010 |
| APRI | 0.887 (0.831-0.930) | 0.025 | 0.106 |
| FIB4 | 0.858 (0.798-0.906) | 0.031 | 0.349 |
| FI | 0.805 (0.737-0.861) | 0.047 | 0.995 |
| FCI | 0.750 (0.677-0.813) | 0.053 | 0.372 |
CHC is a major cause of progressive liver disease, which often leads to cirrhosis and HCC[37]. Monitoring of liver fibrosis is crucial for the clinical management of patients but its precise determination is only possible by histological examination of liver biopsies[28]. Since vascular remodelling has repeatedly been observed during the evolution of diverse CLD[13,34,35,38], the levels of main related factors, such as angiopoietins, might help to evaluate the progression of these diseases. Previous evidences suggested the possible pathogenic role of the Angiopoietins/Tie-2 system on cirrhosis development, thus highlighting its potential to detect the degree of liver injury[34,35,38]. In this regard, some reports described the significant elevation of Ang2 serum levels in patients with liver cirrhosis[39]. Pauta et al[40] also reported higher levels of Ang2 in the systemic and suprahepatic circulation of cirrhotic patients with alcoholic liver disease and established the inverse correlation of Ang1/Ang2 with prognostic models of the disease. Accordingly, our data indicate that circulating levels of angiopoietins in CHC patients are notably related to fibrosis. Moreover, a significant direct association between Ang2/Ang1 ratio and liver cirrhosis has also been observed. These findings concur with those of Vespasiani-Gentilucci et al[38] who reported a close relationship between fibrosis stage and peripheral levels of Ang1 and Ang2. Therefore, all these data highlight the useful role of these angiogenic factors as non-invasive markers of CHC progression. Furthermore, although ROC analysis revealed high accuracy of both, Ang1 and Ang2, (AUC-ROC > 0.7) to identify cirrhosis, Ang2/Ang1 ratio displayed the highest value of AUC-ROC (0.810) and showed valuable sensitivity and specificity for the diagnosis of cirrhosis.
In addition, it must be pointed out that Ang2/Ang1 ratio displays similar or superior precision than other proposed tests (AAR, APRI, FIB4, FI, and FCI). In spite the initial outstanding performance of recently defined index, enhanced liver fibrosis (ELF), which combines several variables (hyaluronic acid, tissue inhibitor of matrix metalloproteinases-1, and amino-terminal propeptide of type III procollagen[41], the limited sample size of cirrhotic patients in that cohort (n = 29) could lead to overestimate its diagnostic potential[42]. Indeed, angiopoietins ratio displays better AUC-ROCs for cirrhosis identification when ELF is analyzed in a larger cohort of patients (0.81 vs 0.78). Finally, it must be noted that Ang2/Ang1 is also simplier and cheaper than other costly and undisclosed procedures such as FibroTest[43,44].
Taken together, these findings suggest that Ang2/Ang1 ratio might constitute a useful minimally invasive indicator of cirrhosis in CHC patients, which could notably help clinical decision-making during patient follow-up. However, the application of this novel biomarker in clinical practice might benefit from further validation.
The authors wish to thank Manuel Gómez Gutiérrez, PhD, for editing the manuscript and each of patients who generously consented to participate.
Hepatitis C virus (HCV) infection often progresses to chronic hepatitis C (CHC), one of the leading causes of cirrhosis and hepatocellular carcinoma (HCC). Angiogenesis is closely related to the pathogenesis of chronic liver disease and its progression to HCC.
Several biomarkers have been investigated to predict liver cirrhosis in patients with CHC; however, few studies have evaluated the usefulness of angiogenic factors to identify cirrhosis in these patients despite angiogenesis often concurs with liver fibrosis.
This study shows that the peripheral value of angiopoietin-2 (Ang2)/angiopoitein-1 (Ang1) was significantly associated with liver fibrosis in patients with CHC, highlighting its potential as novel biomarker for the non-invasive diagnosis of liver fibrosis.
The laudable discriminatory accuracy displayed by Ang2/Ang1 for fibrosis staging might replace other complex and expensive test for monitoring CHC progression.
The unbalance between Ang1 and Ang2 is present in many tumors such as HCC as well as in diverse chronic liver diseases underlining their potential pathogenic role and their impact as targets for therapeutic intervention.
This is a study regarding the role of Ang2/Ang1 ratio as a non-invasive biomarker of fibrosis in CHC. Overall, the manuscript was well-written and all tables and figures were appropriate. The main findings about Ang2/Ang1 ratio was quite novel. The idea of assessment of Ang2/Ang1 ratio as a noninvasive biomarker in cirrhosis in chronic hepatitis C is quite interesting.
| 1. | Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335-1374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2320] [Cited by in RCA: 2245] [Article Influence: 132.1] [Reference Citation Analysis (2)] |
| 2. | European Association for Study of Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2014;60:392-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 658] [Article Influence: 54.8] [Reference Citation Analysis (2)] |
| 3. | Poynard T, Bedossa P. Age and platelet count: a simple index for predicting the presence of histological lesions in patients with antibodies to hepatitis C virus. METAVIR and CLINIVIR Cooperative Study Groups. J Viral Hepat. 1997;4:199-208. [PubMed] |
| 4. | Seeff LB. The history of the “natural history” of hepatitis C (1968-2009). Liver Int. 2009;29 Suppl 1:89-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 172] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 5. | Bertino G, Ardiri A, Proiti M, Rigano G, Frazzetto E, Demma S, Ruggeri MI, Scuderi L, Malaguarnera G, Bertino N. Chronic hepatitis C: This and the new era of treatment. World J Hepatol. 2016;8:92-106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 6. | Chopp S, Vanderwall R, Hult A, Klepser M. Simeprevir and sofosbuvir for treatment of hepatitis C infection. Am J Health Syst Pharm. 2015;72:1445-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Gremion C, Cerny A. Hepatitis C virus and the immune system: a concise review. Rev Med Virol. 2005;15:235-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Blackard JT, Shata MT, Shire NJ, Sherman KE. Acute hepatitis C virus infection: a chronic problem. Hepatology. 2008;47:321-331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Rehermann B. Hepatitis C virus vs innate and adaptive immune responses: a tale of coevolution and coexistence. J Clin Invest. 2009;119:1745-1754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 422] [Article Influence: 24.8] [Reference Citation Analysis (1)] |
| 10. | Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655-1669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2139] [Cited by in RCA: 2191] [Article Influence: 121.7] [Reference Citation Analysis (1)] |
| 11. | Cannito S, Paternostro C, Busletta C, Bocca C, Colombatto S, Miglietta A, Novo E, Parola M. Hypoxia, hypoxia-inducible factors and fibrogenesis in chronic liver diseases. Histol Histopathol. 2014;29:33-44. [PubMed] |
| 12. | Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3533] [Cited by in RCA: 3485] [Article Influence: 193.6] [Reference Citation Analysis (0)] |
| 13. | Paternostro C, David E, Novo E, Parola M. Hypoxia, angiogenesis and liver fibrogenesis in the progression of chronic liver diseases. World J Gastroenterol. 2010;16:281-288. [PubMed] |
| 14. | Pinzani M, Rombouts K, Colagrande S. Fibrosis in chronic liver diseases: diagnosis and management. J Hepatol. 2005;42 Suppl:S22-S36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 192] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 15. | Gabriel A, Kukla M, Wilk M, Liszka Ł, Petelenz M, Musialik J. Angiogenesis in chronic hepatitis C is associated with inflammatory activity grade and fibrosis stage. Pathol Res Pract. 2009;205:758-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Chaparro M, Sanz-Cameno P, Trapero-Marugan M, Garcia-Buey L, Moreno-Otero R. Mechanisms of angiogenesis in chronic inflammatory liver disease. Ann Hepatol. 2007;6:208-213. [PubMed] |
| 17. | Medina J, Sanz-Cameno P, García-Buey L, Martín-Vílchez S, López-Cabrera M, Moreno-Otero R. Evidence of angiogenesis in primary biliary cirrhosis: an immunohistochemical descriptive study. J Hepatol. 2005;42:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Valfrè di Bonzo L, Novo E, Cannito S, Busletta C, Paternostro C, Povero D, Parola M. Angiogenesis and liver fibrogenesis. Histol Histopathol. 2009;24:1323-1341. [PubMed] |
| 19. | Thabut D, Shah V. Intrahepatic angiogenesis and sinusoidal remodeling in chronic liver disease: new targets for the treatment of portal hypertension? J Hepatol. 2010;53:976-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 223] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 20. | Jones N, Chen SH, Sturk C, Master Z, Tran J, Kerbel RS, Dumont DJ. A unique autophosphorylation site on Tie2/Tek mediates Dok-R phosphotyrosine binding domain binding and function. Mol Cell Biol. 2003;23:2658-2668. [PubMed] |
| 21. | Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009;10:165-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 995] [Cited by in RCA: 1098] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 22. | London NR, Smith MC, Li DY. Emerging mechanisms of vascular stabilization. J Thromb Haemost. 2009;7 Suppl 1:57-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Fiedler U, Augustin HG. Angiopoietins: a link between angiogenesis and inflammation. Trends Immunol. 2006;27:552-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 461] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 24. | Korff T, Ernst E, Nobiling R, Feldner A, Reiss Y, Plate KH, Fiedler U, Augustin HG, Hecker M. Angiopoietin-1 mediates inhibition of hypertension-induced release of angiopoietin-2 from endothelial cells. Cardiovasc Res. 2012;94:510-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Fiedler U, Reiss Y, Scharpfenecker M, Grunow V, Koidl S, Thurston G, Gale NW, Witzenrath M, Rosseau S, Suttorp N. Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat Med. 2006;12:235-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 656] [Cited by in RCA: 748] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 26. | Scharpfenecker M, Fiedler U, Reiss Y, Augustin HG. The Tie-2 ligand angiopoietin-2 destabilizes quiescent endothelium through an internal autocrine loop mechanism. J Cell Sci. 2005;118:771-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 300] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 27. | Diaz-Sanchez A, Matilla A, Nuñez O, Lorente R, Fernandez A, Rincón D, Campos R, Bañares R, Clemente G. Serum angiopoietin-2 level as a predictor of tumor invasiveness in patients with hepatocellular carcinoma. Scand J Gastroenterol. 2013;48:334-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. Liver biopsy. Hepatology. 2009;49:1017-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1449] [Cited by in RCA: 1635] [Article Influence: 96.2] [Reference Citation Analysis (2)] |
| 29. | Muir AJ, Trotter JF. A survey of current liver biopsy practice patterns. J Clin Gastroenterol. 2002;35:86-88. [PubMed] |
| 30. | Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1193] [Cited by in RCA: 1408] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 31. | Guido M, Rugge M. Liver biopsy sampling in chronic viral hepatitis. Semin Liver Dis. 2004;24:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 104] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 32. | Manning DS, Afdhal NH. Diagnosis and quantitation of fibrosis. Gastroenterology. 2008;134:1670-1681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 310] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 33. | Zhou K, Lu LG. Assessment of fibrosis in chronic liver diseases. J Dig Dis. 2009;10:7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Hernández-Bartolomé A, López-Rodríguez R, Rodríguez-Muñoz Y, Martín-Vílchez S, Borque MJ, García-Buey L, González-Moreno L, Real Y, Moreno-Otero R, Sanz-Cameno P. Angiopoietin-2 Serum Levels Improve Noninvasive Fibrosis Staging in Chronic Hepatitis C: A Fibrogenic-Angiogenic Link. PLoS One. 2013;8:e66143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Salcedo X, Medina J, Sanz-Cameno P, García-Buey L, Martín-Vilchez S, Borque MJ, López-Cabrera M, Moreno-Otero R. The potential of angiogenesis soluble markers in chronic hepatitis C. Hepatology. 2005;42:696-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 36. | Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2860] [Cited by in RCA: 3129] [Article Influence: 104.3] [Reference Citation Analysis (1)] |
| 37. | Fattovich G, Llovet JM. Risk factors for hepatocellular carcinoma in HCV-cirrhosis: what we know and what is missing. J Hepatol. 2006;44:1013-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (2)] |
| 38. | Vespasiani-Gentilucci U, Galati G, Mazzarelli C, D’Avola D, Spataro S, Gallo P, Rigon A, Pellicelli A, Dicuonzo G, Afeltra A. Angiogenic cytokines in patients undergoing antiviral treatment for chronic hepatitis C virus infection. J Interferon Cytokine Res. 2011;31:207-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 39. | Scholz A, Rehm VA, Rieke S, Derkow K, Schulz P, Neumann K, Koch I, Pascu M, Wiedenmann B, Berg T. Angiopoietin-2 serum levels are elevated in patients with liver cirrhosis and hepatocellular carcinoma. Am J Gastroenterol. 2007;102:2471-2481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 40. | Pauta M, Ribera J, Melgar-Lesmes P, Casals G, Rodríguez-Vita J, Reichenbach V, Fernandez-Varo G, Morales-Romero B, Bataller R, Michelena J. Overexpression of angiopoietin-2 in rats and patients with liver fibrosis. Therapeutic consequences of its inhibition. Liver Int. 2015;35:1383-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 41. | Lichtinghagen R, Pietsch D, Bantel H, Manns MP, Brand K, Bahr MJ. The Enhanced Liver Fibrosis (ELF) score: normal values, influence factors and proposed cut-off values. J Hepatol. 2013;59:236-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 277] [Article Influence: 21.3] [Reference Citation Analysis (10)] |
| 42. | Fernandes FF, Ferraz ML, Andrade LE, Dellavance A, Terra C, Pereira G, Pereira JL, Campos F, Figueiredo F, Perez RM. Enhanced liver fibrosis panel as a predictor of liver fibrosis in chronic hepatitis C patients. J Clin Gastroenterol. 2015;49:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 43. | Shaheen AA, Wan AF, Myers RP. FibroTest and FibroScan for the prediction of hepatitis C-related fibrosis: a systematic review of diagnostic test accuracy. Am J Gastroenterol. 2007;102:2589-2600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 257] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 44. | Baranova A, Lal P, Birerdinc A, Younossi ZM. Non-invasive markers for hepatic fibrosis. BMC Gastroenterol. 2011;11:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 212] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Bunchorntavakul C, Kohla MAS, Komolmit P, Lee HC S- Editor: Yu J L- Editor: A E- Editor: Wang CH